Abstract
Background
Antenatal factors and environmental exposures contribute to recurrent wheezing in early childhood.
Aim
To identify antenatal and environmental factors associated with recurrent wheezing in children from birth to 48 months in the mother and child in the environment cohort, using time-to-event analysis.
Method
Maternal interviews were administered during pregnancy and postnatally and children were followed up from birth to 48 months (May 2013–October 2019). Hybrid land-use regression and dispersion modelling described residential antenatal exposure to nitrogen dioxide (NO2) and particulate matter of 2.5 µm diameter (PM2.5). Wheezing status was assessed by a clinician. The Kaplan-Meier hazard function and Cox-proportional hazard models provided estimates of risk, adjusting for exposure to environmental tobacco smoke (ETS), maternal smoking, biomass fuel use and indoor environmental factors.
Results
Among 520 mother–child pairs, 85 (16%) children, had a single wheeze episode and 57 (11%) had recurrent wheeze. Time to recurrent wheeze (42.9 months) and single wheeze (37.8 months) among children exposed to biomass cooking fuels was significantly shorter compared with children with mothers using electricity (45.9 and 38.9 months, respectively (p=0.03)). Children with mothers exposed to antenatal ETS were 3.8 times more likely to have had recurrent wheeze compared with those not exposed (adjusted HR 3.8, 95% CI 1.3 to 10.7). Mean birth month NO2 was significantly higher among the recurrent wheeze category compared with those without wheeze. NO2 and PM2.5 were associated with a 2%–4% adjusted increased wheezing risk.
Conclusion
Control of exposure to ETS and biomass fuels in the antenatal period is likely to delay the onset of recurrent wheeze in children from birth to 48 months.
Keywords: Smoking, Child
WHAT IS ALREADY KNOWN ON THIS TOPIC
Maternal and environmental tobacco smoke exposure, exposure to biomass or fossil fuels and ambient pollution in the antenatal period increases the risk for adverse respiratory outcomes in infants. The risk for recurrent wheeze in early infancy associated with these exposures is less well established. Recurrent wheeze is an important predictor of childhood asthma.
WHAT THIS STUDY ADDS
Biomass fuel exposure and environmental tobacco smoke exposure increased the risk for recurrent wheeze in infants from birth to 4 years of age. There was a suggestion that ambient pollution, as measured at birth, may be a risk factor for this outcome.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Low-cost interventions to reduce environmental exposures must be implemented in the antenatal period while cleaner fuel usage strategies are necessary in low socioeconomic communities. Further evidence is necessary to understand the relationship between ambient air pollution on recurrent wheeze in infancy.
Introduction
Low-income and middle-income countries (LMICs) experience the highest burden of childhood respiratory disorders.1 Studies have shown that the prenatal and the immediate postnatal period are critical windows for harmful effects from different types of exposures on respiratory health.2 Wheeze in the perinatal and infant stages can be an important predictor of later childhood respiratory health.3
Childhood wheezing comprises a spectrum of presentations, ranging from transient to recurrent or multiple trigger wheeze, with a substantial proportion associated with asthma.4 The prevalence trends of childhood and adolescent current wheeze adolescents have varied in LMICs but have been stable in high-income countries.5 In Europe, studies have reported the prevalence of ever wheezing children diagnosed with asthma by the age of 4 years ranged from 15.9% in Spain to 39.5% in England.6 The prevalence of wheezing in infants has been reported at 15.9% (95% CI 14.0% to 18.0%) in African countries.7 These wheezing episodes are, in the majority, mild, episodic and responsive to therapy. Approximately 30% of children experience recurrent wheezing during the first 5 years of life, and this can be associated with significant morbidity.8
Persistent wheeze at school age is associated with a range of predictors. These include colds and respiratory tract infections, exercise, parental asthma or allergy, eczema, allergic rhinitis, allergic sensitisation and eosinophilia.9 Maternal smoking during pregnancy and exposure to tobacco smoke antenatally and postnatally has been shown to alter lung development, increase susceptibility to lower respiratory tract infections and increase the prevalence of wheezing.10 The presentation of preschool persistent wheeze, in the absence of colds or exercise-induced asthma, may be an important indicator of some environmental trigger. In the Drakenstein Child Health Study, antenatal and early life exposure to environmental tobacco smoke (ETS) was associated with lower respiratory tract infection with associated wheeze in 43% of cases.11
Early-life exposure to biomass fuel has been shown to affect children’s respiratory health outcomes both in the short term and long term.12 In a meta-analysis of eight studies, the risk of acute respiratory tract infection in children exposed to biomass fuels was three times greater than those not exposed.13 Children exposed to smoke biomass fuels in two Nigerian studies had an 8.7% and 5.1%, 12-month prevalence of reported wheezing, respectively.14 Few studies have investigated biomass fuel exposure and wheezing frequency in infancy.
Air pollution is associated with adverse respiratory health, particularly in early infancy when rapid lung growth occurs. Postnatal exposure to air pollution (nitrogen dioxide (NO2), fine particulate matter 2.5 µm diameter (PM2.5, PM10) and sulfur dioxide (SO2)) were associated with an increased incidence of wheezing in European children.15 16 Antenatal and early life exposure to tobacco smoke resulted in an increased risk of infant wheezing in the presence of exposure to increased levels of PM10 and NO2.17 Indoor air quality has been reported with increased risk of adverse respiratory outcomes among infants.18 Other maternal risk factors associated with infant respiratory health include alcohol consumption during pregnancy.19
Recurrent wheeze in infancy, an established marker of childhood asthma8 and associations with environmental factors have not been extensively reported in the literature. The ‘time-to-event’20 and the HR (the ratio of two instantaneous rates of an event at any time during follow-up)21 provide estimates of association in a cohort analysis. In a recent time-to-event study among 5788, Chinese children aged 3–5 years, an increased HR for antenatal and PM exposure in the first year of life was reported for asthma, but not for wheeze.22 Time to wheeze studies are limited, particularly those investigating potential risk factors in children from birth through to the first few years of life.
