Abstract
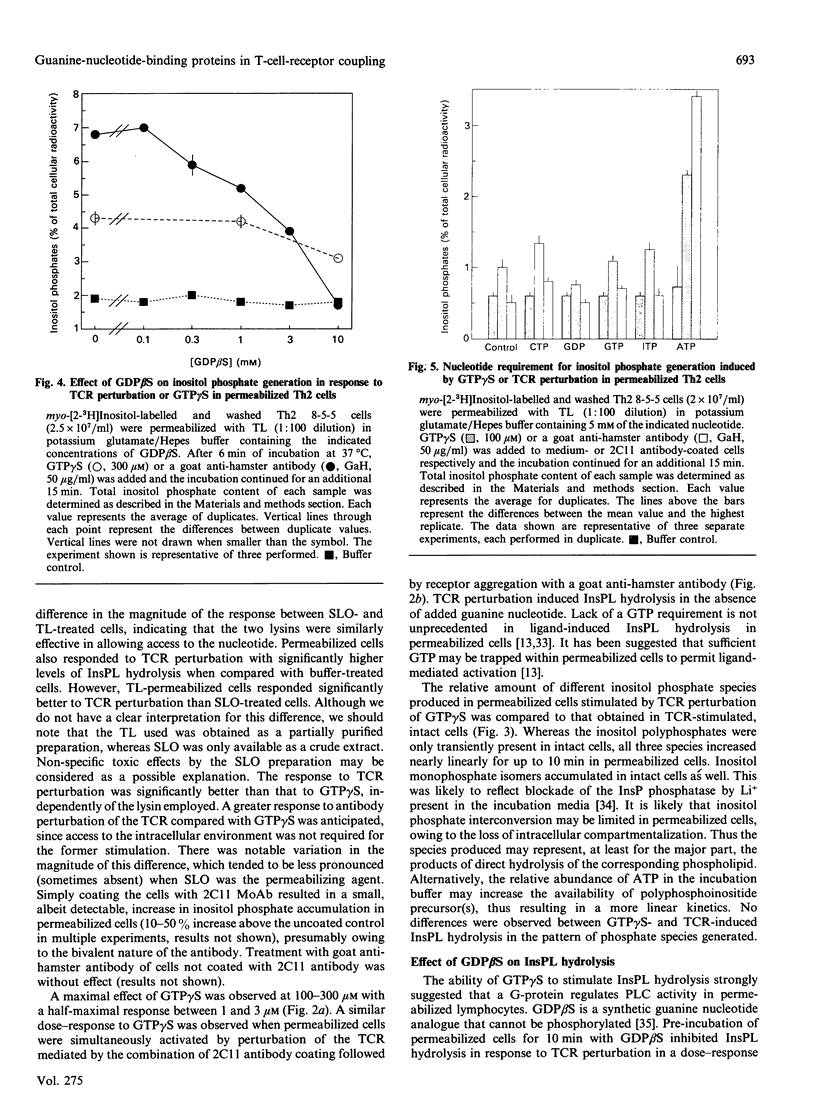
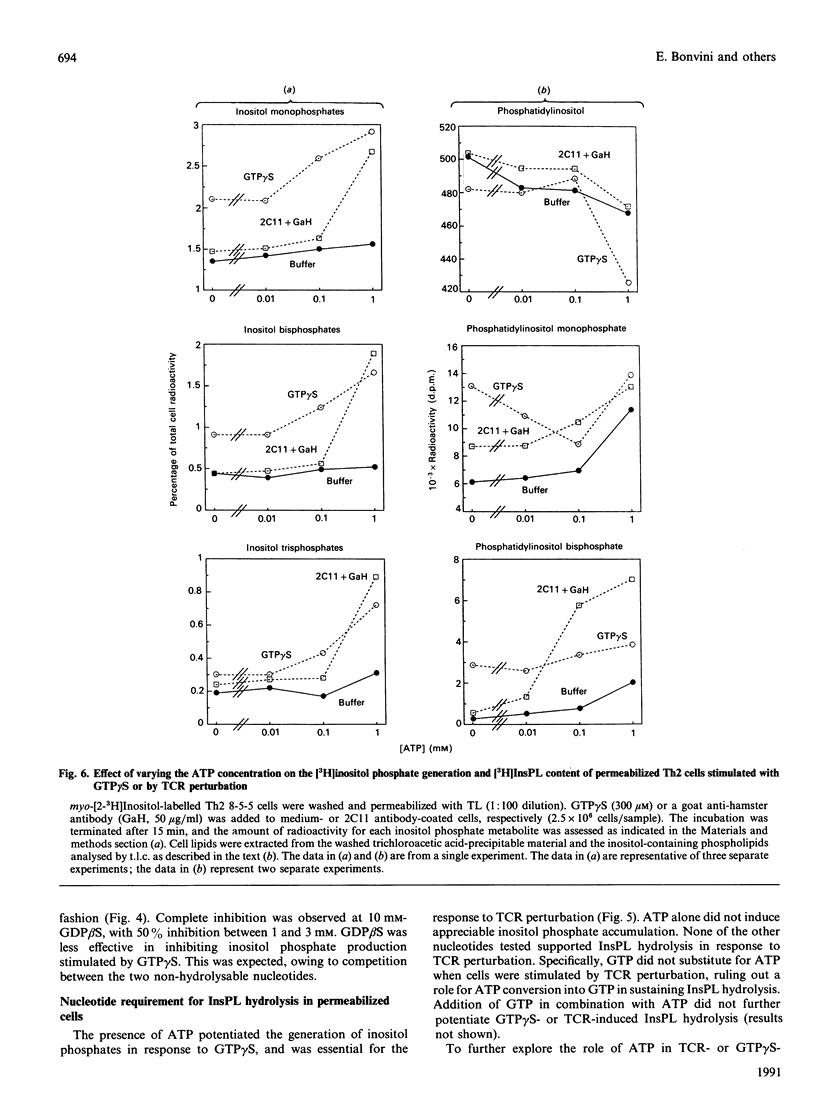
Perturbation of the T-cell receptor (TCR) complex is followed by the rapid hydrolysis of inositol phospholipids (InsPL) by phospholipase C (PLC), producing diacylglycerol and inositol phosphates, which act as second messengers in signal transduction. The mechanism coupling the TCR to InsPL hydrolysis is not clearly defined, and no information is available on this mechanism in the CD4+ helper subset of T-lymphocytes (Th). We have tested the hypothesis that guanine-nucleotide-binding proteins (G-proteins) may couple the TCR to PLC in a murine Th type II (Th2) cell clone. Cell permeabilization with streptolysin O (SLO) or tetanolysin (TL) was used to allow membrane-impermeable nucleotides access to intracellular sites of action. Exposure of permeabilized Th2 cells to guanosine 5'-[gamma-thio]triphosphate (GTP gamma S), a non-hydrolysable GTP analogue, resulted in a 2.1-2.5-fold increase in inositol phosphate generation. Similarly, perturbation of the TCR with the monoclonal antibody 145.2C11 (directed against the epsilon-chain of the CD3 component of the TCR) resulted in a 3.1-4.2-fold increase in InsPL hydrolysis by permeabilized cells. Both lysins were similarly effective in allowing GTP gamma S induction of InsPL hydrolysis, but TL-permeabilized cells responded better to TCR perturbation than SLO-treated cells. A role for G-proteins in TCR coupling to PLC was further supported by the inhibition of TCR-induced InsPL hydrolysis by guanosine 5'-[beta-thio]diphosphate (GDP beta S), a guanine nucleotide analogue that inhibits G-protein function. ATP was required for TCR-mediated InsPL hydrolysis, and potentiated GTP gamma S-induced hydrolysis. Other nucleotides (i.e. CTP, GDP, GTP, ITP) did not affect the response. These data indicate that G-proteins may contribute to the regulation of PLC activation in Th2 cells, coupling it to the TCR.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Hodes R. J. T cell regulation of b cell activation. Cloned Lyt-1+2-T suppressor cells inhibit the major histocompatibility complex-restricted interaction of T helper cells with B cells and/or accessory cells. J Exp Med. 1983 Oct 1;158(4):1178–1190. doi: 10.1084/jem.158.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aussel C., Mary D., Peyron J. F., Pelassy C., Ferrua B., Fehlmann M. Inhibition and activation of interleukin 2 synthesis by direct modification of guanosine triphosphate-binding proteins. J Immunol. 1988 Jan 1;140(1):215–220. