Abstract
The importance of influenza viruses as worldwide pathogens in humans, domestic animals, and poultry is well recognized. Discerning how influenza viruses interact with the host at a cellular level is crucial for a better understanding of viral pathogenesis. Influenza viruses induce apoptosis through mechanisms involving the interplay of cellular and viral factors that may depend on the cell type. However, it is unclear which viral genes induce apoptosis. In these studies, we show that the expression of the nonstructural (NS) gene of influenza A virus is sufficient to induce apoptosis in MDCK and HeLa cells. Further studies showed that the multimerization domain of the NS1 protein but not the effector domain is required for apoptosis. However, this mutation is not sufficient to inhibit apoptosis using whole virus.
Apoptosis is essential in many physiological processes, including tissue atrophy, development of the immune system, and tumor biology (19, 21, 28, 73). Apoptosis also plays an important role in the pathogenesis of many infectious diseases, including those caused by viruses (4, 27, 46, 48). Many virus infections result in apoptosis of host cells, and several viruses have evolved mechanisms to inhibit apoptosis (52, 62). Although there is no obvious advantage for the induction of apoptosis by a cytopathogenic virus, influenza viruses induce apoptosis in numerous cell types both in vivo (29) and in vitro (6, 18, 24, 30, 39, 49, 50, 57).
Influenza viruses induce apoptosis in cells that are permissive for virus replication like macrophages, Madin-Darby Canine Kidney (MDCK) and mink lung epithelial (Mv1Lu) cells (18, 24, 30, 37), and cells which do not support viral replication, such as HeLa cells or lymphocytes. The mechanism of influenza virus-induced apoptosis is not known in detail. However, it appears to involve both cellular and viral factors and may depend on the cell type. Influenza virus-induced apoptosis is inhibited by bcl-2 (37), v-FLIP, and crmA (59) and involves caspase activation (59). There is also evidence for indirect activation of apoptosis during infection. In HeLa cells, Fas antigen, a transmembrane protein belonging to the tumor necrosis factor receptor superfamily (36), and the Fas ligand are upregulated during influenza virus infection and are partially responsible for apoptosis in infected cells (10, 54–56, 67). Through these studies, we have a better understanding of which cellular pathways may be involved in influenza virus-induced apoptosis. However, it is still unclear which viral genes induce apoptosis in cells that support productive viral replication.
It is possible that the expression of any of the individual influenza virus genes may induce apoptosis in the infected cell. The neuraminidase protein (NA) appears to induce apoptosis through indirect and direct mechanisms. Indirectly, NA activates transforming growth factor β (TGF-β) in vivo and in vitro (49). TGF-β is a multifunctional growth-regulatory protein that induces apoptosis in many cell types, including lymphocytes (23) and MDCK cells (45, 49). Neutralizing antibodies against TGF-β only partially inhibit influenza virus-induced apoptosis, suggesting that NA can also induce apoptosis directly. These findings were further supported by Morris et al. (30), who showed that NA induces apoptosis in different cell lines by a TGF-β-independent, virus-dependent mechanism.
In MDCK cells, apoptosis occurs early in the course of viral replication (18). Therefore, it is likely that viral genes expressed early in replication and that interfere with normal cellular processes or associate with cell proteins involved in apoptosis may induce apoptosis directly. In these studies, we focused on the role of the nonstructural (NS) gene.
The NS gene is the smallest segment of the influenza A virus genome and is transcribed into a colinear mRNA encoding two proteins, NS1 and NS2 (also called NEP) (22, 38). Unlike NEP, NS1 is found only in infected cells. NS1 regulates numerous cellular functions during influenza virus infection by binding to polyadenylated mRNAs, inhibiting nuclear export (3, 16, 26, 40, 43); binding to small nuclear RNAs (snRNA), specifically to key components of the spliceosome, blocking pre-mRNA splicing (2, 8, 25, 44, 69) and inhibiting the polyadenylation of host cell mRNA (31); and interacting with several host cell proteins (26, 31, 71, 72). The RNA-binding activities of NS1 are based on the interaction of two functional domains: an RNA-binding domain at the amino end of the protein (amino acids 19 to 38) that binds to poly(A) sequences in mRNAs (43), and an effector domain (amino acids 134 to 161) that interacts with cellular proteins to inhibit mRNA nuclear export (40). These domains are highly conserved within the NS1 gene (20, 68), suggesting that NS1 is evolutionarily conserved.
Arguably, one of NS1's most important functions is inhibiting the activation of the double-stranded RNA (dsRNA) kinase (PKR), thus preventing the interferon (IFN)-mediated antiviral response (11, 12, 17, 26). Takizawa et al. showed that a mutation in the catalytic domain of PKR partially suppresses influenza virus-induced cell death (58). Based on the ability of NS1 to interfere with host cell RNA functions and block the activation of PKR, we propose that NS1 is involved in influenza virus-induced apoptosis. These studies show that expression of NS1 in different cell types is sufficient to induce cell death. Further, using NS1 mutants, we show that the RNA-binding/dimerization domain but not the effector domain is required for NS1-induced apoptosis in cell culture.
MATERIALS AND METHODS
Virus growth and cell culture.
A/Turkey/Ontario/7732/66 (A/Ty/Ont/66) (H5N9; University of Wisconsin influenza virus repository) was propagated in the allantoic cavities of 10- or 11-day old embryonated chicken eggs for 48 h at 35°C. The allantoic fluid was harvested, centrifuged for clarification, and stored at −70°C.
