Abstract
Background
Individuals who are newly diagnosed with clinically isolated syndrome (CIS) or relapsing–remitting multiple sclerosis (RRMS) may choose not to undergo disease-modifying therapies (DMTs) due to concerns about expenses or potential adverse effects. Thus, many individuals will opt for alternative therapies, such as dietary modifications. Among these dietary approaches, the modified Paleolithic elimination diet has shown promise for improving MS-related symptoms; however, restriction of certain food groups can lead to inadequate intake of nutrients.
Methods
Three-day self-reported 24-h dietary recalls using the Automated Self-Administered 24-h (ASA24) Dietary Assessment Tool were assessed during a 12-month quasi-experimental (i.e., non-randomized) trial among individuals who either voluntarily declined DMTs and received health behavior (HB) intervention, (n = 29) or included DMTs and opted for the standard of care (SOC; n = 15). Participants in the HB group received a multimodal intervention that included dietary modifications, a walking program, and breathing exercises. Usual intake of each micronutrient was estimated and then evaluated with the estimated average requirement (EAR)-cut point method.
Results
At 12 months, >80% of both HB and SOC groups completed 3 days of the self-reported 24-h recalls, indicating the potential feasibility of ASA24. From baseline to 12 months, the HB group had a decreased mean ratio in total grains (0.64; 95% CI 0.43–0.93; p = 0.02) and added sugars (0.52; 95% CI 0.35–0.75; p ≤ 0.001), and an increased mean ratio intake of cured meats (1.74; 95% CI 1.05–2.90; p = 0.04); whereas, the SOC group had a decreased mean ratio intake for beef, veal, pork, lamb, and game meat (0.60; 95% CI 0.40–0.90; p = 0.01). At baseline, both groups had high proportions with inadequate intake of vitamin E and calcium. The SOC group also had a high proportion with inadequate intake of vitamin D. By 12-months, the HB group exhibited severe proportions of nutrient inadequacies (>20% of the group) for vitamin D (43.5%), vitamin E (29.1%), calcium (69.9%), and copper (27.8%). The SOC group, following their own diet, had inadequacies for all the same micronutrients, except for copper, as the HB group. The SOC group also had additional inadequacies: vitamin A (21.3%), thiamin (26.3%), riboflavin (24.2%), folate (24.8%), vitamin B12 (27.8%), and zinc (28.2%).
Conclusion
Compared to the usual diet, adhering to the modified Paleolithic elimination diet, as a component of a 12-month multimodal intervention, may lead to reduced consumption of specific food groups, such as added sugars, as well as decreased risk of severe proportions of inadequacy for certain nutrients. The utilization of the ASA24 for acquiring dietary recalls from participants with MS may be feasible for future studies.
Clinical trial registration
clinicaltrials.gov identifier NCT04009005.
Keywords: multiple sclerosis, modified Paleolithic diet, physical activity, mindfulness-based breathing, quasi-experimental
1. Introduction
Multiple sclerosis (MS) is a neurodegenerative disease of the central nervous system characterized by demyelination and inflammation (1). Newly diagnosed individuals often develop a single demyelinating event of the central nervous system, termed clinically isolated syndrome (CIS); with a subsequent demyelinating event, called a relapse, the individual can be described as having relapsing–remitting MS (RRMS) (2). Following a new diagnosis, standard of care (SOC) practices suggest starting a pharmacological disease-modifying therapy (DMT) for individuals with CIS, with unfavorable prognostic factors, or RRMS, with at least one relapse in the previous 2 years (3). While DMTs are efficacious and FDA-approved for reducing relapse risk and slowing disease progression (4); some individuals diagnosed with MS may voluntarily decline DMTs due to the high costs (5), concerns about adverse effects (6), or a lack of health insurance coverage (7). As such, there is a growing interest in more holistic approaches to treatments, such as dietary modifications, among individuals with MS (8). Therefore, there is a need to understand the effect of alternative or adjunctive programs among people with MS who are voluntarily DMT-naïve on their clinical progression.
People with MS have expressed great interest in diet (8) and a desire for evidence-based dietary guidelines and support for better managing symptoms during the MS disease course (9–12). While evidence for specific therapeutic diets has been inconsistent (13), recent meta-analyses (14, 15) of randomized dietary intervention trials in MS found that several dietary interventions, including the modified Paleolithic diet, may lead to improved quality of life (QoL) and reduced fatigue. The modified Paleolithic elimination diet, based on Paleolithic principles, eliminates specific dietary antigens (gluten, casein, and lectins) and enhances micronutrient density (16). Previous modified Paleolithic elimination diet intervention studies have demonstrated favorable improvements among individuals with MS for MS-related symptoms, including fatigue and QoL (17, 18). Given the restrictive intake of food groups on the modified Paleolithic elimination diet, people with MS have potential risks related to nutrient deficiencies. Inadequate intake of certain nutrients, such as folate, magnesium, and vitamin D, are associated with more severe MS symptoms (19, 20). Thus, ensuring individuals following the modified Paleolithic elimination diet are meeting their nutrient requirements is of great importance.
Previous studies have explored the nutrient content of the modified Paleolithic elimination diet (21). Examination of experimental menus for the modified Paleolithic diet suggested its sufficiency in meeting all micronutrient requirements, except for vitamin D, calcium, potassium, and choline across various life stage groups, as well as iron among women of reproductive age (16). In addition, in a randomized control trial of dietary intervention, a three-day weighed food record analysis revealed that adhering to the modified Paleolithic elimination diet had significant reductions in the proportion with inadequate intake of food for calcium, thiamin, and vitamin B12 (22). Similarly, in a pilot study, individuals with MS who reported following the Paleolithic diet were found to be below the estimated adequacy ratio (EAR) for vitamins E and D (23). Therefore, the aim of this study was to evaluate food groups and nutrient intakes among newly diagnosed individuals with CIS or RRMS, who are following the modified Paleolithic elimination diet as part of a remote multimodal intervention, compared to individuals following their usual diet and standard of care, which includes DMTs. In addition, although various methods have been used to assess dietary intake among people with MS, there is a need to assess the feasibility of an online self-reported dietary recall methodology among newly diagnosed people with CIS and RRMS.
2. Methods
2.1. Study design
The study design and primary outcome of QoL results have been described elsewhere (24). In brief, this was a 12-month, quasi-experimental trial conducted at the Prevention Intervention Center at the University of Iowa Hospitals and Clinics. Given the ethical concerns of withholding FDA-approved DMTs from individuals with MS, a quasi-experimental study design was selected to avoid randomization of participants into a non-DMT treatment group (25). Individuals newly diagnosed with CIS and RRMS who followed the standard of care (SOC group), which included DMTs, were compared to individuals who voluntarily declined DMTs and were eligible to receive a multimodal health behavior (HB group) modification intervention that was comprised of diet, a walking program, and breathing exercise. Prior to enrollment, informed consent was obtained from all participants. The University of Iowa Institutional Review Board approved the study protocol (IRB #201908778). The study is registered at clinicaltrials.gov, with the identifier: NCT04009005.
2.2. Participants
Participants were recruited from the continental United States through social media posts, email blasts, and flyers sent to local neurology clinics, and were eligible for enrollment if they had: (1) a diagnosis of RRMS or CIS according to the 2017 McDonald criteria (2), (2) confirmed by the treating neurologist no more than 12 months before the first study visit was completed; (3) between 18 and 55 years of age at the time of consent; (4) consent to share the clinical notes from the primary care and neurology providers during the study period; (5) residence within the continental United States; (6) approval of enrollment by the treating neurologist. Major exclusion criteria for all participants included: (1) moderate or severe mental impairment as measured by the Short Portable Mental Health Questionnaire (26); (2) use of insulin or Coumadin medication; (3) history of oxalate kidney stones, schizophrenia, or active diagnosis of an eating disorder. The complete list of inclusion and exclusion criteria can be found elsewhere (24).
2.3. Study procedures
Upon enrollment, participants in the HB group (n = 29) received the multimodal intervention, which consisted of the following components: a modified Paleolithic elimination diet, 4–7–8 breathing exercises, and a moderate-intensity walking program. At baseline, the participants were provided with an educational module that encompassed an in-depth exploration of the interplay between diet, aerobic exercise, and stress reduction mechanisms, elucidating their potential impacts on both patient-reported symptoms and concurrent comorbid disease processes. Subsequently, the participants were contacted by the study-registered dietitian nutritionist (RDN; LB), who is trained in motivational interviewing and self-determination theory (27–29), to provide an orientation session via Zoom or video call on the study’s intervention diet, walking program, and stress reduction. The RDN answered questions and consulted with the study PI (TW), physical therapist (PT; BB), and other study team members regarding the walking or stress reduction components and motivation for sustaining the behavior changes required by the study. Upon completion of the first month, the RDN scheduled monthly video group support calls or individual coaching calls, based on the participant’s preference. Participants received emails and text support from the study RDN, as needed, as well as regular group emails.
