Abstract
Abstract
Objective
HIV-1 management has advanced significantly with antiretroviral therapy (ART), yet challenges persist, including low-level HIV-1 viraemia (LLV). LLV presents a complex scenario, with varied definitions in the literature, reflecting uncertainties in its clinical interpretation. Questions arise regarding the underlying mechanisms of LLV, whether it signifies ongoing viral replication or stems from other factors. This study aimed to systematically review strategies for LLV management, providing insights into optimal clinical approaches.
Methods
MEDLINE, EMBASE, Cochrane Library, Web of Science and Canadian Agency for Drugs and Technologies in Health were searched for relevant literature on LLV management. We included studies published between 2004 and 2024, assessing interventions such as ART modification, genotypic resistance testing, adherence assessment, performing therapeutic drug monitoring, testing for chronic coinfections and assessing the viral reservoir via HIV DNA quantification. Meta-analyses were conducted where feasible.
Results
The systematic review identified 48 eligible records. Findings indicated limited evidence supporting the effectiveness of ART regimen modification in achieving virological suppression among individuals with LLV. However, studies assessing genotypic resistance testing revealed a significant association between resistance-associated mutations and virological suppression during LLV. Adherence to ART emerged as a critical determinant of treatment efficacy, with interventions showing promise in achieving viral suppression. The clinical utility of therapeutic drug monitoring in managing LLV remained inconclusive. Gaps in the literature were identified regarding follow-up scheduling, managing concurrent chronic infections and assessing inflammatory markers in LLV management.
Conclusions
While ART modification may not consistently achieve virological suppression, genotypic resistance testing may offer insights into treatment outcomes. Adherence to ART emerged as a crucial factor, necessitating tailored interventions. However, further research is needed to elucidate the clinical utility of therapeutic drug monitoring and other management strategies. The study highlights the importance of ongoing research to refine therapeutic approaches and improve patient outcomes in LLV management.
PROSPERO registration number
CRD42024511492.
Keywords: HIV, Viral Load, SYSTEMATIC REVIEW, META-ANALYSIS, Anti-HIV Agents
WHAT IS ALREADY KNOWN ON THIS TOPIC
Low-level HIV-1 viraemia (LLV) remains a challenge, with its clinical interpretation being uncertain.
WHAT THIS STUDY ADDS
The study systematically reviewed the available strategies for LLV management and identified gaps in current research concerning follow-up protocols, management of co-infections, and inflammatory marker assessments in LLV patients.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
The findings highlight the need for tailored interventions to improve adherence to ART, which could influence clinical practice and the use of genotypic resistance testing in guiding LLV management strategies.
Introduction
HIV-1 management has evolved significantly during the last years, primarily due to the near-universal effectiveness of antiretroviral therapy (ART) in controlling viral replication.1 2 However, challenges, such as reducing the burden of non-AIDS-related morbidity and mortality, combating stigma and ensuring universal access to care, still persist. One of these conundrums is represented by low-level HIV-1 viraemia (LLV).3
The literature defines LLV so heterogeneously that it is impossible to refer to all its possible definitions without adopting one of a specific expert or guideline.2,8 Broadly, LLV could be characterised as low-copy HIV-1 replication occurring either below the threshold for suppression (eg, residual viraemia (RV)) or above it but below the threshold for defining viral failure. Nonetheless, even these definitions vary tremendously across the literature.2,8
Prevalence estimates of LLV range from 5% to 30% among people living with HIV (PWH) undergoing ART, depending on the definition used.9 This lack of consensus on the definitions represents a significant challenge in dealing with LLV. Without a clear lower limit definition, determining when no action is required becomes difficult. Moreover, lacking knowledge of the upper limit, that is, virological failure, impedes a clear understanding of the need for an ART modification.
The semantic problem of LLV’s exact definition possibly stems from a dissonance among experts on its virological identity and aetiology. Ongoing exploration into whether LLV stems from actual ongoing viral replication, viral resurgence, persistent viral release from HIV-1 reservoir or a yet-to-be-defined phenomenon contributes to its ambiguity.2 3 While LLV’s clinical consequences remain incompletely elucidated, accumulating and yet contrasting evidence links it to elevated virological failure rates and resistance-associated mutations (RAM) development, although this is less reported in the case of newer first-line antiretroviral drugs.10 11 Additionally, LLV may foster residual immune activation and inflammation, potentially heightening the risk of non-AIDS-related morbidity, such as cardiovascular complications.12
Amidst the emergence of novel ART strategies, such as the long-acting (LA) injectable strategies, and drug toxicity mitigation strategies, uncertainty remains due to concerns regarding LLV and its role in RAM development, blips and viral failure. Furthermore, the correlation between specific ART regimens, treatment adherence, the role of vaccinations or chronic coinfections and LLV development remains inconclusive, necessitating further investigation.13,15 Nevertheless, globally, the detrimental impact of prolonged LLV on clinical outcomes is undeniable.2 3 16 To date, no definitive evidence is available on the optimal management of this fairly common clinical finding. In this context, the present work aimed at providing a Grading of Recommendations, Assessment, Development and Evaluations (GRADE)-based systematic review of the outcomes of several possible management strategies of LLV.
Context and scope
The present work is part of an editorial project comprising a systematic review and meta-analysis of the literature and a modified Delphi consensus.17 The project was aimed at providing GRADE-based and expert recommendations on the management of LLV.
Methods
Search question
In order to guide the building of a search strategy for our systematic review, the following PIECOST (population, intervention/exposure, comparator, outcome, study design, time frame) format question was formulated: ‘In Adult PWH on fully active antiretroviral therapy (ART) presenting residual HIV replication (residual, low level viremia and viral blips) (P), does (1) modifying the ART regimen; (2) performing a genotypic resistance testing (GRT); (3) ensuring adherence to the prescribed regimen; (4) performing therapeutic drug Monitoring (TDM); (5) scheduling an earlier follow-up; (6) reconsidering the presence of chronic coinfections; (7) assessing the patient’s inflammatory markers; (8) conducting peripheral blood HIV DNA quantification (I), compared to no such interventions (C) represent any clinical benefit (in terms of viral suppression, CD4+ and CD8+ cell counts, CD4+/CD8+ ratio, prevention of HIV drug resistance) (O), in the last 20 years (T) in all countries (S)?’
