Abstract
ABSTRACT
Background
The Neuroform Atlas Stent System is an established treatment modality for unruptured anterior and posterior circulation intracranial aneurysms. Location-specific results are needed to guide treatment decision-making. However, it is unclear whether there are differences in safety and efficacy outcomes between carotid and more distal anterior circulation aneurysms.
Methods
The ATLAS IDE trial was a prospective, multicenter, single-arm, open-label interventional study that evaluated the safety and efficacy of the Neuroform Atlas Stent System. We compared differences in efficacy and safety outcomes of proximal internal carotid artery (ICA) versus distal and bifurcation anterior circulation aneurysms.
Results
Of 182 cases, there were 70 aneurysms in the ICA and 112 in the distal anterior circulation (including ICA terminus/bifurcation). There were no significant differences in the primary efficacy endpoint (85.5% vs 83.9%, p=0.78) and complete aneurysm occlusion rates (88.7% vs 87.9%, p=0.78) between proximal ICA aneurysms and distal aneurysms, respectively. Complications were more often encountered in distal and bifurcation aneurysms, but the overall rate of major safety events was low and comparable between the two groups (1.4% vs 6.3%, p=0.14). Recanalization and retreatment rates were also similar between the groups.
Conclusion
The results of this study suggest that the Neuroform Atlas Stent System is a safe and efficacious treatment modality for unruptured anterior circulation intracranial aneurysms, regardless of aneurysm location.
Trial registration number
Keywords: Aneurysm, Device, Intervention, Stent, Coil
WHAT IS ALREADY KNOWN ON THIS TOPIC
Prior to the ATLAS IDE trial, several studies had been conducted on the use of the Neuroform Atlas Stent System for the treatment of anterior circulation aneurysms. These studies demonstrated high rates of successful aneurysm occlusion and acceptable safety profiles. Comparisons of outcomes between proximal internal carotid artery (ICA) aneurysms and distal and bifurcation aneurysms in patients undergoing stent-assisted coiling are scarce.
WHAT THIS STUDY ADDS
This study adds to the existing body of literature on the safety and efficacy of the Neuroform Atlas Stent System for different aneurysm locations. This subanalysis of the ATLAS IDE trial was the first independently adjudicated study to compare safety and efficacy outcomes between proximal and distal anterior circulation aneurysms treated with the Neuroform Atlas Stent System.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
This study supports the device as an effective strategy in treating both proximal ICA and distal aneurysms, posing the device as a viable treatment option for a broad range of aneurysms in the anterior circulation.
INTRODUCTION
Stent-assisted coiling (SAC) has emerged as a strategy for treating wide-neck and complex intracranial aneurysms. The scaffolding provided by the stent mesh has allowed the treatment of lesions previously not amenable to coiling alone. Further evidence suggests that the mesh structure facilitates the achievement of higher coil packing density and stability, along with stimulating re-endothelization.1 The stent technology has been refined over the years, with multiple iterations designed to improve performance and outcomes.2
The Neuroform Atlas Stent System (Stryker Neurovascular, Fremont, CA, USA) is a newer generation of the Neuroform stent featuring a self-expanding, hybrid cell design. It was manufactured to improve navigability, device apposition, and conformability to the vessel wall. The system was initially approved under Humanitarian Device Exemption (HDE) in 2017, and full approval by the Food and Drug Administration (FDA) was granted in 2019. The Pivotal Trial of the Neuroform Atlas Stent for treatment of Anterior Circulation Aneurysms (ATLAS IDE trial) formally investigated the safety and efficacy of the Atlas device for anterior circulation intracranial aneurysms. One-year findings indicated a primary efficacy rate of 84.7% and a 12-month adjusted major complication rate (major ipsilateral stroke or neurological death) of 4.4%, meeting both the primary and secondary performance goals.3 Similar results were reported by the Multi-centric European post-market follow-up study of the Neuroform Atlas Stent System (ATLAS EU PMCF study), with a complete occlusion rate at 1-year follow-up of 91.3%. The study also reported a 4.9% major complication rate, with only 1% resulting in permanent morbidity or mortality.4
Aneurysm location, along with vasculature morphology, may impact the outcome of endovascular stenting. The hemodynamics of sidewall aneurysms can differ from aneurysms in which sharp angulation between the parent vessel and aneurysm ostium exists.5 6 The deployment of a stent in bifurcation aneurysms can significantly modify local vasculature architecture and shear forces, which may interfere with aneurysm recanalization and occlusion.7,10 Still, studies evaluating potential differences in outcomes of proximal versus distal intracranial aneurysms undergoing SAC are scarce in the literature. Our study aimed to compare the safety and efficacy of SAC using the Neuroform Atlas device for proximal versus distal anterior circulation aneurysms.
METHODS
Patients and study design
The ATLAS IDE trial was a prospective, multicenter, single-arm, open-label interventional trial designed to demonstrate the safety and effectiveness of the next-generation Neuroform Atlas Stent System with any approved embolic coils on the market for the treatment of intracranial aneurysms. Eligible patients: (1) aged between 18 and 80 years; (2) documented wide-neck (neck ≥4 mm or dome-to-neck ratio of <2 mm), saccular, intracranial aneurysm located within the anterior circulation (excluding the petrous internal carotid artery (ICA) to the superior hypophyseal ICA region); and (3) parent vessel with a diameter of ≥2 mm and ≤4.5 mm, which could be treated with bare metal coils. Main exclusion criteria included the presence of multiple untreated intracranial aneurysms, an acutely ruptured aneurysm (within 14 days of enrollment), premorbid modified Rankin Scale ≥4 or premorbid Hunt and Hess score ≥3, and prior treatment of the same aneurysm with stent-assisted coil embolization. Patients were recruited from 25 sites across the United States. The modified intent-to-treat (mITT) population with anterior circulation aneurysms used for safety and efficacy analyses was defined as patients who signed the informed consent form and in whom a procedure was attempted, regardless of the success of implantation. Patients were grouped according to aneurysm location into proximal (ICA group; from superior hypophyseal up to the anterior choroidal ICA regions) and distal (non-ICA group; including ICA terminus/bifurcation) aneurysm groups.
