Abstract
Context: Stretching is commonly used as a technique for injury prevention in the clinical setting. Our findings may improve the understanding of the neuromuscular responses to stretching and help clinicians make decisions for rehabilitation progression and return to play.
Objective: To examine the short-term effects of static and proprioceptive neuromuscular facilitation stretching on peak torque (PT), mean power output (MP), active range of motion (AROM), passive range of motion (PROM), electromyographic (EMG) amplitude, and mechanomyographic (MMG) amplitude of the vastus lateralis and rectus femoris muscles during voluntary maximal concentric isokinetic leg extensions at 60 and 300°·s−1.
Design: A randomized, counterbalanced, cross-sectional, repeated-measures design.
Setting: A university human research laboratory.
Patients or Other Participants: Ten female (age, 23 ± 3 years) and 9 male (age, 21 ± 3 years) apparently healthy and recreationally active volunteers.
Intervention(s): Four static or proprioceptive neuromuscular facilitation stretching exercises to stretch the leg extensor muscles of the dominant limb during 2 separate, randomly ordered laboratory visits.
Main Outcome Measure(s): The PT and MP were measured at 60 and 300°·s−1, EMG and MMG signals were recorded, and AROM and PROM were measured at the knee joint before and after the stretching exercises.
Results: Static and proprioceptive neuromuscular facilitation stretching reduced PT (P = .051), MP (P = .041), and EMG amplitude (P = .013) from prestretching to poststretching at 60 and 300°·s−1 (P < .05). The AROM (P < .001) and PROM (P = .001) increased as a result of the static and proprioceptive neuromuscular facilitation stretching. The MMG amplitude increased in the rectus femoris muscle in response to the static stretching at 60°·s−1 (P = .031), but no other changes in MMG amplitude were observed (P > .05).
Conclusions: Both static and proprioceptive neuromuscular facilitation stretching caused similar deficits in strength, power output, and muscle activation at both slow (60°·s−1) and fast (300°·s−1) velocities. The effect sizes, however, corresponding to these stretching-induced changes were small, which suggests the need for practitioners to consider a risk-to-benefit ratio when incorporating static or proprioceptive neuromuscular facilitation stretching.
Keywords: peak torque, electromyography, mechanomyography, range of motion, isokinetics, static stretching
Stretching is traditionally used as part of a warm-up to increase flexibility or pain-free range of motion (ROM) about a joint in an attempt to promote better performances1,2 and/or reduce the risk of injury.3–7 Athletic trainers and other rehabilitation professionals also recommend that their athletes or patients stretch before performing strengthening exercises or strength assessment tests.8 However, authors of recent systematic reviews9,10 and many original studies11–19 have suggested that preexercise stretching may temporarily compromise a muscle's ability to produce force. It is possible that this short-term effect of stretching on muscle force production may affect the performance of various rehabilitation strengthening exercises. More importantly, preexercise stretching may adversely affect the results obtained by muscle strength assessments and, in turn, influence a clinician's decisions regarding rehabilitation progression or return to play.
Two primary hypotheses have been developed to explain this so-called stretching-induced strength deficit11–21: mechanical factors, such as changes in muscle stiffness, and neuromuscular factors, such as altered motor control strategies. Clinically, the simultaneous measurements of mechanomyography (MMG) and electromyography (EMG) can provide unique information about the mechanical properties and motor control strategies during various types of muscle actions. The MMG signal records and quantifies the low-frequency lateral oscillations of active skeletal muscle fibers and provides a noninvasive method to examine muscle function.22–24 The MMG signal has been referred to by many names, including acoustic myography, sound myography, phonomyography, and vibromyography; however, the 1995 CIBA Foundation Symposium25 proposed the term surface mechanomyogram. It has been suggested23 that the lateral oscillations detected by MMG are generated by (1) a gross lateral movement of the muscle at the initiation of a contraction that is generated by nonsimultaneous activations of muscle fibers, (2) smaller subsequent lateral oscillations that occur at the resonant frequency of the muscle, and (3) dimensional changes of the active skeletal muscle fibers. The lateral oscillations produced by contracting muscles may reflect the “mechanical counterpart” of the muscle activation as measured by surface EMG.26–31 Conversely, surface EMG reflects the algebraic sum of electric muscle action potentials that pass within the recording areas of the EMG electrodes. Therefore, EMG amplitude quantifies muscle activation, which can be altered by the number of motor units recruited and the firing rates of the activated motor units.
Recent evidence32–40 has indicated that MMG amplitude is inversely proportional to the stiffness of an active muscle, whereas EMG amplitude may reflect the stretching-induced decreases in muscle activation. Therefore, MMG and EMG measurements may provide useful information about the mechanical and electric factors, respectively, that may explain the stretching-induced strength deficit.
Previous authors have examined the effects of stretching on maximal strength,16,41 explosive force production,19,42,43 vertical jump performance,17,19,21,43 concentric isokinetic peak torque,12–14,18 isometric force production,11,15,43–45 and balance.44 To our knowledge, only 2 previous groups19,42 have compared the effects of static and proprioceptive neuromuscular facilitation (PNF) stretching on human performance measures, and they reported conflicting results. For example, one group42 reported that the vertical jump heights after PNF stretching were lower than after the static stretching and/or control conditions. The other group,19 however, demonstrated no significant differences in jump performances between the PNF stretching and control conditions but significant decreases as a result of static stretching. Thus, limited and inconclusive data are available regarding the effects of static and PNF stretching on muscle strength and power output. The purposes of our study, therefore, were to examine the short-term effects of static and PNF stretching on peak torque (PT), mean power output (MP), active ROM (AROM), passive ROM (PROM), EMG amplitude, and MMG amplitude of the vastus lateralis (VL) and rectus femoris (RF) muscles during voluntary maximal concentric isokinetic leg extensions at 60 and 300°·s−1.
We hypothesized that the static stretching would cause decreases in PT and MP, but the PNF stretching would not.19 This study was also designed to test the hypothesis that stretching-induced decreases in PT are velocity specific18 (affecting PT measurements at slow but not fast angular velocities). We also hypothesized that any stretching-induced decreases in PT and MP would be accompanied by decreases in EMG amplitude due to decreases in muscle activation11,13,15,20 and increases in MMG amplitude due to decreases in muscle stiffness.14 Finally, to examine the effect of the static and PNF stretching protocols, we included ROM measurements and hypothesized that both the static and PNF stretching would cause increases in AROM and PROM values. Thus, overall, we hypothesized that examining the stretching-induced changes in PT, MP, AROM, PROM, EMG amplitude, and MMG amplitude would provide information regarding the effects of both static and PNF stretching and the underlying mechanisms responsible for any short-term performance deficits.
