Abstract
Context: Although a variety of theories and studies have been cited to support the use of joint mobilization in the spine as an integral part of the treatment and rehabilitation process, information about the short-term effects of joint mobilization on acute low back injury with respect to patient pain and strength changes has been limited.
Objective: To examine the short-term effects of grade 1 and 2 posteroanterior joint mobilizations at the lumbar spine on subject pain and muscle force after an episode of acute, mechanical low back pain.
Design: Group (2) by time (2 or 3).
Setting: Athletic training clinic.
Patients or Other Participants: Male collegiate athletes (n = 19) with mechanical low back pain as assessed through a standardized evaluation were randomly assigned to a control (n = 10) or experimental (n = 9) group.
Intervention(s): All subjects underwent a standardized treatment protocol of cryotherapy and stretching during data collection. Subjects completed the McGill Pain Questionnaire and a visual analog scale (the latter to assess pain levels during range-of-motion activities) and, using a handheld dynamometer, performed 3 maximum voluntary isometric contractions to determine muscle force. Grade 1 and 2 joint mobilizations were administered to the experimental group, whereas the control group was placed in a prone position of comfort for the time it took to perform the joint mobilizations.
Main Outcome Measure(s): Baseline, immediate posttreatment, and 24-hour posttreatment measurements of pain and muscle force were taken.
Results: Compared with the control group, the experimental group demonstrated significant decreases in the sensory subscale scores of the McGill Pain Questionnaire and in pain during lumbar extension and a significant increase in force production.
Conclusions: Grade 1 and 2 joint mobilizations reduced subjects' pain and increased force production in the short-term stages of mechanical low back pain.
Keywords: functional assessment, spine, rehabilitation
Various theories and studies support the use of joint mobilization in the spine as an integral part of the treatment and rehabilitation process after low back injury.1–3 However, studies of the specific short-term effects of joint mobilization on acute low back injury with respect to patient pain and strength changes have been limited. An intricate relationship exists between the paraspinal musculature of the lumbar spine and the mechanical structures involved in the movement of the spinal segments. The zygapophyseal joints of the lumbar spine have been suggested to transmit nociceptive output, because local anesthesia administered at the joint capsule has relieved patient pain during motion.4 The annular fibers of the intervertebral disks have also been shown to be densely innervated with both nociceptors and mechanoreceptors that are related to the zygapophyseal joints and the paraspinal musculature.5 Stimulation of the annulus fibrosus at the lumbar spine results in contractions of the multifidus and longissimus muscles, whereas concurrent saline injection of the facet joint leads to a reduction in the response. These results suggest a close relationship among the nerve supplies of the 3 structures.5 Mechanical dysfunctions that result in tissue damage and inflammation can increase the sensitization of surrounding nerve fibers, leading to contractions of the surrounding musculature in response to neural stimulation. This sensitization is thought to result in persistent spinal pain through an increase in muscle activity and sometimes muscle spasm.5 The same mechanism of pain and spasm occurs with the ligamentous structures of the spinal column in relation to the associated musculature. Authors6,7 have shown that although the spinal ligaments remain the primary constraints against joint instability, the paraspinal musculature can be a significant factor in maintaining stability. Additionally, the stimulation of nociceptors in the supraspinous ligament has been shown to result in increased levels of multifidi muscle activation.6
Thus, several neural mechanisms appear to influence paraspinal muscle activation, which may in turn result in increased pain and an exacerbation of symptoms. This muscle activation may persist in the presence of neural stimulation, even when the original injury has healed.5
Joint mobilization techniques are thought to benefit patients with lumbar mechanical dysfunction through the stimulation of joint mechanoreceptors. These receptors are believed to alter the pain-spasm cycle through the presynaptic inhibition of nociceptive fibers in associated structures and the inhibition of hypertonic muscles, which ultimately improves functional abilities.1 The short-term effects of joint mobilization in the lumbar spine have been shown to decrease patient pain on subjective assessment testing; however, objective improvements in the measurements of muscle force and functional assessments have yet to be studied after an acute episode of mechanical low back pain.2 Our purpose was to obtain pain and strength measurements after grade 1 and 2 joint mobilizations administered at the lumbar spine for treatment of acute low back pain and mechanical dysfunction.