We hypothesised that environmental factors, such as air pollution, indoor air quality, maternal smoking and ETS, are likely to be associated with recurrent wheeze in early childhood, resulting in an earlier diagnosis of this outcome. We used a time-to-event analysis to determine the impact of antenatal and environmental risk factors associated with recurrent wheezing in children (birth to 48 months) from the mother and child in the environment (MACE) birth cohort in Durban, South Africa.
Method
Setting
The MACE birth cohort is an ongoing study designed to investigate the risk of environmental pollutant exposure commencing in utero on the long-term respiratory health of children.23 The study is located in communities in the south (high industry/residential mix) and north (less industrialised) of Durban, South Africa.
Sample selection
The selection of pregnant women into the MACE cohort has been described previously.23 In brief, pregnant women attending the public sector antenatal clinics in the south and north communities of Durban, South Africa, meeting the inclusion criteria, were selected. The inclusion criteria included residence in the geographical area for the duration of the pregnancy and the period of follow-up. Those with multiple pregnancies were excluded, however, no pregnancy complications or health conditions were used as exclusion criteria. Mother and child pairs (n=520), who had attended one or more clinical follow-up visits, were included in this analysis. The children were followed up from birth to 48 months at the local hospitals. The mother–child pairs who did not attend any clinic visit were excluded (n=80), and they did not significantly differ from the included pairs (online supplemental table 1 and online supplemental figure 1).
Data sources
Pregnant females participated in interviews at recruitment and during each trimester of pregnancy. Interviews included questions on antenatal risk factors (age, general health and reproductive history; education, income, residential, housing type, alcohol and smoking history, indoor mould and dampness); antenatal ETS exposure (exposure to passive smoking at home and/or work), biomass and fossil fuel exposure, childhood respiratory problems, family history of asthma and tuberculosis. These instruments were based on previously validated questionnaires, such as the National Health and Nutrition Examination Survey.24 Postnatal child data included infant sex, age, birth weight, gestational age and HIV status.
Clinical assessments
Clinical assessments were conducted at 6, 12, 24 and 48 months by a paediatric pulmonologist. Developmental and nutritional status was assessed with the aid of the WHO growth charts and standards.25
The main outcome variables in this study were the presence of wheezing episodes which was assessed through multiple approaches: (1) maternal reporting at each clinical visit (in response to the specific question, ‘Did your child ever have a wheeze (whistling sound from the chest) in the last 12 months?’; (2) review of the child’s ‘Road-to–Health’ record (a comprehensive documentation of primary healthcare and health services visit of every South African child from birth) supported by the reported use of nebulisations and (3) a clinical assessment performed by specialist medical officers at the clinic visit. The medical officers conducted the detailed wheeze interviews and the assessment of the corroborating evidence as outlined above, including determining whether the wheeze episode was associated with any viral infections. Single wheeze was defined as one episode of wheeze determined at any clinical visit during the 48-month follow-up and recurrent wheeze was defined as two or more episodes of wheezing over this follow-up period. The timing of each wheezing episode was documented but recorded as per the date of the clinical assessment.
Environmental monitoring
A hybrid atmospheric dispersion/land use regression model for the prediction of air pollution concentrations in Durban, South Africa was developed for the MACE cohort.26 Statistically significant hybrid models (incorporating geographical and dispersion modelling inputs) were developed for the various pollutants of interest, NO2 and PM2.5. The specific methodology, identified predictor variables and the goodness of fit of these hybrid models have been previously described.26
This model was applied to estimate an annual average ambient exposure level at the address of each MACE participant. These annual average concentrations were adjusted for an estimate of maternal exposure during the first trimester of the pregnancy and the month of birth of the child using continuous monitoring values (based on successive 2 week exposures of Radiello tubes over the course of a year) at a reference site.27 The adjustment was to cover the period of the pregnancy, which would fall across seasons. The weighting was based on continuous ambient measurements for one full year.
The rationale for this adjustment was to better characterise exposure during the pregnancy, which may be biased by the use of an annual average, as the monthly variation in air pollution is not captured in the latter metric. The ratio of the average pollutant concentrations at the reference site during each participant’s first trimester period and month of birth, to the annual average concentration at the reference site was used to adjust the annual average exposure value calculated for the participant’s address to represent the first trimester (online supplemental equation 1) and month of birth (online supplemental equation 2), respectively, as described further in online supplemental material. This adjustment procedure assumes that variation in ambient air quality at the participant’s addresses are consistent with those at the reference site. This is a reasonable assumption given the proximity of the reference site to the participant addresses and that this reference site was selected to reflect general fluctuations in ambient concentrations, as opposed to capturing local emission patterns (eg, as a traffic site or industrial reference site would).
Statistical analysis
Data management, descriptive statistics and time-to-event analyses were performed using STATA V.15.0 for Windows (STATA). Descriptive statistics were used to describe participants’ characteristics related to the outcome variables (single and recurrent episodes of wheezing). Variables that were investigated included maternal risk factors (age, maternal health, education, income, residential, housing type); neonatal risk factors (infant sex, age, birth weight, preterm birth, HIV exposure) and childhood environmental exposures (maternal alcohol history, antenatal ETS exposure, indoor mould and dampness, energy source for cooking and heating, family history of asthma). Two air pollution exposure metrics were applied for each outcome for each participant: a proxy measure for the first trimester and another at the month of birth. χ2 tests for categorical variables and Student’s t-tests for continuous variables were applied in the univariate analysis.
