Abstract
Background
Landmark thrombectomy trials have provided evidence that selected patients with large ischemic stroke benefit from successful endovascular therapy, commonly defined as incomplete (modified Thrombolysis In Cerebral Infarction (mTICI) 2b) or complete reperfusion (mTICI 3). We aimed to investigate whether mTICI 3 improves functional outcomes compared with mTICI 2b in large ischemic strokes.
Methods
This retrospective multicenter cohort study was conducted to compare mTICI 2b versus mTICI 3 in large ischemic strokes in the anterior circulation. Patients enrolled in the German Stroke Registry between 2015–2021 were analyzed. Large ischemic stroke was defined as an Alberta Stroke Program Early CT Score (ASPECTS) of 3–5. Patients were matched by final mTICI grade using propensity score matching. Primary outcome was the 90-day modified Rankin Scale (mRS) score.
Results
After matching, 226 patients were included. Baseline and imaging characteristics were balanced between mTICI 2b and mTICI 3 patients. There was no shift on the mRS favoring mTICI 3 compared with mTICI 2b in large ischemic strokes (adjusted common odds ratio (acOR) 1.12, 95% confidence interval (95% CI) 0.64 to 1.94, P=0.70). The rate of symptomatic intracranial hemorrhage was higher in mTICI 2b than in mTICI 3 patients (12.6% vs 4.5%, P=0.03). Mortality at 90 days did not differ between mTICI 3 and mTICI 2b (33.6% vs 37.2%; adjusted OR 0.69, 95% CI 0.33 to 1.45, P=0.33).
Conclusions
In endovascular therapy for large ischemic strokes, mTICI 3 was not associated with better 90-day functional outcomes compared with mTICI 2b. This study suggests that mTICI 2b might be warranted as the final angiographic result, questioning the benefit/risk ratio of additional maneuvers to seek for mTICI 3 in large ischemic strokes.
Trial registration number
Keywords: thrombectomy, stroke, intervention, angiography, brain
WHAT IS ALREADY KNOWN ON THIS TOPIC
Prior studies indicate superior functional outcomes for complete successful reperfusion (modified Thrombolysis In Cerebral Infarction (mTICI) 3) compared with incomplete successful reperfusion (mTICI 2b)—largely including patients with small-to-moderate ischemic strokes. It remains unclear whether the subdivision of mTICI 2b and mTICI 3 is clinically relevant in endovascular therapy for large ischemic strokes.
WHAT THIS STUDY ADDS
In this retrospective study on endovascular therapy for large ischemic strokes, there was no significant shift on the modified Rankin Scale at 90 days toward better functional outcomes in mTICI 3 compared with mTICI 2b reperfusion. Mortality at 90 days did not differ between mTICI 3 and mTICI 2b patients.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Interventionalists should target mTICI 3 reperfusion in endovascular therapy for large ischemic strokes. However, this study suggests that mTICI 2b might be warranted as the final angiographic result, questioning the benefit/risk ratio of additional maneuvers to seek for mTICI 3 in large ischemic strokes. This study might support intraprocedural decision making in endovascular therapy for large ischemic strokes, considering that mTICI 2b represents the final angiographic outcome in about 40% of patients.
Introduction
There is more and more first-level evidence that endovascular therapy (EVT) is safe, effective, and superior to best medical treatment in patients with large ischemic anterior circulation strokes, which account for approximately 20% of all large vessel occlusions.1,3 In light of these landmark thrombectomy trials, current treatment guidelines will presumably be amended and endorse EVT for selected patients with extensive baseline infarction. Consequently, the question arises whether well-established treatment concepts of EVT fully apply to large ischemic strokes.
Since the 2000s, the Thrombolysis In Cerebral Infarction (TICI) scale and subsequent modifications (mTICI) have become standard practice to determine the technical success of EVT.4,6 The mTICI score grades the extent of tissue reperfusion based on the angiographic appearance of the occluded target artery territory after intervention, ranging from no to complete reperfusion. Successful reperfusion is commonly defined as mTICI grade of 2b or 3, which corresponds to reperfusion of at least 50% of the affected vascular territory.7 8 Despite technical advancements in EVT, mTICI 2b remained the final angiographic outcome in about 40% of patients in recent thrombectomy trials.9
Multiple studies have suggested superior functional outcomes for mTICI 3 compared with mTICI 2b reperfusion—largely including patients with small-to-moderate ischemic infarction on admission.10,12 Accordingly, the European Stroke Organisation–European Society for Minimally Invasive Neurological Therapy (ESO-ESMINT) guidelines strongly recommend to attempt an mTICI 3 reperfusion, if achievable with reasonable safety.13 To date, however, it remains unclear whether the differentiation between mTICI 2b and mTICI 3 is also of clinical relevance for patients with large ischemic strokes.
This study aimed to provide an in-depth comparison of functional outcomes and safety measures among patients with mTICI 2b and mTICI 3 reperfusion after EVT for large ischemic strokes. We hypothesized that mTICI 3 reperfusion is clinically superior to mTICI 2b reperfusion in patients with extensive baseline infarction.
