Abstract
The zona incerta (ZI) predominantly consists of gamma-aminobutyric acid (GABAergic) neurons, located adjacent to the lateral hypothalamus. GABA, acting on GABAA receptors, serves as a crucial neuromodulator in the initiation and maintenance of general anesthesia. In this study, we aimed to investigate the involvement of ZI GABAergic neurons in the general anesthesia process. Utilizing in-vivo calcium signal optical fiber recording, we observed a decrease in the activity of ZI GABAergic neurons during isoflurane anesthesia, followed by a significant increase during the recovery phase. Subsequently, we selectively ablated ZI GABAergic neurons to explore their role in general anesthesia, revealing no impact on the induction of isoflurane anesthesia but a prolonged recovery time, accompanied by a reduction in delta-band power in mice under isoflurane anesthesia. Finally, through optogenetic activation/inhibition of ZI GABAergic neurons during isoflurane anesthesia, we discovered that activation of these neurons facilitated emergence without affecting the induction process, while inhibition delayed emergence, leading to fluctuations in delta band activity. In summary, these findings highlight the involvement of ZI GABAergic neurons in modulating the emergence of isoflurane anesthesia.
Keywords: Zona Incerta, Isoflurane Anesthesia, GABAergic Neurons, Emergence time, Optogenetic
Introduction
General anesthesia is utilized to induce unconsciousness, amnesia, analgesia, and immobility in patients during the perioperative period, a practice that has been prevalent for over 170 years. Recent research has increasingly shown that the temporary loss of consciousness induced by general anesthetics shares similarities with the natural state of unconsciousness experienced during sleep [1–3]. Despite extensive research spanning decades aimed at elucidating the neural mechanisms underlying general anesthesia-induced unconsciousness, a definitive understanding remains elusive. It is widely acknowledged that GABA plays a critical role in both the initiation and maintenance of general anesthesia, as a majority of anesthetics act on GABAA receptors [4]. Despite ongoing efforts, the specific GABAergic neuron populations responsible for mediating the positive effects of GABA on general anesthesia have yet to be definitively identified. This task is challenging due to the widespread distribution of GABAergic neurons throughout various brain structures. There is a growing body of evidence implicating various subpopulations of GABAergic neurons in the mechanisms of general anesthesia [5–8].
The zona incerta (ZI), situated between the hypothalamus and thalamus, has historically been associated with behaviors that are dependent on elevated arousal levels, including food intake, defensive responses, pain processing, and motor function. [9–11]. The zona incerta (ZI) predominantly comprises gamma-aminobutyric acid (GABAergic) neurons and maintains reciprocal projections with arousal-related nuclei, including the lateral hypothalamus (LH), basal forebrain (BF), ventral tegmental area (VTA), paraventricular thalamus (PVT), ventral periaqueductal gray (vPAG), among others [9, 10, 12]. The general anesthesia-induced unconscious state commonly coincides with the suppression of arousal-related behaviors, indicating a potential interconnection between the zona incerta (ZI) and the loss of consciousness (LOC) induced by general anesthesia (GA). Recent studies have further evidenced the significant involvement of GABAergic neurons within the ZI in the regulation of sleep-wake cycles [13]. However, the precise involvement of the zona incerta (ZI) in modulating states of consciousness during general anesthesia (GA) remains largely ambiguous. To address these inquiries, we conducted in-vivo fiber photometry recordings to monitor population activities of ZI GABAergic neurons during the induction and emergence phases of isoflurane anesthesia, elucidating the alterations in ZI GABAergic neuron activity. Subsequently, we selectively ablated GABAergic neurons to ascertain the specific contribution of ZI GABAergic neurons to isoflurane anesthesia. Additionally, optogenetic stimulation of ZI GABAergic neurons was employed to investigate their function during isoflurane anesthesia. Our study revealed a crucial role of ZI GABAergic neurons in facilitating emergence from general anesthesia.
Materials and methods
Animals
This research endeavor obtained approval from the Animal Care and Use Committees of Zunyi Medical University and adhered to the guidelines outlined in the Guide for the care and use of laboratory animals in China (No. 14924, 2001). The VGAT-IRES-Cre mouse strains were acquired from the Jackson Laboratory (JAX Mice and Services), while the C57BL/6J strain was procured from Changsha Tianqin Technology Co., Ltd (Changsha, China). Mice were housed in standard facilities following a 12 h light-dark cycle, with continuous access to food and water. To mitigate potential confounding influences of circadian rhythm, all behavioral assessments and EEG experiments were conducted between 10:00 a.m. and 4:00 p.m.
AAV Vectors and Drugs
The viruses utilized in this study were sourced from Brain-VTA (China), encompassing Adeno-associated viruses (AAVs) expressing GCaMP (rAAV-Eflα-DIO-Gcamp6s-WPRE-pA, PT-0091), optogenetic tools (rAAV-Eflα-DIO-hChR2-mCherry-WPRE-pA, PT-0002; rAAV-Eflα-DIO-eNpHR-mCherry-WPRE-pA, PT-0007), mCherry (rAAV-Eflα-DIO-mCherry-WPRE-pA, PT-0013), and diphtheria toxin A (DTA) (rAAV-Eflα-DIO-DTA-WPRE-pA, PT-0775). After the injection of the neurotoxic virus, no behavioral tests beyond anesthetic behavioral assessments were conducted. Isoflurane was procured from RWD Life Science (China), while pentobarbital and lidocaine were obtained from Chaohui Pharmaceutical (Shanghai, China). The rabbit anti-GABA antibody was manufactured by Sigma Corporation (USA), and CY3 Donkey anti-rabbit IgG was sourced from Abcam Corporation (USA).
