Abstract
Since the start of Afghanistan combat operations in 2001, there has been an increase in complaints of respiratory illnesses in deployed soldiers with no previous history of lung disorders. It is postulated that deployment-related respiratory illnesses are the result of inhalation of desert particulate matter (PM) potentially acting in combination with exposure to other pro-inflammatory compounds. Why some, but not all, soldiers develop respiratory diseases remains unclear. Our goal was to investigate if human airway epithelial cells primed with IL-13, a type 2 inflammatory cytokine, demonstrate stronger pro-inflammatory responses to Afghanistan desert PM (APM). Primary human brushed bronchial epithelial cells from non-deployed, healthy subjects were exposed to APM, both with and without IL-13 pretreatment. APM exposure in conjunction with IL-13 resulted in significantly increased expression of IL-8, a pro-inflammatory cytokine involved in neutrophil recruitment and activation. Furthermore, expression of TLR2 mRNA was increased after combined IL-13 and APM exposure. siRNA-mediated TLR2 knockdown dampened IL-8 production after exposure to APM with IL-13. APM with IL-13 treatment increased IRAK-1 (a downstream signaling molecule of TLR2 signaling) activation, while IRAK-1 knockdown effectively eliminated the IL-8 response to APM and IL-13. Our data suggest that APM exposure may promote neutrophilic inflammation in airways with a type 2 cytokine milieu.
Keywords: particulate matter, Afghanistan, TLR2, type 2 inflammation, IL-13
Upon returning from tours of duty in Afghanistan and Iraq, a subset of soldiers who previously had no diagnosed history of major respiratory illness are presenting with symptoms of a disease now termed Iraq/Afghanistan War-Lung Injury (IAW-LI). Affected soldiers exhibit diverse symptoms including dyspnea, exercise-induced shortness of breath, cough, wheezing, and chest tightness (Szema et al., 2014). Roughly 14% soldiers experienced new-onset respiratory symptoms after deployment, with 6.6% of symptomatic soldiers experiencing new-onset asthma (Szema et al., 2017). According to the United States Army’s Standards of Medical Fitness, a confirmed diagnosis of asthma at any age is grounds for rejection for appointment, enlistment, and induction (Army). Because of this, the percentage of soldiers with diagnosed asthma prior to deployment is near zero. The increasing number of soldiers presenting asthma-like symptoms post deployment is clinically relevant in a population that is designed to be made up of healthy young adults. However, why some healthy soldiers develop new-onset respiratory illnesses remains unclear. In healthy non-asthmatic subjects, a low-grade type 2 inflammation can exist, as IL-13 protein is detectable in bronchoalveolar lavage (BAL) fluid (Gour and Wills-Karp, 2015; Hosoki et al., 2015). Type 2 inflammation is characterized by increased type 2 cytokines like IL-13, eosinophils, mast cells, and basophils (Fahy, 2015). while asthmatics may present with high levels of type 2 airway inflammation, non-asthmatics with low-grade type 2 inflammation may be asymptomatic. However, it is unclear whether pre-existing type 2 inflammation may interact with the environmental factors such as particulate matter (PM) and subsequently enhance airway inflammation.
Exposure to PM, including desert dust, has been identified as a trigger of asthma exacerbations, where both the composition of PM and host cell immune status may play a key role in the disease pathogenesis (Becker et al., 2002; Fahy, 2015). Individual elements of PM may be harmful by themselves or carriers of other toxic materials (Engelbrecht et al., 2009; He et al., 2017). For example, Afghan PM (APM) is known to contain sand, silt, and clay, along with other metals and toxins (Engelbrecht et al., 2009). Whether APM inhalation induces airway inflammatory responses is not clear. However, previous studies in alveolar macrophages suggest that fine and coarse ambient air PM induces inflammatory responses through Toll-like receptor (TLR) signaling (eg, TLR2) (Becker et al., 2002; Shoenfelt et al., 2009). For example, there is a significant decrease in IL-8 production when TLR2 is inhibited using a neutralizing TLR2 antibody followed by air pollution particle treatment (Becker et al., 2005). Upon activation of TLRs, a signaling cascade including IRAK-1 and NF-κB is initiated, ultimately leading to production of inflammatory cytokines such as IL-8. To date, it is unclear whether PM interacts with type 2 cytokines, and subsequently affects TLR signaling and airway inflammation.
Our study was designed to address the mechanism of inflammation involved in asthma-like symptoms experienced by soldiers deployed in Afghanistan. We hypothesized that soldiers with asymptomatic type 2 inflammation during the deployment demonstrated enhanced pro-inflammatory response upon airway exposure to Afghanistan particulate matter (APM). Here, we focus on the effect of APM in type 2 cytokine primed airways in the production of IL-8, a chemokine involved in neutrophil recruitment and activation. Airway neutrophilic inflammation has been shown as a biomarker for a subset of late-onset severe asthma (Shaw et al., 2007; Wenzel, 2012). Often, asthma may develop after repeated exposure to irritants like cigarette smoke and air pollution in subjects that already exhibit atopy (Fahy, 2015). The goal of this study is to determine if exposure to APM exaggerates IL-8 production by primary human airway epithelial cells and the role of TLR2 signaling in the pro-inflammatory effect of APM in IL-13 exposed cells. We observed that APM exposure promotes IL-8 production in IL-13-primed airway epithelial cells thus providing a potential mechanism underlying an asthma-like condition in deployed soldiers.