Methods
Study design
For this retrospective multicenter cohort study, patients were assessed for eligibility who were enrolled in the German Stroke Registry–Endovascular Treatment (GSR) between May 1, 2015 and December 31, 2021. The GSR is an ongoing, prospective, open-label, multicenter registry including patients who underwent EVT in 25 comprehensive stroke centers in Germany (ClinicalTrials.gov identifier: NCT03356392).14 15 The GSR was approved by the responsible ethics committee of the Ludwig Maximilian University, Munich, Germany (689-15). The local ethics committee of each participating center gave approval to contribute fully anonymized data to the GSR. Informed consent for this study was waived after review of the central ethics committee. This study was reported using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.16
Study cohort
Inclusion criteria were defined as follows: (1) acute ischemic stroke in the anterior circulation due to an isolated occlusion of the intracranial internal carotid artery or of the M1 or M2 segment of the middle cerebral artery; (2) large ischemic stroke on pretreatment non-contrast CT defined as Alberta Stroke Program Early CT Score (ASPECTS) values of 3 to 5 according to RESCUE-Japan LIMIT, ANGEL-ASPECT, SELECT2 and TENSION1,317—ASPECTS values of 0 to 2 were not included due to the extremely poor clinical prognosis1,318; (3) age of at least 18 years, prestroke modified Rankin Scale (mRS) score of 0 to 2, and admission National Institutes of Health Stroke Scale (NIHSS) score of 25 or lower according to the inclusion criteria of the TENSION study17; (4) treatment with EVT with successful reperfusion (defined as grade 2b or 3 on the mTICI scale); (5) available data on the mRS score at 90 days. Exclusion criteria were defined as follows: (1) occlusion of the extracranial internal carotid artery or tandem occlusion; (2) concomitant stenting therapy. Online supplemental figure S1 provides a detailed flowchart of the inclusion and exclusion criteria.
Clinical and radiologic assessment
Patient, imaging, and treatment characteristics were retrieved from the GSR. Baseline imaging, digital subtraction angiograms, and follow-up imaging were assessed by local investigators at each participating center. The extent of baseline infarction was determined using ASPECTS in 196 (86.7%) patients on non-contrast head CT and in 30 (13.3%) patients on MRI. Final angiographic outcome was evaluated using the mTICI scale, ranging from 0 to 3 (without the 2c category), with higher grades indicating increased reperfusion. Grade 2b indicates reperfusion of ≥50% and grade 3 indicates reperfusion of 100% of the occluded middle cerebral artery territory at the end of EVT. According to the Second European-Australasian Acute Stroke Study (ECASS II), symptomatic intracranial hemorrhage (sICH) was defined as the presence of any intracranial hemorrhage within 24 hours and a neurological worsening of 4 or more points on the NIHSS.19 Patients underwent clinical assessment at baseline and at 90 days using the NIHSS and mRS.
Outcomes and safety measures
The primary outcome was the mRS score at 90 days. The secondary outcome was independent ambulation defined as mRS score of 0 to 3 at 90 days and functional independence defined as mRS score of 0 to 2 at 90 days. Safety outcomes were the occurrence of sICH within 24 hours and mortality at 90 days.
Statistical analysis
We conducted a 1:1 propensity score matching without replacement to reduce the possible influence of selection bias and confounders on the outcome and safety measures as described previously.15 The propensity scores were calculated using a multivariable regression model in which the treatment status (final mTICI grade of 2b or 3) was regressed on the following covariates: age, sex, NIHSS score on admission, and prestroke mRS. Online supplemental figure S2 provides more detailed information about the propensity score matching.
Descriptive statistics were performed to compare treatment groups (mTICI 2b and mTICI 3) before and after propensity score matching (tables1 2). Categorical variables were reported as counts and percentages and compared between treatment groups using the χ2 test. Continuous variables were reported as median and IQR and compared between treatment groups using the Mann-Whitney U test after assessment for normal distribution.
Table 1. Baseline, imaging, and treatment characteristics compared between mTICI 2b and mTICI 3 patients before and after propensity score matching.
| Before propensity score matching | After propensity score matching | |||||||
| Patients, number (%) | Patients, number (%) | |||||||
| All(n=290) | mTICI 2b(n=132) | mTICI 3(n=158) | P value* | All(n=226) | mTICI 2b(n=113) | mTICI 3(n=113) | P value* | |
| Baseline characteristics | ||||||||
| Age (years), median (IQR) | 74 (62–81) | 74 (61–81) | 74 (63–82) | 0.50† | 74 (62–81) | 73 (60–81) | 74 (63–81) | 0.69† |
| Male sex | 145/290 (50.0%) | 64/132 (48.5%) | 81/158 (51.3%) | 0.64‡ | 117/226 (51.8%) | 57/113 (50.4%) | 60/113 (53.1%) | 0.69‡ |
| Medical history | ||||||||
| Atrial fibrillation | 132/290 (45.5%) | 54/132 (40.9%) | 78/158 (49.4%) | 0.15‡ | 100/226 (44.2%) | 45/113 (39.8%) | 55/113 (48.7%) | 0.18‡ |
| Arterial hypertension | 208/289 (72.0%) | 90/131 (68.7%) | 118/158 (74.7%) | 0.26‡ | 163/225 (72.4%) | 76/112 (67.9%) | 87/113 (77.0%) | 0.13‡ |
| Diabetes | 58/290 (20.0%) | 21/132 (15.9%) | 37/158 (23.4%) | 0.11‡ | 42/226 (18.6%) | 17/113 (15.0%) | 25/113 (22.1%) | 0.17‡ |