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best L. A role for calcium in the breakdown of inositol phospholipids in intact and digitonin-permeabilized pancreatic islets. Biochem J. 1986 Sep 15;238(3):773–779. doi: 10.1042/bj2380773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvini E., DeBell K. E., Kolber M. A., Hoffman T., Hodes R. J., Taplits M. S. Hydrolysis of inositol phospholipids induced by stimulation of the T cell antigen receptor complex in antigen-specific, murine helper T cell clones. Requirement for exogenous calcium. J Immunol. 1989 Jul 15;143(2):587–595. [PubMed] [Google Scholar]
- Brando C., Hoffman T., Bonvini E. High-performance liquid chromatographic separation of inositol phosphate isomers employing a reversed-phase column and a micellar mobile phase. J Chromatogr. 1990 Jul 13;529(1):65–80. doi: 10.1016/s0378-4347(00)83808-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987 Jan 15;241(2):409–414. doi: 10.1042/bj2410409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Joels L. A., Hughes P. J. The interaction of lithium ions with inositol lipid signalling systems. Biochem Soc Trans. 1987 Feb;15(1):32–35. doi: 10.1042/bst0150032. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gardner S. D., Milligan G., Rice J. E., Wakelam M. J. The effect of cholera toxin on the inhibition of vasopressin-stimulated inositol phospholipid hydrolysis is a cyclic AMP-mediated event at the level of receptor binding. Biochem J. 1989 May 1;259(3):679–684. doi: 10.1042/bj2590679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore W., Weiner L. P. The effects of pertussis toxin and cholera toxin on mitogen-induced interleukin-2 production: evidence for G protein involvement in signal transduction. Cell Immunol. 1988 May;113(2):235–250. doi: 10.1016/0008-8749(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Goldman D. W., Chang F. H., Gifford L. A., Goetzl E. J., Bourne H. R. Pertussis toxin inhibition of chemotactic factor-induced calcium mobilization and function in human polymorphonuclear leukocytes. J Exp Med. 1985 Jul 1;162(1):145–156. doi: 10.1084/jem.162.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves J. D., Lucas S. C., Alexander D. R., Cantrell D. A. Guanine nucleotide regulation of inositol phospholipid hydrolysis and CD3-antigen phosphorylation in permeabilized T lymphocytes. Biochem J. 1990 Jan 15;265(2):407–413. doi: 10.1042/bj2650407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Hasegawa-Sasaki H., Lutz F., Sasaki T. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of phosphatidylinositol 4,5-bisphosphate in a human T cell line. Microbiol Immunol. 1988;32(3):293–304. doi: 10.1111/j.1348-0421.1988.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Protein kinase C and calcium ion in mitogenic response of macrophage-depleted human peripheral lymphocytes. J Biol Chem. 1985 Feb 10;260(3):1366–1369. [PubMed] [Google Scholar]
