Abstract
Introduction
Imaging fibroid vascularity may predict fibroid growth and aid to determine most appropriate therapy. Microvascular (MV) flow imaging is relatively new and is able to detect slow flow in small vessels. Data on feasibility, reproducibility, and reliability of MV‐flow imaging in fibroids is lacking. The purpose of our study was to determine the reproducibility of MV‐flow imaging and to explore this technique for clinical practice for assessing blood flow in fibroids.
Material and Methods
Thirty patients with one or multiple fibroids (diameter 1.5–12.0 cm) were prospectively included. Transvaginal ultrasound scanning was performed in B‐mode, 2D MV‐Flow™, 2D and 3D power Doppler mode (HERA W10, Samsung) by two experienced gynecologists at a tertiary care clinic from February to December 2021. The primary outcome was intra‐ and interobserver agreement of the vascular index (VI) and color score (CS). The following parameters: ‘2D MV‐flow VI’, ‘3DPD VI’, ‘2D MV‐flow CS’ and ‘2DPD CS’ were measured offline in the center, pseudocapsule, and entire fibroid. Secondary offline outcomes for exploring 2D MV‐flow for clinical practice, included (1) ability to discern vascular structures, (2) assessing the degree of vascularity via CS and calculating a VI, and (3) determining penetration depth of the ultrasound signal in both power Doppler and MV‐flow imaging.
Results
All scans of the 30 included patients were of sufficient quality to analyze. Inter‐ and intra‐observer correlations of all studied parameters were good to excellent, both for 2D MV‐flow and 2D power Doppler (intercorrelation coefficient 0.992–0.996). Using 2D MV‐flow different vascular structures were visible in detail, in contrary to using 2D and 3D power Doppler. In significantly more fibroids central flow could be visualized using 2D MV‐flow (63%) than with 2D power Doppler (13%, p = 0.001). Finally, penetration of the ultrasound signal was deeper using 2D MV‐flow (3.92 cm) than with 2D power Doppler (2.95 cm, p = 0.001).
Conclusions
Using 2D MV‐flow imaging for determining vascularity is highly reproducible. It has potential added value for clinical practice as it depicts detailed vascular structures and the degree of vascularity, especially in the center of the fibroid.
Keywords: Doppler, fibroids, microvascular flow, sonography, ultrasound, vascularity
Microvascular flow imaging is relatively new and is able to detect slow flow in small vessels. In 30 patients, we showed good reproducibility between microvascular flow and conventional power Doppler imaging. Microvascular flow imaging is easy to use in clinical practice as it depicts detailed vascular structures and differences in tissue penetration, especially in the center of the fibroid.
Abbreviations
- 2D
two‐dimensional
- 3D
three‐dimensional
- CS
color score
- ICC
intercorrelation coefficient
- MV
microvascular
- PD
power Doppler
- VI
vascular index
Key message.
2D microvascular‐flow imaging is highly reproducible and has potential added value for clinical practice as it depicts detailed vascular structures and the degree of vascularity, especially in the center of the fibroid.
1. INTRODUCTION
Uterine fibroids are the most common benign tumors in gynecology. 1 The majority of fibroids is asymptomatic. 2 However, when symptomatic, they may substantially impact women's health and quality of life. 3 Whether fibroids are symptomatic depends on their size, location, and vascularity. 4 , 5 Fibroid growth is influenced by steroid hormones and growth factors, 6 , 7 which reach the fibroid via the uterine artery, through the arcuate arteries, and towards the fibroid capillaries. 8 Fibroids typically have a highly vascularized ‘pseudocapsule’ with larger perifibroid vessels, while the center mainly contains slow‐flow capillaries. 9 , 10 , 11 The extent of fibroid's vascularization is likely to predict its growth and whether the fibroid will become symptomatic. 5 , 12 Measuring the vascularity of uterine lesions, particularly the microvascularity, may also become useful for differentiating atypical uterine fibroids from adenomyosis or uterine sarcomas based on different vascular patterns. 5 , 13 , 14 Accurate diagnosis of myometrial tumors is essential to plan appropriate treatment. Moreover, measuring microvascularity may be useful for evaluating the efficacy of minimally invasive treatments pursuing microvascular ischemia, such as uterine artery embolization. 15 , 16
Ultrasound is a first‐line modality to evaluate fibroids in the outpatient clinic. 17 The macrovascularity of fibroids can be visualized using color or power Doppler. A subjective qualitative color score can describe the degree of circumferential and intralesional vascularization. Vascularity can also be quantified using three‐dimensional (3D) power Doppler resulting in a vascular index. 13 Limitations of both two‐dimensional (2D) and 3D power Doppler measurements are the penetration depth of the ultrasound signal and the inability to visualize microvascularity.
Microvascular (MV) flow imaging is a relatively new Doppler technique that allows visualization of small vessels with slow blood flow velocity. Compared with conventional Doppler imaging techniques, MV‐flow uses a lower pulse repetition frequency, higher frame rate and advanced filtering mode. 18 The high frame rate provides high‐resolution details showing both macro‐ and microvascular blood flow. The advanced filtering modes can, for example, filter noise and tissue movement, while still showing low‐velocity blood flow. 18 , 19 , 20 MV‐flow images can be evaluated subjectively, resulting in a 2D MV‐flow color score (CS), or a 2D MV‐flow vascular index (VI) can be calculated. This technique of measuring slow blood flow is available for multiple ultrasound manufacturers. 18
Qualitatively assessing vascularity using 3D power Doppler vascular index is a widely used technique with a well‐reported reproducibility and good discriminating ability related to histology. 21 , 22 , 23 However, data concerning the reproducibility of these read‐out parameters (CS and VI) determined using 2D MV‐flow is lacking. Therefore, our primary objective was to determine reproducibility and reliability for measuring the vascular index (VI) and CS using 2D MV‐flow imaging and 2D and 3D power Doppler. In addition, we hypothesized that 2D MV‐flow imaging may be capable of imaging more vascular structures than conventional 2D and 3D power Doppler. Therefore, our secondary objectives involved exploring 2D MV‐flow for clinical practice in terms of (1) display of vascular structures, (2) assessing the degree of vascularity in the fibroid, and (3) determining the penetration of the ultrasound signal in both power Doppler and MV‐flow imaging.