In contrast, the SOC group (n = 15) did not receive any guidance or instruction regarding dietary or behavioral interventions. Nonetheless, to sustain participants’ involvement with the research, the SOC group received monthly emails containing updates on the most recent MS research unrelated to diet, physical activity, or mindfulness practices. The study team did not impose any limitations on the diets or behavior-related endeavors of SOC participants.
2.4. Intervention diet and supplements
The study RDN provided guidance to the HB group on following the modified Paleolithic elimination diet. The study diet emphasized consuming 6–9 servings of combined fruits and vegetables daily, along with 9–12 ounces of meat for petite women and 12–21 ounces of meat for men and tall women, adjusting according to the individual’s gender and size. Notably, the study diet excluded all gluten-containing grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee. Additionally, nightshade vegetables, such as tomatoes, white potatoes, eggplants, peppers, as well as seeds and spices, were eliminated from months 3 to 6 of the diet. If desired by the participants, the reintroduction of nightshades, as well as seeds and spices, occurred during months 7–9, with the process closely supervised by the research team to assess tolerance and offer recommendations if adjustments were needed. Following the identification of problematic foods during months 7–9, participants proceeded to follow a personalized modified Paleolithic elimination diet during months 10–12.
In addition to dietary modification, participants were also recommended to take the following supplements: fish oil (2 g), vitamin B12/methyl folate/pyridoxyl-5-phosphate (1,000 mcg/400 mcg/1.5 mg per day, respectively), vitamin D3 (2,500 IUs per day), N-acetyl-l-cysteine (500 mg per day), phosphatidylcholine (420 mg).
2.5. Three-day automated self-administered dietary recalls
Developed by the National Cancer Institute (NCI), the Automated Self-Administered 24-Hour Dietary Assessment Tool (ASA24; version 2020) (30), was utilized to assess the participant’s dietary intake through a secure online website. The ASA24 is a self-administered multi-pass 24-h recall tool for recalling food intake from the prior 24-h period, where participants are led through a series of steps to document all the food and drinks consumed in the past 24 h. Detailed information about the initial validation study for ASA24 has been previously published (31, 32).
The Study Coordinator (MAE) sent an email at the scheduled times to each participant (baseline, months 3, 6, 9, and 12), which included the website for the ASA24 Recalls1 and the participant’s login information. These recalls were to be completed on three non-consecutive days, with two during weekdays (Monday to Thursday), and one during the weekend (Friday to Sunday). In addition, the Study Coordinator monitored the completion of the recalls by logging into the ASA24 website and seeing if participants had been able to complete the recalls. On occasion, when a participant was having trouble with the program, the study RDN was asked to log into a specific participant’s account to troubleshoot the issue or complete the recalls over the phone. The study RDN also contacted NCI support for assistance with issues. In addition, due to the lack of certain foods or ingredients consumed in the study diet, participants were provided with suggested food substitutions. For instance, any type of leafy green vegetable eaten would be entered in the ASA24 as the variable “greens (other kind)”. A list of food alternatives provided to participants can be found in Supplementary Table 1.
Data from the recalls was not used for diet intervention coaching and was not analyzed until the conclusion of the study. Instead, the self-monitoring smartphone application, in which answers are integrated into the respective Research Electronic Data Capture (REDCap) (33, 34), a secure web-based software platform designed to support data capture for research studies, was used to assist the study RDN in coaching individuals and groups regarding their ongoing diet adherence. During the monthly remote group meetings via Zoom, HB group adherence averages were presented, providing group-level feedback on food intake. Subsequently, each participant received their personal averages via a private email message. Specific counseling sessions related to individual participant averages were not conducted.
2.6. Statistical analysis
Baseline characteristics of treatment groups were summarized using counts and percentages or means and standard deviations. Between-treatment comparisons were made using Fisher’s exact test for categorical variables and using a two-sample t-test for continuous variables. Feasibility of the ASA24 assessment (i.e., the number of recalls completed by each participant) was reported as study-time stratified counts and percentages. The number of recalls completed for the HB and SOC groups was assessed for differences using Fisher’s exact test.
ASA24 dietary food and supplement recall data was checked for accuracy and possible entry errors. Based on previous research (35), any days in which implausible energy intake below 500 kcal/day or above 4,000 kcal/day were considered erroneous and were excluded. The mean intake of each nutrient from the ASA24 dietary recalls at each study time point (i.e., months 0, 3, 6, 9, and 12) was calculated for each participant and adjusted for age, gender, and BMI, as well as weekday vs. weekend recall values using the NCI method to estimate the usual intake (36). While 3-day 24-h recalls were requested from participants at each time point, the NCI Method handles missing 24-h dietary recalls by using analytical techniques to estimate the distribution of usual intake even when only 1 or 2 days of 24-h recalls are available. Within- and between-treatment changes in outcomes over time were tested using the generalized linear mixed modeling (GLMM) (37) framework. All models include fixed effects for the treatment group, time, and their interaction. The models also specify a random effect for participants to account for repeated measures. Point estimates, 95% confidence intervals, and p-values for within- and between-treatment mean differences/ratios over time were generated for each outcome. Graphical observation was used to evaluate the normality of data. If data had moderately to severely right-skewed distributions, a negative binomial distribution was used to assess mean change over time.
The usual intake of each nutrient was then compared to the estimated average requirement (EAR) for each life stage group using the EAR-cut point method (38) and combined by weighted means to assess the proportion of each group with inadequate micronutrient intake. Bioconversion of provitamin A carotenoids was accounted for by using retinol activity equivalents (RAEs) for vitamin A. The combination of ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3) was used to assess vitamin D. The bioconversion of tocopherols was accounted for by using alpha-tocopherol equivalents for vitamin E. To account for the differences in the absorption of food folate and of synthetic folic acid obtained from dietary supplements or food fortified with folic acid, Dietary Folate Equivalents (DFE) were used to assess folate (39). Niacin equivalents (NE) were used to assess niacin, which accounts for the contribution of dietary intake of all the forms of niacin that are available to the body, such as tryptophan (40). Given that iron recommendations are not normally distributed among women of reproductive age and are different for postmenopausal women, the proportion of iron inadequate intake was estimated using the probability approach recommended by the National Academy of Medicine (formerly the Institute of Medicine) (41). As previously described (42), the threshold for severe inadequate intake of micronutrients was defined as ≥20% of the group. Usual intake was also compared to Tolerable Upper Intake Levels (ULs) to determine the proportion of excessive intakes.
All analyses were performed with two-sided tests (α = 0.05) using SAS software (version 9.4, SAS Institute, Inc.) and Microsoft Excel (Version 16.86).
3. Results
At baseline, 29 individuals enrolled in the HB group (n = 29) and 15 in the SOC (n = 15) groups, for a total of 44 participants in the trial. At baseline, none of the characteristics assessed differed significantly between the groups (Table 1).
Table 1.
Baseline characteristics of study participants in the health behaviors (HB) and standard of care (SOC) groups.1
| Characteristic | Health behaviors | Standard of care | p-value2 |
|---|---|---|---|
| n | 29 | 15 | |
| Age (years) | 38.0 ± 1.1 | 41.1 ± 2.3 | 0.19 |
| Female (% males) | 26 (89.7%) | 15 (100%) | 0.54 |
| MS duration (years) | 0.29 ± 0.04 | 0.34 ± 0.08 | 0.57 |
| BMI | 25.80 ± 1.0 | 24.3 ± 1.0 | 0.32 |
| Race | |||
| White | 23 (79.3%) | 13 (86.7%) | >0.99 |
| Black | 1 (3.5%) | 0 | |
| Latin or Hispanic | 1 (3.5%) | 0 | |
| Two or more races | 2 (6.9%) | 1 (6.7%) | |
| Unknown or Not Reported | 2 (6.9%) | 1 (6.7%) |
Adapted from (24).
BMI, body mass index; MS, multiple sclerosis.
Data are shown as mean ± SE or n (%).
Significance determined using Fisher’s exact test for categorical variables or two-sample t-test for continuous variables.
Baseline food group equivalent mean intakes were compared to within-group average consumption during the study time point intervals (Table 2). At the 12-month primary endpoint, the HB group exhibited a decreased intake of total grains (Mean Ratio 0.64; 95% CI 0.43–0.93; p = 0.02) and added sugars (Mean Ratio 0.52; 95% CI 0.35–0.75; p ≤ 0.001) and an increased intake of cured meats (Mean Ratio 1.74; 95% CI 1.05–2.90; p = 0.04). Notably, added sugars were the only food group equivalent in which the mean intake had significantly decreased from baseline in the HB group throughout all the time point intervals (i.e., months 3, 6, 9, and 12, p < 0.05 for all) during the study (Supplementary Table 2). Conversely, the SOC group demonstrated a decrease in food group equivalents from baseline to 12 months only for beef, veal, pork, lamb, and game meat (Mean Ratio 0.60; 95% CI 0.40–0.90; p = 0.01). The only significant between-group assessments of intake change from baseline to 12-months were observed in other starchy vegetables (excludes white potatoes) (p = 0.04), total whole grains (p = 0.05), and beef, veal, pork, lamb, and game meat (p = 0.03).