| Dyslipidemia | 106/288 (36.8%) | 47/130 (36.2%) | 59/158 (37.3%) | 0.84‡ | 89/224 (39.7%) | 42/111 (37.8%) | 47/113 (41.6%) | 0.57‡ |
| Prestroke mRS score | 0.23‡ | 0.88‡ | ||||||
| 0 | 208/290 (71.7%) | 89/132 (67.4%) | 119/158 (75.3%) | 164/226 (72.6%) | 82/113 (72.6%) | 82/113 (72.6%) | ||
| 1 | 42/290 (14.5%) | 24/132 (18.2%) | 18/158 (11.4%) | 28/226 (12.4%) | 13/113 (11.5%) | 15/113 (13.3%) | ||
| 2 | 40/290 (13.8%) | 19/132 (14.4%) | 21/158 (13.3%) | 34/226 (15.0%) | 18/113 (15.9%) | 16/113 (14.2%) | ||
| Admission NIHSS score, median (IQR)§ | 17 (13–19) | 17 (14–19) | 16 (12–19) | 0.23† | 17 (13–19) | 17 (14–19) | 17 (12–20) | 0.77† |
| Imaging characteristics | ||||||||
| Occlusion site | 0.58‡ | 0.71‡ | ||||||
| ICA | 89/290 (30.7%) | 42/132 (31.8%) | 47/158 (29.7%) | 78/226 (34.5%) | 38/113 (33.6%) | 40/113 (35.4%) | ||
| MCA – M1 | 171/290 (59.0%) | 79/132 (59.8%) | 92/158 (58.2%) | 127/226 (56.2%) | 66/113 (58.4%) | 61/113 (54.0%) | ||
| MCA – M2 | 30/290 (10.3%) | 11/132 (8.3%) | 19/158 (12.0%) | 21/226 (9.3%) | 9/113 (8.0%) | 12/113 (10.6%) | ||
| Baseline ASPECTS, median (IQR) | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.88† | 5 (4–5) | 5 (4–5) | 5 (4–5) | 0.99† |
| Workflow times | ||||||||
| Last known well/symptom, onset to admission (min), median (IQR) | 225 (108–613) | 230 (98–662) | 217 (112–598) | 0.90† | 237 (104–644) | 243 (93–684) | 226 (118–603) | >0.99† |
| Missing data, number. | 34 | 14 | 20 | 24 | 11 | 13 | ||
| Groin to flow restoration (min), median (IQR) | 35 (24–51) | 35 (25–51) | 35 (23–51) | 0.42† | 35 (24–50) | 37 (26–51) | 32 (23–48) | 0.13† |
| Missing data, number | 10 | 6 | 4 | 10 | 6 | 4 | ||
| Treatment characteristics | ||||||||
| Administration of IVT | 105/290 (36.2%) | 47/132 (35.6%) | 58/158 (36.7%) | 0.85‡ | 76/226 (33.6%) | 37/113 (32.7%) | 39/113 (34.5%) | 0.78‡ |
| General anesthesia | 224/288 (77.8%) | 100/131 (76.3%) | 124/157 (79.0%) | 0.59‡ | 172/224 (76.1%) | 84/112 (74.3%) | 88/112 (77.9%) | 0.53‡ |
| Recanalization attempts during EVT, median (IQR) | 2 (1–3) | 2 (1–3) | 1 (1–3) | 0.005† | 2 (1–3) | 2 (1–3) | 1 (1–2) | <0.001† |
Characteristics were compared between mTICI 2b and mTICI patients.
Mann-Whitney U test for continuous variables
χ2 test for categorical variables.
ASPECTSAlberta Stroke Program Early CT ScoreEVTendovascular therapyICAinternal carotid arteryIVTintravenous thrombolysisMCAmiddle cerebral arterymRSmodified Rankin ScalemTICImodified Thrombolysis In Cerebral InfarctionNIHSSNational Institutes of Health Stroke Scale
Table 2. Functional and safety outcomes after propensity score matching.
| After propensity score matching | OR (95% CI) | |||||||
| Patients, number (%) | ||||||||
| All(n=226) | mTICI 2b(n=113) | mTICI 3(n=113) | P value* | Unadjusted†† | P value | Adjusted‡‡ | P value | |
| Primary outcome | ||||||||
| 90-day mRS score, median (IQR) | 4 (3–6) | 4 (3–6) | 4 (2–6) | 0.71† | 1.09 (0.69 to 1.74)§ | 0.71 | 1.12 (0.64 to 1.94)§ | 0.70 |
| Secondary outcomes | ||||||||
| Independent ambulation (90-day mRS score 0–3) | 89/226 (39.4%) | 45/113 (39.8%) | 44/113 (38.9%) | 0.89‡ | 0.96 (0.56 to 1.64) | 0.89 | 0.82 (0.38 to 1.72) | 0.60 |
| Functional independence (90-day mRS score 0–2) | 55/226 (24.3%) | 26/113 (23.0%) | 29/113 (25.7%) | 0.64‡ | 1.16 (0.63 to 2.13) | 0.64 | 1.13 (0.51 to 2.52)¶ | 0.77 |
| Safety outcomes | ||||||||
| sICH within 24 hours | 19/221 (8.6%) | 14/111 (12.6%) | 5/110 (4.5%) | 0.03‡ | 0.33 (0.10 to 0.90) | 0.04 | NA** | NA** |
| Mortality within 90 days | 80/226 (35.4%) | 42/113 (37.2%) | 38/113 (33.6%) | 0.58‡ | 0.86 (0.50 to 1.48) | 0.58 | 0.69 (0.33 to 1.45) | 0.33 |
Characteristics were compared between mTICI 2b and mTICI 3 patients.
Mann-Whitney U test for continuous variables.
χ2 test for categorical variables.
Common ORs derived from ordinal logistic regression analysis. Values >1 indicate a shift in the distribution of 90-day mRS scores toward lower values (better functional outcomes) favoring mTICI 3 compared with mTICI 2b.
All patients with sICH did not achieve functional independence at 90 days (perfect prediction). Thus, the occurrence of sICH was not considered as covariate for this model.
A multivariable regression analysis for sICH was not performed given the small number of cases (n=19).
Univariable regression analysis with final mTICI grade (mTICI 2b vs mTICI 3) as independent variable. mTICI 2b was used as reference level.
Results were adjusted for age, sex, interval from last known well/symptom onset to hospital admission, admission NIHSS, ASPECTS, number of recanalization attempts, and occurrence of sICH within 24 hours.
mRSmodified Rankin ScalemTICImodified Thrombolysis In Cerebral InfarctionsICHsymptomatic intracranial hemorrhage
Unadjusted odds ratios (ORs) and adjusted common odds ratios (acORs) were estimated by ordinal logistic regression indicating the shift in the direction of lower values (better functional outcomes) on the mRS (table 2 and online supplemental table S1 and S2). The proportional odds assumption was met (Brant test, P=0.83). In addition, unadjusted and adjusted ORs (aORs) were estimated for binary outcome measures by logistic regression analysis (table 2 and online supplemental table S2). ORs with 95% confidence intervals (95% CI) were adjusted for age, sex, interval from symptom onset or last known well to hospital admission, NIHSS score on admission, baseline ASPECTS, and the number of recanalization attempts. We performed complete-case analysis for all regression models. Patients with missing values are specified in online supplemental figure S1.