METHODS
Subjects
Nineteen subjects, all male collegiate athletes (age, 20.3 years; height, 185.4 cm; mass, 92.0 kg) with acute low back pain for less than 48 hours volunteered to participate. Subjects were excluded if they had any conditions (eg, neurologic deficit or suspected disk herniation) for which joint mobilization techniques were contraindicated. Prior treatment of the lumbar spine did not exclude subjects from participation based on the comparison of findings immediately before and after the administration of the treatment protocol. The subjects were introduced to the testing procedures and instrumentation before data collection. Each subject was informed of the risks associated with the study and was required to sign a consent form. The investigation was approved by the university's investigational review board.
Instrumentation
We used the McGill Pain Questionnaire (MPQ) to assess pain during daily activities of living before and after the treatment session. The MPQ is a valid and reliable tool that is widely used in disability evaluations to determine the qualities of the pain experienced and the effects of the pain on the subject.8 Various authors have used the MPQ to determine the level of low back pain in subjects with acute lumbar dysfunction9–11 and the efficacy of various modalities used to treat spine dysfunction.11,12 The visual analog scale (VAS) was also implemented during range-of-motion activities before and after treatment to rate the subject's pain during lumbar flexion and extension and while in a neutral position. The VAS is the most common and feasible tool for the subjective assessment of pain. It is reliable and valid in assessing the intensity of pain a subject is experiencing.13 Based on the subject's prior pain experience as a comparison, the scale rates pain from minimal or none to the most severe pain or incapacitating pain the patient has ever experienced.8 Each centimeter on the 10-cm line was given a numeric value for the purpose of recording pain levels, with 10 cm being the highest possible level of pain. Measurements were recorded to the nearest 0.5 cm.
We used a Nicholas Manual Muscle Tester handheld dynamometer (model 01160; Lafayette Instrument, Lafayette, IN) to determine the effects of joint mobilization on force generation in the erector spinae muscles. Computer-based dynamometers have been used in various studies to determine the functional capacity of the lumbar spine in the research setting.14–16 For the purposes of our research, manual muscle testing was performed with the subject prone.17 The clinician stabilized the subject's lower extremity across the pelvic region while the subject was instructed to raise his head, neck, and upper torso off the table with as much force as possible. The recording of muscle force was taken during a maximum voluntary isometric contraction of lumbar extension, with the amplitude of the response of the muscles reported in kilograms. During the recording of the contraction, the clinician resisted the subject's spinal extension at the thoracic spine between the superior medial angles of the scapulae while recording the force of the resistance applied by the subject.
Joint Mobilization Treatment
Grade 1 and 2 joint mobilizations as defined by Maitland3 were performed by a certified athletic trainer and used for all treatments within the experimental group. Grade 1 joint mobilizations are small-amplitude movements used at the beginning of the joint's range of motion in an attempt to decrease or control patient pain levels. Grade 1 joint mobilizations should be performed before a progression to grade 2 joint mobilizations is attempted in the treatment protocol, and the hand placement during the techniques is identical.3
Grade 2 joint mobilizations are large-amplitude movements that carry halfway into the joint range of motion, occupying any part of the range and yet not reaching the end range. This technique can be used to treat joint stiffness by increasing range of motion and joint pain by stimulating mechanoreceptors.3 During the administration of posteroanterior mobilizations to the lumbar spine, it is important that the patient remain relaxed. The clinician can accurately assess the range of motion of a joint only when the patient is comfortable and relaxed.
