In September 2004, the members of the International Committee of Medical Journal Editors (ICMJE) published a joint editorial aimed at promoting registration of all clinical trials.1 We stated that we will consider a trial for publication only if it has been registered before the enrolment of the first patient. This policy applies to trials that start recruiting on or after July 1, 2005. Because many ongoing trials were not registered at inception, we will consider for publication ongoing trials that are registered before Sept. 13, 2005. Our goal then and now is to foster a comprehensive, publicly available database of clinical trials. A complete registry of trials would be a fitting way to thank the thousands of participants who have placed themselves at risk by volunteering for clinical trials. They deserve to know that the information that accrues from their altruism is part of the public record, where it is available to guide decisions about patient care, and deserve to know that decisions about their care rest on all of the evidence, not just the trials that authors decide to report and that journal editors decide to publish.
We are not alone in pursuing this goal. The World Health Organization (WHO), through meetings in New York, Mexico City and Geneva, has brought us close to the goal of a single worldwide standard for the information that trial authors must disclose. Around the world, governments are beginning to legislate mandatory disclosure of all trials. For example, among the bodies considering new legislation is the US Congress, where the proposed Fair Access to Clinical Trials (FACT) Act would expand the current mandate for registration of clinical trials. Many other journals have adopted our policy of requiring trial registration. These initiatives show that trial registration has become a public issue. But, as our deadline for registration approaches, trial authors and sponsors want to be sure that they understand our requirements, so that reports of their research will be eligible for editorial review. The purpose of this joint and simultaneously published editorial is to answer questions about the ICMJE initiative and to bring our position into harmony with that of others who are working toward the same end.
Our definition of a clinical trial remains essentially the same as in our September 2004 editorial: “Any research project that prospectively assigns human subjects to intervention and comparison groups to study the cause-and-effect relationship between a medical intervention and a health outcome.” By “medical intervention” we mean any intervention used to modify a health outcome. This definition includes drugs, surgical procedures, devices, behavioural treatments, process-of-care changes and the like. We update our 2004 editorial to state that a trial must have at least one prospectively assigned concurrent control or comparison group in order to trigger the requirement for registration.
Among the trials that meet this definition, which need to be registered? The ICMJE wants to ensure public access to all “clinically directive” trials — trials that test a clinical hypothesis about health outcomes (e.g., “Is drug X as effective as drug Y in treating heart failure?”). We have excluded trials from our registration requirement if their primary goal is to assess major unknown toxicity or to determine pharmacokinetics (phase 1 trials). In contrast, we think the public deserves to know about trials that could shape the body of evidence about clinical effectiveness or adverse effects. Therefore, we require registration of all trials whose primary purpose is to affect clinical practice (phase 3 trials). Between these 2 extremes are some clinical trials whose prespecified goal is to investigate the biology of disease or to provide preliminary data that may lead to larger, clinically directive trials.
We recognize that requiring public registration of trials whose prespecified goal is to investigate the biology of disease or to direct further research might slow the forces that drive innovation. Therefore, each journal editor will decide on a case-by-case basis about reviewing unregistered trials in this category. Authors whose trial is unregistered will have to convince the editor that they had a sound rationale when they decided not to register their trial. The ICMJE will maintain this policy for the next 2 years. We will then review our experience.
Our September 2004 editorial specified the information that we would require for trial registration. Attendees at a recent meeting of the WHO registration advisory group identified a minimal registration data set of 20 items (Table 1). The WHO-mandated items collectively address every key requirement that we established in our September 2004 editorial. The ICMJE supports the WHO minimal data set and has adopted it as the ICMJE's requirement: we will consider a trial for publication if the authors register it at inception by completing all 20 fields in the WHO minimal data set. As individual editors, we will review the data in the registration fields when we decide whether to consider the trial for publication. We will consider a registration data set inadequate if it has missing fields or fields that contain uninformative terminology. If an investigator has already registered a clinical trial in a publicly owned, publicly accessible registry using the data fields that we specified in our 2004 editorial, we will consider that registration to be complete as long as each field contains useful information.