Stereotaxic Surgery
Mice were anesthetized with 1.4% isoflurane, and local anesthesia was administered via subcutaneous injection of lidocaine (1%). Subsequently, the mice were positioned on a stereotaxic apparatus (RWD Life Science, Shenzhen, China). Utilizing a microsyringe pump, AAV viruses were bilaterally injected into the Zona Incerta (ZI) at coordinates of anterior-posterior [AP]: − 1.50 mm, medial-lateral [ML]: ±0.95 mm, and dorsal-ventral [DV]: − 4.50 mm through a glass micropipette (150 nL/side; infusion rate: 30 nL/min). The micropipette was retracted at least 10 min post-viral injection. For in-vivo fiber photometry recordings and optogenetic investigations, optical fibers were unilaterally implanted above the ZI at coordinates of AP: − 1.50 mm, ML: ± 0.95 mm, DV: − 4.20 mm, and anchored with three cranial screws and dental cement. Following the implantation procedure, mice were granted a minimum of 3 weeks for post-surgical recovery before the commencement of experiments.
In-vivo Fiber Photometry Recordings
In our prior investigations, a multi-channel fiber photometry system from ThinkerTech Nanjing Bioscience (Nanjing, China) was employed. The fluorescence signals emanating from GCaMP were captured utilizing multifunction data acquisition software provided by Thinker Tech Nanjing Bioscience Inc. Optical transmission between the commutator and the implanted fiber was facilitated through an optical fiber manufactured by Newton (China). Mice were initially situated within an acrylic glass chamber coupled to an isoflurane vaporizer originating from RWD Life Science (Shenzhen, China). An anesthesia monitor (Vamos; Dräger Company, Germany) was integrated with the chamber to oversee isoflurane levels. A baseline period of 5 min was observed prior to anesthesia induction. Subsequently, mice were anesthetized with 1.4% isoflurane, and the instances of loss of righting reflex (LORR) and recovery of righting reflex (RORR) were recorded, with data collection terminating 5 min post-RORR. Continuous delivery of gas for 20 min post-LORR ensured equilibration of isoflurane concentration in the brain. MATLAB 2019a (MathWorks, Cambridge, US) was utilized for fiber photometry data analysis. The values of fluorescence change (ΔF/F) were calculated via the formula: (F - F0)/F0, where F denoted the test fluorescence signal and F0 represented the mean and standard deviation of the baseline signal [14].
Behavioral Testing
The loss of righting reflex (LORR) serves as an indicator of isoflurane anesthesia induction time, while the recovery of righting reflex (RORR) time is commonly utilized as a standardized measure for assessing the emergence time from general anesthesia. To achieve selective depletion of GABAergic neurons within the Zona Incerta (ZI), VGAT -IRES-Cre mice underwent bilateral injections of rAAV-Eflα-DIO-DTA-WPRE-pA into the ZI using aseptic procedures. As depicted in Fig. 1B, the mice were acclimated in an anesthesia chamber for 5 min. Subsequently, 1.4% isoflurane along with oxygen (O2) at a rate of 1 L/min was administered into the chamber, inducing isoflurane-related unconsciousness in mice. The period from the initiation of isoflurane treatment to LORR onset was defined as the LORR duration. Post this, anesthetic maintenance was sustained for 20 min to ensure brain isoflurane concentration equilibrium. Following removal from the chamber, mice were allowed to recover from anesthesia, with the interval from the conclusion of isoflurane infusion to RORR denoting the RORR duration. Throughout the experiment, isoflurane levels within the anesthesia chamber were monitored using an anesthesia monitor (Vamos; Dräger Company, Germany). An electric blanket equipped with a rectal temperature probe was positioned at the base of the anesthesia chamber to uphold the mice’s body temperature at 37 ℃. For optogenetic investigations, rAAV-EF1a-DIO-ChR2-mCherry/rAAV-EF1a-DIO-NpHR-mCherry/rAAV-EF1a-DIO-mCherry were administered into the ZI of VGAT -IRES-Cre mice, with an optic fiber implanted into the ZI region. Optical power was fine-tuned to 5mW using an optical power meter (PM100D, Thorlabs). Light pulses of 473 nm with a width of 150 ms at 2 Hz and 589 nm with a width of 20 ms at 0.1 Hz, as outlined in prior research [15], were employed during the LORR and RORR phases, respectively. Post experimentation, mice were euthanized, and immunofluorescence assessments were conducted to confirm virus expression and specific transfection.
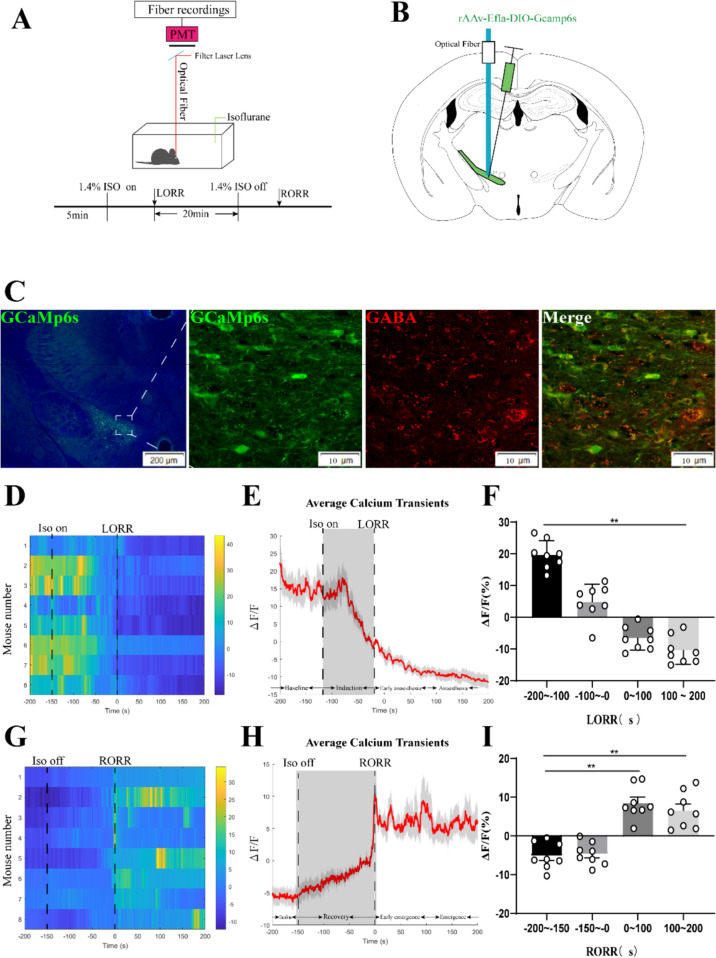
Fig. 1.