2. MATERIAL AND METHODS
2.1. Study design and patient selection
We performed a prospective cohort study in Amsterdam UMC, a tertiary university hospital, from February to December 2021. Sample size was based on Samanci et al. 24 and calculated using a sample size calculator for reliability studies: expected intercorrelation coefficient (ICC) 0.95; accepted lower limit of the 95% confidence interval 0.85; two raters; expected drop‐out 15%: n = 30. 25
We prospectively enrolled patients with uterine fibroids who visited our outpatient clinic. Eligible patients were women with one or multiple fibroids, with a diameter between 1.5 and 12 cm. The presence of multiple uterine fibroids was allowed, but only if one specific target fibroid was easily recognizable as either the largest fibroid or the fibroid closest to the ultrasound probe. Fibroids were classified according to the International Federation of Gynecology and Obstetrics. 26 Exclusion criteria were: younger than 18 years, pregnancy, uterine or cervical malignancy, and the suspicion of adenomyosis. 13 Current or past use of hormonal therapy was not an exclusion criterion. Patients with only good quality ultrasound images suitable for analysis, and with recordings from all ultrasound techniques successfully performed and stored, were included in the study.
2.2. Ultrasound
2D power Doppler, 3D power Doppler and 2D MV‐flow recordings were made during outpatient consultation by an experienced gynecologist (JH or RL) with more than 10 years of experience in advanced ultrasound evaluation of fibroids. During a single consultation 2D B‐mode, 2D power Doppler and 2D MV‐flow cine loops and 3D power Doppler volumes were recorded and stored.
The ultrasound scans were made using a standardized protocol, 11 , 27 including 2D B‐mode and power Doppler evaluation of the uterus and fibroids according to the Morphological Uterus Sonographic Assessment (MUSA) criteria. 13 3D power Doppler scans were made of the region of interest, framing the entire fibroid. In the case of noise artifacts, the fine‐tuning technique was applied conform Nieuwenhuis et al., by increasing the gain until an obvious artifact was visible and then lowering the gain until the artifacts had just vanished. 11 , 27 Patients received instructions to minimize movement artifacts.
Ultrasound scanning was performed using a HERA W10 system (Samsung Medison Co., Ltd., Seoul, South Korea) with a 3D EV2‐10A or EV3‐10B endovaginal probe (2–10 MHz). The settings of the HERA W10 ultrasound included a 2D frequency of 2.0–8.2 MHz, frame average 8–9, gain 50–86 dB, dynamic range 50, power 90 (maximal). Power Doppler pulse repetition frequency of 0.54–58 kHz, sensitivity of 10, filter 3, gain 50. Microvascular flow mode pulse repetition frequency 0.13 kHz, gain 38–50; 3D quality ‘high2’.
2.3. Offline evaluation
The ultrasound examiners, who made the images in the real‐time phase, did not participate in the offline evaluation because of logistic reasons in a research setting. The stored 2D, 3D power Doppler and 2D MV‐flow images were evaluated offline on the same ultrasound machine, by two different and independent observers (MF and BS), both with more than 5 years' experience in advanced ultrasound evaluation of fibroids. The observers were blinded to all patients' demographics and clinical information. From the original cine loop recording, the 2D plane containing the maximal diameter of the fibroid, with clearly present vascularization and no artifacts, was selected for further offline analysis in consensus by both observers (MF, BS). The observers independently drew regions of interest offline. The fibroids pseudocapsule was measured 5 mm from the outline of the entire fibroid. 27 The fibroids' center was a subjective estimation of the inner one‐third of the fibroid's largest diameter. This is schematically depicted in Figure 1.
FIGURE 1.
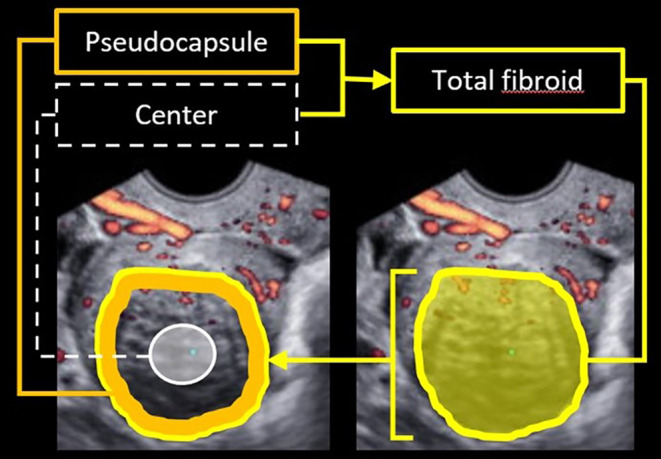
Schematic drawing of the three contours for analysis: Total fibroid (surface within bright‐yellow contour); pseudocapsule (solid orange line), measured 5 mm from the outline of the total fibroid; and the center (surface white circle), as subjective estimation of the inner one‐third of the surface of the the total fibroid.
2.4. Qualitative analysis—Color score
A subjective CS was determined offline on a single 2D power Doppler image and on a 2D MV‐flow image, using the criteria of the Morphological Uterus Sonographic Assessment (MUSA) criteria. No color corresponded with a CS of 1, minimal color = 2, moderate color = 3, and abundant color = 4. 13 All measurements were independently performed by both authors (MF and BS).
2.5. Quantitative analysis—Vascular index
A vascular index (VI) was calculated offline on 3D power Doppler volumes and 2D MV‐flow images. The VI in 3D power Doppler represents the percentage of colored voxels divided by all voxels (colored and gray voxels), the same formula in pixels accounts for 2D MV‐flow images. 13 Offline vascular index using 3D power Doppler was performed using Virtual Organ Computer‐aided AnaLysis (VOCAL) software Sonoview Pro‐1.6.2 (Samsung Medison Co., Ltd., Seoul, South Korea), previously described by Nieuwenhuis et al. The contour of the fibroid was drawn manually for both 3D power Doppler and 2D MV‐flow vascular index. 11 , 27 In short, six rotation steps of 30 degrees were applied to define the total fibroid resulting in a 3D volume. The shell off mode or the inner shell mode (5 mm) was selected corresponding to respectively the entire fibroid or the vascular capsule. The vascular index was drawn automatically for the fibroid center.