Table 2.
Food group equivalent outcomes at baseline and 12-month means and absolute/proportional mean change for the health behaviors (HB) intervention and standard of care.
| Health behavior | Standard of care | HB vs SOC | |||||
|---|---|---|---|---|---|---|---|
| Baseline Mean ± SE1 |
Month 12 Mean ± SE1 |
Mean Diff/Ratio (95% CI)2 |
Baseline Mean ± SE1 |
Month 12 Mean ± SE1 |
Mean Diff/Ratio (95% CI)2 |
p-value3 | |
| Fruits categories (cup eq.) | |||||||
| Total fruits | 1.55 ± 0.15 | 1.52 ± 0.15 | 0.98 (0.69–1.39) | 1.85 ± 0.23 | 2.12 ± 0.24 | 1.15 (1.15–1.52) | 0.86 |
| Whole or cut citrus, melons, berries (excludes juices) | 0.64 ± 0.09 | 0.59 ± 0.08 | 0.91 (0.59–1.40) | 0.48 ± 0.10 | 0.58 ± 0.14 | 1.22 (0.76–1.95) | 0.37 |
| Whole or cut other fruits (excludes juices) | 0.73 ± 0.09 | 0.75 ± 0.12 | 1.02 (0.62–1.70) | 0.94 ± 0.16 | 0.93 ± 0.15 | 0.99 (0.60–1.63) | 0.93 |
| Fruit juices | 0.18 ± 0.03 | 0.19 ± 0.04 | 1.07 (0.57–2.00) | 0.44 ± 0.13 | 0.61 ± 0.22 | 1.40 (0.87–2.26) | 0.50 |
| Vegetables categories (cup eq.) | |||||||
| Total vegetable (excludes legumes) | 4.71 ± 0.37 | 5.14 ± 0.38 | 1.09 (0.90–1.33) | 3.82 ± 0.40 | 3.67 ± 0.36 | 0.96 (0.71–1.31) | 0.49 |
| Dark green vegetables | 1.74 ± 0.24 | 2.24 ± 0.24 | 1.29 (0.98–1.69) | 1.15 ± 0.18 | 1.18 ± 0.15 | 1.03 (0.73–1.44) | 0.31 |
| Total red and orange vegetables | 0.71 ± 0.08 | 0.66 ± 0.08 | 0.94 (0.72–1.23) | 0.69 ± 0.13 | 0.59 ± 0.09 | 0.86 (0.54–1.35) | 0.72 |
| Tomatoes and tomato products | 0.21 ± 0.03 | 0.19 ± 0.04 | 0.93 (0.53–1.63) | 0.43 ± 0.09 | 0.22 ± 0.05 | 0.51 (0.25–1.03) | 0.19 |
| Other red and orange vegetables (excludes tomatoes) | 0.50 ± 0.07 | 0.47 ± 0.07 | 0.94 (0.62–1.43) | 0.26 ± 0.07 | 0.37 ± 0.08 | 1.43 (0.92–2.21) | 0.18 |
| Total starchy vegetables | 0.22 ± 0.05 | 0.21 ± 0.05 | 0.96 (0.58–1.60) | 0.33 ± 0.08 | 0.35 ± 0.10 | 1.04 (0.44–2.45) | 0.89 |
| White potatoes | 0.18 ± 0.05 | 0.12 ± 0.03 | 0.69 (0.38–1.26) | 0.26 ± 0.08 | 0.31 ± 0.10 | 1.18 (0.45–3.05) | 0.35 |
| Other starchy vegetables (excludes white potatoes) | 0.04 ± 0.02 | 0.09 ± 0.04 | 2.07 (0.80–5.33) | 0.07 ± 0.02 | 0.04 ± 0.02 | 0.55 (0.25–1.23) | 0.04 |
| Other vegetables not listed above | 2.04 ± 0.18 | 2.02 ± 0.21 | 0.99 (0.73–1.34) | 1.65 ± 0.28 | 1.56 ± 0.23 | 0.94 (0.58–1.55) | 0.87 |
| Legumes (beans and peas) computed as vegetables | 0.06 ± 0.02 | 0.02 ± 0.01 | 0.31 (0.08–1.15) | 0.10 ± 0.03 | 0.13 ± 0.06 | 1.33 (0.40–4.43) | 0.11 |
| Grain category (oz. eq.) | |||||||
| Total grains | 1.98 ± 0.27 | 1.26 ± 0.21 | 0.64 (0.43–0.93)* | 2.26 ± 0.36 | 2.78 ± 0.54 | 1.23 (0.64–2.36) | 0.08 |
| Total whole grains | 0.12 ± 0.04 | 0.24 ± 0.07 | 1.95 (0.89–4.24) | 0.46 ± 0.13 | 0.33 ± 0.11 | 0.71 (0.38–1.33) | 0.05 |
| Total refined grains | 1.86 ± 0.26 | 1.02 ± 0.19 | 0.55 (0.34–0.89) | 1.79 ± 0.33 | 2.45 ± 0.53 | 1.37 (0.61–3.06) | 0.06 |
| Protein category (oz. eq.) | |||||||
| Total protein foods (excludes legumes) | 9.61 ± 0.60 | 10.2 ± 0.70 | 1.05 (0.85–1.28) | 8.06 ± 1.09 | 5.96 ± 0.59 | 0.76 (0.51–1.14) | 0.17 |
| Beef, veal, pork, lamb, game meat | 2.23 ± 0.39 | 2.50 ± 0.46 | 1.12 (0.74–1.69) | 1.51 ± 0.40 | 0.91 ± 0.32 | 0.60 (0.40–0.90)* | 0.03 |
| Cured meats | 0.53 ± 0.12 | 0.92 ± 0.29 | 1.74 (1.05–2.90)* | 0.25 ± 0.07 | 0.73 ± 0.35 | 2.95 (0.73–11.91) | 0.49 |
| Organ meat5 | 0.12 ± 0.06 | 0.22 ± 0.10 | 0.09 (−0.07 to 0.26) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 (0.00–0.00) | 0.27 |
| Poultry | 2.52 ± 0.44 | 2.89 ± 0.45 | 1.15 (0.73–1.81) | 1.48 ± 0.48 | 1.75 ± 0.44 | 1.19 (0.57–2.46) | 0.94 |
| Eggs and egg substitutes | 0.21 ± 0.06 | 0.38 ± 0.12 | 1.80 (0.77–4.20) | 0.25 ± 0.09 | 0.19 ± 0.10 | 0.77 (0.23–2.65) | 0.27 |
| Soy products excluding soy milk4 | 0.02 ± 0.02 | 0.06 ± 0.03 | 0.04 (−0.6 to 0.13) | 0.21 ± 0.10 | 0.32 ± 0.18 | 0.11 (−0.17–0.39) | 0.63 |
| Peanuts, tree nuts, seeds (excludes coconut) | 0.24 ± 0.08 | 0.08 ± 0.05 | 1.00 (0.57–1.73) | 0.40 ± 0.13 | 0.54 ± 0.23 | 0.60 (0.34–1.05) | 0.21 |
| Dairy category (cup eq.) | |||||||
| Total dairy | 0.54 ± 0.08 | 0.28 ± 0.06 | 0.52 (0.29–0.94) | 0.78 ± 0.12 | 0.58 ± 0.12 | 0.75 (0.42–1.35) | 0.38 |
| Milk (includes calcium-fortified soy milk) | 0.25 ± 0.04 | 0.14 ± 0.03 | 0.54 (0.24–1.22) | 0.45 ± 0.09 | 0.28 ± 0.07 | 0.61 (0.33–1.16) | 0.80 |
| Yogurt | 0.07 ± 0.03 | 0.02 ± 0.01 | 0.30 (0.06–1.59) | 0.04 ± 0.02 | 0.02 ± 0.02 | 0.49 (0.14–1.76) | 0.65 |
| Cheese | 0.18 ± 0.05 | 0.10 ± 0.04 | 0.54 (0.16–1.78) | 0.21 ± 0.08 | 0.15 ± 0.05 | 0.75 (0.23–2.42) | 0.70 |
| Other | |||||||
| Added sugars (tsp. eq.) | 7.46 ± 0.76 | 3.84 ± 0.56 | 0.52 (0.35–0.75)*** | 8.78 ± 1.57 | 7.04 ± 0.89 | 0.80 (0.50–1.35) | 0.16 |
| Alcoholic beverages (no. of drinks) | 0.26 ± 0.08 | 0.12 ± 0.04 | 0.45 (0.19–1.08) | 0.70 ± 0.28 | 0.48 ± 0.18 | 0.55 (0.30–1.00) | 0.71 |
Data are shown as mean ± standard error (SE).
Absolute/proportional mean change from baseline to 12-months.
Significance was determined using generalized linear models with an identity or negative binomial link function.
Data normally distributed.
Within-group statistical significance compared to baseline values indicated by * for (p ≤ 0.05), ** for (p ≤ 0.01), and *** for (p ≤ 0.001).