MDCK and HeLa cells were grown in modified Eagle's medium (MEM; Life Technologies, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS; Life Technologies) and 2 mM glutamine. All cells were maintained at 37°C in 5% CO2.
Construction of plasmids.
Full-length NS gene from A/Ty/Ont/66 was PCR amplified, ligated into the TA vector (Invitrogen, Carlsbad, Calif.), and then subcloned into the expression plasmid pUHD 10-3 (kindly provided by Hermann Bujard) at the EcoRI site. Expression vector pUHD 10-3 contains the heptamerized tetracycline repressor gene operators upstream of the cytomegalovirus promoter in two different orientation sites (15, 47, 51). Clones were screened for proper orientation by restriction enzyme digestion and sequence analysis.
All of the A/Udorn/72 NS1 genes described in this paper were expressed using an NS1 gene containing a 3′ splice site mutation (NS13′SS) ligated into the pBC12 vector via the BamHI site as described previously (2, 40, 68, 70). All mutations were confirmed by dideoxynucleotide sequencing. The cDNAs of NS13′SS (NS1), NS13′SS DM (no NS1 produced), NS1 M2 mutant (R19 and K20 changed to A [RK 19/20 AA]) and NS1Δ117-161 (Table 1) were cloned into the BamHI site of pUHD 10-3. Plasmids were transformed into Escherichia coli DH5α competent cells (Life Technologies), amplified, and purified using a Qiagen (Valencia, Calif.) purification kit.
TABLE 1.
Description of NS mutants
| Mutant | Position of mutation | aaa change (phenotype) | Cellular localization |
|---|---|---|---|
| NS13′SS | Only NS1 protein | Nuclear | |
| NS13′SS DM | No NS1 protein | ||
| M2 | aa 19 and 20 | RK → AA (−) | Nuclear |
| NSΔ117-161 | aa 117–161 | Deletion | Nuclear |
aa, amino acid.
Reassortant virus.
Two viruses containing a mutation in the NS1 RNA-binding domain (RK 19/20 AA or RK 19/20 AD) were constructed in an A/WSN/33 backbone by reverse genetics as described elsewhere (33, 34). Parental WSN and mutant viruses were propagated in MDCK cells grown in MEM containing 5% bovine serum albumin (BSA) and 1 μg of l-1-p-tosylamino-2-phenylethyl chloromethyl ketone–trypsin (Sigma Immunochemicals, St. Louis, Mo.) per ml. Viral titers were determined by 50% tissue culture infective dose.
Stable expression system.
The pUHD 10-3 expression plasmid containing full-length NS, NS1, and NS1 mutants and the transcriptional transactivator (pUHD 15-1) expression plasmid containing the tetracycline repressor gene fused with the viral VP16 coding sequence containing the neomycin cassette (kindly provided by Wen-Hwa Lee) (51) were electroporated into MDCK or HeLa cells. Briefly, cells were electroporated with 10 μg of DNA of both plasmids, and G418-resistant cell lines expressing the protein of interest were selected. The cell lines were maintained in MEM containing 10% FBS in the presence of 2.5 μg of anhydrotetracycline per ml (14) and 400 μg of G418 per ml. Incubating the cells in tetracycline-free medium induced expression of the full-length or mutant NS gene.
DNA fragmentation assay.
Fragmentation of cellular DNA into the characteristic apoptotic ladder was assessed as previously described (49), with minor modifications. Briefly, confluent monolayers of cells expressing pUHD 10-3 expression vector, NS gene, or NS1 mutants were washed with phosphate-buffered saline (PBS) and incubated for various times in tetracycline-free MEM containing 1% FBS. Prior to processing of the DNA, cell monolayers were trypsinized and cell numbers were determined. DNA was harvested by centrifuging the cells, resuspending the cell pellet in 300 μl of cold cell lysis buffer (10 mM Tris, 0.5% Triton X-100 [pH 7.5]), and incubating it on ice for 30 min. The lysates were centrifuged at 12,500 rpm for 10 min at 4°C, and the supernatants were extracted once with buffered phenol and once with chloroform. The DNA was precipitated with 300 mM NaCl and ethanol. DNA samples were resuspended in 15 μl of Tris-EDTA buffer (10 mM Tris, 1 mM EDTA [pH 7.5]) treated with RNase A (Life Technologies). Equal cell numbers were loaded and electrophoresed through a 2% SeaKem GTG agarose gel FMC Bioproducts, Rockland, Maine). The gel was stained with ethidium bromide to visualize DNA fragmentation. Cell viability was also assessed by trypan blue exclusion. Uninfected cells and cells infected with A/Ty/Ont/66 collected at identical times served as controls.
Western blot analysis.
Infected or DNA-transfected cell monolayers were lysed, and total protein concentration was determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.). Equal protein concentrations were heated for 3 min at 100°C in sample buffer containing β-mercaptoethanol and resolved by sodium dodecyl sulfate–5 to 20% gradient polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to nitrocellulose, the proteins were blocked with 1% powdered milk in Tris-buffered saline with 0.1% Tween 20 (TTBS; Sigma Immunochemicals) for 30 min at room temperature. The nitrocellulose was probed for NS protein expression using mouse monoclonal or rabbit polyclonal antibodies diluted 1:1,000 in TTBS and incubated for 1 h at room temperature. Proteins were detected by incubation with a secondary antibody conjugated to horseradish peroxidase followed by enhanced chemiluminescence (Amersham, Arlington Heights, Ill.) according to the manufacturer's protocols.
