Abstract
The prophylactic papillomavirus vaccines currently in clinical trials are composed of viral L1 capsid protein that is synthesized in eukaryotic expression systems and purified in the form of virus-like particles (VLPs). To evaluate whether VLPs are necessary for effective vaccination, we expressed the L1 protein as a glutathione S-transferase (GST) fusion protein in Escherichia coli and assayed its immunogenic activity in an established canine oral papillomavirus (COPV) model that previously validated the efficacy of VLP vaccines. The GST-COPV L1 fusion protein formed pentamers, but these capsomere-like structures did not assemble into VLPs. Despite the lack of VLP formation, the GST-COPV L1 protein retained its native conformation as determined by reactivity with conformation-specific anti-COPV antibodies. Most importantly, the GST-COPV L1 pentamers completely protected dogs from high-dose viral infection of their oral mucosa. L1 fusion proteins expressed in bacteria represent an economical alternative to VLPs as a human papillomavirus vaccine.
Genital human papillomavirus (HPV) infection is a common sexually transmitted disease that is the primary cause of cervical cancer, resulting in approximately 400,000 deaths per year worldwide (30). An effective vaccine against HPV infection would potentially prevent the development of most human cervical dysplasias and carcinomas (4). In addition, a vaccine would also reduce the cost (estimated at $6 billion annually in the United States) of screening and treating premalignant cervical disease (16).
Since HPV cannot replicate in other animal species, evaluation of potential HPV vaccines requires the use of related animal papillomaviruses. The mucosotropic, oncogenic canine oral papillomavirus (COPV) closely mimics the biology of HPV, and the capsid proteins of COPV are closely related to those of HPV, making COPV a relevant and accepted animal model for testing the efficacy of prophylactic vaccine candidates (2, 19, 26, 27).
The L1 capsid protein of papillomaviruses self-assembles into virus-like particles (VLPs) when expressed in insect cells (11, 14) or yeast (12, 23). These L1 VLPs are morphologically similar to virions, being comprised of 72 pentamers (i.e., capsomeres) of L1 arranged in a T=7 icosahedral lattice, but lacking the L2 capsid protein and the viral genome. Previous studies have shown that immunization with purified VLPs protects against experimental papillomavirus infection in rabbits (5, 8), cows (15), and dogs (27). Conformational epitopes on VLPs appear critical for the induction of neutralizing immunoglobulin G (IgG) and for successful vaccination, since denatured L1 protein fails to generate neutralizing antibodies or protect against experimental infection (10, 18, 27).
Although early attempts to use bacteria for producing papillomavirus L1 protein vaccines were unsuccessful due to poor immunogenicity or inefficient expression (1, 9, 13, 28, 29), recent studies have shown that the HPV type 11 (HPV-11) and HPV-16 L1 proteins can be expressed in Escherichia coli in a capsomeric form that assembles into VLPs in vitro (6, 17). Capsomeres of HPV-11 L1 react with conformation-specific antibodies, including neutralizing monoclonal antibodies, and induce neutralizing antibodies in rabbits (21). To determine whether capsomeric/pentameric forms of L1 protein could induce protective immunity in the host, we evaluated the use of cleaved and noncleaved glutathione S-transferase (GST)–COPV L1 fusion proteins expressed in E. coli as a potential immunogen. Our findings indicate that bacterially expressed GST-COPV L1 protein is an excellent candidate for an economical, second-generation papillomavirus vaccine.
MATERIALS AND METHODS
DNA constructs.
To clone the COPV L1 gene into a bacterial expression vector, an EcoRI restriction enzyme site was added to the 5′ end of the L1 gene by PCR amplification of a 567-bp DNA fragment using primers 5′-ACTGACTCGAGAATTCCTGCACAGAATAAATTTTAC-3′ and 5′-ATTGTCCTGCAGTGTGTACC-3′. The resulting DNA fragment was digested with XhoI and PstI and cloned into pBlueBac-COPVL1 (7) at the XhoI-PstI sites. Full-length COPV L1 then was subcloned into pGEX4T2 (Pharmacia Biotech, Piscataway, N.J.) at the EcoRI-NotI sites, so that GST is linked to the 5′ end of COPV L1. The clone was verified by dideoxy-DNA sequencing.