Phase-dependent calcium alterations in ZI GABAergic neurons during isoflurane anaesthesia. A Top: Schematic diagram of fiber photometry recording during isoflurane anesthesia in freely moving mice. Bottom: Timeline for quantifying the LORR and RORR of isoflurane anesthesia. (PMT, Photomultiplier tube is a kind of detection device that can convert weak light signals into electronic signals and amplify them). B Schematic of calcium signal recording model into the ZI of a VGAT -Cre mouse. C Expression of GCaMP6f in the ZI of a VGAT -Cre mouse. Viral expression (GCaMP6s, green) in the ZI and colabeling with GABA neurons (GABA immunofluorescence, red). D Individual transitions from wakefulness to isoflurane-induced LORR with color-coded fluorescent intensities (LORR were represented the point of 0). E Average responses from the state transitions during the process of induction expressed as the mean (red) ± SEM (shaded). (F) The fluorescence calcium signals significantly reduced after isoflurane-induced unconsciousness (The baseline (wake: − 200 to − 100 s) vs. anaesthesia period (100 to 200 s), P = 0.0475; n = 8), the paired Student’s t-tests). G Individual transitions from isoflurane anesthesia state to arousal with color-coded fluorescent intensities (RORR were represented the point of 0). H Average responses from the state transitions during the process of recovery expressed as the mean (red) ± SEM (shaded). I The fluorescence calcium signals ascended after RORR (The baseline (anaesthesia: − 200 to − 150 s) vs. early emergence period (0 to 100 s); P = 0.0055; The baseline: (anaesthesia: − 200 to − 150s) vs. emergence period (100 to 200 s), P = 0.0026; the paired Student’s t-tests; n = 8, *P < 0.05, **P < 0.01)
Histological Localization of Cannula Position and Immunohistochemistry
All mice were euthanized under deep anesthesia induced by 2% isoflurane and lidocaine (2%). Subsequent to achieving deep anesthesia, transcranial infusion of 200 mL of 0.01 M PBS followed by 50 mL of 4% PFA was conducted. Post infusion, the brains were promptly extracted for histological examination. The brains were coronally sectioned into 30 µm frozen sections using a cryostat (CM1950; Leica, Germany) to pinpoint the injection virus localization in accordance with the Paxinos & Franklin mouse brain atlas. GABAergic neurons within the Zona Incerta (ZI) were subject to immunohistochemical staining with anti-GABA antibody generated in rabbit (A2052, Sigma, USA). For lesion experiments, the areas of GABA fluorescence in the ZI were quantified blindly by evaluating positively immunostained neurons in ImageJ (USA). GABA-positive neurons were tallied within a 0.5 mm x 0.5 mm region. The proportions of GABA fluorescence area were determined across three adjacent sections (each spaced 90 μm apart) of the brain, with the mean count per section serving as the data representation. All imaging was performed via the Olympus BX63 virtual microscopy system.
EEG Recording and Analysis
EEG acquisition was synchronized with the behavioral testing procedures. The EEG signals were acquired and filtered within the frequency range of 0.1 to 300 Hz. Relative powers in specific frequency bands (δ: 1–4 Hz, θ: 4–8 Hz, α: 8–12 Hz, β: 12–25 Hz, and γ: 25–60 Hz) were calculated by averaging the signal power across each band’s frequency spectrum and then normalizing it by the total power within the 1–60 Hz range, consistent with methodologies detailed in prior studies [2, 16].Spectrograms were generated through bandpass filtration spanning from 1 to 60 Hz, utilizing multitaper techniques implemented in MATLAB 2016a (R2016a; MathWorks).An initial 5-minute EEG signal recording session was conducted prior to induction. Subsequently, continuous EEG signal recording was maintained throughout the lesion experiments, covering the stages of induction, maintenance, and recovery. Following this, EEG signals were continuously captured starting from 5 min after the mice regained consciousness from isoflurane anesthesia. Power spectrum analysis, as illustrated in Fig. 2C, was carried out on data obtained during the induction and recovery phases under isoflurane anesthesia within the framework of optogenetic experiments. The burst suppression rate (BSR) analysis during the recovery phase was performed using MATLAB 2016a to reduce redundancy and enhance technical terminology clarity.
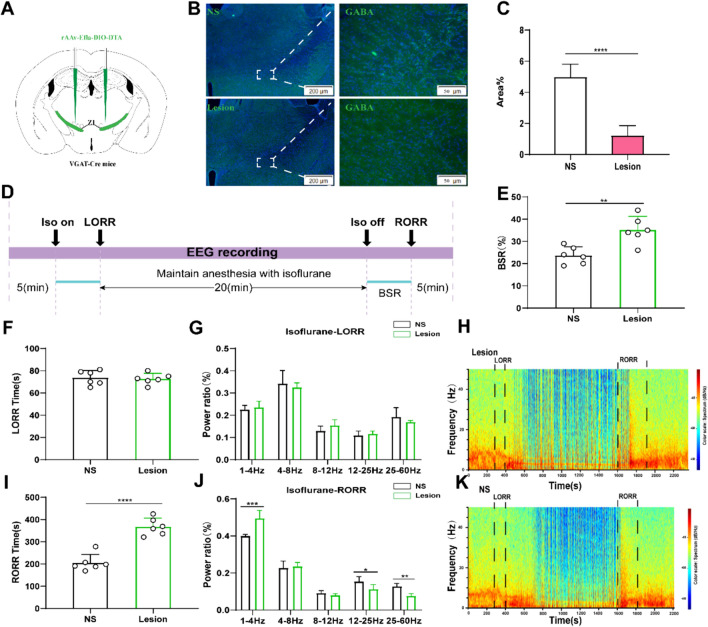
Fig. 2.