Protocol and registration
Patients provided written informed consent prior to study participation. Each participating center obtained institutional review board approval of the investigational device exemption study protocol. The trial was registered at clinicaltrials.gov (NCT02340585). A full description of the trial protocol is available on request, and details are otherwise available in the Pivotal Trial of the Neuroform Atlas Stent for Treatment of Anterior Circulation Aneurysms: One-Year Outcomes publication.3
Device and procedure
The Neuroform Atlas is a self-expanding, open-cell, nitinol stent designed to provide support for the coil mass within the aneurysm and minimize stent deflection. Protocol required dual antiplatelet administration of oral aspirin (81–325 mg/day) and clopidogrel (75 mg/day) for at least 5 days prior to the procedure. Assessment of antiplatelet activity was not required per protocol, and all procedures were carried out under general anesthesia. Placement of a single device was preferably recommended, but a second device was allowed if judged necessary to improve wall apposition and adequately cover the aneurysm neck.
Efficacy outcomes
The primary efficacy outcome of the study was to compare the rate of complete aneurysm occlusion (100% occlusion) at 12-month angiographic follow-up between proximal versus distal anterior circulation aneurysms, in the absence of retreatment or parent vessel stenosis (>50%) at the target location, and no neurological death. Raymond–Roy occlusion classification was used to adjudicate the primary effectiveness outcome.11 Digital subtraction angiography was mandatory at 1-year follow-up to assess efficacy outcomes, and all radiological images were reviewed and adjudicated by an independent imaging core lab. Secondary efficacy outcomes included differences in the incidence of target aneurysm retreatment and rates of aneurysm recanalization. Additionally, the occurrence of implant migration and the incidence of parent artery stenosis (>50%) were also evaluated.
Safety outcomes
The primary safety outcome was to compare the rate of major ipsilateral stroke (increase in National Institutes of Health Stroke Scale (NIHSS) score ≥4) or neurological death between proximal and distal aneurysms at 12-month follow-up. Secondary safety outcomes included worsening major ipsilateral stroke, rate of subarachnoid hemorrhage, and rate of target aneurysm rupture. A clinical events committee evaluated any serious device-related events and prespecified safety event endpoints.
Statistical analyses
Categorical data were summarized using frequencies and percentages, while continuous variables were presented as mean and SD. All analyses comparing location groups accounted for clustering within site. Generalized linear mixed models with a random effect were used for continuous variables and chi-square tests accounting for clustering were used for categorical variables. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Baseline and procedural characteristics
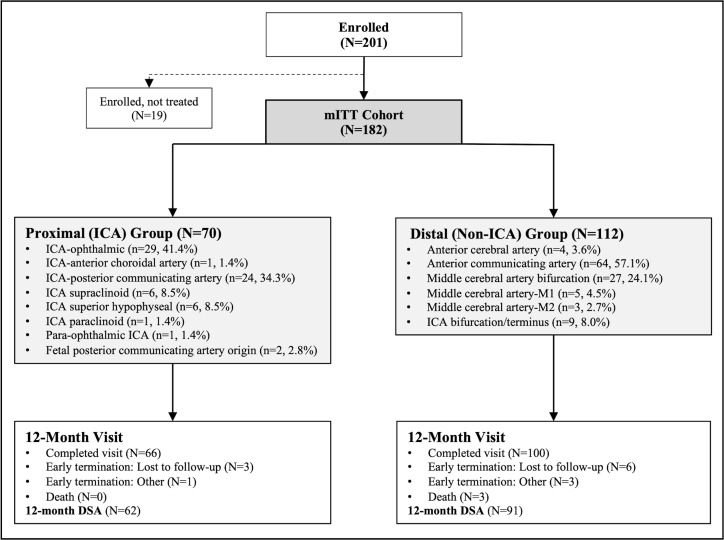
Of the 201 participants enrolled in the study between June 2015 and October 2016, investigational device implantation was attempted in 182 patients (mITT cohort) who were included in the analysis. The mean age of the cohort was 60.3±11.4 years, mostly female (73.1%), and white (80.8%). Twenty-two (12.1%) patients had experienced a previous rupture of the targeted aneurysm and were previously treated with coiling (72.7%, 16/22), balloon-assisted coiling (4.5%, 1/22), or other means (22.7%). Of 182 patients, 153 had one stent implanted (84.1%) and 29 had two stents implanted (15.9%). A total of 70 (38.5%) patients composed the proximal group and 112 (61.5%) the distal aneurysm group. When comparing the groups (table 1), patients with distal aneurysms were more likely to be older, male, and have smaller vessel diameters. Figure 1 provides a visual representation of the enrollment process and details the aneurysm groups with the breakdown of aneurysm locations.
Table 1. Baseline and procedural characteristics.
| Characteristic | Proximal (ICA) group (n=70) | Distal (non-ICA) group(n=112) | P value |
| Demographics | |||
| Age (years), mean (SD) | 57.4 (11.0) | 62.1 (11.3) | 0.03 |
| Male, n (%) | 10 (14.3) | 39 (34.8) | 0.006 |
| White, n (%) | 55 (78.6) | 92 (82.1) | 0.44 |
| Aneurysm characteristics, mean (SD) | |||
| Aneurysm neck width (mm) | 4.0 (1.1) | 4.2 (1.3) | 0.27 |
| Aneurysm size (mm) | 0 (2.0) | 6.2 (2.3) | 0.35 |
| Dome-to-neck ratio | 1.2 (0.3) | 1.1 (0.3) | 0.25 |
| Parent vessel diameter proximal to the aneurysm neck (mm) | 3.7 (0.5) | 2.6 (0.5) | <0.0001 |
| Parent vessel diameter distal to the aneurysm neck (mm) | 3.4 (0.5) | 2.3 (0.4) | <0.0001 |
| Previously ruptured aneurysm treatment, n (%) | 6 (8.6) | 16 (14.3) | 0.23* |
| Coiling only | 5 (83.3) | 11 (68.8) | |
| Balloon-assisted coiling | 1 (16.7) | 0 (0.0) | |
| Other | 0 (0.0) | 5 (31.2) | |
| Procedural technical success, n (%) | 70 (100.0) | 112 (100.0) | N/A |
| Stents implanted, n (%) | 0.07 | ||
| 1 | 66 (94.3) | 87 (77.7) | |
| 2 | 4 (5.7) | 25 (22.3) | |
| Immediate Raymond Class (core lab), n (%) | 0.26 | ||
| 1 | 52 (76.5) | 88 (78.6) | |
| 2 | 14 (20.6) | 14 (12.5) | |
| 3 | 2 (2.9) | 10 (8.9) | |
| Raymond Class 1 and 2 combined, n (%) | 66 (97.1) | 102 (91.1) | 0.14 |
Values in bold type indicate statistical significance.
