Abstract
Objectives
To investigate the frequency and factors associated with disease flare following vaccination against SARS-CoV-2 in people with inflammatory/autoimmune rheumatic and musculoskeletal diseases (I-RMDs).
Methods
Data from the European Alliance of Associations for Rheumatology Coronavirus Vaccine physician-reported registry were used. Factors associated with flare in patients with I-RMDs were investigated using multivariable logistic regression adjusted for demographic and clinical factors.
Results
The study included 7336 patients with I-RMD, with 272 of 7336 (3.7%) experiencing flares and 121 of 7336 (1.6%) experiencing flares requiring starting a new medication or increasing the dosage of an existing medication. Factors independently associated with increased odds of flare were: female sex (OR=1.40, 95% CI=1.05 to 1.87), active disease at the time of vaccination (low disease activity (LDA), OR=1.45, 95% CI=1.08 to 1.94; moderate/high disease activity (M/HDA), OR=1.37, 95% CI=0.97 to 1.95; vs remission), and cessation/reduction of antirheumatic medication before or after vaccination (OR=4.76, 95% CI=3.44 to 6.58); factors associated with decreased odds of flare were: higher age (OR=0.90, 95% CI=0.83 to 0.98), non-Pfizer/AstraZeneca/Moderna vaccines (OR=0.10, 95% CI=0.01 to 0.74; vs Pfizer), and exposure to methotrexate (OR=0.57, 95% CI=0.37 to 0.90), tumour necrosis factor inhibitors (OR=0.55, 95% CI=0.36 to 0.85) or rituximab (OR=0.27, 95% CI=0.11 to 0.66), versus no antirheumatic treatment. In a multivariable model using new medication or dosage increase due to flare as the dependent variable, only the following independent associations were observed: active disease (LDA, OR=1.47, 95% CI=0.94 to 2.29; M/HDA, OR=3.08, 95% CI=1.91 to 4.97; vs remission), cessation/reduction of antirheumatic medication before or after vaccination (OR=2.24, 95% CI=1.33 to 3.78), and exposure to methotrexate (OR=0.48, 95% CI=0.26 to 0.89) or rituximab (OR=0.10, 95% CI=0.01 to 0.77), versus no antirheumatic treatment.
Conclusion
I-RMD flares following SARS-CoV-2 vaccination were uncommon. Factors associated with flares were identified, namely higher disease activity and cessation/reduction of antirheumatic medications before or after vaccination.
Keywords: Antirheumatic Agents, Autoimmune Diseases, Covid-19, Epidemiology, Vaccination
WHAT IS ALREADY KNOWN ON THIS TOPIC
Post-SARS-CoV-2 vaccination disease flares are not frequent in people with inflammatory/autoimmune rheumatic and musculoskeletal diseases (I-RMDs).
Previous studies have been smaller and mainly analysed patient-reported flares, with flare rates typically higher compared with physician-reported flares, often failing to distinguish I-RMD flares from short-term vaccine reactogenicity.
WHAT THIS STUDY ADDS
In this large international physician-reported registry, I-RMD flares and flares requiring medication following vaccination against SARS-CoV-2 were infrequent (3.7 and 1.6%, respectively).
Higher disease activity at the time of SARS-CoV-2 vaccination and cessation/reduction of antirheumatic medications before or after vaccination were associated with an increased probability of flare, while exposure to certain medications such as methotrexate and rituximab was associated with a decreased probability of flare.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These data will aid patients, clinicians and other healthcare professionals making shared informed decisions regarding I-RMD management in the context of vaccination.
These findings will help to inform vaccination strategies in patients with I-RMDs, specifically decisions regarding stopping or reducing antirheumatic medications around the time of vaccination.
Introduction
The COVID-19 pandemic caused unprecedented pressure on healthcare systems and resulted in a dramatic loss of human life worldwide.1 2 The development of highly effective vaccines against SARS-CoV-2 changed the course of the pandemic, with many lives saved by immunisation against SARS-CoV-2, including the lives of patients with inflammatory/autoimmune rheumatic and musculoskeletal diseases (I-RMDs).3,10
Using early data from the international observational European Alliance of Associations for Rheumatology (EULAR) Coronavirus Vaccine (COVAX) physician-reported registry, we previously reported that COVID-19 vaccines were well tolerated by patients with I-RMD with infrequent reports of I-RMD flare (4.4%, 1.5% requiring medication changes) and very infrequent reports of serious adverse events (AEs) (0.4%).8 Other studies have mainly analysed patient-reported flares, with flare rates typically being higher compared with physician-reported flares, and often failing to distinguish I-RMD flares from short-term vaccine reactogenicity.11,23
The possibility of I-RMD flares has raised concerns, not only because there might be specific demographic or clinical features associated with increased risk of post-vaccination flare, but also because some organisations have recommended conventional and targeted synthetic disease-modifying antirheumatic drugs (cs/tsDMARDs) to be withheld ‘for 1–2 weeks (as disease activity allows) after each COVID-19 vaccine dose’, a strategy that could contribute to increased frequency of post-vaccination I-RMD flares.24
Our aim was to investigate the frequency and factors associated with I-RMD flare following vaccination against SARS-CoV-2 in people with I-RMD.
Methods
Data collection
The EULAR COVAX physician-reported registry (https://www.eular.org/eular-covax-registry)8 was an observational registry of patients with a pre-existing I-RMD or non-I-RMD who received one or more doses of any vaccine against SARS-CoV-2. The registry was launched in February 2021 and closed in October 2022. Data were recorded voluntarily by rheumatologists or other members of the clinical rheumatology team directly into an online data entry system (using REDCap,25 26 which is a secure web application for building and managing online surveys and databases) or transferred from a national registry (for Portugal). Cases included in this study had a pre-existing I-RMD diagnosis and received one or two doses of the same COVID-19 vaccine. Patients receiving a combination of vaccines were excluded. Patients receiving more than two doses of the same vaccine were also excluded because disease activity data were only collected at baseline (ie, at the time of first dose of primary vaccination schedule).
Data collected included patients’ age (years), sex, country of residence, primary I-RMD diagnosis, COVID-19 vaccine received, number of doses and dates, physician global assessment of disease activity at the time of first dose of primary vaccination schedule (categorised as remission, low, moderate or high disease activity), exposure to immunomodulatory/immunosuppressive treatments at the time of vaccination, cessation/reduction of antirheumatic medication before or after vaccination, development of post-vaccination I-RMD flares and their characteristics, and prescription of new antirheumatic medication or dosage increase due to flare.
Providers were asked to report as many cases as possible of patients with RMDs vaccinated against SARS-CoV-2, with or without flares. Cases could be collected in outpatient, day care or inpatient settings, with the number of reported cases per session varying depending on feasibility. When reporting only a subset of patients from, for example, a full clinic list, providers were asked to select cases randomly, to avoid selection bias. Furthermore, the time from vaccination to the reporting of the case/outcome/flare was allowed to vary between individuals. Providers were also asked to distinguish between I-RMD flares and AEs, namely AEs within 7 days from vaccination (reactogenicity) and AEs of special interest.
Diagnostic groups
Diagnostic groups were defined based on the physician-reported primary I-RMD diagnosis: (1) inflammatory joint diseases (IJD), (2) connective tissue diseases (CTDs), (3) vasculitis and (4) other I-RMDs. Rheumatic diseases included in each category are listed in table 1.
