Abstract
Flaviviruses comprise a positive-sense RNA genome that replicates exclusively in the cytoplasm of infected cells. Whether flaviviruses require an activated nuclear factor(s) to complete their life cycle and trigger apoptosis in infected cells remains elusive. Flavivirus infections quickly activate nuclear factor kappa B (NF-κB), and salicylates have been shown to inhibit NF-κB activation. In this study, we investigated whether salicylates suppress flavivirus replication and virus-induced apoptosis in cultured cells. In a dose-dependent inhibition, we found salicylates within a range of 1 to 5 mM not only restricted flavivirus replication but also abrogated flavivirus-triggered apoptosis. However, flavivirus replication was not affected by a specific NF-κB peptide inhibitor, SN50, and a proteosome inhibitor, lactacystin. Flaviviruses also replicated and triggered apoptosis in cells stably expressing IκBα-ΔN, a dominant-negative mutant that antagonizes NF-κB activation, as readily as in wild-type BHK-21 cells, suggesting that NF-κB activation is not essential for either flavivirus replication or flavivirus-induced apoptosis. Salicylates still diminished flavivirus replication and blocked apoptosis in the same IκBα-ΔN cells. This inhibition of flaviviruses by salicylates could be partially reversed by a specific p38 mitogen-activated protein (MAP) kinase inhibitor, SB203580. Together, these results show that the mechanism by which salicylates suppress flavivirus infection may involve p38 MAP kinase activity but is independent of blocking the NF-κB pathway.
Flavivirus is a genus of the Flaviviridae family, which consists of more than 68 members. Most flaviviruses are arthropod borne and are capable of infecting their vertebrate hosts through persistently infected mosquito or tick vectors, such as Japanese encephalitis virus (JEV), tick-borne encephalitis virus, and dengue viruses (DEN). The enveloped virion of a flavivirus contains a single-stranded, positive-sense RNA genome of approximately 11 kb, whose replication is primarily cytoplasmic and membrane associated. All known viral proteins are derived from a polyprotein that is expressed from the single open reading frame (ORF) on the flavivirus genome. The structural proteins of flaviviruses, including those of the capsid (C), membrane (M), and envelope (E), are encoded in the 5′ quarter of the ORF, and the nonstructural proteins, namely, NS1 through NS5, are encoded in the rest. In common with other animal positive-sense RNA viruses with a single ORF, the first event of flavivirus replication in infected cells after uncoating is to translate the message-sense RNA genome into a polyprotein, which is co- and/or posttranslationally processed into 10 individual proteins by cellular and viral proteases. Flavivirus particles mature predominantly on intracellular membranes of the cytosol, but not on plasma membranes, of infected cells. Pirating the same intrinsic secretory pathway normally employed by cells to discharge macromolecules, flaviviruses bud from membranes of the endoplasmic reticulum and Golgi apparatus in infected cells to release the mature virions (for reviews, see references 12 and 66). As a result of infection, some flaviviruses cause the infected host cells severe cytopathic effects (CPE). JEV (43) and DEN (2, 21, 22), for example, have been shown to induce infected cells to undergo apoptotic cell death, which is one of the mechanisms that contribute to flavivirus-induced CPE.
As a part of the cellular response system, nuclear factor kappa B (NF-κB) acts as an inducible transcription factor that can be quickly activated in response to many noxious stimulants, including virus infections. Normally sequestered by forming a complex with one of its cytoplasmic inhibitors, NF-κB actually preexists in the cytoplasm of most cell types as an inactive homodimer or heterodimer comprised of one or two of five different subunits, including p50 (NF-κB1), p52, p65 (RelA), c-Rel, and RelB. Upon signaling by different stimuli, the activation cascade of NF-κB involves phosphorylation and degradation of the IκB (inhibitor of NF-κB) proteins, thereby releasing NF-κB from cytoplasmic complexes; NF-κB then translocates into the nucleus and binds to its cognate DNA sequences. Consequently, the expression of NF-κB-dependent genes results in the generation of numerous mediators important for malignant transformation, cell adhesion, cell growth control, apoptosis control, immune functions, and embryonic development (for reviews, see references 3 and 5). In addition, NF-κB may also play a crucial role against viruses, as NF-κB activation can induce expression of the genes for beta interferon, major histocompatibility complex class I, and several inflammatory cytokines (for a review, see reference 4). On the other hand, a wide variety of viruses from various viral families are capable of activating NF-κB in infected cells, including cytomegalovirus (CMV) (69), human immunodeficiency virus type 1 (68), Sendai virus (25, 68), adenovirus (17), hepatitis B virus (15, 50), human T-lymphotropic virus type 1 (26, 28, 54, 58), Epstein-Barr virus (32, 40), influenza virus (60), Sindbis virus (44, 45), and DEN serotype 1 (DEN-1) (52). For Sindbis virus or DEN-1, both of which are single-stranded, positive-sense RNA viruses, apoptotic cell death triggered by infections is facilitated by NF-κB activation (44, 45, 52). Because NF-κB activation is an active cellular process, it is conceivable that some of these viruses may derive certain replication advantages from expression of NF-κB-dependent genes in infected cells. Nevertheless, little is known about whether a cytoplasmic RNA virus requires activation of other nuclear-factors to complete its life cycle in infected cells. This prompted us to investigate in the present study whether NF-κB activation in infected cells is essential for flavivirus replication and for flavivirus-induced apoptosis.
Salicylates are widely employed as nonsteroidal anti-inflammatory drugs. In addition to blockage of prostaglandin production by inhibition of cyclooxygenase (COX), sodium salicylate (NaSal) and acetylsalicylic acid (aspirin) also inhibit NF-κB activation induced by tumor necrosis factor (TNF) and some other agents, most likely by preventing the phosphorylation of IκBα and its subsequent degradation by the ubiquitin-proteasome pathway (6, 9, 29, 39, 59, 61, 62). Furthermore, the inhibitory effects of salicylates on TNF-induced phosphorylation and degradation of IκBα appear to also involve the activation of p38 mitogen-activated protein kinase (MAPK) (70), which is a member of the three structurally related MAPK families identified in mammalian cells. Treatment with salicylates alone was shown sufficient to produce strong p38 MAPK activation in human FS-4 fibroblasts and African green monkey kidney COS-1 cells (70, 71). Hence, besides COX inhibition, the inhibition of NF-κB activation or the induction of p38 MAPK activation might be equally important in the pharmacological actions of salicylates.
Both NaSal and aspirin attenuate the gene expression and infectivity of human CMV in coronary artery smooth muscle cells, probably through the COX-2- or NF-κB-dependent pathway (73). However, the mechanism by which salicylates act against the replication of RNA viruses remains poorly defined and may utilize strategies fundamentally different from those for antagonizing DNA viruses such as CMV.
In this study, we explored the effects of NaSal and aspirin on the replication of JEV and DEN-2 in cultured cells. Our results revealed that salicylates not only suppress the replication of JEV and DEN-2 in a dose-dependent manner but also prevent apoptosis of infected cells. Salicylates had only trivial effects on the replication of Sindbis virus, a prototype of the Alphavirus family, under the same conditions. The inhibitory effects of salicylates on flaviviruses did not appear to result from suppression of NF-κB activation but instead were at least partially due to the activation of p38 MAPK given that the pyridinyl imidazole compound SB-203580, a highly selective p38 MAPK inhibitor, was able to alleviate the antiflavivirus effects of salicylates. In addition, the flaviviruses seemed not to require NF-κB activation in order to replicate in infected cells, since several blocking approaches all failed to influence flavivirus production. Our results suggest that salicylates utilize an NF-κB-independent pathway, which may involve p38 MAPK activity, to interfere with flavivirus replication and thereby delay the process of virus-associated apoptosis.
MATERIALS AND METHODS
Viruses, cell lines, and chemicals.
A plaque-purified Taiwanese JEV strain, RP-9 (13, 14), was employed throughout this study. The propagation of JEV was carried out in baby hamster kidney (BHK)-21 cells utilizing RPMI 1640 medium containing 2% fetal calf serum (FCS; GIBCO). A local Taiwanese strain of DEN-2, PL046, isolated from a dengue fever patient was generously provided by the National Institute of Preventive Medicine, Taiwan, Republic of China (ROC). DEN propagation was carried out in C6/36 cells utilizing RPMI 1640 medium containing 5% FCS (GIBCO). In some experiments Sindbis virus (Ar-339; American Type Culture Collection) was used to infect the target cells. The propagation of Sindbis virus was carried out in BHK-21 or Chinese hamster ovary (CHO) cells utilizing RPMI 1640 medium containing 2% FCS. N18, a mouse neuroblastoma cell line (1) (kindly provided by D. E. Griffin, Johns Hopkins University, Baltimore, Md.), was grown in RPMI 1640 medium containing 10% FCS (GIBCO). NaSal and aspirin were purchased from Sigma. The NF-κB peptide inhibitor SN50, its ineffective analogue SN50M, and phorbol 12-myristate 13-acetate (PMA) were obtained from Calbiochem. Lactacystin and indomethacin were acquired from Biomol.
