Abstract
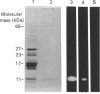
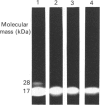
The calcium-dependent regulatory protein calmodulin is a critical element in the machinery regulating exocytosis at nerve terminals. Okabe & Sobue [(1987) FEBS Lett. 213, 184-188] showed that calmodulin interacts with one of the proteins intimately connected with the neuronal exocytotic process, i.e. synapsin 1. We have investigated the site at which calmodulin interacts with synapsin 1. We find that it is possible to generate chemically cross-linked Ca2(+)-dependent complexes between synapsin 1 and calmodulin in vitro, and have used covalent cross-linking in conjunction with calmodulin affinity chromatography to identify fragments of synapsin 1 that interact with calmodulin. Ca2(+)-dependent calmodulin binding is restricted to the 'head' domain (residues 1-453 in bovine synapsin 1). Within this domain the binding site is located in a unique 11 kDa Staphylococcus aureus V8 proteinase generated fragment. This fragment does not contain the site for cyclic-AMP-dependent phosphorylation and therefore does not represent the N-terminus of the protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Gardner K., Bennett V. Association between human erythrocyte calmodulin and the cytoplasmic surface of human erythrocyte membranes. J Biol Chem. 1983 May 25;258(10):6258–6265. [PubMed] [Google Scholar]
- Andreasen T. J., Keller C. H., LaPorte D. C., Edelman A. M., Storm D. R. Preparation of azidocalmodulin: a photoaffinity label for calmodulin-binding proteins. Proc Natl Acad Sci U S A. 1981 May;78(5):2782–2785. doi: 10.1073/pnas.78.5.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert-Foucher E., Font B. Limited proteolysis of synapsin I. Identification of the region of the molecule responsible for its association with microtubules. Biochemistry. 1990 Jun 5;29(22):5351–5357. doi: 10.1021/bi00474a021. [DOI] [PubMed] [Google Scholar]
- Baines A. J. Synapsin I and the cytoskeleton. Nature. 1987 Apr 16;326(6114):646–646. doi: 10.1038/326646a0. [DOI] [PubMed] [Google Scholar]
- Benfenati F., Greengard P., Brunner J., Bähler M. Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers. J Cell Biol. 1989 May;108(5):1851–1862. doi: 10.1083/jcb.108.5.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Kennedy M. B. Deduced primary structure of the beta subunit of brain type II Ca2+/calmodulin-dependent protein kinase determined by molecular cloning. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1794–1798. doi: 10.1073/pnas.84.7.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Baines A. J., Davis J. Purification of brain analogs of red blood cell membrane skeletal proteins: ankyrin, protein 4.1 (synapsin), spectrin, and spectrin subunits. Methods Enzymol. 1986;134:55–69. doi: 10.1016/0076-6879(86)34075-8. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Geisow M. J., Barron J. Dissection of stages in exocytosis in the adrenal chromaffin cell with use of trifluoperazine. Proc R Soc Lond B Biol Sci. 1982 Aug 23;216(1202):111–115. doi: 10.1098/rspb.1982.0064. [DOI] [PubMed] [Google Scholar]
- Burns N. R., Gratzer W. B. Interaction of calmodulin with the red cell and its membrane skeleton and with spectrin. Biochemistry. 1985 Jun 4;24(12):3070–3074. doi: 10.1021/bi00333a040. [DOI] [PubMed] [Google Scholar]