- Kammer G. M., Boehm C. A., Rudolph S. A., Schultz L. A. Mobility of the human T lymphocyte surface molecules CD3, CD4, and CD8: regulation by a cAMP-dependent pathway. Proc Natl Acad Sci U S A. 1988 Feb;85(3):792–796. doi: 10.1073/pnas.85.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Parsons M., Martin P. J., Hansen J. A., Rabinovitch P. S., June C. H. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J Immunol. 1986 Nov 15;137(10):3299–3305. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A., Jacobson B., Miller R. A. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol. 1988 Feb 1;140(3):936–940. [PubMed] [Google Scholar]
- Merritt J. E., Taylor C. W., Rubin R. P., Putney J. W., Jr Evidence suggesting that a novel guanine nucleotide regulatory protein couples receptors to phospholipase C in exocrine pancreas. Biochem J. 1986 Jun 1;236(2):337–343. doi: 10.1042/bj2360337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire-Sluis A. R., Hoffbrand A. V., Wickremasinghe R. G. Evidence that guanine-nucleotide binding regulatory proteins couple cell-surface receptors to the breakdown of inositol-containing lipids during T-lymphocyte mitogenesis. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1223–1231. doi: 10.1016/s0006-291x(87)80263-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Mustelin T. GTP dependence of the transduction of mitogenic signals through the T3 complex in T lymphocytes indicates the involvement of a G-protein. FEBS Lett. 1987 Mar 9;213(1):199–203. doi: 10.1016/0014-5793(87)81491-6. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Pösö H., Andersson L. C. Role of G-proteins in T cell activation: non-hydrolysable GTP analogues induce early ornithine decarboxylase activity in human T lymphocytes. EMBO J. 1986 Dec 1;5(12):3287–3290. doi: 10.1002/j.1460-2075.1986.tb04641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Rhee S. G., Carpenter G. Tyrosine phosphorylation of phospholipase C-II in vitro by the epidermal growth factor receptor. J Biol Chem. 1989 Jun 25;264(18):10335–10338. [PubMed] [Google Scholar]
