ABSTRACT
Background
Guideline-recommended hyperkalaemia management includes dietary potassium (K+) restriction, bicarbonate correction, diuretics and K+ binders with dose reduction of renin–angiotensin–aldosterone system inhibitors as a last resort. The extent to which these recommendations are implemented is uncertain, as real-world data on hyperkalaemia management are limited. The Tracking Treatment Pathways in Adult Patients with Hyperkalemia (TRACK) study is a multinational, prospective, longitudinal study that is being conducted to address this knowledge gap. We report the design and baseline cohort characteristics of this real-world study of hyperkalaemia management decision-making.
Methods
This study enrolled participants within 21 days of an episode of hyperkalaemia in four European countries (UK, Spain, Germany, Italy) and the USA. During the 12-month follow up, data collected will include participant and healthcare provider characteristics (specialty and practice setting), hyperkalaemia treatment objectives and strategies, rationale for management decisions and indicators of response and patient-reported perceptions of their hyperkalaemia treatment.
Results
The enrolled cohort includes 1330 participants, mean age 68 years, of whom 31% were women. At baseline, 6% reported heart failure, 55% chronic kidney disease, 29% both and 9% neither. Most participants (57%) were taking an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker or angiotensin receptor/neprilysin inhibitor at baseline. Mineralocorticoid receptor antagonist use was lower (14%).
Conclusions
The prospective TRACK study will shed light on practitioners’ hyperkalaemia management decision-making and assess the impact of their decisions on hyperkalaemia recurrence. Understanding practitioners’ underlying thought processes will facilitate efforts to improve hyperkalaemia management.
ClinicalTrials.gov: NCT05408039
Keywords: chronic kidney disease, heart failure, hyperkalaemia management, RAASi, real-world evidence
KEY LEARNING POINTS.
What was known:
Although professional societies provide recommendations for managing hyperkalaemia, little is known about practitioners’ real-world decision-making. The TRACK study aims to address this knowledge gap.
This study adds:
We describe the design of the TRACK study, which collects information about healthcare providers, their hyperkalaemia management decision-making, response to treatment, participant characteristics and perceptions of their hyperkalaemia care.
Potential impact:
Improved understanding of the basis for hyperkalaemia treatment choices may support the medical community in optimizing the use of evidence-based therapies such as potassium binders in patients with recurrent hyperkalaemia or maintaining renin–angiotensin–aldosterone system inhibitors in those with heart failure and/or chronic kidney disease.
INTRODUCTION
In a general outpatient population, the prevalence of hyperkalaemia is 1.3% [1], increasing 5-fold among patients with heart failure and up to 20-fold among those with chronic kidney disease (CKD) [2]. Hyperkalaemia predicts poorer survival as well as increased risk of kidney and cardiovascular events, including life-threatening arrhythmias, higher healthcare costs [3, 4] and poorer quality of life [5].
Hyperkalaemia recurrence is common. Following an index episode of hyperkalaemia in patients with CKD stage 3–4, recurrence was identified in 37% within 1 month and 56% within 6 months [6]. One of the major risk factors for recurrent or persistent hyperkalaemia is the use of renin–angiotensin–aldosterone system inhibitors (RAASis), an important therapy demonstrated to slow CKD progression and lower the risk of cardiovascular events. International best practice recommendations for management of hyperkalaemia include maintaining RAASi medications where possible and the use of newer potassium (K+) binders to enable maximal dosing of these disease-modifying therapies [7–9]. The extent to which these recommendations are implemented is uncertain, as real-world data on hyperkalaemia management are limited. We designed and are conducting the Tracking Treatment Pathways in Adult Patients with Hyperkalemia (TRACK) study to better understand hyperkalaemia management, treatment patterns and provider treatment decision-making during routine clinical care. This report describes the study design and baseline characteristics of the fully enrolled cohort.
MATERIALS AND METHODS
TRACK is a prospective cohort study that enrolled patients in the USA and four European countries (UK, Spain, Germany and Italy) within 21 days following an episode of hyperkalaemia, defined as serum/plasma K+ concentration >5.0 mmol/l, with a planned 1-year follow-up. The primary objective is to describe hyperkalaemia management decisions, their rationale and treatment expectations. The secondary objective is to describe baseline characteristics and longitudinal clinical variables in patients with hyperkalaemia. The exploratory objective is to describe patient awareness and satisfaction with their hyperkalaemia management. The study was approved by central and local institutional review boards/independent ethics committees and all participants provided informed consent.