METHODS
Design
We used a randomized, counterbalanced, within-subjects experimental design to compare the short-term effects of static and PNF stretching on PT, MP, AROM, PROM, EMG amplitude, and MMG amplitude. The independent variables were time (prestretching versus poststretching), stretch (static versus PNF), velocity (60 versus 300°·s−1), and muscle (VL versus RF). The dependent variables were PT, MP, AROM, PROM, EMG amplitude, and MMG amplitude. The within-subjects design helped to control for subject variability, such as individual differences in flexibility, strength, prior stretching knowledge, and experience, whereas the counterbalancing technique (as well as a familiarization trial) helped to control for a practice effect that resulted from repeated isokinetic testing, stretching, and measures of joint ROM. Each subject experienced both the static and PNF stretching protocols, but the counterbalancing technique randomly assigned the order of stretching protocols. During both laboratory trials, each subject completed 6 activities: (1) warm-up, (2) prestretching isokinetic assessments, (3) prestretching ROM measurements, (4) static or PNF stretching procedure, (5) poststretching ROM measurements, and (6) poststretching isokinetic assessments. The average duration of each laboratory trial was 58.0 ± 7.0 minutes.
Subjects
Nineteen subjects, 10 women (age, 23 ± 3 years; body mass, 63.3 ± 9.9 kg; height, 160.5 ± 11.6 cm) and 9 men (age, 21 ± 3 years; body mass, 85.7 ± 16.2 kg; height, 183.7 ± 10.5 cm), volunteered to participate. The subjects were healthy and recreationally active (engaging in 1 to 5 hours of regular physical activity per week) and indicated no current or recent knee-, hip- or ankle-related injuries and no apparent limits in knee ROM. The study was approved by the university's institutional review board for human subjects, and all subjects completed a health history questionnaire and signed informed consent forms before testing.
Instrumentation
A calibrated Biodex System 3 isokinetic dynamometer (Biodex Medical Systems Inc, Shirley, NY) was used to measure voluntary maximal torque production during the isokinetic assessments. The participants were seated with restraining straps over the pelvis and torso in accordance with the Biodex Pro Manual.46 The input axis of the dynamometer was aligned with the axis of the knee. A standard handheld goniometer was used to assess ROM, and the surface EMG and MMG signals were recorded with a Biopac data acquisition system (MP150WSW, Biopac Systems Inc, Santa Barbara, CA). The EMG signals (recorded in microvolts) were differentially amplified with a bandwidth of 1 to 5000 Hz, input impedance of 2MΩ (differential), common mode rejection ratio of 110 dB, maximum input voltage of ±10 V, sampling rate of 1000 Hz, and gain of 1000 (EMG100C, Biopac Systems). Pregelled, disposable EMG electrodes containing a 1-cm-diameter Ag-AgCl disc (Moore Medical, New Britain, CT) were used for this study. The MMG signals were detected with active miniature accelerometers (EGAS-FS-10-/VO5, Entran Inc, Fairfield, NJ) that were preamplified with a gain of 200, frequency response of 0 to 200 Hz, sensitivity of 70 mV/m·s−2, and range of ±98 m·s−2.
Peak Torque and Mean Power Output Measurements
Before the initial isokinetic testing, each subject completed a 5-minute warm-up at 50 W on a stationary cycle ergometer. Before and after the static or PNF stretching protocols, maximal concentric isokinetic PT for extension of the dominant leg (based on kicking preference) was measured at randomly ordered velocities of 60 and 300°·s−1. Three or four submaximal warm-up trials preceded 3 maximal muscle actions at both velocities. A 2-minute rest was allowed between tests at the velocities. The number of maximal muscle actions and the rest-period durations were chosen to avoid the potential for musculoskeletal fatigue, which is unlikely with only 3 consecutive maximal muscle actions at 2 separate velocities (60 and 300°·s−1) and a 2-minute rest between velocities.
During the isokinetic tests, torque was sampled by the Biodex dynamometer at a frequency of 100 Hz, stored on a personal computer, and processed offline using custom-written software (LabVIEW version 6.1; National Instruments, Austin, TX). The repetition that yielded the highest PT was selected for analysis. The torque signal was low-pass filtered at 10 Hz, and the PT was recorded as the highest torque value during the approximate middle 30° of ROM from approximately 120° to 150° of extension at the knee (180° indicated full leg extension). The MP was calculated as the time-averaged integrated area under the angle-torque curve during the middle 30° of ROM. This ROM was selected for 3 reasons: (1) to allow comparisons between velocities that were based on a standardized 30° of ROM, (2) to select an area of the torque signal that occurred during the load range (ie, constant angular velocity) at both 60 and 300°·s−1,47 and (3) to be consistent with previous recommendations48 for calculating MP during a constant velocity.
Range-of-Motion Measurements
Immediately after the prestretching isokinetic tests, AROM and PROM were assessed for flexion of the dominant leg. To measure AROM and PROM, the goniometer was positioned along the lateral aspect of the dominant leg using the landmarks described by previous authors.49 The fulcrum was centered over the lateral epicondyle of the femur, with the proximal arm secured along the femur using the greater trochanter as a reference. The distal arm was aligned with the lower leg using the lateral malleolus as a reference. For the AROM measurement, the subject lay prone and actively flexed the leg as far as possible without assistance from the investigator. The PROM was measured with the assistance of the investigator by flexing the subject's leg until there was a verbal acknowledgement of discomfort but not pain. The average of 2 measurements was used for the AROM and PROM scores. Permanent markers identified the anatomical landmarks during the initial prestretching AROM and PROM assessments for consistency during the poststretching assessments.
Stretching Exercises
Once the ROM was recorded, each subject performed 4 stretching exercises designed to stretch the leg extensor muscles of the dominant limb, according to the procedures of several previous studies.12,13,18 During 2 laboratory visits, the subjects performed either the static or the PNF stretching protocols in random order. The PNF stretches used a modified technique, similar to the procedures of a previous study,19 in which the subjects maintained maximal isometric tension of the leg extensors against a manual resistance (applied by the investigator) for 5 seconds, followed by a 30-second passive stretch. Four repetitions of each stretching exercise (static or PNF) were held for 30 seconds at a point of discomfort but not pain, as acknowledged by the subject. Between repetitions, the leg was returned to a neutral position for a 20-second rest period. The average time of each stretching period (both static and PNF) was 16.9 ± 2.3 minutes.