Expression and purification of GST-COPV L1 and L1.
Recombinant COPV L1 was expressed in E. coli as a GST fusion protein and purified from the supernatant of disrupted cells by glutathione-Sepharose chromatography as previously described (6, 17).
Immunization and challenge of dogs.
Twenty beagles (Marshall Farm, North Rose, N.Y.) were randomly distributed into five groups (four per group). Groups A, B, C, D, and E received phosphate-buffered saline (PBS) and 0.05, 1, 20, and 400 ng of GST-L1 per dosage as vaccine, respectively. Intradermal injection into the accessory carpal footpads of 9-week-old beagles was carried out as described (27). For COPV challenge, the maxillary buccal mucosae of the dogs were abraded with a sterile wire brush. Wart homogenate was then applied to the excoriated mucosa with a cotton swab. All animal experiments were approved by Institution Animal Care and Use Committee, and the procedures are consistent with Public Health Service guidelines (20).
Electron microscopy.
The GST-COPV L1 and COPV L1 proteins were absorbed to glow-discharged, carbon-coated grids (EM Sciences, Fort Washington, Pa.) and stained with 2% uranyl acetate. A JEOL 100-CX electron microscope was used for visualization.
Sucrose gradient sedimentation of recombinant proteins.
Two hundred microliters of either GST-COPV L1 or COPV L1 protein was layered onto 4 ml of a 5 to 30% sucrose gradient in PBS and centrifuged at 31,000 rpm for 18 h in a SW55Ti rotor. Fractions (0.3 ml) were collected from the top, and the residual pellet was resuspended into 0.3 ml of PBS. The fractions were assayed for GST-COPV L1 or COPV L1 protein by immunoblotting with anti-AU1 monoclonal antibody (27). Hemoglobin (4.5S), catalase (11S), and β-galactosidase (19S) were used for sedimentation markers.
Calculation of GST-COPV L1 and L1 concentration and immunoblot analysis.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% gel (10% SDS-PAGE) and stained with Coomassie brilliant blue R (Sigma, St. Louis, Mo.). A known amount of IgG was loaded on the same gel to permit quantitation of GST-COPV L1 or L1. For immunoblots, proteins were separated by 4 to 20% gradient SDS-PAGE and then electrophoretically transferred to a polyvinylidenedifluoride membrane. The primary antibody, a mouse anti-AU1 monoclonal antibody (27), was used at 1:1,000 dilution. The secondary antibodies, alkaline phosphatase-conjugated goat anti-mouse and anti-rabbit antibodies (Tropix, Bedford, Mass.), were also used at 1:1,000 dilution. Immunoblots were developed using the CDP-Star chemiluminescent substrate (Tropix).
Enzyme-linked immunosorbent assays (ELISA).
Equivalent amounts of GST-COPV L1 and L1 proteins were serially diluted in PBS and distributed into a 96-well plate. The plate was incubated at 37°C for 1 h, washed with PBS, and then blocked with 1% bovine serum albumin at 37°C for 1 h. The rabbit anti-intact COPV antiserum (1:1,000) or anti-AU1 antibody (1:1,000) was added to the wells, incubated with antigen at 37°C for 1 h, and washed with PBST (PBS plus 0.05% Tween). Horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:15,000; Pierce, Rockford, Ill.) or horseradish peroxidase-conjugated rabbit anti-mouse IgG antibody (1:10,000; Pierce) was then added to the wells reacted with anti-intact COPV antiserum or anti-AU1 antibody, respectively. After washing with PBST, peroxidase substrate (KPL, Gaithersburg, Md.) was added. The reaction was terminated by the addition of stop solution (KPL). The A450 was measured by a Dynatech Miro-ELISA reader within 10 min.
RESULTS
Purification of GST-COPV L1 after expression in E. coli.