Table 1
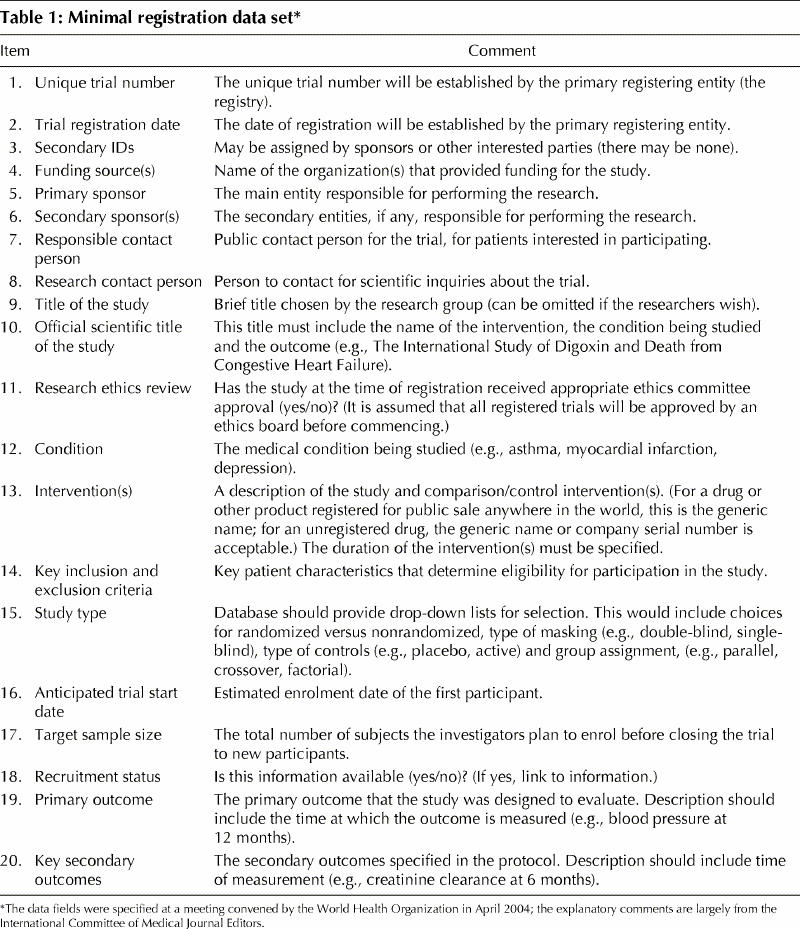
Acceptable completion of data fields is an important concern. It shouldn't be, but it is. Many entries in the publicly accessible clinicaltrials.gov database do not provide meaningful information in some key data fields. A search conducted on May 4, 2005 (Deborah Zarin, MD, personal communication) indicates that certain pharmaceutical-company entries list a meaningless phrase (e.g., “investigational drug”) in place of the actual name of the drug, even though a US law requires trial registrants to provide “intervention name” (www.fda.gov/cder/guidance/4856fnl.htm). Many companies and other entities are completing the data fields in a meaningful fashion. Data entries must include information that will be of value to patients and health professionals; the intervention name is needed if one is to search on that intervention.
We recognize that clinical trial registries have many uses, but, whatever the use, a worldwide uniform standard for a minimal database is necessary. We have participated in the WHO effort to establish a clinically meaningful trial registration process. The ICMJE supports this ongoing project. When it is complete we will evaluate the process and, if it meets our primary objectives, we will adopt it.
We stated our requirements for an acceptable trial registry in the September 2004 editorial, and they remain the same. The registry must be electronically searchable and accessible to the public at no charge. It must be open to all registrants and not for profit. It must have a mechanism to ensure the validity of the registration data.
The purpose of a clinical trials registry is to promote the public good by ensuring that everyone can find key information about every clinical trial whose principal aim is to shape medical decision-making. We will do what we can to help reach this goal. We urge all parties to register new and ongoing clinical trials. If in doubt about whether a trial is “clinically directive,” register it. Don't use meaningless phrases to describe key information. Every trial participant and every investigator should be asking, “Is this clinical trial fully registered?”
Supplementary Material
Footnotes
A French translation of this article is available at www.cmaj.ca/cgi/content/full/172/13/1700/DC1
Published at www.cmaj.ca on May 23, 2005.
Correspondence to: Dr. John Hoey, Editor, CMAJ, 1867 Alta Vista Dr., Ottawa ON K1G 3Y6; fax 613 565-5471; john.hoey@cma.ca
Reference
- 1.De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors [editorial]. CMAJ 2004;171(6):606-7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


