Abstract
ABSTRACT
Background
Patient research partners (PRPs) are people with a disease who collaborate in a research team as partners. The aim of this systematic literature review (SLR) was to assess barriers and facilitators to PRP involvement in rheumatology research.
Methods
The SLR was conducted in PubMed/Medline for articles on PRP involvement in rheumatology research, published between 2017 and 2023; websites were also searched in rheumatology and other specialties. Data were extracted regarding the definition of PRPs, their role and added value, as well as barriers and facilitators to PRP involvement. The quality of the articles was assessed. Quantitative data were analysed descriptively, and principles of thematic content analysis was applied to qualitative data.
Results
Of 1016 publications, 53 articles were included; the majority of these studies were qualitative studies (26%), opinion articles (21%), meeting reports (17%) and mixed-methods studies (11%). Roles of PRPs ranged from research partners to patient advocates, advisors and patient reviewers. PRPs were reported/advised to be involved early in the project (32% of articles) and in all research phases (30%), from the conception stage to the implementation of research findings. The main barriers were challenges in communication and support for both PRPs and researchers. Facilitators of PRP involvement included more than one PRP per project, training of PRPs and researchers, a supportive environment for PRPs (including adequate communication, acknowledgement and compensation of PRPs) and the presence of a PRP coordinator.
Conclusion
This SLR identified barriers and facilitators to PRP involvement, and was key to updating the European Alliance of Associations for Rheumatology recommendations for PRP–researcher collaboration based on scientific evidence.
Keywords: Health services research; Health-Related Quality Of Life; Outcome and Process Assessment, Health Care; Quality Indicators, Health Care
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patient research partners (PRPs) are increasingly integrated into medical research, particularly in rheumatology.
Major global health organisations recognise the central role of PRPs’ involvement in research.
Previous recommendations have guided researchers and PRPs to build collaborative relations but lack a strong evidence base.
WHAT THIS STUDY ADDS
This systematic literature review provides for the first time a comprehensive overview of the emerging role of PRPs in rheumatology research, emphasising their expanding roles, contributions and the value they bring to the research process.
The review identified key barriers to PRP involvement, ranging from personal factors to challenges in training, communication and collaboration, and also identified strategies to enhance PRP involvement.
Early and sustained involvement of PRPs, as well as a supportive environment and effective communication, were found to be essential to enhance the relevance and impact of PRP contribution to research.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Recognising and addressing the barriers to PRP involvement can lead to better support for PRPs, enhancing their involvement in research.
Some facilitators identified include involvement of PRPs since the early stages of research, a supportive environment for PRPs and encouraging researchers to adopt more flexible strategies and behaviours to maximise the benefit of PRP involvement.
This literature review informed European Alliance of Associations for Rheumatology recommendations, highlighting the importance of active collaboration, training, mutual respect, and transparent communication between PRPs and researchers.
Introduction
Patient research partners (PRPs) are described as individuals living with a health condition who ‘provide input to research, through active collaboration as equal partners with researchers’.1 Their involvement is essential to make research more patient centred, for instance, by capturing outcomes that matter to patients. Over the past two decades, the magnitude of PRP involvement and their roles in research has grown substantially.2,8 Patients have transitioned from passive subjects to active collaborators and equal partners, bringing their unique perspectives and valuable insights to the forefront of medical research.5 This change has not only profoundly modified research practices but has also underscored the integral role PRPs play in shaping the future of medical practice.9 The importance of PRP involvement in research has become widely recognised as an essential component of high-quality patient care, highlighted by organisations such as the WHO4 and European Medicine Agency (EMA),10 and is acknowledged across various medical specialties.11,13
In rheumatology, this paradigm shift has been significant. In 2011, the European Alliance of Associations for Rheumatology (EULAR) developed recommendations for the involvement of patient representatives in scientific projects based on expert opinion.14 These recommendations marked a pivotal step, setting the stage for the involvement of PRPs in research projects. Since then, these EULAR recommendations have guided other organisations such as Outcome Measures in Rheumatology (OMERACT), Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Foundation for Research in Rheumatology (FOREUM), to recognise the important role of PRPs or to develop their own guidelines for collaborative research.15,18
As the landscape of patient involvement in research evolves, the literature has witnessed a great surge in data and studies concerning PRP involvement.24 5 15 19,25 These studies not only shed light on the benefits of PRP participation but also highlighted the challenges encountered in this collaborative effort and solutions proposed to overcome barriers.2122 25,29
In 2022, EULAR decided to update the 2011 recommendations for PRP involvement in research, focusing specifically on PRPs in the context of chronic conditions.14 In accordance with the EULAR standardised operational procedures (SOPs) supporting this update, we conducted a systematic literature review (SLR) to inform the EULAR taskforce.
Methods
To support the update of the EULAR recommendations, we conducted in 2023 an SLR that encompassed both qualitative and semiquantitative analyses of recent publications in rheumatology, with the goal of identifying factors that affect PRP involvement, including barriers and facilitators.
Literature search
The SLR aimed to identify publications reporting PRP involvement in rheumatology research published between 1 January 2017 and 1 January 2023. We searched the electronic database PubMed MEDLINE using the terms “patient research partner”, “patient expert”, “patient and public involvement (PPI)”, their synonyms and related concepts. Details of the search terms and search strategy can be found in online supplemental table 1. Two authors (KA, LG) independently assessed the title, abstract and keywords of every publication identified. In the event of disagreement between the reviewers, disparities were discussed and resolved. Additionally, we performed a scoping review of databases to assess PRP involvement and explored six websites from rheumatology: OMERACT, GRAPPA, American College of Rheumatology, EULAR, FOREUM and Osteoarthritis Research Society International. We also searched 2 regulator websites: Food and Drug Administration and EMA, and 10 websites of three selected specialties recognised for significant PRP involvement: cardiology, oncology, endocrinology (diabetes) (online supplemental table 2). A specific search was done in two websites focusing on patient and public involvement: INVOLVE UK by the National Institute for Health Research and Education that empowers (European Patients’ Academy on Therapeutic Innovation), and in orphan diseases to answer specific research questions about training, involvement in grant applications and remuneration of PRPs (online supplemental table 3).
The scope of the literature search was defined by the EULAR taskforce steering group,1 and addressed 11 specific research questions (Box 1).
Box 1. Research topics included in the systematic literature review.
1. Definition of patient research partners (PRPs)
How to define a PRP? Is the current definition of PRPs still adequate?