2.6. Outcome measurements
To investigate reproducibility and reliability, the primary outcomes were intra‐ and inter‐observer agreements, which were calculated for (1) 2D MV‐flow vascular index, (2) 3D power Doppler vascular index, (3) 2D MV‐flow color score, and (4) 2D power Doppler color score in the center of the fibroid, its capsule and the entire fibroid. For the intra‐observer analysis, all variables were calculated twice by observer 1 (MF), with at least 1 week between the two measurements that were assessed in a random order. For the inter‐observer analysis, all variables were measured independently by observer 2 (BS). An ICC of 0.75–0.90 indicates a good agreement, ICC >0.90 an excellent agreement. 28
Secondary outcomes for exploring 2D MV‐flow imaging in the clinic concerned (1) display of vascular structures, (2) the degree of vascularity, and (3) the penetration of the Doppler signal. All secondary outcomes were measured offline. Different vascular structures, including (a) uterine vascular arcade, consisting of arcuate arteries, (b) radial arteries, (c) fibroid's pseudocapsule, consisting of branches from arcuate and radial vascular capsule, (d) vascular branches penetrating the center of the fibroid, were visually identified according to the Morphological Uterus Sonographic Assessment (MUSA) criteria on 2D MV‐flow images. 13 The degree of vascularity is expressed as (I) subjective color score on 2D power Doppler and 2D MV‐flow images and (II) as objective vascular index on 3D power Doppler and 2D MV‐flow images. Measurements were performed in the fibroid center and the fibroid capsule and entire fibroid. The penetration of the Doppler signal was measured in centimeters on 2D power Doppler images, 3D power Doppler images and 2D MV‐flow images. The penetration depth was defined along the direction of the ultrasound wave, from the probe to the deepest ultrasound signal visible.
2.7. Statistical analyses
All statistical analyses were performed using SPSS Statistics 22.0 software package (IBM, New York, NY, USA). Normality of data was and tested by Shapiro Wilk test. Intra‐observer agreement as well as inter‐observer agreement were evaluated with: (1) the intraclass ICC with 95% confidence interval (CI) using a two‐way mixed model, an ICC value of 0.75–0.90 indicates a good agreement, ICC >0.90 indicates an excellent agreement 28 and (2) Bland–Altman plots.
Wilcoxon rank test was used to assess the difference in appearance of central flow between 2D MV‐flow and 2D power Doppler, as well as the penetration depth between 2D MV‐flow and 2D power Doppler. The penetration depth between 2D MV‐flow and 3D power Doppler was compared using an one sample t‐test (two‐sided). A p‐value of <0.05 was considered to be statistically significant.
3. RESULTS
Thirty patients (equal to 30 target fibroids) were included, and images of all target fibroids could be used for evaluation. Table 1 presents the baseline characteristics. 14 Patients showed multiple fibroids on ultrasound, of which one was determined as the target fibroid. The target fibroids' size ranged from 1.5 to 12 cm, with a mean of 5.9 cm. Body‐mass index (BMI) of the patients ranged from 17 to 43 kg/m2, no adverse effect of high BMI was seen on transvaginal ultrasound image quality. At the moment of the ultrasound, nine patients were using leuprolide acetate, five used other hormonal treatments, mostly oral contraceptives, and 16 had no hormonal treatment. The descriptive values for color score and vascular index are shown in Table 2.
TABLE 1.
Baseline population characteristics.
| All patients (n = 30) | |
|---|---|
| Age (years), mean (SD) | 40.3 (7.3) |
| BMI (kg/m2), median (range) a | 24.0 (17.0–43.0) |
| Parity, n (%) | |
| Nullipara | 18 (60.0) |
| Multipara | 12 (40.0) |
| Ethnicity, n (%) | |
| Caucasian | 17 (56.7) |
| Black | 5 (16.7) |
| Different | 3 (10.0) |
| Unknown | 5 (16.7) |
| Main symptom, n (%) | |
| Heavy menstrual blood loss | 12 (40.0) |
| Bulk symptoms | 7 (23.3) |
| Pain | 4 (13.3) |
| None | 7 (23.3) |
| Number of fibroids | |
| 1 | 16 (53.3) |
| 2–4 | 8 (26.7) |
| ≥5 | 6 (20.0) |
| Largest dm target fibroid, mean cm (SD) | 5.9 (2.5) |
| Volume target fibroid, median cm3 (IQR) b | 124.9 (160.1) |
| FIGO type target fibroid, n (%) | |
| 0 | 0 (0) |
| 1 | 0 (0) |
| 2 | 2 (6.7) |
| 3 | 15 (50) |
| 4 | 1 (3.3) |
| 5 | 2 (6.7) |
| 2–5 | 2 (6.7) |
| 6 | 4 (13.3) |
| 7 | 2 (6.7) |
| Other c | 2 (6.7) |
Abbreviations: cm, centimeter; cm3, cubic cm; dm, diameter; FIGO, classification of International Federation of Gynecology and Obstetrics; IQR, interquartile range; kg, kilogram; m, meter; n, number; SD, standard deviation.
Severe obesity (BMI > 40) n = 2.
Volume target fibroid based on 3D volume.
Intraligamental and retrovaginal fibroid.
TABLE 2.
Color score and vascular index for MV‐flow and power Doppler.
| Center | Capsule | Fibroid | |
|---|---|---|---|
| MVF VI (med, IQR) | 4.53 (14.4) | 20.11 (22.7) | 16.20 (14.2) |
| 3DPD VI (med, IQR) | 0.33 (3.7) | 7.56 (8.1) | 5.81 (7.5) |
| MVF CS (med, IQR) | 2 (2) | 3 (1) | 2 (1) |
| No color (n, %) | 11 (36.7) | 0 (0) | 1 (3.3) |
| Minimal color (n, %) | 11 (36.7) | 11 (36.7) | 15 (50.0) |
| Moderate color (n, %) | 3 (10.0) | 14 (46.7) | 10 (33.3) |
| Abundant color (n, %) | 5 (16.7) | 5 (16.7) | 4 (13.3) |
| 2DPD CS (med, IQR) | 1 (0) | 2 (1) | 2 (1) |
| No color (n, %) | 26 (86.7) | 1 (3.3) | 1 (3.3) |
| Minimal color (n, %) | 3 (10.0) | 19 (63.3) | 21 (70.0) |
| Moderate color (n, %) | 0 (0.0) | 9 (30.0) | 7 (23.3) |
| Abundant color (n, %) | 1 (3.3) | 1 (3.3) | 1 (3.3) |
Abbreviations: 2DPD, two dimensional power Doppler; 3DPD, three dimensional power Doppler; CS, color score; IQR, interquartile range; med, median; MVF, microvascular flow; n, number; VI, vascular index.