All definitions of ‘residual viremia’, ‘low-level viremia’ and ‘viral blips’ were included, also in accord with definitions provided by current guidelines available.12 4,8
Inclusion and exclusion criteria
We limited our search to fully published records reporting the outcome of one of the following interventions in HIV-1 only: (1) immediate need to modify the ART regimen; (2) perform GRT; (3) assess adherence to the ART regimen; (4) perform TDM; (5) schedule an earlier follow-up before considering a therapeutic switch; (6) evaluate chronic coinfections; (8) quantify HIV DNA levels in peripheral blood; (7) assess patient’s inflammatory markers, published between 2004 and 2024. Outcomes of interest were viral suppression, improvement of CD4+, CD8+ cell count and ratio or emergence of HIV drug resistance.
Case reports/case series, conference abstracts, conference papers, reviews, meta-analysis, editorials, commentaries, references concerning elite controllers or individuals treated with LA injectables, and descriptive or association studies where no outcome of an intervention was reported were excluded. No further restrictions in terms of country, setting or language were made.
Search and selection process
The electronic databases of MEDLINE, Web of Science, EMBASE, Cochrane Library, ClinicalTrials.gov and Canadian Agency for Drugs and Technologies in Health were searched on 29 January 2024. A search string for PubMed, consisting of Medical Subject Headings terms and free text words, was developed (online supplemental material 1).
Results were merged in the computerised database Rayyan for deduplication and screening by title and abstract.18 Screening was performed in blind by LVR and DZ. When conflicts arose, these were solved by contacting the project coordinator, LS or MC.
After screening by title and abstract, remaining records were assessed for inclusion by reading the study full text, obtaining an inclusion list of records proceeding to the extraction phase, performed in blind by LVR and DZ. Conflicts were solved by LS or MC. Abstracted information was reported on a dedicated computerised module, including author names, record title, publication year, country, study design, objectives, participants, intervention and outcomes. No part of deduplication or selection was done automatically.
Data analysis
Data were grouped according to the outcome reported and the type of intervention implemented. If sufficient data belonging to the same outcome for the same intervention were available, we performed random effects meta-analyses, reporting pooled data with 95% CIs. Heterogeneity was measured by I2 statistics. The meta-analyses were performed on Stata V.15.0 software (StataCorp, College Station, Texas, USA). Studies that reported as outcome virological suppression defined as <20 and <50 cp/mL (copies per millilitre) were separately meta-analysed. When possible, values such as means and SDs were calculated for HIV RNA and CD4 levels in order to use mean difference with 95% CI in the meta-analysis.19 20
We presented and summarised the evidence deriving from the systematic review and meta-analysis according to the GRADE framework in order to rank its certainty. Whenever such approach was not feasible, we reported the systematic review findings without a strength of recommendation. All phases of the present study were performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.21 The protocol for the systematic review was registered on PROSPERO (CRD42024511492).
Quality appraisal of included studies
All studies were assessed for risk of bias, in blind, by LVR and DZ. Conflicts were solved by LS or MC. Randomised studies were assessed by the Cochrane risk-of-bias tool v.222; non-randomised interventional studies (NRIS) were evaluated by ‘Risk Of Bias In Non-randomised Studies - of Interventions’ (ROBINS-I) tool,23 while observational studies were assessed by the Newcastle-Ottawa Scale.24
Results
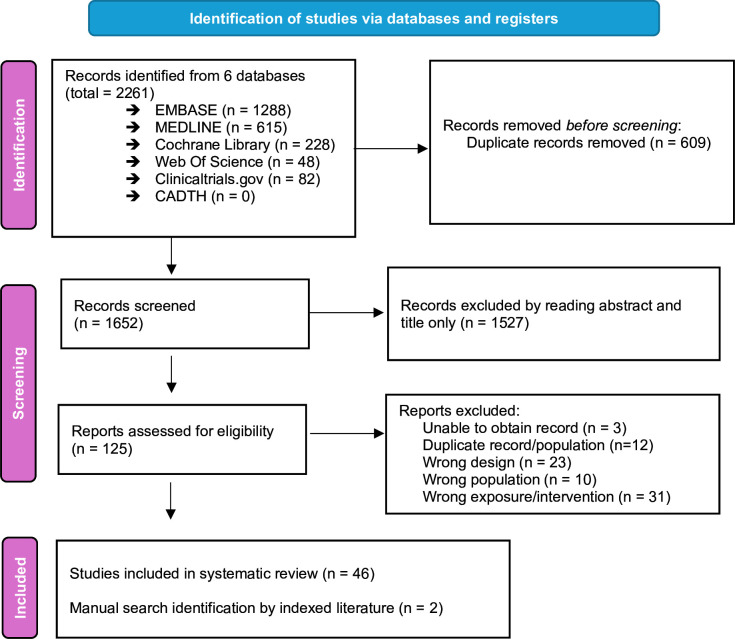
The study selection process is depicted in figure 1.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of the included studies.
48 eligible records published between 2004 and 2023, including nine interventional studies and 39 observational studies, were identified. 10 studies were based in the USA, 6 in France, 6 in Italy, 5 in Canada, 4 in South Africa, 4 in Spain, 2 in Belgium and China, and 1 each in Botswana, Kenya, Peru, Sweden, Switzerland, Taiwan, UK or Uganda. One study involved patients from multiple countries.
Appraisal of included literature
Randomised controlled trials (RCT) exhibited a low risk of bias in 80% of cases (4/5). NRIS demonstrated a higher risk, with 1 out of 4 studies deemed to have high risk, 2 out of 4 with moderate risk and 1 out of 4 with low risk. Observational studies met more than 75% of the evaluation criteria in 71% of cases (27 out of 38) (online supplemental material 2).