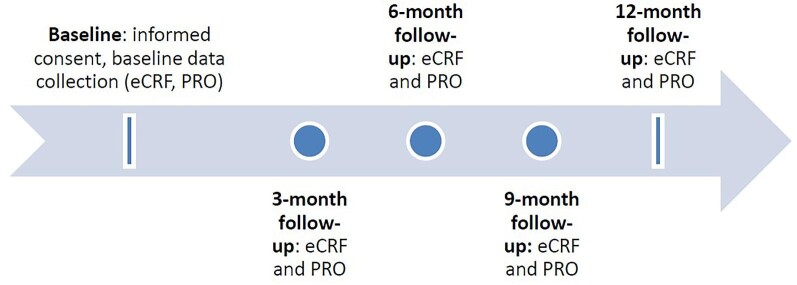
As a pragmatic real-world evidence study, eligibility criteria were streamlined and selected to reflect a broad range of patients with a recent episode of hyperkalaemia (Table 1). No in-person visits and no specific procedures are required. Data describing current real-world management of hyperkalaemia are collected by medical records review and from healthcare providers (Table 2) via electronic case reports at baseline and 3, 6, 9 and 12 months following enrolment (Fig. 1).
Table 1:
Eligibility criteria.
| Inclusion criteria | • Age ≥18 years • [K+] >5.0 mmol/l collected during standard of care within 21 days prior to the date of enrolment • Provision of informed consent |
| Exclusion criteria | • Concurrent participation in any trial that includes the use of K+ binders as an investigational medicinal product • Patients with pseudo-hyperkalaemia • Acute causes of hyperkalaemia, such as infections and/or trauma, to be determined by the principal investigator • Life expectancy of <6 months, based on physician judgement • Kidney transplant anticipated or planned during the study period • Involvement in the planning and/or conduct of the study |
Table 2:
Study objectives and data collected.
| Objectives | Data collected |
|---|---|
| Primary objective: to describe hyperkalaemia management decisions, their rationale and treatment expectations | Healthcare provider-related variables • Hyperkalaemia management decision • Hyperkalaemia management objective(s) (e.g. to achieve GDMT target doses of RAASi for CKD and HF) • Expected hyperkalaemia management duration Indicators of response • Normalization in [K+] levels • Time to [K+] normalization • Hyperkalaemia recurrence frequency • GDMT target doses of RAASi • Time to achieve GDMT target doses of RAASi • Occurrences of hyperkalaemia complications such as arrythmia, muscle weakness, and metabolic acidosis • Healthcare resource utilization |
| Secondary objective: to describe the baseline and longitudinal clinical variables of patients with established hyperkalaemia irrespective of previous hyperkalaemia diagnoses | Demographics (age, sex, and in the USA, race/ethnicity) Medical history • Comorbidities coded to MedDRA terms • Hyperkalaemia treatment history (including use of diet, K+ binders and reduction in RAASi dosing) Medication history (especially RAASi therapy and doses) Laboratory • [K+] • Serum creatinine • Urine albumin:creatine ratio Left ventricular ejection fraction Healthcare resource utilization—overall, heart failure and CKD-specific Dialysis-specific information |
| Exploratory objective: to describe patient awareness and satisfaction with their hyperkalaemia management | Patient-reported outcomes • Patient awareness of their hyperkalaemia management • FACIT-TS-G • Patient awareness of dietary recommendations |
GDMT: guideline-directed medical treatment; MedDRA: Medical Dictionary for Regulatory Activities.
Figure 1:
Study schema. Participants were enrolled within 21 days of an episode of hyperkalaemia, defined as [K+] >5.0 mmol/l. Baseline data were recorded from the medical record and directly from healthcare providers. Follow-up contacts at 3, 6, 9 and 12 months recorded healthcare utilization, medical diagnoses and laboratory results from the medical record.
Patient and healthcare provider characteristics
We recorded participants’ demographics. concomitant medications and medical history (Table 2), including the presence and stage of CKD assessed from calculated estimated glomerular filtration rate (eGFR) using the 2021 Chronic Kidney Disease Epidemiology Collaboration equation [10] and albumin:creatinine ratio, presence of heart failure and reported left ventricular ejection fraction.
Healthcare providers were asked to record their specialty (nephrology, cardiology, primary care, other), demographics, years of experience and number of patients with hyperkalaemia managed each month.
Data collection during follow-up
Participants are followed for 12 months by electronic medical records review at 3-month intervals for hyperkalaemia recurrence. Healthcare providers record their hyperkalaemia treatment objectives, management decisions and indicators of response for the index episode of hyperkalaemia and all subsequent episodes during the 12-month follow-up period (Table 2). Participants are invited to complete an online questionnaire about awareness of their hyperkalaemia management, including whether they received advice about a low K+ diet, medications for hyperkalaemia or adjustments to medications taken for other health conditions (see supplementary material). For those indicating receipt of dietary recommendations, additional questions are asked about how this was provided and their satisfaction with the dietary advice. Participants prescribed medications for hyperkalaemia are asked to complete the validated eight-item Functional Assessment of Chronic Illness Therapy—Treatment Satisfaction—General (FACIT-TS-G) survey [11].
Statistical methods
Assuming a dropout rate of 5%, a sample size of 1250 patients would provide a margin of error of <3% to estimate an endpoint of interest at month 12, providing adequate precision in the point estimation.