We performed subgroup analyses to investigate modifications to the clinical value of mTICI 3 over mTICI 2b based on age, sex, time from last known well or symptom onset to hospital admission, NIHSS score on admission, site of arterial occlusion, and baseline ASPECTS (online supplemental figure S3). Results are only adjusted for significant variables from the mRS shift analysis to avoid overfitting given the smaller group sizes (online supplemental table S1). The results should be considered as explorative (hypothesis-generating).
Sensitivity analyses were conducted to support the robustness of the main findings (1) by excluding patients with occlusion of the M2 segment of the middle cerebral artery (online supplemental table S3), (2) by comparison with patients exhibiting small-to-moderate baseline infarction (online supplemental table S4), and (3) after stratification for the number of recanalization attempts (online supplemental figure S4).
A two-tailed P value <0.05 was considered significant for all statistical tests. All analyses were performed using R statistical software (version 4.1.2, R Project for Statistical Computing) and RStudio statistical software (version 2021.09.1+372, Rstudio).
Results
Patient characteristics
A total of 13 082 patients from the GSR were screened, of which 290 met the inclusion criteria (online supplemental figure S1). After 1:1 propensity score matching, 226 patients were included for subsequent analyses. Of those, 113 patients had a final mTICI grade of 2b (incomplete reperfusion) and 113 patients had a final mTICI grade of 3 (complete reperfusion). Across all patients, the median age was 74 years (IQR 62–81) and 51.8% were male. The median NIHSS score on admission was 17 (IQR 13–19) and the median baseline ASPECTS was 5 (IQR 4–5). Intravenous thrombolysis was administered in 33.6% of cases. Patients with mTICI 2b showed a higher number of recanalization attempts during EVT compared with patients with mTICI 3 (median, 2 vs 1, P<0.001). All other baseline, imaging, and treatment characteristics were balanced between the two treatment groups after propensity score matching (table 1).
Primary and secondary outcomes
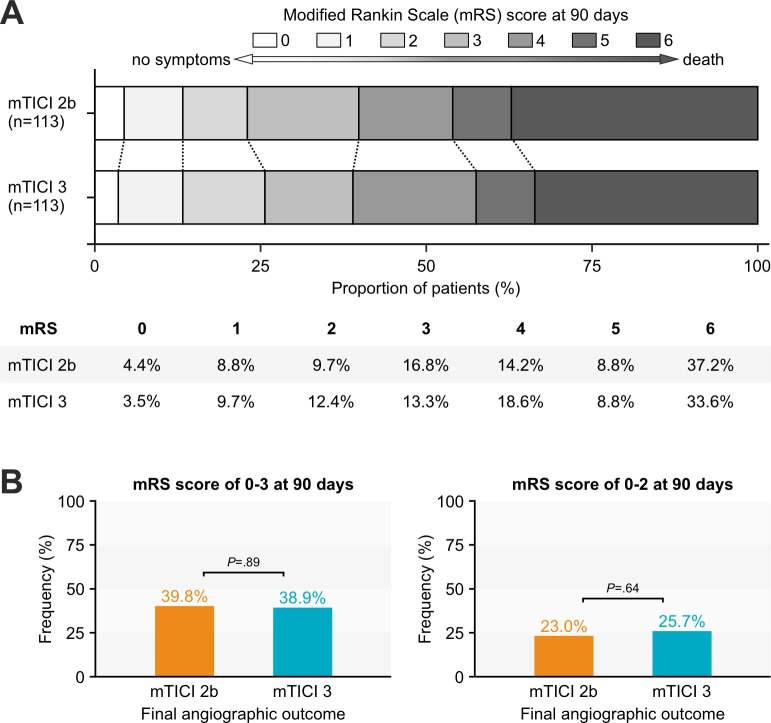
The median 90-day mRS score was 4 (IQR 3–6) after mTICI 2b reperfusion and 4 (IQR 2–6) after mTICI 3 reperfusion in large ischemic strokes (P=0.71). There was no significant shift in the distribution of 90-day mRS scores toward lower values (better functional outcomes) in mTICI 3 patients compared with mTICI 2b patients (acOR 1.12 95% CI 0.64 to 1.94, P=0.70) (table 2). The distributions of 90-day mRS scores stratified by final mTICI grade are shown in figure 1A. Among patients with large ischemic stroke and successful reperfusion, higher age and the occurrence of sICH were also associated with worse functional outcomes at 90 days (online supplemental table S1).
Figure 1. Distribution of modified Rankin Scale (mRS) scores at 90 days stratified by final modified Thrombolysis In Cerebral Infarction (mTICI) grade. (A) Multivariable ordinal regression analysis did not show a significant shift on the mRS toward better functional outcomes in mTICI 3 patients (adjusted common OR 1.12, 95% CI 0.64 to 1.94, P=0.70). (B) There was no difference in the rate of independent ambulation at 90 days (left: 39.8% vs 38.9%, P=0.89) and functional independence at 90 days (right: 23.0% vs 25.7%, P=0.64) between mTICI 2b and mTICI 3 patients.
Compared with mTICI 2b, a final angiographic outcome of mTICI 3 was neither associated with higher odds of achieving independent ambulation (38.9% vs 39.8%, P=0.89; aOR 0.82, 95% CI 0.38 to 1.72, P=0.60) nor functional independence at 90 days (25.7% vs 23.0%, P=0.64; aOR 1.13, 95% CI 0.51 to 2.52, P=0.77) (figure 1B). Results for primary and secondary outcomes were highly similar before and after propensity score matching (Supplementary Table S2) and after exclusion of patients with large ischemic stroke due to M2 segment occlusion (Supplementary Table S3). In contrast, functional outcomes at 90 days differed in EVT for small-to-moderate baseline infarction favoring mTICI 3 reperfusion (Supplementary Table S4).