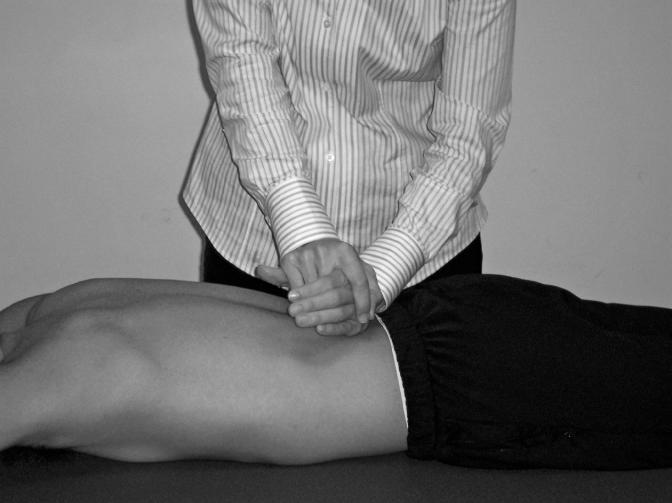
During posteroanterior mobilizations in the lumbar spine, the patient was instructed to lie prone with his hands either by his side on the treatment table or above his head and with his head turned comfortably to one side. Because the implementation of large-amplitude, oscillating movements requires small forces, the clinician used his or her hands rather than thumbs when applying pressure to the patient. Standing on the right side of the patient, the clinician placed the left hand on the patient's back so that the ulnar border of the hand between the pisiform and hook of the hamate was directly over the spinous process of the vertebra to be mobilized. The clinician's shoulders were directly over the point of contact, and full wrist extension was maintained with the forearm in neutral between supination and pronation. Correct positioning of the wrist and forearm of the clinician is the key to sustaining the accuracy of the contact point and the localization of the maneuver. The clinician's right hand then reinforced the left by placing the carpus of the right hand over the radial aspect of the left carpus at the base of the left index finger through the approximation of the right thenar and hypothenar eminences. This placed the right middle, ring, and little fingers between the left index finger and thumb, while the right index finger and thumb were over the back of the left hand. Stability was maintained through grasping the palm of the clinician's left hand between the thenar eminence and the middle, ring, and little fingers of the left hand and through sustained extension of his right wrist. The clinician's shoulders were directly over the contact point on the patient's spinous process, while the elbows were slightly flexed. The oscillating movement that accompanies joint mobilization of the vertebra is obtained by a rocking motion of the upper trunk in an up-and-down direction in the vertical plane, with the transmission of pressure coming through the clinician's arms and shoulders as they act as springs.3 The direction of the applied force was downward, avoiding any variations in either the caudal or cranial directions. This technique was administered once, with a protocol consisting of grade 1 and 2 joint oscillations for 30 seconds each. Grade 1 joint mobilizations were administered consecutively to the 3 spinous processes that surround the pathologic area with 30 seconds of rest in between, followed by grade 2 joint mobilizations performed in the same manner (Figure 1), for a total of 6 repetitions of joint mobilizations.
Figure 1. Joint mobilization technique.
Testing Procedures
Subjects were from athletic teams at a National Collegiate Athletic Association Division III institution. On initial injury, the athlete was evaluated by a researcher to confirm that the low back pain was mechanical and not radicular in nature. The researcher used a specific evaluation procedure in an attempt to identify a mechanical cause. The general lumbar spine evaluation determined the location and patterns of the existing pain, the presence of any functional deficits related to single-plane or combination movements, and a neurologic examination that included reflexes, myotomes, and strength and special tests for the purposes of ruling out radicular, disk, or fracture involvement. All patients received an initial protocol that was implemented for the first 24 to 48 hours after injury until data collection could take place. The protocol consisted of cryotherapy treatment for 15 minutes, followed by a stretching routine for the hamstrings, hip rotators, and low back. The stretching routine consisted of the subject's holding both knees against the chest while in a supine position, followed by 1 knee to the chest at a time. The patient then stretched his lower back by placing his hip in 90° of flexion and pulling 1 leg across his body, inducing pelvic rotation, while both shoulders remained against the treatment table. Finally, the subject was instructed to stretch the piriformis muscle by placing the lateral side of 1 foot against the bent knee of the opposite leg while in a supine position. The subject gently applied pressure to the medial side of his knee, pushing the hip into external rotation. The treatment protocol was implemented once before and once after practice during treatment hours in the athletic training clinic.