The Kaplan-Meier (KM) hazard function was used to estimate the probability of time (age in months at clinical visit) to document a single wheeze episode and time to document recurrent wheezing. The proportional hazard assumption was assessed using the log-negative log-survival plot (the ‘parallel lines test’). The assumption was satisfied. Patients were censored on the last visit time if lost to follow-up or if no wheeze was recorded at the end of the 48-month follow-up. The advantage of this analytical strategy meant that those who attended a single visit over the 4-year period could still contribute data up to the point of their attendance and did not need to be excluded from the sample a priori.
The following variables were considered in the multivariable models: child gender, antenatal ETS, maternal alcohol consumption, family history of asthma, sources of energy at home and the predicted average ambient exposure level PM2.5 and NO2 during the first trimester of the pregnancy and the month of birth of the child, as described above and in online supplemental material.
The proportional hazard assumption was assessed, and all the independent variables satisfied the assumption. The Cox-proportional hazard model was used to assess the relationship between the predictor variables and single and recurrent wheezing episodes. Each variable was tested to see if it was a significant predictor of the occurrence of single and recurrent wheezing using univariate χ2, ANOVA and Cox regression. The univariate Cox-regression analysis was used to estimate the unadjusted HRs. All variables with p<0.25 in the univariate analysis were candidates in the stepwise (backward likelihood ratio) multicovariate Cox regression model. The association of single and recurrent wheezing with ambient exposure to NO2 and PM2.5 was also analysed using Cox-proportional hazards regression models. The adjusted HR was expressed as the effect of a 1 increase in ambient exposure in the child’s residential address. We ran all two-way interactions in the final model and none of them were found to be significant.
Results
Of the 760 live births in the cohort, 520 children attended at least one clinical visit: 442 (85%) at 6 months, 396 (76%) at 12 months, 254 (49%) at 24 months, 153 (29%) at 36 months and 114 (22%) at 48 months. Relocation from the study area (n=171 (21.7%)) was the most common reason for loss to follow-up. There were no demographic differences between those included in this analysis and those excluded (online supplemental table 1). Participants were from low socioeconomic backgrounds (50% had completed high school and a similar percentage with no annual income) with a mean maternal age at recruitment of 26.1 (SD=5.9) years. Of the 520 clinic attendees, 378 (73%) reported no wheezing episodes, 85 (16%) reported a single wheeze and 57 (11%) reported recurrent wheeze. The mean current weight and height of the children in the three groups were similar with 86.9% having a normal BMI and 8.3% being overweight (table 1). There were no meaningful differences between the two wheeze groups when compared with the no-wheeze group.
Table 1. Maternal and infant demographics in MACE birth cohort (n=520).
| Characteristics | Wheezing (%) | |||
| No wheeze n=378 | Single wheeze n=85 | Recurrent wheeze n=57 | P value | |
| Mother education | 0.707 | |||
| <Secondary school education | 24.9 | 31.8 | 24.6 | |
| Matric (high school graduate) | 58.2 | 55.3 | 57.9 | |
| College/technikon/university | 16.9 | 12.9 | 17.5 | |
| Mother’s yearly gross income (US$) | 0.459 | |||
| None | 47.6 | 51.8 | 56.1 | |
| <US$650 | 20.4 | 20.0 | 10.5 | |
| US$650–US$2000 | 18.0 | 18.8 | 17.5 | |
| >US$2000 | 10.8 | 7.1 | 15.8 | |
| Refused to answer | 3.2 | 2.4 | 0.0 | |
| Housing type | 0.986 | |||
| Formal | 83.3 | 83.5 | 84.2 | |
| Informal | 16.7 | 16.5 | 15.8 | |
| Maternal age (mean (SD)) | 26.2 (5.9) | 26.0 (6.4) | 26.3 (6.2) | 0.938 |
| Infant sex (male) | 52.1 | 56.5 | 59.6 | 0.483 |
| Infant age | ||||
| 6 | 21.7 | 8.2 | 0.0 | 0.000 |
| 12 | 23.5 | 30.6 | 24.6 | |
| 24 | 22.5 | 24.7 | 15.8 | |
| 36 | 14.0 | 15.3 | 21.1 | |
| 48 | 18.3 | 21.2 | 38.6 | |
| Birth weight (mean (SD)) | 3149.2 (538.6) | 3050.4 (599.6) | 3184.8 (502.1) | 0.251 |
MACEmother and child in the environment
Among the recurrent wheeze group, a higher proportion of children were exposed to antenatal ETS, maternal obesity and antenatal alcohol consumption when compared with the no wheeze and single wheeze groups. Antenatal maternal alcohol consumption was 3-fold more prevalent among the children with recurrent wheeze when compared with the non-wheezing group while antenatal ETS exposure was almost 1.6-fold higher across these categories (table 2). A family history of asthma was similar among those with a single (20%) and recurrent episodes of wheezing (21%), but higher than those without wheeze (14%). The indoor mould mildew (15%–20%) did not vary across the wheeze categories (table 2).