MATERIALS AND METHODS
Characterization of Afghanistan desert PM (APM)
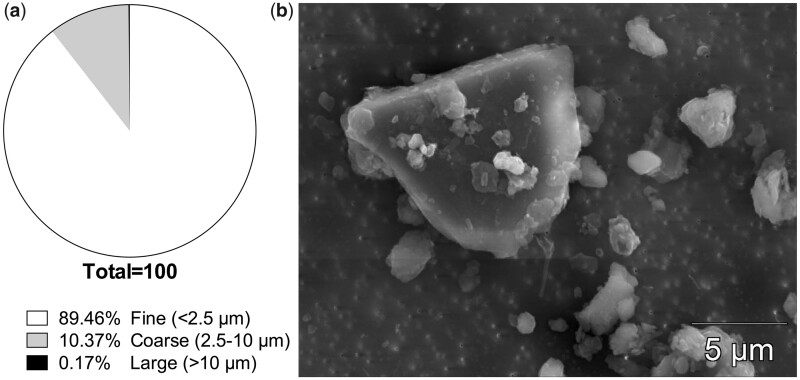
Topsoil from Bagram Air Force Base in the Parwan Province of Afghanistan was collected in August of 2009 and shipped to the United States in lined plastic drums. Particulate samples were irradiated and autoclaved in accordance with United States Department of Agriculture requirements. Characterization of Afghanistan desert PM was performed by the United States Geological Survey (USGS) in Lakewood, Colorado. Samples were analyzed via mass spectroscopy and scanning electron microscope for mineralogical composition and particle size and shape. Briefly, particulate samples were sonicated with isopropanol for 5 min. After sonication, samples were added to a 0.1 µm Nuclepore filter (Whatman, Maidstone, United Kingdom) and placed on a carbon coated scanning electron microscopy (SEM) stub and analyzed at 20 kv and with a current of approximately 1 nA. Size distribution of the APM is as follows: 89.5% less than 2.5 µm (fine particles) and 10.4% between 2.5 µm and 10 µm (coarse particles). Approximately 0.1% of particles are larger than 10 µm, which are not considered a respirable size (Figure 1A). Particle shape was not uniform, as shown in Figure 1B. Mineral content includes carbonates (calcite, dolomite), sheet silicates (clay, kaolinite, illite, chlorite, muscovite, talc, biotite), feldspar, quartz, oxides, zircon, titanite, synchysite, and monazite.
Figure 1.
Size distribution and SEM image of Afghan particulate matter. Samples of Afghan PM from Bagram Air Force Base were analyzed for size distribution and particle shape via scanning electron microscope. Particles are classified as one of three sizes: Fine (<2.5 µm), Coarse (2.5–10 µm), and Large (>10 µm).
Primary human airway epithelial cell cultures
Primary bronchial epithelial cells from a diverse age and gender background of normal, non-deployed subjects were obtained from the Human Primary Cell Core at National Jewish Health (NJH) through our institutional honest broker system (Table 1). Cells were collected from healthy volunteers as well as subjects who needed bronchoscopy to confirm or exclude respiratory diseases. Using our de-identified database, we chose cells from healthy subjects with no known asthma or other respiratory diseases. Patients with any indications of allergies or asthma were excluded from our study. Only healthy research volunteers were compensated for donating airway epithelial cells and all subjects provided written informed consent. The Institutional Review Board (IRB) at National Jewish Health approved collection and use of these cells and all patients consented to donate samples. Cells at passage 1 were cultured and expanded in collagen-coated 60 mm tissue culture dishes containing complete BronchiaLife bronchial epithelial cell growth medium (Lifeline Cell Technology, Frederick, Maryland) at 37°C, 5% CO2, until 90% confluence. Epithelial cells at passage 2 were seeded into culture plates at approximately 0.5 × 105 cells/well under submerged conditions, and treated with IL-13 and APM as described below.
Table 1.
Healthy Human Subject Characteristics
| Patient No. | Age | Gender | FEV1 | FVC | FEV1/FVC |
|---|---|---|---|---|---|
| 1 | 65 | F | 67 | 79 | 84 |
| 2 | 69 | F | 117 | N/A | N/A |
| 3 | 52 | M | 106 | 95 | 112 |
| 4 | 35 | F | N/A | N/A | N/A |
| 5 | 48 | M | N/A | N/A | N/A |
| 6 | 55 | M | 79 | 74 | 81 |
| 7 | 79 | F | 63 | 70 | 91 |
Optimization of IL-13 or APM treatment
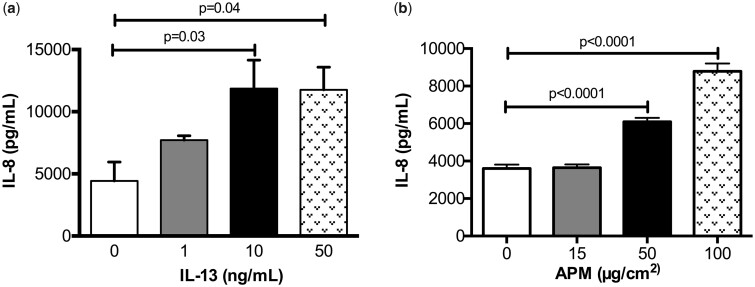
A dose response to optimize IL-13 (R&D Systems, Minneapolis, Minnesota) concentrations (1–50 ng/ml) was performed using primary normal HBECs. After 24 and 48 h of treatment, IL-8 production was measured. IL-13 at both 10 ng/ml and 50 ng/ml significantly increased IL-8 production after 48 h (Figure 2A, p = .03 and p = .04, respectively). We found that maximum induction of IL-8 occurs with the 10 ng/ml dose and there are no further increases at 50 ng/ml. The 48 h timepoint corresponds to the combined treatment times of the IL-13 pre-treatment and the following 24 h period, with and without APM co-treatment. The 10 ng/ml concentration of IL-13 in this study is supported by multiple publications of this cytokine treatment in human airway epithelial cells (Dickinson et al., 2016; Wen et al., 2002).
Figure 2.
IL-13 and APM dose optimizations. Brushed bronchial cells from a normal subject (n = 1) were exposed to control (−) and IL−13 (1, 10, 50 ng/ml) (A) or control (−) and APM (15, 50, 100 ug/cm2) (B) and IL−8 was measured at each timepoint. A one-way ANOVA with Dunn’s multiple comparisons test was used to analyze differences between the treatment groups compared with the control (−) condition.