Comparison of whether there was previous treatment, regardless of treatment modality.
N/Anot available
Figure 1. Patient flow diagram and aneurysm location distribution from ATLAS IDE trial subanalysis groups. DSA, digital subtraction angiography; ICA, internal carotid artery; mITT, modified intent-to-treat.
Effectiveness outcomes
Of 155 patients, composite effectiveness endpoint of complete aneurysm occlusion without clinically significant stenosis or retreatment and no neurological death was met by 85.5% and 83.9% of patients in the proximal and distal groups, respectively. When comparing angiographic follow-up availability at 1 year, the proportions were akin (88.6% for the proximal group vs 81.3% for the distal group, p=0.14). There were no statistically significant differences in the primary efficacy endpoint, Raymond–Roy aneurysm occlusion rates, recanalization rates, and incidence of parent artery stenosis between the groups. Patients from the distal group tended to have increased rates of worsening aneurysm occlusion (6.6% vs 3.2%) and retreatment (4.5% vs 2.9%) compared with the ICA group, but the differences were not statistically significant. Table 2 details the results of effectiveness outcomes between the groups.
Table 2. Effectiveness outcomes.
| Parameter | Proximal (ICA) group | Distal (non-ICA) group | P value |
| Primary efficacy endpoint, n (%) | 53/62 (85.5) | 78/93 (83.9) | 0.78 |
| Secondary efficacy endpoints | |||
| Raymond Class (core lab), n (%) | 0.78 | ||
| 1 | 55/62 (88.7) | 80/91 (87.9) | |
| 2 | 4/62 (6.5) | 8/91 (8.8) | |
| 3 | 3/62 (4.8) | 3/91 (3.3) | |
| Raymond Class 1 and 2 combined, n (%)* | 59/62 (95.2) | 88/91 (96.7) | 0.65 |
| Recanalization, n (%)† | 4/62 (6.5) | 5/92 (5.4) | 0.76 |
| Progressive occlusion of target aneurysm (core lab), n (%) | 0.61 | ||
| Same | 37/62 (59.7) | 55/91 (60.4) | |
| Better | 23/62 (37.1) | 30/91 (33.0) | |
| Worse | 2/62 (3.2) | 6/91 (6.6) | |
| Parent artery stenosis >50% (core lab), n (%) | 1/62 (1.6) | 1/91 (1.1) | 0.78 |
| Incidence of stent migration (core lab), n (%) | 0/68 (0.0) | 0/112 (0.0) | N/A |
| Incidence of retreatment (site-reported), n (%)‡ | 2/70 (2.9) | 5/112 (4.5) | 0.55 |
Composite outcome: complete aneurysm occlusion (100% occlusion – Raymond Class 1) of the treated target lesion on 12-month angiography, in the absence of retreatment, or parent artery stenosis (>50%) at the target location, and no neurological death.
Recanalization is defined as a Raymond score of 3 at 12-month visit or retreatment due to recanalization.
Two of the seven subjects had pre-planned staged procedures.
N/Anot available
Safety outcomes
When comparing proximal versus distal with respect to primary safety composite outcome, the non-ICA group tended to have a higher rate of events than the ICA group, although the difference was not statistically significant (6.3% vs 1.4%, p=0.14). Similarly, a higher trend for subarachnoid hemorrhage and aneurysm rupture was encountered for the non-ICA group, but there were no significant differences between the groups. The results of safety outcome analyses in both groups are shown in table 3.
Table 3. Safety endpoints.
| Parameter | Proximal (ICA) group | Distal (non-ICA) group |
| Primary safety endpoint, n (%)* | 1/70 (1.4) | 7/112 (6.3) |
| Secondary safety endpoints | ||
| New or worsening major ipsilateral stroke (CEC adjudicated), n (%) | 1/70 (1.4) | 7/112 (6.3) |
| Subarachnoid hemorrhage (CEC-adjudicated), n (%) | 1/70 (1.4) | 6/112 (5.4) |
| Aneurysm rupture (CEC-adjudicated), n (%) | 1/70 (1.4) | 4/112 (3.6) |
Any major ipsilateral stroke or neurological death within 12 months. Note that one subject experienced both major ipsilateral stroke and neurological death.
CECclinical events committee
DISCUSSION
This is the first independently adjudicated study comparing differences in safety and efficacy outcomes of proximal versus distal anterior circulation aneurysms in patients undergoing SAC. The ATLAS IDE trial cohort included 70 (38.5%) ICA aneurysms and 112 (61.5%) distal anterior circulation aneurysms (including ICA terminus/bifurcation) successfully treated with the Neuroform Atlas Stent System. At 1-year follow-up, aneurysms treated with the Atlas device demonstrated high complete occlusion rates, with similar efficacy results in proximal and distal intracranial aneurysms. Patients from the non-ICA group had a trend for higher complications, but the difference in safety outcomes was not statistically significant.