RESULTS
Expression of NS proteins induces apoptosis.
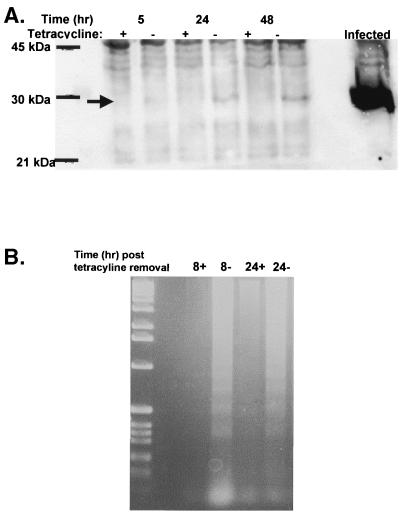
Previous studies showed that HeLa cells undergo apoptosis during influenza virus infection. To determine if the expression of NS1 and NS2 was sufficient to induce apoptosis, HeLa cells were transfected with the full-length NS gene from A/Ty/Ont/66 in a tetracycline-regulated system, and cells expressing NS proteins were selected and screened for protein expression by Western blot analysis. NS protein expression was evident 24 h after tetracycline withdrawal and increased slightly at 48 h. NS protein levels in the NS-expressing cells were much lower than levels in infected cells (Fig. 1A). In spite of low protein levels, DNA fragmentation was observed in NS-expressing cells within 8 h after the removal of tetracycline (Fig. 1B). DNA fragmentation was not observed in HeLa cells in the presence of tetracycline.
FIG. 1.
Expression of NS induces apoptosis in HeLa cells. (A) Confluent cultures of HeLa cells expressing pUHD 10-3 containing the NS gene from A/Ty/Ont/66 were washed two times with PBS and then incubated for 5, 24, or 48 h in medium with (+) or without (−) anhydrotetracycline. Cell monolayers were lysed, and 5 mg of total protein was loaded per lane under reducing conditions and resolved by SDS-PAGE. After being transferred to nitrocellulose, proteins were probed for NS with a mouse monoclonal antibody against NS. Bands were detected by enhanced chemiluminescence as instructed by the manufacturer. The arrow indicates the location of NS1. Molecular size markers are indicated on the left. MDCK cells infected with A/Ty/Ont/66 at an MOI of 0.5 for 24 h served as a positive control (virus lane). (B) Confluent cultures of HeLa cells in 25-cm2 flasks expressing pUHD 10-3 containing the NS gene from A/Ty/Ont/66 were washed two times with PBS and then incubated for 8 or 24 h in medium containing anhydrotetracycline (+) or anhydrotetracycline-free medium (−) containing 1% FBS at 37°C in 5% CO2. DNA was collected and analyzed for DNA fragmentation by agarose gel analysis.
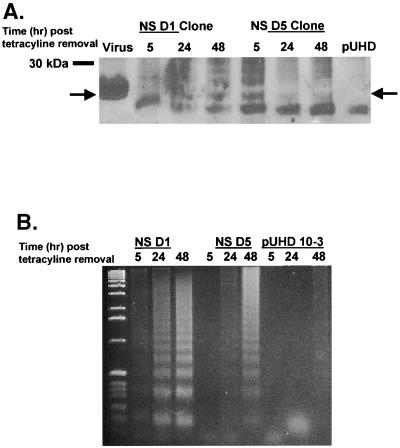
Similar studies were performed with MDCK cells. MDCK cells were transfected with the full-length NS gene from A/Ty/Ont/66 in a tetracycline-regulated system, and two clones (NS D1 and NS D5) expressing NS proteins were selected. Similar to the case for HeLa cells, the levels of NS protein were much lower in all clones tested than in infected cells (Fig. 2A). In spite of low protein levels, NS D1 and NS D5 were tested for DNA fragmentation after the removal of tetracycline. Increased fragmentation was observed in both NS D1 and NS D5 in a time-dependent manner (Fig. 2B). DNA fragmentation was observed as early as 5 h after the removal of tetracycline in clone D1; however, clone D5 had slower kinetics, and laddering was not evident until 24 h after tetracycline removal (Fig. 2B). No DNA fragmentation was observed in MDCK cells expressing only vector after the removal of tetracycline. Similar results were observed in mink lung epithelial and chicken embryo fibroblast (CEF) cells (data not shown). These results show that NS induces apoptosis in different cell types regardless of their ability to support productive viral replication.
FIG. 2.
Expression of NS induces apoptosis. (A) Confluent cultures of MDCK cells expressing pUHD 10-3 containing the NS gene from A/Ty/Ont/66 were washed two times with PBS and then incubated for 5, 24, or 48 h in medium without anhydrotetracycline. Cell monolayers were lysed, and 5 mg of total protein was loaded per lane under reducing conditions and resolved by SDS-PAGE. After being transferred to nitrocellulose, proteins were probed for NS with a mouse monoclonal antibody against NS. Bands were detected by enhanced chemiluminescence as instructed by the manufacturer. The arrow indicates the location of NS1. Molecular size markers are indicated on the left. MDCK cells infected with A/Ty/Ont/66 at an MOI of 0.5 for 24 h served as a positive control (virus lane), and cells expressing empty vector (pUHD) served as a negative control. Results for two different clones (D1 and D5) are shown. (B) Confluent cultures of MDCK cells in 25-cm2 flasks expressing pUHD 10-3 containing the NS gene from A/Ty/Ont/66 or empty vector (pUHD) were washed two times with PBS and then incubated for 5, 24, or 48 h in anhydrotetracycline-free MEM containing 1% FBS at 37°C in 5% CO2. DNA was collected and analyzed for DNA fragmentation by agarose gel analysis. Two different clones, D1 and D5, expressing NS protein were analyzed.