Table 1. Patients’ demographics and clinical characteristics.
| IJDn=5207(71%) | CTDn=1320(18%) | Vasculitisn=686(9.4%) | OIRMDn=123(1.7%) | All patientsn=7336 | ||
| Age, years | Mean (SD) | 58.2 (15) | 54.5 (15.9) | 67.7 (15.1) | 53.3 (13.7) | 58.3 (15.5) |
| Range | 18–96 | 18–90 | 19–95 | 18–87 | 18–96 | |
| Sex | Female | 3356 (64.5) | 1153 (87.3) | 407 (59.3) | 67 (54.5) | 4983 (67.9) |
| Male | 1851 (35.5) | 167 (12.7) | 279 (40.7) | 56 (45.5) | 2353 (32.1) | |
| Country | Portugal | 1892 (36.3) | 341 (25.8) | 21 (3.1) | 1 (0.8) | 2255 (30.7) |
| France | 1095 (21) | 489 (37) | 326 (47.5) | 67 (54.5) | 1977 (26.9) | |
| Italy | 716 (13.8) | 219 (16.6) | 136 (19.8) | 28 (22.8) | 1099 (15) | |
| Slovakia | 348 (6.7) | 45 (3.4) | 36 (5.2) | 0 | 429 (5.8) | |
| Latvia | 284 (5.5) | 47 (3.6) | 20 (2.9) | 0 | 351 (4.8) | |
| Other countries* | 872 (16.7) | 179 (13.6) | 147 (21.4) | 27 (22) | 1225 (16.7) | |
| Primary I-RMD diagnosis | Rheumatoid arthritis | 2647 (50.1) | NA | NA | NA | 2647 (36.1) |
| Axial spondyloarthritis | 1184 (22.7) | NA | NA | NA | 1184 (16.1) | |
| Psoriatic arthritis | 892 (17.1) | NA | NA | NA | 892 (12.2) | |
| Other peripheral spondyloarthritis (including reactive arthritis) | 178 (3.4) | NA | NA | NA | 178 (2.4) | |
| Non-systemic juvenile idiopathic arthritis | 88 (1.7) | NA | NA | NA | 88 (1.2) | |
| Systemic juvenile idiopathic arthritis | 14 (<1) | NA | NA | NA | 14 (<1) | |
| Gout or other crystal arthritis | 100 (1.9) | NA | NA | NA | 100 (1.4) | |
| Other inflammatory arthritis | 104 (2) | NA | NA | NA | 104 (1.4) | |
| Systemic lupus erythematosus | NA | 546 (41.4) | NA | NA | 546 (7.4) | |
| Primary antiphospholipid syndrome | NA | 33 (2.5) | NA | NA | 33 (<1) | |
| Sjogren’s syndrome | NA | 294 (22.3) | NA | NA | 294 (4) | |
| Systemic sclerosis | NA | 245 (18.6) | NA | NA | 245 (3.3) | |
| Idiopathic inflammatory myopathy | NA | 86 (6.5) | NA | NA | 86 (1.2) | |
| Mixed connective tissue disease | NA | 44 (3.3) | NA | NA | 44 (<1) | |
| Undifferentiated connective tissue disease | NA | 72 (5.5) | NA | NA | 72 (1) | |
| Large vessel vasculitis—Takayasu arteritis | NA | NA | 18 (2.6) | NA | 18 (<1) | |
| Large vessel vasculitis—giant cell arteritis | NA | NA | 157 (22.9) | NA | 157 (2.1) | |
| Polymyalgia rheumatica | NA | NA | 270 (39.4) | NA | 270 (3.7) | |
| Medium vessel vasculitis—polyarteritis nodosa, Kawasaki disease | NA | NA | 14 (2) | NA | 14 (<1) | |
| ANCA-associated vasculitis—MPA, GPA, EGPA | NA | NA | 139 (20.3) | NA | 139 (1.9) | |
| Immune complex small vessel vasculitis | NA | NA | 9 (1.3) | NA | 9 (<1) | |
| Bechet’s disease | NA | NA | 50 (7.3) | NA | 50 (<1) | |
| Other vasculitis | NA | NA | 29 (4.2) | NA | 29 (<1) | |
| Monogenic autoinflammatory syndrome | NA | NA | NA | 17 (13.8) | 17 (<1) | |
| Non-monogenic autoinflammatory syndrome | NA | NA | NA | 15 (12.2) | 15 (<1) | |
| IgG4-related disease | NA | NA | NA | 16 (13) | 16 (<1) | |
| Sarcoidosis | NA | NA | NA | 63 (51.2) | 63 (<1) | |
| Relapsing polychondritis | NA | NA | NA | 9 (7.3) | 9 (<1) | |
| Chronic recurrent multifocal osteomyelitis | NA | NA | NA | 3 (2.4) | 3 (<1) |
Other countries include Albania, Australia, Austria, Belgium, Croatia, Czechia, Estonia, Germany, Greece, Hungary, Ireland, Lithuania, Luxembourg, Monaco, Netherlands, Poland, Republic of Moldova, Romania, Russian Federation, Slovenia, Spain, Switzerland, Turkey, Ukraine, USA and UK.
ANCAantineutrophil cytoplasmic antibodyCTDconnective tissue diseaseEGPAeosinophilic granulomatosis with polyangiitisGPAgranulomatosis with polyangiitisIJDinflammatory joint diseaseI-RMDinflammatory rheumatic and musculoskeletal diseaseMPAmicroscopic polyangiitisNAnot applicableOIRMDother inflammatory rheumatic and musculoskeletal disease
Antirheumatic medications
Exposure to the following immunomodulatory/immunosuppressive treatments at the time of COVID-19 vaccination was gathered: (1) csDMARDs: antimalarials (hydroxychloroquine and chloroquine), leflunomide, methotrexate and sulfasalazine; (2) biological DMARDs (bDMARDs): abatacept, belimumab, rituximab, interleukin (IL)-1 inhibitors (anakinra, canakinumab and rilonacept), IL-6 inhibitors (tocilizumab, sarilumab), IL-12/23 inhibitors (ustekinumab), IL-23 inhibitors (guselkumab, risankizumab and tildrakizumab), IL-17 inhibitors (secukinumab, ixekizumab and brodalumab) and tumour necrosis factor (TNF) inhibitors (adalimumab, certolizumab, etanercept, golimumab, infliximab and TNF inhibitor biosimilars); (3) tsDMARDs: apremilast and Janus kinase (JAK) inhibitors (tofacitinib, baricitinib and upadacitinib); (4) immunosuppressants: glucocorticoids, azathioprine/6-mercaptopurine, cyclophosphamide, ciclosporin, mycophenolate mofetil and tacrolimus; (5) other drugs, including intravenous immunoglobulin and antifibrotics.
I-RMD flare characteristics and definition
Flare was defined by physician report (signs and symptoms interpreted by the local physician as being suggestive of post-vaccination I-RMD flare). The following detailed information about flares was collected: (1) type of flare (including fever, weight loss, increase in fatigue, increase in dryness, enlarged lymph nodes, arthralgia, arthritis flare, cutaneous, pulmonary, renal, neurological, muscular, cardiac, gastrointestinal or haematological flare, or other types of flare); (2) severity of flare (mild, moderate, severe without hospitalisation and severe with hospitalisation); (3) information about changes in antirheumatic medication (including dosage increase or new medication) due to the flare; and (4) period of time between vaccination and the flare.
Statistical analyses
Data were reported descriptively for the entire cohort of patients with I-RMDs and for each diagnostic group. Factors associated with COVID-19 vaccination-related disease flares were estimated using univariable and multivariable logistic regression analyses and reported as OR and 95% CI. Two separate multivariable models were built, one with ‘I-RMD flare’ as dependent variable and one with ‘new antirheumatic medication or dosage increase due to flare’ as dependent variable.
Covariates included in the models were the following: age (per decade of life), sex, rheumatic disease diagnostic group (IJD (reference), CTD, vasculitis and other inflammatory rheumatic diseases), disease activity (remission (reference), low disease activity, and moderate or high disease activity), vaccine type (Pfizer/BioNTech (reference), Moderna, AstraZeneca and other vaccines), rheumatic disease treatment and cessation/reduction of antirheumatic medications at the time of vaccination.
For patients being treated with more than one of the medications of interest (except glucocorticoids), we created a medication hierarchy based on clinical expertise to categorise patients, as previously reported.10 This process creates disjoint categories, allowing a clear reference group for interpretation of the regression models and avoiding collinearities. The following hierarchy of treatment allocation was used: immunosuppressants (azathioprine/6-mercaptopurine, cyclophosphamide, ciclosporin, mycophenolate mofetil and tacrolimus)>methotrexate>leflunomide>sulfasalazine>antimalarials. Patients receiving b/tsDMARDs were considered solely in the b/tsDMARD group. Glucocorticoids were analysed separately in the model.