Rules and conventions to be used in the presentation and analysis of the study objectives as defined in the study protocol were prespecified in a statistical analysis plan. As the study will not be testing formal statistical hypotheses, no confirmatory testing is planned. Continuous variables are presented as mean and standard deviation (SD). Categorical variables are presented by numbers and percentages. P-values are calculated by Fisher's exact test or Pearson's chi-squared test.
RESULTS
Between 14 July 2022 and 15 December 2023, 1376 patients were screened and 1330 enrolled in the TRACK study from 93 sites in five countries: Germany, Italy, Spain, UK and USA. Cohort demographics and baseline characteristics, overall and by country, are summarized in Table 3. For 37% of participants, the qualifying episode of hyperkalaemia was their first. For patients qualifying with a recurrent episode of hyperkalaemia, the most recent episode occurred 5.6 months (mean) and 1.7 months (median) prior. Race and ethnicity were only reported for US participants, of whom 66% were White, 29% Black and 8% Hispanic/Latino. Participants were enrolled across the spectrum of CKD severity, with 7% in CKD stage 1 or 2 and 14%, 18% and 26% in stages 3a, 3b and 4, respectively. Among 387 participants (35%) with CKD stage 5, 282 (73%) were receiving chronic dialysis (96% haemodialysis, 4% peritoneal dialysis).
Table 3:
Baseline characteristics of the TRACK cohort by country.
| Characteristics | Overall (N = 1330) |
Germany (n = 230) |
Italy (n = 309) |
Spain (n = 259) |
UK (n =301) |
USA (n = 231) |
|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 67.7 (13.6) | 72.8 (12.3) | 69.3 (13.2) | 69.2 (11.4) | 62.0 (14.5) | 66.4 (13.8) |
| Missing, n | 1 | 1 | ||||
| Female, n (%) | 416 (31) | 74 (32) | 102 (33) | 84 (32) | 90 (30) | 66 (29) |
| Body mass index (kg/m2), mean (SD) | 28.3 (6.4) | 27.9 (5.8) | 26.2 (5.2) | 28.2 (5.6) | 29.0 (7.1) | 30.6 (7.3) |
| Missing | 42 | 2 | 12 | 5 | 22 | 1 |
| History of CKD/heart failure, n (%) | ||||||
| CKD | 736 (55) | 50 (22) | 170 (55) | 137 (53) | 225 (75) | 154 (67) |
| Heart failure | 83 (6) | 23 (10) | 19 (6) | 21 (8) | 13 (4) | 7 (3) |
| CKD and heart failure | 385 (29) | 87 (38) | 94 (30) | 96 (37) | 49 (16) | 59 (26) |
| Neither CKD nor heart failure | 122 (9) | 70 (30) | 22 (7) | 5 (2) | 14 (5) | 11 (5) |
| Missing | 4 | 4 | ||||
| Patients with CKD, n (%) | 1121 | 136 | 264 | 233 | 274 | 213 |
| Stage 1 or 2 | 73 (7) | 25 (18) | 12 (5) | 19 (8) | 8 (3) | 9 (4) |
| Stage 3a | 159 (14) | 33 (24) | 26 (10) | 33 (14) | 27 (10) | 40 (19) |
| Stage 3b | 205 (18) | 35 (26) | 41 (16) | 36 (16) | 31 (11) | 62 (29) |
| Stage 4 | 296 (26) | 27 (20) | 53 (20) | 86 (37) | 83 (30) | 47 (22) |
| Stage 5, not on chronic dialysis | 105 (9) | 0 | 38 (14) | 20 (8) | 48 (18) | 2 (1) |
| Stage 5, on chronic dialysis | 282 (25) | 16 | 94 (36) | 39 (15) | 77 (28) | 53 (25) |
| Missing | 1 | 1 | ||||
| Medications, n (%) | ||||||
| ACEi/ARB/ARNI | 764 (57) | 164 (71) | 148 (48) | 183 (71) | 150 (50) | 119 (52) |
| At target ACEi/ARB/ARNI dose | 532 (70) | 129 (79) | 96 (65) | 97 (53) | 120 (81) | 90 (76) |
| Missing | 1 | 1 | ||||
| MRA | 183 (14) | 41 (18) | 42 (14) | 68 (26) | 24 (8) | 8 (4) |
| At target MRA dose | 115 (63) | 34 (83) | 22 (52) | 39 (57) | 13 (54) | 7 (88) |
| [K+] (mmol/l), n (%) | ||||||
| ≤5.0 | 5 (0.4) | 1 (0.4) | 1 (0.3) | 1 (0.4) | 0 | 2 (1) |
| >5.0–≤5.5 | 809 (62) | 158 (70) | 191 (66) | 155 (61) | 153 (52) | 152 (66) |
| >5.5–≤6.0 | 361 (27) | 48 (21) | 72 (23) | 77 (30) | 106 (36) | 58 (25) |
| >6.0–≤6.5 | 83 (6) | 7 (3) | 19 (6) | 17 (7) | 28 (9) | 12 (5) |
| >6.5 | 43 (3) | 13 (6) | 8 (3) | 6 (2) | 10 (3) | 6 (3) |
| Missing | 29 | 3 | 18 | 3 | 4 | 1 |
Most participants (57%) were taking an angiotensin-converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB) or angiotensin receptor neprilysin inhibitor (ARNI) at baseline, although the proportion at target dose varied by country. Mineralocorticoid receptor antagonist (MRA) use was lower (14%) and potassium binder use infrequent [sodium zirconium cyclosilicate (SZC) 2%, patiromer 0.4%, sodium/calcium polystyrene sulfonate 1%]. Sodium-glucose co-transporter-2 inhibitor use was reported by 26% of participants at baseline.