Bold values indicate within-group statistical significance.
Baseline nutrient intake from food and supplements was evaluated, followed by subsequent within-group comparisons to the study time point intervals (Table 3). By the 12-month primary endpoint, the HB group exhibited a decreased intake of carbohydrates (0.83; 95% CI 0.70–0.99, p = 0.04) and vitamin D (0.54; 95% CI 0.31–0.0.3; p = 0.03). In comparison, the SOC group observed decreases in energy (0.84; 95% CI 0.71–0.99; p = 0.04), total fat (0.73; 95% CI 0.62–0.86; p = 0.0001), vitamin E (0.78, 95% CI 0.65–0.94; p = 0.01). Additional mean nutrient intake from food and supplements for months 3, 6, and 9 can be found in the supplemental materials (Supplementary Table 3). The only significant between-group assessments of intake change from baseline to 12-month were observed for total fat (p = 0.01), vitamin E (p = 0.04), and choline (p = 0.04).
Table 3.
Food and supplement intake from baseline and 12-month means and proportion of mean change for the health behaviors (HB) intervention and standard of care.
| Health behavior | Standard of care | HB vs SOC | |||||
|---|---|---|---|---|---|---|---|
| Baseline Mean ± SE1 |
Month 12 Mean ± SE1 |
Mean Ratio (95% CI) 2 |
Baseline Mean ± SE1 |
Month 12 Mean ± SE1 |
Mean Ratio (95% CI)2 |
p-value3 | |
| Macronutrients | |||||||
| Energy (kcal/day) | 1,832.09 ± 63.20 | 1,679.07 ± 69.75 | 0.91 (0.81–1.04) | 1,783.11 ± 104.04 | 1,509.09 ± 81.98 | 0.84 (0.71–0.99)* | 0.42 |
| Protein (g/day) | 90.03 ± 4.06 | 89.58 ± 4.74 | 1.00 (0.87–1.13) | 76.65 ± 7.41 | 65.45 ± 4.59 | 0.85 (0.65–1.13) | 0.33 |
| Carbohydrate (g/day) | 168.58 ± 7.22 | 140.25 ± 7.39 | 0.83 (0.70–0.99)* | 181.13 ± 12.88 | 175.78 ± 13.04 | 0.97 (0.80–1.18) | 0.24 |
| Total fat (g/day) | 91.80 ± 4.41 | 89.15 ± 4.98 | 0.97 (0.83–1.12) | 82.15 ± 7.19 | 61.49 ± 5.44 | 0.73 (0.62–0.86)*** | 0.01 |
| Total water (g/day) | 2,988.22 ± 129.68 | 3,173.98 ± 122.88 | 1.06 (0.95–1.19) | 2,790.44 ± 186.79 | 2,724.19 ± 190.72 | 0.98 (0.85–1.13) | 0.36 |
| Total dietary fiber (g/day) | 27.01 ± 1.75 | 28.00 ± 2.02 | 1.04 (0.84–1.23) | 24.41 ± 1.96 | 24.09 ± 1.88 | 1.00 (0.78–1.27) | 0.81 |
| Micronutrients | |||||||
| Vitamins | |||||||
| Vitamin A4 (mcg RAE/day) | 1,974.08 ± 255.78 | 2,122.00 ± 245.72 | 1.09 (0.86–1.37) | 1,139.47 ± 137.05 | 1,226.47 ± 207.19 | 1.10 (0.76–1.61) | 0.95 |
| Vitamin C (mg/day) | 251.77 ± 30.22 | 224.47 ± 20.86 | 1.07 (0.82–1.38) | 317.26 ± 62.04 | 173.93 ± 19.15 | 0.94 (0.72–1.25) | 0.54 |
| Vitamin D5 (mcg/day) | 67.50 ± 8.89 | 39.55 ± 6.52 | 0.54 (0.31–0.93)* | 65.76 ± 11.59 | 53.97 ± 14.87 | 0.48 (0.12–1.28) | 0.86 |
| Vitamin E6 (mg/day) | 32.42 ± 6.30 | 23.91 ± 4.47 | 1.06 (0.85–1.33) | 15.01 ± 1.46 | 10.62 ± 0.99 | 0.78 (0.65–0.94)** | 0.04 |
| Vitamin K (mcg/day) | 937.93 ± 193.72 | 1,047.23 ± 183.31 | 1.12 (0.84–1.49) | 523.57 ± 89.99 | 485.73 ± 71.91 | 0.93 (0.70–1.25) | 0.39 |
| Thiamin (mg/day) | 5.25 ± 1.92 | 6.08 ± 1.84 | 0.97 (0.80–1.70) | 18.79 ± 5.49 | 18.13 ± 5.26 | 1.13 (0.85–1.151) | 0.37 |
| Riboflavin (mg/day) | 3.94 ± 0.94 | 6.74 ± 1.88 | 0.99 (0.87–1.12) | 19.32 ± 5.47 | 18.30 ± 5.26 | 0.90 (0.75–1.08) | 0.41 |
| Niacin7 (mg/day) | 31.26 ± 1.97 | 32.34 ± 2.61 | 0.98 (0.86–1.11) | 47.95 ± 8.99 | 45.50 ± 9.08 | 0.89 (0.67–1.18) | 0.57 |
| Vitamin B6 (mg/day) | 4.52 ± 0.91 | 8.14 ± 1.88 | 1.07 (0.90–1.28) | 24.39 ± 7.44 | 23.90 ± 7.66 | 0.93 (0.72–1.21) | 0.38 |
| Folate8 (mcg DFE/day) | 705.20 ± 57.41 | 839.80 ± 78.92 | 1.11 (0.88–1.40) | 922.77 ± 149.35 | 909.44 ± 159.34 | 1.10 (0.86–1.41) | 0.95 |
| Vitamin B12 (mcg/day) | 312.45 ± 88.48 | 229.35 ± 50.77 | 0.93 (0.68–1.27) | 214.86 ± 129.64 | 94.70 ± 46.32 | 0.62 (0.38–1.04) | 0.19 |
| Choline (mg/day) | 385.56 ± 17.13 | 426.53 ± 24.39 | 1.09 (0.92–1.29) | 361.27 ± 42.20 | 273.20 ± 20.94 | 0.76 (0.56–1.02) | 0.04 |
| Minerals | |||||||
| Calcium (mg/day) | 955.71 ± 70.90 | 858.56 ± 61.09 | 0.91 (0.74–1.11) | 864.31 ± 69.95 | 766.99 ± 67.48 | 0.85 (0.72–1.01) | 0.62 |
| Copper (mg/day) | 2.37 ± 0.28 | 2.32 ± 0.28 | 1.02 (0.86–1.22) | 1.69 ± 0.13 | 1.39 ± 0.09 | 0.85 (0.71–1.01) | 0.14 |
| Iron (mg/day) | 18.63 ± 1.67 | 16.30 ± 1.50 | 0.94 (0.80–1.12) | 17.42 ± 2.68 | 17.11 ± 2.82 | 0.90 (0.76–1.02) | 0.73 |
| Magnesium (mg/day) | 493.65 ± 31.83 | 407.98 ± 24.24 | 1.00 (0.83–1.21) | 423.91 ± 33.73 | 360.89 ± 24.60 | 0.92 (0.82–1.04) | 0.50 |
| Phosphorus (mg/day) | 1,343.77 ± 52.13 | 1,280.91 ± 63.38 | 0.95 (0.82–1.10) | 1,252.17 ± 88.87 | 1,071.87 ± 63.42 | 0.85 (0.70–1.04) | 0.39 |
| Selenium (mcg/day) | 135.10 ± 13.17 | 169.69 ± 29.94 | 1.29 (0.91–1.81) | 145.73 ± 20.95 | 106.83 ± 13.93 | 0.79 (0.46–1.35) | 0.13 |
| Zinc (mg/day) | 15.16 ± 1.30 | 16.05 ± 1.66 | 0.98 (0.81–1.19) | 13.83 ± 2.93 | 19.74 ± 6.75 | 0.86 (0.69–1.06) | 0.34 |
| Potassium (mg/day) | 3,831.98 ± 184.95 | 3,764.55 ± 177.79 | 0.98 (0.86–1.13) | 3,542.87 ± 262.30 | 3,366.46 ± 201.71 | 0.95 (0.80–1.14) | 0.78 |
| Sodium (mg/day) | 3,302.71 ± 137.22 | 3,229.49 ± 164.67 | 0.98 (0.85–1.14) | 3,050.60 ± 259.84 | 2,537.84 ± 219.61 | 0.83 (0.68–1.01) | 0.19 |
Data are shown as mean ± standard error (SE).
Proportion of mean change from baseline to 12-months.
Significance was determined using generalized linear models with a negative binomial link function.
As retinol activity equivalents (RAEs). 1 RAE = 1 μg retinol, 12 μg β-carotene, 24 μg α-carotene, or 24 μg β-cryptoxanthin. The RAE for dietary provitamin A carotenoid is two-fold greater than retinol equivalents (REs), whereas the RAE for preformed vitamin A is the same as RE.