Stent-assisted techniques were initially conceptualized to treat wide-neck aneurysms by preventing coiling herniation into the parent vessel and achieving high packing density. Additional mechanisms contributing to high occlusion rates with SAC compared with coiling alone have been identified, including the mesh serving as a scaffold for neointima formation and changes in local flow dynamics.12 Tortuous vascular architecture can increase the risk of incomplete stent apposition, posing challenges to stent technology depending on the aneurysm’s location.13 The Atlas device is a self-expandable stent, allowing for better performance in curvatures and in cases of vessel size discrepancy between proximal and distal landing zones. Its hybrid design offers stability with closed cells at the proximal end, and open cells at the distal end provide conformability to the vessel wall.14 The laser-cut manufacturing also contributes to mitigating device foreshortening, providing an advantage compared with braided stents.15 These features, along with a low-profile mesh and a 0.0165 delivery system, have made the device an optimal tool for bifurcation and distal aneurysms treatment compared with other stents.16 17
Local shear forces and spatial gradients at the apex of bifurcation aneurysms are highly dependent on the bifurcation and inflow vessel geometry and may contribute to the development of brain aneurysms.18 Conflicting results and hypotheses have been encountered over the years regarding the association and impact of vascular architecture and stent remodeling on bifurcation aneurysms.78 10 18,21 Still, these studies reinforce the geometric and hemodynamic differences of proximal sidewall aneurysms compared with more distal, bifurcation aneurysms undergoing SAC. A previous comparison between sidewall and terminal-type aneurysms (mainly consisting of bifurcation aneurysms) showed a trend for higher symptomatic complication rates in terminal-type aneurysms (5.7% vs 1.2%) but the difference was not significant (p=0.15).22 Similarly, non-ICA aneurysm patients in the ATLAS IDE cohort showed a trend for higher safety primary outcomes (6.3% vs 1.4%) but the difference was also not statistically significant (p=0.14). While the difference in complication rates between groups was not found to be statistically significant, there was a noticeable trend towards increased occurrences of stroke, vessel perforation, subarachnoid hemorrhage, and aneurysm rupture, which is easy to be rationalized by considering the more distal nature of the aneurysm, smaller vessel caliber, greater stress on vessel walls, larger ratio of microcatheter size to vessel size, among others.
Complete aneurysm occlusion in the non-ICA group was achieved by 87.9% of patients, and 4.5% underwent retreatment. Close to 90% of all anterior circulation aneurysms from the non-ICA group were located at bifurcations, with the anterior communicating artery (57%) and middle cerebral artery (24%) as the most common sites. Different endovascular modalities have been employed for the treatment of bifurcation aneurysms, including coiling, SAC, intrasaccular flow modifier, and flow diverters. The ATLAS IDE subanalysis of MCA aneurysms demonstrated higher rates of complete aneurysm occlusion (80.8%) compared with results from a meta-analysis of unruptured MCA aneurysms treated by different endovascular modalities (60%),23 and comparable to studies focusing on MCA aneurysm treatment with SAC (78.9–90.6%).24 25 In a previous experience including 184 Acomm aneurysms undergoing SAC, 86.9% showed complete occlusion and 13.1% recanalization during long-term surveillance; the overall procedural complication rate in the unruptured cohort was 5.7%.26 When considering treatment with the Woven EndoBridge (WEB) device, a series of 48 anterior communicating artery aneurysms reported that complete occlusion was achieved in 62.5% of cases.27 In an early experience evaluating flow diverters for intracranial bifurcation aneurysm treatment, 97.3% of cases demonstrated no aneurysm filling at 18 months follow-up, with new permanent neurologic deficits occurring in 9.4% of cases.28 In a systematic review of flow diversion treatment of unruptured saccular anterior communicating artery aneurysms, complete occlusion was seen in 84.9% of cases, with an overall treatment-related complication rate of 8.6% (3.5% permanent complications).29 Direct comparison among the techniques can be challenging due to variable aneurysm location, morphology, and flow dynamics. However, the ATLAS IDE cohort demonstrated high occlusion rates and an acceptable safety profile for distal and bifurcation anterior circulation aneurysms compared with historical data and different treatment modalities, emphasizing the potential for broader adoption of the device.
Additionally, at 1-year follow-up of the ATLAS IDE trial, 88.7% of ICA aneurysm patients were completely occluded, and 2.9% underwent retreatment. The safety endpoint was met by 1.4% of patients, corresponding to a single case of major ipsilateral stroke. Previous studies have described the feasibility of SAC for treating ICA aneurysms.30 31 In contemporary ICA aneurysm treatment, however, flow diverters have become one of the mainstay strategies. PREMIER was the largest prospective trial evaluating the pipeline embolization device (PED) for treating small and medium-sized unruptured intracranial aneurysms (95% located at the ICA). At 1-year follow-up, 81.9% with aneurysms had complete occlusion, retreatment rate was 2.9%, and safety endpoint was met by 2.1%.32 In a cohort comparing ophthalmic aneurysms treated with SAC (n=62) and PED (n=106), there was no significant difference in complete occlusion (76% vs 81%, p=0.52), retreatment rates (6.5% vs 0.9%, p=0.06), and neurologic complications (4.8% vs 9.4%, p=0.38) between the groups.33 In a similar study comparing differences in outcomes involving communicating ICA segment aneurysms, there were no significant differences in complete occlusion (70.6% vs 81%, p=0.45) and complications between SAC and PED.34 A propensity score matching was used to compare the outcomes of 309 patients with ICA aneurysms undergoing treatment with Atlas SAC and PED. There were no significant differences in the rates of aneurysm occlusion (89.9% vs 86.5%, p=0.486), total complications (5.6% vs 11.2%, p=0.177), or favorable functional outcome (96.6% vs 97.8%, p=1.0) between the Atlas and PED groups.35 The high occlusion rates in our study and the available literature suggest that SAC with the Atlas device remains an efficacious strategy for ICA aneurysm treatment.