Subjects were then randomly assigned to either a control (n = 10) or experimental (n = 9) group. On meeting with the clinician, each subject was instructed to fill out an MPQ concerning his assessment of pain. Pain during range of motion and muscle force were then measured. The VAS was used to determine pain during the measurements of flexion and extension. The subjects were instructed to bend forward as far as possible during the recording of flexion and to place their hands on their hips and bend backward as far as possible during the recording of extension. The VAS was taken at the end ranges of both flexion and extension.
The recordings of muscle force were determined with a handheld dynamometer. The subject was instructed to lie prone on the treatment table with his hands at his sides while lifting his head, neck, and upper torso off the table with as much force as possible. The clinician placed the handheld dynamometer at the level of the superior medial angle of both scapulae as the subject performed a 5-second maximal voluntary isometric contraction against resistance. This process was repeated 3 times with a 25-second rest between repetitions.
The joint mobilization treatment was administered to the experimental group as previously described, and the control group was placed in a prone position on the treatment table for the time it took to administer 1 joint mobilization treatment session. Immediately after the completion of the assigned treatment protocol, the subject was instructed to repeat the assessments of range of motion and muscle activity in the same order. Follow-up data collection was also conducted 24 hours after the administration of the joint mobilization. The subject completed the MPQ, followed by range-of-motion and muscle activity assessments in the order previously recorded. All subjects continued to participate in competition as tolerated during the period of data acquisition.
Data Analysis
We determined mean test scores and SEs for measurements of overall pain, pain during range of motion, and force generation for both the control and experimental groups. All statistical analyses were calculated using a personal computer and SPSS statistical software (version 10.0 for Windows; SPSS Inc, Chicago, IL). Repeated-measures analyses of variance were performed to determine the differences between the scores of the control and experimental groups. Data findings were considered statistically significant when revealing an α of .05 or less. The Tukey Honestly Significant Difference test was used for post hoc comparisons.
RESULTS
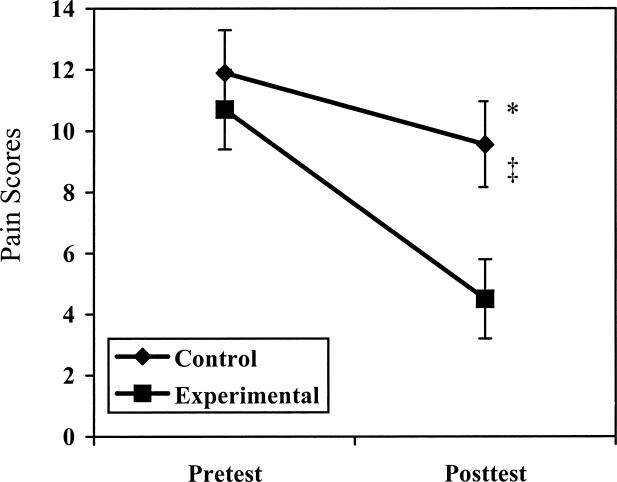
Overall pain decreased for all subjects over time as scored by the MPQ (P = .001, F1,17 = 21.440). Pain decreased for the sensory pain subscale (P = .000, F1,17 = 22.077), and a difference was noted between groups and tests (P = .048, F1,17 = 4.533). The Tukey Honestly Significant Difference test revealed a significant decrease for the sensory pain subscale for both groups and a significant decrease for posttreatment values in the experimental group compared with the control group (Figure 2). Pain decreased for the affective subscale (P = .005, F1,17 = 10.188), evaluative subscale (P = .008, F1,17 = 8.922), and miscellaneous subscale (P = .019, F1,17 = 6.671), with no significant interactions noted between group and test.
Figure 2. McGill Pain Questionnaire sensory subscale comparison of control and experimental groups.