Virus infection and titration.
For infection with JEV or DEN, monolayers of the indicated cells grown in 6- or 12-well plates were initially adsorbed with virus at a multiplicity of infection (MOI) of 5 for 1 h at 37°C. After adsorption, the unbound viruses were removed by three gentle washings with serum-free RPMI 1640 medium. Fresh medium containing 2% FCS was added to each plate for further incubation at 37°C. At the end of infection, the culture media were harvested for a plaque-forming assay to determine virus titers. Briefly, a virus dilution was added to 80% confluent BHK-21 cells and incubated at 37°C for 1 h. After adsorption, the cells were washed and overlaid with 1% agarose (SeaPlaque; FMC BioProducts) containing RPMI 1640 with 1% FCS. After incubation for 4 (for JEV) or 7 (for DEN) days, the resulting cells were fixed with 10% formaldehyde and stained with 0.5% crystal violet for plaque counting. Virus titers were expressed as PFU per milliliter.
Western immunoblot analysis.
Cell lysates were prepared in a lysis buffer (1% Nonidet P-40, 150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1 mM EDTA) containing a cocktail of protease inhibitors (20 μg of phenylmethysulfonyl fluoride per ml, 2 μg of leupeptin per ml, 2 μg of aprotinin per ml). Lysates were mixed with an equal volume of sample buffer with 2-mercaptoethanol, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a nitrocellulose membrane (Hybond-C Super; Amersham). The nonspecific antibody-binding sites were blocked with 5% skim milk in phosphate-buffered saline (PBS), and the membranes were reacted with monoclonal antibodies specific for JEV E or NS1 (13). The resulting blot was treated with a horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Cappel) and developed with ECL reagent (Amersham).
MTT assay.
An MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay was used to measure mitochondrial functions, which served as an index of living cells. The assay was carried out as previously described (46, 71) with minor modifications. Briefly, MTT (Sigma) was dissolved in 0.1 M Tris-buffered saline to make a 5-mg/ml solution, which was then filtered to remove insoluble residues. Fifty microliters of MTT solution was added to each well containing tested cells in a 12-well plate and incubated at 37°C for 4 h. At the end of incubation, the MTT solution was removed, and 500 μl of isopropanol containing 0.04 N HCl was added to dissolve the dark-blue crystals precipitated in the wells. A 100-μl portion of the resulting solution was removed from each well and read at 540 nm on a microplate reader (Dynatech MR5000).
DNA fragmentation assay.
Low-molecular-weight DNA was extracted from apoptotic cells following the published method (65). Briefly, cell suspensions in Hanks buffered salt solution (HBSS) were incubated with 70% ethanol for 24 h at −20°C. The resulting cells were centrifuged to remove ethanol, and the cell pellets were resuspended and incubated in 40 μl of PC buffer (192 mM Na2HPO4 mM citric acid [pH 7.8]) at room temperature (RT) for 30 min. After centrifugation at 1,000 × g for 5 min, the supernatants were collected and vacuum concentrated in new microcentrifuge tubes for 15 min using a SpeedVac. Three microliters of NP-40 solution (0.25%) and 3 μl of RNase A solution (1 mg/ml) were then added and incubated at 37°C for 30 min. After incubation, 3 μl of proteinase K solution (1 mg/ml) was added and the mixture was further incubated at 37°C for 30 min. The resulting DNA-containing extracts were then analyzed by 2% agarose gel electrophoresis in 1× Tris-borate-EDTA (TBE) buffer using ethidium bromide (EtBr) staining.
Cellular DNA fragmentation ELISA.
Levels of apoptotic cell death were measured by a quantitative sandwich enzyme immunoassay using a commercial kit (Cellular DNA Fragmentation ELISA; Boehringer Mannheim). Cells were labeled with bromodeoxyuridine (BrdU) overnight prior to virus infection. At the various indicated time points postinfection, cells were permeabilized to release the cytoplasmic DNA fragments into the supernatant. The amounts of BrdU-labeled DNA released were measured by an enzyme-linked immunosorbent assay (ELISA) reader (Microplate reader; Molecular Devices) using antibodies against DNA and BrdU.
RNA dot blotting and [3H]uridine incorporation.
For RNA preparation, the published method (16) was followed; briefly, cell monolayers in 60-mm dishes were first incubated with RNA lysis buffer (4 M guanidine thiocyanate, 25 mM sodium citrate [pH 7.0], 10 mM β-mercaptoethanol, 0.5% N-lauroylsarcosine) for 15 min at 4°C. Cell lysates were extracted twice with acid pheno-chloroform (1:1), and RNA in the aqueous phase was then collected and precipitated by alcohol. Appropriate amounts of RNA from each sample were twofold serially diluted with RNA dilution buffer (diethylpyrocarbonate [DEPC]-treated H2O–20× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate {pH 7.0}]–formaldehyde [5:3:2]) and then applied to a nylon membrane (Boehringer Mannheim) using a dot blotting manifold (Pierce). The resulting RNA samples on the membrane were fixed by UV cross-linking, blotted, and detected by a digoxigenin (DIG) nonradioactive nucleic acid labeling and detection system (Boehringer Mannheim) according to the manufacturer's instructions. Briefly, the filter was prehybridized with DIG Easy Hyb solution (Boehringer Mannheim) at 50°C for 1 h before the probe was added and hybridized overnight. DIG-11-dUTP was incorporated into the DNA probe by Taq polymerase during PCR. To obtain the DIG-labeled DNA probe specific for JEV, a plasmid containing the JEV NS1 gene was used as the template for PCR with a primer set hybridized to nucleotides 2478 (positive sense) (5′-GCGATCCAGACACTGGATGTGCCA-3′) and 3534 (negative sense) (5′-GCGGATCCTAAGCATCAACCTGTGA-3′) of the JEV cDNA. After overnight hybridization, the filter was washed twice for 5 min in 2× SSC–0.1% SDS at RT and twice for 15 min in 0.1× SSC–0.1% SDS at 68°C. To detect the RNA-DNA binding signal on the filter, an anti-DIG antibody-alkaline phosphatase conjugate was used to bind to the hybridized probe, and the signal was detected by exposing the filter to X-ray film in the presence of the chemiluminescence substrate CSPD (Roche Molecular Biochemicals).
To incorporate [3H]uridine, infected cells were first treated with actinomycin D at 2 μg/ml for 1 h and then labeled with [3H]uridine (Amersham) at 10 μCi/ml in culture medium for another 1 h. Actinomycin D was used to reduce polII RNA synthesis so that the signal from polII-independent RNA synthesis and viral RNA replication could be readily detected. The total cellular RNAs were isolated with NET buffer (50 mM Tris-HCl [pH 7.75], 150 mM NaCl, 0.1% NP-40, 1 mM EDTA) plus 2% SDS and precipitated on glass fiber disκs (GF/C; Whatman) with a trichloroacetic acid (TCA) solution (5% TCA and 20 mM sodium pyrophosphate). The disks were washed with 70% ethanol and dried at RT. [3H]uridine incorporation was measured with a β-counter (Beckman) using scintillation fluid (Biofluor; Dupont, NEN).
LDH assay.
Cell viability was assessed by the release of the cytoplasmic enzyme lactate dehydrogenase (LDH) using a commercial kit (Cytotoxicity Detection Kit; Boehringer Mannheim) following the manufacturer's instructions. Briefly, the culture supernatants from cell samples were clarified by centrifugation, mixed with the reaction mixture (diaphorase-NADH+, tetrazolium salt INT-sodium lactate), incubated at RT for about 30 min, and then read by an ELISA reader at 490 nm (Microplate reader; Molecular Devices).
Determination of luciferase activity.