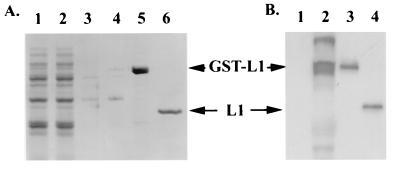
Full-length COPV L1 was cloned into the pGEX4T2 plasmid vector and then transfected into E. coli strain DH5α. Expression of the 80-kDa GST-COPV L1 protein was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) (Fig. 1B, compare lanes 2 and 1). The supernatant of disrupted cells (Fig. 1B, lane 1) was loaded on a glutathione-Sepharose column, and after a stepwise wash procedure, GST-COPV L1 was eluted with 10 mM reduced glutathione (Fig. 1A, lane 5). Alternatively, the GST moiety was removed by thrombin cleavage while the GST-COPV L1 was bound to the column (Fig. 1A, lane 6). The identities of the GST-COPV L1 and L1 proteins were confirmed by immunoblotting using the anti-AU1 monoclonal antibody (Fig. 1B, lanes 3 and 4).
FIG. 1.
Purification of GST-COPV L1 protein after expression in E. coli. GST-COPV L1 protein was expressed and purified from bacteria by glutathione-Sepharose affinity chromatography. The indicated fractions were separated by SDS-PAGE and either stained with Coomassie blue (A) or immunoblotted with anti-L1 monoclonal antibody (B). (A) Lanes: 1, whole-cell lysate after IPTG induction; 2, flowthrough from glutathione-Sepharose column; 3, ATP-Mg2+ wash; 4, 2.5 M urea wash; 5, 10 mM reduced glutathione eluant; 6, eluant after thrombin digestion. (B) Lanes: 1, uninduced cell lysate; 2, cell lysate after induction with IPTG; 3, GST-L1 fraction; 4, L1 fraction after thrombin cleavage.
GST-COPV L1 and COPV L1 assemble into pentamers.
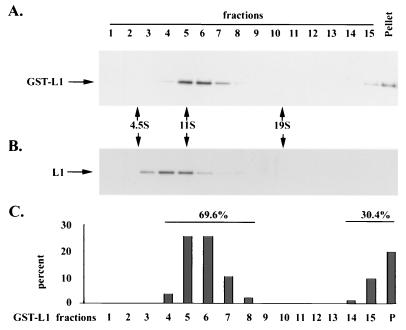
Velocity sedimentation analysis has been used to assess HPV pentamer and VLP formation (17). Given the molecular masses of the recombinant proteins, pentamers of GST-COPV L1 (400 kDa) and COPV L1 (280 kDa) might be expected to sediment at 12 to 16S and 10 to 14S, respectively. VLPs are estimated to sediment at approximately 140S, compared to polyomavirus capsids at 240S (22). As shown in Fig. 2A and C, the majority of GST-COPV L1 had a sedimentation value of 11 to 13S, which is consistent with that expected for pentamers. About 30% of GST-COPV L1 was found in the last fraction and the pellet, suggesting some aggregate formation. A shorter-timed sedimentation analysis showed that the pelleted GST-COPV L1 fractions consisted of aggregates of heterogeneous size (data not shown), with only a small percentage of GST-COPV L1 sedimenting at 140S. When GST was removed, COPV L1 sedimented with a predominant species between 9 and 11S at pentamer size (Fig. 2B). Thus, velocity sedimentation analysis showed that both GST-COPV L1 and COPV L1 form pentamers after purification from bacteria.
FIG. 2.
Sucrose gradient sedimentation of GST-COPV L1 and COPV L1 proteins. GST-COPV L1 or COPV L1 was analyzed by sedimentation ultracentrifugation on a 5 to 30% sucrose gradient. The fractions were assayed for GST-COPV L1 (A) or COPV L1 (B) by immunoblotting with anti-AU1 monoclonal antibody. The amount of GST-COPV L1 in the fractions was determined by densitometry and plotted as the percentage of total GST-COPV L1 protein (C). Hemoglobin (4.5S), catalase (11S), and β-galactosidase (19S) were used as sedimentation markers.
When expressed in eukaryotic or yeast expression systems, COPV L1 spontaneously assembles into VLPs with a diameter of 55 nm (27). When purified from E. coli, however, the thrombin-cleaved GST-COPV L1 preparation consisted of pentameric capsomere structures, as determined by electron microscopy (Fig. 3B). The noncleaved GST-COPV L1 preparation (Fig. 3A) also appeared to be in pentameric form, as judged by the presence of characteristic 11- to 12-nm structures, some of which exhibited a stain-filled central axis. The typical “donut” appearance of capsomeres was less apparent in the noncleaved L1 preparations than the cleaved L1 preparations, most likely as a consequence of the N-terminal appended GST moiety. No VLP-like structures could be detected by EM in these preparations. In addition, noncleaved GST-COPV L1 could not be induced to self-assemble in vitro into capsid-like structures under any buffer conditions (data not shown), whereas thrombin-cleaved L1 of other papillomaviruses does self-assemble in vitro into a variety of structures (6), including VLPs. Thus, the fused GST moiety affects both the morphology and assembly properties of COPV L1.