To identify the optimal concentrations of APM, a dose response was performed by treating cultured normal human bronchial epithelial cells (HBECs) with APM at 15, 50, and 100 µg/cm2 for 6, 24, and 48 h. There was minimal induction of IL-8 after 6 h at all doses and at the lower dose (15 µg/cm2) after 24 h, compared with the control (no APM). At 50 and 100 µg/cm2 after 24 h, there was a significant dose dependent increase in IL-8 (Figure 2B, p < .0001 for both). The 50 µg/cm2 is well in line with previous PM toxicity studies and a more physiologically relevant dose for in vitro modeling of PM exposures of deployed soldiers (Breznan et al., 2016; Sayes et al., 2007; Wang et al., 2016). Ultimately, we selected the 50 µg/cm2 dose over 100 µg/cm2 because there was minimal cell death seen at this timepoint and the higher dose (100 µg) was more representative of a particle-overload/sustained sandstorm exposure.
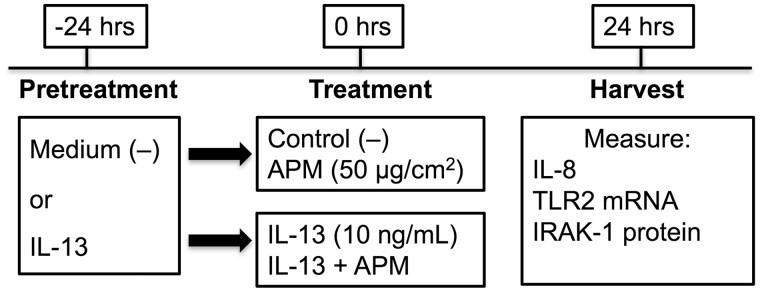
In vitro IL-13 and APM exposure
An overview of the cell culture conditions and treatment scheme is shown in Figure 3. Cells in culture plates were pretreated with medium control (−) or IL-13 (10 ng/ml) (R&D Systems) for 24 h prior to treatment with PM. APM was suspended in cell culture medium immediately before treatment and added to cells pretreated with medium or IL-13 at 50 µg/cm2. Supernatants were collected for analysis of inflammatory cytokines by ELISA and cells were harvested for Western blotting and qPCR.
Figure 3.
Overview of cell culture treatments. Schematic demonstrating cell culture conditions and treatments for in vitro APM exposures of bronchial epithelial cells.
Prior to treatment, dried APM was measured and suspended in cell culture medium. The cell culture medium suspension with APM was vortexed before addition to each well and pipetted vigorously. The APM was not sonicated to avoid the potential toxic effect associated with the sonicated PM. No precautions outside of autoclaving for shipment were taken that would alter the bacteria within the sample, including LPS. After all wells had been treated, plates were placed on an orbital rotator for 10 min to allow dust to be evenly distributed throughout the well. Cells were then placed in the incubator until harvest.
siRNA-mediated knockdown of TLR2 in human bronchial epithelial cells
HBECs were seeded and cultured under submerged condition to confluency of 50%–70%. Cells were transfected for 16 h with either TLR2 siRNA or scrambled siRNA control (Life Technologies, Carlsbad, California) in the presence of lipofectamine in cell culture medium without antibiotics. Cells were washed twice with PBS and allowed to recover in medium or medium plus IL-13 for 24 h before treatment with APM, IL-13, and the combination. Cells were harvested in Lysis Buffer 17 (R&D Systems) for confirmation of TLR2 protein knockdown via ELISA.
Enzyme-linked-immunosorbent assay (ELISA)
IL-8 levels in cell supernatants and TLR2 levels in cell lysates were determined with the DuoSet development ELISA kits (R&D Systems).
Quantitative real-time RT-PCR
Quantitative gene expression assay for TLR2 was custom made from Integrated DNA Technologies (IDT, Coralville, Iowa). The specific primers and probes for TLR2 were: Forward (GGC-CAGCAAATTACCTGTGTG), Reverse (AGGCGGACATCCTGAACCT), and Probe (TCCATCCCATGTGCG-TGGCC). The TaqMan gene expression assay for IL-8 was obtained from Applied Biosystems (Life Technologies, Foster City, California). The housekeeping gene GAPDH (Life Technologies) was evaluated as internal positive controls. Quantitative real-time PCR was performed on the CFX96TM real-time PCR Detection System (Bio-Rad, Hercules, California). The comparative cycle of threshold (ΔΔCt) method was used to demonstrate the relative levels of target genes.
Western blot analysis
Cells were lysed in RIPA lysis buffer with protease and phosphatase inhibitors (Fisher Scientific, Waltham, Massachusetts). The same amount of protein lysate (eg, 30 µg) was electrophoresed on an 8% SDS-PAGE gel, transferred onto a nitrocellulose membrane, blocked with 2.5% nonfat milk, and incubated with antibodies against pIRAK-1 (OABF01186, AVIVA Biosystem, San Diego, California), total IRAK-1 (sc-55530, Santa Cruz Biotech, Inc, Santa Cruz, California), and GAPDH (sc-32233, Santa Cruz Biotech, Inc) overnight at 4°C. After washing, membranes were incubated with the appropriate HRP-linked secondary antibodies and Pierce ECL Prime Western blotting substrate (Fisher Scientific). Membranes were developed using the Fotodyne Foto/Analyst FX system (FOTODYNE Incorporated, Hartland, Wisconsin). Densitometry was performed using NIH ImageJ software. pIRAK-1 levels were normalized to total IRAK-1 protein, with GAPDH used as a loading control.
Generation of IRAK-1 knockdown primary human tracheobronchial epithelial cells
A stable IRAK1-deficient primary human tracheobronchial epithelial (HTBE) cell line was generated by lentiviral shRNA knockdown using cells isolated from a deceased individual who donated lungs and trachea for research purposes to National Jewish Health. HTBE cells were transduced with IRAK1-specific shRNA or control (scrambled) shRNA using lentivirus expressing puromycin resistance gene. After 48 h in culture, the cells were harvested and seeded onto irradiated, puromycin-resistant 3T3 fibroblasts for expansion and selection of cells with puromycin (1 µg/ml). IRAK1 knockdown was assessed by Western blot. Cells were seeded in 24-well plates at 0.5 × 105 and then pretreated with medium control or IL-13 and exposed to APM with and without IL-13. Supernatants were collected for IL-8 ELISA and cells were harvested for mRNA expression analysis and confirmation of knockdown via Western blot.