Lesion of ZI GABAergic neurons delayed arousal from isoflurane anesthesia. A Schematic showing bilateral injection of Cre-independent AAVs into the ZI. B Image showing GABA staining from a mouse with specific ZI lesion using AAV-CAG-DIO-DTA (NS represents normal saline). C Proportions of GABA-fluorescent area in ZI, indicating that the lesion group animals were selectively ablated ZI GABAergic neurons (P = 0.0001 by independent-samples t-test; n = 6 per group). D Protocol for behavioral and electroencephalogram (EEG) recording of induction time and emergence time. Spectrograms of EEG power during the isoflurane anaesthesia period in the control group. E BSR at recovery process of isoflurane in control group and lesion group (P = 0.0001 by independent-samples t-test; n = 6 per group). F The induction time in control group and lesion group during the maintenance of 1.4% isoflurane anesthesia. G Lesion of ZI GABAergic neurons displaying no significant change in the power ratios of the EEG. Spectrograms of EEG power during the isoflurane anaesthesia period in lesion group (H) and the control group (K). I Lesion of ZI GABAergic neurons peolonged the recovery time from 1.4% isoflurane anesthesia. (J) During the recovery time, lesion of ZI GABAergic neurons significantly altered the power ratios of the EEG. (δ band: lesion group vs. control group; P = 0.0003 by independent-samples t-test;β band: lesion group vs. control group; P = 0.018 by independent-samples t-test, γ band: lesion group vs. control group; P = 0.01 by independent-samples t-test; n = 6 per group)
Data Analysis
Statistical analyses were carried out using the GraphPad Prism software package (version 6.0; GraphPad Software Inc., San Diego, CA, USA). Normality tests were applied to all datasets. Paired Student’s t-tests were utilized to assess differences in calcium signals pre- and post-events, LORR and RORR durations, and changes in burst suppression rate (BSR) within groups (ChR2-light-on vs. ChR2-light-off or NpHR-light-on vs. NpHR-light-off). Independent-samples t-tests were employed to compare proportions of GABA-fluorescent area, LORR and RORR times, as well as EEG power between lesion and sham groups. Additionally, independent-samples t-tests were conducted to compare LORR and RORR times and alterations in EEG power bands in optogenetic experiments between mcherry-light-on vs. ChR2-light-on or mcherry-light-on vs. NpHR-light-on groups.Data were represented as mean ± standard deviation (SD) or mean ± standard error of the mean (SEM). Statistical significance was established at p < 0.05 for all analyses.
Result
Population Activities of ZI GABAergic Neurons in Response to Isoflurane Anesthesia in mice
In order to investigate the potential correlation between population dynamics of ZI GABAergic neurons and isoflurane anesthesia, we employed AAV (rAAv-Ef1α-DIO-Gcamp6s-WPRE-pA) injections into the ZI region of VGAT-Cre mice (Fig. 1A–C). Real-time assessment of ZI GABAergic neuron activity during both induction and emergence phases of 1.4% isoflurane anesthesia was conducted. The induction period was divided into four sequential time segments: pre-anesthesia baseline (-200 to -100 s; -100 s denoted the initiation of 1.4% isoflurane), induction phase (− 100 to 0 s), early anesthesia phase (0 to 100 s), and anesthesia maintenance phase (100 to 200 s). Notably, ZI GABAergic neuronal activity showed a partial increase followed by a robust decline during the induction period preceding LORR (induction period vs. pre-anesthesia baseline, 24.74% ± 10.67% vs. 4.892% ± 5.488%; P = 0.0004; Fig. 1D, E and F). Post-LORR, neuronal activity further decreased compared to the pre-anesthesia baseline (early anesthesia vs. pre-anesthesia baseline, − 6.638% ± 3.716% vs. 24.74% ± 10.67%; P = 0.0001; Fig. 1D, E and F) or the anesthesia maintenance phase (anesthesia vs. pre-anesthesia baseline, − 10.5% ± 4.345% vs. 24.74% ± 10.67%; P = 0.0001; Fig. 1E and F). In the recovery phase, Ca2+ signals were assessed across four consecutive time periods: anesthesia baseline (− 200 to − 150 s), recovery phase (− 150 to 0 s; − 150 s represented the cessation of 1.4% isoflurane), early emergence phase (0 to 100 s), and emergence phase (100 to 200 s). Notably, Ca2+ signals during the recovery phase exhibited a gradual increment compared to the anesthesia baseline, with no statistical significance between the two groups. A marked surge in Ca2+ signals was observed at the moment of consciousness recovery (Fig. 1G and H). Furthermore, Ca2+ signals during the emergence phase significantly surpassed those of the anesthesia baseline (early emergence vs. anesthesia baseline, 8.968% ± 5.21% vs. − 5.121% ± 3.444%; P = 0.0001; emergence vs. anesthesia baseline, 6.825% ± 7.571% vs. − 5.121% ± 3.444%; P = 0.001; Fig.1H and I). In essence, our findings suggest a modulation in the activity of ZI GABAergic neurons under isoflurane anesthesia, showcasing a decrease during anesthesia and subsequent recovery with increased activity, reflecting dynamic alterations in GABA neuron functionality within the ZI region.