Subgroup analyses
The subgroup analyses for independent ambulation are displayed in Supplementary Figure S3) and were generally supportive of the primary analysis. Compared with incomplete reperfusion (mTICI 2b), complete reperfusion (mTICI 3) was not associated with a higher likelihood of independent ambulation in patients of older and younger age, different sex, shorter and longer time between symptom onset or last known well and hospital admission, lower and higher NIHSS scores on admission, and various sites of vessel occlusion.
For further subanalysis, the study cohort was stratified for the number of recanalization attempts. There was neither a superior rate of independent ambulation when complete reperfusion was achieved at first pass (mTICI 3 at attempt 1 vs mTICI 2b at attempt ≥2, P=0.92), nor when complete reperfusion was achieved after multiple attempts (mTICI 3 at attempt ≥2 vs mTICI 2b at attempt 1, P=0.74) (see Supplementary Figure S4).
Safety outcomes
The rate of sICH was higher in mTICI 2b than in mTICI 3 patients (12.6% vs 4.5%, P=0.03). There was no difference in death within 90 days between the two treatment groups (33.6% vs 37.2%, P=0.58; aOR 0.69, 95% CI 0.33 to 1.45, P=0.33).
Discussion
This retrospective multicenter cohort study provides an in-depth comparison between incomplete (mTICI 2b) and complete successful reperfusion (mTICI 3) in EVT for large ischemic strokes in the anterior circulation. We found that in patients with extensive baseline infarction and successful EVT, functional outcomes at 90 days did not differ between mTICI 2b and mTICI 3 reperfusion. The rate of sICH was higher in patients in which the final angiographic outcome was mTICI 2b. Mortality at 90 days was comparable between both groups. This study adds new insights into EVT for large ischemic strokes, suggesting that the radiological subdivision of mTICI 2b and mTICI 3 reperfusion might have minor clinical implications in this subgroup of patients.
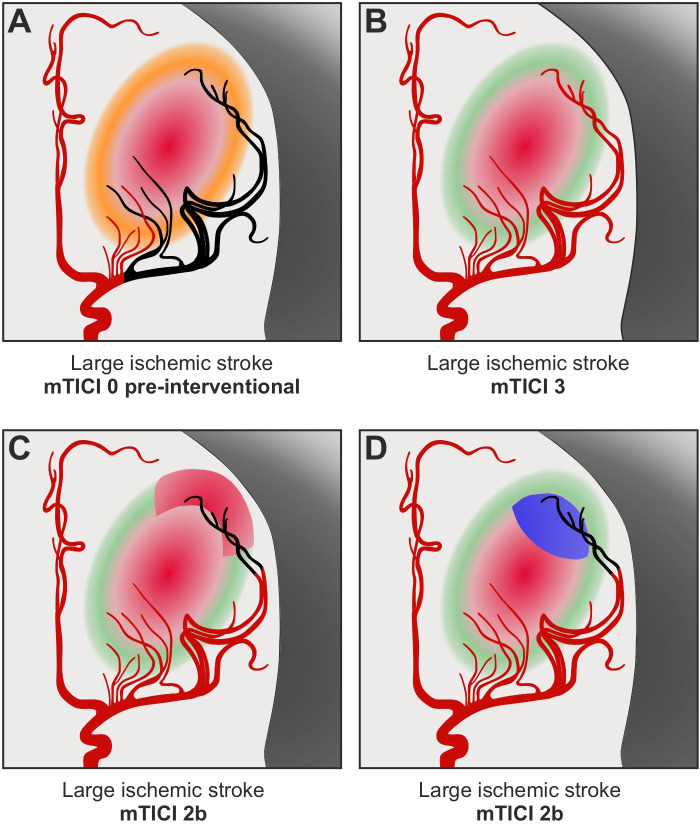
Multiple studies found that the rate of post-stroke functional independence gradually increased with higher grades of vessel reperfusion across the TICI scale—largely including patients with small-to-moderate ischemic infarction on admission.20 21 Our data reveal that in patients with large ischemic strokes, mTICI 2b and mTICI 3 reperfusion did not show meaningful differences in functional outcomes at 90 days. This lack of differentiation with respect to functional outcomes may appear contradictory to the key assumption underlying the treatment effect of EVT: The more affected vascular territory is reperfused, the more ischemic brain tissue is rescued from irreversible infarction, and the less likely are disabling long-term consequences after stroke (figure 2A–B). In the following, we present alternative approaches which might contribute to the missing translation of higher reperfusion into better functional outcomes among patients with large ischemic strokes and mTICI 2b/3 reperfusion.
Figure 2. Comparison of incomplete and complete reperfusion in endovascular therapy for large ischemic strokes. (A) Illustration of a large vessel occlusion with extensive baseline infarction. Before endovascular therapy, there is already irreversible ischemic injury in major parts of the occluded middle cerebral artery territory (red area), besides penumbral tissue indicating still salvageable brain tissue at risk (orange area). (B) Optimal scenario of mTICI 3 reperfusion. Penumbral tissue is saved by complete reperfusion of the affected vascular territory (green area), limiting the ischemic core to the initial injury (red area). (C, D) Alternative scenario of mTICI 2b reperfusion. The incomplete distal branch filling might lead to (C) progressive infarction in addition to the initial ischemic injury (additional red area) and/or (D) persisting occlusion supplying already irreversibly damaged brain tissue (blue area). mTICI, modified Thrombolysis In Cerebral Infarction.
First, the lack of differentiation between mTICI 2b and mTICI 3 might be due to futile angiographic improvement in EVT for large ischemic strokes. Theoretically, the incomplete distal branch filling in mTICI 2b patients could lead to progressive infarction in addition to the ischemic injury on admission (figure 2C), but could also represent a persisting occlusion which supplies already irreversibly damaged brain tissue—without clinical benefit if reperfused (figure 2D). Notably, the likelihood of futile angiographic improvement should increase with greater baseline infarction of the affected vascular territory. Furthermore, previous studies suggest that the clinical benefit of mTICI 3 over mTICI 2b reperfusion progressively decreases over time as a result of diminishing salvageable penumbra,22 and ultimately disappears in the late time window,23 in which many low ASPECTS patients arrive at hospital. Both correlated variables, greater baseline infarction and later hospital admission, could partially explain the inconsistent treatment effects of mTICI 3 over mTICI 2b reperfusion in small-to-moderate compared with large ischemic strokes.