Vitamin D, D2 + D3 (mcg).
Vitamin E, alpha-tocopherol (mg).
NE, Niacin Equivalents.
DFE, Dietary Folate Equivalents (mcg).
Within-group statistical significance compared to baseline values indicated by * for (p ≤ 0.05), ** for (p ≤ 0.01), and *** for (p ≤ 0.001).
Bold values indicate within-group statistical significance.
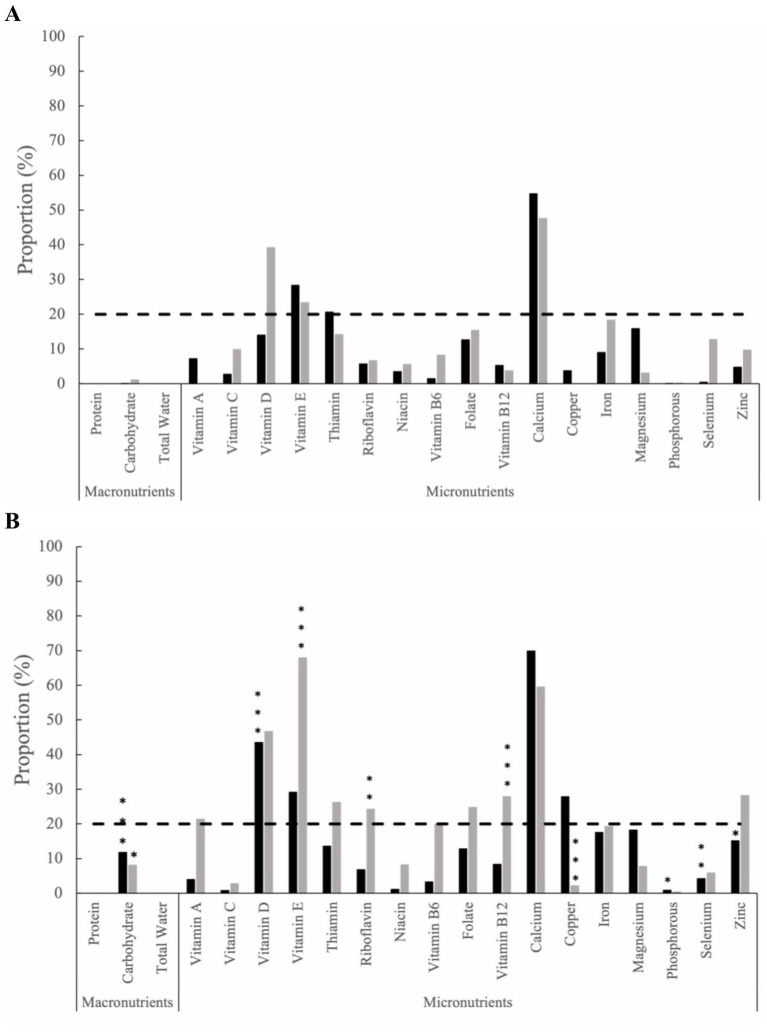
The proportion of inadequate nutrient intake from food and supplements was assessed at baseline (Figure 1A), and within-group comparisons were made throughout the study time intervals. Within the HB group, an increased proportion of inadequate nutrient intake was observed from baseline to month 12 for carbohydrates (11.78%; p ≤ 0.001), vitamin D (43.53%, p ≤ 0.001), phosphorus (0.82%, p = 0.04), selenium (4.17%, p ≤ 0.01), and zinc (15.11%, p = 0.02) (Figure 1B). In contrast, the SOC group exhibited differing proportions of inadequate nutrient intakes. At 12-months, an increased proportion of inadequate nutrient intake from baseline was observed for carbohydrates (8.10%; p = 0.02), vitamin E (67.88%, p ≤ 0.001), riboflavin (24.22%, p ≤ 0.01), vitamin B12 (27.83%, p ≤ 0.001), and copper (2.17%; p ≤ 0.001) (Figure 1B). Notably, at 12 months, the HB group exhibited levels above the severe nutrient inadequacy threshold (defined as ≥20% of the group) for calcium (69.88%), vitamin D (43.53%), vitamin E (29.12%), and copper (27.83%). In contrast, in the SOC group, the following micronutrients were found to exceed the severe inadequacy threshold: vitamin E (67.88%), calcium (59.49%), vitamin D (46.64%), zinc (28.18%), vitamin B12 (27.83%), thiamin (26.25%), riboflavin (24.22%), folate (24.75%), and vitamin A (21.27%). An additional proportion of inadequate nutrient intake from food and supplements for months 3, 6, and 9 can be found in the supplemental materials (Supplementary Figure 1).
Figure 1.
The proportion of inadequate nutrient intake among the health behaviors group (black bars) and standard of care group (grey bars) from food and supplements at (A) baseline and (B) Month 12. The dashed line represents severe micronutrient inadequate intake, defined as ≥20% of the group. Statistical significance is determined by two sample z-tests. Within-group statistical significance compared to baseline values are indicated by * for (p ≤ 0.05), ** for (p ≤ 0.01), and *** for (p ≤ 0.001).
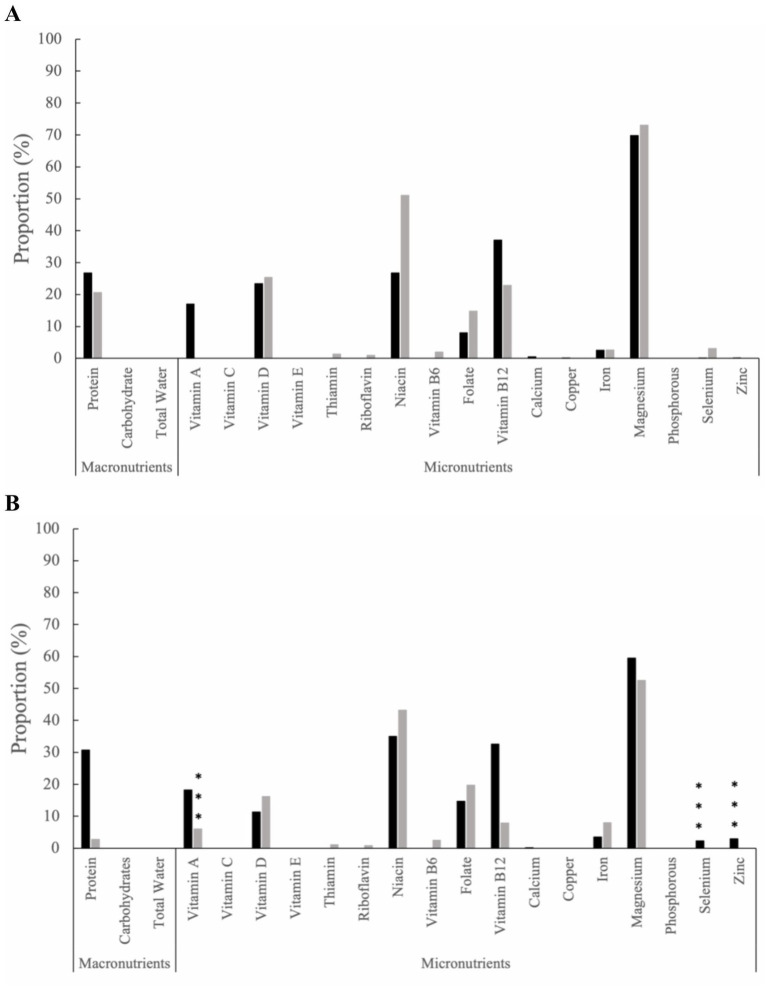
Excessive nutrient intake from food and supplements was evaluated from baseline (Figure 2A), with subsequent intra-group comparisons conducted throughout the study time intervals. By 12 months (Figure 2B), the HB group had increased excess nutrient intakes for selenium (2.31%, p ≤ 0.001) and zinc (2.92%, p ≤ 0.001). In contrast, the SOC group demonstrated excessive nutrient intake solely for vitamin A from baseline to 12 months (6.06%, p ≤ 0.001). Additional excessive nutrient intake from food and supplements for months 3, 6, and 9 can be found in the supplemental materials (Supplementary Figure 2).
Figure 2.
The proportion of excess nutrient intake among the health behaviors group (black bars) and standard of care group (grey bars) from food and supplements at (A) baseline and (B) Month 12. Statistical significance is determined by two sample z-tests. Within-group statistical significance compared to baseline values are indicated by * for (p ≤ 0.05), ** for (p ≤ 0.01), and *** for (p ≤ 0.001).