Lesion of ZI GABAergic Neurons Slowed down the Emergence Process of Isoflurane Anesthesia
To explore the role of Zona Incerta (ZI) GABAergic neurons in isoflurane anesthesia, the AAV (rAAv-Eflα-DIO-DTA-WPRE-pA) was administered into the ZI of VGAT-cre mice to selectively ablate ZI GABAergic neurons (Fig. 2A). Following a 3-week period post-virus injection, the proportion of GABA-fluorescent area in the ZI was notably reduced in the lesion group compared to the control group, indicating a significant decrease in the number of ZI GABAergic neurons (Fig. 2B and C). So, we started the experiment (Fig. 2D). During the induction phase, there were no statistically significant differences in Loss of Righting Reflex (LORR) time between the lesion group and the control group (Fig. 2F). Additionally, cortical EEG readings and Spectrograms of EEG power showed no discernible distinctions between the control and lesion groups (Fig. 2G, H and K). Noteworthy findings emerged during the recovery period from isoflurane anesthesia, where the ablation of ZI GABAergic neurons led to increased Burst-Suppression Ratio (BSR) compared to the control group (Fig. 2C). In terms of Recovery of Righting Reflex (RORR) duration, the lesion group exhibited longer recovery times than the control group (Fig. 2I). Moreover, the δ wave amplitude was significantly higher in the lesion group than in the control group, while the power ratio of β and γ waves was lower in the lesion group compared to the control group (Fig. 2J). Similarly, it can be seen in Spectrograms of EEG power, the high frequency waves in the lesion group were significantly less than those in the control group (Fig. 2H and K).These findings indicate that ZI GABAergic neurons influence the process of emergence from isoflurane anesthesia, specifically during the recovery phase rather than the induction phase.
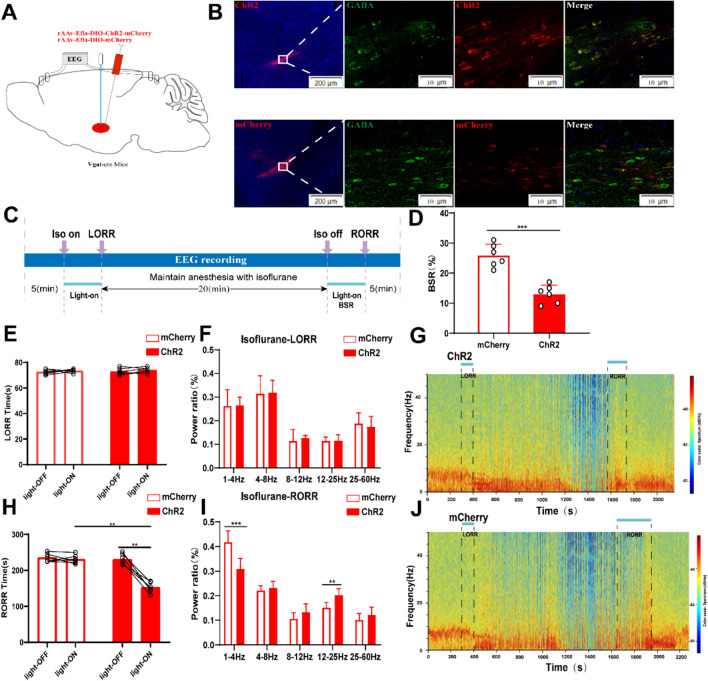
Activation of the ZI GABAergic Neurons Accelerated the Recovery from Isoflurane Anesthesia
To specifically activate Zona Incerta (ZI) GABAergic neurons, we employed optogenetics to manipulate these neurons’ activity in VGAT-Cre mice by injecting the virus (rAAV-Eflα-DIO-hChR2-mCherry-WPRE-pA and rAAV-Eflα-DIO-mCherry-WPRE-pA) into ZI (Fig. 3A and B). Start the experiment as shown in Figure C, In comparison to the mCherry control group, optogenetic stimulation of GABAergic neurons in the ZI initiated at the onset of isoflurane exposure until the Loss of Righting Reflex (LORR) point did not impact LORR duration or the EEG power spectrum during LORR (Fig. 3E and F). Notably, when stimulation commenced at the conclusion of isoflurane inhalation and persisted until Recovery of Righting Reflex (RORR), the optical activation of ZI GABAergic neurons led to a shortened arousal duration (152.7 [14.75] s vs. 229.8 [9.745] s, p = 0.0001, Fig. 3H). Furthermore, EEG recordings revealed significant differences in the power spectrum between the ChR2 (optogenetic activation) group and the mCherry group during the RORR phase, with a marked decrease in the δ band ratio (30.8 ± 4.3% vs. 41.8 ± 4.5%, P = 0.0017), and an increase in the β band ratio (20.3 ± 2.5% vs. 15.2 ± 2.1%, P = 0.0031, Fig. 3I). Noteworthy enhancements in high-frequency wave bands and reductions in low-frequency wave bands were observed later in the ChR2 group during the recovery process (Fig. 3G and J). Additionally, the Burst-Suppression Ratio (BSR) during the RORR period was diminished with optogenetic activation of ZI GABAergic neurons compared to the mCherry-light-on group, as depicted in Fig. 3D. In summary, these outcomes indicate that the activation of ZI GABAergic neurons modulates the processes involved in emerging from isoflurane anesthesia.
Fig. 3.