Second, arterial collateralization might contribute to the highly comparable functional outcomes between incomplete and complete reperfusion in large ischemic strokes. Again, patients with extensive baseline infarction exhibit on average prolonged intervals between last known to be well and hospital admission. At the time of baseline imaging, the affected brain tissue, which until then did not sustain irreversible ischemic injury, might receive sufficient arterial blood supply by collateral flow. Thus, in the presence of arterial collaterals, increasing reperfusion from mTICI 2b to mTICI 3 could be more likely to represent needless angiographic improvement, since the reperfused tissue would not have died either way.24 In line with this hypothesis, a retrospective Swiss study demonstrated a diminishing clinical benefit of mTICI 3 over mTICI 2b in patients with favorable arterial collateralization.25
Third, the mRS might be insensitive to detect possible, but most probably subtle, long-term sequelae of mTICI 2b compared with mTICI 3 reperfusion in large ischemic strokes. Although the mRS represents a global disability measure which allows the clinician to consider non-physical attributes such as cognition, language, and social functioning, the mRS remains heavily weighted toward physical disability and the need for assistance.26 Since even after successful EVT, the median 90-day mRS score is 4 and therefore indicates a generally high degree of physical disability, the distinction between for example moderately severe and severe disability might be difficult.
Our analysis revealed that in patients with large ischemic stroke, mTICI 2b reperfusion showed higher rates of sICH compared with mTICI 3 reperfusion. This inverse association between higher reperfusion grades and occurrence of sICH has been described in previous studies11 27 28 and might be mediated by longer procedure time and more recanalization attempts in case of persisting vessel occlusion,29 which were also observed in our data. These lower rates of sICH after mTICI 3 suggest that reperfusion injury might not represent the only pathophysiological mechanism of sICH, even in patients with a pre-existing large ischemic core.
Two recent studies found that in large ischemic strokes, mTICI 2b/3 reperfusion is only associated with favorable functional outcomes when restored within two thrombectomy maneuvers.30 31 The question arises whether EVT can be stopped as soon as mTICI 2b is obtained or whether EVT should be continued to achieve mTICI 3. Our study does not suggest that mTICI 3 after the first or after multiple thrombectomy maneuvers improves functional outcomes compared with mTICI 2b reperfusion in extensive baseline strokes. These findings challenge the clinical value of additional rescue maneuvers after mTICI 2b reperfusion in these highly affected patients. Rescue maneuvers to achieve mTICI 3 might entail the risk of jeopardizing the treatment success of mTICI 2b reperfusion by adverse events.24 32 33 Finally, our results do not allow for a clear recommendation of stopping EVT after mTICI 2b in large ischemic strokes, which would eventually require a randomized controlled trial. However, this study encourages interventionalists to perform a very careful risk-benefit assessment of additional maneuvers as soon as mTICI 2b reperfusion is restored in EVT for large ischemic strokes. In mTICI 2b patients, a matching of the hypoperfused territory with the ischemic region on baseline imaging might further improve intraprocedural decision making and warrants thorough investigation in future studies.
This study has certain limitations. First, the retrospective design might lead to selection bias and reduce the generalizability of the results, even after propensity score matching to balance the treatment groups for baseline characteristics. Second, the number of included patients might be insufficient to detect potentially small group differences at a given significance level of P<0.05. Third, the final angiographic outcome was determined using a TICI scale without a finer subdivision of TICI 2b and without the TICI 2c category as proposed by the expanded Thrombolysis In Cerebral Infarction (eTICI) score.20 Subsuming TICI 2b and TICI 2c spans a wide range of angiographic outcomes and could reduce potential differences in clinical outcomes between incomplete and complete reperfusion in EVT for large ischemic strokes. However, previous studies in small-to-moderate strokes also used the mTICI scale and could demonstrate superior functional outcomes in mTICI 3 compared with mTICI 2b reperfusion, suggesting important differences with respect to the size of baseline infarction.11 12 Fourth, baseline ASPECTS and final mTICI were assessed by local investigators at each participating center, raising concerns about interrater reliability. Fifth, the GSR does not provide detailed information about perfusion imaging, which precludes the analysis of ischemic core and penumbra volumes. Moreover, the treatment benefit of mTICI 3 reperfusion might increase in the presence of a target mismatch profile derived from perfusion imaging.
In summary, future studies should compare mTICI 2b and mTICI 3 reperfusion in extensive baseline infarction in a larger patient population using the eTICI scale, measures of arterial collateralization, and perfusion imaging.
Conclusion
This study did not find clinically meaningful differences in 90-day functional outcomes between mTICI 2b and mTICI 3 reperfusion in EVT for large ischemic strokes. Our data suggest that mTICI 2b might be warranted as the final angiographic result in large ischemic strokes. Moreover, this study questions the clinical benefit/risk profile of additional rescue maneuvers to treat persisting distal occlusions targeting mTICI 3 in these severely affected patients. An individual patient data meta-analysis of the landmark large core thrombectomy trials would be of great interest to improve intraprocedural decision making in EVT for large ischemic strokes.
supplementary material
Acknowledgements
The authors acknowledge the GSR investigators and the GSR steering committee.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: Data may be obtained from a third party (GSR) and are not publicly available. The data that support the findings of this study are available from the GSR.