Mean baseline [K+], assessed within 21 days of the index hyperkalaemia episode, was 5.5 mmol/l (SD 0.44) and ranged from 4 to 9 mmol/l. Mean baseline creatinine was 308 mmol/l (SD 278).
Healthcare providers
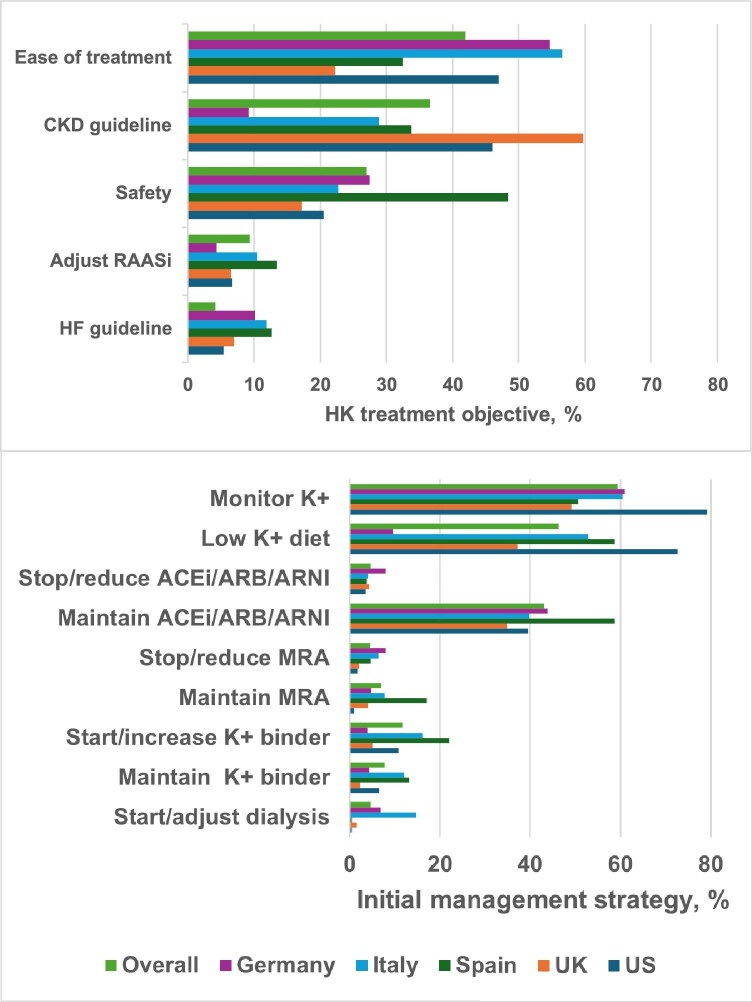
The most commonly reported hyperkalaemia treatment objectives were ease of treatment (42%) and CKD guideline adherence (37%), followed by safety, ACEi/ARB/ARNI adjustment and adherence to heart failure guidelines (Fig. 2). Variation by country was observed, with ease of treatment being the most common objective in Germany, Italy and the USA, while safety was the most common in Spain. The most frequently reported initial management strategy was [K+] monitoring (59% overall), followed by adoption of a low K+ diet (46%) and maintenance of ACEi/ARB/ARNI (43%). Discontinuation or dose reduction of ACEi/ARB/ARNI or MRA was infrequent, 5% for each. Country-specific strategies included higher K+ binder use in Spain, Italy and the USA; dialysis adjustment or initiation was more frequently reported in Italy.
Figure 2:
Hyperkalaemia treatment objectives and initial management strategy by country. The most common hyperkalaemia treatment objectives (upper panel) and initial management strategies (lower panel) are shown by country. Providers could record multiple responses for each participant.