- O'Shea J. J., Urdahl K. B., Luong H. T., Chused T. M., Samelson L. E., Klausner R. D. Aluminum fluoride induces phosphatidylinositol turnover, elevation of cytoplasmic free calcium, and phosphorylation of the T cell antigen receptor in murine T cells. J Immunol. 1987 Nov 15;139(10):3463–3469. [PubMed] [Google Scholar]
- Rottem S., Cole R. M., Habig W. H., Barile M. F., Hardegree M. C. Structural characteristics of tetanolysin and its binding to lipid vesicles. J Bacteriol. 1982 Nov;152(2):888–892. doi: 10.1128/jb.152.2.888-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S. H., Cho K. S., Lee K. Y., Suh P. G., Rhee S. G. Purification and characterization of two immunologically distinct phosphoinositide-specific phospholipases C from bovine brain. J Biol Chem. 1987 Sep 15;262(26):12511–12518. [PubMed] [Google Scholar]
- Samelson L. E., O'Shea J. J., Luong H., Ross P., Urdahl K. B., Klausner R. D., Bluestone J. T cell antigen receptor phosphorylation induced by an anti-receptor antibody. J Immunol. 1987 Oct 15;139(8):2708–2714. [PubMed] [Google Scholar]
- Schrezenmeier H., Ahnert-Hilger G., Fleischer B. A T cell receptor-associated GTP-binding protein triggers T cell receptor-mediated granule exocytosis in cytotoxic T lymphocytes. J Immunol. 1988 Dec 1;141(11):3785–3790. [PubMed] [Google Scholar]
- Smith C. D., Cox C. C., Snyderman R. Receptor-coupled activation of phosphoinositide-specific phospholipase C by an N protein. Science. 1986 Apr 4;232(4746):97–100. doi: 10.1126/science.3006254. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Sussman J. J., Merćep M., Saito T., Germain R. N., Bonvini E., Ashwell J. D. Dissociation of phosphoinositide hydrolysis and Ca2+ fluxes from the biological responses of a T-cell hybridoma. Nature. 1988 Aug 18;334(6183):625–628. doi: 10.1038/334625a0. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Uhl J., Lutz F., Roka L. Pseudomonas aeruginosa cytotoxin stimulates prostacyclin production in cultured pulmonary artery endothelial cells: membrane attack and calcium influx. J Cell Physiol. 1985 Apr;123(1):64–72. doi: 10.1002/jcp.1041230111. [DOI] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Ueda T., Ichikawa Y., Kusaka I. Guanine nucleotide-induced Ca2+ release in permeabilized murine thymocytes. FEBS Lett. 1988 Jul 18;234(2):272–274. doi: 10.1016/0014-5793(88)80096-6. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Olashaw N. E., Nishibe S., Rhee S. G., Pledger W. J., Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-gamma in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989 Jul;9(7):2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]