Statistical analysis
Since data from different subjects were not normally distributed, a nonparametric approach was taken for statistical analysis. A Wilcoxon Signed Rank test was used to compare mean rank differences when two groups were present. A Kruskal-Wallis test followed by Dunn’s multiple comparisons test was used when 4 groups were present. A one-way ANOVA with a Dunnett’s multiple comparison test was used to analyze the normally distributed data in the dose optimization preliminary studies. A p value of less than .05 was considered significant.
RESULTS
APM Induces IL-8 Production in a Type 2 Inflammatory Setting
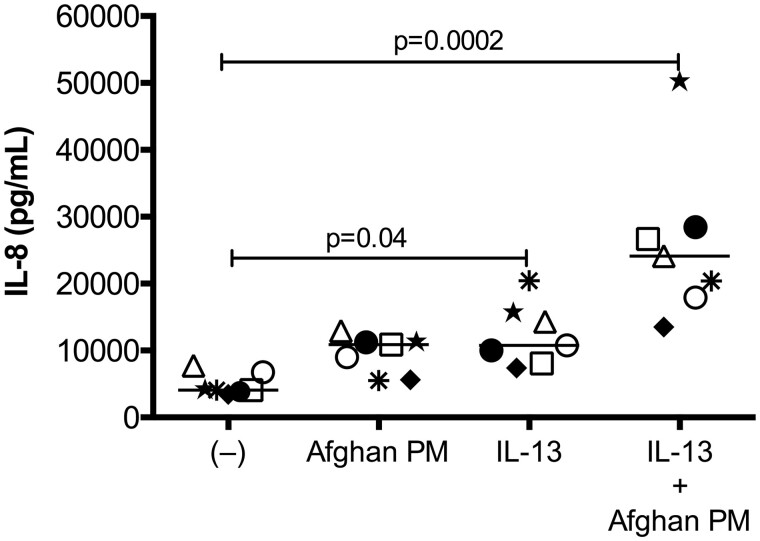
In cultured primary bronchial epithelial cells from a cohort of 7 healthy subjects, IL-13 exposure alone significantly increased production of IL-8 at 24 h (p = .04). The combination of APM with IL-13 further increased production of IL-8 (p = .0002) (Figure 4). The dramatic increase in IL-8 production of combined APM with IL-13 treatments, compared with APM levels alone, suggests synergistic effects of both stimuli.
Figure 4.
Afghan PM induces IL-8 production in a type 2 inflammatory setting. Brushed bronchial cells from normal, non-deployed subjects (n = 7) were exposed to control (−), APM, IL-13, and the combination for 24 h. APM, IL-13, and the combination significantly increased IL-8 compared with medium control-treated cells. Each circle represents a unique patient and bars indicate the median. A paired Wilcoxon test was used to compare mean rank differences between two groups.
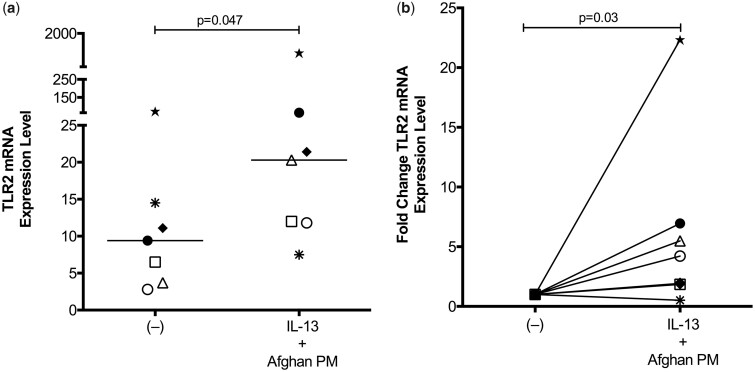
APM Enhances TLR2 mRNA Expression in IL-13-Exposed Airway Epithelial Cells
Given the previous report of TLR2 up-regulation by air pollution particles and the role of TLR2 signaling in the pro-inflammatory responses to PM in epithelial cells, we next evaluated if TLR2 expression was affected by APM exposure (Becker et al., 2005). After 24 h of APM with IL-13 treatment, TLR2 mRNA expression was significantly upregulated in bronchial epithelial cells (p = .047) (Figure 5A). IL-13 or APM alone did not increase TLR2 expression levels compared with untreated control cells (data not shown). Due to the large variations in gene expression levels between human subjects, we also analyzed TLR2 mRNA expression levels by fold change (Figure 5B). IL-13 and APM together significantly increased TLR2 mRNA expression levels by an average of 6-fold compared with control (p = .03).
Figure 5.
APM drives TLR2 mRNA expression in IL-13 exposed epithelial cells. Gene expression analysis of brushed bronchial cells from normal, non-deployed subjects (n = 7) exposed to control (−), APM, IL-13, and the combination for 24 h. Combination treatment of APM and IL-13 lead to significantly increased expression of TLR2 demonstrated via expression level (A) and fold change (B). Each circle represents a unique patient and bars indicate the median. A paired Wilcoxon test was used to compare mean rank differences between two groups.