Missing values for vaccine type and disease activity were derived by multiple imputation using full conditional specification (age, sex, flare, country of residence, disease group, disease activity, vaccine type and exposure to glucocorticoid treatment were included in the imputation model). Results of the logistic regression analyses for 100 imputed datasets were pooled by Rubin’s rules. Secondary models (sensitivity analysis) were performed without data imputation.
Results
General characteristics
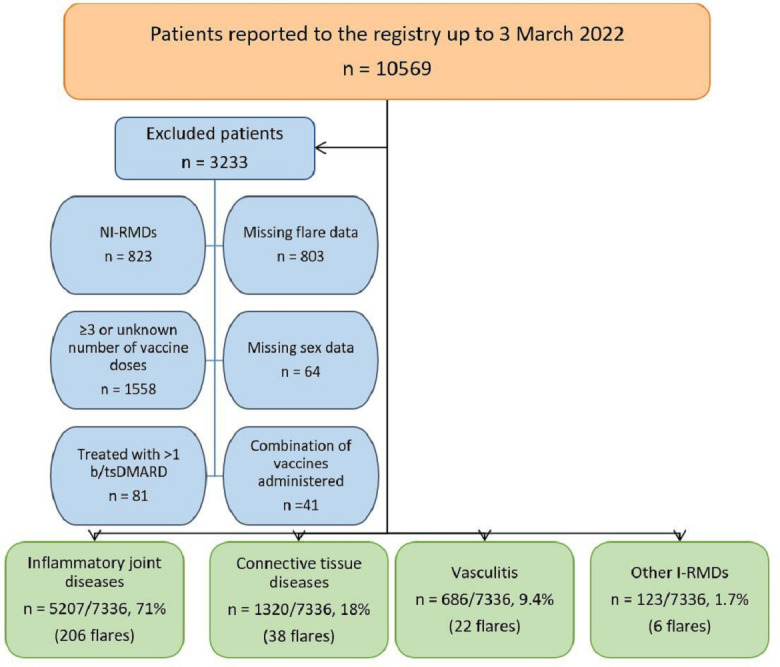
Of 10 569 patients reported to the registry, 7336 patients with I-RMDs were included in this study. Reasons for exclusion are presented in figure 1. The majority were female (68%) and mean age was 58.3 (SD 15.5) years. Most patients had IJD (71%), followed by CTD (18%), vasculitis (9.4%) and other I-RMDs (1.7%). Three-quarters of the diagnoses consisted of rheumatoid arthritis (RA; 36.1%), axial spondyloarthritis (axSpA; 16.1%), psoriatic arthritis (PsA; 12.2%), systemic lupus erythematosus (SLE; 7.4%) and polymyalgia rheumatica (PMR; 3.7%) (table 1).
Figure 1. Study flow chart. Some patients were excluded for more than one reason. bDMARD, biological disease-modifying antirheumatic drug; I-RMDs, inflammatory rheumatic and musculoskeletal diseases; NI-RMD, non-inflammatory rheumatic and musculoskeletal disease; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
While the majority of patients were in remission (39.7%) or had low disease activity (27.4%), 17.8% of all cases had missing data on disease activity, and the remaining patients (15.1%) had moderate or high disease activity. The most frequently used medication groups were csDMARDs (58.2%), ts/bDMARDs (49%) and immunosuppressants (35.2%), respectively. The rate of not using antirheumatic medications was 7% (table 2). The most common individual medications were methotrexate (34.1%), glucocorticoids (30.3%) and TNF inhibitors (30.3%). In terms of disease subgroups, the use of csDMARDs and b/tsDMARDs was highest in IJD (63.2% and 63%, respectively), while the use of immunosuppressants was highest in vasculitis (72.2%).
Table 2. Inflammatory/autoimmune rheumatic and musculoskeletal disease activity and medications.
| IJDn=5207 | CTDn=1320 | Vasculitisn=686 | OIRMDn=123 | All patientsn=7336 | |
| Physician-reported disease activity | |||||
| Remission | 1934 (37.1) | 526 39.8) | 393 (57.3) | 62 (50.4) | 2915 (39.7) |
| Low disease activity | 1487 (28.6) | 304 (23) | 180 (26.2) | 40 (32.5) | 2011 (27.4) |
| Moderate disease activity | 808 (15.5) | 74 (5.6) | 47 (6.9) | 9 (7.3) | 938 (12.8) |
| High disease activity | 147 (2.8) | 12 (0.9) | 8 (1.2) | 1 (0.8) | 168 (2.3) |
| Missing/unknown | 831 (16) | 404 (30.6) | 58 (8.4) | 11 (9) | 1304 (17.8) |
| Antirheumatic medication exposure | |||||
| csDMARDs | 3289 (63.2) | 786 (59.6) | 163 (23.8) | 31 (25.2) | 4269 (58.2) |
| Antimalarials | 323 (6.2) | 632 (47.9) | 11 (1.6) | 8 (6.5) | 974 (13.3) |
| Held before vaccination | 1 | 1 | 0 | 0 | 2 |
| Reduced before vaccination | 0 | 1 | 0 | 0 | 1 |
| Held after vaccination | 1 | 2 | 0 | 0 | 3 |
| Reduced after vaccination | 0 | 2 | 0 | 0 | 2 |
| Leflunomide | 379 (7.3) | 14 (1.1) | 9 (1.3) | 1 (<1) | 403 (5.5) |
| Held before vaccination | 6 | 0 | 0 | 0 | 6 |
| Reduced before vaccination | 1 | 0 | 0 | 0 | 1 |
| Held after vaccination | 2 | 0 | 0 | 0 | 2 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Methotrexate | 2200 (42.3) | 139 (10.5) | 141 (20.6) | 22 (17.9) | 2502 (34.1) |
| Held before vaccination | 66 | 3 | 5 | 0 | 74 |
| Reduced before vaccination | 10 | 0 | 1 | 0 | 11 |
| Held after vaccination | 137 | 6 | 7 | 1 | 151 |
| Reduced after vaccination | 6 | 0 | 0 | 0 | 6 |
| Sulfasalazine | 387 (7.4) | 1 (<1) | 2 (<1) | 0 | 390 (5.3) |
| Held before vaccination | 1 | 0 | 0 | 0 | 1 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 1 | 0 | 0 | 0 | 1 |
| bDMARDs | 3042 (58.4) | 130 (9.8) | 146 (21.3) | 31 (25.2) | 3349 (45.7) |
| Abatacept | 118 (2.3) | 2 (<1) | 1 (<1) | 0 | 121 (1.6) |
| Held before vaccination | 6 | 0 | 0 | 0 | 6 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 3 | 0 | 0 | 0 | 3 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Belimumab | 0 | 38 (2.9) | 0 | 0 | 38 (<1) |
| Held before vaccination | 0 | 2 | 0 | 0 | 2 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Rituximab | 187 (3.6) | 71 (5.4) | 57 (8.3) | 6 (4.9) | 321 (4.4) |
| Held before vaccination | 14 | 1 | 7 | 2 | 24 |
| Reduced before vaccination | 1 | 0 | 0 | 1 | 2 |
| Held after vaccination | 1 | 1 | 0 | 0 | 2 |
| Reduced after vaccination | 1 | 0 | 0 | 0 | 1 |
| IL-1 inhibitors | 19 (<1) | 2 (<1) | 1 (<1) | 7 (5.7) | 29 (<1) |
| Held before vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| IL-6 inhibitors | 278 (5.3) | 9 (<1) | 63 (9.2) | 3 (2.4) | 353 (4.8) |
| Held before vaccination | 13 | 1 | 3 | 0 | 17 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 9 | 0 | 0 | 0 | 9 |
| Reduced after vaccination | 1 | 0 | 0 | 0 | 1 |
| IL-12/23 inhibitors | 46 (<1) | 2 (<1) | 1 (<1) | 0 | 49 (<1) |
| Held before vaccination | 1 | 0 | 1 | 0 | 2 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| IL-23 inhibitors | 6 (<1) | 0 | 0 | 0 | 6 (<1) |
| Held before vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| IL-17 inhibitors | 207 (4.2) | 1 (<1) | 0 | 1 (<1) | 209 (2.8) |
| Held before vaccination | 5 | 0 | 0 | 0 | 5 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 5 | 0 | 0 | 0 | 5 |
| Reduced after vaccination | 1 | 0 | 0 | 0 | 1 |
| TNF inhibitors | 2181 (41.9) | 5 (<1) | 23 (3.4) | 14 (11.4) | 2223 (30.3) |
| Held before vaccination | 67 | 0 | 2 | 0 | 69 |
| Reduced before vaccination | 9 | 0 | 0 | 0 | 9 |
| Held after vaccination | 58 | 1 | 1 | 0 | 60 |
| Reduced after vaccination | 5 | 0 | 0 | 0 | 5 |
| tsDMARDs | 241 (4.6) | 7 (<1) | 1 (<1) | 0 | 249 (3.4) |