2. Roles and activities undertaken by PRPs
What are the roles and activities of PRPs in rheumatic musculoskeletal disease research?
3. Benefits and added value of PRP involvement for PRPs themselves, researchers, the research itself
What is the added value of PRPs in different types of research and groups?
4. Types of scientific projects that involved PRPs and the stages of the projects in which they participated
What types of projects are (or should) PRPs (be) involved in?
What phases of a project are (or should) PRPs (be) involved in?
5. Selection and recruitment processes for PRPs
How are (or should) PRPs (be) recruited and selected?
How many PRPs are (or should be) involved in the research?
6. Insights into the experiences and feedback provided by PRPs
What are the PRP feedback and experiences, in terms of facilitators and barriers to PRP involvement?
How can we improve the PRP experience and involvement overall?
7. Roles of a coordinator for PRPs in research
Are PRP facilitators involved, if so how, and is it useful?
Is a facilitator/PRP coordinator recommended?
What is the reported usefulness of a facilitator ?
8. Training provided to PRPs or researchers
Do the PRPs involved have a specific training (previously/during the study)?
How should researchers be educated, trained, supported to enhance PRP involvement?
9. Evaluation and monitoring related to PRP involvement
How should PRP involvement be monitored or evaluated? At which time points and by whom?
How should PRP involvement evaluation/monitoring be reported?
10. Recognition, compensation and acknowledgement of PRPs during their involvement in a scientific project
How should PRP involvement be recognised and acknowledged?
Is (should) compensation (be) proposed?
11. Barriers encountered and proposed solutions to enhance PRP involvement
What are the barriers encountered during PRP involvement?
Which strategies and contextual factors enable optimal engagement of PRPs?
Inclusion and exclusion criteria
We included all types of articles reporting PRP involvement in all types of research, including trials and observational studies, qualitative studies, mixed-methods studies and reports of meetings, opinion papers and reviews. We did not exclude published articles from any country, aiming to enhance the generalisability of our findings. Recommendations and guidelines on PRPs were also analysed and were used as supportive information. Articles not focused on rheumatology research or not bringing any information on PRPs (ie, not answering one or more research questions), as well as not in English, were excluded. Articles only mentioning PRPs or their involvement, without providing any details (eg, on their roles, contributions or barriers/facilitators), were excluded as well. Articles with duplicate information (ie, multiple publications reporting on a single study) were excluded if they did not provide additional information relevant to our research questions.
We also identified relevant articles by hand search of the references cited in the included studies, extending the inclusion period to the date of publication of the previous recommendations (2011–2023).
Data extraction
Data collection encompassed both quantitative and qualitative data, addressing various aspects of PRP involvement and providing answers to our research questions (Box 1). Data were extracted and checked independently by two authors (KA and MdW). Discrepancies were resolved by discussion among the core team (KA, MdW, PS, LG).
Quality assessment
Papers were assessed for quality only if they reported original data. Review papers, recommendation papers, opinion papers, case studies, study protocols, report papers and qualitative studies not primarily focused on PRPs were excluded from quality assessment. Given the diversity of study types, we used the Critical Appraisal Skills Programme (CASP) checklist for qualitative studies, literature reviews and cross-sectional studies as described in the EULAR SOP.30 31 This tool, originally developed for qualitative studies, assesses elements such as the clarity of research aims, appropriateness of methodology, suitability of the research design, adequacy of data collection and clarity in reporting outcomes. For mixed-methods studies, we used the Mixed Methods Appraisal Tool (MMAT, a critical appraisal tool that is designed for the appraisal stage of systematic mixed-studies reviews, that is, reviews that include qualitative, quantitative and mixed-methods studies32 (see online supplemental tables 4 and 5 for quality assessment). To facilitate interpretation, an overall quality assessment for the level of evidence (LoE) was conducted by evaluating the number of items on the score checklist and on the key items. Subsequently, the authors reached a consensus on classification of the articles’ quality as high, medium or low quality.
This SLR was not considered appropriate by PROSPERO for registration due to the mixed-methods study analyses involved.
Patient and public involvement
This SLR study is the result of a co-production of three PRPs (MdW, CZ, HB) and five researchers, all being members of the EULAR steering committee responsible for updating the EULAR recommendations on PRP involvement.1 The three PRPs actively contributed to all meetings and discussions within the steering committee. They were involved at the early stage of formulating the research questions until reviewing and agreeing on the final manuscript. They were also actively engaged in planning dissemination and implementation of the study findings within the wider community and patient associations. The recruitment of the PRPs was coordinated by one of the PRPs (MdW), the convenor of the project.
Analysis
For quantitative data, a descriptive analysis of findings is reported, including characteristics of studies (study design, population, country, study objectives), characteristics of PRPs, selection process of PRPs, type of involvement, phases of the research where their involvement occurred, with numbers and percentages using frequency tables and charts.
The number of PRPs involved in the studies was quantified using two distinct methods: first, coauthorship count: direct examination of the research articles’ authorship lists. PRPs were identified based on explicit mentioning of their role as ‘PRP’ or other specific identification. Second, participation count: this approach assessed the number of broader involvements of PRPs in activities of the research project. For instance, in a GRAPPA meeting report, the number of PRPs who actively participated was counted.8
Qualitative data were analysed according to the principles of thematic content analysis (more details in online supplemental table 6).33 The results were discussed within the EULAR taskforce,1 and any disagreements on the interpretation of the findings were resolved by a consensus of the core group (MdW, LG, PS, KA).
Results
Search strategy
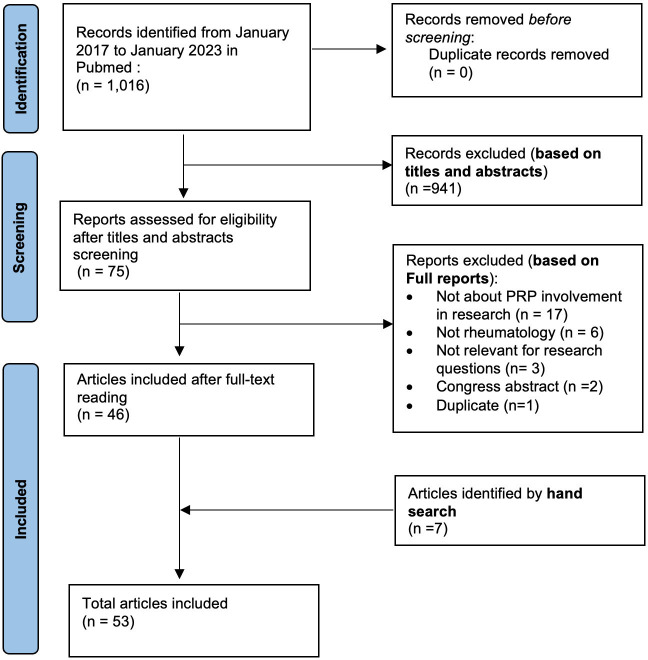
The SLR yielded a total of 1016 records of which 941 (92.6%) were excluded based on titles and abstracts. We conducted a full-text screening of 75 papers and 46 (61.3%) were included. The main reasons for exclusion were papers not related to rheumatology, lacking reports of PRP involvement in research, being irrelevant to our research questions, or being duplicates or conference abstracts (figure 1). Additionally, 7 papers were identified by hand search, resulting in a total number of 53 included articles.