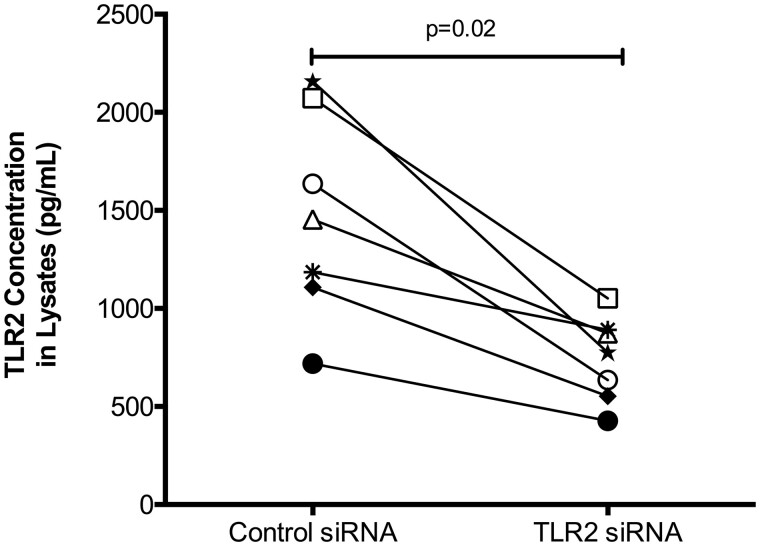
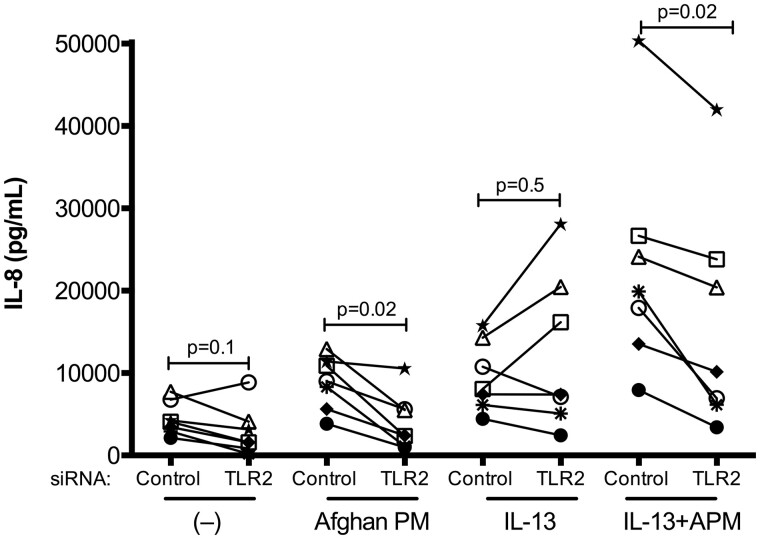
TLR2 Knockdown Dampens IL-8 Production After APM and IL-13 Stimulation
After identifying the association of TLR2 expression and the pro-inflammatory effect of APM with IL-13, RNA interference using TLR2 or control siRNA was performed in bronchial epithelial cells from 7 subjects. Proteins from cell lysates were used to measure TLR2 levels via ELISA, and successful knockdown was verified in all subjects (p = .02, Figure 6). TLR2 siRNA, compared with control (scrambled) siRNA, resulted in significant reduction in IL-8 production in APM plus IL-13 treated cells (p = .02) as well as in APM alone treated cells after 24 h (p = .02) (Figure 7). TLR2 knockdown did not affect baseline levels of IL-8 or IL-13 induced IL-8 production (p = .1 and p = .5, respectively).
Figure 6.
Confirmation of TLR2 siRNA knockdown in cell lysates. Brushed bronchial cells from normal, non-deployed subjects (n = 7) were cultured under submerged conditions then transfected with either TLR2 siRNA or scrambled negative control siRNA. Cells were lysed and a TLR2 ELISA was used to confirm knockdown of the target gene. Each circle represents one unique subject. A paired Wilcoxon test was used to compare mean rank differences between groups.
Figure 7.
Afghan PM-dependent IL-8 production is decreased after TLR2 knockdown. IL-8 ELISA of cells from non-deployed subjects (n = 7) transfected with negative control or TLR2 siRNA, then treated with control (−), APM, IL-13, and the combination. Each circle represents one unique subject. A paired Wilcoxon test was used to compare mean rank differences between control siRNA versus TLR2 siRNA within each treatment.
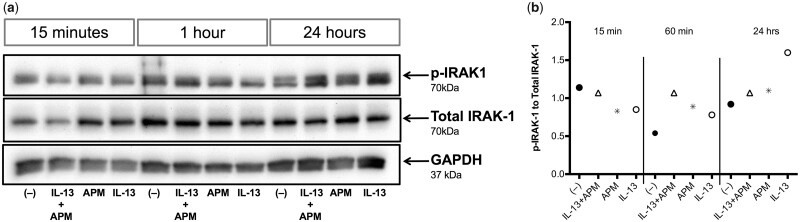
Afghan PM Increases Phosphorylation of IRAK-1
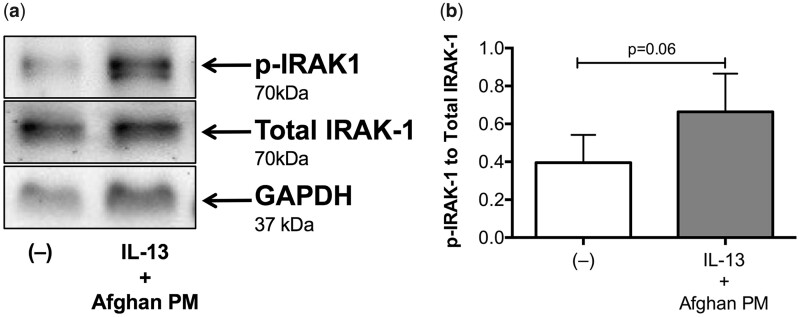
We performed a time course of IRAK-1 activation. There was no change in IRAK-1 activation in response to any of the treatments at 15 min. The combined treatment of APM and IL-13 increased IRAK-1 activity starting at 1 h and lasting through 24 h (Figure 8, n = 1). We then compared IRAK-1 activation in airway epithelial cells under various conditions. The combination treatment of APM with IL-13 resulted in increased IRAK-1 activation as indicated by the ratio of phosphorylation of IRAK-1 to total IRAK-1 protein in bronchial epithelial cells 24 h after stimulation (Figure 9, p = .06).