| Apremilast | 13 (<1) | 0 | 0 | 0 | 13 (<1) |
| Held before vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| JAK inhibitors | 228 (4.4) | 7 (<1) | 1 (<1) | 0 | 236 (3.2) |
| Held before vaccination | 7 | 0 | 0 | 0 | 7 |
| Reduced before vaccination | 2 | 0 | 0 | 0 | 2 |
| Held after vaccination | 26 | 1 | 0 | 0 | 27 |
| Reduced after vaccination | 2 | 0 | 0 | 0 | 2 |
| Immunosuppressants | 1294 (24.9) | 729 (55.2) | 495 (72.2) | 63 (51.2) | 2581 (35.2) |
| Glucocorticoids (systemic) | 1255 (24.1) | 463 (35.1) | 447 (65.2) | 56 (45.5) | 2221 (30.3) |
| Held before vaccination | 2 | 2 | 2 | 0 | 6 |
| Reduced before vaccination | 3 | 1 | 2 | 0 | 6 |
| Held after vaccination | 4 | 0 | 2 | 0 | 6 |
| Reduced after vaccination | 1 | 0 | 7 | 0 | 8 |
| Azathioprine/6-mercaptopurine | 19 (<1) | 94 (7.1) | 32 (4.7) | 4 (3.3) | 149 (2) |
| Held before vaccination | 0 | 2 | 0 | 1 | 3 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 1 | 0 | 0 | 1 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Ciclosporin | 12 (<1) | 17 (1.3) | 0 | 0 | 29 (<1) |
| Held before vaccination | 1 | 1 | 0 | 0 | 2 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Cyclophosphamide | 1 (<1) | 7 (<1) | 5 (<1) | 0 | 13 (<1) |
| Held before vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Mycophenolate mofetil/mycophenolic acid | 5 (<1) | 142 (10.8) | 11 (1.6) | 3 (2.4) | 161 (2.2) |
| Held before vaccination | 1 | 4 | 0 | 0 | 5 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 3 | 0 | 0 | 3 |
| Reduced after vaccination | 0 | 1 | 0 | 0 | 1 |
| Tacrolimus | 2 (<1) | 6 (<1) | 0 | 0 | 8 (<1) |
| Held before vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Other medications | |||||
| Intravenous immunoglobulin | 1 (<1) | 12 (<1) | 2 (<1) | 1 (<1) | 16 (<1) |
| Held before vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 1 | 0 | 0 | 1 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| Antifibrotics | 8 (<1) | 2 (<1) | 0 | 0 | 10 (<1) |
| Held before vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced before vaccination | 0 | 0 | 0 | 0 | 0 |
| Held after vaccination | 0 | 0 | 0 | 0 | 0 |
| Reduced after vaccination | 0 | 0 | 0 | 0 | 0 |
| No DMARD therapy | 201 (3.9) | 224 (17) | 71 (10.3) | 20 (16.3) | 516 (7) |
| Unknown | 46 (<1) | 37 (2.8) | 3 (<1) | 1 (<1) | 87 (1.2) |
All medications including monotherapy and/or combination therapy.
bDMARDsbiological DMARDscsDMARDsconventional synthetic DMARDsCTDconnective tissue diseaseDMARDdisease-modifying antirheumatic drugIJDinflammatory joint diseaseILinterleukinJAKJanus kinaseOIRMDother inflammatory rheumatic and musculoskeletal diseaseTNFtumour necrosis factortsDMARDstargeted synthetic DMARDs
In terms of vaccination, 17.7% of the included cases received one dose, while 82.3% received two doses (table 3). Among those who received two doses, the majority received the Pfizer/BioNTech vaccine (74.1%), followed by the AstraZeneca (13.2%) and Moderna vaccines (9.4%). Similarly, among patients who received only one dose, the Pfizer/BioNTech, AstraZeneca and Moderna vaccines were also the most commonly administered, accounting for 42.8%, 9.2% and 30.8% of cases, respectively.
Table 3. SARS-CoV-2 vaccination and flares in patients with inflammatory/autoimmune rheumatic and musculoskeletal diseases.
| IJDn=5207 | CTDn=1320 | Vasculitisn=686 | OIRMDn=123 | All patientsn=7336 | |
| Vaccine administered | |||||
| Pfizer | 3446 (66.2) | 973 (73.7) | 517 (75.4) | 93 (75.6) | 5029 (68.6) |
| Moderna | 508 (9.8) | 118 (8.9) | 63 (9.2) | 6 (4.9) | 695 (9.5) |
| AstraZeneca | 934 (17.9) | 158 (12) | 94 (13.7) | 14 (11.4) | 1200 (16.4) |
| Janssen | 154 (3) | 25 (1.9) | 4 (0.6) | 0 | 183 (2.5) |
| Sputnik V | 6 (0.1) | 1 (0.1) | 0 | 0 | 7 (0.1) |
| CoronaVac/Sinovac | 31 (0.6) | 22 (1.7) | 6 (0.9) | 10 (8.1) | 69 (0.9) |
| Other | 3 (0.1) | 0 | 1 (0.1) | 0 | 4 (0.1) |
| Number of doses administered | |||||
| One | 935 (18) | 229 (17.3) | 121 (17.6) | 15 (12.2) | 1300 (17.7) |
| Two | 4272 (82) | 1091 (82.7) | 565 (82.4) | 108 (87.8) | 6036 (82.3) |
| Flare following vaccination | |||||
| Yes | 206 (4) | 38 (2.9) | 22 (3.2) | 6 (4.9) | 272 (3.7) |
| No | 5001 (96) | 1282 (97.1) | 664 (96.8) | 117 (95.1) | 7064 (96.3) |
CTDconnective tissue diseaseIJDinflammatory joint diseaseOIRMDother inflammatory rheumatic and musculoskeletal disease
Demographic and clinical characteristics of patients with post-vaccination I-RMD flare
Disease flares were reported in 272 (3.7%) of the 7336 patients. Of these patients, 206 (4%) were in the IJD group, 38 (2.9%) in the CTD group, 22 (3.2%) in the vasculitis group and 6 (4.9%) in the other I-RMD group. Mean age of patients with post-vaccination flare was 56.2 (SD 14.3) years and most patients were female (204 of 272, 75%). Most patients had low disease activity (37.9%) before vaccination, followed by remission (36%), moderate (18.8) and high disease activity (1.5%). The most recent vaccine dose before flare was the first in 38.6% and the second in 36% of patients, although these data were missing in 25% of cases. Mean time between flare and the most recent vaccine dose was 7.2 days (SD 8.2). Mean time between vaccination and case reporting was 115 days (SD 98). Most flares were mild (34.2%) or moderate (45.6%), although severe flares with or without hospitalisation occurred in 4.4% and 9.2% of the flare cases, respectively. Flare of arthritis (49.6%), polyarthralgia (37.9%) and increase in fatigue (12.1%) were the most common types of flare. In addition, 44.5% of patients with a flare (1.6% of all patients) started on a new medication or increased the dosage of an existing medication because of the flare (table 4 and online supplemental table 1). TNF inhibitors (30.5), methotrexate (30.1%) and glucocorticoids (24.6%) were the most commonly used drugs, when a flare was observed (online supplemental table 2).