Cells were transiently transfected with a reporter plasmid, pNFκB-Luc (Stratagene), carrying the luciferase gene downstream of the NF-κB promoter. After 18 h, the cells were infected by JEV at an MOI of 5, and at 6 h postinfection the resulting cells were harvested and lysed to determine luciferase activity by using a Luciferase Assay System kit purchased from Promega. To examine the salicylate effect, in some indicated experiments the cells were also treated with 5 mM NaSal. Luciferase activity was expressed as relative light units.
Establishment of cell clones permanently expressing IκBα-ΔN.
All cell lines stably expressing IκBα-ΔN were cloned from single cells by the limiting-dilution method as previously described (13). To establish cell clones stably expressing IκBα-ΔN, 60% confluent BHK-21 cells were transfected by Lipofectamine (BRL) with the human IκBα-ΔN expression plasmid pCMV4/IκBα-ΔN (11). The transfected cells were selected and cloned in the presence of Geneticin (GIBCO). The expression of IκBα-ΔN in cell clones was assessed by Western blotting, using an antibody specific for the human IκBα-ΔN protein (Santa Cruz). The resultant clones were cultured in RPMI 1640 medium containing 5% FCS.
RESULTS
NaSal and aspirin inhibit the replication of flavivirus, but not that of Sindbis virus, in BHK-21 cells and N18 mouse neuroblastoma cells.
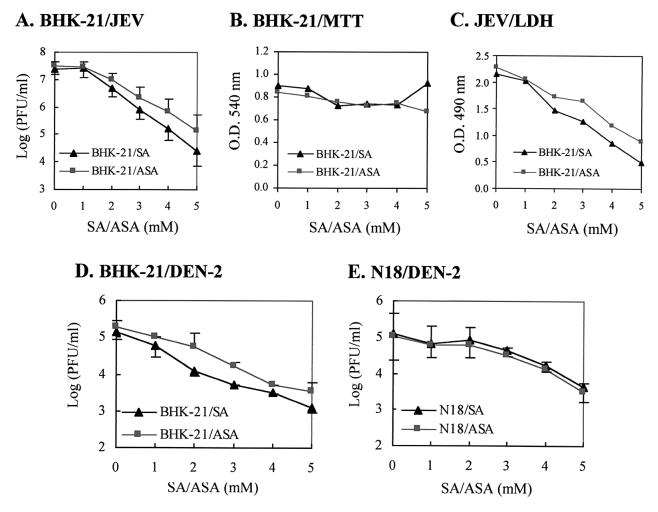
Both BHK-21 and N18 cells can support the productive replication of JEV and DEN-2 (13, 47), and the severe CPE induced by the viruses lead to cell death through a mechanism probably involving apoptosis (43). To study the effects of NaSal and aspirin on JEV replication, BHK-21 fibroblasts were first infected with RP-9, a neurovirulent strain of JEV (14), at an MOI of 5, and after 1 h of virus adsorption the infected cells were washed and incubated in the presence of varying amounts of salicylates for 48 h. A concentration of salicylates between 1 and 5 mM was chosen for subsequent experiments because this is the actual serum range suggested for patients treated for chronic inflammatory diseases, and concentrations higher than 6.5 mM have been proven to be too toxic for clinical use (34). As Fig. 1A indicates, within the range of 1 to 5 mM the addition of NaSal or aspirin to the culture medium inhibited JEV replication in a dose-dependent manner. To determine whether or not the antiviral effects of NaSal and aspirin were due merely to the cytotoxic effect of salicylates on BHK-21 cells, an MTT assay was performed to evaluate mitochondrial activity (46) as a viability index for salicylate-treated cells. Figure 1B shows that no significant difference in mitochondrial function was detectable among cells treated with salicylates at concentrations ranging from 1 to 5 mM. In addition, salicylate by itself did not appear to have any direct anti-JEV capability, since pretreatment of JEV stocks with 5 mM NaSal or aspirin for 1 h at 37°C did not affect virus infectivity (data not shown). These results indicated that it was the infected BHK-21 cells that were targeted by salicylates to result in an inhibitory effect on JEV replication. Since we previously demonstrated that JEV could cause severe CPE of infected cells (43), the effects of salicylates on JEV-induced CPE were examined. Morphologically, at 36 h postinfection, CPE resulting from JEV infection were evident in the BHK-21 cells without salicylate treatment, whereas the infected, salicylate-treated BHK-21 cells appeared to be more resistant to virus-induced cytopathic damage (data not shown). The magnitude of cellular damage was then quantified by measuring the amount of LDH, a cytoplasmic enzyme, that had leaked into the culture medium from tested cells. As shown in Fig. 1C, at 36 h postinfection, the release of LDH from JEV-infected cells gradually declined as the concentration of NaSal or aspirin increased, consistent with the antiviral phenomenon seen in Fig. 1A. This result suggested that salicylates alleviated the JEV-triggered CPE in the infected BHK-21 cells in a dose-dependent fashion.
FIG. 1.
NaSal and aspirin inhibit flavivirus replication in BHK-21 and N18 cells. (A) BHK-21 cells were infected by JEV at an MO1 of 5, and at 1 h postinfection, the infected cells were treated with varying concentrations of NaSal (SA) or aspirin (ASA). After a 48-h incubation at 37°C, the virus titers (expressed as PFU per milliliter) in the culture media were determined by a plaque forming assay as described in Materials and Methods. Values of virus titers are shown as means ± standard errors of the means for three independent experiments. (B) MTT assay of salicylate-treated BHK-21 cells. BHK-21 cells were treated with SA or ASA at the indicated concentrations for 48 h at 37°C, and the viabilities of the resulting cells were determined by an MTT assay. (C) LDH release assay. At 32 h postinfection, amounts of LDH released from the JEV-infected BHK-21 cells treated with varying amounts of salicylates were measured by an ELISA plate reader at 490 nm. Optical densities at 490 mm are shown as means for representative experiments performed in triplicate. (D) BHK-21 cells were infected by DEN at an MO1 of 5 and were treated with varying concentrations of SA or ASA as indicated. After a 48-h incubation at 37°C, the virus titers (in PFU per milliliter) in the culture media were determined as described for panel A). (E) N18 cells were infected by DEN and treated with SA or ASA. After a 48-h incubation at 37°C, the virus titers (in PFU per milliliter) in the culture media were determined as described for panel A.
We also investigated the effects of salicylates on JEV replication in a murine neuroblastoma cell line N18. Within the 1 to 5 mM range, the presence of NaSal or aspirin in the media of JEV-infected cells exhibited an antiviral effect in a dose-dependent manner, and at higher concentrations (3 to 5 mM), aspirin seemed to be slightly more effective than NaSal as an inhibitor of JEV replication (data not shown). In addition, in agreement with our observations for BHK-21 cells, salicylates also appeared to reduce JEV-induced CPE in N18 cells; likewise, a similar inhibitory effect of salicylates on JEV replication was noticed with other cell types tested, including Vero, COS-7, and mouse astrocytoma DBT cells (data not shown). Since this inhibition was not restricted to one cell type, the salicylate-mediated anti-JEV effect seemed to operate in a cell type-independent manner.
We next explored whether salicylates can also block the replication of DEN, another member of the mosquito-borne flaviviruses, in cultured cells. BHK-21 or N18 cells were infected by a human DEN-2 isolate, PL046, at an MOI of 5, and the infected cells were then incubated for appropriate periods with various concentrations of NaSal or aspirin. At 48 h postinfection the number of infectious virus particles released into the culture medium was determined. As Fig. 1D shows, both NaSal and aspirin hindered the replication of DEN-2 in BHK-21 cells in a concentration-dependent fashion. Similarly, salicylates also inhibited the replication of DEN-2 in N18 cells (Fig. 1E). Taken together, these results showed that salicylates possessed an antiflavivirus effect on this cell culture system. This observation raises the possibility that salicylates may also be capable of hampering the replication of other RNA viruses in addition to flaviviruses in cultured cells. To address this issue, another positive-sense RNA virus, Sindbis virus (Ar-339; American Type Culture Collection), which is a prototype of the Alphavirus family, was examined under the conditions described above. We found that after 18 h of incubation, neither NaSal nor aspirin had an inhibitory effect on Sindbis virus replication in BHK-21 or N18 cells. There was no apparent difference in virus production between infected cells that were treated with salicylates and those that were not, even at a concentration of 5 mM (data not shown). These salicylates also failed to suppress Sindbis virus-induced CPE in both cell types (data not shown). These results indicate that the inhibitory effects of salicylates are not universal among RNA viruses. The inhibition of flaviviruses, but not alphaviruses, by salicylates is likely due to the difference in the modes of virus replication between flaviviruses and alphaviruses in infected cells.