3.1. Intra‐observer and inter‐observer agreement
The intra‐observer and inter‐observer agreements were good to excellent for both 2D MV‐flow color score and 2D power Doppler color score (Table 3), as well as for 2D MV‐flow vascular index and 3D power Doppler vascular index (Table 4). Bland–Altman plots regarding the vascular index measured by 2D MV‐flow and 3D power Doppler are presented in the Supplemental information (Figures S1 and S2). Overall, the differences between the measurements were small, with little random noise. The difference in vascular index between the observers was constant, even while the mean in vascular index increased.
TABLE 3.
Intra‐observer and inter‐observer coefficients for color score.
| Observer | MVF CS | 2DPD CS | |
|---|---|---|---|
| Center | 1A (med, IQR) | 2 (2) | 1 (1) |
| 1B (med, IQR) | 2 (2) | 1 (0) | |
| 2 (med, IQR) | 2 (1) | 1 (0) | |
|
Intra‐observer Cohen's κ (SE) |
0.906 (0.182) |
0.772 (0.171) |
|
|
Inter‐observer Cohen's κ (SE) |
0.903 (0.182) |
0.812 (0.175) |
|
| Capsule | 1A (med, IQR) | 3 (1) | 2 (1) |
| 1B (med, IQR) | 3 (1) | 2 (1) | |
| 2 (med, IQR) | 3 (1) | 2 (1) | |
|
Intra‐observer Cohen's κ (SE) |
0.894 (0.182) |
0.954 (0.182) |
|
|
Inter‐observer Cohen's κ (SE) |
0.834 (0.811) |
0.873 (0.182) |
|
| Total fibroid | 1A (med, IQR) | 3 (1) | 2 (1) |
| 1B (med, IQR) | 2 (1) | 2 (1) | |
| 2 (med, IQR) | 2 (1) | 2 (0) | |
|
Intra‐observer Cohen's κ (SE) |
0.971 (0.182) |
0.955 (0.181) |
|
|
Inter‐observer Cohen's κ (SE) |
0.919 (0.181) |
0.766 (0.178) |
Note: Observer 1A (MF, first measurement), observer 1B (MF, second measurement) and observer 2 (BS). Intra‐observer: observer 1A vs 1B. Inter‐observer: observer 1A vs observer 2. A Cohen's κ value of 0.61–0.81 indicates a substantial agreement, Cohen's κ > 0.81 an almost perfect agreement.
Abbreviations: 3DPD, three dimensional power Doppler; CI, confidence interval; CS, color score; ICC, intercorrelation coefficient; IQR, interquartile range; med, median; MVF, Microvascular flow; SE, standard error; VI, vascular index; κ, kappa.
TABLE 4.
Intra‐observer and inter‐observer coefficients for vascular index.
| Observer | MVF VI | 3DPD VI | |
|---|---|---|---|
| Center | 1A (med, IQR) | 4.15 (15.28) | 0.30 (4.69) |
| 1B (med, IQR) | 4.85 (15.25) | 0.15 (3.26) | |
| 2 (med, IQR) | 4.30 (14.18) | 0.41 (4.37) | |
|
∆ Observers 1A‐1B (±SD) Intra‐observer ICC (95% CI) |
0.68 (±2.44) 0.992 (0.983‐0.996) |
0.11 (±5.13) 0.901 (0.804‐0.951) |
|
| ∆ Observers 1A‐2 | 0.29 (±3.54) | 0.37 (±5.37) | |
|
Inter‐observer ICC (95% CI) |
0.984 (0.969‐0.993) |
0.875 (0.757‐0.938) |
|
| Capsule | 1A (med, IQR) | 17.96 (21.29) | 7.50 (8.80) |
| 1B (med, IQR) | 21.15 (18.80) | 6.81 (6.81) | |
| 2 (med, IQR) | 23.86 (19.30) | 7.32 (8.18) | |
|
∆ Observers 1A‐1B Intra‐observer ICC (95% CI) |
0.75 (±3.90) 0.959 (0.917‐0.980) |
0.79 (±1.97) 0.915 (0.831‐0.959) |
|
| ∆ Observers 1A‐2 | 0.66 (±5.02) | 0.55 (±1.63) | |
|
Inter‐observer ICC (95% CI)) |
0.940 (0.879‐0.971) |
0.946 (0.891‐0.974) |
|
| Total fibroid | 1A (med, IQR) | 15.95 (15.88) | 5.88 (7.83) |
| 1B (med, IQR) | 15.85 (13.65) | 4.99 (7.31) | |
| 2 (med, IQR) | 16.45 (13.75) | 4.89 (6.95) | |
|
∆ Observers 1A‐1B Intra‐observer ICC (95% CI) |
0.64 (±1.47) 0.995 (0.989‐0.997) |
0.37 (±1.60) 0.971 0.940‐0.986) |
|
| ∆ Observers 1A‐2 | 0.64 (±1.75) | 0.52 (±1.08) | |
|
Inter‐observer ICC (95% CI) |
0.993 (0.985‐0.996) |
0.983 (0.964‐0.992) |
Note: Observer 1A (MF, first measurement), observer 1B (MF, second measurement) and observer 2 (BS). Intra‐observer: observer 1A vs 1B. Inter‐observer: observer 1A vs observer 2. ∆ Observers: mean difference. An ICC value of 0.75–0.90 indicates a good agreement, ICC > 0.90 an excellent agreement.
Abbreviations: 3DPD, three dimensional power Doppler; CI, confidence interval; CS, color score; ICC, intercorrelation coefficient; IQR, interquartile range; med, median; MVF, Microvascular flow; SD, standard deviation; VI, vascular index; κ, kappa.