Table 4. Demographic and clinical characteristics of patients with post-vaccination flare.
| IJDn=206 | CTDn=38 | Vasculitisn=22 | OIRMDn=6 | All patientsn=272 | |
| Age, years, mean (SD) | 56.2 (13.4) | 50.8 (13.1) | 66.6 (18.9) | 53.7 (17.7) | 56.2 (14.3) |
| Sex | |||||
| Female | 156 (75.7) | 32 (84.2) | 14 (63.6) | 2 (33.3) | 204 (75) |
| Male | 50 (24.3) | 6 (15.8) | 8 (36.4) | 4 (66.7) | 68 (25) |
| Disease activity | |||||
| Remission | 69 (33.5) | 15 (39.5) | 13 (59.1) | 1 (16.7) | 98 (36) |
| Low disease activity | 86 (41.7) | 10 (26.3) | 5 (22.7) | 2 (33.3) | 103 (37.9) |
| Moderate disease activity | 36 (17.5) | 8 (21.1) | 4 (18.2) | 3 (50) | 51 (18.8) |
| High disease activity | 4 (1.9) | 0 | 0 | 0 | 4 (1.5) |
| Missing/unknown | 11 (5.4) | 5 (13.1) | 0 | 0 | 16 (5.9) |
| Vaccine dose before flare | |||||
| First | 80 (38.8) | 13 (34.2) | 8 (36.4) | 4 (66.7) | 105 (38.6) |
| Second | 73 (35.4) | 15 (39.5) | 9 (40.9) | 1 (16.7) | 98 (36) |
| Unknown/missing | 53 (25.7) | 10 (26.3) | 5 (22.7) | 1 (16.7) | 69 (25) |
| Vaccine type before flare | |||||
| Pfizer | 140 (68) | 29 (76.3) | 18 (81.8) | 4 (66.7) | 191 (70.2) |
| Moderna | 20 (9.7) | 3 (7.9) | 0 | 1 (16.7) | 24 (8.8) |
| AstraZeneca | 43 (20.9) | 6 (15.8) | 4 (18.2) | 0 | 53 (19.5) |
| CoronaVac/Sinovac | 0 | 0 | 0 | 1 (16.7) | 1 (0.4) |
| Unknown/missing | 3 (1.5) | 0 | 0 | 0 | 3 (1.1) |
| Time between vaccine and flare, days, mean (SD) | 6.8 (7.9)(n=141) | 7.8 (7.5)(n=27) | 11.1 (11.4)(n=16) | 1 (1)(n=3) | 7.2 (8.2)(n=187) |
| Type of flare | |||||
| Fever | 15 (7.3) | 4 (10.5) | 2 (9.1) | 1 (16.7) | 22 (8.1) |
| Weight loss | 0 | 0 | 0 | 1 (16.7) | 1 (0.4) |
| Increase in fatigue | 16 (7.8) | 10 (26.3) | 4 (18.2) | 3 (50) | 33 (12.1) |
| Increase in dryness | 0 | 3 (7.9) | 0 | 1 (16.7) | 4 (1.5) |
| Enlarged lymph nodes | 2 (1) | 3 (7.9) | 0 | 0 | 5 (1.8) |
| Polyarthralgia | 77 (37.4) | 14 (36.8) | 9 (40.9) | 3 (50) | 103 (37.9) |
| Arthritis flare | 118 (57.3) | 9 (23.7) | 7 (31.8) | 1 (16.7) | 135 (49.6) |
| Cutaneous flare | 9 (4.4) | 6 (15.8) | 2 (9.1) | 3 (50) | 20 (7.4) |
| Pulmonary flare | 1 (0.5) | 1 (2.6) | 2 (9.1) | 0 | 4 (1.5) |
| Renal flare | 0 | 0 | 2 (9.1) | 0 | 2 (0.7) |
| Neurological flare | 0 | 2 (5.3) | 1 (4.5) | 0 | 3 (1.1) |
| Muscular flare | 6 (2.9) | 4 (10.5) | 3 (13.6) | 1 (16.7) | 14 (5.1) |
| Cardiac flare | 1 (0.5) | 1 (2.6) | 1 (4.5) | 0 | 3 (1.1) |
| Gastrointestinal flare | 1 (0.5) | 0 | 0 | 0 | 1 (0.4) |
| Haematological flare | 0 | 3 (7.9) | 0 | 0 | 3 (1.1) |
| Other | 16 (7.8) | 3 (7.9) | 1 (4.5) | 2 (33.3) | 22 (8.1) |
| Severity of flare | |||||
| Mild | 69 (33.5) | 17 (44.7) | 6 (27.3) | 1 (16.7) | 93 (34.2) |
| Moderate | 101 (49) | 12 (31.6) | 10 (45.5) | 1 (16.7) | 124 (45.6) |
| Severe without hospitalisation | 19 (9.2) | 2 (5.3) | 3 (13.6) | 1 (16.7) | 25 (9.2) |
| Severe with hospitalisation | 2 (1) | 4 (10.5) | 3 (13.6) | 3 (50) | 12 (4.4) |
| Unknown | 8 (3.9) | 2 (5.3) | 0 | 0 | 10 (3.7) |
| New medication or dosage increase due to flare | |||||
| Yes | 86 (41.7) | 19 (50) | 13 (59.1) | 3 (50) | 121 (44.5) |
| No | 110 (53.4) | 15 (39.5) | 8 (36.4) | 3 (50) | 136 (50) |
| Unknown | 10 (4.9) | 4 (10.5) | 1 (4.5) | 0 | 15 (5.5) |
CTDconnective tissue diseaseIJDinflammatory joint diseaseOIRMDother inflammatory rheumatic and musculoskeletal disease
Factors associated with I-RMD flare
In univariable analyses, the following variables were found to be associated with I-RMD flare: age, female sex, low disease activity (vs remission), other vaccine types (vs Pfizer/AstraZeneca/Moderna vaccines) and cessation/reduction of antirheumatic medications at the time of vaccination. Furthermore, moderate/high disease activity (vs remission), exposure to methotrexate, exposure to rituximab and cessation/reduction of antirheumatic medications at the time of vaccination were associated with new medication or dosage increase due to flare (online supplemental table 3).
Two separate multivariable models were built, one with ‘I-RMD flare’ as dependent variable and one with ‘new medication or dosage increase due to flare’ as dependent variable (table 5).
Table 5. Multivariable logistic regression analysis of factors associated with flare, using either ‘I-RMD flare‘ or ‘new antirheumatic medication or dosage increase due to flare’ as the dependent variable.