Healthcare providers were invited to provide their specialty: 597 (45%) participants were managed by nephrologists, 237 (18%) by cardiologists and 90 (7%) by another specialist; 30% did not specify their specialty. Nephrologists were more likely than non-nephrologists to cite CKD guideline compliance as an objective, prescribe a low-K+ diet and manage K+ binder therapy (both P < .0001 versus non-nephrologists) and less likely to manage, i.e. prescribe, adjust dose, decide to maintain dose or discontinue RAASi therapy as an initial strategy (P < .0001; Table 4). Cardiologists were more likely than non-cardiologists to manage RAASi therapy as an initial strategy (P < .05 for ACEi/ARB/ARNI, P < .0001 for MRA) and less likely to manage K+ binder therapy (P < .05).
Table 4:
Hyperkalaemia treatment objective and initial management strategy by provider specialty.
| Characteristics | Nephrologists | Non-nephrologists | Cardiologists | Non-cardiologists |
|---|---|---|---|---|
| Participants with provider specialty reported, n | 597 | 327 | 237 | 687 |
| Treatment objective, n (%) | ||||
| Number with response | 553 | 313 | 227 | 639 |
| Ease of treatment | 196 (35) | 145 (46) | 77 (34) | 264 (41) |
| Safety | 170 (31) | 97 (31) | 92 (41) | 175 (27) |
| Heart failure guidelines | 18 (3) | 72 (23) | 66 (29) | 24 (4) |
| CKD guidelines | 285 (52) | 26 (8) | 14 (6) | 297 (47) |
| Adjust RAASi | 50 (9) | 36 (12) | 34 (15) | 52 (8) |
| Initial management strategy, n (%) | ||||
| Number with response | 588 | 327 | 237 | 678 |
| Low K+ diet | 400 (68)b | 71 (22) | 42 (18)b | 429 (63) |
| ACEi/ARB/ARNIa | 265 (45)b | 194 (59) | 42 (18)c | 429 (63) |
| Discontinued | 12 (5) | 8 (4) | 8 (6) | 12 (4) |
| Dose reduced | 8 (3) | 5 (3) | 5 (4) | 8 (2) |
| Dose maintained | 237 (88) | 170 (88) | 115 (83) | 292 (91) |
| Dose increased | 4 (2) | 6 (3) | 6 (4) | 4 (1) |
| Started | 7 (3) | 8 (4) | 7 (5) | 8 (2) |
| MRAa | 26 (4)b | 99 (30) | 90 (38)b | 35 (5) |
| Discontinued | 5 (19) | 17 (17) | 15 (17) | 7 (20) |
| Dose reduced | 2 (8) | 13 (13) | 12 (13) | 3 (9) |
| Dose maintained | 18 (69) | 65 (66) | 59 (66) | 24 (69) |
| Dose increased | 1 (4) | 1 (1) | 1 (1) | 1 (3) |
| Started | 0 | 5 (5) | 5 (6) | 0 |
| K+ bindera | 152 (26)b | 45 (14) | 39 (16)c | 158 (23) |
| Discontinued | 1 (0.7) | 0 | 0 | 1 (1) |
| Dose reduced | 1 (0.7) | 0 | 0 | 1 (1) |
| Dose maintained | 58 (38) | 10 (22) | 8 (21) | 60 (38) |
| Dose increased | 21 (14) | 2 (4) | 1 (3) | 22 (14) |
| Started | 71 (47) | 33 (73) | 30 (77) | 74 (47) |
| Dialysis (started, unscheduled, changed prescription) | 24 (4)b | 0 | 0b | 24 (4) |
Participants can be counted in more than one row. Not all response options are shown. P-value calculated by Fisher's exact test for dialysis comparisons or by Pearson's chi-squared test for other comparisons.
For medication dose change or discontinuation, n is the number of participants for whom a response is provided for the medication category. Denominator for calculating the percentage is the number of participants for whom any initial management strategy is provided.
P < .0001 versus non-nephrologists or non-cardiologists.
P < .05 versus non-cardiologists.
DISCUSSION
TRACK is a prospective, pragmatic, observational study designed to shed light on healthcare provider decision-making in patients with a diagnosis of hyperkalaemia, the rationale underpinning providers’ management choices and treatment expectations. By including providers from a variety of specialties in Europe and the USA, the study is expected to provide clinical insights across a broad spectrum of real-world practice settings. Another strength is its enrolment of subjects across the range of CKD, including patients on dialysis, which will shed light on important differences in hyperkalaemia management and provider attitudes across stages of CKD.
Prospective, contemporaneous longitudinal data on hyperkalaemia management decision-making are needed to support the implementation of evidence-based therapies by improving our understanding of providers’ treatment objectives, acute versus chronic management strategies and the effectiveness of these strategies. For example, identifying predictors of hyperkalaemia recurrence may facilitate targeted chronic use of newer K+ binders. Knowledge of the steps practitioners take to achieve or maintain optimal doses of RAASis [12–14] in different countries and across provider subspecialties can help professional societies and health systems shape treatment protocols and supportive messaging. Understanding patient perceptions about their treatment may identify barriers to lifestyle change and medication adherence [15].