NS1 induces apoptosis.
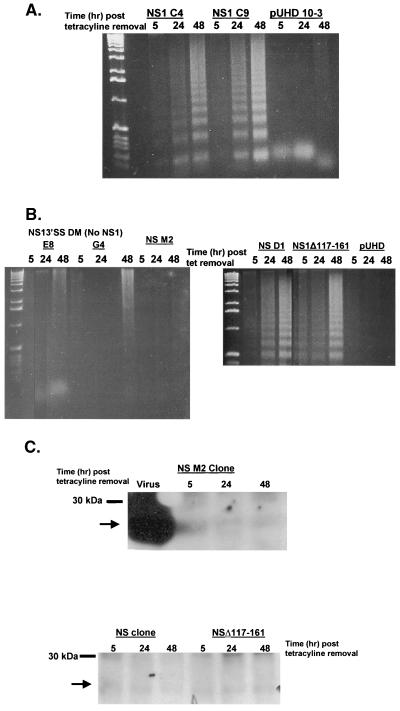
The NS gene of influenza virus encodes two proteins, NS1 and NEP (NS2). Attempts to express NEP in the tetracycline system were unsuccessful. Therefore, we examined the induction of DNA laddering in two different MDCK clones expressing NS1. Similar to expression of the NS proteins, NS1 expression induced DNA laddering in a time-dependent manner (Fig. 3A). Both clones (NS1 C4 and NS1 C9) showed clear apoptosis 24 h after the removal of tetracycline, with more intense laddering observed at 48 h. The expression of NS1 protein is required for apoptosis in this system. Two cellular clones expressing an NS1 splice site mutation containing two stop sites (E8 and G4), which makes no NS1 protein, failed to induce DNA laddering after tetracycline withdrawal (Fig. 3B). No fragmentation was observed in the MDCK cells expressing vector alone. Similar to the NS protein analysis, the clones expressing NS1 had significantly less NS1 protein than did infected cells.
FIG. 3.
The RNA-binding domain of NS1 is required for apoptosis. (A) Confluent cultures of MDCK cells expressing pUHD 10-3 containing the NS1 gene from A/Udom/72 or empty vector (pUHD) were washed two times with PBS and then incubated for 5, 24, or 48 h in anhydrotetracycline-free MEM containing 1% FBS at 37°C in 5% CO2. DNA was collected and analyzed for DNA fragmentation by agarose gel analysis. Two different clones, C4 and C9, expressing NS1 protein were analyzed. (B) Confluent cultures of MDCK cells expressing pUHD 10-3 containing an NS mutant that fails to produce NS1 protein (NS13′SS DM clones E8 and G4), a mutation in the RNA-binding domain (M2), full-length NS (clone D1), or a deletion of the effector domain (NS1Δ117-161) were washed two times with PBS and then incubated for 5, 24, or 48 h in anhydrotetracycline-free MEM containing 1% FBS at 37°C in 5% CO2. DNA was collected and analyzed for DNA fragmentation by agarose gel analysis. (C) Confluent cultures of MDCK cells expressing pUHD 10-3 containing the NS M2 (RNA-binding domain mutation), full-length NS gene from A/Ty/Ont/66, or NS1Δ117-161 were washed two times with PBS and then incubated for 5, 24, or 48 h in medium without anhydrotetracycline. Cell monolayers were lysed, and 5 mg of total protein was loaded per lane under reducing conditions and resolved by SDS-PAGE. After being transferred to nitrocellulose, proteins were probed for NS with a rabbit polyclonal antibody against NS. Bands were detected by enhanced chemiluminescence as instructed by the manufacturer. The arrow indicates the location of NS. Molecular size markers are indicated on the left. MDCK cells infected with A/Ty/Ont/66 at an MOI of 0.5 for 24 h served as a positive control (virus lane).
The effector domain of NS1 is not required for apoptosis.
NS1 has a number of described functional domains, including an RNA-binding/dimerization domain and an effector domain that interacts with cellular proteins (3, 25, 26, 32, 40–44, 53, 68). To determine if either of these domains is required for the induction of apoptosis, MDCK cells expressing NS1 proteins with a mutation in the functional RNA-binding domain/dimerization (amino acids 19 and 20 mutated from RK to AA [M2]) or a deletion of the effector domain (amino acids 117 to 161) were tested for DNA fragmentation. The deletion of the effector domain had no effect on DNA laddering (Fig. 3B). Fragmentation was observed at 5 h and then increased through 48 h, similar to clones expressing full-length NS1. In contrast, a mutation in the RNA-binding/dimerization functional domain resulted in no DNA fragmentation even at 48 h after tetracycline removal (Fig. 3B). This result could be explained by the loss of NS1 protein expression in cells expressing the RNA-binding/dimerization domain. However, Western blot analysis shows that this clone was expressing NS1 protein within 5 h after removal of tetracycline and that protein levels were still detectable at 48 h (Fig. 3C). These studies show that expression of the NS1 protein is sufficient to induce apoptosis in numerous cell types by a mechanism that is independent of the effector domain.