| Covariates | Outcome: flare | Outcome: new medication or dosage increase due to flare | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, per decade of life | 0.90 (0.83 to 0.98) | 0.015 | 0.92 (0.81 to 1.04) | 0.18 |
| Female sex | 1.40 (1.05 to 1.87) | 0.021 | 1.24 (0.82 to 1.88) | 0.31 |
| Diagnostic group | ||||
| Inflammatory joint diseases | Reference | Reference | ||
| Connective tissue diseases | 0.68 (0.43 to 1.08) | 0.10 | 0.84 (0.45 to 1.57) | 0.59 |
| Vasculitis | 0.96 (0.57 to 1.62) | 0.88 | 0.93 (0.47 to 1.85) | 0.84 |
| Other I-RMD | 1.34 (0.56 to 3.21) | 0.51 | 1.09 (0.32 to 3.70) | 0.89 |
| Disease activity | ||||
| Remission | Reference | Reference | ||
| Low disease activity | 1.45 (1.08 to 1.94) | 0.013 | 1.47 (0.94 to 2.29) | 0.093 |
| Moderate/high disease activity | 1.37 (0.97 to 1.95) | 0.075 | 3.08 (1.91 to 4.97) | <0.001 |
| Vaccine | ||||
| Pfizer | Reference | Reference | ||
| Moderna | 0.87 (0.56 to 1.34) | 0.52 | 1.37 (0.77 to 2.45) | 0.29 |
| AstraZeneca | 1.13 (0.83 to 1.56) | 0.44 | 1.41 (0.90 to 2.22) | 0.13 |
| Other | 0.10 (0.01 to 0.74) | 0.023 | 0 | 1 |
| Antirheumatic medication | ||||
| None | Reference | Reference | ||
| Immunosuppressants | 0.32 (0.07 to 1.35) | 0.12 | 0 | 1 |
| Methotrexate | 0.57 (0.37 to 0.90) | 0.014 | 0.48 (0.26 to 0.89) | 0.019 |
| Leflunomide | 1.24 (0.60 to 2.57) | 0.57 | 0.76 (0.25 to 2.25) | 0.62 |
| Sulfasalazine | 0.85 (0.35 to 2.07) | 0.71 | 0.75 (0.22 to 2.62) | 0.65 |
| Antimalarials | 0.78 (0.43 to 1.40) | 0.41 | 0.47 (0.19 to 1.18) | 0.11 |
| TNFi | 0.55 (0.36 to 0.85) | 0.007 | 0.60 (0.33 to 1.09) | 0.096 |
| tsDMARDs | 0.56 (0.28 to 1.13) | 0.11 | 0.39 (0.13 to 1.18) | 0.094 |
| Rituximab | 0.27 (0.11 to 0.66) | 0.004 | 0.10 (0.01 to 0.77) | 0.027 |
| Other bDMARDs | 0.62 (0.38 to 1.01) | 0.055 | 0.57 (0.28 to 1.14) | 0.11 |
| Glucocorticoids | 0.69 (0.46 to 1.04) | 0.076 | 0.96 (0.56 to 1.65) | 0.89 |
| Cessation/reduction of antirheumatic medications at the time of vaccination | 4.76 (3.44 to 6.58) | <0.001 | 2.24 (1.33 to 3.78) | 0.002 |
For ‘Outcome: flare’, the total N was 7336 (272 flares); for ‘Outcome: new medication or dosage increase due to flare’, some outcome data were missing, and the total N was 5287 (121 flares). Missing values for vaccine type and disease activity were derived by multiple imputation using full conditional specification.
bDMARDsbiological disease-modifying antirheumatic drugsI-RMDinflammatory rheumatic and musculoskeletal diseaseTNFitumour necrosis factor inhibitorstsDMARDstargeted synthetic disease-modifying antirheumatic drugs
Independent factors associated with I-RMD flare were: higher age (per decade of life: OR=0.90, 95% CI=0.83 to 0.98), female sex (OR=1.40, 95% CI=1.05 to 1.87), active disease (low disease activity, OR=1.45, 95% CI=1.08 to 1.94; moderate/high disease activity, OR=1.37, 95% CI=0.97 to 1.95; vs remission), non-Pfizer/AstraZeneca/Moderna vaccines (OR=0.10, 95% CI=0.01 to 0.74; vs Pfizer), cessation/reduction of antirheumatic medication before or after vaccination (OR=4.76, 95% CI=3.44 to 6.58), and exposure to methotrexate (OR=0.57, 95% CI=0.37 to 0.90), TNF inhibitors (OR=0.55, 95% CI=0.36 to 0.85) and rituximab (OR=0.27, 95% CI=0.11 to 0.66), compared with no antirheumatic treatment.
In the multivariable model with new medication or dosage increase due to flare as the dependent variable, the only independent associations observed were: active disease (low disease activity, OR=1.47, 95% CI=0.94 to 2.29; moderate/high disease activity, OR=3.08, 95% CI=1.91 to 4.97; vs remission), cessation/reduction of antirheumatic medication before or after vaccination (OR=2.24, 95% CI=1.33 to 3.78), and exposure to methotrexate (OR=0.48, 95% CI=0.26 to 0.89) and rituximab (OR=0.10, 95% CI=0.01 to 0.77), compared with no antirheumatic treatment.
Secondary models (sensitivity analysis) performed without data imputation yield very similar results to the primary analysis (online supplemental table 4).
Discussion
The EULAR COVAX physician-reported registry represents the largest international case series of individuals with I-RMDs who received vaccination against SARS-CoV-2. Within this study, flares were reported in 3.7% of patients with I-RMD, while flares requiring starting a new medication or increasing the dosage of an existing medication were reported in 1.6% of the patients. Higher disease activity and cessation/reduction of antirheumatic medications before or after vaccination were associated with an increased probability of flare, while exposure to certain medications such as methotrexate and rituximab was associated with a decreased probability of flare (both for flares in general as well as for flares requiring starting a new antirheumatic medication or change the dose of a current medication). Flares were also more likely to occur in younger patients and females, and less likely to occur with non-Pfizer/AstraZeneca/Moderna vaccines; however, these associations were only observed for flares in general, but not for flares requiring starting a new antirheumatic medication or change the dose of a current medication.
The reported incidences of flare and need to start a new antirheumatic medication or to increase the dosage of an existing medication are in line with the initial results of the EULAR COVAX Registry, where we reported incidences of 4.4% and 1.5%, respectively.8 Other studies have reported similar or higher incidences, up to 26.7%, depending on the definition of flare (including patient or physician reported), study methodology and study population investigated.11,23 The highest incidence of flare (26.7%) was reported in a small group of 191 individuals with autoimmune rheumatic diseases who reported minimal clinically important worsening in the 10-item PROMIS physical function form.17 Importantly, the reported incidences of flare and need to start a new antirheumatic medication or to increase the dosage of an existing medication are not beyond the expected underlying risk of flare based on the natural history of I-RMDs.27 28
Post-vaccination flares in individuals with I-RMDs may occur with any vaccination, possibly due to non-specific adjuvant effects or molecular mimicry triggering immune activation, leading to dysregulation of both adaptive and innate immune responses. This dysregulation may involve disordered nucleic acid metabolism and abnormal interferon-induced gene signatures via the TLR-7/9 pathways, contributing to robust early innate immune responses.29 Our findings confirm that while flares in I-RMDs following SARS-CoV-2 vaccination are possible, they are infrequently observed.
In our study, younger age and female sex were found to be more likely associated with flare occurrence. Several studies have explored the association between demographic factors and post-SARS-CoV-2-vaccination flare. For example, in a patient-reported study assessing risk factors for flare requiring treatment after COVID-19 vaccination, female sex emerged as a significant predictor, while age did not show a significant association with flare.20 Other studies have reported the association of age and sex with SARS-CoV-2 vaccination-related flare as non-significant factors.12 15 17 These discrepancies should be interpreted taking into account differences in mean age, sex distribution, disease types, and other study-related methods and characteristics.
o specific disease group was identified as associated with flare. Likewise, another study15 found that specific I-RMD subgroups were not a risk factor for flare. However, a patient-reported study suggested that certain diseases, such as SLE, PsA and PMR, were associated with higher odds of flare, while idiopathic inflammatory myopathies were associated with lower odds of flare compared with RA.20 Data regarding which rheumatic diseases or disease groups are more prone to flare are limited.
The relationship between vaccine type and flare has been depicted differently across various studies. In our study, we showed that vaccination with other vaccines (excluding Pfizer, AstraZeneca and Moderna) was less likely to be associated with flare. This observation should be interpreted with caution, given the low number of non-Pfizer/AstraZeneca/Moderna vaccines administered and the potential reporting bias. Some studies reported higher flare incidence with AstraZeneca20 and Moderna,17 while others did not report a relationship between vaccine type and flare.15 30
Importantly, we found that higher disease activity and cessation/reduction of antirheumatic medications before or after vaccination were associated with an increased probability of flare, both for flares in general as well as for flares requiring starting a new antirheumatic medication or change the dose of a current medication. This finding highlights the importance of I-RMD control when considering vaccination schedules. There is limited knowledge on post-vaccination disease flare in patients with active disease. The potential mechanism underlying post-vaccination flare in such patients may result from persistent immune pathway dysregulation, even during periods of low disease activity, further exacerbated by vaccination. While EULAR recommendations suggest that vaccination should preferably be administered during quiescent disease states,31 in the context of a severe pandemic like COVID-19, vaccination is crucial irrespective of disease activity state.