* Indicates significant decrease (P < .05) in sensory pain scale for the control group over time; †, significant decrease in sensory pain scale for the experimental group over time; and ‡, significant difference between the control and experimental groups over time for the sensory pain scale
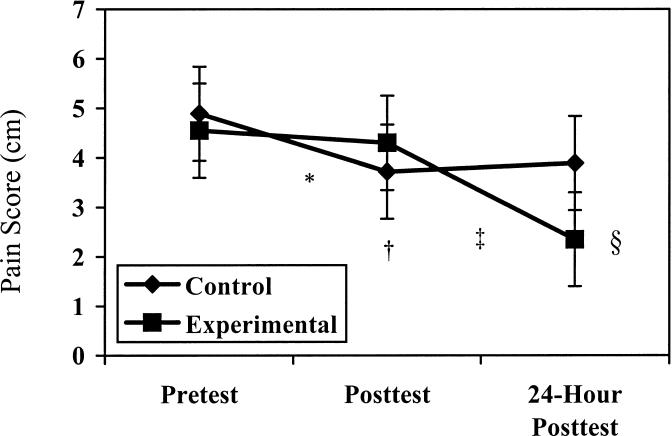
Using the VAS, we found no differences in pain while the subject was in the neutral position. Pain during flexion decreased on the VAS (P = .012, F2,34 = 5.096), with no significant interactions seen between group and test. Pain during extension decreased on the VAS (P = .001, F2,34 = 10.063), and a difference between group and test was observed (P = .030, F2,34 = 4.410). The Tukey Honestly Significant Difference test revealed a significant decrease in pain between the pretest and immediate posttest in the control group, the pretest and 24-hour posttest in the experimental group, the immediate posttest and the 24-hour posttest in the experimental group, and the 24-hour posttest in the control group and the 24-hour posttest in the experimental group (Figure 3).
Figure 3. Visual analog scale results during extension for control and experimental groups.
* Indicates significant decrease (P < .05) in pain between the pretest and the immediate posttest in the control group; †, significant decrease in pain between the pretest and 24-hour test for the experimental group; ‡, significant decrease in pain between the immediate posttest and 24-hour posttest for the experimental group; and §, significant difference in pain between the 24-hour posttest for the experimental and control groups
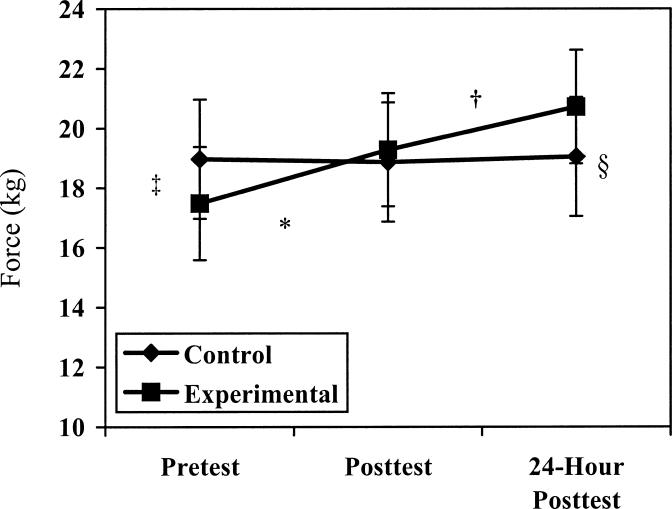
Force increased over time (P = .000, F2,34 = 9.839), and an interaction was seen between group and test (P = .001, F2,34 = 9.083). The Tukey Honestly Significant Difference test revealed a significant increase in force for the experimental group between the pretest and both the immediate and 24-hour posttests and between the immediate posttest and the 24-hour posttest. Significant differences in force production were also seen between the groups for the pretest measures and for the 24-hour posttest measures (Figure 4).
Figure 4. Force production for control and experimental groups.
* Indicates significant increase (P < .05) in force production between the pretest and the immediate posttest in the experimental group; †, significant increase in force production between the immediate posttest and the 24-hour posttest in the experimental group; ‡, significant difference in force production between the pretest force groups; and §, significant difference in force production between the posttest force groups
DISCUSSION
McGill Pain Questionnaire
We hypothesized that overall pain would decrease significantly, as measured by the MPQ, in the experimental group when compared with the treatment group. Although overall pain decreased significantly in both groups across time during the 24-hour period, no difference was recorded between the experimental and control groups. The highest score possible on the MPQ is a 76, signifying the highest level of pain. All subjects were determined to have an acute injury of a mechanical nature and were allowed to continue athletic participation during the period of this study. Therefore, at the time of baseline testing, the subjects' scores did not display a high level of pain.