- Bähler M., Benfenati F., Valtorta F., Czernik A. J., Greengard P. Characterization of synapsin I fragments produced by cysteine-specific cleavage: a study of their interactions with F-actin. J Cell Biol. 1989 May;108(5):1841–1849. doi: 10.1083/jcb.108.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernik A. J., Pang D. T., Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Nemeth E. F. On the calcium receptor activating exocytosis: inhibitory effects of calmodulin-interacting drugs on rat mast cells. J Physiol. 1982 Feb;323:229–244. doi: 10.1113/jphysiol.1982.sp014070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R. Protease from Staphyloccus aureus. Methods Enzymol. 1976;45:469–475. doi: 10.1016/s0076-6879(76)45041-3. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V. Calmodulin-binding proteins from brain and other tissues. Biochem J. 1979 Nov 1;183(2):285–295. doi: 10.1042/bj1830285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Sobue K., Kanda K., Harada A., Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989 Jan;108(1):111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper J. E., Kelly R. B. Calmodulin is tightly associated with synaptic vesicles independent of calcium. J Biol Chem. 1984 Jan 10;259(1):148–153. [PubMed] [Google Scholar]
- Husain A., Howlett G. J., Sawyer W. H. Analysis of the calcium-dependent interaction of calmodulin with bovine serum albumin. Anal Biochem. 1985 Mar;145(2):217–221. doi: 10.1016/0003-2697(85)90352-5. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Purification of the Ca2+-stimulated ATPase activator from human erythrocytes. Its membership in the class of Ca2+-binding modulator proteins. J Biol Chem. 1978 Jul 10;253(13):4676–4682. [PubMed] [Google Scholar]
- Kenigsberg R. L., Côté A., Trifaró J. M. Trifluoperazine, a calmodulin inhibitor, blocks secretion in cultured chromaffin cells at a step distal from calcium entry. Neuroscience. 1982;7(9):2277–2286. doi: 10.1016/0306-4522(82)90138-5. [DOI] [PubMed] [Google Scholar]
- Kenigsberg R. L., Trifaró J. M. Microinjection of calmodulin antibodies into cultured chromaffin cells blocks catecholamine release in response to stimulation. Neuroscience. 1985 Jan;14(1):335–347. doi: 10.1016/0306-4522(85)90183-6. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Toscano W. A., Jr, Storm D. R. Cross-linking of iodine-125-labeled, calcium-dependent regulatory protein to the Ca2+-sensitive phosphodiesterase purified from bovine heart. Biochemistry. 1979 Jun 26;18(13):2820–2825. doi: 10.1021/bi00580a021. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momayezi M., Lumpert C. J., Kersken H., Gras U., Plattner H., Krinks M. H., Klee C. B. Exocytosis induction in Paramecium tetraurelia cells by exogenous phosphoprotein phosphatase in vivo and in vitro: possible involvement of calcineurin in exocytotic membrane fusion. J Cell Biol. 1987 Jul;105(1):181–189. doi: 10.1083/jcb.105.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R. A., Sihra T. S., Czernik A. J., Nairn A. C., Greengard P. Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature. 1990 Feb 15;343(6259):647–651. doi: 10.1038/343647a0. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Okabe T., Sobue K. Identification of a new 84/82 kDa calmodulin-binding protein, which also interacts with actin filaments, tubulin and spectrin, as synapsin I. FEBS Lett. 1987 Mar 9;213(1):184–188. doi: 10.1016/0014-5793(87)81488-6. [DOI] [PubMed] [Google Scholar]
- Ouimet C. C., McGuinness T. L., Greengard P. Immunocytochemical localization of calcium/calmodulin-dependent protein kinase II in rat brain. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5604–5608. doi: 10.1073/pnas.81.17.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin D., Langley O. K., Aunis D. Anti-alpha-fodrin inhibits secretion from permeabilized chromaffin cells. Nature. 1987 Apr 2;326(6112):498–501. doi: 10.1038/326498a0. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Czernik A. J., Kao H. T., Takei K., Johnston P. A., Horiuchi A., Kanazir S. D., Wagner M. A., Perin M. S., De Camilli P. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989 Sep 29;245(4925):1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M., Fournier S., Novas M. L. The p65 protein is a calmodulin-binding protein present in several types of secretory vesicles. Neuroscience. 1989;29(1):1–8. doi: 10.1016/0306-4522(89)90327-8. [DOI] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]