FIG. 3.
Morphology of purified GST-COPV L1 and COPV L1 preparations. The GST-L1 (A) and thrombin-cleaved L1 (B) proteins were negatively stained and examined by electron microscopy as described in Materials and Methods. Both preparations formed 11- to 12-nm capsomeric structures, with the characteristic donut appearance being less obvious in GST-COPV L1. Bars, 100 nm.
GST-COPV L1 displays both conformational and nonconformational epitopes.
Previous studies have shown that conformational epitopes on the papillomavirus virion are essential for generating neutralizing antibodies in host animals (10, 25). VLPs also display conformational epitopes and induce high titers of neutralizing antibodies in vaccinated animals. In contrast, SDS-disrupted VLPs lack native conformation and fail to generate neutralizing antibodies or protect against experimental infection (10). To determine whether the GST-COPV L1 and cleaved L1 proteins would potentially be suitable as a vaccine, we evaluated their display of conformational epitopes.
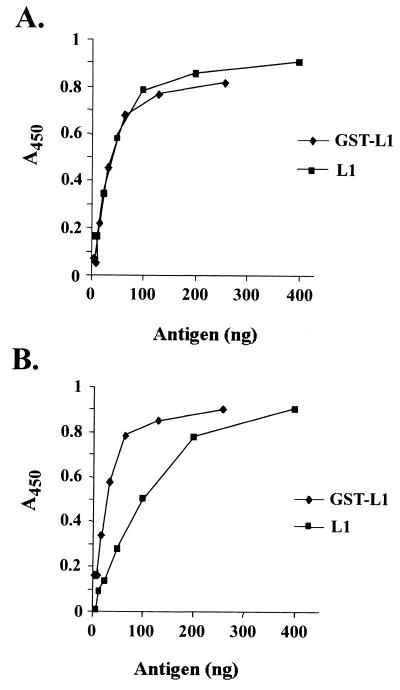
ELISAs of GST-COPV L1 and L1 were performed with a rabbit polyclonal antiserum (generated against intact COPV virions) which recognizes type-specific, conformation-dependent epitopes on both COPV virions and VLPs (7). Both the GST-COPV L1 fusion protein and the purified L1 protein reacted strongly with this conformation-dependent antiserum (Fig. 4A). In contrast to findings with COPV VLPs, GST-COPV L1 and L1 reacted with the conformation-independent AU-1 antibody (Fig. 4B), indicating that GST-COPV L1 and L1 proteins also display nonconformational epitopes. Our results indicate that the assembled COPV VLP structure is not essential to display conformational epitopes detected by neutralizing antiserum, a finding consistent with previous results for the HPV-11 L1 protein (21).
FIG. 4.
Display of conformational and nonconformational epitopes by the GST-COPV L1 and COPV L1 proteins. Equivalent amounts of GST-COPV L1 and COPV L1 (based on L1 content) were tested for immunoreactivity in ELISAs with the conformation-dependent, rabbit polyclonal anti-COPV antiserum (A) and the conformation-independent, mouse anti-AU1 monoclonal antibody (B). Both protein preparations showed similar reactivity with the antibodies.
GST-COPV L1 protects beagles against papillomavirus challenge.