Each subject performed 1 unassisted stretching exercise followed by 3 assisted stretching exercises. For the unassisted stretching exercise, the subject stood upright with one hand against a wall for balance (Figure 1). The subject then flexed the dominant leg to a knee-joint angle of 90°. For the contract phase of the PNF stretch, a padded chair was placed beneath the foot so the subject could apply maximal isometric tension at a 90° knee-joint angle without the assistance of the investigator. During the stretch phase of the PNF protocol and the static stretching, the ankle of the flexed leg was grasped by the ipsilateral hand, and the foot was raised so the heel of the dominant foot approached the buttocks. After the unassisted stretching exercise, the remaining stretches were completed with the assistance of the primary investigator. The first assisted stretching exercise was performed with the subject lying prone on a padded table and the legs fully extended (Figure 2). The dominant leg was flexed at the knee joint and slowly pressed down so the subject's heel approached the buttocks. If the heel was able to contact the buttocks, the knee was gently lifted off the supporting surface, causing a slight hyperextension at the hip joint, to complete the stretch. To perform the second assisted stretching exercise, the subject stood with the back to a table and rested the dorsal surface of the dominant foot on the table by flexing the leg at the knee joint (Figure 3). From this position, the dominant leg extensors were stretched by gently pushing back on both the knee of the flexed leg and the corresponding shoulder. The final assisted stretching exercise began with the subject lying supine along the edge of the padded table and the dominant leg hanging off the table (Figure 4). The dominant leg was flexed at the knee, and the thigh was slightly hyperextended at the hip by gently pressing down on the knee. All stretching exercises (unassisted and assisted) were performed with either the static or PNF protocol. Immediately after the stretching exercises, AROM and PROM were reassessed. After the poststretching AROM and PROM measurements, the maximal concentric isokinetic testing was also repeated. Time elapsed from the end of the stretching to the beginning of the isokinetic testing was 10.5 ± 2.8 minutes.
Figure 1. The initial unassisted stretching exercise.

Figure 2. The first assisted stretching exercise.

Figure 3. The second assisted stretching exercise.

Figure 4. The final assisted stretching exercise.
Electromyography Measurements
Bipolar surface electrode arrangements were placed along the longitudinal axes of the VL and RF muscles (Figure 5). For the VL, electrodes were placed at 66% of the distance from the anterior superior iliac spine to the lateral border of the patella with interelectrode distances of 3.6 ± 0.3 cm. For the RF, electrodes were placed at 50% of the distance from the anterior superior iliac spine to the superior aspect of the patella with a mean interelectrode distance of 4.4 ± 0.2 cm. Electrodes were placed in accordance with the recommendations of Hermens et al50 to avoid overlap with the innervation zones and to reduce the risk of cross-talk between muscles. Interelectrode impedance for each muscle was kept below 2000 Ω by swabbing with isopropyl alcohol and carefully abrading the skin with 8 to 12 light strokes over the electrode site with emery paper (3M Red Dot Trace Prep, 3M Canada Inc, London, Ontario, Canada).
Figure 5. An example of the electromyographic electrode and mechanomyographic sensor placements.

Mechanomyography Measurements
To record the MMG signals, accelerometers were placed perpendicular to the longitudinal axes of the VL and RF muscles (see Figure 5). For the VL, the accelerometer was placed at 50% of the distance from the anterior superior iliac spine to the lateral border of the patella, superior to the active EMG electrodes. For the RF, the accelerometer was placed at 50% of the distance from the anterior superior iliac spine to the superior border of the patella, between the active EMG electrodes. Both accelerometers were affixed to the skin with 3M double-sided foam tape and microporous surgical tape to ensure consistent contact pressure.13,32–34,51–53
Signal Processing
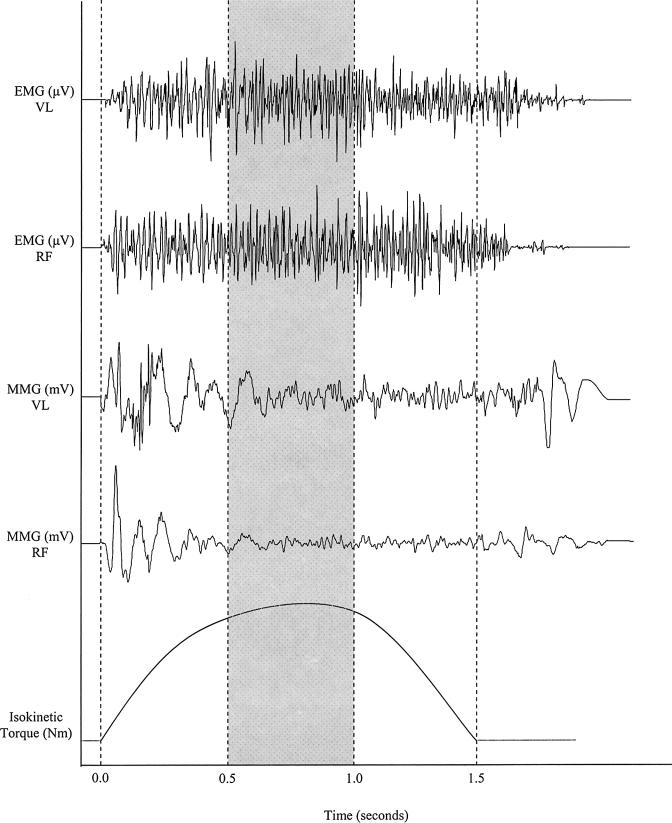
The analog EMG and MMG signals were sampled at a frequency of 1 KHz, stored on a personal computer, and expressed as root mean square amplitude values by software (AcqKnowledge III, Biopac Systems). The EMG and MMG signals were bandpass filtered (second-order Butterworth filter) at 10 to 500 Hz and 5 to 100 Hz, respectively. All subsequent analyses used the filtered EMG and MMG amplitude values. Like the PT and MP calculations, EMG and MMG amplitude values corresponding to the approximate middle 30° of ROM (approximately 120° to 150° of extension at the knee) were analyzed to help avoid the acceleration and deceleration phases of movement that are typical with isokinetic dynamometers.47 This allowed for comparisons between the velocities based on a standardized ROM. To obtain the EMG and MMG signal epochs during the middle 30° of ROM, the durations for each subject's isokinetic contractions at 60 and 300°·s−1 were obtained from custom software (LabVIEW). One third of the isokinetic contraction duration was used to calculate the middle third of the corresponding EMG and MMG signal bursts, starting with the onset of the EMG signal (Figure 6).