Impact of ART regimen modification on virological suppression and CD4 levels
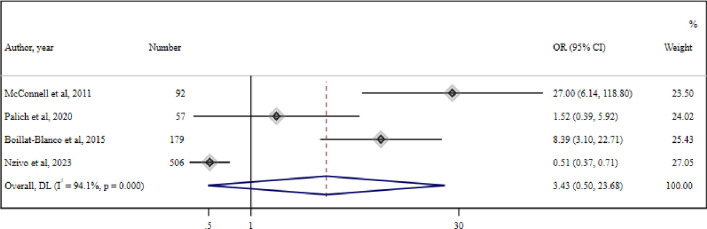
14 studies evaluating the role of ART switch during LLV in PWH were included, three of which were RCTs.25,27 The meta-analysis of four cohort studies,1128,30 reporting virological suppression (defined as <20 cp/mL) among 435 PWH with LLV who switched therapy and 532 PWH with LLV who did not, reported no significant association between therapeutic switch and virological suppression (OR=3.43 (95% CI 0.5 to 23.68) (I2=94.1%, p<0.001)) (figure 2).
Figure 2. Meta-analysis of four studies reporting association of virological suppression (<20 cp) and therapeutic switch. DL, DerSimonian and Laird method random-effect metanalysis.
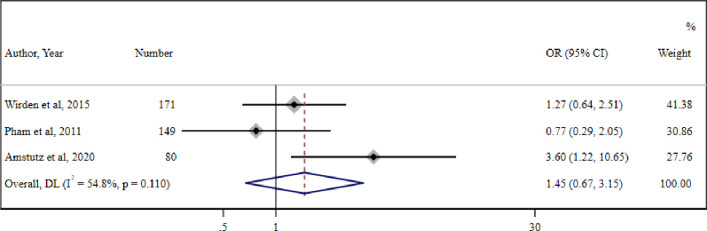
The meta-analysis of three studies27 31 32 (one RCT, one cohort and one case–control) reporting virological suppression (defined as <50 cp/mL) in 121 PWH with LLV who switched therapy and 192 PWH with LLV who continued therapy also reported no significant association between therapeutic switch and virological suppression (OR=1.45 (95% CI 0.67 to 3.15) (I2=54.8%, p=0.1)) (figure 3).
Figure 3. Meta-analysis of three studies reporting association of virological suppression (<50 cp) and therapeutic switch. DL-DerSimonian and Laird method random-effect metanalysis.
Among the studies that could not be meta-analysed, a non-randomised study and an RCT, both based in the USA, report that treatment intensification of ART with raltegravir did not decrease the rate of RV in subjects on ART.26 33 A French study investigating switch to a dual therapy, based on maraviroc and raltegravir in 16 PWH with RV at baseline and 26 weeks after switch, did not find a reduction in RV. Additionally, a decrease in CD4/CD8+ ratio was observed.34 Another US non-randomised study found that, in nine PWH, RV was not reduced by ART intensification with any of efavirenz, ritonavir/boosted lopinavir and ritonavir/boosted atazanavir.35 Conversely, a beneficial effect of ART switch in PWH with RV (HIV RNA <50 cp/mL) was reported in two studies.25 36 The first study found a reduction in HIV DNA and RV at week 96 in the switch arm among 50 PWH with RV, randomised either to continue a regimen with dolutegravir plus one reverse transcriptase inhibitor (RTI) or switch to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF).25 The second study investigated the efficacy of switching to dolutegravir/lamivudine as a maintenance therapy in 41 PWH with RV, reporting a significant increase on the rate of plasma HIV RNA target not detected (TND, HIV RNA <50 cp/mL) from 42.1% at baseline to 86.5% at week 144.36 A cohort study on PWH with LLV found a virological suppression in 20/27 cases after ART modification.37
As for the level of CD4 lymphocytes, the meta-analysis of only two studies reported a mean difference of CD4 T cell count before and after therapeutic switch of +18.26 (95% CI −111.4 to 43.34 (I2=29.2%, p=0.3)) (online supplemental material 3).
An NRIS among 10 patients reported median CD4 counts prior to and 4 weeks after raltegravir intensification of 0.580 and 0.605 x 10∧9 cells/L, respectively.33 The grading of evidence using GRADE-pro documented a low or very low certainty of evidence for the studies that could be meta-analysed mainly because of serious risk of bias, inconsistency and imprecision (online supplemental material 4).
Performing GRT
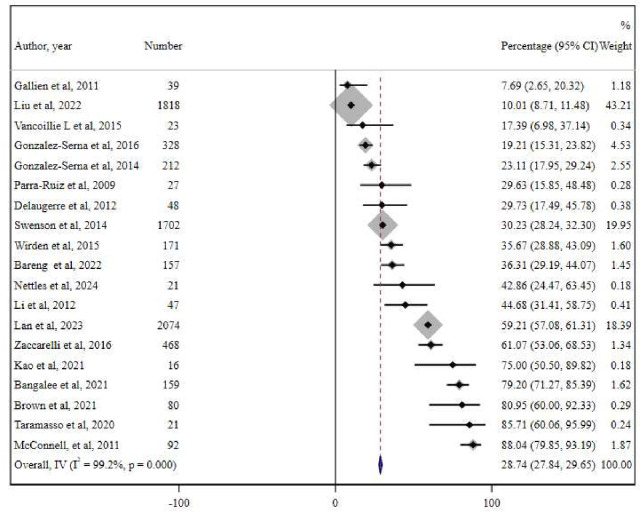
Included data derive from observational studies, conducted only on PWH with LLV undergoing GRT on plasma.1028 31 37,50 The meta-analysis of 19 cohort studies including 7508 PWH on ART with LLV showed an overall drug resistance of 28.74% (95% CI 27.84% to 29.65%) (I2=99.2%, p<0.001) (figure 4).
Figure 4. Meta-analysis of 19 studies reporting the percentage of drug resistance in people living with HIV (PWH) with low-level HIV-1 viraemia (LLV) as documented by genotypic resistance testing (GRT) conducted to manage LLV. IV, inverse variance metanalysis.69 70.
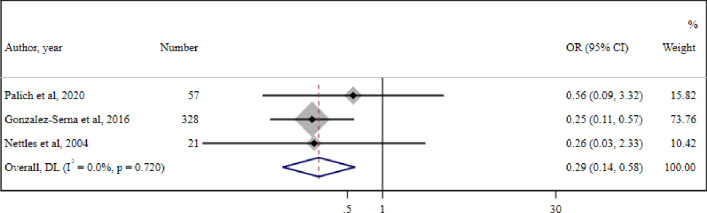
A meta-analysis of three cohort studies conducted in 406 participants with LLV concluded that PWH with LLV who have drug resistance documented by GRT are significantly less likely to achieve virological suppression compared with PWH with LLV without any drug resistance (OR=0.29 (95% CI 0.14 to 0.58) (I2=0.0%, p=0.7)) (figure 5).