Although both GST-COPV L1 and L1 proteins appear to have similar antigenic properties, we decided to focus on the use of GST-COPV L1 as a vaccine due to its simpler purification scheme as well as its inability to self-assemble beyond the pentamer form. A vaccine study with 20 beagles was performed with different dosages of GST-COPV L1 protein, which were administered without adjuvant. Groups of four beagles were vaccinated at 9 weeks of age with an intradermal injection of 1 ml of PBS (control) or 0.05, 1, 20, and 400 ng purified GST-COPV L1. All animals were boosted with the same protein dosage 2 weeks later (Table 1). Two weeks after boosting, the dogs were challenged with infectious COPV bilaterally on their oral buccal mucosae. Dogs were evaluated weekly for 14 weeks after challenge for the appearance and size of oral papillomas. Control dogs and dogs receiving 0.05 ng of protein developed papillomas 5 weeks postchallenge. Dogs from groups C and D that received midrange concentrations of GST-COPV L1 protein (1 and 20 ng) developed papillomas 1 to 2 weeks later than the PBS control group. On average, these papillomas was also smaller than those observed in groups A and B. Complete protection from virus-induced papillomas was observed with the 400-ng dose of GST-COPV L1. All four dogs in this group remained free of oral papillomas throughout the experiment (14 weeks following viral challenge).
TABLE 1.
GST-L1 vaccine trial in dogsa
The five groups (four dogs per group) were challenged with COPV according to established protocols. Viral challenge was performed 2 weeks following the vaccine boost, and the appearance and size of tumors was monitored weekly.
These animals had smaller tumors that appeared 1 to 2 weeks later than in the controls.
GST-COPV L1-vaccinated beagles develop COPV-specific antibodies.
Earlier studies showed that VLP-vaccinated beagles develop COPV-specific antibodies (27). To determine whether the GST-COPV L1 protein induced the development of COPV-specific antibodies in vaccinated beagles, the dogs in the study described above were bled every week for the duration of the experiment. Aliquots of serum from dogs in each group were pooled, diluted 1:100, and evaluated for the presence of COPV-specific IgG by ELISA. Antibodies specific for intact COPV were detected only in dogs that had been protected from viral challenge, i.e., in dogs immunized with 400 ng of GST-COPV L1 (Fig. 5). In this group of dogs, the titer of COPV-specific IgG increased following primary immunization (week 0) and boosting (week 2) and peaked after virus challenge (week 4). In contrast, dogs immunized with either PBS or lower dosages of GST-COPV L1 showed no significant increase in COPV-specific antibodies. Complete protection from experimental challenge therefore correlated with the presence of detectable IgG in the serum of vaccinated animals. However, it appears that even low titers of neutralizing antibodies (presumably present in dogs receiving the low-dose L1 vaccines) can alter the outcome of viral infection and might even be protective against low-dose natural infections.
FIG. 5.
Induction of COPV-specific antibodies in immunized dogs. Serum samples were taken at the indicated times from dogs immunized with 0.05 to 400 ng of GST-L1 protein. The serum samples were pooled according to group and assayed by ELISA for COPV-specific IgG as described in Materials and Methods. Groups: A, 0 ng of GST-L1; B, 0.05 ng of GST-L1; C, 1 ng of GST-L1; D, 20 ng of GST-L1; E, 400 ng of GST-L1.
DISCUSSION
This report describes an effective prophylactic vaccine against papillomaviruses which is generated from a bacterial expression system. The study demonstrates that the papillomavirus L1 capsid protein expressed as a fusion protein, in this case with GST, which greatly simplifies its purification, maintains its immunogenic properties. Compared to existing methodologies for producing VLP-based papillomavirus vaccines in eukaryotic cells, the bacterial system is potentially more economical and offers the possibility of generating a low-cost vaccine for use in developing countries.
Using a canine model, we have shown that GST-COPV L1, comprised predominantly of pentamers, is sufficient to protect against infection of mucosal surfaces. Thus, neutralizing epitopes do not necessarily need to be displayed in a complex, assembled structure (e.g., VLP) for effective recognition by the host immune system. Rather, as suggested by the atomic structure of the HPV-16 L1 pentamer (6), such epitopes may be properly configured within the context of the pentameric capsomere. Although some neutralizing sites may bridge capsomeres (3), the epitopes retained on the GST-COPV L1 pentamers are clearly sufficient for inducing protective neutralizing antibodies.