Figure 6. Examples of electromyographic (EMG, measured in microvolts), mechanomyographic (MMG, measured in millivolts), and isokinetic torque (measured in Newton-meters) signals measured simultaneously from the vastus lateralis (VL) and rectus femoris (RF) muscles during a maximal concentric isokinetic leg extension at 60°·s−1.
For this example, the duration of the isokinetic contraction was 1.5 seconds; therefore, the shaded area represents the signal epochs used for analysis
Statistical Analysis
Two separate, 3-way, repeated-measures analyses of variance (stretch [static versus PNF] × time [prestretching versus poststretching] × velocity [60 versus 300°·s−1]) were used to analyze the PT and MP data. Two separate, 4-way, repeated-measures analyses of variance (stretch [static versus PNF] × time [prestretching versus poststretching] × velocity [60 versus 300°·s−1] × muscle [VL versus RF]) were used to analyze the EMG and MMG amplitude data. Two separate, 2-way, repeated-measures analyses of variance (stretch [static versus PNF] × time [prestretching versus poststretching]) were used to analyze the AROM and PROM measurements. When appropriate, follow-up analyses included additional lower-order analyses of variance and paired-samples t tests. In addition, effect size estimates were calculated based on the method of Cohen54 for any differences observed. We used SPSS (version 11.5; SPSS Inc, Chicago, IL) and Excel (version 2003; Microsoft Corp, Seattle, WA) for all statistical analyses. A type I error rate of 5% was considered acceptable for all statistical comparisons.
RESULTS
Peak Torque and Mean Power Output
The mean values for PT and MP are reported in Table 1. For PT and MP, no 3-way interactions (time × stretch × velocity), no 2-way interactions (time × stretch, stretch × velocity, time × velocity), and no main effect for stretch were noted, but significant main effects were seen for time and velocity. The marginal means for PT (collapsed across stretch and velocity) decreased from prestretching to poststretching (P = .051, effect size = 0.08). In addition, the marginal means for PT (collapsed across time and stretch) decreased from 60 to 300°·s−1 (P < .001, effect size = 1.02). The marginal means for MP (collapsed across stretch and velocity) also decreased from prestretching to poststretching (P = .041, effect size = .08), whereas the marginal means for MP (collapsed across time and stretch) increased from 60 to 300°·s−1 (P < .001, effect size = 1.48). Overall, these results indicated that PT and MP decreased from prestretching to poststretching at 60 and 300°·s−1 in response to both the static and PNF stretching. In addition, PT decreased, whereas MP increased from 60 to 300°·s−1, which was consistent with the traditional force-velocity55 and power-velocity56 relationships during maximal concentric isokinetic muscle actions.
Table 1. Peak Torque and Mean Power Output Values*.
Range of Motion
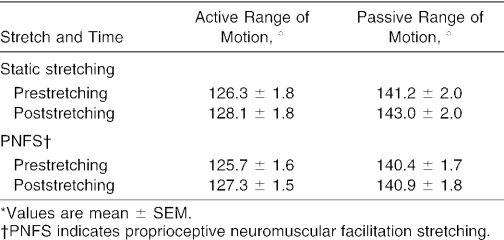
The mean values for AROM and PROM are reported in Table 2. For both AROM and PROM, no 2-way interactions (time × stretch) and no main effects for stretch were noted, but significant main effects for time were seen. The marginal means for AROM (collapsed across stretch) increased from prestretching to poststretching (P < .001, effect size = 0.28). Similarly, the marginal means for PROM (collapsed across stretch) increased from prestretching to poststretching (P = .001, effect size = 0.18). Thus, AROM and PROM increased from prestretching to poststretching in response to both the static and PNF stretching.
Table 2. Active Range-of-Motion and Passive Range-of-Motion Values*.
Electromyographic Amplitude
The mean values for EMG amplitude are reported in Table 3. The analysis indicated no 4-way interactions (time × stretch × velocity × muscle), no 3-way interactions (stretch × time × velocity, stretch × time × muscle, stretch × velocity × muscle, time × velocity × muscle), no 2-way interactions (time × stretch, time × muscle, time × velocity, stretch × velocity, stretch × muscle, velocity × muscle), and no main effects for stretch or velocity but significant main effects for time and muscle. The marginal means for EMG amplitude (collapsed across stretch, muscle, and velocity) decreased from prestretching to poststretching (P = .013, effect size = 0.30). The marginal means for EMG amplitude (collapsed across time, stretch, and velocity) were greater for the RF than the VL (P = .008, effect size = 0.47). Overall, these findings indicated that EMG amplitude for the VL and RF muscles decreased from prestretching to poststretching at 60 and 300°·s−1 in response to both the static and PNF stretching.
Table 3. EMG Amplitude and MMG Amplitude Values*.
Mechanomyographic Amplitude
The mean values for MMG amplitude are reported in Table 3. The analysis indicated a 4-way interaction (time × stretch × velocity × muscle). Subsequent paired-samples t tests indicated that MMG amplitude increased from prestretching to poststretching (static only) at 60°·s−1 for the RF (P = .031, effect size = 0.43). However, no other changes were seen in MMG amplitude from prestretching to poststretching. Furthermore, MMG amplitude increased from 60 to 300°·s−1 for all assessments (P < .001, effect size = 1.47), which was consistent with our previous findings regarding the velocity-related increases in MMG amplitude.32–34,51,52
DISCUSSION
We found a 2.8% decrease in PT and a 3.2% decrease in MP as a result of the static and PNF stretching exercises. These results were consistent with previous reports of short-term decreases in performance after a bout of stretching.11–19,43–45,57–60 Our results are unique, however, in that we observed decreases in muscle strength and power as a result of both the static and PNF stretching exercises. Conflicting evidence exists regarding the effects of PNF stretching on jumping performance.19,42 Vertical jump heights after PNF stretching were lower than after the static stretching and/or control conditions.42 Yet, another group19 demonstrated no significant differences in jump performances between the PNF stretching and control conditions. These conflicting results19,42 may be due to the differences among stretching protocols and/ or the types of jumping tests performed (vertical jump versus drop jump versus concentric-only jump). For example, a contract-relax, agonist-contract PNF method involving two 10-second isometric contractions for each of the stretching repetitions was used in one study,42 whereas the other19 used a contract-relax method involving one 5-second isometric contraction followed by a 15-second passive stretch for each stretching repetition. Furthermore, it has been suggested that the magnitude of the performance decrement may be in direct proportion to the magnitude of the stretching exercises.15 It is possible, therefore, that either the magnitude of the stretching or fatigue may have influenced the vertical jumping abilities after the PNF stretching conducted in these previous studies.19,42 Nevertheless, our results extend the findings of previous studies19,42 and suggest that both PNF and static stretching reduce the force- and power-producing capabilities of the leg extensors during voluntary maximal concentric isokinetic muscle actions at 60 and 300°·s−1.