The feasibility of participants completing the requested 3-day dietary recalls on ASA24 was measured. At baseline, all participants in the HB and SOC groups completed at least 1 day of a 24-h dietary recall, which decreased at the 12-month timepoint, with 95.8% of participants in the HB group and 92.3% of participants in the SOC group completing at least 1 day (Supplementary Table 4). However, the completion percentage of participants declined for the requested 3 days of a 24-h dietary recall using ASA24 for the HB group. At baseline, 86.2% of participants in the HB group and 66.7% of participants in the SOC group completed 3 days of dietary recalls. At 12 months, 83.3% of the participants in the HB group had completed the requested 3 days of 24-h dietary recalls, and 84.6% in the SOC group, surpassing the predefined threshold for success, which was set at greater than 80% completion. There were no statistically significant differences in the percent of completion between the HB and SOC groups at any time point and the number of 24-h recalls completed (p > 0.05 for all).
4. Discussion
Following a 12-month, remotely delivered, multimodal intervention that included the modified Paleolithic elimination diet, the HB group reduced its intake of added sugars, total grains, refined grains, total dairy, peanuts, tree nuts, and seeds, as well as legumes from baseline to month 12. Moreover, at month 12, using the EAR-cut point method, the proportion of participants with inadequate intake of micronutrients exceeded the threshold for severe inadequacy (defined as ≥20% of the group) in key nutrients, including calcium, vitamin D, vitamin E, and copper. Conversely, within the SOC group, adhering to their typical dietary patterns, the proportion with inadequate intake of micronutrients exceeded the threshold for severe inadequacy for vitamin E, calcium, vitamin D, zinc, and additional nutrients, including vitamin B12, thiamin, riboflavin, folate, and vitamin A. These findings suggest that individuals recently diagnosed with RRMS or CIS who adopt a modified Paleolithic elimination diet as part of a lifestyle behavioral intervention may reduce their risk of severe inadequacy for certain nutrients, as compared to adhering to their usual dietary practices.
Participants following the intervention diet significantly reduced their intake of added sugars and total grains while increasing cured meats by 12 months compared to their baseline diet. The observed reductions in food group intakes can be attributed to HB group participants’ high adherence (95.8% adherence) to the modified Paleolithic elimination diet, which has been previously noted (24). The modified Paleolithic diet recommends an intake of meat based on sex and size of the individual, limited intake of gluten-free grains and legumes while strictly avoiding dairy, gluten-containing grains, eggs, and sweeteners/sweet foods, which corresponds with the present study participants’-reduced intake of the respective food groups. Most notably, participants in the HB group consistently maintained a lowered intake of added sugars throughout the entire 12-month duration compared to their baseline usual diet, which was not observed in the SOC group. Added sugars refer to sugars that are added in food preparation or manufacturing, such as glucose, fructose, sucrose (a sugar molecule made from glucose and fructose combined), and hydrogenated starch hydrolysates (high-fructose corn syrup) (43), often used to sweeten or enhance the flavor of foods. Excessive sugar consumption has been associated with various health concerns, including obesity, metabolic disorders, diabetes, cardiovascular disease, cancer, depression, and cognitive impairment (43). Considering the accumulating evidence linking increased added sugar intake and chronic diseases, both the American Heart Association and World Health Organization recommend limiting added sugars to no more than 10% of total calories; which, for the average adult, translates to approximately 200 calories, 50 g, or 12 teaspoons (43), as well as aligning with the modified Paleolithic elimination diet recommendations of one teaspoon or less. Specifically in MS, findings from an animal MS mouse model, experimental autoimmune encephalomyelitis, an animal model of MS, fed a high-fructose diet for up to 12 weeks, found that the abundance of beneficial gut bacteria decreased while potentially pro-inflammatory bacteria were enriched (44). Furthermore, the study revealed increased expression of intestinal immune markers across the small intestine, colon, and spleen (44), suggesting a potential link between high-sugar intake in MS and the alteration of gut microbiota, triggering unfavorable immune responses within both the gut and the periphery, potentially contributing to MS-related symptoms; however, further research is necessary to understand the influence of high-sugar intake among a human MS population. Understanding the food groups eaten by individuals with MS is important, as significant associations of healthy dietary habits, such as increased consumption of fruit, vegetables, and dietary fat food groups, are associated with improved QoL and less likelihood of increased disability when compared to individuals following a less nutritious diet (45).
At 12-months, the HB group exhibited severe inadequacies (>20% of the group) for the micronutrients, vitamin D, vitamin E, calcium, and copper. The micronutrient intakes noted in the HB group share some similarities with a previous randomized control trial involving individuals with RRMS who followed a modified Paleolithic elimination diet, finding that by 12- and 24-weeks, participants had inadequate intake from food and supplements of calcium and iron (22). Since the modified Paleolithic elimination diet restricts dairy, consumption of calcium-fortified foods such as fortified juices or milk alternatives or calcium supplementation may be beneficial (22). Supplementation of vitamin D is recommended for individuals with MS (46), given the lack of vitamin D-rich food. The addition of vitamin E-rich food, such as sunflower seeds, almonds, and avocados, and copper-rich foods, such as oysters and mushrooms, may be beneficial to avoid deficiencies.
Alarmingly, the SOC group, following their own diet, had inadequacies for all the same micronutrients, except for copper, as the HB group, in addition to vitamin A, thiamin, riboflavin, folate, vitamin B12, and zinc. This is similar to some of the micronutrients found to be in lower intake among people with MS in the Netherlands, compared to the general population (47). Furthermore, the SOC group inadequacies are further corroborated in the general U.S. adult population, in which inadequacies in vitamin A, vitamin C, vitamin D, vitamin E, calcium, and magnesium intake have been noted both from food and supplements (42). The SOC group’s inadequacies in B vitamins are of concern, as deficiencies in vitamin B12 and folate have been linked with increased fatigue (19), lower physical ability (48), and can contribute to the pathogenesis of MS (49). Among individuals with RRMS, vitamin B12 and folic acid supplementation have revealed the potential role in improving the QoL (50). In the current study, the supplementation of vitamin B12, methyl folate, and pyridoxal-5-phosphate in the HB group may have mitigated deficiencies in these micronutrients, as inadequacies were only present in the SOC group. Thus, suggesting the importance of targeted micronutrient supplementation for individuals with MS to avoid nutrient insufficiencies and that dietary modifications along with nutrient supplementation may benefit individuals with MS (51).
The potential for excess intake of certain nutrients is of concern when combining dietary modifications and nutrient supplementation. By month 12, the HB group had increased excess intake from baseline of selenium and zinc; whereas, the SOC group only had an excess of vitamin A. Excessive intake of certain micronutrients, specifically for fat-soluble vitamins, such as vitamin A, which was observed in the SOC group, are of great concern as accumulation in the body can result in toxicity. On the other hand, excess water-soluble vitamins, such as B vitamins, are of less concern as they can excreted from the body through urine. Mineral excess, such as selenium and zinc observed in the HB group, can result in adverse health events. Selenium toxicity can lead to symptoms such as nausea, vomiting, nail discoloration, hair loss, fatigue, irritability, and foul breath odor (often described as “garlic breath”) (52). Although uncommon, zinc toxicity can lead to nausea, vomiting, epigastric pain, lethargy, and fatigue (53). While no statistically significant changes were observed from baseline to 12 months for both HB and SOC groups, it is noteworthy that magnesium levels exhibited the greatest mineral excess at both time points. This can possibly be attributed to participants taking additional magnesium supplements, given that dietary sources of magnesium, such as dark green vegetable intake, did not change by 12 months and excess. Worth noting, that although excess magnesium intake has not been associated with toxicity, it may lead to gastrointestinal distress and discomfort, such as diarrhea (54). While upper limits of certain nutrients have been created by the Food and Agriculture Organization of the United Nations/World Health Organization to avoid the increased likelihood of adverse effects, excessive intake of nutrients should be monitored, especially in vulnerable populations, such as individuals with MS, to avoid chronic adverse effect outcomes (55).
The feasibility of using ASA24 for conducting 24-h dietary recall among individuals newly diagnosed with MS as a means of dietary assessment was assessed in the present study. Remarkably, at 12 months, both study groups achieved a completion rate of over 80% for the requested 3 days of 24-h recalls; the completion rate increased to above 90% when considering the completion of at least a single 1-day 24-h recall. This high completion rate can potentially be attributed to the proactive approach taken by the Study Coordinator, who sent email reminders to all participants to complete the 24-h recalls at the specific study interval time points, as well as the continuous support from the study RDN for the HB group. While completion of dietary recalls was notably high, it is important to note that three participants required assistance from the study RDN to complete the ASA24 recalls over the phone due to cognitive impairment. While moderate to severe cognitive impairment, as measured by the Short Portable Mental Status Questionnaire (SPMSQ) (26), was an exclusion criterion for the present study, these findings are similar to a prior study that obtained dietary recalls through ASA24 from individuals diagnosed with MS (56). The need for assistance among participants in completing 24-h dietary recalls may be a consideration for future research studies, given that the incidence of cognitive impairment can occur among individuals with MS (57). Potential accommodations could be explored, such as the inclusion of a support individual within the study who could act as a proxy to complete the recalls on behalf of the participant, which could help alleviate the burden associated with recalling dietary intake for individuals with MS. In addition, not all foods and ingredients recommended in the modified Paleolithic elimination diet were available as selectable options within the ASA24 platform. Although suggested food substitutions were provided to HB group participants, this additional step could potentially introduce further participant burden or confusion, particularly for participants with cognitive impairment, as well as not accurately represent the participant’s dietary intake. Searching for alternative objective strategies to assess the dietary intake for people with MS could enhance the accuracy and reliability of dietary assessments for future studies (30).