Although hyperkalaemia management recommendations are widely available, reports of real-world management among boots-on-the-ground practitioners are limited. A retrospective analysis of data from the Veterans Affairs Corporate Data Warehouse (2016–2018) identified 288 patients with [K+] ≥5.1 mmol/l who started patiromer or sodium polystyrene sulfonate (SPS) and were not on chronic dialysis. SZC was not available during the study time frame. Of patients who started patiromer, 97%, 40% and 25% remained on therapy at 1, 3 and 6 months, respectively. Of those who started SPS, 25%, 5% and 2% remained on therapy at 1, 3 and 6 months, respectively. More than 70% of participants had [K+] <5.1 mmol/l up to 6 months post-index hyperkalaemia episode. At baseline, 32% were taking an RAASi; ≈80% continued their RAASi therapy during the 6-month follow-up period [16]. In this population, chronic therapy with a K+ binder lowered [K+] and a high proportion of RAASi users were able to continue RAASi therapy.
In a retrospective analysis of US claims data (2016–2017) that included patients with a diagnosis of hyperkalaemia who were treated with patiromer (n = 610), SPS (n = 5556) or no K+ binder (n = 21 282), RAASi use at the time of the index hyperkalaemia event was reported for 35%, 43% and 40% of patients in the respective groups [17]. During the 6 months following the index hyperkalaemia event, persistent RAASi utilization was numerically higher among patients with persistent patiromer use (patiromer 78%, SPS 57%, no K+ binder 57%).
In a retrospective, propensity-matched analysis of claims data from patients with an episode of hyperkalaemia taking RAASi medication in the USA (n = 582), Japan (n = 888) and Spain (n = 104), RAASi dose maintenance or up-titration was more frequent at 6 months post-index hyperkalaemia episode among those taking SZC compared with no K+ binder [18]. Odds ratios for maintaining RAASi therapy were 2.02 [95% confidence interval (CI) 1.65–2.46] in the USA, 3.14 (95% CI 2.58–3.82) in Japan and 2.83 (95% CI 1.46–5.46) in Spain for those treated with SZC relative to no K+ binder treatment.
As retrospective studies, these and similar reports do not provide insights into the thought processes underlying healthcare providers’ decision-making. As seen with our prospective data collection, considerable variation in hyperkalaemia management is apparent by country and provider specialty. As follow-up data become available, patient views of their health condition and hyperkalaemia treatment will provide additional insights not obtainable through retrospective analysis. Better understanding of provider and patient views is anticipated to inform health system, professional society and payer efforts to improve the quality of hyperkalaemia care.
The proposed study design has some limitations. Selection of healthcare providers interested in a hyperkalaemia study may introduce bias; the study sought to include a variety of specialties and types of clinical practices to mitigate this risk. Healthcare providers were not required to report their specialty and 30% chose not to do so. The by-specialty differences observed in treatment objectives and management strategies should be interpreted in light of the missing data. Nonetheless, these findings may help professional societies to tailor messages encouraging their members to adopt evidence-based therapies.
Uptake of guideline-directed medical treatment into clinical practice is generally slow [19, 20] and hyperkalaemia management is no exception. Adoption of evidence-based therapies will potentially reduce hyperkalaemia recurrence with associated health risks and resource utilization and facilitate maintenance of optimal RAASi dosing with its established benefits. Understanding the thought processes underpinning providers’ management decisions is a necessary prerequisite for identifying and implementing measures to improve the care of patients with hyperkalaemia.
Supplementary Material
ACKNOWLEDGEMENTS
Ethics committees/institutional review boards are listed in the supplementary material.
Contributor Information
Judith Hsia, CPC Clinical Research, University of Colorado, Aurora, CO, USA.
Nitin Shivappa, AstraZeneca, Wilmington, DE, USA.
Ameet Bakhai, Royal Free NHS Hospital, London, UK.
Jordi Bover, Hospital Germans Trias i Pujol, Badalona, Spain.
Javed Butler, Baylor Scott & White Research Institute, Dallas, TX, and University of Mississippi, Jackson, MI, USA.
Pietro Manuel Ferraro, Università degli Studi di Verona, Verona, Italy.
Linda Fried, Pittsburgh Healthcare System, Pittsburgh, PA, USA.
Markus P Schneider, University of Erlangen-Nürnberg, Erlangen, Germany.
Navdeep Tangri, University of Manitoba, Winnipeg, MB, Canada.
Wolfgang C Winkelmayer, Baylor College of Medicine, Houston, TX, USA.
Meredith Bishop, AstraZeneca, Gaithersburg, MD, USA.
Hungta Chen, AstraZeneca, Wilmington, DE, USA.
Anna-Karin Sundin, AstraZeneca, Gothenburg, Sweden.
Marc P Bonaca, CPC Clinical Research, University of Colorado, Aurora, CO, USA.
FUNDING
TRACK is funded by AstraZeneca.
AUTHORS’ CONTRIBUTIONS
NT, MB, AKS, JH contributed to design of the study; HC oversaw data analysis; JH drafted the manuscript; all co-authors provided editorial review and final approval of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to privacy laws, such as the General Data Protection Regulation.