Table 2. Risk factors associated with child wheezing (n=520).
| Characteristics | Wheezing | ||
| No wheeze n=378% | Single wheeze n=85% | Recurrent wheeze n=57% | |
| Current maternal smoking | 4.2 | 5.9 | 7.0 |
| Antenatal ETS | 48.9 | 57.6* | 77.2** |
| Maternal antenatal alcohol consumption | 7.6 | 4.9** | 21.4** |
| Low birth weight | 10.3 | 17.6* | 5.3 |
| Preterm birth | 12.4 | 11.8 | 8.8 |
| Infant HIV exposed | 35.2 | 34.1 | 33.3 |
| Syphilis positive | 5.9 | 10.6* | 1.8 |
| Family history of asthma | 14.3 | 20.0 | 21.1 |
| Maternal BMI | |||
| Underweight | 4.0 | 1.2 | 7.0 |
| Normal | 34.0 | 42.4 | 29.8 |
| Overweight | 30.8 | 30.6 | 21.1 |
| Obese | 31.3 | 25.9 | 42.1 |
| Child BMI | |||
| Normal | 79.2 | 86.9 | 86.0 |
| Underweight | 4.9 | 3.6 | 3.5 |
| Overweight | 10.4 | 8.3 | 3.5 |
| Obese | 5.5 | 1.2 | 5.3 |
| Mould mildew | |||
| Yes | 12.3 | 20.0 | 15.8 |
| Energy sources for cooking | |||
| Electricity | 97.9 | 96.5 | 94.7 |
| Biomass fuels | 0.8 | 1.2 | 1.8 |
| Paraffin | 1.1 | 1.2 | 1.8 |
| Other | 0.3 | 1.2 | 1.8 |
| Air pollution exposure mean (SD) | |||
| Birth month NO2 () | 14.9 (6.6) | 16.7 (8.0)** | 16.6 (7.3)* |
| First trimester NO2 () | 17.5 (5.8) | 16.5 (5.6) | 16.3 (5.4) |
| Birth month PM2.5 () | 11.4 (5.0) | 12.6 (5.4)* | 12.4 (5.3) |
| First trimester PM2.5 () | 13.5 (4.3) | 12.7 (4.4) | 12.3 (3.9)* |
Calculated as per equation s1 and 2 shown in the online supplemental material.
p-value<0.05; ** p-value p<0.0051 when compared to the ‘“no wheeze’” group.
BMIbody mass indexETS, environmental tobacco smoking
Both NO2 and PM2.5 calculated at birth were higher among the recurrent and single wheeze categories than those without wheezing. This relationship was reversed when using exposure metrics in the first trimester. The NO2 measure at birth between the recurrent wheeze category and no wheeze reached a statistically significant difference (16.7 (SD 7.3) and 14.9 (SD 6.6), respectively) (table 2).
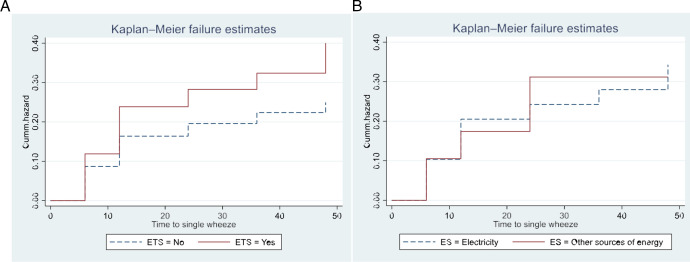
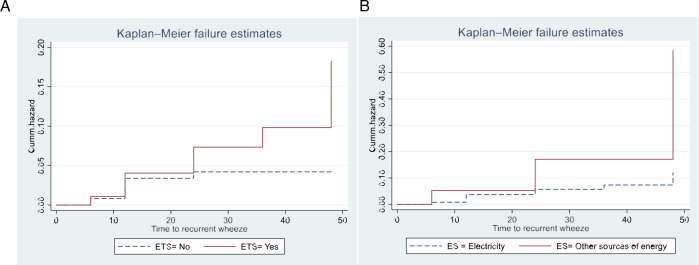
The time to having single and recurrent wheezing among children with antenatal ETS exposure was shorter relative to those children without exposure to antenatal ETS, as shown in the hazard (KM) curves in (figure 1A and figure 2A). For children from a mother exposed to antenatal ETS, the mean time to single wheeze and to recurrent wheeze was 37.1 months and 45.4 months, respectively. Similarly, the KM curves showed a shorter time to single and recurrent wheeze for children whose household used biomass or fossil fuels, compared with those using electrical sources only (figure 1B and figure 2B and online supplemental table 2). These differences were statistically significant (p<0.05) (online supplemental table 2).
Figure 1. Kaplan-Meier curves for the hazard of single wheezing according to (A) antenatal environmental tobacco smoke (ETS), (B) energy sources (ES). (Cum hazard is the accumulated risk of experiencing single wheezing).
Figure 2. Kaplan-Meier curves for the hazard of recurrent wheezing according to (A) antenatal environmental tobacco smoke (ETS), (B) energy sources (ES). (Cum hazard is the accumulated risk of experiencing recurrent wheezing).
Children from mothers exposed to antenatal ETS had an approximately 1.7 times higher risk of single wheeze (adjusted HR 1.69 (95% CI 1.1 to 2.6)) and 3.8 times higher risk of recurrent wheeze adjusted (HR 3.76 (95% CI 1.3 to 10.6)) (table 3) than children with mothers not exposed to antenatal ETS. Exposure to biomass fuels showed a threefold adjusted increase in risk for recurrent wheeze (HR 3.09 (95% CI 0.91 to 10.45)) (table 3). Neither single nor recurrent wheezing was significantly associated with exposure to NO2 or PM2.5. Per unit increase in pollutant (both NO2 and PM2.5) measured in the month of birth, resulted in a 2%–4% adjusted increase in risk for single or recurrent wheeze compared with no wheeze, although these did not reach statistical significance. This pattern was absent for the exposure in the first trimester of pregnancy (table 3).