Optogenetic activation of ZI GABAergic neurons facilitates arousal from isoflurane anesthesia. A Diagram of the optogenetic virus injection and stimulation sites in ZI. B Expression of virus (ChR2 and mCherry, red) in ZI GABAergic neurons and colabeling with GABA (GABA immunofluorescence, green). C Protocol for optogenetic activation during isoflurane anesthesia. D Optical activation of ZI GABAergic neurons reduced the ratio of BSR in the recovery period. E Optical activation of ZI GABAergic neurons did not change induction time (LORR: mcherry-light-on vs. ChR2-light-on, P = 0.6, independent-simples t-test; ChR2-light-on vs. ChR2-light-off. P = 0.2, paired t-test). F, I Comparison of each EEG frequency band between the two groups during optogenetic activation of ZI GABAergic neurons. Spectrograms of EEG power during the isoflurane anaesthesia period in ChR2 group G and the mCherry group (J). H Optical activation of ZI GABAergic neurons shortened emergence time from 1.4% isoflurane anesthesia (RORR: mcherry-light-on vs. ChR2-light-on, P = 0.0009, independent-simples t-test; ChR2-light-on vs. ChR2-light-off. P = 0.0002, paired t-test). Representative EEG heatmap of the process in the two groups
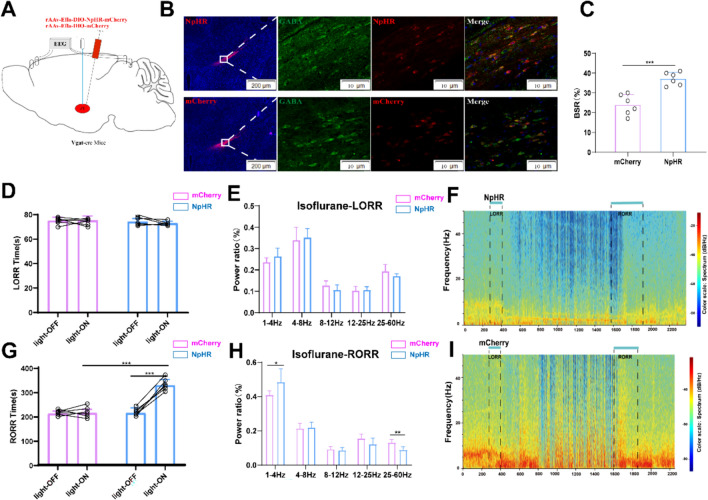
Inhibition of ZI GABAergic Neurons Slows down Recovery from Isoflurane Anesthesia
In order to explore inhibitory ZI GABAergic neurons in the anesthesia effect, we introduced AAV-EF1a-DIO-NpHR-mCherry or AAV-EF1a-DIO-mCherry virus into the ZI. In comparison to the control groups without laser stimulation, photoinhibition of GABAergic neurons in the ZI post-isoflurane administration extended the Recovery of Righting Reflex (RORR) duration (215.8 ± 20.01 s vs. 329.7 ± 24.40 s, P = 0.002), with no effect on the Loss of Righting Reflex (LORR) time. Likewise, when compared to the mCherry control group, photoinhibition also heightened RORR time (213.5 ± 10.78 vs. 329.7 ± 24.4 s, P = 0.0001) without altering LORR time in the ChR2 group (Fig. 4D and G). While photoinhibition during the LORR phase did not yield statistically significant results, it notably raised the power of the δ band (40.9 ± 2.3 vs. 48.4 ± 7.80%, P = 0.05) and reduced the power of the γ band (13.1 ± 1.7% vs. 8.8 ± 1.7%, P = 0.02) in the ChR2 group during the RORR period (Fig. 4H). Consistent with the findings from the lesion experiments, the Burst-Suppression Ratio (BSR) during the recovery phase was elevated by optogenetic inhibition of ZI GABAergic neurons compared to the mCherry control, suggesting the involvement of ZI GABAergic neurons in the emergence processes of isoflurane anesthesia to some extent.
Fig. 4.
Optogenetic inhibition of ZI GABAergic neurons slow down recovery from isoflurane anesthesia. A Schematic of optogenetic inhibition of NpHR-expressing ZI GABAergic neurons with EEG recordings B Expression of NpHR (red) and GABA immunofluorescence (green) in the ZI. C Optical inhibition of ZI GABAergic neurons reduced the ratio of BSR in the recovery period. D Optical stimulation of ZI GABAergic neurons did not alter the induction time of isoflurane anesthesia. E Optical inhibition of ZI GABAergic neurons did not change the band distribution of EEG power during the induction time. F Representative EEG heatmap of NpHR group. G Optogenetic inhibition of ZI GABAergic neurons delayed the emergence from isoflurane anesthesia. H Optogenetic inhibition of ZI GABAergic neurons regulated the band distribution of EEG power during RORR processes of isoflurane anesthesia. I Representative EEG heatmap of control group
Discussion
Revealing the underlying mechanisms of general anesthesia poses a significant challenge within the fields of anesthesiology and neuroscience. Our latest investigation suggests that Zona Incerta (ZI) GABAergic neurons function as promoters of wakefulness, playing a crucial role in reducing anesthesia depth and facilitating recovery from general anesthesia. The neural activity of ZI GABAergic neurons exhibits a gradual decrease during the induction of isoflurane anesthesia, followed by a sharp increase during the emergence phase, closely correlating with the anesthetized states induced by isoflurane. Lesions or optogenetic inhibition of ZI GABAergic neurons delays emergence from isoflurane anesthesia and cortical arousal, while optogenetic activation of these neurons accelerates emergence and promotes cortical arousal.
To explore the involvement of ZI in general anesthesia, the use of calcium fiber photometry is highlighted as a sensitive and effective method for monitoring ZI neuronal responses to anesthetics in vivo [2, 14]. ZI GABAergic neurons are stimulated during arousal and suppressed during anesthesia, In vivo fiber photometry recordings conducted by Zhao et al. [15] revealed that Zona Incerta (ZI) GABAergic neurons exhibit excitatory responses during hunting-related behaviors, underscoring their involvement in activities reliant on elevated arousal levels. Furthermore, the study observed a progressive increase in neuronal activity of ZI GABAergic neurons leading up to arousal during the recovery phase. A peak in Ca2 + signaling was detected during the transition from anesthesia to arousal, highlighting the active engagement of ZI GABAergic neurons in arousal processes, particularly at the moment of emergence. These findings emphasize the pivotal role of ZI GABAergic neurons in regulating arousal states and their heightened activation during the switch from anesthesia to wakefulness.