When measuring vascularity in the center of the fibroid, the interval between the lower and upper 95% limits of agreement was smaller when 2D MV‐flow VI was used, indicating less variance between the two observers. Measuring vascularity in the capsule, this interval was smaller using 3DPD.
3.2. Vascular structures
In general, on 2D MV‐flow images distinctive vascular structures were visible, such as the radial arteries branching from the uterine vascular arcade and the vascular branches of the leading vessels towards the fibroid. On power Doppler images, both 2D and 3D, these vascular structures were not visualized by the power Doppler signal and only larger vessels, such as the uterine vascular arcade and the fibroid's pseudocapsule were visible. Representative images of a fibroid's vascular structures depicted by 2D MV‐flow, 2D power Doppler, and 3D power Doppler are displayed in Figure 2. The vascular capsule was clearly visible using 2D MV‐flow, only slightly visible using 3D power Doppler and partly visible using 2D power Doppler. In addition, 2D MV‐flow imaging was able to depict the smaller, slow‐flow vessels within the fibroids' center, which were not visible using 2D and 3D power Doppler.
FIGURE 2.
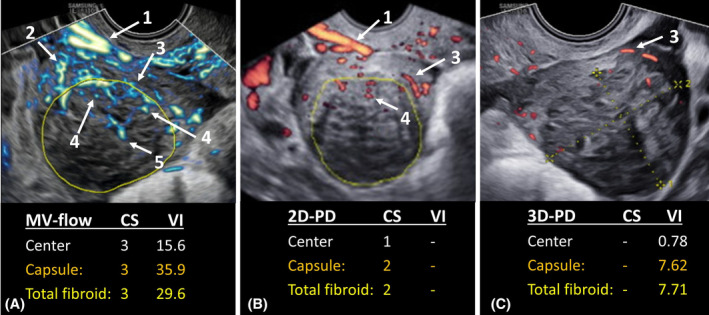
Representative sagittal ultrasound images of one single fibroid obtained with (A) microvascular flow (MV‐flow) imaging; (B) two‐dimensional power Doppler (2D‐PD); and (C) three‐dimensional power Doppler (3D‐PD). Reported outcomes are: Color socore (CS) obtained by MV‐flow or 2D‐PD; and vascular index (VI) obtained by MV‐flow or 3D‐PD. Visible vascular structures are: (1) uterine vascular arcade, consisting of arcuate arteries, (2) radial arteries, (3) fibroid's pseudocapsule, (4) vascular branches penetrating the fibroid, (5) central vascularization.
3.3. Degree of vascularity
Qualitative assessment of the images showed the ability of 2D MV‐flow imaging to visualize central flow in significantly more fibroids (63.3%; 19 of 30), compared with 2D power Doppler (13.3%; 4 of 30; p < 0.001). Of the 26 fibroids scoring no color in their center using 2D power Doppler, 15 fibroids did show color signal using 2D MV‐flow imaging (minimal color n = 10; moderate color n = 3; abundant color n = 2), Table 2 and Figure 3. The relation between the qualitative and quantitative assessments is shown in Figure 4. The higher the subjective 2D MV‐Flow color score, the higher the 2D MV‐Flow vascular index for the target fibroid, in both the center as the capsule. The same accounts for 2D power Doppler color score and 3D power Doppler vascular index, in both center as capsule. In addition, based on the depicted vascularity in the center, 2D MV‐flow imaging could distinguish all four categories, that is, no flow, minimal flow, moderate flow and abundant flow. 2D power Doppler could only distinguish between no flow and minimal flow.
FIGURE 3.
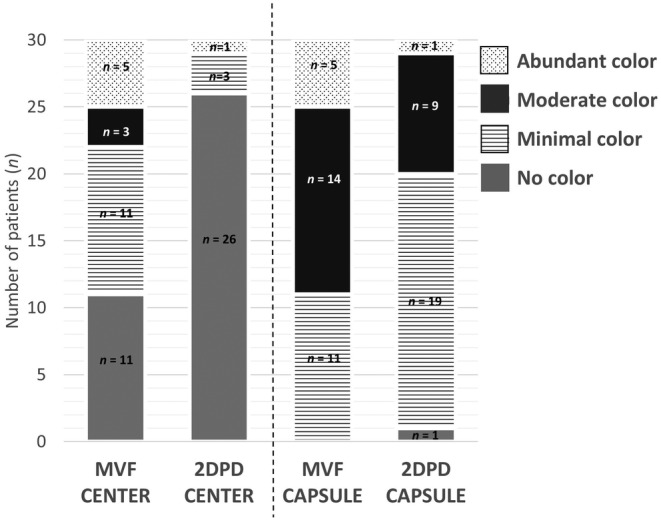
Color score per fibroid. Data shown as number (n) of fibroids displaying a specific color score in the center and capsule as determined by MV‐flow (MVF) and 2D power Doppler (2DPD).
FIGURE 4.
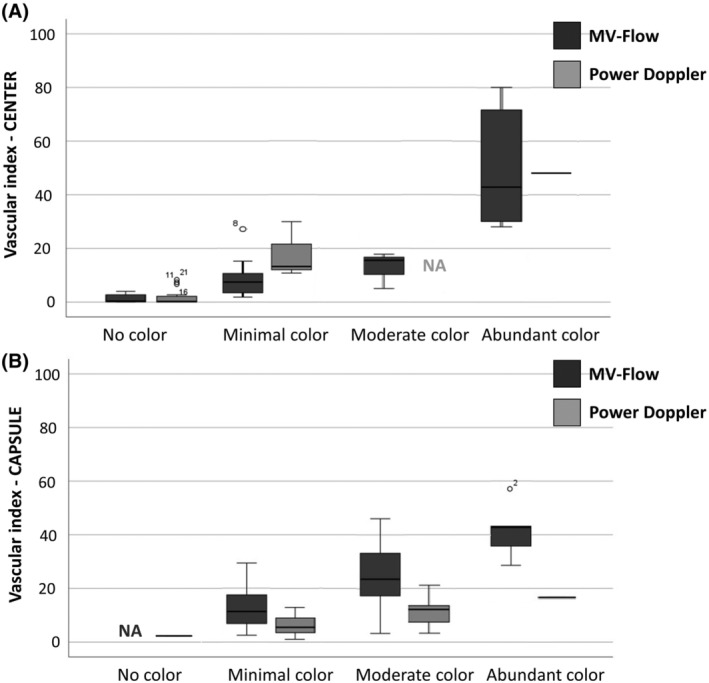
Boxplots showing the relation between Color Score and Vascular Index for the fibroids' center (A) and the vascular capsule (B). The higher the subjective color score, the higher the vascular index for the target fibroid. This graph also shows, based on the depicted vascularity in the center, 2D MV‐flow imaging could distinguish all four categories, that is, no flow, minimal flow, moderate flow and abundant flow. 2D Doppler could only distinguish between no flow and minimal flow in the center. Data shown as median with interquartile range. MV, microvascular; 2D, two‐dimensional; PD, power Doppler; NA, not applicable (n = 0).