Limitations
This analysis has some limitations that need to be acknowledged. There is a potential for selection bias since the study was non-randomized. This means that there could be other factors influencing the results that were not captured in the dataset. Heterogeneity within the groups may limit the evaluation of the primary intervention effect on primary outcomes between proximal and distal aneurysms, including location-specific effects. Loss of follow-up was noted in a subset of patients, which could potentially introduce uncertainty to the generalizability of our findings. However, it is reassuring to observe that the proportion of patients lost to follow-up was evenly distributed between the groups, which might mitigate some of the associated risks of bias. It is worth noting that challenges in securing complete angiographic follow-up are not uncommon in prospective studies of this nature. Despite these limitations, this study also had strengths. The study design included an independent central imaging core lab and an independent clinical event committee, which helped reduce bias and validate the discussion on approach options for anterior circulation aneurysm treatment.
CONCLUSIONS
Our study demonstrated no significant differences in effectiveness and safety outcomes between proximal ICA aneurysms compared with distal and bifurcation aneurysms in patients treated with the Neuroform Atlas Stent System. These findings reemphasize the Atlas stent as a safe and efficacious treatment modality for unruptured anterior circulation intracranial aneurysms. A trend towards higher complications was observed for more distal aneurysms. Larger studies are needed to assess this possible difference.
Acknowledgements
The authors acknowledge Patricia Morgan and the Stryker team for their assistance.
Footnotes
Funding: The ATLAS trial (Safety and Effectiveness of the Treatment of Wide Neck, Saccular Intracranial Aneurysms with the Neuroform Atlas Stent System) was funded by Stryker Neurovascular.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Christiana Care (Christiana Care Institutional Review Board): CCC# 35146; DDD# 602973Cedars Sinai Medical Center (Office of Research Compiance and Quality Improvement): MOD00002885; Pro00040978Cleveland Clinic (Cleveland Clinic Institutional Review Board): #15-777MUSC (Institutional Review Board for Human Research): Pro00043019RUSH (Rush University Medical Center Institutional Review Board) 15010903-IRB01-AM13SSM DePaul (SSMSTL IRB): #15-03-0633St Luke’s Episcopal (Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals): H-37845Univ of KY Hosp (WIRB): #1-1342680-1Univ of Mass (UMass Chan Medical School): MODCR00000071Harborview (WIRB): 1-1516101-1Vanderbilt University (Human Research Protections Program – HRPP): #151918VCU (WIRB): #1-1516101-1Methodist Hospital (HMRI IRB): CR00001111Swedish Medical Ctr (HCA - HealthONE Institutional Review Board): #718984-48UPMC (WIRB): #1-1380703-1Wellstar Kennestone Hospital (WIRB): #1-1516101-1Beth Israel (Committee on Clinical Investigations): #2015P000153Hosp of the Univ of Penn (University of Pennsylvania IRB): #822253Methodist HC (UTHSC IRB): #15-03792-FBJohns Hopkins (Office of Human Subjects Research IRB): IRB00061212/CIR00060978Los Robles Medical Center (WIRB): #1-1516101-1Lyerly Baptist (Baptist Health IRB): #15-19Tufts (Tufts Health Sciences IRB): MOD-02-11615Buffalo-Kaledia (UBIRB): MOD00008600St Vincent Mercy Hospital (Mercy Health North Institutional Review Board): #2016-1Lutheran Medical Center (SCL Health - Front Range IRB): #201621 Participants gave informed consent to participate in the study before taking part.
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: For the ATLAS Investigators: Lyerly Neurosurgery-Baptist (19) - Ricardo Hanel (PI), Eric Sauvageau (Sub-I), Amin Nima Aghaebrahim (Sub-I), Nancy Ebreo (CRC), Karen Bell (CRC), Lanaya Lewis (CRC); Mercy St. Vincent (18) - Osama Zaidat (PI), Eugene Lin (Sub-I), Tina M Steinhauser (CRC), Dee Tilley (CRC), Julie Goins-Whitmore (past CRC), Brandi Breseman (past CRC), Melissa A Thomas (past CRC); University of Pittsburgh (17) - Ashutosh Jadhav (PI and past Sub-I), Bradley Gross (Sub-I), Lisa Baxendell (CRC), Patricia Feineigle (CRC), Vicki Gilchrist (CRC), Brian Jankowitz (past Sub-I and past PI), Andrew Ducruet (past Sub-I), David Panczkowski (past Sub-I), Hazem Shoirah (past Sub-I), Alhamza Al-Bayati (past Sub-I), Amin Aghaebrahim (past Sub-I), Tudor Jovin (past Sub-I), Greg Weiner (past Sub-I), Cynthia Kenmuir (past Sub-I), Prasanna Tadi (past Sub-I), Gregory Walker (past Sub-I), Kelsea Haibach (past CRC), Carlynn Graves (past CRC), Yvonne Cannon (past CRC); WellStar Research Institute (16) - Ahmad Khaldi (PI), Rishi Gupta (Sub-I), Marianne Bain (CRC), Laura Murphy (CRC), Andrew K Johnson (past Sub-I), Barbara A Foster (past CRC), Tasha Futch (past CRC), Portia Thomas (past CRC); RIA/Swedish (14) - Don