Collaborators: The GSR investigators and the GSR steering committee: Dr. med. Anna Allegiani (Asklepios Klinik Altona); Prof. Dr. med. Jörg Berrouschot (Klinikum Altenburger Land); PD Dr. med. Tobias Boeckh-Behrens (Klinikum rechts der Isar der Technischen Universität München); Dr.med. Georg Bohner (Charité Berlin); Prof. Dr. med. Jan Borggrefe (Johannes Wesling Klinikum Minden; Universitätsklinikum RUB); Dr. med. Albrecht Bormann (Klinikum Altenburger Land); Dr. med. Michael Braun (Bezirkskrankenhaus Günzburg); Prof. Dr. med. Franziska Dorn (Universitätsklinikum Bonn); Prof. Dr. med. Bernd Eckert (Asklepios Hamburg Altona); Prof. Dr. med. Ulrike Ernemann (Universitätsklinik Tübingen); PD Dr. med. Marielle Ernst (Georg-August-Universität Göttingen); Prof. Dr. med. Jens Fiehler (UKE Hamburg-Eppendorf); Prof. Dr. med. Christian Gerloff (UKE Hamburg-Eppendorf); Prof. Dr. med. Klaus Gröschel (Uniklinik Mainz); Prof. Dr. med. Gerhard F. Hamann (Bezirkskrankenhaus Günzburg); Dr. med. Jörg Hattingen (Klinikum Nordstadt); Dr. med. Karl-Heinz Henn (Klinikum Offenbach); Dr. med. Fee Keil (Uniklinik Frankfurt/Main); Prof. Dr. med. Lars Kellert (LMU Klinikum); Dr. med. Christoffer Kraemer (Klinikum Lüneburg); PD Dr. med. Ruben Mühl-Benninghaus (Klinikum Lüneburg); Dr. med. Alexander Ludolph (Klinikum Offenbach); Prof. Dr. med. Christian Nolte (Charité Berlin); Prof. Dr. med. Omid Nikoubashman (Uniklinik RWTH Aachen); Dr. med. Martina Petersen (Klinikum Osnabrück); Prof. Dr. med. Gabor Petzold (Universitätsklinikum Bonn); PD Dr. med. Sven Poli (Universitätsklinik Tübingen); PD Dr. med. Arno Reich (Uniklinik RWTH Aachen); Prof. Dr. med. Christian Riedel (Georg-August-Universität Göttingen); Prof. Dr. med. Joachim Röther (Asklepios Hamburg Altona); Dr. med. Jan Hendrik Schäfer (Uniklinik Frankfurt/Main); Maximilian Schell (UKE Hamburg-Eppendorf); Prof. Dr. med. Peter Schellinger (Johannes Wesling Klinikum Minden; Universitätsklinikum RUB); PD Dr. med Eberhard Siebert (Charité Berlin); Prof. Dr. med. Florian Stögbauer (Klinikum Osnabrück); Prof. Dr. med. Götz Thomalla (UKE Hamburg-Eppendorf); Dr. med. Steffen Tiedt (LMU Klinikum); Prof. Dr. med. Christoph Trumm (LMU Klinikum); Dr. med. Timo Uphaus (Uniklinik Mainz); Dr. med. Silke Wunderlich (Klinikum rechts der Isar der Technischen Universität München); and Dr. med. Sarah Zweynert (Charité Berlin).
Contributor Information
Laurens Winkelmeier, Email: l.winkelmeier@uke.de.
Tobias D Faizy, Email: t.faizy@uke.de.
Caspar Brekenfeld, Email: c.brekenfeld@uke.de.
Christian Heitkamp, Email: c.heitkamp@uke.de.
Gabriel Broocks, Email: g.broocks@uke.de.
Matthias Bechstein, Email: m.bechstein@uke.de.
Paul Steffen, Email: pa.steffen@uke.de.
Maximilian Schell, Email: m.schell@uke.de.
Susanne Gellissen, Email: s.gellissen@uke.de.
Helge Kniep, Email: H.kniep@uke.de.
Goetz Thomalla, Email: thomalla@uke.de.
Jens Fiehler, Email: fiehler@uke.de.
Fabian Flottmann, Email: f.flottmann@uke.de.
for the German Stroke Registry – Endovascular Treatment (GSR):
Anna Allegiani, Jörg Berrouschot, Tobias Boeckh-Behrens, Georg Bohner, Jan Borggrefe, Albrecht Bormann, Michael Braun, Franziska Dorn, Bernd Eckert, Ulrike Ernemann, Marielle Ernst, Jens Fiehler, Christian Gerloff, Klaus Gröschel, Gerhard F. Hamann, Jörg Hattingen, Karl-Heinz Henn, Fee Keil, Lars Kellert, Christoffer Kraemer, Ruben Mühl-Benninghaus, Alexander Ludolph, Christian Nolte, Omid Nikoubashman, Martina Petersen, Gabor Petzold, Sven Poli, Arno Reich, Joachim Röther, Jan Hendrik Schäfer, Maximilian Schell, Peter Schellinger, Eberhard Siebert, Florian Stögbauer, Götz Thomalla, Steffen Tiedt, Christoph Trumm, Timo Uphaus, Silke Wunderlich, Sarah Zweynert, and Christian Riedel
Data availability statement
The data that support the findings of this study are available upon reasonable request after approval of the ethics committee and all participating centers.