3.4. Penetration depth
The penetration of the Doppler signal was significantly deeper in 2D MV‐flow imaging (3.92 cm (IQR 1.61)) compared with 3D power Doppler (3.16 cm (IQR 2.33); p < 0.001) and 2D power Doppler (2.95 cm (IQR 1.66); p = 0.001). Representative images of the Doppler penetration are shown in Figure 2.
4. DISCUSSION
This is the first study in gynecology to show high reproducibility for measuring vascularity in uterine fibroids using 2D MV‐flow imaging, as demonstrated by good‐to‐excellent intra‐ and interobserver agreements.
Interestingly, 2D MV‐flow was able to visualize more central flow in more fibroids compared with 2D and 3D power Doppler. Due to the visualization of more central flow and a deeper penetration of the MV‐flow signal, we showed that color scores will generally be higher when based on 2D MV‐flow images than based on 2D power Doppler images. Additionally, more specific vascular structures can be visualized using 2D MF‐flow compared with 2D and 3D power Doppler imaging. Previously, in an exploratory feasibility study we demonstrated the ability of MV‐flow to image the microvasculature of several uterine disorders, that is, fibroids, adenomyosis, endometriosis and a uterine niche. 18 Together with this current study, we showed the promising application of imaging microvascularity.
The ability of 2D MV‐flow to show low velocities at a great distance from the probe depends on ultrasound settings and advanced motion filters. 18 Both 2D or 3D power Doppler and 2D MV‐flow are pulsed‐wave ultrasound techniques; i.e. sending a frequency with a certain pulse repetition frequency (PRF). The PRF of 2D MV‐flow (0.13 kHz) is lower than 2D or 3D power Doppler (0.55 kHz) and can therefore measure lower velocities by the formula: velocity = distance * PRF. A lower PRF results in more time in between pulses to travel forward and back to the probe, thus a higher penetration. A disadvantage of a low PRF is artifacts, since all slow movements are considered to be blood flow. Therefore, 2D MV‐flow has a higher frame rate compared with 2D or 3D power Doppler to optimize the spatial resolution, as well as advanced noise filters. Information received on slow flow is displayed sharp, and allows better distinguish between noise and true blood flow as input for the advanced filters. 2D or 3D power Doppler can also be performed using a PRF of 0.1–0.2 kHz, however, lacking the advanced motion filters, this will result in a poor signal‐to‐noise ratio. Settings influencing VI outcomes, which are extensively discussed by Frijlingh et al. also apply for MV‐flow settings. 11 As part of the current study we did not optimized machine settings, such as PRF, for MV‐flow. When performing research using MV‐flow imaging, it is necessary to mention the machine settings to compare all published results in literature.
To date, there is a lack of studies comparing 2D MV‐flow imaging with 2D or 3D power Doppler in the field of uterine pathology. In other fields, such as carpal tunnel syndrome or imaging of the ovaries, 2D MV‐flow imaging showed promising results with higher sensitivity compared with 2D power Doppler. 29 , 30 2D MV‐flow imaging already showed good intra‐observer agreements in other fields, such as ablation treatment of thyroid nodules, 31 breast masse, 32 and in detecting synovial vascularity in rheumatoid arthritis, 33 but there are no studies on the intra‐observer agreement for 2D MV‐flow imaging in gynecology. This imaging technique may also be used in gynecology for monitoring the effectiveness of minimally invasive therapies such as ablation or vascular occlusion, nowadays the non‐perfused volume on magnetic resonance imaging (MRI) is used as outcome. 34 MRI is more expensive than ultrasound techniques, 13 may have a long waiting list, or may not be available in every medical center. The growing trend towards non‐surgical uterine fibroid treatments urges the need for a cost‐effective, easily applicable technique that quantifies vascularization such as 2D MV‐flow imaging.
2D MV‐flow imaging is a novel technique that is designed to be more sensitive in depicting microvascular slow‐flow in the center fibroids than 2D or 3D power Doppler, where fibroids typically show an avascular or hypo‐perfused center. 35 A detailed display of the blood flow in a fibroid's center is necessary to be able to assess the growth potential of fibroids, to select the correct treatment and predict their responsiveness to therapy. To predict fibroid response to embolization therapy, Samanci et al. used 2D MV‐flow imaging prior to therapy to assess the vasculature. 24 The authors confirm the excellent inter‐observer agreement for 28 women when comparing 2D MV‐flow imaging with 2D power Doppler ultrasound. They conclude that 2D MV‐flow imaging is more sensitive than 2D power Doppler ultrasound in showing microvessels and is a better diagnostic tool for predicting response to embolization. Histology often shows that the center contains mainly slow‐flow capillaries which are dispersed, uniform and smaller compared with the perifibroid arteries, which is in line with 2D MV‐flow imaging, but not accurately displayed by 2D or 3D power Doppler imaging. 10 , 11
One of the strengths of this study was the quality and homogeneity of the ultrasound images. Ultrasound was performed using a standardized method by two experienced gynecologists using advanced equipment. To evaluate ultrasound images, predefined and strict criteria were applied. In case of multiple fibroids, we applied a subjective criterion, to analyze the best recognizable fibroid based on size and/or position. We defined the criteria for offline analysis of vascularity based on literature as much as possible, however for the center of the fibroid no method of assessment exists. Therefore we arbitrarily took the one‐third of the entire fibroid, since normally you see the rich vascularized pseudo‐capsule on both sides, being the other two‐third. Selection bias could have occurred since we applied strict criteria and included only patients with easily recognizable fibroids, and with whom all ultrasound modalities had led to good image quality. This resulted in quite a long inclusion period, as in our tertiary referral center mostly complicated cases with multiple fibroids, with or without additional adenomyosis visit the outpatient clinic. The included patients ranged in BMI (17–43 kg/cm2), which may have influenced the image quality due to the presence of for example visceral fat around the uterus. In our population six patients were obese; four patients had a BMI 30–35 and two patients had a BMI >35, however, they still met the inclusion criteria regarding image quality. Finally, because of the small sample size the data must be interpreted carefully, 30 patients evaluated by 2 operators can cause selection bias. Therefore, this study should be considered a first attempt to evaluate the reproducibility of 2D MV‐flow imaging.