The knowledge regarding the use, reduction or discontinuation of antirheumatic drugs in the context of SARS-CoV-2 vaccination is still scarce. Both EULAR and the American College of Rheumatology (ACR) provided recommendations on these drugs. Unlike the ACR, which recommends withholding certain drugs, including methotrexate, JAK inhibitors, abatacept, mycophenolate mofetil and rituximab, in patients with controlled disease,24 EULAR does not recommend temporarily reducing or holding any of these drugs except for rituximab.6 32 A recent randomised trial investigated the effects of a 2-week discontinuation of methotrexate in patients with RA following administration of an inactivated SARS-CoV-2 vaccine. The study found that discontinuing methotrexate improved the anti-SARS-CoV-2 IgG response; however, methotrexate discontinuation was also associated with an increase in disease flares after vaccination compared with patients who continued methotrexate.33 Similarly, in an open-label, multicentre, randomised-controlled superiority trial involving individuals with immune-mediated inflammatory diseases, including RA, psoriasis with or without arthritis, axSpA, atopic dermatitis, PMR and SLE, a 2-week interruption of methotrexate therapy resulted in increased antibody responses against the receptor-binding domain of the SARS-CoV-2 spike protein, although with a short-term significant increase in the risk of disease flare after COVID-19 booster vaccination compared with continued methotrexate therapy.34 The clinical significance of interruption of methotrexate after vaccination and improved humoral response is yet to be determined (ie, the clinical impact of this strategy in terms of protection against SARS-CoV-2 infection or prevention of worse COVID-19 outcomes is still unknown), and a shared decision process is needed to weigh the potential benefit of enhancing the humoral response against SARS-CoV-2 against the potential risk of I-RMD exacerbation.
Finally, in our study, patients exposed to methotrexate, TNF inhibitors (overall flare rate only) and rituximab had a lower incidence of flares in our study. In another study,12 receiving csDMARDs or biological therapy was associated with a lower incidence of flares, while in another study, use of mycophenolate mofetil and glucocorticoids was also linked to lower flare incidences.17 However, a cross-sectional study of patients with SLE found no significant association between flare and SLE medications.14 It is known that some immunosuppressive or immunomodulatory drugs reduce seroconversion rates, rendering the vaccine less immunogenic and potentially impacting flare rates.4
This study has limitations. First, there is potential selection bias since the COVAX Registry relies on voluntary case reporting; however, this could in principle have led to over-reporting of flares and AEs; therefore, the low rate of flares consistent with other publications is reassuring. Second, as the data are collected from multiple centres across Europe, they represent subjective evaluations across different settings that could influence results, particularly regarding disease activity and flare assessment. Third, time between vaccination and case reporting is also variable, limiting data interpretation and not allowing us to draw any conclusions regarding the long-term safety profile of vaccines against SARS-CoV-2. Fourth, for some signs/symptoms, it can be difficult to determine if the event should be considered an I-RMD flare or simply a transient reactogenicity effect of the vaccine (eg, polyarthralgia); in our study, this decision was left to the reporting physician, which can be considered a study limitation. Importantly, no causal conclusions regarding vaccination and the development of flares can firmly be drawn from this dataset. The strengths of the current study lie in its clinician-reported nature, the large number of patients, provision of multicentre and international real-life data, and detailed examination of clinical and demographic factors associated with flare across various rheumatic diseases.
In conclusion, our findings suggest that disease flare following SARS-CoV-2 vaccination in individuals with immune-mediated rheumatic diseases (I-RMDs) is uncommon. Higher disease activity and cessation/reduction of antirheumatic medications before or after vaccination were associated with an increased probability of flare, while exposure to certain medications such as methotrexate and rituximab was associated with a decreased probability of flare. These findings will assist patients, clinicians and other healthcare professionals in making informed decisions regarding the management of I-RMDs in the context of SARS-CoV-2 vaccination and contribute to the development of the most appropriate vaccination strategies for patients with I-RMDs.
supplementary material
Acknowledgements
We wish to thank all healthcare providers who entered data into the registry
Footnotes
Funding: Financial support from the European Alliance of Associations for Rheumatology (EULAR).
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Josef S Smolen
Patient consent for publication: Not applicable.
Data availability free text: Applications to access the data will be considered by the European Alliance of Associations for Rheumatology (EULAR) on a case-by-case basis, and data will be shared if the request is considered reasonable, of scientific interest, and legally and ethically possible.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Collaborators: In addition to the authors listed above, the following colleagues also contributed to the EULAR COVAX Registry by submitting at least 10 cases each: Viviane Queyrel, Julien Henry, Raphaele Seror, Eric Toussirot, Emoke Stenova, Azeddine Dellal, Vanda Mlynarikova, Romain Forestier, François Lamer, Hélène Maillard, Amélie Leurs, Thierry Zenone, Daniel Wendling, Amélie Florent, Theodoros Dimitroulas, Simona Rednic, Bernard Combe, Yves Piette, Jozef Odnoga, Giovanna Cuomo, Ioannis Raftakis, Jean-Camille Meric, Sylvain Lanot, Marion Mirabel, Mikhail Protopopov, Katalin Törõcsik, John Brockbank, Marion Jacob, Pascal Coquerelle, Christophe Richez, Elisabeth Gervais, Séverine Verlinden, Antoine Froissart, Fabienne Roux, Marion Couderc, Renaud Desbarbieux, Alojzija Hocevar, Pierre-Yves Jeandel, Sophie Rivière, Luciana Popa, Fabienne Coury, Inita Bulina, Jean-Jacques Dubost, Lionel Spielmann, Marie-Hélène Guyot, Nicolas Deseyne, Isabelle Amigues, Dagmar Mičeková, Loraine Gauzere, Gaëlle Viadere, Natalia de la Torre-Rubio, Victor Strotz.
Presented at: The work reported in this manuscript has previously been presented at ACR Convergence 2022: Farisoğulları B, et al. Factors Associated with Disease Flare Following SARS-CoV-2 Vaccination in People with Inflammatory Rheumatic and Musculoskeletal Diseases–Results from the Physician-Reported EULAR Coronavirus Vaccine (COVAX) Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/factors-associated-with-disease-flare-following-sars-cov-2-vaccination-in-people-with-inflammatory-rheumatic-and-musculoskeletal-diseases-results-from-the-physician-reported-eular-coronaviru/. Accessed 14 May 2024.
Ethics approval: Given the registry collects anonymous non-interventional data, the UK Health Research Authority (HRA) does not class the registry as a research study (in line with the HRA decision tool) and patient consent is not required.
Contributor Information
Bayram Farisogullari, Email: bayramfarisogullari@gmail.com.
Saskia Lawson-Tovey, Email: saskia.lawson-tovey@manchester.ac.uk.
Kimme L Hyrich, Email: kimme.hyrich@manchester.ac.uk.
Laure Gossec, Email: laure.gossec@aphp.fr.
Loreto Carmona, Email: loreto.carmona@inmusc.eu.
Anja Strangfeld, Email: strangfeld@drfz.de.
Elsa F Mateus, Email: elsafrazaomateus@gmail.com.
Martin Schäfer, Email: martin.schaefer@drfz.de.
Ana Rodrigues, Email: anamfrodrigues@gmail.com.
Eric Hachulla, Email: eric.hachulla@chru-lille.fr.
Jose A Gomez-Puerta, Email: JAGOMEZ@clinic.cat.
Marta Mosca, Email: marta.mosca@med.unipi.it.
Patrick Durez, Email: patrick.durez@uclouvain.be.
Ludovic Trefond, Email: ltrefond@chu-clermontferrand.fr.
Tiphaine Goulenok, Email: tiphaine.goulenok@aphp.fr.
Martina Cornalba, Email: martinacornalba2@gmail.com.
Emoke Stenova, Email: e.stenova@hotmail.com.
Inita Bulina, Email: inita.bulina@stradini.lv.
Eva Strakova, Email: strakova.eva@pobox.sk.
Julija Zepa, Email: julija.zepa@rsu.lv.
Nicolas Roux, Email: nicolas.roux@uneos.fr.
Olivier Brocq, Email: olivier.brocq@chpg.mc.