References
- 1.Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386:1303–13. doi: 10.1056/NEJMoa2118191. [DOI] [PubMed] [Google Scholar]
- 2.Sarraj A, Pujara DK, Campbell BC. Endovascular thrombectomy for acute large ischemic strokes. Reply. N Engl J Med. 2023;389:89–90. doi: 10.1056/NEJMc2305915. [DOI] [PubMed] [Google Scholar]
- 3.Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388:1272–83. doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
- 4.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–37. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 5.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the interventional management of stroke II trial. AJNR Am J Neuroradiol. 2008;29:582–7. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650–63. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 8.Yoo AJ, Simonsen CZ, Prabhakaran S, et al. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke. 2013;44:2509–12. doi: 10.1161/STROKEAHA.113.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill MD, Goyal M, Menon BK, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-Na1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395:878–87. doi: 10.1016/S0140-6736(20)30258-0. [DOI] [PubMed] [Google Scholar]
- 10.Kaesmacher J, Dobrocky T, Heldner MR, et al. Systematic review and meta-analysis on outcome differences among patients with TICI2b versus TICI3 reperfusions: success revisited. J Neurol Neurosurg Psychiatry . 2018;89:910–7. doi: 10.1136/jnnp-2017-317602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dargazanli C, Consoli A, Barral M, et al. Impact of modified TICI 3 versus modified TICI 2B reperfusion score to predict good outcome following endovascular therapy. AJNR Am J Neuroradiol. 2017;38:90–6. doi: 10.3174/ajnr.A4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleine JF, Wunderlich S, Zimmer C, et al. Time to redefine success? TICI 3 versus TICI 2B recanalization in middle cerebral artery occlusion treated with thrombectomy. J Neurointerv Surg. 2017;9:117–21. doi: 10.1136/neurintsurg-2015-012218. [DOI] [PubMed] [Google Scholar]
- 13.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO) - European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2023;15:e8. doi: 10.1136/neurintsurg-2018-014569. [DOI] [PubMed] [Google Scholar]
- 14.Alegiani AC, Dorn F, Herzberg M, et al. Systematic evaluation of stroke thrombectomy in clinical practice. The German stroke registry endovascular treatment. Int J Stroke. 2019;14:372–80. doi: 10.1177/1747493018806199. [DOI] [PubMed] [Google Scholar]
- 15.Faizy TD, Broocks G, Heit JJ, et al. Association between intravenous thrombolysis and clinical outcomes among patients with ischemic stroke and unsuccessful mechanical reperfusion. JAMA Netw Open . 2023;6:e2310213. doi: 10.1001/jamanetworkopen.2023.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simera I, Moher D, Hoey J, et al. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35–53. doi: 10.1111/j.1365-2362.2009.02234.x. [DOI] [PubMed] [Google Scholar]
- 17.Bendszus M, Bonekamp S, Berge E, et al. A randomized controlled trial to test efficacy and safety of thrombectomy in stroke with extended lesion and extended time window. Int J Stroke. 2019;14:87–93. doi: 10.1177/1747493018798558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkelmeier L, Broocks G, Kniep H, et al. Venous outflow profiles are linked to clinical outcomes in ischemic stroke patients with extensive baseline infarct. J Stroke. 2022;24:372–82. doi: 10.5853/jos.2022.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–51. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 20.Liebeskind DS, Bracard S, Guillemin F, et al. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11:433–8. doi: 10.1136/neurintsurg-2018-014127. [DOI] [PubMed] [Google Scholar]
- 21.LeCouffe NE, Kappelhof M, Treurniet KM, et al. 2B, 2C, or 3: what should be the angiographic target for endovascular treatment in ischemic stroke. Stroke. 2020;51:1790–6. doi: 10.1161/STROKEAHA.119.028891. [DOI] [PubMed] [Google Scholar]
- 22.Kitano T, Todo K, Yoshimura S, et al. Futile complete recanalization: patients characteristics and its time course. Sci Rep. 2020;10:4973. doi: 10.1038/s41598-020-61748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maïer B, Finitsis S, Mazighi M, et al. The benefit of a complete over a successful reperfusion decreases with time. Ann Neurol. 2023;93:934–41. doi: 10.1002/ana.26599. [DOI] [PubMed] [Google Scholar]
- 24.Kaesmacher J, Ospel JM, Meinel TR, et al. Thrombolysis in cerebral infarction 2B reperfusions: to treat or to stop. Stroke. 2020;51:3461–71. doi: 10.1161/STROKEAHA.120.030157. [DOI] [PubMed] [Google Scholar]
- 25.Kurmann CC, Mujanovic A, Piechowiak EI, et al. Heterogeneity of the relative benefits of TICI 2C/3 over TICI 2B50/2B67: are there patients who are less likely to benefit? Clin Neuroradiol. 2022;32:817–27. doi: 10.1007/s00062-021-01131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 27.Lee YB, Yoon W, Lee YY, et al. Predictors and impact of hemorrhagic transformations after endovascular thrombectomy in patients with acute large vessel occlusions. J Neurointerv Surg. 2019;11:469–73. doi: 10.1136/neurintsurg-2018-014080. [DOI] [PubMed] [Google Scholar]
- 28.Desai SM, Tonetti DA, Morrison AA, et al. Relationship between reperfusion and intracranial hemorrhage after thrombectomy. J Neurointerv Surg. 2020;12:448–53. doi: 10.1136/neurintsurg-2019-015337. [DOI] [PubMed] [Google Scholar]
- 29.Maros ME, Brekenfeld C, Broocks G, et al. Number of retrieval attempts rather than procedure time is associated with risk of symptomatic intracranial hemorrhage. Stroke. 2021;52:1580–8. doi: 10.1161/STROKEAHA.120.031242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namitome S, Uchida K, Shindo S, et al. Number of passes of endovascular therapy for stroke with a large ischemic core: secondary analysis of RESCUE-Japan LIMIT. Stroke. 2023;54:1985–92. doi: 10.1161/STROKEAHA.123.042552. [DOI] [PubMed] [Google Scholar]
- 31.Winkelmeier L, Faizy TD, Broocks G, et al. Association between recanalization attempts and functional outcome after thrombectomy for large ischemic stroke. Stroke. 2023;54:2304–12. doi: 10.1161/STROKEAHA.123.042794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flottmann F, van Horn N, Maros ME, et al. Early TICI 2B or late TICI 3 - is perfect the enemy of good? Clin Neuroradiol. 2022;32:353–60. doi: 10.1007/s00062-021-01048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steffen P, Van Horn N, McDonough R, et al. Continuing early mTICI 2B recanalization may improve functional outcome but is associated with a higher risk of intracranial hemorrhage. Front Neurol. 2022;13:955242. doi: 10.3389/fneur.2022.955242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request after approval of the ethics committee and all participating centers.