Reassortant WSN virus containing a mutation in the NS1 RNA-binding domain induces apoptosis.
The generation of the plasmid-based reverse genetics system provides a powerful opportunity to understand the importance of single gene mutations within the context of whole virus (33, 34). Based on the results described above, the RNA-binding/dimerization region of the NS1 protein is required for NS-induced apoptosis in MDCK cells. To determine if this mutation is sufficient to delete influenza virus-induced apoptosis, we attempted to generate viruses whose NS1 proteins have mutations that abolish RNA-binding/dimerization activity. Two different mutations were generated, RK 19/20 AA and RK 19/20 AD. Both of the mutant viruses were infectious, although they were highly attenuated in MDCK cells compared to parental WSN virus, averaging a 3-log-lower virus titer. To determine if the mutant viruses could induce apoptosis in MDCK cells, cells were infected with a multiplicity of infection (MOI) of 2 and examined for DNA laddering at 24 and 48 h postinfection (p.i.). A high MOI was used to ensure maximum levels of apoptosis independent of replication differences between the parental strain and the mutant viruses. At 24 h p.i., the RK 19/20 AA mutant virus induced laddering similar to that observed with WSN virus (Fig. 4). In contrast, no laddering was observed at 24 hr p.i. with the RK 19/20 AD virus; however, both viruses induced apoptosis by 48 h, similar to the WSN parental virus. Similar results were seen in mink lung epithelial and CEF cells. These results suggest that while the RNA-binding/dimerization domain is required for NS1-induced apoptosis, a mutation in this region is not sufficient to inhibit influenza virus-induced apoptosis.
FIG. 4.
Influenza viruses containing mutated NS1 RNA-binding sites induce apoptosis. Confluent cultures of MDCK cells were washed two times with PBS, incubated with MEM alone (lane 1) or MEM with A/WSN parental virus (lane 2), A/WSN NS1 RK 19/20 AA (lane 3), or NS1 RK 19/20 AD (lane 4) at an MOI of 2 and incubated for 1 h at 37°C in 5% CO2. After the 1-h binding period, the cells were washed with PBS to remove residual virus and incubated for 24 or 48 h in MEM containing 5% BSA. DNA was collected and analyzed for DNA fragmentation by agarose gel analysis.
DISCUSSION
The above results show that the expression of the NS1 protein of influenza virus in MDCK and HeLa cells is sufficient to induce apoptosis. Western blot analysis showed that very little NS1 protein was expressed in the NS clones compared to influenza virus-infected cells. MDCK cells are difficult to transfect, and even after electroporation, only 1 to 10% of the surviving cells expressed NS. Additionally, the NS1 protein proved to be very toxic to MDCK cells. We were unable to generate stable cell lines expressing NS1 protein even under the control of the tetracycline-regulated system. The system was slightly leaky even in the presence of high concentrations of anhydrotetracycline, a stable tetracycline derivative. During selection of clones, cells expressing high concentrations of NS died; therefore, the G418 selection time was shortened from 2 weeks to 1 week to allow analysis of NS-expressing clones. Attempts were also made to express NS1 in MDCK cells by using the ecdysone system, with similar results (data not shown). Similar problems were observed in mink lung epithelial and CEF cells but not HeLa cells. It is unclear why the HeLa cells were better able to support expression of the NS protein.
Further studies using well-described NS mutants showed that the RNA-binding domain, but not the effector domain, is essential for the induction of apoptosis. Previous studies showed that mutating amino acids R19 and K20 to alanine resulted in a loss of dsRNA binding and U6 snRNA binding (32). More recently, Wang et al. showed that R19 is essential for dimerization of the NS1 protein (70). These results suggest that RNA binding and/or dimerization of NS1 protein may be required for the induction of apoptosis by NS. The mutation of the effector domain would have no effect on dimerization or RNA binding. In support of these findings, a laboratory variant of A/Turkey/Oregon/71 virus, which encodes an NS1 protein that is only 125 amino acids long and lacks an effector domain (35), also induces apoptosis (data not shown).
Using a WSN backbone, influenza viruses containing mutations in the RNA-binding domain of the NS1 protein were generated and examined for apoptosis. The growth of the mutated viruses was highly attenuated compared to the WSN parental strain in MDCK cells (data not shown). However, the mutated and parental viruses grew to equal titers in Vero cells and CEF cells prepared from 6-day-old embryos (unpublished data). Similar results were shown with viruses containing long deletions in the NS1 protein or lacking the NS1 gene entirely (5, 12, 17). These results suggest that mutating the RNA-binding/dimerization region of NS1 protein may inhibit NS1's ability to inhibit PKR activation and IFN response. Despite attenuation in MDCK cells, the mutated viruses induced apoptosis similarly to parental WSN virus when used at an MOI of 2. In addition, apoptosis was inhibited with caspase inhibitors (59), suggesting that the viruses used similar cellular pathways leading to cell death.