Figure 8.
IRAK-1 activation occurs at the later phase after IL-13+APM treatment. Western blot analysis of bronchial epithelial cells from a non-deployed subject (Patient No. 2, n = 1) stimulated with control (−), APM, IL-13, and the combination for 15 min, 1 h, and 24 h (A). Duplicate wells were pooled at harvest in Western lysis buffer. Densitometry data with target proteins normalized to total protein (B).
Figure 9.
Afghan PM and IL-13 signal through IRAK-1. Western blot analysis of bronchial epithelial cells from non-deployed subjects (n = 6) stimulated with control (−) or IL-13+APM. GAPDH was used as an internal loading control. Combination treatment of APM and IL-13 increases activation of p-IRAK1 (A). Densitometry data with target protein normalized to total protein levels (B).
IRAK-1 is Required for APM and IL-13 Induced Pro-Inflammatory Response
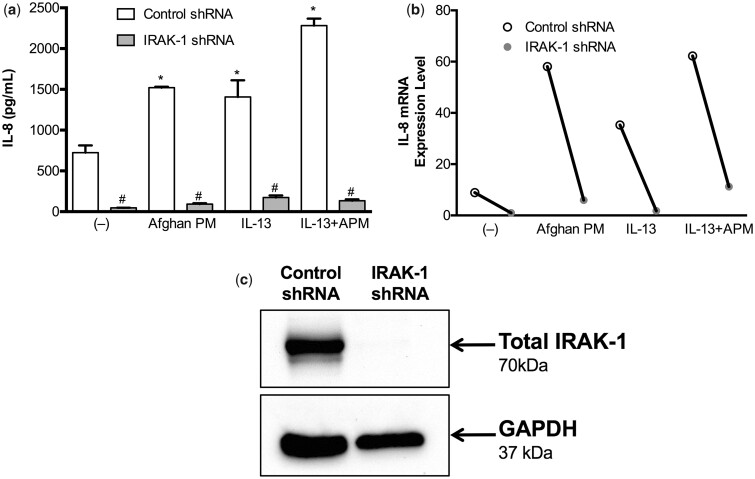
Based on our Western blot data, IRAK-1 appears to be involved in the inflammatory signaling pathway of airway epithelial cells after APM with IL-13 treatment. In control shRNA cells, APM, IL-13, and particularly the combination, results in significantly increased production of IL-8. As compared to the cells transduced with control shRNA, IRAK-1 knocked down cells produced significantly less IL-8 across all conditions (Figure 10). In APM with IL-13 treated IRAK-1 knockdown cells, IL-8 production was significantly reduced to 94% of control levels (p < .0001).
Figure 10.
IRAK-1 is essential for IL-8 production after APM and IL-13 treatment. Control and IRAK-1 shRNA was used to generate IRAK-1 knockdown tracheobronchial epithelial cells from one normal, deceased subject. After IRAK-1 shRNA, IL-8 production is significantly decreased across all conditions (A). IL-8 mRNA levels follow protein levels (B). Confirmation of IRAK-1 knockout via Western blot, with GAPDH as a loading control (C).
DISCUSSION
In this study, we demonstrate that PM from Afghanistan induced IL-8 production by bronchial epithelial cells and that this effect was enhanced by IL-13. While both APM and IL-13 individually increase IL-8, they combine to enhance pro-inflammatory responses stronger than both stimulants alone. By performing gene knockdown studies, we found that the TLR2/IRAK-1 signaling axis is critical to the pro-inflammatory responses to APM with IL-13.
With the rates of respiratory illnesses in post-deployed soldiers increasing, our study presents a clinically relevant model in the United States, where atopy is very common (Faniran et al., 1999; Piccirillo et al., 2016). In our model, APM and IL-13 combine to induce production of IL-8, a neutrophilic chemoattractant. Neutrophilic inflammation is considered one of the many asthma phenotypes, but the inflammatory state without asthma or asthma-like symptoms may represent a feature of this Afghanistan War Lung Injury airway disease. In a military setting, our data may help explain why some, but not all soldiers develop respiratory illnesses post-deployment.
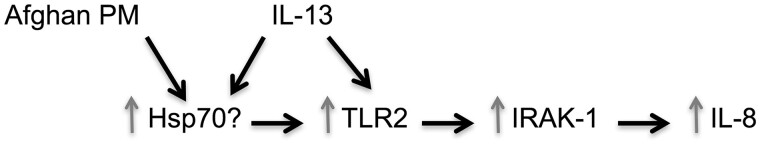
While PM from other sources may bind TLRs (Becker et al., 2005), how Afghanistan PM induces inflammatory responses, particularly in the presence of IL-13, remains unknown. We show that after stimulation with APM and IL-13, TLR2 expression is upregulated, but only when combined. Next we looked into upstream molecules that are known to activate TLR2. Both PM and IL-13/type 2 immunity cytokines have been shown to increase Hsp70, which then upregulates TLR2 (Asea et al., 2002; Becker et al., 2005; Gally et al., 2012; Min et al., 2017). We speculate that Hsp70 may be a converging point for the inflammatory response induced by PM and type 2 immunity. In future studies, we hope to further elucidate the role of Hsp70 in the TLR2/IRAK-1/IL-8 axis after APM and IL-13 stimulation, as well as TLR2 ligands in the APM. Although TLR2 signaling is critical to IL-8 induction by APM with IL-13, TLR2 knockdown did not completely abolish IL-8 induction, suggesting involvement of other pathways.