Table 3. Unadjusted and adjusted Cox-proportional hazards model for risk factors that contribute to single and recurrent episodes of childhood wheezing (n=520).
| Variables | Child wheezing | |||||||
| Single wheezing (n=85) | Recurrent wheezing (n=57) | |||||||
| Unadjusted HR(95% CI) | P value | Adjusted HR(95% CI) | P value | Unadjusted HR(95% CI) | P value | Adjusted HR(95% CI) | P value | |
| Child gender | ||||||||
| Male | 1.27 (0.89 to 1.82) | 0.192 | 1.14 (0.79 to 1.65) | 0.483 | 1.27 (0.63 to 2.54) | 0.493 | ||
| Female | 1 | 1 | ||||||
| Antenatal ETS | ||||||||
| Yes | 1.53 (1.06 to 2.22) | 0.024 | 1.69 (1.11 to 2.56) | 0.014 | 2.28 (1.03 to 5.06) | 0.043 | 3.76 (1.33 to 10.66) | 0.013 |
| No | 1 | 1 | ||||||
| Maternal alcohol consumption | ||||||||
| Yes | 1.45 (0.83 to 2.53) | 0.195 | 1.27 (0.74 to 2.20) | 0.408 | 1.77 (0.68 to 4.58) | 0.241 | 1.07 (0.34 to 3.36) | 0.914 |
| No | 1 | 1 | ||||||
| Family history of asthma | ||||||||
| Yes | 1.30 (0.84 to 2.01) | 0.234 | 1.36 (0.87 to 2.14) | 0.179 | 0.55 (0.20 to 1.57) | 0.264 | 0.59 (0.20 to 1.72) | 0.332 |
| No | 1 | 1 | ||||||
| Energy sources for cooking | ||||||||
| Biomass or fossil fuels | 0.96 (0.35 to 2.59) | 0.932 | 1.00 (0.36 to 2.76) | 0.998 | 3.37 (1.03 to 11.05) | 0.045 | 3.09 (0.91 to 10.45) | 0.069 |
| Electricity | 1 | 1 | ||||||
| Birth month NO2* | 1.01 (1.00 to 1.05) | 0.291 | 1.02 (0.99 to 1.04) | 0.206 | 1.04 (1.00 to 1.08) | 0.080 | 1.03 (1.00 to 1.08) | 0.146 |
| First Trimester NO2* | 0.98 (0.95 to 1.01) | 0.127 | 0.97 (0.94 to 1.00) | 0.078 | 0.97 (0.93 to 1.03) | 0.346 | 0.95 (0.91 to 1.01) | 0.071 |
| Birth Month PM2.5* | 1.01 (0.98 to 1.05) | 0.390 | 1.02 (0.98 to 1.05) | 0.292 | 1.04 (0.98 to 1.11) | 0.210 | 1.04 (0.97 to 1.10) | 0.282 |
| First Trimester PM2.5* | 0.97 (0.93 to1.01) | 0.166 | 0.96 (0.92 to 1.01) | 0.077 | 0.94 (0.86 to 1.01) | 0.106 | 0.95 (0.87 to 1.03) | 0.186 |
HR is significant at α=0.05.
Bold values are statistically significant.
Per one unit increase of pollutant .
ETSenvironmental tobacco smokeNO2nitrogen dioxidePM2.5particulate matter of 2.5 µm diameter
Discussion
In this study, time to single and recurrent wheeze were significantly reduced with exposure to antenatal ETS or biomass or fossil cooking fuels as compared with no exposure. Additionally, the latter agents and ambient pollution at birth showed an increased the risk for these outcomes, although not always statistically significant when adjusting for other covariates.
Few studies have investigated ‘time to recurrent wheeze’, considering environmental exposures at different time points (antenatally and postnatally), within a cohort followed up in infancy. These findings suggest a reduced time to recurrent wheeze, an important predictor of childhood asthma,8 presents with important clinical significance. Low-cost interventions to reduce antenatal ETS exposure and biomass or fossil fuel usage at an individual level are possible, and our findings suggest that these could have important health benefits.
There is evidence that ETS presents with a short-term risk, as well as increasing the risk for longer-term adverse health. Both antenatal and postnatal maternal smoking increased the risk of wheezing and persistent wheeze in early life.28 29 Other studies, from LMICs, have also shown this association.30 31 In addition to our findings providing additional support for the association of antenatal ETS exposure with wheezing, we were able to show that among those with recurrent wheeze, the onset of wheeze is significantly sooner among exposed children.
Biomass fuel exposure is a well-recognised cause of respiratory disease.32 An increase in the prevalence of wheeze among preschoolers has been associated with exposure to biomass fuels in indoor environments in early infancy.33 In our study, the time to recurrent wheeze was significantly related to biomass fuels. Even though a small percentage of participants were exposed to these energy sources, a significant association with recurrent wheezing (OR 3.37 (95% CI 1.03 to 11.05)) was observed. There have been a few studies that have reported exposure to indoor air quality and the association to childhood wheezing.18 Maternal alcohol consumption has been shown to increase the risk of impaired lung function in infants,19 however, in our findings although alcohol consumption was high in mothers of recurrent wheezers there was no association between maternal alcohol intake and recurrent wheezers.