Eric Veillard, Email: eric.veillard@wanadoo.fr.
Bernd Raffeiner, Email: berndraffeiner@yahoo.com.
Gerd R Burmester, Email: gerd.burmester@charite.de.
Xavier Mariette, Email: xavier.mariette@aphp.fr.
Pedro M Machado, Email: p.machado@ucl.ac.uk.
EULAR COVAX Registry:
Viviane Queyrel, Julien Henry, Raphaele Seror, Eric Toussirot, Emoke Stenova, Azeddine Dellal, Vanda Mlynarikova, Romain Forestier, François Lamer, Hélène Maillard, Amélie Leurs, Thierry Zenone, Daniel Wendling, Amélie Florent, Theodoros Dimitroulas, Simona Rednic, Bernard Combe, Yves Piette, Jozef Odnoga, Giovanna Cuomo, Ioannis Raftakis, Jean-Camille Meric, Sylvain Lanot, Marion Mirabel, Mikhail Protopopov, Katalin Törõcsik, John Brockbank, Marion Jacob, Pascal Coquerelle, Christophe Richez, Elisabeth Gervais, Séverine Verlinden, Antoine Froissart, Fabienne Roux, Marion Couderc, Renaud Desbarbieux, Alojzija Hocevar, Pierre-Yves Jeandel, Sophie Rivière, Luciana Popa, Fabienne Coury, Inita Bulina, Jean-Jacques Dubost, Lionel Spielmann, Marie-Hélène Guyot, Nicolas Deseyne, Isabelle Amigues, Dagmar Mičeková, Loraine Gauzere, Gaëlle Viadere, Natalia de la Torre-Rubio, and Victor Strotz
Data availability statement
Data are available upon reasonable request.
References
- 1.Callaway E, Cyranoski D, Mallapaty S, et al. The coronavirus pandemic in five powerful charts. Nature . 2020;579:482–3. doi: 10.1038/d41586-020-00758-2. [DOI] [PubMed] [Google Scholar]
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–60. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. Bnt162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–23.:NEJMoa2101765. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroon FPB, Najm A, Alunno A, et al. Risk and prognosis of SARS-Cov-2 infection and vaccination against SARS-Cov-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann Rheum Dis. 2022;81:422–32. doi: 10.1136/annrheumdis-2021-221575. [DOI] [PubMed] [Google Scholar]
- 5.Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-Cov-2. Ann rheum dis. Ann Rheum Dis . 2020;79:851–8. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
- 6.Landewé RBM, Kroon FPB, Alunno A, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-Cov-2: the November 2021 update. Ann Rheum Dis. 2022;81:1628–39. doi: 10.1136/annrheumdis-2021-222006. [DOI] [PubMed] [Google Scholar]
- 7.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21:475–84. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS-Cov-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus vaccine (COVAX) physician-reported Registry. Ann Rheum Dis. 2022;81:695–709. doi: 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]
- 9.Najm A, Alunno A, Machado PM. COVID - how will it continue to change our lives. Joint Bone Spine. 2023;90:105572. doi: 10.1016/j.jbspin.2023.105572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported Registry. Ann Rheum Dis. 2021;80:930–42. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbhaiya M, Levine JM, Bykerk VP, et al. Systemic rheumatic disease flares after SARS-Cov-2 vaccination among rheumatology outpatients in New York City. Ann Rheum Dis. 2021;80:1352–4. doi: 10.1136/annrheumdis-2021-220732. [DOI] [PubMed] [Google Scholar]
- 12.Connolly CM, Ruddy JA, Boyarsky BJ, et al. Disease flare and Reactogenicity in patients with rheumatic and musculoskeletal diseases following two-dose SARS-Cov-2 messenger RNA vaccination. Arthritis Rheumatol . 2022;74:28–32. doi: 10.1002/art.41924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Geng Y, Wang Y, et al. Safety and disease flare of autoimmune inflammatory rheumatic diseases: a large real-world survey on Inactivated COVID-19 vaccines. Ann Rheum Dis. 2022;81:443–5. doi: 10.1136/annrheumdis-2021-221736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felten R, Kawka L, Dubois M, et al. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the International VACOLUP study. Lancet Rheumatol . 2021;3:e613–5. doi: 10.1016/S2665-9913(21)00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fragoulis GE, Bournia V-K, Mavrea E, et al. COVID-19 vaccine safety and Nocebo-prone associated hesitancy in patients with systemic rheumatic diseases: a cross-sectional study. Rheumatol Int. 2022;42:31–9. doi: 10.1007/s00296-021-05039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaur P, Agrawat H, Shukla A. COVID-19 vaccine hesitancy in patients with systemic autoimmune rheumatic disease: an interview-based survey. Rheumatol Int. 2021;41:1601–5. doi: 10.1007/s00296-021-04938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagtap K, Naveen R, Day J, et al. Flares in autoimmune rheumatic diseases in the post-COVID-19 vaccination period-a cross-sequential study based on COVAD surveys. Rheumatology (Oxford) 2023;62:3838–48. doi: 10.1093/rheumatology/kead144. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81:564–8. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohanasundaram K, Santhanam S, Natarajan R, et al. Covid-19 vaccination in autoimmune rheumatic diseases: A multi-center survey from Southern India. Int J Rheum Dis. 2022;25:1046–52. doi: 10.1111/1756-185X.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rider LG, Parks CG, Wilkerson J, et al. Baseline factors associated with self-reported disease flares following COVID-19 vaccination among adults with systemic rheumatic disease: results from the COVID-19 global rheumatology alliance vaccine survey. Rheumatology (Oxford) 2022;61:SI143–50. doi: 10.1093/rheumatology/keac249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 global rheumatology alliance vaccine survey. RMD Open. 2021;7:e001814. doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tani C, Cardelli C, Depascale R, et al. Long-term outcomes of COVID-19 vaccination in patients with rare and complex connective tissue diseases: the ERN-Reconnet VACCINATE study. J Transl Autoimmun . 2023;7:100221. doi: 10.1016/j.jtauto.2023.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zavala-Flores E, Salcedo-Matienzo J, Quiroz-Alva A, et al. Side effects and flares risk after SARS-Cov-2 vaccination in patients with systemic lupus erythematosus. Clin Rheumatol. 2022;41:1349–57. doi: 10.1007/s10067-021-05980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis JR, Johnson SR, Anthony DD, et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 5. Arthritis Rheumatol. 2023;75:E1–16. doi: 10.1002/art.42372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Minor BL, et al. The Redcap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)—A Metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bykerk VP, Shadick N, Frits M, et al. Flares in rheumatoid arthritis: frequency and management. A report from the BRASS Registry. J Rheumatol. 2014;41:227–34. doi: 10.3899/jrheum.121521. [DOI] [PubMed] [Google Scholar]
- 28.Markusse IM, Dirven L, Gerards AH, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the best study. Arthritis Res Ther. 2015;17:232. doi: 10.1186/s13075-015-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watad A, De Marco G, Mahajna H, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-Cov-2 vaccination. Vaccines . 9:435. doi: 10.3390/vaccines9050435. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinte L, Negoi F, Ionescu GD, et al. COVID-19 vaccine does not increase the risk of disease flare-UPS among patients with autoimmune and immune-mediated diseases. J Pers Med. 2021;11:1283. doi: 10.3390/jpm11121283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furer V, Rondaan C, Heijstek MW, et al. Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 32.Bijlsma JW. EULAR December 2020 viewpoints on SARS-Cov-2 vaccination in patients with Rmds. Ann Rheum Dis. 2021;80:411–2. doi: 10.1136/annrheumdis-2020-219773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araujo CSR, Medeiros-Ribeiro AC, Saad CGS, et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with Inactivated SARS-Cov-2 vaccine: a randomised clinical trial. Ann Rheum Dis. 2022;81:889–97. doi: 10.1136/annrheumdis-2021-221916. [DOI] [PubMed] [Google Scholar]
- 34.Abhishek A, Boyton RJ, Peckham N, et al. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir Med. 2022;10:840–50. doi: 10.1016/S2213-2600(22)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.