The measurements in this study were performed by two experienced observers, resulting in good‐excellent ICCs. Less skills might result in different outcomes. Therefore, the high ICCs may be an overestimation. However, application of color scores is easy and a quick calculation of the 2D MV‐flow vascular index has a steep learning curve. 13 The most complex measurement was obtaining 3D power Doppler vascular index, which needs more experience and is quite time‐consuming. Yet, a 3D volume should be more accurate in measuring a vascular index compared with a 2D MV‐flow image. In larger fibroids, measuring a vascular index using 3D power Doppler can result in a VI = 0, due to physical limitation of tissue penetration by the ultrasound wave. 5 , 11 The physical limitation also applies for 2D MV‐flow imaging, however, 2D MV‐flow is more sensitive in picking up slow‐flow signals, with somewhat higher tissue penetration depth. However, the ROI that was selected using the ‘shell‐off mode’ encompasses the entire fibroid. Due to the penetration limitation, the vascular index shown (both for MV‐flow and power Doppler) is likely to be an underestimation of the actual vascularity. Additionally, there are most likely differences in VIs calculated for small fibroids close to the transducer, and large fibroids situated far away. Assuming similar vascularity, the difference in size and/or location may result in a higher VI for the fibroids smaller in size and/or located closer to the transducer. Due to lower sensitivity and shorter penetration, the margin‐of‐error is larger for VIs determined by 3D power Doppler than by 2D MV‐flow. Despite the downsides of calculating a VI using offline analysis on 3D power Doppler images, it is a technique with well‐reported reproducibility and good discriminating ability related to histology. 21 , 22 , 23
We suggest confirming the accuracy of 2D MV‐flow imaging in a prospective study with histology as a reference to prove true vascularity. The sample size calculation based on this study, as well as additional studies for further exploring the full potential of 2D MV‐flow imaging like in arteriovenous malformations, and its clinical relevance for imaging uterine fibroids related to clinical outcomes of different fibroid treatments.
5. CONCLUSION
2D MV‐flow imaging is highly reproducible and has potential added value for clinical practice as it depicts detailed vascular structures and the degree of vascularity, especially in the center of the fibroid.
AUTHOR CONTRIBUTIONS
Marissa Frijlingh: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing—original draft. Barbara Stoelinga: conceptualization, data curation, investigation, validation, writing—review and editing. Robert A. de Leeuw: conceptualization, methodology, resources, software, supervision, writing—review and editing. Wouter J. K. Hehenkamp: conceptualization, methodology, supervision, writing—review and editing. Jos W. R. Twisk: formal analysis, methodology, writing—review and editing. Thierry van den Bosch: conceptualization, methodology, supervision, writing—review and editing. Lynda J. M. Juffermans: conceptualization, investigation, methodology, project administration, supervision, writing—original draft. Judith A. F. Huirne: conceptualization, data curation, funding acquisition, methodology, project administration, resources, software, supervision, validation, writing—original draft.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the ethics committee of the Amsterdam UMC, location VU University Medical Center (ref. no. 2017/494) on September 20, 2018. The current study is registered in the U.S. Clinical Trial register (ClinicalTrials.gov; reference number NCT05643339).
Supporting information
Figure S1.
Figure S2.
Table S1.
Frijlingh M, Stoelinga B, de Leeuw RA, et al. Microvascular flow imaging of fibroids: A prospective pilot study. Acta Obstet Gynecol Scand. 2024;103:2193‐2202. doi: 10.1111/aogs.14914
REFERENCES
- 1. Woźniak A, Woźniak S. Ultrasonography of uterine leiomyomas. Prz Menopauzalny. 2017;16:113‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bajaj S, Gopal N, Clingan MJ, Bhatt S. A pictorial review of ultrasonography of the FIGO classification for uterine leiomyomas. Abdom Radiol. 2022;47:341‐351. [DOI] [PubMed] [Google Scholar]
- 3. Wegienka G, Baird DD, Hertz‐Picciotto I, et al. Self‐reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101:431‐437. [DOI] [PubMed] [Google Scholar]
- 4. Mavrelos D, Ben‐Nagi J, Holland T, Hoo W, Naftalin J, Jurkovic D. The natural history of fibroids. Ultrasound Obstet Gynecol. 2010;35:238‐242. [DOI] [PubMed] [Google Scholar]
- 5. Nieuwenhuis LL, Keizer AL, Stoelinga B, et al. Fibroid vascularisation assessed with three‐dimensional power Doppler ultrasound is a predictor for uterine fibroid growth: a prospective cohort study. BJOG. 2018;125:577‐584. [DOI] [PubMed] [Google Scholar]
- 6. Blake RE. Leiomyomata uteri: hormonal and molecular determinants of growth. J Natl Med Assoc. 2007;99:1170‐1184. [PMC free article] [PubMed] [Google Scholar]
- 7. Salim R, Lee C, Davies A, Jolaoso B, Ofuasia E, Jurkovic D. A comparative study of three‐dimensional saline infusion sonohysterography and diagnostic hysteroscopy for the classification of submucous fibroids. Hum Reprod. 2005;20:253‐257. [DOI] [PubMed] [Google Scholar]
- 8. Ciarmela P, Delli Carpini G, Greco S, et al. Uterine fibroid vascularization: from morphological evidence to clinical implications. Reprod Biomed Online. 2022;44:281‐294. [DOI] [PubMed] [Google Scholar]
- 9. Pelage JP, Cazejust J, Pluot E, et al. Uterine fibroid vascularization and clinical relevance to uterine fibroid embolization. Radiographics. 2005;25(Suppl 1):S99‐S117. [DOI] [PubMed] [Google Scholar]