Effects of salicylates on synthesis of viral RNA in JEV-infected N18 cells.
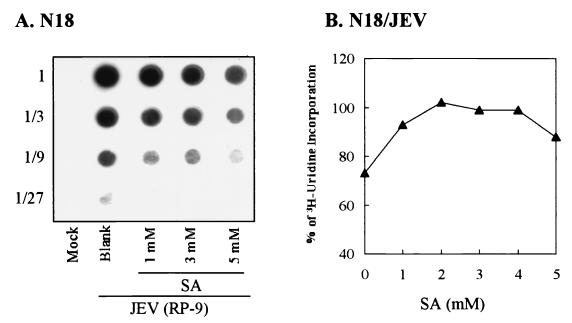
To further characterize the mechanism of salicylate-mediated inhibition, the effects of NaSal on viral RNA synthesis in JEV-infected N18 cells were investigated. Total viral RNA isolated from RP-9-infected, NaSal-treated N18 cells was quantitated by RNA dot blot analysis using a double-stranded DNA probe specific for the JEV NS1 region (46). As Fig. 2A shows, at 18 h postinfection the amounts of total viral RNA were gradually reduced as the dose of NaSal in the culture medium increased, indicating that salicylate decreased the amount of viral RNA accumulation. As a control, the total amounts of polII-independent RNA synthesis, as detected by [3H]uridine incorporation, did not differ significantly among JEV-infected N18 cells treated with varying concentrations of NaSal (Fig. 2B). These results thus suggest that NaSal exerted its antiflavivirus activity by interfering specifically with flavivirus RNA synthesis in the infected cells.
FIG. 2.
Effects of NaSal on viral RNA synthesis in JEV-infected N18 cells. (A) RNA dot blotting. Total RNAs were isolated at 18 h postinfection from JEV-or mock-infected N18 cells treated with various doses of NaSa1 (SA) as indicated. Appropriate amounts of RNA from each sample were threefold serially diluted (marked as 1 to 1/27 on the left of the panel) and applied to a nylon membrane that was then hybridized with the DIG-labeled DNA probe specific for the JEV NS1 region. (B) [3H]uridine incorporation. JEV-infected N18 cells were treated with varying doses of SA and incubated for 17 h at 37°C. After the resulting cells were labeled with [3H]uridine for 1 h, the newly synthesized, actinomycin D-resistant RNA was isolated. One-tenth of the cell lysates from each sample was counted for incorporation of [3H]uridine into RNA as described in Materials and Methods.
Effects of salicylates on synthesis of JEV glycoproteins.
Both E and NS1 proteins of JEV are glycoproteins that can be secreted into culture media during infection (13). To determine whether or not salicylates can influence the synthesis of viral proteins, the amounts of viral glycoproteins accumulating intracellularly or released extracellularly from JEV-infected BHK-21 or N18 cells were determined by Western blot and densitometry analysis using monoclonal antibodies specific for viral E and NS1 glycoproteins. The accumulation of intracellular (Fig. 3A) or extracellular (Fig. 3B) E proteins from both BHK-21 and N18 cells was only slightly affected by different concentrations of NaSal. In contrast, upon NaSal treatment, the amounts of intracellular (Fig. 3A), as well as extracellular (Fig. 3B), NS1 proteins accumulating from JEV-infected cells decreased as the NaSal concentration gradually increased. This inhibitory effect of NaSal on viral protein synthesis was not likely due to its cytotoxic effect on the infected cells, since total protein synthesis, as measured by [35S]methionine incorporation, did not differ significantly among cells treated with varying amounts of NaSal or between treated and untreated cells (data not shown). These data suggest that NaSal may either diminish the amount of NS1 glycoproteins synthesized intracellularly or block the secretion of these glycoproteins from JEV-infected cells. Still, it remains unclear why NaSal selectively influenced the accumulation of JEV NS1 more than that of E or NS1′ proteins in infected cells. Whether NaSal affects the stability of NS1 protein at a posttranslational level during JEV replication requires further study. In addition, why NaSal could inhibit viral RNA synthesis (Fig. 2A) more strongly than viral glycoprotein synthesis (Fig. 3), especially at 1 mM NaSal, remains unclear and needs to be addressed thoroughly in future experiments.
FIG. 3.
Effects of NaSal on synthesis of JEV glycoproteins in infected BHK-21 and N18 cells. Immunoblot analysis of JEV E or NS1 protein in cell lysates (A) or in supernatants (B) from BHK-21 (lanes 1 to 6) or N18 cells (lanes 7 to 12) cells treated with doses of NaSa1 (SA) ranging from 0 to 5 mM as indicated. NS1′ is the longer version of the NS1 protein derived from an aberrant cleavage in the JEV-infected cells (36).
Salicylates suppress flavivirus-induced apoptosis in cultured cells.
Since JEV infection triggers apoptotic cell death (43), we investigated whether salicylates are capable of preventing infected cells from undergoing JEV-induced apoptosis. JEV strain RP-9 (at an MOI of 5) was used to infect BHK-21 or N18 cells. At 32 h postinfection, low-molecular-weight DNA was isolated from mock- or JEV-infected cells treated with varying amounts of salicylates, and cells were then examined using agarose gel electrophoresis. As a control, a DNA sample derived from BHK-21 cells infected with JEV exhibited characteristic internucleosomal size laddering (Fig. 4A, lane 1), whereas this DNA pattern was not seen in the sample obtained from mock-infected cells, even though they had been treated with 5 mM NaSal (Fig. 4A, lane 2) or aspirin (lane 6). Salicylates appeared to inhibit JEV-induced apoptosis of infected BHK-21 cells in a dose-dependent manner, since as the concentration of NaSal (Fig. 4A, lanes 3 to 5) or aspirin (lanes 7 to 9) increased, the extent of virus-induced DNA ladder formation gradually decreased.
FIG. 4.
Salicylates suppress flavivirus-induced apoptosis in BHK-21 and N18 cells. (A) Agarose gel electrophoresis of DNA fragmentation. BHK-21 cells were either mock infected (lanes 2 and 6) or infected with JEV at an MOI of 5 (lanes 1, 3 to 5, and 7 to 9), and cells were then left untreated (lane 1) or treated with NaSal (SA) (lanes 2 to 5) or aspirin (ASA) (lanes 6 to 9) at the indicated concentrations. Low-molecular-weight DNA was isolated from cells at 32 h postinfection and analyzed by 2% agarose gel in the presence of EtBr. Lane M, 100-bp ladders as DNA markers. (B) Gel analysis of DNA ladders from JEV-infected N18 cells that were left untreated (lane 1) or treated with 5 mM SA (lane 2) at 32 h postinfection. (C) Kinetics of DNA fragmentation from JEV-infected N18 cells that were left untreated (filled squares) or treated (open circles) with 5 mM SA, determined by ELISA. Prior to virus infection, cells were labeled with BrdU overnight. At the indicated time points following infection, the cells were permeabilized to release the cytoplasmic DNA fragments into the supernatants. The amounts of BrdU-labeled DNA released were measured by ELISA as optical densities (O.D.) at 450 nm using antibodies against DNA and BrdU (see Materials and Methods).
Similarly, 5 mM NaSal also suppressed JEV-induced apoptosis in infected neuronal N18 cells (Fig. 4B, lane 2), whereas a DNA ladder was observed in infected cells without NaSal treatment (Fig. 4B, lane 1). To further characterize the inhibitory kinetics of NaSal on JEV-induced apoptosis in cell cultures, we performed a DNA fragmentation ELISA (see Materials and Methods) using JEV-infected N18 cells as a model. This quantitative analysis allowed us to measure the amounts of free DNA fragments released in the cytoplasm of apoptotic cells at different time points following infection (43). As shown in Fig. 4C, the amount of DNA fragments detected from control JEV-infected cells increased progressively with time, peaking at 32 h postinfection but declining thereafter; in contrast, when infected cells were treated with 5 mM NaSal, the upturn in DNA fragmentation appeared to a lesser extent, and there was no apparent CPE in these cells during this incubation period. Similarly, both NaSal and aspirin also inhibited DEN-induced apoptosis (data not shown). Together, these results indicate that salicylates can indeed diminish flavivirus-induced apoptosis in cultured cells, likely by blocking virus replication in the infected cells.
The antiflavivirus effects of salicylates were not mediated by blocking NF-κB activation in infected cells.