Figure 1. Flow chart of selected article search on PRP involvement in rheumatology research. PRP, patient research partner.
Quality assessment (LoE) of the papers
Nineteen articles were assessed for quality using the predefined scores according to the study type. Overall, 79% (15 of 19) were classified as high quality, 11% (2 of 19) as medium quality and 11% as low quality (online supplemental table 4).
Study characteristics
The included studies were qualitative studies (14 of 53, 26%), opinion articles (11 of 53, 21%), meeting reports (9 of 53, 17%), mixed-methods studies (6 of 53, 11%), recommendation articles (4 of 53, 8%), reviews (SLR or scoping review; 3 of 53, 6%), cross-sectional (2 of 53, 4%), case studies (2 of 53, 4%), observational (1 of 53, 2%) and study protocol (1 of 53, 2%) (online supplemental tables 4 and 5).
Overall, 62% were published in rheumatology journals. Geographically, most of the studies were from Europe (50%), followed by North America (31%).
Identification of barriers encountered and proposed solutions to enhance PRP involvement
Barriers to PRP involvement (table 1 and online supplemental table 7) included emotional and personal factors, communication and relationship challenges, inadequate training and support, difficulties in the research process and pace, as well as collaboration and engagement issues.2,421 22 24 Effective strategies to enhance PRP involvement (table 1) included early involvement, a supportive environment, effective communication and trust, and providing support and training for PRPs and researchers.7 21 22 26 29 38 40 43 44
Table 1. Barriers and strategies to enhance PRP involvement in rheumatology research.
| Concept | Barriers | Strategies to enhance patient involvement |
| Emotional and personal factors |
|
|
| Communication and relationship |
|
|
| Training and support |
|
|
| Research process and pace |
|
|
| Collaboration and engagement |
|
|
PRPpatient research partner
Definitions of PRP
Among the 53 included papers, 62% provided a definition of PRP. Importantly, a significant portion (30%) of these papers46 15 26 27 34,36 45 46 adopted the 2011 EULAR definition of PRP as ‘persons with a relevant disease who operate as active research team members on an equal basis with professional researchers, adding the benefit of their experiential knowledge to any phase of the project’.14 These papers consistently emphasised the importance of active involvement and fostering equal partnerships between PRP and researchers.
Additionally, seven papers (13%) expanded upon this definition by incorporating informal caregivers into the PRP definition,2028 37 38 47,49 known as persons, usually family members, who provide unpaid care to someone with whom they share a personal relationship.
The roles and activities of PRPs
The roles and activities of PRPs covered a wide spectrum, extending from research partners to patient advocates, advisory roles and participation as patient reviewers (as detailed in table 2 and online supplemental table 8). Their contributions encompassed a diverse range of activities, including providing input in guideline development, shaping research agendas, and actively advocating in scientific and clinical committees.
Table 2. Activities and roles of PRPs.
| Areas of involvement | Activities and roles of PRPs |
| Development/design |
|
| Leadership |
|
| Co-leadership role | |
| Coauthorship | |
| Education | |
| Planning | |
| Facilitation | |
| Reviewer | |
| Recruitment | |
| Evaluation |
|
| Participation |
EULAREuropean Alliance of Associations for RheumatologyFOREUMFoundation for Research in RheumatologyGRAPPAGroup for Research and Assessment of Psoriasis and Psoriatic ArthritisPROpatient-reported outcomePRPspatient research partners
The added value of PRP involvement
The literature reported that PRPs added significant value across various aspects of research (table 3). Specifically, 53% of the articles indicated that PRP involvement brought benefits for the PRP themselves, that is, better understanding of their medical condition, acquisition of practical skills, improved comprehension of the research process and increased self-confidence.2 21 25 36 39 Furthermore, 26% of the articles highlighted advantages for the research process, that is, heightened relevance of the research, enhancement of its overall impact and enrichment of the results by adding experiential knowledge.2 7 21 25 29 36 38 39 45 The positive impact on researchers, reported in 15% of the articles, encompassed deeper insights into research priorities, increased motivation, innovative ideas, awareness of the impact of their work, a comprehensive approach to addressing patients’ needs and improved communication in lay language (table 3).2 21 25 34 36 38 40 The added value of PRP involvement was also reported as advantageous for the wider community by enhancing the acceptance of research that prioritises community benefits.2 21 25 36
Table 3. Articles reporting on added value of PRP involvement in research for PRPs, for researchers and for the research.
| Added value for: | Percentage of articles | Number of articles (N=53) | |
| PRPs | Better knowledge of disease | 30 | 8 |
| Better knowledge of research | 19 | 5 | |
| Acquisition of practical skills | 15 | 4 | |
| Confidence | 15 | 4 | |
| All | Total: 51 | 27 | |
| Researchers | Better understanding of research priorities and needs | 19 | 7 |
| Increased motivation and focus | 9 | 3 | |
| Gain of novel perspectives and ideas | 9 | 3 | |
| Real-life implication of their work | 9 | 4 | |
| Attaining a more holistic view of patients with RMD | 9 | 3 | |
| Better use of lay language | 6 | 2 | |
| All | Total: 15 | 8 | |
| Research | Enhancement of the relevance of research | 50 | 7 |
| Improve the impact of the research | 21 | 3 | |
| Bring experiential knowledge to research | 21 | 3 | |
| All | Total: 26 | 14 |
PRPpatient research partnerRMD, rheumatic musculoskeletal disease
Types of research that involved PRPs
PRPs were actively involved in a wide range of scientific projects, including basic, translational and clinical research.50 Although the benefits of PRP involvement were less apparent in basic and translational research, some researchers and PRPs recognised the substantial advantages of collaborative partnerships in this area.3 25 34 A scoping review highlighted the benefits of PRP engagement in preclinical research, including enhanced understanding of basic science research for PRPs, broadened perspectives for researchers, and positive influence on study questions and methods, along with fostering mutual learning, new collaborations, and improved research quality and efficiency.40 One study reported that researchers were committed to finding more meaningful ways to integrate PRPs into basic scientific research and dissemination of the project results.3 Strategies to enhance PRP involvement (ie, training, support, PRP-focused tasks) were also reported.3
Research phases in which PRP participated
Early involvement of PRPs in the research was reported or recommended in 32% of the included articles, emphasising engaging PRPs from the inception of a research project.219,22 27 This early engagement was reported to enable PRPs to actively shape research questions and methodologies in line with their priorities. Additionally, 30% of the articles stressed the importance of PRPs’ continuous participation throughout all research stages (table 4).415 21 22 26 35 43 52,54
Table 4. Articles reporting or recommending PRP involvement in different phases of the research project.