The GST-COPV L1 preparation differs from VLPs not only because of the GST moiety but also because it displays epitopes not normally displayed on VLPs or on virus particles. The GST-COPV L1 protein reacts strongly with the AU1 antibody, a finding not observed with intact COPV virions or VLPs. With VLP preparations, strong reactivity with AU1 is indicative of protein denaturation and loss of antigenicity. However, it appears possible to expose the L1 AU1 epitope without denaturing the protein since both GST-COPV L1 and L1 pentameric structures expose the AU1 domain while retaining their reactivity with conformation-dependent antibodies. Thus, our studies clearly indicate that a gain in reactivity of GST-COPV L1 to antibodies specific for linear, nonconformational epitopes (commonly detected in denatured L1 protein) does not necessarily indicate that L1 protein has lost its native conformational epitopes. Dissociation of VLP formation from the display of conformation epitopes has also been noted in the opposite direction, i.e., L1 proteins which assemble into VLPs but fail to express conformational epitopes (7).
Although the GST-COPV L1 and the L1 VLP vaccine preparations are effective at nanogram dosages, we cannot yet directly evaluate the precise efficacy of these different vaccine preparations. Our previous study with L1 VLPs in dogs indicates that approximately 50 ng of protein is sufficient for inducing immunity. In the present study, 400 ng of GST-COPV L1 gave complete protection against viral challenge (Table 1) and elicited papillomavirus-specific antibodies in serum (Fig. 5). We did not evaluate any dosages between 20 and 400 ng. However, the partial effect of GST-COPV L1 on tumor size and appearance suggests that there was some immunologic response even at 20 and 1 ng. Effective responses at nanogram levels are also supported by a dose-response study of HPV-11 L1 capsomeres for inducing neutralizing antibodies in rabbits (21). It is possible that low levels of endotoxin associated with bacterially expressed GST-L1 protein may act as adjuvant and contribute to the strong immunogenicity in these preparations. We have measured endotoxin levels in the GST-L1 and L1 protein preparations and found them to be equivalent at 100 endotoxin units per μg of protein (data not shown).
In conclusion, we have described a novel vaccine that protects dogs against papillomavirus infection. The vaccine is based on the expression of a GST-L1 fusion protein in bacteria and offers a simplified, economical alternative to VLPs for producing an HPV vaccine which can be used in developing countries where cervical cancer is a leading cause of cancer deaths among women (24).
ACKNOWLEDGMENTS
We thank Stephanie Kuehn for technical assistance with this study.
Financial support was provided by NIH grants R01CA57994 and R01CA37667, awarded to R.S. and R.L.G., respectively.
REFERENCES
- 1.Banks L, Matlashewski G, Pim D, Churcher M, Roberts C, Crawford L. Expression of human papillomavirus type 6 and type 16 capsid proteins in bacteria and their antigenic characterization. J Gen Virol. 1987;68:3081–3089. doi: 10.1099/0022-1317-68-12-3081. [DOI] [PubMed] [Google Scholar]
- 2.Bell J A, Sundberg J P, Ghim S J, Newsome J, Jenson A B, Schlegel R. A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology. 1994;62:194–198. doi: 10.1159/000163910. [DOI] [PubMed] [Google Scholar]
- 3.Booy F P, Roden R B, Greenstone H L, Schiller J T, Trus B L. Two antibodies that neutralize papillomavirus by different mechanisms show distinct binding patterns at 13 A resolution. J Mol Biol. 1998;281:95–106. doi: 10.1006/jmbi.1998.1920. [DOI] [PubMed] [Google Scholar]
- 4.Breitburd F, Coursaget P. Human papillomavirus vaccines. Semin Cancer Biol. 1999;9:431–444. doi: 10.1006/scbi.1999.0147. [DOI] [PubMed] [Google Scholar]
- 5.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J T, Lowy D R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X S, Garcea R L, Goldberg I, Casini G, Harrison S C. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Ghim S J, Jenson A B, Schlegel R. Mutant canine oral papillomavirus L1 capsid proteins which form virus-like particles but lack native conformational epitopes. J Gen Virol. 1998;79:2137–2146. doi: 10.1099/0022-1317-79-9-2137. [DOI] [PubMed] [Google Scholar]