Recent authors18 suggested that the short-term effects of static stretching on PT are velocity specific. Specifically, stretching-induced decreases in PT during maximal concentric isokinetic leg extensions were reported at the slower velocities (60 and 90°·s−1), but no effects of stretching were observed at the faster velocities (150, 210, or 270°·s−1).18 Our results were similar to those of previous studies12–14 and demonstrated stretching-induced decreases in PT at both slow (60°·s−1) and fast (300°·s−1) velocities. Therefore, it is possible that the stretching-induced decreases in PT may not be as velocity specific as originally suggested.18 Moreover, the suggestion that static stretching before performance is sport specific, affecting strength but not power athletes,18 may also need to be reevaluated.
As we mentioned previously, 2 hypotheses have been developed to account for the stretching-induced decreases in muscular force–producing capacity: (1) mechanical factors, such as alterations in the viscoelastic properties of the musculotendinous unit,12–15,18,45,57 and (2) neurologic factors, such as decreased motor unit activation.11,13,15,20 A previous group15 reported that after 15 minutes of recovery from intense stretching, most of the decreases in muscular force–generating capacity were attributable to intrinsic mechanical properties of the musculotendinous unit rather than neural factors. Specifically, it was hypothesized that stretching may have altered the length-tension relationship and/or the plastic deformation of connective tissues such that the maximal force-producing capabilities of the musculotendinous unit could be limited.15 Investigators18,45 have also suggested that the primary mechanism underlying the stretching-induced decreases in force production (after 10 minutes of recovery) is related to a decrease in musculotendinous stiffness that may alter the length-tension relationship of the muscle fibers. These suggestions are particularly applicable considering the 10-minute recovery we allowed after the stretching protocols. Furthermore, previous researchers have suggested that the angle-torque relationship (ie, the torque curve) during maximal concentric isokinetic muscle actions may provide information regarding the length-tension relationship of the muscle fibers.60–62 It is possible, therefore, that stretching-induced alterations in the length-tension relationship may be manifested through changes in the angle-torque relationship, which, in turn, may be evident by changes in the area under the angle-torque curve. Our results support this hypothesis and suggest that the static and PNF stretching decreased MP and, therefore, reduced the time-averaged area under the angle-torque curve, which implies stretching-induced alterations in the length-tension relationship. One limitation of our results, however, was that we only used a truncated portion of the ROM (middle 30°) to calculate MP. It is still unclear how stretching may affect the area under the angle-torque curve throughout the full ROM. Future authors should carefully examine the effects of stretching on the entire angle-torque relationship to better understand the effects of stretching on the length-tension relationship.
We hypothesized that AROM and PROM would increase as a result of the stretching exercises. Our results support our hypothesis and indicate stretching-induced increases in AROM and PROM (1.6% and 1.0% increases, respectively). These findings suggest, therefore, that static and PNF stretching both cause increases in musculotendinous compliance, which allows for a greater pain-free ROM. The observed increases in PROM may provide information regarding the clinical benefits of static and PNF stretching, because they require the application of an external force on the limb by an examiner. Conversely, the increase in AROM suggested that the internal forces of the antagonist (hamstrings) are able to move the leg through a greater ROM as a result of the static and PNF stretching. Thus, AROM may provide information regarding the performance benefits of static and PNF stretching (due to the absence of external forces applied by an examiner), which may better simulate performance conditions. In addition, little is known about the simultaneous influences of a warm-up in conjunction with stretching on PROM and/or AROM; therefore, future investigators should attempt to distinguish between the individual contributions of a warm-up and stretching exercises to increase joint ROM.
Previous groups22,32–34,36,37,63 have suggested that MMG amplitude is inversely related to muscle stiffness but directly related to MP.32–34,51,52,63 It has been suggested that a stiff muscle may attenuate the lateral oscillations of the individual muscle fibers and, therefore, decrease MMG amplitude.22,63 Thus, we hypothesized that stretching would decrease muscle stiffness and increase MMG amplitude.14,22,32,34,36,37,63,64 Our results indicate an increase in MMG amplitude from prestretching to poststretching (static only) at 60°·s−1 for the RF, which only partially supported our hypothesis that stretching increases MMG amplitude, which may be an indicator of decreases in muscle stiffness. However, the increase in MMG amplitude was observed for only 1 muscle (RF) at 1 velocity (60°·s−1) in response to the static stretching. No other stretching-induced changes were observed for MMG amplitude. These findings are not consistent with those of previous authors,14 who reported increases in MMG amplitude as a result of stretching the biceps brachii muscle. The increases reported were primarily attributed to stretching-induced changes in muscle stiffness.14 This conflicting evidence could be due to the differences in muscle architecture (VL and RF versus biceps brachii) and/or possible competing influences of stretching-induced decreases in MP and increases in muscle compliance, both of which may influence MMG amplitude. Thus, further research is necessary to examine the relationship between MMG amplitude and muscle stiffness and how this relationship is affected by stretching.
It has also been hypothesized that stretching-induced decreases in force production may be due to neural factors such as decreased motor unit activation, firing frequency, and/or altered reflex sensitivity.11,13,15,20,43 Previous groups have demonstrated stretching-induced decreases in muscle activation through the use of surface11,13,15,43 and fine-wire20 EMG in addition to twitch interpolation techniques.11,15,43 Decreases in motor-unit recruitment (EMG amplitude) and firing frequency (zero crossing rate) were observed after repeated passive stretches of the plantar flexors.20 In addition, 60% of the stretching-induced decreases in force production of the triceps surae (up to 15 minutes after stretching) were reported to be due to neural factors.15 Other authors11 also suggested that at least part of the stretching-induced decreases in maximal force production of the leg extensors was a result of decreases in muscle activation. Moreover, our laboratory group13 has reported decreases in PT and EMG amplitude after stretching for the stretched and unstretched (contralateral) leg extensor muscles, which were partially attributed to an unidentified central nervous system inhibitory mechanism. However, stretching-induced decreases in PT, with no changes in surface EMG, have been reported.14,57 Our results support those of previous studies11,13,15,20,43 and indicate stretching-induced decreases in EMG amplitude at 60 and 300°·s−1 for the VL and RF muscles. The conflict of our results with those of previous studies14,57 may be related to the differences among the muscles tested (leg extensors versus triceps surae versus biceps brachii) and/or the stretching protocols.