Figure 5. Meta-analysis of three studies reporting the association of drug resistance in people living with HIV (PWH) with low-level HIV-1 viraemia (LLV) as documented by genotypic resistance testing (GRT) and virological suppression. DL- DerSimonian and Laird method random-effect metanalysis.
A US cohort study including 34 PWH with LLV reported mainly resistances in gag,51 another cohort study analysing 3895 samples from 2200 patients found a resistance prevalence of 74%.52 Another cohort study reporting data on 54 participants of two clinical trials regarding resistance before and after at least 24 weeks of follow-up found that new resistance mutations were detected in 37% of these participants during LLV.53
The grading of evidence using GRADEpro documented a very low certainty of evidence for the studies that could be meta-analysed, downgrading for inconsistency, indirectness and imprecision (online supplemental material 5).
Assessing adherence to the ART regimen
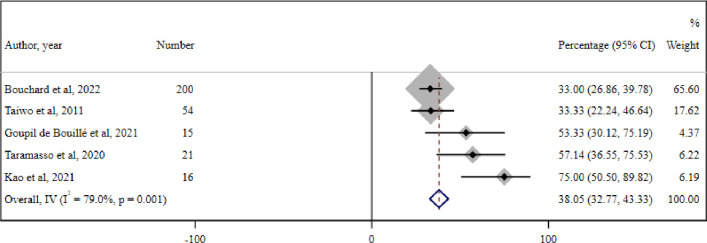
11 studies regarding the role of adherence assessment to ART for the management of LLV were identified. The meta-analysis of five cohort studies among 306 PWH with LLV reported an overall prevalence of suboptimal adherence to ART of 38.05% (95% CI 32.7% to 43.3%) (I2=79%, p=0.001) (figure 6).
Figure 6. Meta-analysis of five cohort studies reporting the overall prevalence of suboptimal adherence to the antiretroviral therapy (ART) regimen among people living with HIV (PWH) with low-level HIV-1 viraemia (LLV). IV-Inverse Variance metanalysis.
An RCT conducted in Uganda, evaluating the effect of adherence counselling in 68 participants with LLV versus those who received the standard of care (n=68 individuals), reported that undetectable viraemia was nearly twice as high in the intervention arm (57.4% vs 29.9%; p=0.037).54 The effects of counselling on improving adherence and in turn reducing LLV (HIV RNA 51–999 cp/mL) were also reported by Konstantopoulos and colleagues.55 LLV was significantly associated to lower adherence in a French case–control study, as well as in a second one conducted in Canada and a third cohort study in Italy.56,58 An Italian cohort study including 281 patients in therapy with a highly forgiving regimen concludes that adherence above 70%, measured through refill rate, was enough to maintain viral suppression, stating that an elevated regimen forgiveness may be an important feature, next to adherence, to improve patient outcome.59 On the contrary, reported adherence was similar among PWH with and without LLV in a prospective cohort study in Peru and a case–control study in the USA.60 61
The grading of evidence using GRADEpro documented a very low certainty of evidence for the studies that could be meta-analysed, downgrading for indirectness and imprecision (online supplemental material 6).
Performing TDM
Three records discussing the impact of TDM on the management of LLV were identified. A Canadian cohort study measured subtherapeutic drug concentrations in 78/328 (24%) treated individuals with HIV-1 RNA levels between 50 and 999 cp/mL.38 In contrast, an observational study in Peru found no difference in nevirapine concentration among 33 adherent individuals with LLV and 49 adherent individuals without LLV, defined as HIV-1 RNA levels of 30–1000 cp/mL.60 Finally, a French prospective cohort study concluded that plasma drug concentrations were adequate in 53/57 (93%) individuals with HIV-1 RNA levels between 21 and 200 cp/mL.11
Scheduling an earlier follow-up before considering a therapeutic switch
The systematic review did not identify eligible records regarding the impact of anticipating follow-up visits on the management of patients with LLV.
Evaluating chronic coinfections
There is a lack of knowledge regarding the role of chronic infections in managing patients with LLV, as no studies addressing this issue were identified.
Assessing patient inflammatory markers
The search identified four relevant observational studies on the topic. A cohort study in the USA (236 individuals) found no correlation between LLV (HIV-1 RNA 20–399 cp/mL) and levels of interleukin 6 (IL-6) and C reactive protein (CRP).62 A study in Africa (95 individuals) similarly found no correlation between LLV (HIV-1 RNA 50–999 cp/mL) and a series of inflammation markers.63 However, a Swedish case–control study found that among 68 participants with HIV-1 RNA levels between 50 and 999 cp/mL, viraemia correlated with levels of growth differentiation factor 15 and D-dimer; no correlation was found with CRP, VCAM-1, interferon-inducible protein 10 or soluble CD14.12 In a Spanish cross-sectional study (n=52 individuals), microbial translocation and levels of tumour necrosis factor-alpha and IL-6 levels were higher in the presence of HIV-1 RNA levels between 20 and 200 cp/mL compared with levels <20 cp/mL.64
Quantify HIV DNA levels in peripheral blood
The search identified limited evidence on this topic. In a single-arm pilot study in the USA involving 10 treated participants with detectable HIV-1 RNA below 200 cp/mL, 24–96 weeks after initiating ART, the level of viraemia positively correlated to the amount of reservoir, measured by infection units per million cells.65 An RCT in Italy assigned 40 virologically suppressed participants to either continue dolutegravir plus one RTI or switch to coformulated E/C/F/TAF. This study showed no significant correlation between HIV-1 DNA levels and detection of HIV-1 RNA levels, in terms of TND and RV development, over a period of 96 weeks.25 In contrast, an observational study in Canada (n=127 individuals) demonstrated a correlation between RV and the frequency of CD4+ cells carrying HIV-1 integrated DNA.66
Discussion
This systematic review explores several strategies for managing individuals with LLV. In assessing the role of treatment switch and intensification, there is lack of evidence supporting the effectiveness in achieving viral suppression among individuals with LLV. Our meta-analyses on viral suppression were divided into two targets to better visualise results from similar target groups (ie, <20 cp/mL, <50 cp/mL), and no significant advantage of an ART switch was noticed in reaching either, possibly due to the very heterogeneous starting and switching therapies, as well as different follow-up periods. Furthermore, an ART switch prompts the need for further investigation to refine treatment approaches tailored to the specific patient before performing the change of regimen, including evaluating other possible explanations for LLV (eg, lack of perfect treatment adherence, new drug–drug interaction or newly developed RAM). Another possible explanation would involve a lack of development of new RAMs during LLV, an assumption in line with only a minority of authors.11 On the other hand, the meta-analysis of 19 studies reporting the prevalence of relevant RAMs in LLV population concludes that in almost one in three individuals, relevant RAMs during LLV are found. Furthermore, the meta-analysis controlling for viral suppression after a GRT (and GRT-guided ART switch in some of the patients) showed a significant association between the presence of RAMs and achieving suppression during LLV. Even if there is a lack of studies that directly assess the effectiveness of conducting or not a GRT on virological suppression, the data thus reported indirectly suggest performing a GRT in LLV, as this phenomenon may be driven by the development of new RAMs. This would, in turn, allow for a broader idea that competent viral replication may be at the base of LLV, rather than simple bouts of release of incompetent viral particles from the reservoir. At the very least, it can be assumed that ordering a GRT in the assessment of LLV would provide a safer framework to rely on, managing the issue with due caution. Nevertheless, many included studies reported data on patients not on current first-line ART regimens.