Intriguingly, targeted lesions of Zona Incerta (ZI) GABAergic neurons did not impact the induction phase of anesthesia but resulted in a delayed emergence characterized by reduced slow wave activity under isoflurane anesthesia. Furthermore, optogenetic manipulation of ZI GABAergic neurons during anesthesia revealed significant effects on arousal states. Inhibition of ZI GABAergic neurons through optogenetics mirrored the outcomes observed with specific lesions, while activation of these neurons produced a wake-promoting effect during isoflurane anesthesia, accompanied by a decrease in slow-wave activity. GABA, traditionally recognized as the primary inhibitory neurotransmitter in the brain, plays a crucial role in modulating general anesthesia. Notably, chemogenetic activation of GABAergic neurons in the rostromedial tegmental nucleus, ventral tegmental area (VTA), and basal forebrain (BF) has been shown to expedite the onset of anesthesia in mouse models [5–7]. However, recent research has shown that GABA is not only inhibited in the brain but also plays a crucial role in activated states of wakefulness. Selective chemogenetic activation of lateral hypothalamus (LH) GABAergic neurons promotes wakefulness, while acute inhibition promotes sleep [8]. Wang et al. discovered that optogenetic activation of dorsal-intermediate lateral septum GABAergic neurons led to heightened arousal and hastened the recovery from anesthesia in mice [3]. Consistent with these findings, ZI GABAergic neurons play a role in modulating the emergence from isoflurane anesthesia. These results emphasize the distinct functions of various subsets of GABAergic neurons and underscore the precise and specific regulation of general anesthesia across different brain regions.
While ZI GABAergic neurons were suppressed during the induction phase, targeted ablation or inhibition of these neurons did not affect the induction duration. This aligns with the cortical EEG data during induction, which showed no discernible variance in cortical activity between mice with activated ZI neurons and those without activation during anesthesia. Liu and colleagues [13] determined that the chemogenetic modulation of Lhx6-positive GABAergic neurons in the ventral ZI specifically influenced sleep duration exclusively in adult mice. In contrast to a previous investigation on the role of ZI in sleep-wake regulation, our findings suggest that the inhibition of ZI GABAergic neurons is not a crucial factor in promoting unconsciousness induced by anesthetics. Recent evidence suggests that the process of emergence from general anesthesia is complex and not merely the inverse of the induction phase. [17–19]. These results align with our prior research, demonstrating that pharmacogenetic stimulation of the parabrachial nucleus, a glutamatergic area, did not affect the onset duration of propofol or isoflurane anesthesia but facilitated the emergence from general anesthesia [6, 19]. Taylor et al. utilized optogenetic methods to activate VTA dopamine neurons and observed that optical stimulation of the VTA led to arousal from isoflurane anesthesia [17]. Furthermore, local administration of norepinephrine (NE) within the central medial thalamic nucleus hastened the recovery from propofol-induced anesthesia without affecting its induction or the animals’ sensitivity to propofol anesthesia in rat models. This highlights the distinct neurobiological mechanisms underlying the processes of induction and emergence from anesthesia [20]. From the aforementioned research and our own findings, it is evident that there exists a unique neurobiological basis for the processes of anesthesia induction and emergence. The induction and recovery phases of anesthesia are not necessarily explained in the same neurobiological way. They could be independent.
The functions of ZI GABAergic neurons in modulating anesthesia could potentially be influenced by their specific projection patterns. The ZI sends projections to various brain regions involved in arousal and motivation, such as the LH, PVT, vPAG, and VTA [9, 10, 12], which may play a role in promoting wakefulness within the ZI. Previous studies have demonstrated that the LH orexinergic system plays a critical role in promoting the emergence from, rather than the induction of, general anesthesia in rodent models [21, 22], while ZI GABAergic neurons specifically target the LH. More recently, Zhang identified a robust inhibitory connection originating from the ZI and projecting to PVT glutamatergic neurons, thereby establishing a link between environmental context and behaviors associated with binge-like eating [12]. A growing body of research suggests that the paraventricular thalamus plays a critical role in promoting wakefulness and is considered essential and capable of inducing wakefulness [23, 24]. Recent investigations have revealed that GABAergic neurons located in the rostral sector of the Zona Incerta (ZI) establish direct synaptic connections with excitatory, rather than inhibitory, neurons within the ventrolateral periaqueductal gray (vPAG) [10]. Our prior investigations have indicated that ventrolateral periaqueductal gray (vPAG) dopaminergic neurons play a role in modulating propofol and isoflurane anesthesia [16, 25]. Moreover, there is consensus in the scientific community regarding the significant involvement of the Ventral Tegmental Area (VTA) in the modulation of general anesthesia [6, 17, 26]. Hence, we hypothesized that the wake-promoting function of Zona Incerta (ZI) GABAergic neurons in general anesthesia is mediated through wake-related downstream pathways.
The current study has certain limitations that need consideration. While there was a significant presence of inhibitory GABAergic projections from the Zona Incerta (ZI) to wake-related downstream regions, direct assessment of the axonal effects of ZI GABAergic neurons on the activity of downstream neurons during isoflurane anesthesia was not conducted using electrophysiological techniques. Future studies should aim to explore the role of ZI in various anesthetic agents to broaden the scope of our findings. Another limitation is that the ZI is implicated in animal locomotion and predation, but the impact of its motor function on the arousal effects of general anesthesia was not investigated in this study. The final limitation is that following the viral injection for the lesion experiments, we did not conduct any additional behavioral tests on the experimental and control groups of mice. Moreover, due to constraints in our experimental conditions, we did not monitor the sleep of the mice; therefore, we cannot ascertain the potential impact on their sleep. Nevertheless, we maintain that despite these limitations, the essential role of ZI in general anesthesia persists. For instance, research has demonstrated that GABAergic neurons expressing GAD2 in the Substantia Nigra Reticulata (SNr) not only regulate movement but also contribute to the transition from wakefulness to slow-wave sleep in mice [27]. Furthermore, existing literature suggests that both noradrenergic neurons in the Locus Coeruleus (LC) and neurons in the Pedunculopontine Nucleus (PPN) are involved in the regulation of movement and arousal [28–31]. While these nuclei exhibit functional parallels with the Zona Incerta (ZI), it is evident that they also contribute to the modulation of the sleep-wake cycle.
In aggregate, our present study reveals the pivotal involvement of Zona Incerta (ZI) GABAergic neurons in regulating emergence from isoflurane anesthesia.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (NSFC, Grant Nos. 81760259, 82301447 and 81971298), The Science and Technology Department of Guizhou Province (Qiankehe JichuZK[2022]597 and Qiankehe JichuZK[2022]630), Clinical Research Center for Acute and Chronic Pain in Hunan Province 2023SK4014.