| Phases of the research project | Number of articles (n) reporting or recommending PRP involvementn (%)(n total=53) |
| Conception of the study and research question | 9 (47) |
| Study design and planning | 9 (47) |
| Patient inclusion in the study | 9 (47) |
| Data collection | 8 (45) |
| Data analysis | 8 (45) |
| Data interpretation | 11 (51) |
| Dissemination | 15 (58) |
| Implementation | 4 (38) |
| Evaluation | 3 (6) |
| All phases | 16 (32) |
PRPpatient research partner
Number of PRPs
The number of PRPs involved in research is shown in online supplemental figure 1. When considering the coauthorship lists, the majority of articles clearly specified the name and identity of PRPs; subsequently, the number of PRPs involved in the writing and reviewing of the article could be easily deducted. Yet, in 19% of cases, the identification of a coauthor as a PRP was unclear. In cases where PRP involvement was explicitly highlighted by coauthorship, 34% of the articles included one or two PRPs per project, 17% of articles included three or four PRPs, and 25% of articles involved more than five PRPs. Notably, single-centre studies commonly involved one or two PRPs as coauthors. One study, which engaged four PRPs, found this number to be beneficial due to the diverse perspectives they brought.45 Larger-scale international consortia projects recruited a higher number of PRPs, with around six PRPs being identified as an effective group size for facilitating participation and decision-making.2
On the other hand, when reporting all PRP involvement and activities in a research project, 36% of the articles reported a number of PRP higher than nine (online supplemental figure 1). Therefore, the number of PRPs involved in research can be higher than the number of PRPs mentioned as coauthors.
Selection and recruitment processes for PRPs
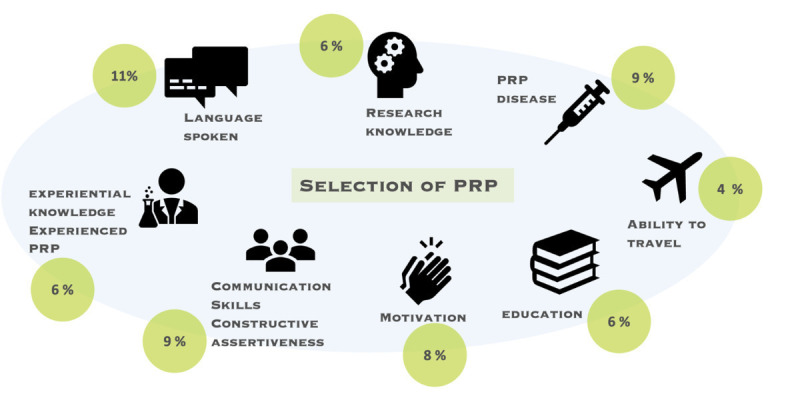
The selection process of PRPs was reported in 34% of articles (figure 2). PRP selection criteria were mainly language proficiency (11%), research knowledge (6%), disease diagnosis (9%), communication skills and constructive assertiveness (9%), motivation (8%), educational background (6%), experiential knowledge and expertise (6%) as well as travel capability (4%).23 15 19 21 23 24 27 34 35 55,58 Recruitment methods for PRPs were diverse, relying on patient organisations, marketing companies, rheumatology associations, social media, community outreach, clinic visits, personal connections with patients or researchers, word-of-mouth referrals and volunteering.2 21 34 38 41 44 53 59 Furthermore, 28% of studies emphasised the importance of clarifying patient roles through clear goal-setting and exchanging mutual expectations early in the project initiation phase.1519,21 27 29 36 42 45 47 Additionally, 28% of studies highlighted the need for inclusivity and diverse representation in PRP recruitment.2 4 15 35 41 42 52
Figure 2. The selection criteria of PRPs reported in the studies. PRP, patient research partner.
Creating a supportive environment for PRPs
A supportive environment for PRPs was reported to depend on several key principles (table 1)4 19 20 25 36 42 52 53 60: ensuring a balanced and manageable workload that respects PRP abilities, providing adequate resources and time for PRP involvement, offering support to overcome language barriers, promoting flexibility and offering accessible accommodation to participate in meetings and scientific conferences.7 21 38 40 43 44 Equal relationships and co-leadership between PRPs and researchers were cited in several papers as crucial, emphasising mutual respect, trust, and open, transparent communication.7 15 19 Building strong team communication, and establishing informal personal relationships between PRPs and researchers were also found to be important factors to enhance collaboration.20 38 47 Regular feedback and discussions about the quality of collaboration, combined with ongoing adjustments to meet the needs and preferences of PRPs, were proposed in two papers.34 45
Roles of a PRP coordinator
A PRP ‘coordinator’ was defined in some papers, as an individual or a role within a research team responsible for facilitating and supporting the collaboration between researchers and PRPs.2 20 25 47 61 The presence of a PRP coordinator was reported or advised in 29% of the included articles.2 3 19 28 34 35 40 42 44 48 61 PRP coordinators were reported to be helpful in facilitating effective communication among PRPs, researchers and stakeholders, aligning expectations, organising logistics, moderating group discussions, providing ongoing education and support, and assisting in the recruitment and selection of PRPs in projects (table 5).2 20 25 35 36 42 47 This role was reported to be taken by a member of the research team, a PRP or a designated person within a patient organisation or academic institution.2 38
Table 5. Potential roles of a PRP coordinator.
| Competencies | Roles of PRP coordinator |
| Communication |
|
| Project coordination and expectation alignment |
|
| Facilitate research discussions |
|
| Continuity and education |
|
| Selection of PRP |
|
PRPpatient research partner
Training of researchers
We found that 34% of the included articles included in the SLR reported or advised training or education of researchers.47 19 21 25 28 29 38,40 44 Researchers could receive training concerning various aspects of working with PRPs (table 6).