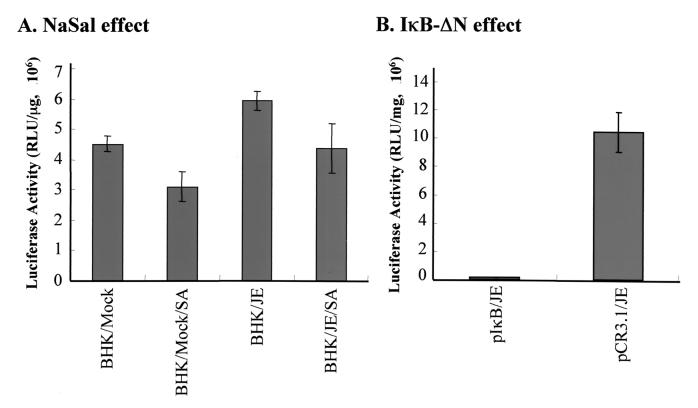
DEN replication activates NF-κB in human hepatoma cells, which, in turn, triggers apoptotic cell death (52). Considering that both NaSal and aspirin have been shown to inhibit NF-κB activation by preventing the degradation of its cytoplasmic inhibitor IκB (39), we investigated whether the NF-κB pathway is essential for flavivirus replication or for the process of virus-induced apoptosis, and whether the antiflavivirus effect of salicylates is mediated by blocking NF-κB activation in infected cells. Using an NF-κB–luciferase reporter plasmid controlled by five NF-κB binding sites (pNFκB-Luc; Stratagene), we found (Fig. 5A) that after 6 h of infection, JEV could induce NF-κB activation compared to the mock infection control (P < 0.005 by the Student t test) and addition of 5 mM NaSal could suppress this activation (P < 0.05 by the Student t test). We next explored the effect of an NF-κB-specific cell-permeating peptide inhibitor, SN50, on JEV replication in infected cells (48). At 1 h post-JEVinfection, the infected BHK-21 and N18 cells were treated with varying concentrations of SN50, and virus yields from these cells were assessed after 36 h of incubation. The results (Fig. 6) show that neither SN50 nor its mutant peptide (SN50M, a negative control) suppressed JEV replication in BHK-21 (Fig. 6A) or N18 (Fig. 6B) cells, whereas the control, 5 mM NaSal did so substantially in both types of cells. In addition, using the NF-κB–luciferase reporter system, treatment of cells with 100 μg of SN50 was found to reduce JEV-induced NF-κB activation by approximately 43% compared to untreated counterparts, whereas the control mutant peptide, SN50M, failed to do so. On the other hand, lactacystin, which is a specific proteasome inhibitor and is also known as a potent inhibitor of NF-κB activation (20), exhibited a negligible effect on JEV replication (Fig. 6C). In addition, neither SN50 nor lactacystin blocked the occurrence of apoptotic cell death in JEV- or DEN-infected cells (data not shown). However, both of these inhibitors potently repressed NF-κB activation when cells were treated with 10 nM PMA or 10 μg of poly (I · C) (data not shown), which has been shown to activate NF-κB, likely through a protein kinase C activation pathway (8, 24, 67, 76).
FIG. 5.
Effect of NaSal or the dominant-negative mutant IκB-ΔN on JEV-induced NF-κB activation. (A) NaSal effect. A total of 8 × 104 BHK-21 cells transfected with pNFκB-Luc were either left untreated or treated with 5 mM NaSa1 or for 18 h. The resulting cells were then either infected with JEV (at an MOI of 5) or mock infected, and at 6 h postinfection, cell lysates were prepared for determination of luciferase activity. (B) IκB-ΔN effect. A total of 8 × 104 BHK-21 cells were transfected either with pNFκB-Luc together with plκB-ΔN or with pNFκB-Luc plus the pCR3.1 vector, and these cells were then infected with JEV at an MOI of 5 for another 6 h. The resulting cell lysates were prepared and assessed for luciferase activity. Values shown are representative of the results from three independent experiments. Luciferase activity is expressed as relative light units.
FIG. 6.
Role of NF-κB activation in JEV replication in BHK-21 and N18 cells. (A and B) Effect of the cell-permeating peptide inhibitor SN50 on JEV replication. At 1 h postinfection, JEV-infected BHK-21 (A) or N18 (B) cells were treated with varying doses of SN50, and virus yields from the cells were determined by a plaque assay after a 36-h incubation. As negative controls, the infected cells were either left untreated (Blank) or, treated with the mutant peptide SN50M; as a positive control, cells were treated with 5 mM NaSal (SA). (C and D) Effect of lactacystin (C) or indomethacin (D) on JEV reproduction in BHK-21 cells. At 1 h postinfection, JEV-infected BHK-21 cells were either left untreated (Blank) or treated with varying doses of lactacystin or indomethacin, and after 20 h of incubation, virus yields from the resulting cells were determined by plaque assay as described for Fig. 1A.
The effectiveness of salicylates in treating inflammation has been attributed to their ability to inhibit prostaglandin production by blocking COX (53, 74). In addition, aspirin has been reported to attenuate CMV replication in cultured cells, partly by inhibiting COX activity (73). We therefore examined whether suppression of COX results in inhibition of flavivirus replication. After 1 h postinfection, JEV-infected BHK-21 cells were treated with varying concentrations of indomethacin (1 to 50 μM), which is a potent COX inhibitor with effective anti-inflammatory function (34). We found that addition of indomethacin, even at concentrations higher than those normally used clinically had no inhibitory effect on JEV replication in BHK-21 cells (Fig. 6D). This result implies that the antiflavivirus effect of salicylates discussed above was not due to inhibition of COX activity in the infected cells.
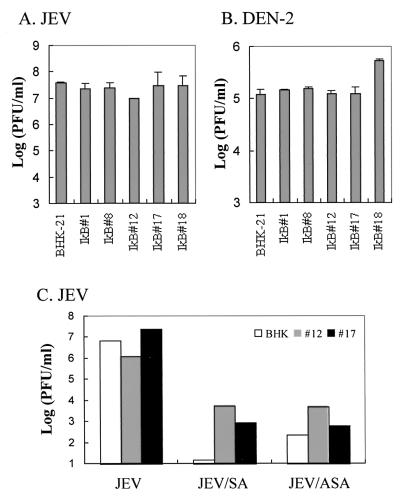
To further confirm that NF-κB activation in the infected cells was not required for flavivirus replication or virus-induced apoptosis, we performed a genetic experiment using cells either transiently or constitutively expressing IκBα-ΔN, a dominant-negative mutant that efficiently blocks NF-κB activation (11). The results in Fig. 5B show that transient expression of IκBα-ΔN could efficiently inhibit JEV-induced NF-κB activation in BHK-21 cells. We established several independent BHK-21 clones stably expressing IκBα-ΔN and chose five of these clones (see below) to study the replication capability of JEV or DEN in an NF-κB–null background. The amounts of IκBα-ΔN expression in the individual clones were found to be similar by Western blot analysis. Using the luciferase reporter plasmid, we examined the status of NF-κB activation in flavivirus-infected cells with or without salicylate treatments. We observed that stable expression of IκBα-ΔN almost completely suppressed constitutive NF-κB activity compared to that in the parental BHK-21 cells, and such IκBα-ΔN-expressing clones indeed led to failure of NF-κB activation in response to poly(I · C) treatment or JEV infection (data not shown). We next examined whether flaviviruses could replicate in these NF-κB–null cells. Figure 7 reveals that after infection with JEV (Fig. 7A) or DEN-2 (Fig. 7B), virus yields from all five IκBα-ΔN-expressing clones were similar to that from wild-type BHK-21 cells. In addition, we observed an apparent virus-induced CPE, including abnormal microscopic appearance and apoptosis indicated by DNA ladder formation, in flavivirus-infected, IκBα-ΔN-expressing clones as readily as in infected BHK-21 cells (data not shown). On the other hand, NaSal or aspirin still substantially inhibited JEV replication (Fig. 7C) and virus-induced CPE (data not shown) in the IκBα-ΔN-expressing cells at 24 h postinfection, even though suppression of virus yields was not as obvious as in wild-type BHK-21 cells. Moreover, in addition to the early time point at 24 h postinfection (Fig. 7C), we also observed the failure of IκB-ΔN to inhibit JEV replication at 36 and 54 h postinfection, similarly, addition of 5 mM NaSal still effectively suppressed virus yields approximately 100-fold in IκB-ΔN-expressing cells compared to those in their untreated counterparts. Taken together, these results suggest that (i) flaviviruses do not seem to require NF-κB activation to complete their replication, (ii) flaviviruses do not have to activate NF-κB in order to trigger apoptosis from the cells we used here, and (iii) salicylates appear to exert antiflavivirus effects by an NF-κB-independent mechanism in infected BHK-21 cells.