Strengths of this study include the use of 3-day 24-h dietary recalls and longitudinal collection of dietary recalls; however, limitations were present in this study. The study’s sample size is below the standard recommendation for the NCI method (n = 50) used to adjust usual intake; although, the dietary data spanned more days than required by the NCI method to mitigate the impact of limited sample size on variability; thus, these results are not generalizable. Additionally, the ASA24 did not contain several of the foods and ingredients found in modified Paleolithic elimination diet recommendation, which may likely not accurately represent the dietary intake of the HB group participants. Considering the study’s timing during the Coronavirus 2019 (COVID-19) pandemic, it is plausible that participants’ eating habits were influenced (58), and potentially affecting vitamin and mineral supplement intake. Social desirability bias may have resulted in more favorable 24-h dietary intake self-reports from the HB group (59). Furthermore, recall bias may have also influenced the accuracy of usual dietary intake records from all participants (60, 61), especially given that some participants in the current study experienced cognitive impairment. While participants in the HB group were asked about their adherence to each component of the study (i.e., study diet, breathing exercises, and 10-min walking regimen), the vitamin regimen intake adherence was not specifically asked under the study diet, as well as not asked from the SOC group. Future studies should highly consider adding objective measurement methods of dietary intake assessment, in addition to the self-reported ASA24, to ensure accurate analysis.
In Memoriam
In memoriam of Dr. John Kamholz, who passed during the peer review process of the manuscript.
Acknowledgments
The authors want to acknowledge the students who supported recruitment and data acquisition, as well as the participants in this study, for their dedication and commitment to the study intervention.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by the Institute for Clinical and Translational Science (ICTS) at the University of Iowa, and University of Iowa institutional funds. The ICTS is supported by the National Institutes of Health Clinical and Translational Science Award program (UM1TR004403). SMS is a research trainee of the Dartmouth Health Primary Care Fellowship (T32HP32520), which is supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS). TJT, SMS, and FS were supported by the Carter Chapman Shreve Family Foundation and the Carter Chapman Shreve Fellowship Fund for diet and lifestyle research conducted by the Wahls Research team at the University of Iowa. In-kind support was provided by the University of Iowa College of Public Health Preventive Intervention Center. The study was supported by PI discretionary funds. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U.S. Government.
Footnotes
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the University of Iowa Institutional Review Board [(IRB #201908778)]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ME: Investigation, Project administration, Writing – review & editing. LB: Investigation, Writing – review & editing. TT: Formal analysis, Software, Validation, Writing – review & editing. PT: Formal analysis, Software, Writing – review & editing. LR: Formal analysis, Writing – review & editing. BB: Investigation, Writing – review & editing. FS: Investigation, Writing – review & editing. CG: Investigation, Writing – review & editing. JK: Investigation, Writing – review & editing. LS: Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing. TW: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Conflict of interest
TW personally follows and promotes the Wahls™ diet. She has equity interest in the following companies: Terry Wahls LLC; TZ Press LLC; The Wahls Institute, PLC; FBB Biomed Inc.; Levels Health Inc., Foogal Inc. and the website http://www.terrywahls.com. She also owns the copyright to the books Minding My Mitochondria (2nd Edition) and The Wahls Protocol, The Wahls Protocol Cooking for Life, and the trademarks The Wahls Protocol® and Wahls™ diet, Wahls Paleo™ diet, and Wahls Paleo Plus™ diets, and Wahls Behavior Change™. She has completed grant funding from the National Multiple Sclerosis Society for the Dietary Approaches to Treating Multiple Sclerosis Related Fatigue Study. She has financial relationships with Vibrant America LLC, Standard Process Inc., MasterHealth Technologies Inc., Foogal Inc., and the Institute for Functional Medicine Inc. She receives royalty payments from Penguin Random House. TW has conflict of interest management plans in place with the University of Iowa and the Iowa City Veteran’s Affairs Medical Center.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SP declared a past co-authorship with the authors TT and TW.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1369700/full#supplementary-material
References
- 1.Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. (2019) 26:27–40. doi: 10.1111/ene.13819 [DOI] [PubMed] [Google Scholar]
- 2.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 3.Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases. (2015) 3:545–55. doi: 10.12998/wjcc.v3.i7.545, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. (2018) 391:1622–36. doi: 10.1016/S0140-6736(18)30481-1 [DOI] [PubMed] [Google Scholar]
- 5.Hartung DM, Bourdette DN, Ahmed SM, Whitham RH. The cost of multiple sclerosis drugs in the us and the pharmaceutical industry: too big to fail? Neurology. (2015) 84:2185–92. doi: 10.1212/WNL.0000000000001608, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratos K, McGarragle K, Thistle J, Vyas MV, Lee L. Non-compliance with disease modifying therapies in patients with multiple sclerosis: a qualitative analysis. Mult Scler Relat Disord. (2020) 41:102016. doi: 10.1016/j.msard.2020.102016, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Marrie RA, Salter AR, Fox R, Cofield SS, Tyry T, et al. Health insurance affects the use of disease-modifying therapy in multiple sclerosis. Neurology. (2016) 87:365–74. doi: 10.1212/WNL.0000000000002887, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn M, Bhargava P, Kalb R. Your patients with multiple sclerosis have set wellness as a high priority-and the national multiple sclerosis society is responding. US Neurology. (2015) 11:80–6. doi: 10.17925/USN.2015.11.02.80 [DOI] [Google Scholar]
- 9.Silveira SL, Richardson EV, Motl RW. Desired resources for changing diet among persons with multiple sclerosis: qualitative inquiry informing future dietary interventions. Int J MS Care. (2022) 24:175–83. doi: 10.7224/1537-2073.2021-052, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean C, Parks S, Titcomb TJ, Arthofer A, Meirick P, Grogan N, et al. Facilitators of and barriers to adherence to dietary interventions perceived by women with multiple sclerosis and their support persons. Int J MS Care. (2022) 24:235–41. doi: 10.7224/1537-2073.2021-051, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell RD, Black LJ, Begley A. Navigating dietary advice for multiple sclerosis. Health Expect. (2021) 24:853–62. doi: 10.1111/hex.13226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkhalii-Wilhelm S, Sippel A, Riemann-Lorenz K, Kofahl C, Scheiderbauer J, Arnade S, et al. Experiences of persons with multiple sclerosis with lifestyle adjustment-a qualitative interview study. PLoS One. (2022) 17:e0268988. doi: 10.1371/journal.pone.0268988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans E, Levasseur V, Cross AH, Piccio L. An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult Scler Relat Disord. (2019) 36:101393. doi: 10.1016/j.msard.2019.101393, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Snetselaar LG, Cheek JJ, Fox SS, Healy HS, Schweizer ML, Bao W, et al. Efficacy of diet on fatigue and quality of life in multiple sclerosis: a systematic review and network meta-analysis of randomized trials. Neurology. (2023) 100:e357–66. doi: 10.1212/WNL.0000000000201371, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Guerrero Aznar MD, Villanueva Guerrero MD, Cordero Ramos J, Eichau Madueño S, Morales Bravo M, López Ruiz R, et al. Efficacy of diet on fatigue, quality of life and disability status in multiple sclerosis patients: rapid review and meta-analysis of randomized controlled trials. BMC Neurol. (2022) 22:388. doi: 10.1186/s12883-022-02913-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chenard CA, Rubenstein LM, Snetselaar LG, Wahls TL. Nutrient composition comparison between a modified Paleolithic diet for multiple sclerosis and the recommended healthy U.S.-style eating pattern. Nutrients. (2019) 11:537. doi: 10.3390/nu11030537, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahls TL, Titcomb TJ, Bisht B, Eyck PT, Rubenstein LM, Carr LJ, et al. Impact of the Swank and Wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: the waves randomized parallel-arm clinical trial. Mult Scler J Exp Transl Clin. (2021) 7:20552173211035399. doi: 10.1177/20552173211035399, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisht B, Darling WG, Shivapour ET, Lutgendorf SK, Snetselaar LG, Chenard CA, et al. Multimodal intervention improves fatigue and quality of life in subjects with progressive multiple sclerosis: a pilot study. Degener Neurol Neuromuscul Dis. (2015) 5:19–35. doi: 10.2147/dnnd.S76523, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitarafan S, Harirchian MH, Nafissi S, Sahraian MA, Togha M, Siassi F, et al. Dietary intake of nutrients and its correlation with fatigue in multiple sclerosis patients. Iran J Neurol. (2014) 13:28–32. PMID: [PMC free article] [PubMed] [Google Scholar]