Adherence to ART emerges as a critical determinant of treatment efficacy and virological outcomes in managing LLV. Interventions aimed at improving adherence show promise in achieving viral suppression in the limited eligible studies.54 Nevertheless, more structured studies on a larger scale are needed. Also, sociocultural factors could represent an additional need for tailoring the approaches to address individual adherence barriers effectively. Finally, it would be interesting to measure, on a larger scale, LLV as a function of compliance with the new, more forgiving, treatments, as their marked forgiveness might be sufficient for allowing a more erratic drug administration schedule.
TDM is another known potential tool for optimising treatment outcomes in HIV care. However, when coming at evaluating the evidence from the literature regarding its clinical utility in managing LLV, results are inconclusive, highlighting the need for further research to elucidate its role in this context.
The impact of scheduling more frequent check-ups in PWH with LLV was not assessable by the records found in this review, but it is safe to assume it could at least show utility in improving the outcomes of those who have problem with retention in care and regimen adherence, as already reported in the literature.67
Similarly, the review identifies literature gaps in assessing the clinical significance of concurrent chronic viral coinfections, or the effect of monitoring inflammatory markers, in the management of these individuals, underscoring the need for definitive evidence on the topic. Despite a potential significance in HIV pathogenesis,12 64 the review highlights the lack of empirical evidence elucidating their utility in this context.
Assessing HIV DNA levels in peripheral blood mononuclear cells (PBMC) as a useful marker in guiding clinical decisions in LLV requires further investigation to fully understand its clinical implications for managing LLV.
On a final note, all the above-mentioned management strategies for LLV could have a role in achieving viral suppression; however, it is crucial to acknowledge that a part of patients presenting with LLV could not suppress their viraemia even after performing all possible management strategies (ie, non-suppressible viraemia).68 Such issue should be addressed by future research to better define its clinical significance.
Furthermore, there is not enough evidence to provide a unique, shared definition of LLV, which calls for high-quality studies to decide a common evidence-based definition.
Strengths and limitations
The results of the present study should be considered in the light of some limitations. First, the nomenclature of LLV in the literature is inconstant, reflecting the diverse nature of multiple virological entities, but also a heterogeneity of study periods and strategies in dealing with this subject. Moreover, virological suppression and failure were heterogeneously defined in all the studies, also due to the different viral load assays used. In the attempt of mitigating the heterogeneity of definitions, the search strategy was designed to report all the possible definitions of LLV, and results were meta-analysed in subgroups according to their outcomes. Nevertheless, heterogeneity of included studies was almost invariably high in the meta-analysis reported.
Second, when it comes to selecting resources reporting an outcome for an intervention in the LLV, evidence is scarce, and interventions included and compared in the present study were frequently not powered to identify our outcome of interest, hence indirectness was an issue in almost all the studies.
Nevertheless, the inclusion of a wide selection of literature, enriched by relevant meta-analysis for similar outcomes and the GRADE evaluation of the evidence, renders this review a useful tool for the clinicians treating LLV.
Conclusion
In conclusion, LLV poses a multifaceted challenge in contemporary HIV care, warranting nuanced approaches to management and underscored the imperative for continued research to refine therapeutic strategies and enhance patient outcomes. The findings of the present systematic review and meta-analysis of the literature, together with the consensus achieved by the Expert Panel, may help in assisting clinical practice and charting future research endeavours in this intricate domain.