The induction of apoptosis in the NS1 mutant viruses may also be due to the activation of the IFN-induced PKR and IFN (10, 54–56, 58, 67). During virus infection, PKR is activated by its interaction with dsRNA, resulting in a cascade of effects including activation of transcriptional factors leading to apoptosis (13). NS1 inhibits the activation of PKR through upregulation of a cellular PKR inhibitor (61), by binding directly to PKR (17), and by inhibiting IFN regulatory factor 3 (60). Takizawa et al. showed that PKR is involved in influenza virus-induced apoptosis (55, 58). The NS1 mutant viruses may be unable to inhibit PKR activation by dsRNA, leading to PKR autophosphorylation and increased IFN levels, resulting in cell death. Studies examining this hypothesis are under way. In the vector system, no dsRNA should be generated and PKR should remain inactive. However, it is possible that dsRNA is generated by the plasmid, and IFN levels may be elevated, resulting in apoptosis. Studies are under way to determine how expressed NS1 is inducing apoptosis and what cellular factors are involved.
We and others showed that NA induces apoptosis directly (30) and through the activation of TGF-β (49). It is probable that a portion of the apoptosis observed with the NS mutant viruses is due to NA, whether directly or through TGF-β activation. Finally, the expression of other viral proteins may induce apoptosis. During the NS studies, we also expressed the matrix (M) and nucleoprotein (NP) genes of A/Ty/Ont/66. Expression of the M gene in the tetracycline-regulated system had no effect on cell viability, and it was possible to make a stable cell line expressing M proteins. In contrast, NP also induced apoptosis in MDCK cells, although with different kinetics than NS1 (data not shown).
The question still remains as to whether virus-induced apoptosis is advantageous for viral spread or is advantageous to the host by possibly reducing viral titers. Depletion of lymphocytes in chickens infected with the highly virulent avian influenza Ty/Ont virus (18, 65, 66) and mice infected with the highly virulent human A/Hong Kong/483/97 virus (64) is associated with apoptosis. It has been suggested that influenza virus-induced apoptosis of lymphocytes may be important in viral pathogenesis in highly pathogenic influenza virus. In addition, cells undergoing influenza virus-induced apoptosis are taken up by dendritic cells, inducing a cytotoxic T-cell response (1). Similarly, macrophages phagocytose infected cells through an apoptosis-dependent mechanism (9). These results suggest that understanding the role of apoptosis in influenza virus pathogenesis will be complex and may be dependent on the cell type and environment. However, these studies will increase our understanding of viral pathogenesis and may lead to new therapies for influenza virus infection.
ACKNOWLEDGMENTS
We are very appreciative to Robert Krug (University of Texas at Austin) for providing the NS1 and NS2 constructs used in these studies, for providing technical assistance in establishing the cell lines, and for critically reading and revising the manuscript. We are also grateful to Martha McGregor and Laura Kelley for expert technical assistance, to Hermann Bujard (University of Heidelberg) for the tetracycline-responsive system, to Robert Webster (St. Jude's Children's Hospital) for the monoclonal antibodies against NS proteins, and to Chris Olsen, Diane Larsen, Matthew Koci, Holly Sellers, and Terry Tumpey for critically reading and revising the manuscript.
This work was supported by Public Health Service research grants from the National Institute of Allergy and Infectious Diseases to V.S.H. and Y.K. S.S-C. was supported initially by a postdoctoral training fellowship in tumor virology through the McArdle Cancer Center at the University of Wisconsin followed by USDA CRIS project 661232000020.
REFERENCES
- 1.Albert M L, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I- restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Caplen F V, Nemeroff M E, Qiu Y, Krug R M. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 1992;6:255–267. doi: 10.1101/gad.6.2.255. [DOI] [PubMed] [Google Scholar]
- 3.Chien C Y, Tejero R, Huang Y, Zimmerman D E, Rios C B, Krug R M, Montelione G T. A novel RNA-binding motif in influenza A virus non-structural protein 1. Nat Struct Biol. 1997;4:891–895. doi: 10.1038/nsb1197-891. [DOI] [PubMed] [Google Scholar]
- 4.Collins M. Potential roles of apoptosis in viral pathogenesis. Am J Respir Crit Care Med. 1995;152:S20–S24. doi: 10.1164/ajrccm/152.4_Pt_2.S20. [DOI] [PubMed] [Google Scholar]
- 5.Egorov A, Brandt S, Sereinig S, Romanova J, Ferko B, Katinger D, Grassauer A, Alexandrova G, Katinger H, Muster T. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J Virol. 1998;72:6437–6441. doi: 10.1128/jvi.72.8.6437-6441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fesq H, Bacher M, Nain M, Gemsa D. Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology. 1994;190:175–182. doi: 10.1016/S0171-2985(11)80292-5. [DOI] [PubMed] [Google Scholar]