CONFLICT OF INTEREST STATEMENT
J.H. and M.P.B. receive salary support from CPC, a non-profit academic research organization affiliated with the University of Colorado, which receives research grant/consulting funding from Agios Pharmaceuticals, Alexion Pharma Good Kaisha, Amgen, Anthos Therapeutics, ARCA Biopharma, AstraZeneca Pharma India, AstraZeneca Pharmaceuticals, AstraZeneca UK, AstraZeneca, Produtos Farmaceuticos, Atentiv, Bayer, Bayer (Proprietary) Limited, Bayer Aktiengesellschaft, Bayer Pharma, Beth Israel Deaconess Medical Center, Better Therapeutics, Bionest Partners, Boston Clinical Research Institute, BMS, CellResearch, Cleerly, Colorado Department of Public Health and Environment, Cook Regentec, CSL Behring, Eidos Therapeutics, EPG Communication Holdings, Esperion Therapeutics, Faraday Pharmaceuticals, HeartFlow, Hummingbird Bioscience, Insmed, Ionis Pharmaceuticals, IQVIA, Janssen Pharmaceuticals, Janssen Research & Development, Janssen Scientific Affairs, Lexicon Pharmaceuticals, LSG, MedImmune, Medpace, Medscape, Merck Sharp & Dohme, Northwell Health, Novartis Pharmaceuticals, Novo Nordisk, Osiris Therapeutics, Pfizer, PPD Development, Prothena Biosciences, Regeneron, Regents of the University of Colorado, Sanifit Therapeutics, Sanofi, Silence Therapeutics, Stanford University, Stealth BioTherapeutics, Brigham & Women's Hospital, Thrombosis Research Institute, UCD iC42 Lab, University of Colorado Denver, University of Pittsburgh, VarmX and WraSer. J.H. also reports owning AstraZeneca stock. N.S., M.B., H.C. and A.-K.S. own AstraZeneca stock. A.B. has received consultant fees and grant/other support from Abbott, Association of the British Pharmaceutical Industry, Accentus Medical, Amgen, Amore Health, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Closer Still Media, Daiichi Sankyo, Health Smart, Janssen, Lattice Point, Lilly, Medtronic, McKinsey, MSD, Napp, Novartis, Novo Nordisk, Pfizer, Remedica, Sanofi Aventis and AirEmail. J.Bover received advisory and/or lecture fees and/or congress travel expenses from AbbVie, Amgen, AstraZeneca, Bayer, CSL-Vifor, GSK, Menarini, Rubió, Sanofi and Theramex. J.Butler has received consultant fees from Abbott, American Regent, Amgen, Applied Therapeutic, AskBio, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, BMS, Cardiac Dimension, Cardiocell, Cardior, CSL Behring, CVRx, Cytokinetics, Daxor, Edwards, Element Science, Faraday, Foundry, G3P, Innolife, Impulse Dynamics, Imbria, Inventiva, Ionis, Levator, Lexicon, Lilly, LivaNova, Janssen, Medtronics, Merck, Occlutech, Owkin, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Prolaio, Pulnovo, Regeneron, Renibus, Roche, Salamandra, Salubris, Sanofi, SC Pharma, Secretome, Sequana, SQ Innovation, Tenex, Tricog, Ultromics, Vifor and Zoll. P.M.F. has received consultant fees and grant/other support from Allena Pharmaceuticals, Alnylam, Amgen, AstraZeneca, Bayer, Gilead, Novo Nordisk, Otsuka Pharmaceuticals, Rocchetta and Vifor Fresenius and royalties as an author for UpToDate. L.F. serves on data safety monitoring boards for Novo Nordisk and Regeneron and owns Amgen stock. M.P.S. has received advisory board fees and honoraria from AstraZeneca, Bayer, Vifor Pharma Group and Boehringer Ingelheim/Lilly. N.T. has received grants/research support from the Canadian Institutes of Health Research, National Institutes of Health, Kidney Foundation of Canada, Bayer, AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceuticals, Research Manitoba, Otsuka Pharmaceutical, Tricida and Lilly; honoraria or consultation fees from AstraZeneca, Bayer, Boehringer Ingelheim, GSK, Janssen Pharmaceuticals, Otsuka Pharmaceutical, Prokidney, Roche, Tricida and Lilly; and owns stock in ClinPredict, Klinrisk, Quanta, Marizyme, Mesentech, Renibus Therapeutics, PulseData and Tricida. W.C.W. has received consultant fees from Akebia, Anthos, Ardelyx, AstraZeneca, Bayer Boehringer Ingelheim, Cadrenal, GSK, Merck, Natera, Novartis, Pharmacosmos, Unicycive, Vera and Zydus.