supplementary material
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Mark Charles Atkins
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Collaborators: Low-level HIV Viremia Consensus Panel: Drieda Zaçe (Department of Systems Medicine, Infectious Disease Clinic, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy), Lorenzo Vittorio Rindi (Department of Systems Medicine, Infectious Disease Clinic, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy), Mirko Compagno (Department of Systems Medicine, Infectious Disease Clinic, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy), Luna Colagrossi (Microbiology and Diagnostic Immunology, Bambino Gesù Children's Hospital, Rome, Italy), Maria Mercedes Santoro (Department of Experimental Medicine, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy), Francesca Ceccherini-Silberstein (Department of Experimental Medicine, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy), Andrea Cossarizza (Department of Medical and Surgical Sciences for Children and Adults, Univ. of Modena and Reggio Emilia School of Medicine, Modena, Italy), Antonio Di Biagio (Infectious Diseases Unit, San Martino Policlinico Hospital, Genoa, Italy - Department of Health Sciences, University of Genoa, Genoa, Italy), Giovanni Di Perri (Unit of Infectious Diseases, Amedeo di Savoia Hospital, Department of Medical Sciences, University of Turin, Italy), Anna Maria Geretti (Department of Systems Medicine, Infectious Disease Clinic, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy, Dept of Infection, North Middlesex University Hospital, London, UK, School of Immunity and Microbial Sciences, King’s College London, London, UK), Nicola Gianotti (Department of Infectious Diseases, IRCCS San Raffaele Hospital, Milan, Italy), Andrea Gori (Department of Infectious Diseases, ASST Fatebenefratelli Sacco University Hospital, 20157 Milan, Italy), Sergio Lo Caputo (Infectious Diseases Unit, University of Foggia, Foggia, Italy), Giordano Madeddu (Unit of Infectious Diseases, Department of Medicine, Surgery, and Pharmacy, University of Sassari, 07100 Sassari, Italy), Giulia Carla Marchetti (Clinic of Infectious and Tropical Diseases, Department of Health Sciences, ASST Santi Paolo e Carlo, University of Milan, Via A. di Rudinì 8, 20142 Milan, Italy), Claudio Mastroianni (Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy), Cristina Mussini (Department of Surgical, Medical, Dental and Morphological Sciences, University of Modena and Reggio Emilia, 41121 Modena, Italy), Maurizio Zazzi (Department of Medical Biotechnologies, University of Siena, 53100 Siena, Italy), Massimo Andreoni (Department of Systems Medicine, Infectious Disease Clinic, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy), Carlo Federico Perno (Unicamillus University, Rome Italy), Loredana Sarmati (Department of Systems Medicine, Infectious Disease Clinic, Tor Vergata University, Via Montpellier, 1 - 00133 Rome, Italy).
Contributor Information
Drieda Zaçe, Email: driedazace@gmail.com.
Lorenzo Vittorio Rindi, Email: l.rindi@gmail.com.
Mirko Compagno, Email: mirkocompagno2@gmail.com.
Luna Colagrossi, Email: luna.colagrossi@opbg.net.
Maria Mercedes Santoro, Email: santormaria@gmail.com.
Carlo Federico Perno, Email: perno@uniroma2.it.
Loredana Sarmati, Email: srmldn00@uniroma2.it.
Low-level HIV Viremia Consensus Panel:
Massimo Andreoni, Francesca Ceccherini-Silberstein, Luna Colagrossi, Mirko Compagno, Andrea Cossarizza, Antonio Di Biagio, Giovanni Di Perri, Anna Maria Geretti, Nicola Gianotti, Andrea Gori, Sergio Lo Caputo, Giordano Madeddu, Giulia Carla Marchetti, Claudio Mastroianni, Cristina Mussini, Carlo Federico Perno, Lorenzo Vittorio Rindi, Maria Mercedes Santoro, Loredana Sarmati, Drieda Zaçe, and Maurizio Zazzi
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Crespo-Bermejo C, de Arellano ER, Lara-Aguilar V, et al. Persistent low-Level viremia in persons living with HIV undertreatment: An unresolved status. Virulence. 2021;12:2919–31. doi: 10.1080/21505594.2021.2004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F, Simonetti FR. Learning from Persistent Viremia: Mechanisms and Implications for Clinical Care and HIV-1 Cure. Curr HIV/AIDS Rep. 2023;20:428–39. doi: 10.1007/s11904-023-00674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryscavage P, Kelly S, Li JZ, et al. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. 2014;58:3585–98. doi: 10.1128/AAC.00076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosioni J, Levi L, Alagaratnam J, et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med. 2023;24:1126–36. doi: 10.1111/hiv.13542. [DOI] [PubMed] [Google Scholar]
- 5.Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651–69. doi: 10.1001/jama.2020.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi RT, Bedimo R, Hoy JF, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2023;329:63–84. doi: 10.1001/jama.2022.22246. [DOI] [PubMed] [Google Scholar]
- 7.WHO Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021. https://www.who.int/publications/i/item/9789240031593 Available. [PubMed]
- 8.Department of Health and Human Services Panel on antiretroviral guidelines for adults N. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV how to cite the adult and adolescent antiretroviral guidelines: panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/ n.d. Available.
- 9.Fleming J, Mathews WC, Rutstein RM, et al. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS. 2019;33:2005–12. doi: 10.1097/QAD.0000000000002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JA, Amstutz A, Nsakala BL, et al. Extensive drug resistance during low-level HIV viraemia while taking NNRTI-based ART supports lowering the viral load threshold for regimen switch in resource-limited settings: a pre-planned analysis from the SESOTHO trial. J Antimicrob Chemother. 2021;76:1294–8. doi: 10.1093/jac/dkab025. [DOI] [PubMed] [Google Scholar]
- 11.Palich R, Wirden M, Peytavin G, et al. Persistent low-level viraemia in antiretroviral treatment-experienced patients is not linked to viral resistance or inadequate drug concentrations. J Antimicrob Chemother. 2020;75:2981–5. doi: 10.1093/jac/dkaa273. [DOI] [PubMed] [Google Scholar]
- 12.Elvstam O, Medstrand P, Jansson M, et al. Is low-level HIV-1 viraemia associated with elevated levels of markers of immune activation, coagulation and cardiovascular disease? HIV Med. 2019;20:571–80. doi: 10.1111/hiv.12756. [DOI] [PubMed] [Google Scholar]