Author Contributions
Hong Chen and Chengxi Liu completed data analysis and writing the manuscript. Wei Shen and Tian Yu were responsible for design. Junxiao Liu was responsible for calcium fiber photometry. Haifeng He, Shouyang Yu and Chengdong Yuan performed the behavioral tests and the electrophysiology recordings. Yu Zhang and Tianyuan Luo guided the experiment. All authors read and approved the final manuscript.
Funding
The authors have not disclosed any funding.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
All experimental and surgical procedures were approved by Committees on Investigations Involving Animals in Zunyi Medical University, China(grant number: ZMU21-2107-091).
Footnotes
Hong Chen and Chengxi Liu are the co-first authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Franks NP, Wisden W (2021) The inescapable drive to sleep: overlapping mechanisms of sleep and sedation. Science 374(6567):556–559 [DOI] [PubMed] [Google Scholar]
- 2.Liu C et al (2021) Lateral habenula glutamatergic neurons modulate isoflurane anesthesia in mice. Front Mol Neurosci 14:628996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D et al (2021) GABAergic neurons in the dorsal-intermediate lateral septum regulate sleep-wakefulness and anesthesia in mice. Anesthesiology 135(3):463–481 [DOI] [PubMed] [Google Scholar]
- 4.Franks NP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9(5):370–386 [DOI] [PubMed] [Google Scholar]
- 5.Vlasov K et al (2021) Activation of GABAergic neurons in the Rostromedial Tegmental Nucleus and other Brainstem regions promotes sedation and facilitates sevoflurane anesthesia in mice. Anesth Analg 132(4):e50–e55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin L et al (2019) Optogenetic/Chemogenetic Activation of GABAergic Neurons in the ventral Tegmental Area facilitates General Anesthesia via projections to the lateral hypothalamus in mice. Front Neural Circuits 13:73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai S et al (2021) Effect of basal forebrain somatostatin and parvalbumin neurons in propofol and isoflurane anesthesia. CNS Neurosci Ther 27(7):792–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venner A et al (2016) A Novel Population of Wake-promoting GABAergic neurons in the ventral lateral hypothalamus. Curr Biol 26(16):2137–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitrofanis J (2005) Some certainty for the zone of uncertainty? Exploring the function of the zona incerta. Neuroscience 130(1):1–15 [DOI] [PubMed] [Google Scholar]
- 10.Chou XL et al (2018) Inhibitory gain modulation of defense behaviors by zona incerta. Nat Commun 9(1):1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shang C et al (2019) A subcortical excitatory circuit for sensory-triggered predatory hunting in mice. Nat Neurosci 22(6):909–920 [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, van den Pol AN (2017) Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356(6340):853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K et al (2017) Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature 548(7669):582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao WW et al (2021) Nucleus accumbens neurons expressing dopamine D1 receptors modulate states of consciousness in sevoflurane anesthesia. Curr Biol 31(9):1893 –1902.e5 [DOI] [PubMed] [Google Scholar]
- 15.Zhao ZD et al (2019) Zona incerta GABAergic neurons integrate prey-related sensory signals and induce an appetitive drive to promote hunting. Nat Neurosci 22(6):921–932 [DOI] [PubMed] [Google Scholar]
- 16.Liu C et al (2020) Dopamine neurons in the ventral periaqueductal gray modulate isoflurane anesthesia in rats. CNS Neurosci Ther 26(11):1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor NE et al (2016) Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A 113(45):12826–12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarnal V, Vlisides PE, Mashour GA (2016) Neurobiol Anesthetic Emergence J Neurosurg Anesthesiol 28(3):250–255 [DOI] [PMC free article] [PubMed]
- 19.Luo T et al (2018) Parabrachial neurons promote Behavior and Electroencephalographic Arousal from General Anesthesia. Front Mol Neurosci 11:420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu B et al (2017) Noradrenergic transmission in the central medial thalamic nucleus modulates the electroencephalographic activity and emergence from propofol anesthesia in rats. J Neurochem 140(6):862–873 [DOI] [PubMed] [Google Scholar]
- 21.Zhang LN et al (2012) Orexin-A facilitates emergence from propofol anesthesia in the rat. Anesth Analg 115(4):789–796 [DOI] [PubMed] [Google Scholar]
- 22.Li J et al (2019) Orexin activated emergence from isoflurane anaesthesia involves excitation of ventral tegmental area dopaminergic neurones in rats. Br J Anaesth 123(4):497–505 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y et al (2021) Norepinephrine modulates wakefulness via α1 adrenoceptors in paraventricular thalamic nucleus. iScience 24(9):103015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren S et al (2018) The paraventricular thalamus is a critical thalamic area for wakefulness. Science 362(6413):429–434 [DOI] [PubMed] [Google Scholar]
- 25.Li J et al (2018) Involvement of Ventral Periaqueductal Gray Dopaminergic Neurons in Propofol Anesthesia. Neurochem Res 43(4):838–847 [DOI] [PubMed] [Google Scholar]
- 26.Gui H et al (2021) Dopaminergic projections from the Ventral Tegmental Area to the Nucleus Accumbens modulate Sevoflurane Anesthesia in mice. Front Cell Neurosci 15:671473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D et al (2020) A common hub for sleep and motor control in the substantia nigra. Science 367(6476):440–445 [DOI] [PubMed] [Google Scholar]
- 28.Caggiano V et al (2018) Midbrain circuits that set locomotor speed and gait selection. Nature 553(7689):455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroeger D et al (2017) Cholinergic, Glutamatergic, and GABAergic neurons of the Pedunculopontine Tegmental Nucleus have distinct effects on Sleep/Wake Behavior in mice. J Neurosci 37(5):1352–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee AM et al (2014) Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron 83(2):455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holstege JC, Kuypers HG (1987) Brainstem projections to spinal motoneurons: an update. Neuroscience 23(3):809–821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.