Frei (current PI and past Sub-I), Richard Bellon (Sub-I), Benjamin Atchie (Sub-I), Ian Kaminsky (Sub-I), Lisa Kodis (CRC), Mark Talley (CRC), Tiffany Talley (CRC), Alex Edinger (CRC), David Loy (past PI and Sub-I), Dan Huddle (past Sub-I), Michelle Lexin (past CRC), Brad Fasbinder (CRC), Alicia Drew (past CRC), Joanna Snead (past CRC), Ashley Bitner (past CRC), Sarah Weiss (past CRC), Nouara Sadaoui (past CRC); Tufts Medical Center (12) - Adel Malek (PI), Emma Jost-Price (CRC), Keri Sullivan (CRC), Haley Huggins (past CRC), Lindsey Soll (past CRC), Sarah Gans (past CRC), Michelle Bettle (past CRC); Cleveland Clinic Foundation (10) - Gabor Toth (PI), Mark Bain (Sub-I), Peter Rasmussen (Sub-I), M Shazam Hussain (Sub-I), Nina Moore (Sub-I), Thomas Masaryk (Sub-I), Mohamed Elgabaly (Sub-I), Erin Bynum (CRC), Russell Cerejo (past Sub-I), Julian Hardman (past Sub-I), Seby John (past Sub-I), Andrew Bauer (past Sub-I), Erin Mayock (past CRC), Vikram Puvenna (past CRC), Jenny Peih-Chir Tsai (past Sub-I); SUNY Buffalo (9) - Adnan Siddiqui (PI), Elad Levy (Sub-I), Kenneth Snyder (Sub-I), Jason Davies (Sub-I), Mary Hartney (CRC), Jonna Sakowski (CRC), Courtney Drozdowski (past CRC), Heather Ross (past CRC), Linda Bookhagen (past CRC); Beth Israel Deaconess (7) - Ajith Thomas (PI), Christopher Ogilvy (Sub-I), Patricia Baum (CRC); Virginia Commonwealth (7) - John Reavey-Cantwell (PI), Dennis Rivet (Co-PI), Charlotte Gilman (CRC); Cedars Sinai Medical Center (6) - Michael Alexander (PI), Franklin Moser (Sub-I), Marcel Maya (Sub-I), Michael Schiraldi (Sub-I), Vicki Manoukian, MA (CRC), Paula Eboli (past Sub-I); Johns Hopkins University (6) - Justin Caplan (current PI), Bowen Jiang (Sub-I), Matthew Bender (Sub-I), Ellen Sheehan (CRC), Jessica Wollett (CRC), Geoffrey Colby (past PI), Lauren Dise (past CRC), Anna Bugaeva (past CRC), Barbara Michniewicz (past CRC), Thomas Hemmingson (past CRC); Christiana Care Health Services (5) - Sudhakar Satti (PI), Thinesh Sivapatham (Sub-I), Robie Zent (CRC), Ann Marie Le Noir (CRC);Hospital of the University of PA (5) - David Kung (current PI and past Sub-I), Bryan Pukenas (Sub-I), Robert Hurst (Sub-I), Timothy Prior (CRC), Whitney Sarchiapone (CRC), Yelena Gorelik (past CRC), Francis Quattrone (past CRC), Michelle J. Smith (past PI);Univ of Massachusetts (4) -Ajit Puri (PI), Francesco Massari (Sub-I), Mary Howk (CRC), David Rex (past Sub-I), Kimberly Ty (past CRC), Jen Donham (past CRC), Wen Li (past CRC); University of Kentucky (4) - Justin Fraser (current PI and past Sub-I), Stephen Grupke (Sub-I), Jennifer Isaacs (CRC), Abdulnasser Alhajeri (past PI), Caroline Rodgers (past CRC); Linda Joyce McCown (past CRC); Houston Methodist Hospital (4) - Richard Klucznik (PI), Orlando Diaz (Sub-I), Gavin Britz (Sub-I), Yi Zhang (Sub-I), Michelle Prystash (CRC), Vivian Escamilla (CRC), Adrienne New (CRC), Liliana Calderon (past CRC), Elmira Ramos (past CRC), Ramon Guardiola (past CRC), Bhavin Shah (past CRC), Lenis Sosa (past CRC), Melissa Whipple (past CRC); Medical University of South Carolina (4) - Alejandro Spiotta (current PI and past Sub-I), Jonathan Lena (Sub-I), Ayesha Vohra (CRC), Meredith Robinson (CRC), Aquilla Turk (past PI), Mohamad Chaudry (past Sub-I), Kyle Fargen (past Sub-I), Raymond Turner (past Sub-I), Emily Young (past CRC), Adrian Parker (past CRC), Angela Robinson (past CRC), Andrew Dippre (past CRC), Anita Deveaux (past CRC), Amora Mayo-Perez (past CRC); Baylor College of Medicine (3) - Peter Kan (PI), Bridget Solis (CRC), Melyssa Fink (CRC), Edward Duckworth (past Sub-I), Samantha Macias (CRC), Gilberto DeFreitas (CRC), Stephen Harold (CRC), Sree Vidya (past CRC); Los Robles Regional (3) - Muhammad Asif Taqi (PI), Anastasia Vechera (CRC), Samuel Hou (past Sub-I), Sajid Suriya (past CRC), Syed Quadri (past CRC); Methodist Healthcare Memphis (2) - Adam S Arthur (PI), Lucas Elijovich (Sub-I), Daniel Hoit (Sub-I), Christopher Nickele (Sub-I), Amanda Nolte (CRC), Jessica Jameson (CRC), Barrett Patel (CRC), Hani Rashed (CRC), Jay Vachhani (past Sub-I), Vinodh Thomas Doss (past Sub-I); Rush University (2) - Richard W Crowley (current PI and past Sub-I), Bartosz Jacher (CRC), Demetrius Lopes (past PI), Carol Macpherson (past CRC), Amanda Arand (past CRC), Christy Anton (past CRC), Michael Chen (past Sub-I), John Dao (past CRC), Francisco Acosta (past CRC); Harborview Medical Center (2) - Danial Hallam (PI), Basavaraj Ghodke (Sub-I), Michael Levitt (Sub-I), Kellie Sheehan (CRC), Louis Kim (Sub-I); SSM DePaul Health Center (2) - Richard Callison (PI), Amer Alshekhlee (Sub-I), Michelle Raymond (CRC), Sushant Kale (past Sub-I); Vanderbilt University (1) - Michael Froehler (PI), Matt Fusco (Sub-I), Rohan Chitale (Sub-I), Drew Anderson (CRC), Natalie Hall (CRC), Sally (Sarah) Baggette (CRC), Dima Sbenaty (past CRC), Kathryn McNabb (past CRC), Morgan A Pittman (past CRC), Joy Grabenstein (past CRC), David McKeel (past CRC).