FIG. 7.
Effects of constitutive IκBα-ΔN expression on antiflavivirus capability of salicylates. The ability of JEV (A) or DEN (B) to replicate in IκBα-ΔN-expressing cell clones (IκB#1, #8, #12, #17, and #18) was compared to that in wild-type BHK-21 cells by measurement of titers of virus released into the culture medium. (C) The anti-JEV effects of salicylates were determined for the IκBα-ΔN-expressing cell clones (IκB#12 and #17) and the wild-type BHK-21 cells. After 1 h of JEV infection, cells were left untreated or treated with 5 mM NaSal (SA) or aspirin (ASA) and then incubated at 37°C for another 24 h. Virus production in the culture medium was determined by a plaque assay. Data are mean virus titers for two independent experiments.
The antiflavivirus effects of salicylates were partially undermined by blocking p38 MAPK activation.
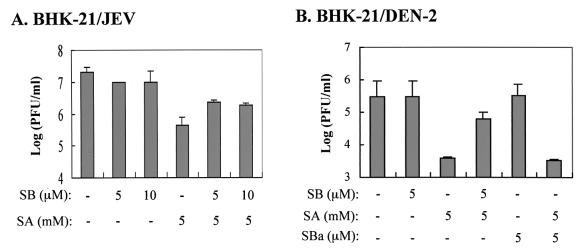
We next investigated whether p38 MAPK might be involved in the antiflavivirus effects of salicylates. Figure 8A shows that 5 μM SB203580, which is a specific p38-MAPK inhibitor, partially reversed the anti-JEV effect originating from 5 mM NaSal, while addition of SB203580 alone to JEV-infected BHK-21 cells had no inhibitory effect on JEV replication. Similarly, SB203580, but not its ineffective analogue SB202474, also partly neutralized the inhibitory effect of NaSal on DEN replication in BHK-21 cells (Fig. 8B). Together, these results suggest that the activity of p38 MAPK may play a role in the inhibition of flavivirus replication by salicylates in cultured cells.
FIG. 8.
Antiflavivirus capability of salicylates in cells treated with the p38 MAPK inhibitor SB203580. (A) BHK-21 cells were infected by JEV at an MOI of 5, and at 1 h postinfection SB203580 (SB) was added to the cells at varying micromolar concentrations in the presence or absence (−) of 5 mM NaSal (SA). After 36 h of incubation at 37°C, virus yields in the culture media were determined by a plaque assay. (B) BHK-21 cells were infected by DEN at an MOI of 5, and at 1 h postinfection varying doses (micromolar concentrations) of SB203580 or its ineffective analogue SB202474 (SBa) were added cells in the presence or absence (−) of 5 mM SA. After 36 h of incubation at 37°C, virus yields in the culture media were determined as described for Fig. 1A.
DISCUSSION
Aspirin and NaSal are commonly prescribed drugs that have wide spectra of pharmacological activities and multiple sites of action. In this study we demonstrated that aspirin and its metabolite NaSal, at concentrations (1 to 5 mM) compatible with the amounts in the sera of patients undergoing anti-inflammatory therapy, specifically inhibited flavivirus replication, as well as virus-induced apoptosis, in infected cells (Fig. 1 to 4). In contrast, salicylates had only a trivial inhibitory effect on Sindbis virus infection under the same conditions, despite the fact that flaviviruses and Sindbis virus, a prototype of Alphavirus family, have several similarities, i.e., they are both positive-sense RNA viruses that replicate primarily in the cytoplasm of infected cells. These observations indicate that to complete the virus life cycle, flaviviruses and Sindbis virus may differ in their mode of replication, especially in what cellular functions to recruit and how to exploit certain cellular factors to form a viral replicase complex, which may or may not be functionally influenced by salicylates. Alternatively, salicylates may be able to directly target enzymes or structural proteins of flaviviruses more profoundly than those of Sindbis virus. In fact, the significant reduction in JEV NS1 proteins, but not viral envelope proteins, by NaSal treatment (Fig. 3) suggests that the antiflavivirus effects of salicylates may occur by direct blocking of the flavivirus replication machinery, because NS1, which can be expressed only in infected cells, has been shown to participate indispensably in both viral replication and morphogenesis (49, 51, 55, 56). Aside from cellular factors, it remains uncertain what other viral factors might also contribute to such a salicylate-inhibitable phenotype for flavivirus infection in a culture system.
Through a COX-2-dependent pathway, human CMV infection of smooth muscle cells generates a large quantity of cellular reactive oxygen species (ROS); these in turn stimulate NF-κB activation, which is critical for CMV replication (73). Aspirin and NaSal have been demonstrated to possess anti-CMV effects, likely by directly scavenging ROS and blocking NF-κB activation; additionally, indomethacin, which, like aspirin, is a nonselective COX-1 and COX-2 inhibitor, can attenuate CMV infection by suppressing COX activities as well as by reducing the amount of virus-induced ROS (73). This observation indicates that to exert anti-CMV effects, salicylates and indomethacin primarily intervene in the process of virus triggering of ROS or NF-κB activation in CMV-infected smooth muscle cells. In contrast, we found that treatment of infected cells with indomethacin failed to suppress either flavivirus replication (Fig. 6D) or virus-induced apoptosis (data not shown), suggesting that COX activity is not required for flavivirus infections in a culture system. In addition, we found that the specific NF-κB inhibitors SN50 and lactacystin had no inhibitory effect on flavivirus replication (Fig. 6A, B, and C), and even with stable expression of dominant-negative IκBα-ΔN in the host cells, flaviviruses were still capable of replicating efficiently (Fig. 7A and B) and of triggering apoptosis (data not shown). These data clearly illustrate that NF-κB activation is not essential either for a flavivirus to reproduce itself or for the infected cells to undergo apoptosis. This result is in contrast to findings from several previous studies concerning DEN-1 (52), DEN-2 (35), Sindbis virus (44, 46), and reovirus (18), in which virus replication activated NF-κB, which is necessary for such viruses to induce apoptosis. This discrepancy reaffirms the notion that whether NF-κB promotes, inhibits, or plays no role in an apoptotic process appears to depend predominantly on the specific cell type and the type of inducer (for a review, see reference 5). Interestingly, however, our data revealed that both aspirin and NaSal not only diminished flavivirus production (Fig. 1) but also blocked virus-induced apoptosis (Fig. 4), even in IκBα-ΔN-expressing cells. This finding strongly suggests that salicylates are able to exert their antiflavivirus effects independently of blocking the NF-κB pathway in a culture system.
NaSal rapidly and persistently activates p38 MAPK, which appears to be essential for NaSal to induce apoptosis in human fibroblasts and for its inhibitory action on TNF-induced IκBα phosphorylation and degradation (70, 71). By use of a specific p38 inhibitor, SB203580, we found that NaSal inhibited flavivirus replication, at least in part through mediation of p38 MAPK activation, whereas SB203580 by itself failed to suppress either flavivirus reproduction (Fig. 8). To elucidate the exact role of p38 kinase in the antiflavivirus effects of salicylates, one needs to further explore whether activation of p38 kinase alone, in the absence of salicylate treatment, still leads to inhibition of flaviviruses in infected cells. Alternatively, p38 kinase activation may operate synergistically with another known or unidentified action(s) of salicylates to inhibit flavivirus infection. Our results shown in Fig. 8 seem to support the latter notion, since a specific p38 inhibitor, SB203580, could only partially attenuate the antiflavivirus effects of NaSal in infected BHK-21 cells. However, since p38 kinases consist of p38α, p38β, p38γ, and p38δ isoforms, and SB203580 specifically inhibits p38α and p38β (36, 42) but not p38γ and p38δ (19, 27, 41, 75), we cannot exclude the possibility that the incomplete suppression by SB203580 of the antiflavivirus effects of NaSal (Fig. 8) may have been due to failure to block the activity of p38γ and/or p38δ. Among the MAPK subfamilies identified in mammalian cells, p38 kinases (30, 42, 64) are often strongly activated by different kinds of stresses, such as UV irradiation, osmotic shock, TNF, and interleukin 1 treatment. Characteristically, p38 kinase activation has been suggested to play an essential role in triggering apoptosis in several systems (10, 33, 57, 71, 78). Salicylates may conceivably be able, via p38 activation, to create a hostile cellular milieu that abrogates flavivirus infection. It will be of interest to investigate whether the mechanism for p38 involved in the antiflavivirus effects of salicylates is similar to that involved in induction of apoptosis by other death stimuli.