- 7.Fodor E, Devenish L, Engelhardt O G, Palese P, Brownlee G G, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortes P, Beloso A, Ortin J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 1994;13:704–712. doi: 10.1002/j.1460-2075.1994.tb06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto I, Pan J, Takizawa T, Nakanishi Y. Virus clearance through apoptosis-dependent phagocytosis of influenza A virus-infected cells by macrophages. J Virol. 2000;74:3399–3403. doi: 10.1128/jvi.74.7.3399-3403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto I, Takizawa T, Ohba Y, Nakanishi Y. Co-expression of Fas and Fas-ligand on the surface of influenza virus-infected cells. Cell Death Differ. 1998;5:426–431. doi: 10.1038/sj.cdd.4400362. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Sastre A, Durbin R K, Zheng H, Palese P, Gertner R, Levy D E, Durbin J E. The role of interferon in influenza virus tissue tropism. J Virol. 1998;72:8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy D E, Durbin J E, Palese P, Muster T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 13.Gil J, Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis. 2000;5:107–114. doi: 10.1023/a:1009664109241. [DOI] [PubMed] [Google Scholar]
- 14.Gossen M, Bujard H. Anhydrotetracycline, a novel effector for tetracycline controlled gene expression systems in eukaryotic cells. Nucleic Acids Res. 1993;21:4411–4412. doi: 10.1093/nar/21.18.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatada E, Fukuda R. Binding of influenza A virus NS1 protein to dsRNA in vitro. J Gen Virol. 1992;73:3325–3329. doi: 10.1099/0022-1317-73-12-3325. [DOI] [PubMed] [Google Scholar]
- 17.Hatada E, Saito S, Fukuda R. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J Virol. 1999;73:2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinshaw V S, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 20.Kawaoka Y, Gorman O T, Ito T, Wells K, Donis R O, Castrucci M R, Donatelli I, Webster R G. Influence of host species on the evolution of the nonstructural (NS) gene of influenza A viruses. Virus Res. 1998;55:143–156. doi: 10.1016/s0168-1702(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 21.Kerr J F, Winterford C M, Harmon B V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. . (Erratum, 73:3108.) [DOI] [PubMed] [Google Scholar]
- 22.Lamb R A, Krug R M. Orthomyxoviridae. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1353–1396. [Google Scholar]
- 23.Lomo J, Blomhoff H K, Beiske K, Stokke T, Smeland E B. TGF-beta 1 and cyclic AMP promote apoptosis in resting human B lymphocytes. J Immunol. 1995;154:1634–1643. [PubMed] [Google Scholar]
- 24.Lowy R J, Dimitrov D S. Characterization of influenza virus-induced death of J774.1 macrophages. Exp Cell Res. 1997;234:249–258. doi: 10.1006/excr.1997.3602. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Qian X Y, Krug R M. The influenza virus NS1 protein: a novel inhibitor of pre-mRNA splicing. Genes Dev. 1994;8:1817–1828. doi: 10.1101/gad.8.15.1817. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Wambach M, Katze M G, Krug R M. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology. 1995;214:222–228. doi: 10.1006/viro.1995.9937. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig S, Pleschka S, Wolff T. A fatal relationship—influenza virus interactions with the host cell. Viral Immunol. 1999;12:175–196. doi: 10.1089/vim.1999.12.175. [DOI] [PubMed] [Google Scholar]
- 28.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 29.Mori I, Komatsu T, Takeuchi K, Nakakuki K, Sudo M, Kimura Y. In vivo induction of apoptosis by influenza virus. J Gen Virol. 1995;76:2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 30.Morris S J, Price G E, Barnett J M, Hiscox S A, Smith H, Sweet C. Role of neuraminidase in influenza virus-induced apoptosis. J Gen Virol. 1999;80:137–146. doi: 10.1099/0022-1317-80-1-137. [DOI] [PubMed] [Google Scholar]
- 31.Nemeroff M E, Barabino S M, Li Y, Keller W, Krug R M. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- 32.Nemeroff M E, Qian X Y, Krug R M. The influenza virus NS1 protein forms multimers in vitro and in vivo. Virology. 1995;212:422–428. doi: 10.1006/viro.1995.1499. [DOI] [PubMed] [Google Scholar]
- 33.Neumann G, Kawaoka Y. Genetic engineering of influenza and other negative-strand RNA viruses containing segmented genomes. Adv Virus Res. 1999;53:265–300. doi: 10.1016/s0065-3527(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 34.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez D R, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norton G P, Tanaka T, Tobita K, Nakada S, Buonagurio D A, Greenspan D, Krystal M, Palese P. Infectious influenza A and B virus variants with long carboxyl terminal deletions in the NS1 polypeptides. Virology. 1987;156:204–213. doi: 10.1016/0042-6822(87)90399-0. [DOI] [PubMed] [Google Scholar]
- 36.Oehm A, Behrmann I, Falk W, Pawlita M, Maier G, Klas C, Li-Weber M, Richards S, Dhein J, Trauth B C, et al. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709–10715. [PubMed] [Google Scholar]
- 37.Olsen C W, Kehren J C, Dybdahl-Sissoko N R, Hinshaw V S. bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J Virol. 1996;70:663–666. doi: 10.1128/jvi.70.1.663-666.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Neill R E, Talon J, Palese P. The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J. 1998;17:288–296. doi: 10.1093/emboj/17.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price G E, Smith H, Sweet C. Differential induction of cytotoxicity and apoptosis by influenza virus strains of differing virulence. J Gen Virol. 1997;78:2821–2829. doi: 10.1099/0022-1317-78-11-2821. [DOI] [PubMed] [Google Scholar]