Contributor Information
Ricardo A Hanel, Email: rhanel@lyerlyneuro.com.
Gustavo M Cortez, Email: gustavomcortez.md@gmail.com.
Brian T Jankowitz, Email: brian.jankowitz@pennmedicine.upenn.edu.
Eric Sauvageau, Email: Eric.Sauvageau@bmcjax.com.
Amin Aghaebrahim, Email: amin.aghaebrahim@bmcjax.com.
Eugene Lin, Email: elin@mercy.com.
Ashutosh P Jadhav, Email: ashutoshpjadhavmd@gmail.com.
Bradley Gross, Email: bgross83@gmail.com.
Ahmad Khaldi, Email: ahmad.khaldi@wellstar.org.
Rishi Gupta, Email: Rishi.gupta@wellstar.org.
Donald Frei, Email: don.frei@riaco.com.
David Loy, Email: David_Loy@bshsi.org.
Lori Lyn Price, Email: lorilyn.price@stryker.com.
Steven W Hetts, Email: steven.hetts@ucsf.edu.
Osama O Zaidat, Email: OOZaidat@Mercy.com.
ATLAS Investigators:
Ricardo Hanel, Eric Sauvageau, Amin Nima Aghaebrahim, Nancy Ebreo, Karen Bell, Lanaya Lewis, Mercy Vincent Zaidat, Eugene Lin, Tina M Steinhauser, Dee Tilley, Julie Goins Whitmore, Brandi Breseman, Melissa A Thomas, Ashutosh Jadhav, Bradley Gross, Lisa Baxendell, Patricia Feineigle, Vicki Gilchrist, Brian Jankowitz, Andrew Ducruet, David Panczkowski, Hazem Shoirah, Alhamza Al Bayati, Amin Aghaebrahim, Tudor Jovin, Greg Weiner, Cynthia Kenmuir, Prasanna Tadi, Gregory Walker, Kelsea Haibach, Carlynn Graves, Yvonne Cannon, Ahmad Khaldi, Rishi Gupta, Marianne Bain, Laura Murphy, Andrew K Johnson, Barbara A Foster, Tasha Futch, Portia Thomas, Richard Bellon Don Frei, Benjamin Atchie, Ian Kaminsky, Lisa Kodis, Mark Talley, Tiffany Talley, Alex Edinger, David Loy, Dan Huddle, Michelle Lexin, Brad Fasbinder, Alicia Drew, Joanna Snead, Ashley Bitner, Sarah Weiss, Nouara Sadaoui, Adel Malek, Emma Jost Price, Keri Sullivan, Haley Huggins, Lindsey Soll, Sarah Gans, Michelle Bettle, Gabor Toth, Mark Bain, Peter Rasmussen, M Shazam Hussain, Nina Moore, Thomas Masaryk, Mohamed Elgabaly, Erin Bynum, Russell Cerejo, Julian Hardman, Seby John, Andrew Bauer, Erin Mayock, Vikram Puvenna, Jenny Peih Tsai, Adnan Siddiqui, Elad Levy, Kenneth Snyder, Jason Davies, Mary Hartney, Jonna Sakowski, Courtney Drozdowski, Heather Ross, Linda Bookhagen, Ajith Thomas, Christopher Ogilvy, Patricia Baum, John Reavey Cantwell, Dennis Rivet, Charlotte Gilman, Michael Alexander, Franklin Moser, Marcel Maya, Michael Schiraldi, Vicki Manoukian, Paula Eboli, Justin Caplan, Bowen Jiang, Matthew Bender, Ellen Sheehan, Jessica Wollett, Geoffrey Colby, Lauren Dise, Anna Bugaeva, Barbara Michniewicz, Thomas Hemmingson, Sudhakar Satti, Thinesh Sivapatham, Robie Zent, Ann Marie Le Noir, David Kung, Bryan Pukenas, Robert Hurst, Timothy Prior, Whitney Sarchiapone, Yelena Gorelik, Francis Quattrone, Michelle J Smith, Ajit Puri, Francesco Massari, Mary Howk, David Rex, Kimberly Ty, Jen Donham, Wen Li, Justin Fraser, Stephen Grupke, Jennifer Isaacs, Abdulnasser Alhajeri, Caroline Rodgers, Linda Joyce McCown, Richard Klucznik, Orlando Diaz, Gavin Britz, Yi Zhang, Michelle Prystash, Vivian Escamilla, Adrienne New, Liliana Calderon, Elmira Ramos, Ramon Guardiola, Bhavin Shah, Lenis Sosa, Melissa Whipple, Alejandro Spiotta, Jonathan Lena, Ayesha Vohra, Meredith Robinson, Aquilla Turk, Mohamad Chaudry, Kyle Fargen, Raymond Turner, Emily Young, Adrian Parker, Angela Robinson, Andrew Dippre, Anita Deveaux, Amora Mayo Perez, Peter Kan, Bridget Solis, Melyssa Fink, Edward Duckworth, Samantha Macias, Gilberto De Freitas, Stephen Harold, Sree Vidya, Muhammad Asif Taqi, Anastasia Vechera, Samuel Hou, Sajid Suriya, Syed Quadri, Adam S Arthur, Lucas Elijovich, Daniel Hoit, Christopher Nickele, Amanda Nolte, Jessica Jameson, Barrett Patel, Hani Rashed, Jay Vachhani, Vinodh Thomas Doss, Richard W Crowley, Bartosz Jacher, Demetrius Lopes, Carol Macpherson, Amanda Arand, Christy Anton, Michael Chen, John Dao, Francisco Acosta, Danial Hallam, Basavaraj Ghodke, Michael Levitt, Kellie Sheehan, Louis Kim, Richard Callison, Amer Alshekhlee, Michelle Raymond, Sushant Kale, Michael Froehler, Matt Fusco, Rohan Chitale, Drew Anderson, Sally Natalie Hall, Sally Sarah Baggette, Dima Sbenaty, Kathryn McNabb, Morgan A Pittman, Joy Grabenstein, and David McKeel
Data availability statement
Data are available upon reasonable request.