- 20.Concerto C, Rodolico A, Ciancio A, Messina C, Natale A, Mineo L, et al. Vitamin D and depressive symptoms in adults with multiple sclerosis: a scoping review. Int J Environ Res Public Health. (2021) 19:199. doi: 10.3390/ijerph19010199, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titcomb TJ, Bisht B, Moore DD, 3rd, Chhonker YS, Murry DJ, Snetselaar LG, et al. Eating pattern and nutritional risks among people with multiple sclerosis following a modified Paleolithic diet. Nutrients. (2020) 12:1844. doi: 10.3390/nu12061844, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Titcomb TJ, Brooks L, Smith KL, Ten Eyck P, Rubenstein LM, Wahls TL, et al. Change in micronutrient intake among people with relapsing-remitting multiple sclerosis adapting the Swank and Wahls diets: an analysis of weighed food records. Nutrients. (2021) 13:3507. doi: 10.3390/nu13103507, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masullo L, Papas MA, Cotugna N, Baker S, Mahoney L, Trabulsi J. Complementary and alternative medicine use and nutrient intake among individuals with multiple sclerosis in the United States. J Community Health. (2015) 40:153–60. doi: 10.1007/s10900-014-9913-z, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Saxby SM, Shemirani F, Crippes LJ, Ehlinger MA, Brooks L, Bisht B, et al. Effects of a remote multimodal intervention involving diet, walking program, and breathing exercise on quality of life among newly diagnosed people with multiple sclerosis: a quasi-experimental non-inferiority pilot study. Degener Neurol Neuromuscul Dis. (2024) 14:1–14. doi: 10.2147/DNND.S441738, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bärnighausen T, Tugwell P, Røttingen JA, Shemilt I, Rockers P, Geldsetzer P, et al. Quasi-experimental study designs series-paper 4: uses and value. J Clin Epidemiol. (2017) 89:21–9. doi: 10.1016/j.jclinepi.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. (1975) 23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 27.Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. (2009) 64:527–37. doi: 10.1037/a0016830, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrick H, Williams GC. Self-determination theory: its application to health behavior and complementarity with motivational interviewing. Int J Behav Nutr Phys Act. (2012) 9:18. doi: 10.1186/1479-5868-9-18, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behavior. Psychol Inq. (2000) 11:227–68. doi: 10.1207/S15327965PLI1104_01 [DOI] [Google Scholar]
- 30.Subar AF, Kirkpatrick SI, Mittl B, Zimmerman TP, Thompson FE, Bingley C, et al. The automated self-administered 24-hour dietary recall (Asa24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. (2012) 112:1134–7. doi: 10.1016/j.jand.2012.04.016, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkpatrick SI, Subar AF, Douglass D, Zimmerman TP, Thompson FE, Kahle LL, et al. Performance of the automated self-administered 24-hour recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr. (2014) 100:233–40. doi: 10.3945/ajcn.114.083238, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell DC, Cheng FW, Still CD, Jensen GL. A validation of automated self-administered 24-hour dietary recalls(Asa24) relative to interviewer-administered recalls using the nutrition data system for research (Ndsr). FASEB J. (2016) 30:43.3. doi: 10.1096/fasebj.30.1_supplement.43.3 [DOI] [Google Scholar]
- 33.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The Redcap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendez MA, Popkin BM, Buckland G, Schroder H, Amiano P, Barricarte A, et al. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am J Epidemiol. (2011) 173:448–58. doi: 10.1093/aje/kwq380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tooze JA, Kipnis V, Buckman DW, Carroll RJ, Freedman LS, Guenther PM, et al. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: the Nci method. Stat Med. (2010) 29:2857–68. doi: 10.1002/sim.4063, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R Springer; (2009). [Google Scholar]
- 38.Institute of Medicine Subcommittee on I, Uses of Dietary Reference I, Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference I . Dri dietary reference intakes: applications in dietary assessment. Washington (DC): National Academies Press (US) (2000). [Google Scholar]
- 39.Suitor CW, Bailey LB. Dietary folate equivalents: interpretation and application. J Am Diet Assoc. (2000) 100:88–94. doi: 10.1016/s0002-8223(00)00027-4 [DOI] [PubMed] [Google Scholar]
- 40.Medicine Io, Board F, Nutrition, Nutrients SoURLo, Standing Committee on the Scientific Evaluation of Dietary Reference I, its Panel on Folate OBV et al. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, D.C: National Academies Press; (2000). [PubMed] [Google Scholar]
- 41.Institute of Medicine Panel on M . Dietary reference intakes for vitamin a, vitamin K, arsenic, boron, chromium, copper, iodine, Iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press (US) (2001). [PubMed] [Google Scholar]
- 42.Agarwal S, Reider C, Brooks JR, Fulgoni VL., 3rd. Comparison of prevalence of inadequate nutrient intake based on body weight status of adults in the United States: an analysis of NHANES 2001-2008. J Am Coll Nutr. (2015) 34:126–34. doi: 10.1080/07315724.2014.901196, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Gillespie KM, Kemps E, White MJ, Bartlett SE. The impact of free sugar on human health-a narrative review. Nutrients. (2023) 15:889. doi: 10.3390/nu15040889, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson SR, Ali S, Shrode RL, Mangalam AK. Effect of a fructose-rich diet on gut microbiota and immunomodulation: potential factors for multiple sclerosis. Immunohorizons. (2023) 7:213–27. doi: 10.4049/immunohorizons.2300008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadgkiss EJ, Jelinek GA, Weiland TJ, Pereira NG, Marck CH, van der Meer DM. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr Neurosci. (2015) 18:125–36. doi: 10.1179/1476830514y.0000000117, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans E, Piccio L, Cross AH. Use of vitamins and dietary supplements by patients with multiple sclerosis: a review. JAMA Neurol. (2018) 75:1013–21. doi: 10.1001/jamaneurol.2018.0611 [DOI] [PubMed] [Google Scholar]
- 47.Ramsaransing GS, Mellema SA, De Keyser J. Dietary patterns in clinical subtypes of multiple sclerosis: an exploratory study. Nutr J. (2009) 8:36. doi: 10.1186/1475-2891-8-36, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bromley L, Horvath PJ, Bennett SE, Weinstock-Guttman B, Ray AD. Impact of nutritional intake on function in people with mild-to-moderate multiple sclerosis. Int J MS Care. (2019) 21:1–9. doi: 10.7224/1537-2073.2017-039, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis. J Clin Neurosci. (2009) 16:399–403. doi: 10.1016/j.jocn.2008.05.015, PMID: [DOI] [PubMed] [Google Scholar]
- 50.Nozari E, Ghavamzadeh S, Razazian N. The effect of vitamin B12 and folic acid supplementation on serum homocysteine, anemia status and quality of life of patients with multiple sclerosis. Clin Nutr Res. (2019) 8:36–45. doi: 10.7762/cnr.2019.8.1.36, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav V, Shinto L, Bourdette D. Complementary and alternative medicine for the treatment of multiple sclerosis. Expert Rev Clin Immunol. (2010) 6:381–95. doi: 10.1586/eci.10.12, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, et al. Acute selenium toxicity associated with a dietary supplement. Arch Intern Med. (2010) 170:256–61. doi: 10.1001/archinternmed.2009.495, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fosmire GJ. Zinc toxicity. Am J Clin Nutr. (1990) 51:225–7. doi: 10.1093/ajcn/51.2.225 [DOI] [PubMed] [Google Scholar]
- 54.Medicine Io, Board F, Nutrition, Intakes SCotSEoDR . Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, D.C: National Academies Press; (2000). [PubMed] [Google Scholar]
- 55.Pike V, Zlotkin S. Excess micronutrient intake: defining toxic effects and upper limits in vulnerable populations. Ann N Y Acad Sci. (2019) 1446:21–43. doi: 10.1111/nyas.13993, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Silveira SL, Jeng B, Gower BA, Motl RW. Feasibility, acceptability, and preliminary validity of self-report dietary assessment in adults with multiple sclerosis: comparison with doubly labeled water measured Total energy expenditure. Nutrients. (2021) 13:1198. doi: 10.3390/nu13041198, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagert B, Camplair P, Bourdette D. Cognitive dysfunction in multiple sclerosis: natural history, pathophysiology and management. CNS Drugs. (2002) 16:445–55. doi: 10.2165/00023210-200216070-00002, PMID: [DOI] [PubMed] [Google Scholar]
- 58.González-Monroy C, Gómez-Gómez I, Olarte-Sánchez CM, Motrico E. Eating behaviour changes during the COVID-19 pandemic: a systematic review of longitudinal studies. Int J Environ Res Public Health. (2021) 18:11130. doi: 10.3390/ijerph182111130, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. (1995) 24:389–98. doi: 10.1093/ije/24.2.389, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Willett W, Willett WC. 5024-hour dietary recall and food record methods In: Willett W, editor. Nutritional epidemiology. Oxford: Oxford University Press; (1998) [Google Scholar]
- 61.Naska A, Lagiou A, Lagiou P. Dietary assessment methods in epidemiological research: current state of the art and future prospects. F1000Res. (2017) 6:926. doi: 10.12688/f1000research.10703.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.