- 40.Qian X Y, Alonso-Caplen F, Krug R M. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J Virol. 1994;68:2433–2441. doi: 10.1128/jvi.68.4.2433-2441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian X Y, Chen Z Y, Zhang J, Rabson A B, Krug R M. New approach for inhibiting Rev function and HIV-1 production using the influenza virus NS1 protein. Proc Natl Acad Sci USA. 1996;93:8873–8879. doi: 10.1073/pnas.93.17.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian X Y, Chien C Y, Lu Y, Montelione G T, Krug R M. An amino-terminal polypeptide fragment of the influenza virus NS1 protein possesses specific RNA-binding activity and largely helical backbone structure. RNA. 1995;1:948–956. [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu Y, Krug R M. The influenza virus NS1 protein is a poly(A)-binding protein that inhibits nuclear export of mRNAs containing poly(A) J Virol. 1994;68:2425–2432. doi: 10.1128/jvi.68.4.2425-2432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu Y, Nemeroff M, Krug R M. The influenza virus NS1 protein binds to a specific region in human U6 snRNA and inhibits U6–U2 and U6–U4 snRNA interactions during splicing. RNA. 1995;1:304–316. [PMC free article] [PubMed] [Google Scholar]
- 45.Quinlan M P. E1A 12S in the absence of E1B or other cooperating oncogenes enables cells to overcome apoptosis. Oncogene. 1993;8:3289–3296. [PubMed] [Google Scholar]
- 46.Razvi E S, Welsh R M. Apoptosis in viral infections. Adv Virus Res. 1995;45:1–60. doi: 10.1016/s0065-3527(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 47.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roulston A, Marcellus R C, Branton P E. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 49.Schultz-Cherry S, Hinshaw V S. Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol. 1996;70:8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz-Cherry S, Krug R M, Hinshaw V S. Induction of apoptosis by influenza virus. Semin Virol. 1998;8:491–495. [Google Scholar]
- 51.Shan B, Lee W H. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 53.Shih S R, Nemeroff M E, Krug R M. The choice of alternative 5′ splice sites in influenza virus M1 mRNA is regulated by the viral polymerase complex. Proc Natl Acad Sci USA. 1995;92:6324–6328. doi: 10.1073/pnas.92.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takizawa T. Induction of apoptosis by influenza virus infection. Uirusu. 1997;47:69–76. doi: 10.2222/jsv.47.69. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 55.Takizawa T. Mechanism of the induction of apoptosis by influenza virus infection. Nippon Rinsho. 1996;54:1836–1841. . (In Japanese.) [PubMed] [Google Scholar]
- 56.Takizawa T, Fukuda R, Miyawaki T, Ohashi K, Nakanishi Y. Activation of the apoptotic Fas antigen-encoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology. 1995;209:288–296. doi: 10.1006/viro.1995.1260. [DOI] [PubMed] [Google Scholar]
- 57.Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993;74:2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 58.Takizawa T, Ohashi K, Nakanishi Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J Virol. 1996;70:8128–8132. doi: 10.1128/jvi.70.11.8128-8132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takizawa T, Tatematsu C, Ohashi K, Nakanishi Y. Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol Immunol. 1999;43:245–252. doi: 10.1111/j.1348-0421.1999.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 60.Talon J, Horvath C M, Polley R, Basler C F, Muster T, Palese P, Garcia-Sastre A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol. 2000;74:7989–7996. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan S L, Katze M G. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J Interferon Cytokine Res. 1998;18:757–766. doi: 10.1089/jir.1998.18.757. [DOI] [PubMed] [Google Scholar]
- 62.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 64.Tumpey T M, Lu X, Morken T, Zaki S R, Katz J M. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Campen H, Easterday B C, Hinshaw V S. Destruction of lymphocytes by a virulent avian influenza A virus. J Gen Virol. 1989;70:467–472. doi: 10.1099/0022-1317-70-2-467. [DOI] [PubMed] [Google Scholar]
- 66.Van Campen H, Easterday B C, Hinshaw V S. Virulent avian influenza A viruses: their effect on avian lymphocytes and macrophages in vivo and in vitro. J Gen Virol. 1989;70:2887–2895. doi: 10.1099/0022-1317-70-11-2887. [DOI] [PubMed] [Google Scholar]
- 67.Wada N, Matsumura M, Ohba Y, Kobayashi N, Takizawa T, Nakanishi Y. Transcription stimulation of the Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J Biol Chem. 1995;270:18007–18012. doi: 10.1074/jbc.270.30.18007. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Krug R M. The RNA-binding and effector domains of the viral NS1 protein are conserved to different extents among influenza A and B viruses. Virology. 1996;223:41–50. doi: 10.1006/viro.1996.0453. [DOI] [PubMed] [Google Scholar]
- 69.Wang W, Krug R M. U6atac snRNA, the highly divergent counterpart of U6 snRNA, is the specific target that mediates inhibition of AT-AC splicing by the influenza virus NS1 protein. RNA. 1998;4:55–64. . (Erratum, 4:348.) [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, Riedel K, Lynch P, Chien C Y, Montelione G T, Krug R M. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA. 1999;5:195–205. doi: 10.1017/s1355838299981621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff T, O'Neill R E, Palese P. Interaction cloning of NS1-l, a human protein that binds to the nonstructural NS1 proteins of influenza A and B viruses. J Virol. 1996;70:5363–5372. doi: 10.1128/jvi.70.8.5363-5372.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolff T, O'Neill R E, Palese P. NS1-binding protein (NS1-BP): a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J Virol. 1998;72:7170–80. doi: 10.1128/jvi.72.9.7170-7180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young L S, Dawson C W, Eliopoulos A G. Viruses and apoptosis. Br Med Bull. 1997;53:509–521. doi: 10.1093/oxfordjournals.bmb.a011627. [DOI] [PubMed] [Google Scholar]