Table 6. Reported training content for researchers and PRPs.
| Soft skills | Training content for researchers | Training content for PRPs |
| General training |
|
|
| Communication skills training |
|
|
| Education |
|
|
| Resources |
|
|
| Confidence building |
|
– |
| Engagement of PRPs |
|
– |
CARRAChildhood Arthritis and Rheumatology Research AllianceEULAREuropean Alliance of Associations for RheumatologyEUPATIEuropean Patients' Academy on Therapeutic InnovationNIHNational Institute of HealthOMERACTOutcome Measures in RheumatologyPCORIPatient-Centered Outcomes Research InstitutePRPpatient research partner
Training of PRPs
Educating and training PRPs was proposed in many papers to enhance the quality of their collaboration with researchers. Notably, nearly half of the publications emphasised the importance of training, with 21% recommending it and 25% providing it.2528 29 35 37 45 51 62,64 PRP training and support included various aspects (table 6). Training of PRPs was reported to foster well-prepared and empowered PRPs ready to engage effectively in research collaborations.22 26 29
Evaluation and monitoring related to PRP involvement
Around 21% of the included publications recommended or reported some form of evaluation,34 15 19,21 25 34 35 with 28% collecting feedback from PRPs on their involvement. Regular discussions and evaluations of the quality and impact of PRPs’ collaboration and contributions were reported to enhance understanding, satisfaction and impact, allowing for adjustments and improvements as needed.4 5 37 60 Some tools were reported for monitoring such as the Patient-Centered Outcomes Research Institute conceptual framework, an evaluative framework for research engagement,19 surveys to evaluate the impact of PRPs in the project,3 26 the Public Involvement Impact Assessment Framework Guidance,53 and the Guidance for Reporting Involvement of Patients and the Public.25 34
Recognition, compensation and acknowledgement of PRPs
Recognising, compensating and acknowledging the contributions of PRPs during their involvement in a scientific project were reported to be essential components of equal and meaningful partnerships.27
In the context of recognition, coauthorship was cited as proof of PRP involvement and equality in research collaborations.5 39 The SLR revealed a growing trend in recognising PRPs through coauthorship in 68% of articles,2,68 15 19 and acknowledgement in 45% of articles.36 7 25 27 28 34 37 43,45 48 51 53 56
Compensation refers to the payment of salary, wages, honorarium, fees or allowances for the time commitment and expertise of PRPs; this is different from reimbursing PRPs for expenses (eg, travel expenses and accommodation).49 Non-compensation for PRPs was reported as a limitation and challenge for their effective involvement.4 While PRPs can opt out of payment, several papers reported that researchers should consider compensation in their budget planning.2 39 49 Some articles advised that institutions should simplify processes for fair PRP payment, and funders should enable researchers to allocate resources for PRP involvement.5
Discussion
The role of PRPs in rheumatology research has significantly expanded over recent years. The findings of this SLR underscore the important roles and contributions of PRPs in research projects, and the added value of PRP involvement, not only in clinical research, but also in basic, translational, registry and longitudinal observational studies. This review also highlighted current challenges and barriers, and pulled together proposals of strategies to overcome them.
The exact definition and roles of PRPs remain unclear for some researchers. A wide proportion of the reviewed studies had adopted the 2011 EULAR definition of PRP which reflects the global acknowledgement of the importance of PRP involvement in rheumatology research and the need for specific recommendations.14 PRPs hold a crucial position in recognising and actively integrating the patient perspective, their voice and needs into research decision-making processes. Diverse roles and activities were undertaken by PRPs in this SLR, from research partners to patient advocates, reflecting the many ways PRPs can contribute. Their involvement, as evident in recent papers shaping research priorities, guideline development, and scientific and clinical committees, suggests a trend towards more inclusive and patient-centred research practices.
Our review revealed specific barriers and challenges in communication, training, research processes and collaboration. These challenges highlight difficulties in communication and relationship dynamics during research, the necessity for training and support for both PRPs and researchers, concerns about the research process and its pace, and obstacles in PRP collaboration, including issues of recognition and diversity. Inclusivity and diversity are important topics for future research. To address these challenges effectively, targeted strategies such as fostering open communication, creating a supportive environment, ensuring early and sustained involvement, using a PRP coordinator and providing appropriate training and support for PRPs and researchers are crucial. These findings underscore the ongoing need for refining and implementing these strategies to enhance PRP involvement more efficiently.26
A key observation from the SLR is the importance of early and sustained PRP involvement in research projects. Engaging PRPs from the research project’s inception ensures that research questions and methodologies are aligned with patients’ priorities and perspectives right from the start. Sustained involvement further reinforces the trust and collaboration between PRPs and researchers, leading to research outcomes that are more relevant and impactful. The OMERACT recommendations proposed that the level and timing of PRP involvement should vary based on the scope and type of project, emphasising adaptability as a key factor for successful involvement.15
Evaluation and monitoring are also integral aspects of PRP involvement. This ongoing reflection and feedback process is vital for fostering effective and meaningful PRP involvement in research. Recognition, compensation and acknowledgement of PRPs stand as key elements for fostering a meaningful partnership. Coauthorship serves not only to document the PRP’s contribution but also reinforces the idea of collaborative research. Of note, we observed disparity between the involvement of PRPs in research activities versus their acknowledgement as coauthors. This disparity may arise from some PRPs not prioritising or desiring coauthorship, or being unable to participate in producing and writing a research paper due to health-related challenges such as disease flare-ups or fatigue. In ensuring equitable recognition, a collective effort is essential to guarantee that PRPs receive due acknowledgement and compensation for their valuable contributions to scientific research.
Our study has strengths and weaknesses. One important strength of this SLR is that the findings will equip researchers, healthcare professionals and other stakeholders with evidence-based solutions to improve PRP involvement in medical research. To this end, the findings have supported the process of updating the EULAR recommendations for PRP involvement and made them more evidence based.1 Another strength is the obtention of a more nuanced understanding of the challenges and complexities surrounding PRP involvement in rheumatology research. Furthermore, our study stands out for its comprehensive approach, analysing a broad spectrum of study types, including quantitative and qualitative studies, reviews, opinion pieces and information from websites. The inclusion of various rheumatic musculoskeletal disease conditions, encompassing both paediatric and adult populations, enhances the robustness of our findings. Another notable strength lies in the co-production of this work by three PRPs. The project was initiated and led by a PRP (MdW) who gave the work direction, participated in article screening, article analysis, overall interpretation and manuscript writing. The two other PRPs brought important insights into PRP roles, facilitators and barriers.