We sought to investigate the levels of IRAK-1 activation, a downstream effector of TLR2. IRAK-1 activation was increased following APM with IL-13 combination, suggesting that TLR2 and IRAK-1 cooperate in the pro-inflammatory response. We further confirmed the role of IRAK-1 in the knockdown study where IL-8 production was drastically decreased. We found that only the combination leads to increased IRAK-1 activation at 24 h, but by this point, the individual effect that may have occurred in the earlier phases may have disappeared. We speculate that the combination of IL-13 and APM may prolong IRAK-1 activation. A recent publication from our group demonstrated that IL-13 increases IRAK-1 activation in macrophages (Ito et al., 2018), however the role of IRAK-1 in epithelial cell response to IL-13 in combination with APM has yet to be defined. Collectively, our study has indicated the TLR2/IRAK-1 signaling axis as a potential mechanism driving the pro-inflammatory response of APM in an airway type 2 cytokine milieu.
This study is not without limitations. We still do not know the components within the APM that directly bind or activate TLR2. In a pilot study, we treated APM and IL-13 exposed cells with a lipase that would neutralize lipoproteins, a known TLR2 ligand (Hashimoto et al., 2006). However, our study did not show any significant differences of IL-8 production in APM with IL-13 treated cells with or with the lipase incubation. This suggests that lipoproteins may not exist in APM or the level of lipoproteins is minimal. Similarly, previous studies show that TLR2 is involved in air pollution induced inflammatory responses, but the exact TLR2 ligands were not identified (Becker et al., 2005; Hiraiwa and van Eeden, 2013).
This study used a frequently employed submerged cell culture system to investigate airway inflammation after APM and IL-13 treatment. This system is widely used to model damaged airways, here representing soldiers with abnormal type 2 immunity. Submerged cultures have been demonstrated to be an appropriate model for particulate exposures by other researchers as well (Ghio et al., 2013; Panas et al., 2014). Air-liquid interface (ALI) cultures represent healthy well-differentiated airway epithelium, but in PM toxicity studies, it has been reported that submerged cultures may be a more effective system. In a study by Ghio et al., submerged cultures produced significantly more IL-8 and IL-6 (another pro-inflammatory cytokine) compared to ALI cultures after treatment with air pollution particles (Ghio et al., 2013). In early optimization experiments with Afghanistan PM in ALI cultures, we obtained similar results, likely because of the mucus produced by this culture method, which causes large clumped aggregates of particulates and minimizes cell contact and stimulation of the epithelial cells.
Instead of using primary samples from actual deployed soldiers, we used a cell culture model to represent airways of post-deployment patients. Our upcoming studies will address this limitation as active recruitment of post-deployment soldiers is ongoing. While the scope of these results is limited to those soldiers already displaying abnormal type 2 immunity, our results can be applied to the larger military population who may not yet be experiencing symptoms. We demonstrated increases in IL-8 production after APM sensitization alone, but the mechanisms of that inflammation, independent of type 2 mediators, remains to be investigated.
We recognize that our cohort uses cells from some subjects that are older than most deployed soldiers. Previous studies indicate that cells from older subjects may have higher pro-inflammatory cytokine responses compared with younger subjects (Wolf et al., 2012), but controversy exists (Maniar-Hew et al., 2013). Our older subjects (60+ years old) responded similarly to the younger age (less than 50 years old) (data not shown). Furthermore, we did not find an impact of gender on IL-8 production, which differs from the previous work showing higher IL-8 production in women than men (Lefevre et al., 2012).
In conclusion, our study provides a possible mechanism whereby deployed atopic military personnel may develop exaggerated inflammatory responses while on deployment to Afghanistan (Figure 11). Defining the role of TLR2 and the subsequent signaling events may improve our understanding of the airway inflammatory responses and clinical symptoms that characterizes the newly identified lung disease. With this information, researchers may be able to find a targetable molecule to guide future treatment.
Figure 11.
Overview of proposed APM and IL-13 signaling pathway.
ACKNOWLEDGMENTS
We would like to thank Heather Lowers and Dr Geoff Plumlee at the United States Geological Survey in Lakewood, Colorado for their efforts in characterizing and analyzing our Afghanistan PM samples. In addition, we would like to thank the other GLIDE investigators at National Jewish Health, Dr Cecile Rose and Dr Max Seibold, for their discussions about clinical manifestations about dust health effects and airway cell biology. Finally, we would like to thank Dr Greg Downey’s laboratory for providing us with the Afghanistan PM and Nicole Roberts in the Chu Lab for providing the primary cells used in this study.
Contributor Information
Reena Berman, Department of Medicine, National Jewish Health, Denver, Colorado 80206.
Gregory P Downey, Department of Medicine, National Jewish Health, Denver, Colorado 80206.
Azzeddine Dakhama, Department of Medicine, National Jewish Health, Denver, Colorado 80206.
Brian J Day, Department of Medicine, National Jewish Health, Denver, Colorado 80206.
Hong Wei Chu, Department of Medicine, National Jewish Health, Denver, Colorado 80206.
FUNDING
This work was supported by a grant from the Department of Defense (R150109 to Drs Downey, Day, and Chu.).
REFERENCES
- Army, U. S. Army Standards of Medical Fitness. Available at: https://www.calculator.net/pdf/r40_501.pdf. Accessed June 14 2017.