Using effect-size values to gauge the size of the effect of the treatments may be an important statistical tool for determining clinical relevance versus statistical significance. The effect-size values54 for the stretching-induced changes in PT, MP, AROM, PROM, EMG amplitude, and MMG amplitude in our study were 0.08, 0.08, 0.28, 0.18, 0.30, and 0.43, respectively. Based on the interpretation guidelines,54 effect-size values ranging from 0.08 to 0.43 are small to small or moderate. Thus, the stretching-induced changes in PT, MP, AROM, PROM, EMG amplitude, and MMG amplitude in our study might be best interpreted based on the context of the application. For example, the decision to stretch elite athletes before performances, in which relatively small differences exist between first and second place, may be affected by our findings. However, in a rehabilitation setting, in which stretching may be used to maintain a functional ROM for an injured muscle or joint65 and for warm-up before muscle strengthening treatments and assessments, the small stretching-induced changes observed in our study may not be as clinically relevant. Therefore, caution should be used when interpreting our findings. When designing treatment programs, clinicians may benefit from considering a risk-to-benefit ratio that weighs the risk of decreases in strength, power, and muscle activation against the benefit of maintaining a functional ROM. For instance, during the early stages of rehabilitation, regaining a functional ROM is more important than maximal muscle-force production. However, as the rehabilitation program progresses and normal ROM is restored, force production and muscle activation become increasingly important, particularly for the redevelopment of strength and power. Based on our results and the results of previous studies,11–19,43–45,57–59 clinicians should consider the possible effect of preexercise stretching on muscle strengthening exercises. More importantly, clinicians should be aware of the possibility for stretching-induced strength deficits when conducting strength tests on their patients, particularly when the results of these tests are used for making decisions for rehabilitation progression and return to play.
In summary, our primary findings were decreases in PT, MP, and EMG amplitude as a result of both static and PNF stretching. These findings were unique in that, to our knowledge, no other authors have examined the effects of static and PNF stretching on isolated muscle performance with a repeated-measures design. In addition, the fact that the effects of the stretching were evident at both 60 and 300°·s−1 suggested that stretching-induced decreases in performance may not be as velocity specific as previously suggested.12–14,18 Regarding the mechanisms underlying the stretching-induced performance deficit, the decreases in PT and MP and the decreases in EMG amplitude we observed in our study tentatively support the hypothesis that stretching may alter the length-tension relationship12,13,16,18,45,57 and the hypothesis that stretching may reduce muscle activation,11–13,15,20 respectively. Furthermore, as indicated by the static stretching-induced increase in MMG amplitude for the RF at 60°·s−1 in our study in conjunction with previous findings,14 MMG may be useful in investigating the mechanical changes that may occur as a result of stretching, such as decreases in muscle stiffness. In addition, the relatively small effect-size values in our study suggest that the application of the stretching-induced changes in performance may be context specific, implying the need for practitioners to consider a risk-to-benefit ratio when designing rehabilitation or training programs that incorporate preexercise stretching. Because our subjects were free of any lower extremity injuries, further research is needed to examine the effect of preexercise stretching on muscle strengthening and/or strength assessments in athletes or patients who have experienced a muscle, tendon, or joint injury.
Acknowledgments
We thank Dr Joseph P. Weir of Des Moines University Osteopathic Medical Center in Des Moines, IA, for writing the software (LabVIEW 6.1, National Instruments, Austin, TX) used to analyze the data obtained from the Biodex dynamometer.
REFERENCES
- Shellock FG, Prentice WE. Warming-up and stretching for improved physical performance and prevention of sports-related injuries. Sports Med. 1985;2:267–278. doi: 10.2165/00007256-198502040-00004. [DOI] [PubMed] [Google Scholar]
- Smith CA. The warm-up procedure: to stretch or not to stretch. A brief review. J Orthop Sports Phys Ther. 1994;19:12–17. doi: 10.2519/jospt.1994.19.1.12. [DOI] [PubMed] [Google Scholar]
- Bixler B, Jones RL. High-school football injuries: effects of a post-halftime warm-up and stretching routine. Fam Pract Res J. 1992;12:131–139. [PubMed] [Google Scholar]
- Ekstrand J, Gillquist J. Soccer injuries and their mechanisms: a prospective study. Med Sci Sports Exerc. 1983;15:267–270. doi: 10.1249/00005768-198315030-00014. [DOI] [PubMed] [Google Scholar]
- Ekstrand J, Gillquist J, Möller M, Oberg B, Liljedahl SO. Incidence of soccer injuries and their relation to training and team success. Am J Sports Med. 1983;11:63–67. doi: 10.1177/036354658301100203. [DOI] [PubMed] [Google Scholar]
- Garrett WE., Jr. Muscle strain injuries: clinical and basic aspects. Med Sci Sports Exerc. 1990;22:436–443. [PubMed] [Google Scholar]
- Safran MR, Seaber AV, Garrett WE., Jr. Warm-up and muscular injury prevention: an update. Sports Med. 1989;8:239–249. doi: 10.2165/00007256-198908040-00004. [DOI] [PubMed] [Google Scholar]
- Perrin DH. Isokinetic Exercise and Assessment. Champaign, IL: Human Kinetics; 1993:51,55.