The MPQ scale can be divided into subscales of pain determination, including sensory, affective, evaluative, and miscellaneous sections. The sensory subscale of the questionnaire demonstrated a significant decrease in pain levels during the 24-hour period within the experimental group compared with the control group. The sensory subscale constitutes half of the entire MPQ, with the highest possible score being 40 and the remaining subscales constituting a highest possible score of 36. The affective, evaluative, and miscellaneous subscales demonstrated no significant interactions between the experimental and control groups.
Our findings may be attributed to the subject population. The sensory subscale strictly deals with the sensation and characteristics of the pain that is felt, whereas the remaining subscales determine the level at which that sensation of pain is affecting the patient emotionally and psychologically. The MPQ attempts to determine the pain experienced in terms of spatial, pressure, and thermal properties, in addition to the degree to which the pain has an effect on a subject's fear and anxiety levels.8 An individual who is competing in athletics at the collegiate level has most likely encountered an injury and experienced the resultant pain in the past and may, therefore, be better equipped to handle such a situation on a psychological level. Previous injury and continued participation in the presence of pain may have affected the findings within these categories. Another factor worth considering is that the pain assessments were obtained from subjects within 48 hours of the acute episode. The evaluative and affective portions of the MPQ target the effects of chronic pain on a subject's well-being and may not be an accurate tool for assessing acute incidences of pain.
These findings are consistent with one previous study on the effect of joint mobilization on sensory pain level. Using the MPQ, Goodsell et al2 found a significant decrease in subject pain assessment in individuals with low back pain after the application of lumbar posteroanterior mobilization. However, the application of treatment was not consistent across subjects, because individuals received joint mobilization treatments of grades 1 to 4, depending on the specific injury.
The significant decrease within the sensory subscale of the MPQ of the experimental group may be attributed to the stimulation of mechanoreceptors at the facet joint and its relationship to the surrounding musculature. Stimulation of mechanoreceptors within the joint capsules of the facet inhibits the nociceptive fibers in the area, thereby disrupting the pain-spasm cycle.1 Several researchers5–7,18–22 have demonstrated that stimulation of spinal ligaments, intervertebral disks, and facet joints within the lumbar spine produces a response within the paraspinal musculature. A close relationship exists among the neurologic structures that supply the ligamentous, muscular, and cutaneous tissues at the lumbar spine and the pain-spasm cycle.23,24 Therefore, the inclusion of manual therapy techniques may influence the joint receptors and disrupt or modulate the pain-spasm cycle.
Force
We hypothesized that force production of the paraspinal muscles would significantly increase after the application of joint mobilizations. We found a significant increase in force production as measured by a handheld dynamometer within the experimental group between the pretest and both the immediate posttest and the 24-hour posttest. Force production also significantly increased between the immediate posttest and the 24-hour posttest. No significant findings were noted regarding force production within the control group; however, we did find a difference in force production between the pretest and posttest measures of the groups.
These findings are consistent with those of Keller and Colloca,24 who observed muscle activity in the lumbar spine through the use of electromyographic recordings after the application of mechanical-force spinal manipulation. The muscle activity was recorded during a maximum voluntary isometric contraction as the subject was instructed to perform trunk extensions before and after manipulation was performed. Muscle activity increased significantly during maximal voluntary isometric contraction after the application of manual therapy, leading one to theorize that the stimulation of the mechanoreceptors within the lumbar spine aided in the restoration of spinal muscle synergy.24 Dishman and Bulbulian25 also observed the effects of spinal manipulation, with and without a thrust, on spinal reflex attentuation and, thus, on the motor neuron pool. They found that stimulation of cutaneous receptors, muscle spindles, and joint mechanoreceptors produced an overall inhibition, thereby reducing the hypertonicity of the surrounding paraspinal musculature. This overall inhibition, although it may seem to contradict the increased force production, may play a role in the reeducation of muscle-fiber recruitment during muscular contractions due to the decreases in muscle spasm. In addition, there was also a significant, yet transient, increase in the α-motoneuron excitability after the application of manual therapy, leading one to suspect a possible increase in the responsiveness of the muscles after the manual therapy.25 The fact that the administration of joint mobilizations in our study decreased sensory components of pain while increasing the ability of the paraspinal musculature to produce force may indicate its role in reorienting the reflex arc of the mobile spinal segment.