There was a statistically significant higher mean NO2 month of birth exposure among the recurrent wheeze category compared with the non-wheeze category. This relationship was not seen when exposures from the first trimester were considered. Similarly, the adjusted HRs for pollutants at the time of birth NO2-related single and recurrent wheeze were increased, suggesting a pollutant-related risk, but with CIs including the null. We did not show any relationship between wheeze to PM2.5 exposure levels. In a recent time-to-event analysis, every 10 mg/m3 rise in prenatal PM exposure, resulted in an increased HR for childhood asthma of 1.6 (95% CI 1.16 to 2.3), 1.3 (95% CI 1.1 to 1.6) and 1.2 (95% CI 0.05 to 1.5) with PM1, PM2.5 and PM10, respectively.20 Significant associations between higher prenatal and early life PM2.5 exposure and ever wheeze (RR 3.76 (95% CI 1.4 to 10.0) per 5 μg/m3 increase in pollutant) and current wheeze in the past year (RR 7.91 (95% CI 1.5 to 41.6) per 5 μg/m3) increase in pollutant) were reported among children born to mothers in a sample of 536 children in Mexico City.34 This is similar to the reported relationship of pollutant levels with recurrent wheezing in other studies, where estimates of risk ranged from OR 1.18 (95% CI 1.1 to 1.3) to OR 3.58 (95% CI 1.2 to 10.8).35 36 To explore whether our models were influenced by our use of a change in 1 ug/m3 unit increase in pollutant as compared with a change in IQR, we performed a sensitivity analysis using the IQR. The estimates from this analysis are shown in online supplemental table 3a,b. The adjusted HR estimates for the exposure variables varied only slightly but did not change in statistical significance.
The lack of a statistically significant association with ambient pollutants in our study may be a result of our exposure characterisation. Although we created regression models to describe ambient pollutant concentration at the household level during the antenatal period, no systematic assessment was performed to determine exposure in the postnatal period during which the outcomes were assessed. To attempt to address this, we developed proxy measures of exposure based on a month of birth and the trimester of conception. These two proxy measures reflect exposure during the period that is most likely to impact the morphology of the growing lung. However, simultaneously, our outcome of interest (wheeze) is likely to be affected by a shorter and more recent exposure measure. This was not captured in this analysis and may account for the absence of a statistically significant pollutant-related effect. This apriori choice of exposure metrics was based primarily on our objective to determine the effect of antenatal exposures on the outcomes. We were post hoc constrained in developing postnatal exposure metrics for specific time points (such as for each clinic visit or each wheeze episode) because of the computational demands in creating new exposure metrics. Of interest though was the differences seen in the first trimester and birth month measures, with the latter suggesting more important relationships. Despite the possibility of these being chance findings, it may imply that more recent postnatal ambient pollutant exposures influence wheeze outcomes as compared with antenatal exposures.
Recurrent wheezing is a common symptom of illness during infancy and early childhood.37,40 The prevalence of recurrent wheezing in infancy has been reported at between 14.3% and 36.6%, declining to 17.3% and 12% in the second and third year, respectively, among infants in Arizona.37 Other reported risk factors for recurrent wheeze include male sex, mycoplasma infection and home smoke exposure,38 severe pneumonia, low birth weight39 and gastro-oesophageal reflux.40 In our sample, approximately 27% reported wheeze, and 11% of recurrent wheeze occurred in children with lower respiratory tract infection (LRTI), and ETS emerging as a key risk factor in keeping with the previously reported findings. Some of these factors, in keeping with our findings, also suggested a shorter time to recurrent wheeze.38
Recurrent wheeze has been shown to be an important determinant of the development of childhood asthma in the European population. It has been reported that 21.6% who present with recurrent wheeze in early childhood progressed to childhood asthma by the age of 7 years,41 with asthma prevalence associated with an increase in the number of family members affected with asthma.42 In our findings, although an increasing trend in prevalence existed for family history of asthma in the different wheeze categories (14% among those without wheezing; 20% among single wheezing and 21% among recurrent wheezing), we were not able to objectively test for asthma.
Our paper has numerous strengths. This mother–child longitudinal birth cohort has rigorously collected outcome and covariate data over the antenatal period and up to 5 years of age. This is the first study in Africa that used a novel data-driven statistical method using KM curves to identify time-to-event (single or recurrent wheezing) episodes. The association between time to single and recurrent wheezing was associated with ETS and biomass fuels and suggestive of a relationship with ambient pollution, and this warrants a continuation of study follow-up to further investigate the possibility of childhood asthma.
Our analytical approach, using a time-to-event strategy meant that we were able to use all data provided by participants in the cohort, and not restricted just to those with multiple visits. To assess the validity of our results, the proportional hazard assumption was assessed using the log-negative-log-survival plot (parallel lines test). This assumption was satisfied.43 44 The cohort design allowed for the collection of key time-related covariates which could be adjusted for in the analysis.
Apart from the exposure characterisation limitation described above, the assessment of outcome (single and recurrent wheeze) may have presented as a limitation. Generally, these are reliant on parent/caregiver reporting wheezing episodes, and this may have been subjected to recall bias. Our use of standardised questions from well-documented epidemiological studies, such as The National Health and Nutrition Examination Survey (NHANES) allows us to compare across studies. We believe that our assessment approach is a strength of our study. Outcomes were assessed by paediatric pulmonologists and experienced clinicians and were not simply dependent on questionnaire responses. Additionally, the child health record, the monthly questionnaire and other medical records together with the physician-diagnosed wheezing that occurred at follow-up visits were used in the classification of the wheezing categories.