- Thacker SB, Gilchrist J, Stroup DF, Kimsey CD., Jr. The impact of stretching on sports injury risk: a systematic review of the literature. Med Sci Sports Exerc. 2004;36:371–378. doi: 10.1249/01.mss.0000117134.83018.f7. [DOI] [PubMed] [Google Scholar]
- Shrier I. Does stretching improve performance? A systematic and critical review of the literature. Clin J Sport Med. 2004;14:267–273. doi: 10.1097/00042752-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Behm DG, Button DC, Butt JC. Factors affecting force loss with prolonged stretching. Can J Appl Physiol. 2001;26:261–272. [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Johnson GO, Miller JM, Coburn JW, Beck TW. Acute effects of static stretching on peak torque in women. J Strength Cond Res. 2004;18:236–241. doi: 10.1519/R-13303.1. [DOI] [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Weir JP, Johnson GO, Coburn JW, Beck TW. The acute effects of static stretching on peak torque, mean power output, electromyography, and mechanomyography. Eur J Appl Physiol. In press. [DOI] [PubMed]
- Evetovich TK, Nauman NJ, Conley DS, Todd JB. Effect of static stretching of the biceps brachii on torque, electromyography, and mechanomyography during concentric isokinetic muscle actions. J Strength Cond Res. 2003;17:484–488. doi: 10.1519/1533-4287(2003)017<0484:eossot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Sale DG, MacDougall JD. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol. 2000;89:1179–1188. doi: 10.1152/jappl.2000.89.3.1179. [DOI] [PubMed] [Google Scholar]
- Kokkonen J, Nelson AG, Cornwell A. Acute muscle stretching inhibits maximal strength performance. Res Q Exerc Sport. 1998;69:411–415. doi: 10.1080/02701367.1998.10607716. [DOI] [PubMed] [Google Scholar]
- McNeal JR, Sands WA. Acute static stretching reduces lower extremity power in trained children. Pediatr Exerc Sci. 2003;15:139–145. [Google Scholar]
- Nelson AG, Guillory IK, Cornwell A, Kokkonen J. Inhibition of maximal voluntary isokinetic torque production following stretching is velocity-specific. J Strength Cond Res. 2001;15:241–246. [PubMed] [Google Scholar]
- Young W, Elliott S. Acute effects of static stretching, proprioceptive neuromuscular facilitation stretching, and maximum voluntary contractions on explosive force production and jumping performance. Res Q Exerc Sport. 2001;72:273–279. doi: 10.1080/02701367.2001.10608960. [DOI] [PubMed] [Google Scholar]
- Avela J, Kyrolainen H, Komi PV. Altered reflex sensitivity after repeated and prolonged passive muscle stretching. J Appl Physiol. 1999;86:1283–1291. doi: 10.1152/jappl.1999.86.4.1283. [DOI] [PubMed] [Google Scholar]
- Knudson D, Bennett K, Corn R, Leick D, Smith C. Acute effects of stretching are not evident in the kinematics of the vertical jump. J Strength Cond Res. 2001;15:98–101. [PubMed] [Google Scholar]
- Barry DT, Cole NM. Fluid mechanics of muscle vibrations. Biophys J. 1988;53:899–905. doi: 10.1016/S0006-3495(88)83171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orizio C. Muscle sound: bases for the introduction of a mechanomyographic signal in muscle studies. Crit Rev Biomed Eng. 1993;21:201–243. [PubMed] [Google Scholar]
- Stokes MJ. Acoustic myography: applications and considerations in measuring muscle performance. Isokinet Exerc Sci. 1993;3:4–15. [Google Scholar]
- Investigating muscle sounds by mechanomyography. Discussion at: CIBA Foundation Symposium; December 12, 1995; London, England.
- Gordon G, Holbourn H. The sounds from single motor units in a contracting muscle. J Physiol. 1948;107:456–464. doi: 10.1113/jphysiol.1948.sp004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Mills KR. The relationship between mean power frequency of the EMG spectrum and muscle fibre conduction velocity. Electroencephalogr Clin Neurophysiol. 1985;60:130–134. doi: 10.1016/0013-4694(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Komi PV, Tesch P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur J Appl Physiol Occup Physiol. 1979;42:41–50. doi: 10.1007/BF00421103. [DOI] [PubMed] [Google Scholar]
- Moritani T, Gaffney FD, Carmichael T, Hargis J. Interrelaionships among muscle fiber types, electromyogram and blood pressure during fatiguing isometric contraction. In: Winter DA, Norman RW, Wells RP, Hayes KC, Patla AE, eds. Biomechanics IXA. Champaign, IL: Human Kinetics; 1985: 287–292.
- Solomonow M, Baratta R, Shoji H, D'Ambrosia R. The EMG-force relationships of skeletal muscle: dependence on contraction rate, and motor units control strategy. Electromyogr Clin Neurophysiol. 1990;30:141–152. [PubMed] [Google Scholar]
- Westbury JR, Shaughnessy TG. Associations between spectral representation of the surface electromyogram and fiber type distribution and size in human masseter muscle. Electromyogr Clin Neurophysiol. 1987;27:427–435. [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Johnson GO, Ebersole KT, Perry SR, Bull AJ. Mechanomyographic amplitude and mean power output during maximal, concentric, isokinetic muscle actions. Muscle Nerve. 2000;23:1826–1831. doi: 10.1002/1097-4598(200012)23:12<1826::aid-mus5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Johnson GO, Ebersole KT, Perry SR, Bull AJ. Mechanomyographic and electromyographic responses of the superficial muscles of the quadriceps femoris during maximal, concentric isokinetic muscle actions. Isokinet Exerc Sci. 2000;8:109–117. [Google Scholar]
- Cramer JT, Housh TJ, Weir JP. Power output, mechanomyographic, and electromyographic responses to maximal, concentric, isokinetic muscle actions in men and women. J Strength Cond Res. 2002;16:399–408. et al. [PubMed] [Google Scholar]
- Ebersole KT, Housh TJ, Weir JP, Johnson GO, Evetovich TK, Smith DB. The effects of leg angular velocity on mean power frequency and amplitude of the mechanomyographic signal. Electromyogr Clin Neurophysiol. 2000;40:49–55. [PubMed] [Google Scholar]
- Evetovich TK, Housh TJ, Johnson GO, Smith DB, Ebersole KT, Perry SR. Gender comparisons of the mechanomyographic responses to maximal concentric and eccentric isokinetic muscle actions. Med Sci Sports Exerc. 1998;30:1697–1702. doi: 10.1097/00005768-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Evetovich TK, Housh TJ, Stout JR, Johnson GO, Smith DB, Ebersole KT. Mechanomyographic responses to concentric isokinetic muscle contractions. Eur J Appl Physiol Occup Physiol. 1997;75:166–169. doi: 10.1007/s004210050142. [DOI] [PubMed] [Google Scholar]