- 13.Raccagni AR, Diotallevi S, Lolatto R, et al. Viral blips and virologic failures following mpox vaccination with MVA-BN among people with HIV. AIDS. 2023;37:2365–9. doi: 10.1097/QAD.0000000000003733. [DOI] [PubMed] [Google Scholar]
- 14.Zhao M, Zhuo C, Li Q, et al. Cytomegalovirus (CMV) infection in HIV/AIDS patients and diagnostic values of CMV-DNA detection across different sample types. Ann Palliat Med. 2020;9:2710–5. doi: 10.21037/apm-20-1352. [DOI] [PubMed] [Google Scholar]
- 15.Tasker SA, O’Brien WA, Treanor JJ, et al. Effects of influenza vaccination in HIV-infected adults: a double-blind, placebo-controlled trial. Vaccine (Auckl) 1998;16:1039–42. doi: 10.1016/s0264-410x(97)00275-2. [DOI] [PubMed] [Google Scholar]
- 16.Ostrowski SR, Katzenstein TL, Thim PT, et al. Low‐Level Viremia and Proviral DNA Impede Immune Reconstitution in HIV‐1–Infected Patients Receiving Highly Active Antiretroviral Therapy. J Infect Dis. 2005;191:348–57. doi: 10.1086/427340. [DOI] [PubMed] [Google Scholar]
- 17.Rindi LV, Zaçe D, Compagno M, et al. Management of low-level HIV viremia during antiretroviral therapy: Delphi consensus statement and appraisal of the evidence. Sex Transm Infect. 2024;100:442–9. doi: 10.1136/sextrans-2024-056199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 20.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scutari R, Galli L, Alteri C, et al. Evaluation of HIV-DNA and residual viremia levels through week 96 in HIV-infected individuals who continue a two-drug or switch to a three-drug integrase strand transfer inhibitor-based regimen. Int J Antimicrob Agents. 2023;61:106771. doi: 10.1016/j.ijantimicag.2023.106771. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amstutz A, Nsakala BL, Vanobberghen F, et al. Switch to second-line versus continued first-line antiretroviral therapy for patients with low-level HIV-1 viremia: An open-label randomized controlled trial in Lesotho. PLoS Med. 2020;17:e1003325. doi: 10.1371/journal.pmed.1003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell MJ, Mier-Mota J, Flor-Parra F, et al. Improved viral suppression after treatment optimization in HIV-infected patients with persistent low-level viremia. J Acquir Immune Defic Syndr. 2011;58:446–9. doi: 10.1097/QAI.0b013e3182364513. [DOI] [PubMed] [Google Scholar]
- 29.Boillat-Blanco N, Darling KE, Schoni-Affolter F, et al. Virological Outcome and Management of Persistent Low-Level Viraemia in HIV-1-Infected Patients: 11 Years of the Swiss HIV Cohort Study. Antivir Ther (Lond) 2015;20:165–75. doi: 10.3851/IMP2815. [DOI] [PubMed] [Google Scholar]
- 30.Nzivo MM, Waruhiu CN, Kang’ethe JM, et al. HIV Virologic Failure among Patients with Persistent Low-Level Viremia in Nairobi, Kenya: It Is Time to Review the >1000 Virologic Failure Threshold. Biomed Res Int. 2023;2023:8961372. doi: 10.1155/2023/8961372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirden M, Todesco E, Valantin M-A, et al. Low-level HIV-1 viraemia in patients on HAART: risk factors and management in clinical practice. J Antimicrob Chemother. 2015;70:2347–53. doi: 10.1093/jac/dkv099. [DOI] [PubMed] [Google Scholar]
- 32.Pham T, Alrabaa S, Somboonwit C, et al. The HIV Virologic Outcomes of Different Interventions Among Treatment-Experienced Patients With 2 Consecutive Detectable Low-Level Viremia. J Int Assoc Physicians AIDS Care (Chic) 2011;10:54–6. doi: 10.1177/1545109710385122. [DOI] [PubMed] [Google Scholar]
- 33.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–9. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campillo-Gimenez L, Assoumou L, Valantin M-A, et al. Switch to maraviroc/raltegravir dual therapy leads to an unfavorable immune profile with low-level HIV viremia. AIDS. 2015;29:853–6. doi: 10.1097/QAD.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 35.Dinoso JB, Kim SY, Wiegand AM, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Miguel Buckley R, Rial-Crestelo D, Montejano R, et al. Long-term Evaluation of Residual Viremia in a Clinical Trial of Dolutegravir Plus Lamivudine as Maintenance Treatment for Participants With and Without Prior Lamivudine Resistance. Open Forum Infect Dis. 2022;9:ofac610. doi: 10.1093/ofid/ofac610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parra-Ruiz J, Álvarez M, Chueca N, et al. Resistencias genotípicas en pacientes con VIH-1 y grados de viremia persistentemente bajos. Enferm Infecc Microbiol Clín. 2009;27:75–80. doi: 10.1016/j.eimc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Serna A, Swenson LC, Watson B, et al. A single untimed plasma drug concentration measurement during low-level HIV viremia predicts virologic failure. Clin Microbiol Infect. 2016;22:1004. doi: 10.1016/j.cmi.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Lan Y, Ling X, Deng X, et al. Drug Resistance Profile Among HIV-1 Infections Experiencing ART with Low-Level Viral Load in Guangdong China During 2011-2022: A Retrospective Study. Infect Drug Resist. 2023;16:4953–64. doi: 10.2147/IDR.S419610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bangalee A, Hans L, Steegen K. Feasibility and clinical relevance of HIV-1 drug resistance testing in patients with low-level viraemia in South Africa. J Antimicrob Chemother. 2021;76:2659–65. doi: 10.1093/jac/dkab220. [DOI] [PubMed] [Google Scholar]
- 41.Nettles RE, Kieffer TL, Simmons RP, et al. Genotypic resistance in HIV-1-infected patients with persistently detectable low-level viremia while receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:1030–7. doi: 10.1086/423388. [DOI] [PubMed] [Google Scholar]
- 42.Zaccarelli M, Santoro MM, Armenia D, et al. Genotypic resistance test in proviral DNA can identify resistance mutations never detected in historical genotypic test in patients with low level or undetectable HIV-RNA. J Clin Virol. 2016;82:94–100. doi: 10.1016/j.jcv.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Bareng OT, Moyo S, Zahralban-Steele M, et al. HIV-1 drug resistance mutations among individuals with low-level viraemia while taking combination ART in Botswana. J Antimicrob Chemother. 2022;77:1385–95. doi: 10.1093/jac/dkac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taramasso L, Magnasco L, Bruzzone B, et al. How relevant is the HIV low level viremia and how is its management changing in the era of modern ART? A large cohort analysis. J Clin Virol. 2020;123:104255. doi: 10.1016/j.jcv.2019.104255. [DOI] [PubMed] [Google Scholar]