A limitation of the study might be the heterogeneity of the included papers. Because of the expected limited reporting of PRP involvement in rheumatology research, we decided to include a diversity of papers in the SLR, varying from qualitative studies, case studies and original research papers to conference reports and opinion articles. This heterogeneity did not allow for any form of meta-analysis, nor for identifying themes that would benefit individual groups of PRPs such as people with rare diseases, children or young adults, or people with different cultural or ethnic backgrounds. Furthermore, quality assessments could not be uniformly applied across all study types. It is important to note that the traditional evidence hierarchy may not be applicable to this SLR, given the expected absence of randomised controlled studies. Despite this, certain papers were assessed to be of high quality of evidence within their respective study types. While the systematic approach ensured a comprehensive gathering of data, there might be relevant grey literature or non-English-language publications that were not included. Another limitation might be the time period of the last 6 years, including data from articles published between January 2017 and January 2023. This time frame was chosen to reflect studies performed after the 2011 EULAR recommendations were published, taking into account the implementation time gap.14 Furthermore, the chosen time span resulted in 53 articles which was deemed sufficient for gathering relevant data related to our research questions.
In conclusion, this SLR identified numerous publications reporting on PRP involvement in rheumatology research. Most authors reported that PRP involvement not only enriches the research process but also ensures that research outcomes are more relevant, meaningful and patient centred. However, for this involvement to be genuinely effective, it is essential to address the barriers and challenges that PRPs and researchers are facing. By updating the EULAR 2011 recommendations, based on the findings of this SLR, we can look forward to a future where research is more inclusive, collaborative, and aligned with patient needs and perspectives.
supplementary material
Footnotes
Funding: Funded by EULAR grant RES005.
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Kimme L Hyrich
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Data availability free text: All data relevant to the study are included in the article or uploaded as supplemental information. Additional data are available on reasonable request.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Krystel Aouad, Email: krystel.aouad@hotmail.com.
Maarten de Wit, Email: martinusdewit@hotmail.com.
Muriel Elhai, Email: muriel.elhai@usz.ch.
Diego Benavent, Email: d_benavent@hotmail.com.
Heidi Bertheussen, Email: heidi.bertheussen@gmail.com.
Condruta Zabalan, Email: codruta.filip@gmail.com.
Jette Primdahl, Email: jprimdahl@danskgigthospital.dk.
Paul Studenic, Email: paul.studenic@meduniwien.ac.at.
Laure Gossec, Email: laure.gossec@aphp.fr.
Data availability statement
Data are available upon reasonable request.
References
- 1.de Wit M, Aouad K, Elhai M, et al. EULAR updated recommendations for the involvement of patient research partners in rheumatology research. 2023. [DOI] [PMC free article] [PubMed]
- 2.Taylor J, Dekker S, Jurg D, et al. Making the patient voice heard in a research consortium: experiences from an EU project (IMI-APPROACH) Res Involv Engagem. 2021;7:24. doi: 10.1186/s40900-021-00267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch R, Simons G, Wähämaa H, et al. Development and formative evaluation of patient research partner involvement in a multi-disciplinary European translational research project. Res Involv Engagem. 2020;6:6. doi: 10.1186/s40900-020-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wit M, Cooper C, Tugwell P, et al. Practical guidance for engaging patients in health research, treatment guidelines and regulatory processes: results of an expert group meeting organized by the World Health Organization (WHO) and the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO) Aging Clin Exp Res. 2019;31:905–15. doi: 10.1007/s40520-019-01193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Wit M, Adebajo A. Unique role of rheumatology in establishing collaborative relationships in research. Past, present and future of patient engagement. Ann Rheum Dis. 2019;78:293–6. doi: 10.1136/annrheumdis-2018-214387. [DOI] [PubMed] [Google Scholar]
- 6.Schöpf AC, Schlöffel M, Amos T, et al. Development and formative evaluation of a communication skills training program for persons with rheumatic and musculoskeletal diseases. Health Commun. 2019;34:680–8. doi: 10.1080/10410236.2018.1431760. [DOI] [PubMed] [Google Scholar]
- 7.Pollock J, Raza K, Pratt AG, et al. Patient and researcher perspectives on facilitating patient and public involvement in rheumatology research. Musculoskeletal Care. 2017;15:395–9. doi: 10.1002/msc.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Sullivan DP, Steinkoenig I. GRAPPA patient research partner network: update to the GRAPPA 2020 annual meeting. J Rheumatol. 2021;97:64. doi: 10.3899/jrheum.201680. [DOI] [PubMed] [Google Scholar]
- 9.Nikiphorou E, Alunno A, Carmona L, et al. Patient-physician collaboration in rheumatology: a necessity. RMD Open. 2017;3:e000499. doi: 10.1136/rmdopen-2017-000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EMA Getting involved. 2018. [18-Sep-2023]. https://www.ema.europa.eu/en/partners-networks/patients-consumers/getting-involved Available. Accessed.
- 11.Costa Alencar AB, Selig WKD, Geissler J, et al. Adopting recommendations for implementing patient involvement in cancer research: a Funder’s approach. Res Involv Engagem. 2023;9:6. doi: 10.1186/s40900-023-00410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ESC patient engagement. [18-Sep-2023]. https://www.escardio.org/The-ESC/What-we-do/esc-patient-engagement Available. Accessed.
- 13.Diabetes UK Patient and public involvement (PPI) in your study. [18-Sep-2023]. https://www.diabetes.org.uk/research/for-researchers/apply-for-a-grant/help-with-involving-participants Available. Accessed.
- 14.de Wit MPT, Berlo SE, Aanerud GJ, et al. European League against rheumatism recommendations for the inclusion of patient representatives in scientific projects. Ann Rheum Dis. 2011;70:722–6. doi: 10.1136/ard.2010.135129. [DOI] [PubMed] [Google Scholar]
- 15.Cheung PP, de Wit M, Bingham CO, 3rd, et al. Recommendations for the involvement of patient research partners (PRP) in OMERACT working groups. A report from the OMERACT 2014 working group on PRP. J Rheumatol. 2016;43:187–93. doi: 10.3899/jrheum.141011. [DOI] [PubMed] [Google Scholar]
- 16.OMERACT patient research partner network. [18-Sep-2023]. https://omeractprpnetwork.org/ Available. Accessed.