- Asea A., Rehli M., Kabingu E., Boch J. A., Bare O., Auron P. E., Stevenson M. A., Calderwood S. K. (2002). Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277, 15028–15034. [DOI] [PubMed] [Google Scholar]
- Becker S., Dailey L., Soukup J. M., Silbajoris R., Devlin R. B. (2005). TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol. Appl. Pharmacol. 203, 45–52. [DOI] [PubMed] [Google Scholar]
- Becker S., Fenton M. J., Soukup J. M. (2002). Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am. J. Respir. Cell Mol. Biol. 27, 611–618. [DOI] [PubMed] [Google Scholar]
- Breznan D., Karthikeyan S., Phaneuf M., Kumarathasan P., Cakmak S., Denison M. S., Brook J. R., Vincent R. (2016). Development of an integrated approach for comparison of in vitro and in vivo responses to particulate matter. Part. Fibre Toxicol. 13, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J. D., Alevy Y., Malvin N. P., Patel K. K., Gunsten S. P., Holtzman M. J., Stappenbeck T. S., Brody S. L. (2016). IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy 12, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht J. P., McDonald E. V., Gillies J. A., Jayanty R. K., Casuccio G., Gertler A. W. (2009). Characterizing mineral dusts and other aerosols from the Middle East—Part 2: Grab samples and re-suspensions. Inhal. Toxicol. 21, 327–336. [DOI] [PubMed] [Google Scholar]
- Fahy J. V. (2015). Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 15, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faniran A. O., Peat J. K., Woolcock A. J. (1999). Prevalence of atopy, asthma symptoms and diagnosis, and the management of asthma: Comparison of an affluent and a non-affluent country. Thorax 54, 606–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally F., Minor M. N., Smith S. K., Case S. R., Chu H. W. (2012). Heat shock factor 1 protects against lung mycoplasma pneumoniae infection in mice. J. Innate Immun. 4, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio A. J., Dailey L. A., Soukup J. M., Stonehuerner J., Richards J. H., Devlin R. B. (2013). Growth of human bronchial epithelial cells at an air-liquid interface alters the response to particle exposure. Part. Fibre Toxicol. 10, 25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gour N., Wills-Karp M. (2015). IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M., Tawaratsumida K., Kariya H., Aoyama K., Tamura T., Suda Y. (2006). Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int. Immunol. 18, 355–362. [DOI] [PubMed] [Google Scholar]
- He M., Ichinose T., Yoshida Y., Arashidani K., Yoshida S., Takano H., Sun G., Shibamoto T. (2017). Urban PM2.5 exacerbates allergic inflammation in the murine lung via a TLR2/TLR4/MyD88-signaling pathway. Sci. Rep. 7, 11027.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa K., van Eeden S. F. (2013). Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm. 2013, 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki K., Ying S., Corrigan C., Qi H., Kurosky A., Jennings K., Sun Q., Boldogh I., Sur S. (2015). Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One 10, e0126035.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Schaefer N., Sanchez A., Francisco D., Alam R., Martin R. J., Ledford J. G., Stevenson C., Jiang D., Li L., et al. (2018). Toll-interacting protein, tollip, inhibits IL-13-mediated pulmonary eosinophilic inflammation in mice. J. Innate Immun. 10, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre N., Corazza F., Duchateau J., Desir J., Casimir G. (2012). Sex differences in inflammatory cytokines and CD99 expression following in vitro lipopolysaccharide stimulation. Shock 38, 37–42. [DOI] [PubMed] [Google Scholar]
- Maniar-Hew K., Clay C. C., Postlethwait E. M., Evans M. J., Fontaine J. H., Miller L. A. (2013). Innate immune response to LPS in airway epithelium is dependent on chronological age and antecedent exposures. Am. J. Respir. Cell Mol. Biol. 49, 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H. J., Kim K. S., Yoon J. H., Kim C. H., Cho H. J. (2017). T-helper 2 cytokine-induced heat shock protein 70 secretion and its potential association with allergic rhinitis. Int. Forum Allergy Rhinol. 7, 530–535. [DOI] [PubMed] [Google Scholar]
- Panas A., Comouth A., Saathoff H., Leisner T., Al-Rawi M., Simon M., Seemann G., Dossel O., Mulhopt S., Paur H. R., et al. (2014). Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J. Nanotechnol. 5, 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccirillo A. L., Packnett E. R., Cowan D. N., Boivin M. R. (2016). Epidemiology of asthma-related disability in the U.S. Armed Forces: 2007–2012. J. Asthma 53, 668–678. [DOI] [PubMed] [Google Scholar]
- Sayes C. M., Reed K. L., Warheit D. B. (2007). Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 97, 163–180. [DOI] [PubMed] [Google Scholar]
- Shaw D. E., Berry M. A., Hargadon B., McKenna S., Shelley M. J., Green R. H., Brightling C. E., Wardlaw A. J., Pavord I. D. (2007). Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest 132, 1871–1875. [DOI] [PubMed] [Google Scholar]
- Shoenfelt J., Mitkus R. J., Zeisler R., Spatz R. O., Powell J., Fenton M. J., Squibb K. A., Medvedev A. E. (2009). Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J. Leukoc. Biol. 86, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szema A., Mirsaidi N., Patel B., Viens L., Forsyth E., Li J., Dang S., Dukes B., Giraldo J., Kim P., et al. (2017). Proposed Iraq/Afghanistan War-Lung Injury (IAW-LI) Clinical Practice Recommendations: National Academy of Sciences' Institute of Medicine Burn Pits Workshop. Am. J. Mens Health 11, 1653–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szema A. M., Reeder R. J., Harrington A. D., Schmidt M., Liu J., Golightly M., Rueb T., Hamidi S. A. (2014). Iraq dust is respirable, sharp, and metal-laden and induces lung inflammation with fibrosis in mice via IL-2 upregulation and depletion of regulatory T cells. J. Occup. Environ. Med. 56, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li N., Deng F., Buglak N., Park G., Su S., Ren A., Shen G., Tao S., Guo X. (2016). Human bronchial epithelial cell injuries induced by fine particulate matter from sandstorm and non-sandstorm periods: Association with particle constituents. J. Environ. Sci. (China) 47, 201–210. [DOI] [PubMed] [Google Scholar]
- Wen F. Q., Kohyama T., Liu X., Zhu Y. K., Wang H., Kim H. J., Kobayashi T., Abe S., Spurzem J. R., Rennard S. I. (2002). Interleukin-4- and interleukin-13-enhanced transforming growth factor-beta2 production in cultured human bronchial epithelial cells is attenuated by interferon-gamma. Am. J. Respir. Cell Mol. Biol. 26, 484–490. [DOI] [PubMed] [Google Scholar]
- Wenzel S. E. (2012). Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 18, 716–725. [DOI] [PubMed] [Google Scholar]
- Wolf J., Weinberger B., Arnold C. R., Maier A. B., Westendorp R. G., Grubeck-Loebenstein B. (2012). The effect of chronological age on the inflammatory response of human fibroblasts. Exp. Gerontol. 47, 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]