We were not able to include the entire cohort in our analysis, as some participants (n=80) did not attend any clinic visits during the 48-month review period. However, we do not believe that this non-participation biased our findings, as there were no meaningful differences between these two groups (online supplemental table 1).
In conclusion, prenatal exposure to passive smoking and use of biomass energy sources were risk factors associated with time to recurrent wheezing in this birth cohort over the first 48 months of life. At the time of birth, NO2 was related to single and recurrent wheeze were increased, suggesting a pollutant-related risk. These findings, therefore, support emerging evidence that antenatal ETS and exposure to air pollution might influence the development of recurrent wheezing in children.
supplementary material
Acknowledgements
We wish to acknowledge the Mother and Child in the Environment (MACE) cohort study participants, and all study fieldworkers and nursing staff at the participating clinics and hospitals. We also wish to acknowledge the Institute of Risk Assessment Sciences (IRAS), University of Utrecht (Kees de Hoogh and Bert Brunekreef) and the Swiss TPH (Martin Röösli and Kees Meliefste) for their roles in exposure assessment and characterisation within the cohort
Footnotes
Funding: The Mother and Child in the Environment (MACE) cohort study is funded by the following research funding agencies in South Africa: National Research Foundation (NRF) (grant number: 90550), Medical Research Council (MRC) and the AstraZeneca Research Trust and the University of KwaZulu-Natal Flagships Programme.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Biomedical Research Ethics Committee, University of KwaZulu Natal (Ethics ID: BF263/12). Participants gave informed consent to participate in the study before taking part.
Contributor Information
Kareshma Asharam, Email: ramchar4@ukzn.ac.za.
Aweke A Abebaw Mitku, Email: aweke92@yahoo.com.
Lisa Ramsay, Email: ramsaylf@gmail.com.
Prakash Mohan Jeena, Email: jeena@ukzn.ac.za.
Rajen N Naidoo, Email: naidoon@ukzn.ac.za.
Data availability statement
Data are available on reasonable request.
References
- 1.Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014;49:430–4. doi: 10.1002/ppul.23030. [DOI] [PubMed] [Google Scholar]
- 2.Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372:905–13. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwong CG, Bacharier LB. Phenotypes of wheezing and asthma in preschool children. Curr Opin Allergy Clin Immunol. 2019;19:148–53. doi: 10.1097/ACI.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigelman A, Bacharier LB. Infection-induced wheezing in young children. J Allergy Clin Immunol. 2014;133:603–4. doi: 10.1016/j.jaci.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asher MI, Rutter CE, Bissell K, et al. Worldwide trends in the burden of asthma symptoms in school-aged children: global asthma network phase I cross-sectional study. Lancet. 2021;398:1569–80. doi: 10.1016/S0140-6736(21)01450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uphoff EP, Bird PK, Antó JM, et al. Variations in the prevalence of childhood asthma and wheeze in Medall cohorts in Europe. ERJ Open Res. 2017;3:00150-2016. doi: 10.1183/23120541.00150-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez-Alvarez I, Niu H, Guillen-Grima F, et al. Meta-analysis of prevalence of wheezing and recurrent wheezing in infants. Allergol Immunopathol (Madr) 2018;46:210–7. doi: 10.1016/j.aller.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Tenero L, Piazza M, Piacentini G. Recurrent wheezing in children. Transl Pediatr. 2016;5:31–6. doi: 10.3978/j.issn.2224-4336.2015.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Martínez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Factors predicting persistence of early wheezing through childhood and adolescence: a systematic review of the literature. J Asthma Allergy. 2017;10:83–98. doi: 10.2147/JAA.S128319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maritz GS, Harding R. Life-long programming implications of exposure to tobacco smoking and nicotine before and soon after birth: evidence for altered lung development. Int J Environ Res Public Health. 2011;8:875–98. doi: 10.3390/ijerph8030875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanker A, Barnett W, Workman L, et al. Early-life exposure to indoor air pollution or tobacco smoke and lower respiratory tract illness and wheezing in African infants: a longitudinal birth cohort study. Lancet Planet Health. 2017;1:e328–36. doi: 10.1016/S2542-5196(17)30134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Air pollution and child health: prescribing clean air: summary. Contract No.: WHO/CED/PHE/18.01. Geneva: World Health Organization; 2018. [Google Scholar]
- 13.Po JYT, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–9. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 14.Thacher JD, Emmelin A, Madaki AJK, et al. Biomass fuel use and the risk of asthma in Nigerian children. Respiratory Medicine. 2013;107:1845–51. doi: 10.1016/j.rmed.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Khreis H, Kelly C, Tate J, et al. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31. doi: 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Veras MM, de Oliveira Alves N, Fajersztajn L, et al. Before the first breath: prenatal exposures to air pollution and lung development. Cell Tissue Res. 2017;367:445–55. doi: 10.1007/s00441-016-2509-4. [DOI] [PubMed] [Google Scholar]