- 17.FOREUM – involving PRP. [18-Sep-2023]. https://www.foreum.org/involving_prp.cfm Available. Accessed.
- 18.GRAPPA Network Patient research partners. [9-Oct-2023]. https://www.grappanetwork.org/prp-network/ Available. Accessed.
- 19.Kirwan JR, de Wit M, Frank L, et al. Emerging guidelines for patient engagement in research. Value in Health. 2017;20:481–6. doi: 10.1016/j.jval.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Belton J, Hoens A, Scott A, et al. Patients as partners in research: it’s the right thing to do. J Orthop Sports Phys Ther. 2019;49:623–6. doi: 10.2519/jospt.2019.0106. [DOI] [PubMed] [Google Scholar]
- 21.Goel N. Enhancing patient research partner engagement: research in psoriatic arthritis. Best Pract Res Clin Rheumatol. 2021;35:101685. doi: 10.1016/j.berh.2021.101685. [DOI] [PubMed] [Google Scholar]
- 22.Mikdashi J. The meaningful role of patients, and other stakeholders in clinical practice guideline development. Rheum Dis Clin North Am. 2022;48:691–703. doi: 10.1016/j.rdc.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 23.de Wit M, Abma T, Koelewijn-van Loon M, et al. Involving patient research partners has a significant impact on outcomes research: a responsive evaluation of the International OMERACT conferences. BMJ Open. 2013;3:e002241. doi: 10.1136/bmjopen-2012-002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Wit M, Abma T, Koelewijn-Van Loon M, et al. Facilitating and inhibiting factors for long-term involvement of patients at outcome conferences--lessons learnt from a decade of collaboration in OMERACT: a qualitative study. BMJ Open. 2013;3:e003311. doi: 10.1136/bmjopen-2013-003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wit MPT, Koenders MI, Neijland Y, et al. Patient involvement in basic rheumatology research at Nijmegen: a three year’s responsive evaluation of added value, pitfalls and conditions for success. BMC Rheumatol . 2022;6:66. doi: 10.1186/s41927-022-00296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studenic P, Sekhon M, Carmona L, et al. Unmet need for patient involvement in rheumatology registries and observational studies: a mixed methods study. RMD Open. 2022;8:e002472. doi: 10.1136/rmdopen-2022-002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel N. Conducting research in psoriatic arthritis: the emerging role of patient research partners. Rheumatology (Oxford) 2020;59:i47–55. doi: 10.1093/rheumatology/kez338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello W, Dorris E. Laying the groundwork: building relationships for public and patient involvement in pre-clinical paediatric research. Health Expect. 2020;23:96–105. doi: 10.1111/hex.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tunis SR, Maxwell LJ, Graham ID, et al. Engaging stakeholders and promoting uptake of OMERACT core outcome instrument SETS. J Rheumatol. 2017;44:1551–9. doi: 10.3899/jrheum.161273. [DOI] [PubMed] [Google Scholar]
- 30.EULAR Project grant application. [9-Oct-2023]. https://www.eular.org/project-grant-application Available. Accessed.
- 31.Long HA, French DP, Brooks JM. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Research Methods in Medicine & Health Sciences. 2020;1:31–42. doi: 10.1177/2632084320947559. [DOI] [Google Scholar]
- 32.Hong QN, Pluye P, Fàbregues S, et al. Improving the content validity of the mixed methods appraisal tool: a modified E-Delphi study. J Clin Epidemiol. 2019;111:49–59. doi: 10.1016/j.jclinepi.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res . 2005;15:1277–88. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 34.de Souza S, Johansson EC, Karlfeldt S, et al. Patient and public involvement in an international rheumatology translational research project: an evaluation. BMC Rheumatol . 2022;6:83. doi: 10.1186/s41927-022-00311-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Wit M, Kirwan JR, Tugwell P, et al. Successful stepwise development of patient research partnership: 14 years' experience of actions and consequences in outcome measures in rheumatology (OMERACT) Patient. 2017;10:141–52. doi: 10.1007/s40271-016-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Wit M, Campbell W, FitzGerald O, et al. Patient participation in psoriasis and psoriatic arthritis outcome research: a report from the GRAPPA 2013 annual meeting. J Rheumatol. 2014;41:1206–11. doi: 10.3899/jrheum.140171. [DOI] [PubMed] [Google Scholar]
- 37.Carr ECJ, Patel JN, Ortiz MM, et al. Co-design of a patient experience survey for arthritis central intake: an example of meaningful patient engagement in healthcare design. BMC Health Serv Res. 2019;19:355. doi: 10.1186/s12913-019-4196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Gaizo V, Kohlheim M. Patient engagement in pediatric rheumatology research. Rheum Dis Clin North Am. 2022;48:1–13. doi: 10.1016/j.rdc.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Haribhai-Thompson J, Dalbeth N, Stewart S, et al. Involving people with lived experience as partners in musculoskeletal research: lessons from a survey of Aotearoa/New Zealand musculoskeletal researchers. J Orthop Sports Phys Ther. 2022;52:307–11. doi: 10.2519/jospt.2022.10986. [DOI] [PubMed] [Google Scholar]
- 40.Fox G, Fergusson DA, Daham Z, et al. Patient engagement in preclinical laboratory research: a scoping review. EBioMedicine. 2021;70:103484. doi: 10.1016/j.ebiom.2021.103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golenya R, Chloros GD, Panteli M, et al. How to improve diversity in patient and public involvement. Br J Hosp Med (Lond) 2021;82:1–8. doi: 10.12968/hmed.2021.0176. [DOI] [PubMed] [Google Scholar]
- 42.de Wit M, Campbell W, Coates LC, et al. Let’s talk about inclusion: a report on patient research partner involvement in the GRAPPA 2015 annual meeting. J Rheumatol. 2016;43:970–3. doi: 10.3899/jrheum.160117. [DOI] [PubMed] [Google Scholar]
- 43.de Wit M, Guillemin F, Grimm S, et al. Patient engagement in health technology assessment (HTA) and the regulatory process: what about rheumatology? RMD Open. 2020;6:e001286. doi: 10.1136/rmdopen-2020-001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons S, Thomson W, Cresswell K, et al. What do young people with rheumatic conditions in the UK think about research involvement? A qualitative study. Pediatr Rheumatol Online J. 2018;16:35. doi: 10.1186/s12969-018-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schöpf-Lazzarino AC, Böhm P, Garske U, et al. Involving patients as research partners exemplified by the development and evaluation of a communication-skills training programme (KOKOS-Rheuma) Z Rheumatol. 2021;80:132–9. doi: 10.1007/s00393-020-00839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Stewart S, Darlow B, et al. Patient research partner involvement in rheumatology clinical trials: analysis of journal articles 2016-2020. Ann Rheum Dis. 2021;80:1095–6. doi: 10.1136/annrheumdis-2021-220138. [DOI] [PubMed] [Google Scholar]