One limitation with the use of the handheld dynamometer for the measurement of back extension is that the reliability of the measures of force production has not been assessed in the literature. Our 3 measurements were reliable within each session (intraclass correlation coefficients >0.93), but the intersession reliability of the technique and the validity of this method have not been documented.
Range of Motion With the Visual Analog Scale
We hypothesized that the VAS score would significantly decrease after the application of joint mobilizations. Significant differences were noted across both groups on the VAS while the subjects were in both lumbar flexion and extension and also between immediate posttest measures for the control group and during 24 hours for the treatment group and between the posttest scores for the 2 groups. This demonstrates that the addition of joint mobilizations may lead to a greater decrease in pain over time. This finding also supports the findings for the sensory subset in the MPQ. Joint mobilization techniques for the lumbar spine add only minimal time to a treatment plan and will be beneficial for the patient over time with regard to decreasing pain.
CONCLUSIONS
Few authors in the area of joint mobilization have examined the effects of manual therapy on the injured lumbar spine. Low back pain that results from mechanical dysfunction is a frequent occurrence in the athletic population and demands a great deal of care from a clinician, encompassing a thorough evaluation and comprehensive treatment plan. In this study, we examined the effects of grade 1 and 2 joint mobilizations on a group of collegiate athletes with relatively minor mechanical lumbar injuries and found that the mobilization techniques resulted in immediate and 24-hour increases in the ability of the paraspinal musculature to produce force, a 24-hour decrease in pain as measured by the sensory subscale of the MPQ, and a decrease in pain during lumbar extension as measured by the VAS when compared with subjects in the control group. The joint mobilization method implemented in this study is a simple, low-risk technique when properly incorporated into a treatment protocol for patients assessed with low back pain of mechanical origin. The administration of this particular mobilization technique is quick and does not add to the cost of treatment. Grade 1 and 2 joint mobilizations, when applied to the specific population in this study, seemed to aid patients in the short-term stages of injury.
We attempted to examine the use of joint mobilizations on the lumbar spine as an effective technique that may address issues within the reflex arc of the mobile segment that are commonly neglected during the treatment and rehabilitation of low back pain. Conventional rehabilitation techniques implemented at the lumbar spine may not address stimulation of the mechanoreceptors in the mobile segment as joint mobilizations seem to do. Our results also provide a baseline for researchers to expand on in the future. Future investigators should examine the effects of different grades of joint mobilization and different methods of application, measuring the effects of such techniques with various instruments.
REFERENCES
- Colloca CJ, Keller TS. Electromyographic reflex responses to mechanical force, manually assisted spinal manipulative therapy. Spine. 2001;26:1117–1124. doi: 10.1097/00007632-200105150-00005. [DOI] [PubMed] [Google Scholar]
- Goodsell M, Lee M, Latimer J. Short-term effects of lumbar posteroanterior mobilization in individuals with low back pain. J Manipulative Physiol Ther. 2000;23:332–342. [PubMed] [Google Scholar]
- Maitland GD. Vertebral Manipulation. 4th ed. Boston, MA: Butterworths; 1977.