- 10. Walocha JA, Litwin JA, Miodoński AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088‐1093. [DOI] [PubMed] [Google Scholar]
- 11. Frijlingh M, Juffermans L, de Leeuw R, et al. How to use power Doppler ultrasound in transvaginal assessment of uterine fibroids. Ultrasound Obstet Gynecol. 2022;60:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keizer AL, Niewenhuis LL, Hehenkamp WJK, Twisk JWR, Brolmann HAM, Huirne JAF. Fibroid vascularisation assessed with 3D power Doppler as predictor for fibroid related symptoms and quality of life; a pilot study. Facts Views Vis Obgyn. 2021;13:387‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46:284‐298. [DOI] [PubMed] [Google Scholar]
- 14. Exacoustos C, Romanini ME, Amadio A, et al. Can gray‐scale and color Doppler sonography differentiate between uterine leiomyosarcoma and leiomyoma? J Clin Ultrasound. 2007;35:449‐457. [DOI] [PubMed] [Google Scholar]
- 15. Moss J, Christie A. Uterine artery embolization for heavy menstrual bleeding. Womens Health. 2016;12:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krämer B, Hahn M, Taran FA, Kraemer D, Isaacson KB, Brucker SY. Interim analysis of a randomized controlled trial comparing laparoscopic radiofrequency volumetric thermal ablation of uterine fibroids with laparoscopic myomectomy. Int J Gynaecol Obstet. 2016;133:206‐211. [DOI] [PubMed] [Google Scholar]
- 17. Shwayder J, Sakhel K. Imaging for uterine Myomas and Adenomyosis. J Minim Invasive Gynecol. 2014;21:362‐376. [DOI] [PubMed] [Google Scholar]
- 18. Frijlingh M, de Leeuw RA, Juffermans LJM, van de Bosch T, Huirne JAF. Visualisation of microvascular flow in benign uterine disorders: a pilot study of a new diagnostic technique. Facts Views Vis Obgyn. 2023;15:115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mack LM, Mastrobattista JM, Gandhi R, Castro EC, Burgess APH, Lee W. Characterization of placental microvasculature using superb microvascular imaging. J Ultrasound Med. 2019;38:2485‐2491. [DOI] [PubMed] [Google Scholar]
- 20. Hasegawa J, Yamada H, Kawasaki E, Matsumoto T, Takahashi S, Suzuki N. Application of superb micro‐vascular imaging (SMI) in obstetrics. J Matern Fetal Neonatal Med. 2018;31:261‐263. [DOI] [PubMed] [Google Scholar]
- 21. Nieuwenhuis LL, Betjes HE, Hehenkamp WJ, Heymans MW, Brolmann HA, Huirne JA. The use of 3D power Doppler ultrasound in the quantification of blood vessels in uterine fibroids: feasibility and reproducibility. J Clin Ultrasound. 2015;43:171‐178. [DOI] [PubMed] [Google Scholar]
- 22. Minsart AF, Ntoutoume Sima F, Vandenhoute K, Jani J, Van Pachterbeke C. Does three‐dimensional power Doppler ultrasound predict histopathological findings of uterine fibroids? A preliminary study. Ultrasound Obstet Gynecol. 2012;40:714‐720. [DOI] [PubMed] [Google Scholar]
- 23. Raine‐Fenning NJ, Nordin NM, Ramnarine KV. Determining the relationship between three‐dimensional power Doppler data and true blood flow characteristics: an in‐vitro flow phantom experiment. Ultrasound Obstet Gynecol. 2008;32:540‐550. [DOI] [PubMed] [Google Scholar]
- 24. Samanci C, Ozkose B, Ustabasioglu FE, et al. Diagnostic value of superb microvascular imaging in prediction of uterine artery embolization treatment response in uterine leiomyomas. J Ultrasound Med. 2021;40:2607‐2615. [DOI] [PubMed] [Google Scholar]
- 25. Arifin WN. Sample size calculator (web). 2023. Available from: http://wnarifin.github.io
- 26. Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders . FIGO classification system (PALM‐COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3‐13. [DOI] [PubMed] [Google Scholar]
- 27. Nieuwenhuis LL, Hehenkamp WJK, Brolmann HAM, Huirne JAF. 3D power Doppler in uterine fibroids; influence of gain, cardiac cycle and off‐line measurement techniques. J Obstet Gynaecol. 2018;38:103‐109. [DOI] [PubMed] [Google Scholar]
- 28. Koo TK, Li MY. A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayaz E, Aslan A, İnan İ, Yıkılmaz A. Evaluation of ovarian vascularity in children by using the “superb microvascular imaging” ultrasound technique in comparison with conventional Doppler ultrasound techniques. J Ultrasound Med. 2019;38:2751‐2760. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, Chen L, Wu L, et al. Value of superb microvascular imaging ultrasonography in the diagnosis of carpal tunnel syndrome: compared with color Doppler and power Doppler. Medicine. 2017;96:e6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lan Y, Li N, Song Q, Zhang MB, Luo YK, Zhang Y. Correlation and agreement between superb micro‐vascular imaging and contrast‐enhanced ultrasound for assessing radiofrequency ablation treatment of thyroid nodules: a preliminary study. BMC Med Imaging. 2021;21:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee EJ, Chang YW, Oh E, Hwang J, Kim HJ, Hong SS. Reproducibility and diagnostic performance of the vascular index of superb microvascular imaging in real‐time breast ultrasonography for evaluating breast masses. Ultrasonography. 2021;40:398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diao XH, Shen Y, Chen L, et al. Superb microvascular imaging is as sensitive as contrast‐enhanced ultrasound for detecting synovial vascularity in rheumatoid arthritis. Quant Imaging Med Surg. 2022;12:2866‐2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szkodziak P, Szkodziak F, Trzeciak K, Czuczwar P. Minimally invasive procedures in the management of uterine fibroids. Prz Menopauzalny. 2017;16:122‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weintraub JL, Romano WJ, Kirsch MJ, Sampaleanu DM, Madrazo BL. Uterine artery embolization: sonographic imaging findings. J Ultrasound Med. 2002;21:633‐637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Table S1.