- 45.Liu P, You Y, Liao L, et al. Impact of low-level viremia with drug resistance on CD4 cell counts among people living with HIV on antiretroviral treatment in China. BMC Infect Dis. 2022;22:426. doi: 10.1186/s12879-022-07417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaugerre C, Gallien S, Flandre P, et al. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS One. 2012;7:e36673. doi: 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez-Serna A, Min JE, Woods C, et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis. 2014;58:1165–73. doi: 10.1093/cid/ciu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JZ, Gallien S, Do TD, et al. Prevalence and significance of HIV-1 drug resistance mutations among patients on antiretroviral therapy with detectable low-level viremia. Antimicrob Agents Chemother. 2012;56:5998–6000. doi: 10.1128/AAC.01217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kao S-W, Liu Z-H, Wu T-S, et al. Prevalence of drug resistance mutations in HIV-infected individuals with low-level viraemia under combination antiretroviral therapy: an observational study in a tertiary hospital in Northern Taiwan, 2017-19. J Antimicrob Chemother. 2021;76:722–8. doi: 10.1093/jac/dkaa510. [DOI] [PubMed] [Google Scholar]
- 50.Swenson LC, Min JE, Woods CK, et al. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS. 2014;28:1125–34. doi: 10.1097/QAD.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferretti F, Mackie NE, Singh GKJ, et al. Characterization of low level viraemia in HIV-infected patients receiving boosted protease inhibitor-based antiretroviral regimens. HIV Res Clin Pract. 2019;20:107–10. doi: 10.1080/25787489.2020.1716159. [DOI] [PubMed] [Google Scholar]
- 52.Santoro MM, Fabeni L, Armenia D, et al. Reliability and clinical relevance of the HIV-1 drug resistance test in patients with low viremia levels. Clin Infect Dis. 2014;58:1156–64. doi: 10.1093/cid/ciu020. [DOI] [PubMed] [Google Scholar]
- 53.Taiwo B, Gallien S, Aga E, et al. Antiretroviral drug resistance in HIV-1-infected patients experiencing persistent low-level viremia during first-line therapy. J Infect Dis. 2011;204:515–20. doi: 10.1093/infdis/jir353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanyeenya N, Nakanjako D, Makumbi F, et al. Effectiveness of intensive adherence counselling in achieving an undetectable viral load among people on antiretroviral therapy with low-level viraemia in Uganda. HIV Med. 2024;25:245–53. doi: 10.1111/hiv.13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konstantopoulos C, Ribaudo H, Ragland K, et al. Antiretroviral regimen and suboptimal medication adherence are associated with low-level human immunodeficiency virus viremia. Open Forum Infect Dis. 2015;2:ofu119. doi: 10.1093/ofid/ofu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goupil de Bouillé J, Collignon M, Capsec J, et al. Low-level HIV viremia is associated with low antiretroviral prescription refill rates and social deprivation. AIDS Care. 2021;33:1445–50. doi: 10.1080/09540121.2020.1806198. [DOI] [PubMed] [Google Scholar]
- 57.Bouchard A, Bourdeau F, Roger J, et al. Predictive Factors of Detectable Viral Load in HIV-Infected Patients. AIDS Res Hum Retroviruses. 2022;38:552–60. doi: 10.1089/AID.2021.0106. [DOI] [PubMed] [Google Scholar]
- 58.Maggiolo F, Di Filippo E, Comi L, et al. Reduced adherence to antiretroviral therapy is associated with residual low-level viremia. Pragmat Obs Res. 2017;8:91–7. doi: 10.2147/POR.S127974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maggiolo F, Valenti D, Teocchi R, et al. Real World Data on Forgiveness to Uncomplete Adherence to Bictegravir/ Emtricitabine/Tenofovir Alafenamide. J Int Assoc Provid AIDS Care. 2022;21:23259582221140208. doi: 10.1177/23259582221140208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bull ME, Mitchell C, Soria J, et al. Monotypic low-level HIV viremias during antiretroviral therapy are associated with disproportionate production of X4 virions and systemic immune activation. AIDS. 2018;32:1389–401. doi: 10.1097/QAD.0000000000001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller LG, Golin CE, Liu H, et al. No evidence of an association between transient HIV viremia (“Blips”) and lower adherence to the antiretroviral medication regimen. J Infect Dis. 2004;189:1487–96. doi: 10.1086/382895. [DOI] [PubMed] [Google Scholar]
- 62.Eastburn A, Scherzer R, Zolopa AR, et al. Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One. 2011;6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Botha-Le Roux S, Elvstam O, De Boever P, et al. Cardiovascular Profile of South African Adults with Low-Level Viremia during Antiretroviral Therapy. J Clin Med. 2022;11:2812. doi: 10.3390/jcm11102812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reus S, Portilla J, Sánchez-Payá J, et al. Low-level HIV viremia is associated with microbial translocation and inflammation. J Acquir Immune Defic Syndr. 2013;62:129–34. doi: 10.1097/QAI.0b013e3182745ab0. [DOI] [PubMed] [Google Scholar]
- 65.Gandhi RT, Bosch RJ, Aga E, et al. Residual plasma viraemia and infectious HIV-1 recovery from resting memory CD4 cells in patients on antiretroviral therapy: results from ACTG A5173. Antivir Ther. 2013;18:607–13. doi: 10.3851/IMP2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chun T-W, Murray D, Justement JS, et al. Relationship between residual plasma viremia and the size of HIV proviral DNA reservoirs in infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2011;204:135–8. doi: 10.1093/infdis/jir208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palma A, Lounsbury DW, Messer L, et al. Patterns of HIV service use and HIV viral suppression among patients treated in an academic infectious diseases clinic in North Carolina. AIDS Behav. 2015;19:694–703. doi: 10.1007/s10461-014-0907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Esteban-Cantos A, Montejano R, Pinto-Martínez A, et al. Non-suppressible viraemia during HIV-1 therapy: a challenge for clinicians. Lancet HIV. 2024;11:e333–40. doi: 10.1016/S2352-3018(24)00063-8. [DOI] [PubMed] [Google Scholar]
- 69.Gallien S, Delaugerre C, Charreau I, et al. Emerging integrase inhibitor resistance mutations in raltegravir-treated HIV-1-infected patients with low-level viremia. AIDS. 2011;25:665–9. doi: 10.1097/QAD.0b013e3283445834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vancoillie L, Mortier V, Demecheleer E, et al. Drug Resistance is Rarely the Cause or Consequence of Long-Term Persistent Low-Level Viraemia in HIV-1-Infected Patients on ART. Antivir Ther (Lond) 2015;20:789–94. doi: 10.3851/IMP2966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.