REFERENCES
- 1. Humphrey T, Davids MR, Chothia MY et al. How common is hyperkalaemia? A systematic review and meta-analysis of the prevalence and incidence of hyperkalaemia reported in observational studies. Clin Kidney J 2022;15:727–37. 10.1093/ckj/sfab243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mclean A, Nath M, Sawhney S. Population epidemiology of hyperkalemia: cardiac and kidney long-term health outcomes. Am J Kidney Dis 2022;79:527–538.e1. 10.1053/j.ajkd.2021.07.008 [DOI] [PubMed] [Google Scholar]
- 3. Betts KA, Woolley JM, Mu F et al. The cost of hyperkalemia in the United States. Kidney Int Rep 2018;3:385–93. 10.1016/j.ekir.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haas JS, Krinke KS, Maas C et al. The burden of hyperkalemia in Germany—a real world evidence study assessing the treatment and costs of hyperkalemia. BMC Nephrol 2020;21:332. 10.1186/s12882-020-01942-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grandy S, Jackson J, Moon R et al. Health-related quality of life and lifestyle changes in patients with chronic kidney disease and hyperkalaemia: real-world data from the US, five European countries and China. Int J Clin Pract 2021;75:e14326. 10.1111/ijcp.14326 [DOI] [PubMed] [Google Scholar]
- 6. Rowan CG, Agiro A, Chan KA et al. Hyperkalemia recurrence following medical nutrition therapy in patients with stage 3-4 chronic kidney disease: the REVOLUTIONIZE I real-world study. Adv Ther 2024;41:2381–98. 10.1007/s12325-024-02835-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burton JO, Coats AJS, Kovesdy CP et al. An international Delphi consensus regarding best practice recommendations for hyperkalaemia across the cardiorenal spectrum. Eur J Heart Fail 2022;24:1467–77. 10.1002/ejhf.2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes CKD Work Group . KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024;105(4 Suppl):S117–S314. [DOI] [PubMed] [Google Scholar]
- 9. Alfonzo A, Harrison A, Baines R et al. Clinical practice guidelines. Treatment of acute hyperkalaemia in adults. https://ukkidney.org/sites/renal.org/files/FINAL%20VERSION%20-%20UKKA%20CLINICAL%20PRACTICE%20GUIDELINE%20-%20MANAGEMENT%20OF%20HYPERKALAEMIA%20IN%20ADULTS%20-%20191223_0.pdf (accessed 8 August 2024).
- 10. Inker LA, Eneanya ND, Coresh J et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FACIT Group . FACIT-TS-G. Functional Assessment of Chronic Illness Therapy – Treatment Satisfaction – General. https://www.facit.org/measures/facit-ts-g (accessed 8 August 2024).
- 12. Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. https://www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec (accessed 8 August 2024). [Google Scholar]
- 13. Bolaños JA, Seliger SL. Recurrent hyperkalaemia in renin-angiotensin-aldosterone system inhibitor (RAASi) treatment: stuck between a rock and a hard place. Clin J Am Soc Nephrol 2021;16:345–7. 10.2215/CJN.00950121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis J, Israni R, Betts KA et al. Real-world management of hyperkalaemia in the emergency department: an electronic medical record analysis. Adv Ther 2022;39:1033–44. 10.1007/s12325-021-02017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Da Silva Menolli PV, Brummel AMI, Guidoni CM et al. Patient's perception of medication and non-adherence to chronic disease treatments. A population-based study in Brazil. J Pharm Health Serv Res 2024;15:rmae001. 10.1093/jphsr/rmae001 [DOI] [Google Scholar]
- 16. Kovesdy CP, Gosmanova EO, Woods SD et al. Real-world management of hyperkalemia with patiromer among United States veterans. Postgrad Med 2020;132:176–83. 10.1080/00325481.2019.1706920 [DOI] [PubMed] [Google Scholar]
- 17. Desai NR, Rowan CG, Alvarez PJ et al. Hyperkalemia treatment modalities: a descriptive observational study focused on medication and healthcare resource utilization. PLoS One 2020;15:e0226844. 10.1371/journal.pone.0226844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rastogi A, Pollack CV, Lázaro IJS et al. Maintained renin–angiotensin–aldosterone system inhibitor therapy with sodium zirconium cyclosilicate following a hyperkalaemia episode: a multicountry cohort study. Clin Kidney J 2024;17:sfae083. 10.1093/ckj/sfae083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nicholas SB, Daratha KB, Alicic RZ et al. Prescription of guideline-directed medical therapies in patients with diabetes and chronic kidney disease from the CURE-CKD Registry, 2019–2020. Diabetes Obes Metab 2023;25:2970–9. 10.1111/dom.15194 [DOI] [PubMed] [Google Scholar]
- 20. Shahid I, Khan MS, Fonarow GC et al. Bridging gaps and optimizing implementation of guideline-directed medical therapy for heart failure. Prog Cardiovasc Dis 2024;82:61–9. 10.1016/j.pcad.2024.01.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to privacy laws, such as the General Data Protection Regulation.