There are other possible actions of salicylates that may contribute to the antiflavivirus effects of salicylates and that can be confirmed by future experiments. NaSal inhibits the activation of p42 and p44 kinases (also referred to as extracellular signal-regulated kinase [ERK]) induced by TNF (72) and also inhibits the activation of ERK in neutrophils for integrin-mediated responses (63). However, by use of PD098059, a specific inhibitor of MEK (the proximal kinase activating ERK), we found that ERK activity may not participate in the antiflavivirus effects of salicylates (data not shown). In addition, in an activator protein-1 (AP-1)–luciferase transgenic mouse model, aspirin or NaSal suppressed UV-induced AP-1 activity in a dose-dependent manner, likely by blocking the activation of ERK, c-Jun N-terminal kinase kinases, and p38 kinase (31). Similarly, aspirin or salicylic acid inhibited AP-1 activation, as well as tumor promoter-induced transformation, in a J6 mouse epidermal cell line; these mechanisms might involve elevation of intracellular H+ concentrations (23). Whether flavivirus infections activate AP-1, whether AP-1 activation is required for flavivirus replication, and which AP-1-dependent genes may be pharmacological targets for salicylates in the process of flavivirus inhibition are interesting issues that remain to be studied. In addition, NaSal treatment, which is biochemically distinguishable from heat treatment, has been found to partially trigger a human heat shock response (38) and induce the DNA binding state of human heat shock transcription factor (HSF) (37). In Drosophila melanogaster, NaSal decreases intracellular ATP levels and induces HSF binding, as well as chromosomal puffing (77). Thus, it can be envisioned that salicylates may utilize a heat shock pathway or deplete intracellular ATP levels to render host cells unsuitable for infection by flaviviruses.
In summary, we found that salicylates inhibit flavivirus replication and virus-induced apoptosis in a dose-dependent manner and that this inhibition is apparently not mediated by blocking either COX activities or NF-κB activation. On the other hand, a specific p38 inhibitor, SB203580, partially rescued flavivirus replication from the antiflavivirus effects of salicylates, indicating that p38 kinase activation by salicylates is somewhat responsible for the antiflavivirus phenomenon. We also observed that the processes of flavivirus replication and virus-triggered apoptosis did not appear to require NF-κB activation, at least in the cell lines used in this study. Together, we conclude that the mechanism by which salicylates suppress flavivirus replication, although it may involve p38 MAPK activity, is independent of blocking the NF-κB activation pathway. However, one must exercise caution in interpreting or applying our results concerning how salicylates inhibit DEN infections, since salicylates are notorious for anti-platelet function, giving rise to an increase in bleeding time, a situation that can be extremely hazardous for a dengue hemorrhagic fever or dengue shock syndrome patient inappropriately treated with salicylates. Nevertheless, the results of the present study using salicylates as molecular probes in a culture system contribute to our understanding of flavivirus-host interactions and shed some light on the identification of potential new pharmacological targets for the control of flavivirus infections.
ACKNOWLEDGMENTS
The kind gifts of the N18 cell line from D. E. Griffin, pCMV4/IκBα-ΔN from D. W. Ballard, and DEN-2 strain PL046 from the National Institute of Preventive Medicine are deeply appreciated.
C.-L.L. was supported by two grants (NSC 89-2320-B-016-089 and NSC 89-2323-B-016-001) from the National Science Council (NSC), ROC, and one (DOD-89-18) from the Department of Defense (DOD), ROC. Y.-L.L. was supported by a grant from the NSC (NSC-89-2320-B-001-018).
REFERENCES
- 1.Amano T, Richelson E, Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones. Proc Natl Acad Sci USA. 1972;69:258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20.20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 5.Barkett M, Gilmore T D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand F, Philippe C, Antoine P J, Baud L, Groyer A, Capeau J, Cherqui G. Insulin activates nuclear factor kappa B in mammalian cells through a Raf-1-mediated pathway. J Biol Chem. 1995;270:24435–24441. doi: 10.1074/jbc.270.41.24435. [DOI] [PubMed] [Google Scholar]
- 7.Bi Z, Reiss C S. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitar R, Beauparlant P, Lin R, Pitha P, Hiscott J. Retrovirus-mediated transfer of nuclear factor-kappa B subunit genes modulates IκB α and interferon beta expression. Cell Growth Differ. 1995;6:965–976. [PubMed] [Google Scholar]
- 9.Bitko V, Velazquez A, Yang L, Yang Y C, Barik S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology. 1997;232:369–378. doi: 10.1006/viro.1997.8582. [DOI] [PubMed] [Google Scholar]
- 10.Brenner B, Koppenhoefer U, Weinstock C, Linderkamp O, Lang F, Gulbin E. Evidence for a novel function of the CD40 ligand as a signaling molecule in T-lymphocytes. J Biol Chem. 1997;272:22173–22181. doi: 10.1074/jbc.272.35.22173. [DOI] [PubMed] [Google Scholar]
- 11.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 13.Chen L-K, Liao C-L, Lin C-G, Lai S-C, Liu C-I, Ma S-H, Huang Y-Y, Lin Y-L. Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology. 1996;217:220–229. doi: 10.1006/viro.1996.0109. [DOI] [PubMed] [Google Scholar]
- 14.Chen L-K, Lin Y-L, Liao C-L, Lin C-G, Huang Y-L, Yeh C-T, Lai S-C, Jan J-T, Chin C. Generation and characterization of organ-tropism mutants of Japanese encephalitis virus in vivo and in vitro. Virology. 1996;223:79–88. doi: 10.1006/viro.1996.0457. [DOI] [PubMed] [Google Scholar]
- 15.Chirillo P, Falco M, Puri P L, Artini M, Balsano C, Levrero M, Natoli G. Hepatitis B virus pX activates NF-κB-dependent transcription through a Raf-independent pathway. J Virol. 1996;70:641–646. doi: 10.1128/jvi.70.1.641-646.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomezynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Clesham G J, Adam P J, Proudfoot D, Flynn P D, Efstathiou S, Weissberg P L. High adenoviral loads stimulate NFκB-dependent gene expression in human vascular smooth muscle cells. Gene Ther. 1998;5:174–180. doi: 10.1038/sj.gt.3300576. [DOI] [PubMed] [Google Scholar]
- 18.Connolly J L, Rodgers S E, Clarke P, Ballard D W, Kerr L D, Tyler K L, Dermody T S. Reovirus-induced apoptosis requires activation of transcription factor NF-κB. J Virol. 2000;74:2981–1989. doi: 10.1128/jvi.74.7.2981-2989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuenda A, Cohen P, Buee-Scherrer V, Goedert M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO J. 1997;16:295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui H, Matsui K, Omura S, Schauer S L, Matulka R A, Sonenshein G E, Ju S T. Proteasome regulation of activation-induced T cell death. Proc Natl Acad Sci USA. 1997;94:7515–7520. doi: 10.1073/pnas.94.14.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Despres P, Flamand M, Ceccaldi P E, Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Despres P, Frenkiel M P, Ceccaldi P E, Duarte Dos Santos C, Deubel V. Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J Virol. 1998;72:823–829. doi: 10.1128/jvi.72.1.823-829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Z, Huang C, Brown R E, Ma W-Y. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. J Biol Chem. 1997;272:9962–9970. doi: 10.1074/jbc.272.15.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finco T S, Baldwin A S., Jr Kappa B site-dependent induction of gene expression by diverse inducers of nuclear factor kappa B requires Raf-1. J Biol Chem. 1993;268:17676–17679. [PubMed] [Google Scholar]
- 25.Garoufalis E, Kwan I, Lin R, Mustafa A, Pepin N, Roulston A, Lacoste J, Hiscott J. Viral induction of the human beta interferon promoter: modulation of transcription by NF-κB/rel proteins and interferon regulatory factors. J Virol. 1994;68:4707–4715. doi: 10.1128/jvi.68.8.4707-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Jr, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKα and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16:3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Good L, Sun S C. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type 1 Tax involves degradation of IκBβ. J Virol. 1996;70:2730–2735. doi: 10.1128/jvi.70.5.2730-2735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-κB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 30.Han J, Lee J-D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 31.Huang C, Ma W-Y, Hanenberger D, Cleary M P, Bowden G T, Dong Z. Direct evidence for an important role of sphingomyelinase in ultraviolet-induced activation of c-Jun N-terminal kinase. J Biol Chem. 1997;272:26325–26331. doi: 10.1074/jbc.272.44.27753. [DOI] [PubMed] [Google Scholar]