- Evetovich TK, Housh TJ, Weir JP, Johnson GO, Smith DB, Ebersole KT. Mean power frequency and amplitude of the mechanomyographic signal during maximal eccentric isokinetic muscle actions. Electromyogr Clin Neurophysiol. 1999;39:123–127. [PubMed] [Google Scholar]
- Smith DB, Housh TJ, Johnson GO, Evetovich TK, Ebersole KT, Perry SR. Mechanomyographic and electromyographic responses to eccentric and concentric isokinetic muscle actions of the biceps brachaii. Muscle Nerve. 1998;21:1438–1444. doi: 10.1002/(sici)1097-4598(199811)21:11<1438::aid-mus11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Smith DB, Housh TJ, Stout JR, Johnson GO, Evetovich TK, Ebersole KT. Mechanomyographic responses to maximal eccentric isokinetic muscle actions. J Appl Physiol. 1997;82:1003–1007. doi: 10.1152/jappl.1997.82.3.1003. [DOI] [PubMed] [Google Scholar]
- Nelson AG, Kokkonen J. Acute ballistic muscle stretching inhibits maximal strength performance. Res Q Exerc Sport. 2001;72:415–419. doi: 10.1080/02701367.2001.10608978. [DOI] [PubMed] [Google Scholar]
- Church JB, Wiggins MS, Moode FM, Crist R. Effect of warm-up and flexibility treatments on vertical jump performance. J Strength Cond Res. 2001;15:332–336. [PubMed] [Google Scholar]
- Power K, Behm D, Cahill F, Carroll M, Young W. An acute bout of static stretching: effects on force and jumping performance. Med Sci Sports Exerc. 2004;36:1389–1396. doi: 10.1249/01.mss.0000135775.51937.53. [DOI] [PubMed] [Google Scholar]
- Behm DG, Bambury A, Cahill F, Power K. Effect of acute static stretching on force, balance, reaction time, and movement time. Med Sci Sports Exerc. 2004;36:1397–1402. doi: 10.1249/01.mss.0000135788.23012.5f. [DOI] [PubMed] [Google Scholar]
- Nelson AG, Allen JD, Cornwell A, Kokkonen J. Inhibition of maximal voluntary isometric torque production by acute stretching is joint-angle specific. Res Q Exerc Sport. 2001;72:68–70. doi: 10.1080/02701367.2001.10608934. [DOI] [PubMed] [Google Scholar]
- Biodex Pro Manual. Shirley, NY: Biodex Medical Systems Inc; 1998.
- Brown LE, Whitehurst M, Gilbert R, Buchhalter DN. The effect of velocity and gender on load range during knee extension and flexion exercise on isokinetic device. J Orthop Sports Phys Ther. 1995;21:107–112. doi: 10.2519/jospt.1995.21.2.107. [DOI] [PubMed] [Google Scholar]
- Iossifidou AN, Baltzopoulos V. Peak power assessment in isokinetic dynamometry. Eur J Appl Physiol. 2000;82:158–160. doi: 10.1007/s004210050667. [DOI] [PubMed] [Google Scholar]
- Funk DC, Swank AM, Mikla BM, Fagan TA, Farr BK. Impact of prior exercise on hamstring flexibility: a comparison of proprioceptive neuromuscular facilitation and static stretching. J Strength Cond Res. 2003;17:489–492. doi: 10.1519/1533-4287(2003)017<0489:iopeoh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Feriks B, Merletti R. European Recommendations for Surface Electromyography. et al. Enschede, The Netherlands: Roessingh Research and Development; 1999.
- Cramer JT, Housh TJ, Evetovich TK. The relationships among peak torque, mean power output, mechanomyography, and electromyography in men and women during maximal, eccentric isokinetic muscle actions. Eur J Appl Physiol. 2002;86:226–232. doi: 10.1007/s00421-001-0529-5. et al. [DOI] [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Weir JP. Mechanomyographic and electromyographic amplitude and frequency responses from the superficial quadriceps femoris muscles during maximal, eccentric isokinetic muscle actions. Electromyor Clin Neurophysiol. 2002;42:337–346. et al. [PubMed] [Google Scholar]
- Ebersole KT, Housh TJ, Johnson GO, Evetovich TK, Smith DB, Perry SR. MMG and EMG responses of the superficial quadriceps femoris muscles. J Electromyogr Kinesiol. 1999;9:219–227. doi: 10.1016/s1050-6411(98)00036-4. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988.
- Perrine JJ, Edgerton VR. Muscle force-velocity and power-velocity relationships under isokinetic loading. Med Sci Sports Exerc. 1978;10:159–166. [PubMed] [Google Scholar]
- Faulkner JA, Claflin DR, McCully KK. Power output of fast and slow fibers from human skeletal muscles. In: Jones NL, McCartney N, McComas AJ, eds. Human Muscle Power. Champaign, IL: Human Kinetics; 1986:81–91.
- Cornwell A, Nelson AG, Sidaway B. Acute effects of stretching on the neuromechanical properties of the triceps surae muscle complex. Eur J Appl Physiol. 2002;86:428–434. doi: 10.1007/s00421-001-0565-1. [DOI] [PubMed] [Google Scholar]
- Nelson AG, Cornwell A, Heise GD. Acute stretching exercises and vertical jump stored elastic energy. Med Sci Sports Exerc. 1996;28:S156. [Google Scholar]
- Young WB, Behm DG. Effects of running, static stretching and practice jumps on explosive force production and jumping performance. J Sports Med Phys Fitness. 2003;43:21–27. [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Proske U. Human hamstring muscles adapt to eccentric exercise by changing optimum length. Med Sci Sports Exerc. 2001;33:783–790. doi: 10.1097/00005768-200105000-00017. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Tetro DT. Changes in the relationship between joint angle and torque production associated with the repeated bout effect. J Sports Sci. 2003;21:927–932. doi: 10.1080/0264041031000140400. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Hogan DE. Effect of knee flexion angle on active joint stiffness. Acta Physiol Scand. 2004;180:294–254. doi: 10.1046/j.0001-6772.2003.01240.x. [DOI] [PubMed] [Google Scholar]
- Orizio C, Perini R, Veicsteinas A. Changes of muscular sound during sustained isometric contraction up to exhaustion. J Appl Physiol. 1989;66:1593–1598. doi: 10.1152/jappl.1989.66.4.1593. [DOI] [PubMed] [Google Scholar]
- Bodor M. Mechanomyographic and electromyographic muscle responses are related to power. Muscle Nerve. 1999;22:649–650. doi: 10.1002/(sici)1097-4598(199905)22:5<649::aid-mus18>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Houglum PA. Therapeutic Exercise for Athletic Injuries. Champaign, IL: Human Kinetics; 2001.