- 8.Christensen N D, Reed C A, Cladel N M, Han R, Kreider J W. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doorbar J, Gallimore P H. Identification of proteins encoded by the L1 and L2 open reading frames of human papillomavirus 1a. J Virol. 1987;61:2793–2799. doi: 10.1128/jvi.61.9.2793-2799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghim S, Christensen N D, Kreider J W, Jenson A B. Comparison of neutralization of BPV-1 infection of C127 cells and bovine fetal skin xenografts. Int J Cancer. 1991;49:285–289. doi: 10.1002/ijc.2910490224. [DOI] [PubMed] [Google Scholar]
- 11.Hagensee M E, Yaegashi N, Galloway D A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann K J, Cook J C, Joyce J G, Brown D R, Schultz L D, George H A, Rosolowsky M, Fife K H, Jansen K U. Sequence determination of human papillomavirus type 6a and assembly of virus-like particles in Saccharomyces cerevisiae. Virology. 1995;209:506–518. doi: 10.1006/viro.1995.1283. [DOI] [PubMed] [Google Scholar]
- 13.Jenison S A, Firzlaff J M, Langenberg A, Galloway D A. Identification of immunoreactive antigens of human papillomavirus type 6b by using Escherichia coli-expressed fusion proteins. J Virol. 1988;62:2115–2123. doi: 10.1128/jvi.62.6.2115-2123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirnbauer R, Booy F, Cheng N, Lowy D R, Schiller J T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirnbauer R, Chandrachud L M, O'Neil B W, Wagner E R, Grindlay G J, Armstrong A, McGarvie G M, Schiller J T, Lowy D R, Campo M S. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 16.Kurman R J, Henson D E, Herbst A L, Noller K L, Schiffman M H. Interim guidelines for management of abnormal cervical cytology. The 1992 National Cancer Institute Workshop. JAMA. 1994;271:1866–1869. [PubMed] [Google Scholar]
- 17.Li M, Cripe T P, Estes P A, Lyon M K, Rose R C, Garcea R L. Expression of the human papillomavirus type 11 L1 capsid protein in Escherichia coli: characterization of protein domains involved in DNA binding and capsid assembly. J Virol. 1997;71:2988–2995. doi: 10.1128/jvi.71.4.2988-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Y L, Borenstein L A, Selvakumar R, Ahmed R, Wettstein F O. Progression from papilloma to carcinoma is accompanied by changes in antibody response to papillomavirus proteins. J Virol. 1993;67:382–389. doi: 10.1128/jvi.67.1.382-389.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholls P K, Stanley M A. The immunology of animal papillomaviruses. Vet Immunol Immunopathol. 2000;73:101–127. doi: 10.1016/s0165-2427(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 21.Rose R C, White W I, Li M, Suzich J A, Lane C, Garcea R L. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J Virol. 1998;72:6151–6154. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salunke D M, Caspar D L, Garcea R L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986;46:895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- 23.Sasagawa T, Pushko P, Steers G, Gschmeissner S E, Hajibagheri M A, Finch J, Crawford L, Tommasino M. Synthesis and assembly of virus-like particles of human papillomaviruses type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology. 1995;206:126–135. doi: 10.1016/s0042-6822(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 24.Schiller J T, Lowy D R. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol. 1996;7:373–382. doi: 10.1006/scbi.1996.0046. [DOI] [PubMed] [Google Scholar]
- 25.Steele J C, Gallimore P H. Humoral assays of human sera to disrupted and nondisrupted epitopes of human papillomavirus type 1. Virology. 1990;174:388–398. doi: 10.1016/0042-6822(90)90092-6. [DOI] [PubMed] [Google Scholar]
- 26.Sundberg J P, Schlegel R, Jenson A B. Mucosotropic papillomavirus infections. Lab Anim Sci. 1998;48:240–242. [PubMed] [Google Scholar]
- 27.Suzich J A, Ghim S J, Palmer-Hill F J, White W I, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11537. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson G H, Roman A. Expression of human papillomavirus type 6 E1, E2, L1 and L2 open reading frames in Escherichia coli. Gene. 1987;56:289–295. doi: 10.1016/0378-1119(87)90146-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Carmichael J, Ferguson J, Inglis S, Ashrafian H, Stanley M. Expression of human papillomavirus type 16 L1 protein in Escherichia coli: denaturation, renaturation, and self-assembly of virus-like particles in vitro. Virology. 1998;243:423–431. doi: 10.1006/viro.1998.9050. [DOI] [PubMed] [Google Scholar]
- 30.zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]