- 17.Sonnenschein-van der Voort AMM, de Kluizenaar Y, Jaddoe VWV, et al. Air pollution, fetal and infant tobacco smoke exposure, and wheezing in preschool children: a population-based prospective birth cohort. Environ Health. 2012;11:91. doi: 10.1186/1476-069X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patelarou E, Tzanakis N, Kelly FJ. Exposure to indoor pollutants and wheeze and asthma development during early childhood. Int J Environ Res Public Health. 2015;12:3993–4017. doi: 10.3390/ijerph120403993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray D, Willemse L, Visagie A, et al. Determinants of early-life lung function in African infants. Thorax. 2017;72:445–50. doi: 10.1136/thoraxjnl-2015-207401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedgwick P, Joekes K. Kaplan-Meier survival curves: interpretation and communication of risk. BMJ. 2013;347:f7118. doi: 10.1136/bmj.f7118. [DOI] [Google Scholar]
- 21.Sedgwick P, Joekes K. Interpreting hazard ratios. BMJ. 2015;351:h4631. doi: 10.1136/bmj.h4631. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wei J, Shi Y, et al. Early-life exposure to Submicron particulate air pollution in relation to asthma development in Chinese preschool children. J Allergy Clin Immunol. 2021;148:771–82. doi: 10.1016/j.jaci.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Jeena PM, Asharam K, Mitku AA, et al. Maternal demographic and antenatal factors, low birth weight and preterm birth: findings from the mother and child in the environment (MACE) birth cohort, Durban, South Africa. BMC Pregnancy Childbirth. 2020;20:628. doi: 10.1186/s12884-020-03328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey. analytic guidelines, 1999-2010. Vital Health Stat 2. 2013:1–24. [PubMed] [Google Scholar]
- 25.World Health Organisation . WHO child growth standards : length/height-for-age, weight-for-age, weight-for-length, weight -for-height and body mass index-for-age : methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 26.Tularam H, Ramsay LF, Muttoo S, et al. A hybrid air pollution / land use regression model for predicting air pollution concentrations in Durban, South Africa. Environ Pollut. 2021;274:116513. doi: 10.1016/j.envpol.2021.116513. [DOI] [PubMed] [Google Scholar]
- 27.Tularam H, Ramsay LF, Muttoo S, et al. Harbor and intra-city drivers of air pollution: findings from a land use regression model, Durban, South Africa. Int J Environ Res Public Health. 2020;17:5406. doi: 10.3390/ijerph17155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 29.den Dekker HT, Voort AMMS der, de Jongste JC, et al. Tobacco smoke exposure, airway resistance, and asthma in school-age children: the generation R study. CHEST. 2015;148:607–17. doi: 10.1378/chest.14-1520. [DOI] [PubMed] [Google Scholar]
- 30.le Roux DM, Myer L, Nicol MP, et al. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: the Drakenstein Child Health Study. Lancet Glob Health. 2015;3:e95–103. doi: 10.1016/S2214-109X(14)70360-2. [DOI] [PubMed] [Google Scholar]
- 31.Karki S, Fitzpatrick AL, Shrestha S. Risk factors for pneumonia in children under 5 years in a teaching hospital in Nepal. Kathmandu Univ Med J (KUMJ) 2014;12:247–52. doi: 10.3126/kumj.v12i4.13729. [DOI] [PubMed] [Google Scholar]
- 32.Lall O, Bowatte G, Dharmaratne S, et al. Household use of Biomass fuel, especially traditional stove is associated with childhood wheeze and eczema: a cross sectional study of rural communities in Kandy, Sri Lanka. J Asthma. 2023;60:235–43. doi: 10.1080/02770903.2022.2043360. [DOI] [PubMed] [Google Scholar]
- 33.Norbäck D, Lu C, Zhang Y, et al. Sources of indoor particulate matter (PM) and outdoor air pollution in China in relation to asthma, wheeze, rhinitis and eczema among pre-school children: synergistic effects between antibiotics use and PM10 and second hand smoke. Environ Int. 2019;125:252–60. doi: 10.1016/j.envint.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Rivera Rivera NY, Tamayo-Ortiz M, Mercado García A, et al. Prenatal and early life exposure to particulate matter, environmental tobacco smoke and respiratory symptoms in Mexican children. Environ Res. 2021;192:110365. doi: 10.1016/j.envres.2020.110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Q, Lu C, Li Y, et al. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 2016;150:119–27. doi: 10.1016/j.envres.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 36.Sbihi H, Tamburic L, Koehoorn M, et al. Perinatal air pollution exposure and development of asthma from birth to age 10 years. Eur Respir J. 2016;47:1062–71. doi: 10.1183/13993003.00746-2015. [DOI] [PubMed] [Google Scholar]
- 37.Mallol J, García-Marcos L, Solé D, et al. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65:1004–9. doi: 10.1136/thx.2009.115188. [DOI] [PubMed] [Google Scholar]
- 38.Geng L, Tang X, Hua L, et al. The analysis of risk factors for recurrent wheezing in infants and clinical intervention. Transl Pediatr. 2023;12:1810–22. doi: 10.21037/tp-23-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Chen L, Miao Y, et al. An analysis of risk factors associated with recurrent wheezing in the pediatric population. Ital J Pediatr. 2023;49:31. doi: 10.1186/s13052-023-01437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupu VV, Miron IC, Lupu A, et al. The relationship between gastroesophageal reflux disease and recurrent wheezing in children. Medicine (Baltimore) 2021;100:e27660. doi: 10.1097/MD.0000000000027660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce N, Aït-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International study of asthma and allergies in childhood (ISAAC) Thorax. 2007;62:758–66. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ly NP, Gold DR, Weiss ST, et al. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117:e1132–8. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Han D, Hou Y, et al. Statistical inference methods for two crossing survival curves: a comparison of methods. PLoS ONE. 2015;10:e0116774. doi: 10.1371/journal.pone.0116774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rulli E, Ghilotti F, Biagioli E, et al. Assessment of proportional hazard assumption in aggregate data: a systematic review on statistical methodology in clinical trials using time-to-event endpoint. Br J Cancer. 2018;119:1456–63. doi: 10.1038/s41416-018-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.