- Jackson RP, Jacobs RR, Montesano PX. Volvo award in clinical sciences: facet joint injection in low back pain: a prospective statistical analysis. Spine. 1988;1988;13:966–971. doi: 10.1097/00007632-198809000-00002. [DOI] [PubMed] [Google Scholar]
- Indahl A, Kaigle AM, Reikeras O, Holm SH. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine. 1997;22:2834–2840. doi: 10.1097/00007632-199712150-00006. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Zhou BH, Harris M, Lu Y, Baratta RV. The ligamento-muscular stabilizing system of the spine. Spine. 1998;23:2552–2562. doi: 10.1097/00007632-199812010-00010. [DOI] [PubMed] [Google Scholar]
- Stubbs M, Harris M, Solomonow M, Zhou B, Lu Y, Baratta RV. Ligamento-muscular protective reflex in the lumbar spine of the feline. J Electromyogr Kinesiol. 1998;8:197–204. doi: 10.1016/s1050-6411(97)00012-6. [DOI] [PubMed] [Google Scholar]
- Nordin M, Andersson GBJ, Pope MH. Musculoskeletal Disorders in the Workplace. Baltimore, MD: Mosby; 1997.
- Hurley DA, Dusoir TA, McDonough SM, Moore AP, Baxter GD. How effective is the acute low back pain screening questionnaire for predicting 1-year follow-up in patients with low back pain? Clin J Pain. 2001;17:256–263. doi: 10.1097/00002508-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763–2769. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- Katz J, Jackson MB, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12:50–55. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Ceccherelli F, Rigoni MT, Gagliardi G, Ruzzante L. Comparison of superficial and deep acupuncture in the treatment of lumbar myofascial pain: a double-blind randomized controlled study. Clin J Pain. 2002;18:149–153. doi: 10.1097/00002508-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–184. [PubMed] [Google Scholar]
- Parks KA, Crichton KS, Goldford RJ, McGill SM. A comparison of lumbar range of motion and functional ability scores in patients with low back pain: assessment for range of motion validity. Spine. 2003;28:380–384. doi: 10.1097/01.BRS.0000048466.78077.A6. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Oleys DM, Edwards RR, Lowery D. Clinical characteristics of chronic back pain as a function of gender and oral opioid use. Spine. 2003;28:143–150. doi: 10.1097/00007632-200301150-00010. [DOI] [PubMed] [Google Scholar]
- Verna JL, Mayer JM, Mooney V, Pierra EA, Robertson VL, Graves JE. Back extension endurance and strength: the effect of variable-angle roman chair exercise training. Spine. 2002;27:1772–1777. doi: 10.1097/00007632-200208150-00016. [DOI] [PubMed] [Google Scholar]
- Hislop HJ, Montgomery J. Daniels and Worthingham's Muscle Testing. 6th ed. Philadelphia, PA: WB Saunders; 1995.
- Pickar JG, McLain RF. Responses of mechanosensitive afferents to manipulation of the lumbar facet in the cat. Spine. 1995;20:2379–2385. doi: 10.1097/00007632-199511001-00002. [DOI] [PubMed] [Google Scholar]
- McLain RF, Pickar JG. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine. 1998;23:168–173. doi: 10.1097/00007632-199801150-00004. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Minaki Y, Oota I, Yokogushi K, Ishii S. Mechanosensitive afferent units in the lumbar intervertebral disc and adjacent muscle. Spine. 1993;18:2252–2256. doi: 10.1097/00007632-199311000-00018. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Takahashi K, Takahashi Y, Morinaga T, Shimada Y, Moriya H. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine. 1996;21:917–924. doi: 10.1097/00007632-199604150-00003. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Cavanaugh JM, el-Bohy AA, Getchell TV, King AI. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg Am. 1990;72:865–870. [PubMed] [Google Scholar]
- Matre DA, Sinkjaer T, Svensson P, Arendt-Nielsen L. Experimental muscle pain increases the human stretch reflex. Pain. 1998;75:331–339. doi: 10.1016/s0304-3959(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Keller TS, Colloca DC. Mechanical force spinal manipulation increases trunk muscle strength assessed by electromyography: a comparative clinical trial. J Manipulative Physiol Ther. 2000;23:585–595. doi: 10.1067/mmt.2000.110947. [DOI] [PubMed] [Google Scholar]
- Dishman JD, Bulbulian R. Spinal reflex attenuation with spinal manipulation. Spine. 2000;25:2519–2525. doi: 10.1097/00007632-200010010-00015. [DOI] [PubMed] [Google Scholar]