- 32.Huen D S, Henderson S A, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 33.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoch M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/INK and p38 signalling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 34.Insel P. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout. In: Hardman J G, Molinoff P B, Rudden R W, Gilman A G, editors. The pharmacological basis of therapeutics. New York, N.Y: McGraw-Hill; 1996. pp. 617–657. [Google Scholar]
- 35.Jan J T, Chen B H, Ma S H, Liu C I, Tsai H P, Wu H C, Jiang S Y, Yang K D, Shaio M F. Potential dengue virus-triggered apoptosis pathway in human neuroblastoma cells: arachidonic acid, superoxide anion, and NF-κB are sequentially involved. J Virol. 2000;74:8680–8691. doi: 10.1128/jvi.74.18.8680-8691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Chen C, Li Z, Guo W, Gegner J A, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 37.Jurivich D A, Pachetti C, Qiu L, Welk J F. Salicylate triggers heat shock factor differently than heat. J Biol Chem. 1995;270:24489–24495. doi: 10.1074/jbc.270.41.24489. [DOI] [PubMed] [Google Scholar]
- 38.Jurivich D A, Sistonen L, Kroes R A, Morimoto R I. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 39.Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 40.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 41.Lechner C, Zahalka M A, Giot J-F, Moller N P, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 43.Liao C-L, Lin Y-L, Wang J-J, Huang Y-L, Yeh C-T, Ma S-H, Chen L-K. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol. 1997;71:5963–5971. doi: 10.1128/jvi.71.8.5963-5971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin K I, DiDonato J A, Hoffmann A, Hardwick J M, Ratan R R. Suppression of steady-state, but not stimulus-induced NF-κB activity inhibits alphavirus-induced apoptosis. J Cell Biol. 1998;141:1479–1487. doi: 10.1083/jcb.141.7.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin K I, Lee S H, Narayanan R, Baraban J M, Hardwick J M, Ratan R R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-κB. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Y-L, Huang Y-L, Ma S-H, Yeh C-T, Chiou S-Y, Chen L-K, Liao C-L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin Y-L, Liao C-L, Chen L-K, Yeh C-T, Liu C-I, Ma S-H, Huang Y-Y, Huang Y-L, Kao C-L, King C-C. Study of dengue virus infection in SCID mice engrafted with human K562 cells. J Virol. 1998;72:9729–9737. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y Z, Yao S Y, Veach R A, Torgerson T R, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 49.Lindenbach B D, Rice C M. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol. 1997;71:9608–9617. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucito R, Schneider R J. Hepatitis B virus X protein activates transcription factor NF-κB without a requirement for protein kinase C. J Virol. 1992;66:983–991. doi: 10.1128/jvi.66.2.983-991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackenzie J M, Jones M K, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 52.Marianneau P, Cardona A, Edelman L, Deubel V, Despres P. Dengue virus replication in human hepatoma cells activates NF-κB, which in turn induces apoptotic cell death. J Virol. 1997;71:3244–3249. doi: 10.1128/jvi.71.4.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell J A, Saunders M, Barnes P J, Newton R, Belvisi M G. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappa B) activation: role of arachidonic acid. Mol Pharmacol. 1997;51:907–912. doi: 10.1124/mol.51.6.907. [DOI] [PubMed] [Google Scholar]
- 54.Munoz E, Israel A. Activation of NF-κB by the Tax protein of HTLV-1. Immunobiology. 1995;193:128–136. doi: 10.1016/s0171-2985(11)80535-8. [DOI] [PubMed] [Google Scholar]
- 55.Muylaert I R, Galler R, Rice C M. Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: effects on virus replication and mouse neurovirulence. Virology. 1996;222:159–168. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- 56.Muylaert I R, Galler R, Rice C M. Genetic analysis of the yellow fever virus NS1 protein: identification of a temperature-sensitive mutation which blocks RNA accumulation. J Virol. 1997;71:291–298. doi: 10.1128/jvi.71.1.291-298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase. J Biol Chem. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 58.Nicot C, Tie F, Giam C Z. Persistent activation of NF-κB/Rel by human T-cell leukemia virus type 1 Tax involves degradation of IκBβ. J Virol. 1998;72:6777–6784. doi: 10.1128/jvi.72.8.6777-6784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oeth P, Mackman N. Salicylates inhibit lipopolysaccharide-induced transcriptional activation of the tissue factor gene in human monocytic cells. Blood. 1995;86:4144–4152. [PubMed] [Google Scholar]
- 60.Pahl H L, Baeuerle P A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-κB. J Virol. 1995;69:1480–1484. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palombella V J, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 62.Pierce J W, Read M A, Ding H, Luscinskas F W, Collins T. Salicylates inhibit IκBα phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol. 1996;156:3961–3969. [PubMed] [Google Scholar]
- 63.Pillinger M H, Capodici C, Rosenthal P, Kheterpal N, Hanft S, Philips M R, Weissmann G. Modes of action of aspirin-like drugs: salicylates inhibit ERK activation and integrin-dependent neutrophil adhesion. Proc Natl Acad Sci USA. 1998;95:14540–14545. doi: 10.1073/pnas.95.24.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 65.Ramachandra S, Studzinski G P. Morphological and biochemical criteria of apoptosis. In: Studzinski G P, editor. Cell growth and apoptosis. A practical approach. Oxford, United Kingdom: Oxford University Press, IRL Press; 1995. pp. 119–142. [Google Scholar]
- 66.Rice C M. Flaviviridae: the viruses and their replication, p. 931–959. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott Raven Publishers; 1996. [Google Scholar]
- 67.Rosette C, Karin M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-κB. J Cell Biol. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roulston A, Conti L, McKiel V, Wainberg M A, Hiscott J. Virus induction of NF-κB/Rel proteins and type 1 interferon gene expression in myelomonoblastic cells. Leukemia. 1994;8:S170–S174. [PubMed] [Google Scholar]
- 69.Sambucetti L C, Cherrington J M, Wilkinson G W, Mocarski E S. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwenger P, Alpert D, Skolnik E Y, Vilcek J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor: IκBα necrosis factor-induced IκBα phosphorylation and degradation. Mol Cell Biol. 1998;18:78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwenger P, Bellosta P, Vietor I, Basilico C, Skolnik E Y, Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci USA. 1997;94:2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwenger P, Skolnik E Y, Vildek J. Inhibition of tumor necrosis factor-induced p42/p44 mitogen-activated protein kinase activation by sodium salicylate. J Biol Chem. 1996;271:8089–8094. doi: 10.1074/jbc.271.14.8089. [DOI] [PubMed] [Google Scholar]
- 73.Speir E, Yu Z X, Ferrans V J, Huang E S, Epstein S E. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase-2 in coronary artery smooth muscle cells. Circ Res. 1998;83:210–216. doi: 10.1161/01.res.83.2.210. [DOI] [PubMed] [Google Scholar]
- 74.Vane J R, Flower R J, Botting R M. History of aspirin and its mechanism of action. Stroke. 1990;12:S12–S23. [PubMed] [Google Scholar]
- 75.Wang X S, Diener K, Manthey C L, Wang S, Rosenzweig B, Bray J, Delaney J, Cole C N, Chan-Hui P-Y, Mantlo N, Lichenstein H S, Zukowski M, Yao Z. Molecular cloning and characterization of a novel p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:23668–23674. doi: 10.1074/jbc.272.38.23668. [DOI] [PubMed] [Google Scholar]
- 76.Wesselborg S, Bauer M K A, Vogt M, Schmitz M L, Schulze-Osthoff K. Activation of transcription factor NF-κB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J Biol Chem. 1997;272:12422–12429. doi: 10.1074/jbc.272.19.12422. [DOI] [PubMed] [Google Scholar]
- 77.Winegarden N A, Wong K S, Sopta M, Westwood J T. Sodium salicylate decreases intracellular ATP, induces both heat shock factor binding and chromosomal puffing, but does not induce hsp70-gene transcription in Drosophila. J Biol Chem. 1996;271:26971–26980. doi: 10.1074/jbc.271.43.26971. [DOI] [PubMed] [Google Scholar]
- 78.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]