- 47.Young K, Kaminstein D, Olivos A, et al. Patient involvement in medical research: what patients and physicians learn from each other. Orphanet J Rare Dis. 2019;14:21. doi: 10.1186/s13023-018-0969-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leese J, Macdonald G, Kerr S, et al. ‘Adding another spinning plate to an already busy life’. benefits and risks in patient partner-researcher relationships: a qualitative study of patient partners’ experiences in a Canadian health research setting. BMJ Open. 2018;8:e022154. doi: 10.1136/bmjopen-2018-022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards DP, Cobey KD, Proulx L, et al. Identifying potential barriers and solutions to patient partner compensation (payment) in research. Res Involv Engagem . 2022;8:7. doi: 10.1186/s40900-022-00341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoemaker CG, Richards DP, de Wit M. Matching researchers’ needs and patients’ contributions: practical tips for meaningful patient engagement from the field of rheumatology. Ann Rheum Dis. 2023;82:312–5. doi: 10.1136/ard-2022-223561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morin SN, Djekic-Ivankovic M, Funnell L, et al. Patient engagement in clinical guidelines development: input from >1000 members of the Canadian osteoporosis patient network. Osteoporos Int. 2020;31:867–74. doi: 10.1007/s00198-019-05248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leese J, Kerr S, McKinnon A, et al. Evolving patient-researcher collaboration: an illustrative case study of a patient-led knowledge translation event. J Particip Med. 2017;9:e13. doi: 10.2196/jopm.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esen E, Gnanenthiran S, Lunt L, et al. The your rheum story: involvement of young people in rheumatology research. BMC Rheumatol. 2022;6:43. doi: 10.1186/s41927-022-00273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van der Elst K, De Cock D, Bangels L, et al. ‘More than just Chitchat’: a qualitative study concerning the need and potential format of a peer mentor programme for patients with early rheumatoid arthritis. RMD Open. 2021;7:e001795. doi: 10.1136/rmdopen-2021-001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Sullivan DP, Steinkoenig I. GRAPPA patient research partner network: update to the GRAPPA 2020 annual meeting. J Rheumatol Suppl. 2021;97:64. doi: 10.3899/jrheum.201680. [DOI] [PubMed] [Google Scholar]
- 56.Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the psoriatic arthritis impact of disease (Psaid) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73:1012–9. doi: 10.1136/annrheumdis-2014-205207. [DOI] [PubMed] [Google Scholar]
- 57.Elliott RS, Taylor E, Ainsworth J, et al. Improving communication of the concept of ‘treat-to target’ in childhood lupus: a public and patient (PPI) engagement project involving children and young people. BMC Rheumatol . 2022;6:69. doi: 10.1186/s41927-022-00300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyng KD, Larsen JB, Birnie KA, et al. Participatory research: a priority setting partnership for chronic musculoskeletal pain in Denmark. Scand J Pain . 2023;23:402–15. doi: 10.1515/sjpain-2022-0019. [DOI] [PubMed] [Google Scholar]
- 59.Goodman SM, Miller AS, Turgunbaev M, et al. Clinical practice guidelines: incorporating input from a patient panel. Arthritis Care Res (Hoboken) 2017;69:1125–30. doi: 10.1002/acr.23275. [DOI] [PubMed] [Google Scholar]
- 60.Shoop-Worrall SJW, Cresswell K, Bolger I, et al. Nothing about us without us: involving patient collaborators for machine learning applications in rheumatology. Ann Rheum Dis. 2021;80:1505–10. doi: 10.1136/annrheumdis-2021-220454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jongsma KR, Milota MM. Establishing a multistakeholder research agenda: lessons learned from a James LIND alliance partnership. BMJ Open. 2022;12:e059006. doi: 10.1136/bmjopen-2021-059006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schoemaker CG, Armbrust W, Swart JF, et al. Dutch juvenile idiopathic arthritis patients, carers and clinicians create a research agenda together following the James LIND alliance method: a study protocol. Pediatr Rheumatol Online J. 2018;16:57. doi: 10.1186/s12969-018-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goel N, O’Sullivan D, de Wit M, et al. The patient research partner network matures: a report from the GRAPPA 2017 annual meeting. J Rheumatol Suppl. 2018;94:52–3. doi: 10.3899/jrheum.180138. [DOI] [PubMed] [Google Scholar]
- 64.Pauling JD, Frech TM, Domsic RT, et al. Patient participation in patient-reported outcome instrument development in systemic sclerosis. Clin Exp Rheumatol. 2017;35 Suppl 106:184–92. [PubMed] [Google Scholar]
- 65.Goel N, O’Sullivan D, Steinkoenig I, et al. Tackling patient centricity: a report from the GRAPPA 2016 annual meeting. J Rheumatol. 2017;44:703–5. doi: 10.3899/jrheum.170152. [DOI] [PubMed] [Google Scholar]
- 66.Bywall KS, Esbensen BA, Lason M, et al. Functional capacity vs side effects: treatment attributes to consider when Individualising treatment for patients with rheumatoid arthritis. Clin Rheumatol. 2022;41:695–704. doi: 10.1007/s10067-021-05961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brett J, Staniszewska S, Simera I, et al. Reaching consensus on reporting patient and public involvement (PPI) in research: methods and lessons learned from the development of reporting guidelines. BMJ Open. 2017;7:e016948. doi: 10.1136/bmjopen-2017-016948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helliwell PS, Gladman DD, Gottlieb AB. Prologue: 2016 annual meeting of the group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA) J Rheumatol. 2017;44:658–60. doi: 10.3899/jrheum.170139. [DOI] [PubMed] [Google Scholar]
- 69.PARE-PRP-2326. [16-Oct-2023]. https://esor.eular.org/enrol/index.php?id=398 Available. Accessed.
- 70.EULAR PARE patient research partners. [16-Oct-2023]. https://www.eular.org/pare-patient-research-partners Available. Accessed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.