References
- 1.King B, Vaziri S, Singla A, et al. Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with Enterprise and Neuroform stents: a comparative analysis of the literature. J Neurointerv Surg. 2015;7:905–9. doi: 10.1136/neurintsurg-2014-011457. [DOI] [PubMed] [Google Scholar]
- 2.Oushy S, Rinaldo L, Brinjikji W, et al. Recent advances in stent-assisted coiling of cerebral aneurysms. Expert Rev Med Devices. 2020;17:519–32. doi: 10.1080/17434440.2020.1778463. [DOI] [PubMed] [Google Scholar]
- 3.Zaidat OO, Hanel RA, Sauvageau EA, et al. Pivotal trial of the Neuroform Atlas Stent for treatment of anterior circulation aneurysms: one-year outcomes. Stroke. 2020;51:2087–94. doi: 10.1161/STROKEAHA.119.028418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefevre P-H, Schramm P, Kemmling A, et al. Multi-centric European post-market follow-up study of the Neuroform Atlas Stent System: primary results. J Neurointerv Surg. 2022;14:694–8. doi: 10.1136/neurintsurg-2021-017849. [DOI] [PubMed] [Google Scholar]
- 5.Shojima M. Basic fluid dynamics and tribia related to flow diverter. J Neuroendovasc Ther. 2017;11:109–16. doi: 10.5797/jnet.ra-diverter.2016-0012. [DOI] [Google Scholar]
- 6.Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Ann Biomed Eng. 1997;25:460–9. doi: 10.1007/BF02684187. [DOI] [PubMed] [Google Scholar]
- 7.Gao B, Baharoglu MI, Cohen AD, et al. Stent-assisted coiling of intracranial bifurcation aneurysms leads to immediate and delayed intracranial vascular angle remodeling. AJNR Am J Neuroradiol. 2012;33:649–54. doi: 10.3174/ajnr.A2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan H, Lu G, Ge L, et al. Hemodynamic effects of stent-induced straightening of parent artery vs. stent struts for intracranial bifurcation aneurysms. Front Neurol. 2021;12:802413. doi: 10.3389/fneur.2021.802413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adeeb N, Griessenauer CJ, Patel AS, et al. The use of single stent-assisted coiling in treatment of bifurcation aneurysms: a multicenter cohort study with proposal of a scoring system to predict complete occlusion. Neurosurgery. 2018;82:710–8. doi: 10.1093/neuros/nyx310. [DOI] [PubMed] [Google Scholar]
- 10.Funakoshi Y, Imamura H, Tani S, et al. Effect of straightening the parent vessels in stent-assisted coil embolization for anterior communicating artery aneurysms. World Neurosurg. 2019;126:e410–6. doi: 10.1016/j.wneu.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 12.Aenis M, Stancampiano AP, Wakhloo AK, et al. Modeling of flow in a straight stented and nonstented side wall aneurysm model. J Biomech Eng. 1997;119:206–12. doi: 10.1115/1.2796081. [DOI] [PubMed] [Google Scholar]
- 13.Heller RS, Malek AM. Parent vessel size and curvature strongly influence risk of incomplete stent apposition in Enterprise intracranial aneurysm stent coiling. AJNR Am J Neuroradiol. 2011;32:1714–20. doi: 10.3174/ajnr.A2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jankowitz BT, Hanel R, Jadhav AP, et al. Neuroform Atlas Stent System for the treatment of intracranial aneurysm: primary results of the Atlas Humanitarian Device Exemption cohort. J Neurointerv Surg. 2019;11:801–6. doi: 10.1136/neurintsurg-2018-014455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho S-H, Jo W-I, Jo Y-E, et al. Bench-top comparison of physical properties of 4 commercially-available self-expanding intracranial stents. Neurointervention. 2017;12:31–9. doi: 10.5469/neuroint.2017.12.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteiro A, Cortez GM, Aghaebrahim A, et al. Low-profile Visualized Intraluminal Support Jr braided stent versus Atlas self-expandable stent for treatment of intracranial aneurysms: a single center experience. Neurosurgery. 2021;88:E170–8. doi: 10.1093/neuros/nyaa458. [DOI] [PubMed] [Google Scholar]
- 17.Kato N, Nishimura K, Sonoda S, et al. Comparison of clinical outcomes after stent-assisted coiling with 3 types of self-expanding laser-cut stents in patients with wide-necked intracranial aneurysms. World Neurosurg. 2021;146:e701–7. doi: 10.1016/j.wneu.2020.10.166. [DOI] [PubMed] [Google Scholar]
- 18.Lauric A, Hippelheuser JE, Malek AM. Induction of aneurysmogenic high positive wall shear stress gradient by wide angle at cerebral bifurcations, independent of flow rate. J Neurosurg. 2018;131:442–52. doi: 10.3171/2018.3.JNS173128. [DOI] [PubMed] [Google Scholar]
- 19.Gao B, Baharoglu MI, Malek AM. Angular remodeling in single stent-assisted coiling displaces and attenuates the flow impingement zone at the neck of intracranial bifurcation aneurysms. Neurosurgery. 2013;72:739–48. doi: 10.1227/NEU.0b013e318286fab3. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T, Kakizawa Y, Yoshino M, et al. Numerical analysis of bifurcation angles and branch patterns in intracranial aneurysm formation. Neurosurgery. 2019;85:E31–9. doi: 10.1093/neuros/nyy387. [DOI] [PubMed] [Google Scholar]
- 21.Leng X, Wan H, Li G, et al. Hemodynamic effects of intracranial aneurysms from stent-induced straightening of parent vessels by stent-assisted coiling embolization. Interv Neuroradiol. 2021;27:181–90. doi: 10.1177/1591019921995334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teramoto S, Oishi H, Arai H. Comparative analysis of long-term effect of stent-assisted coiling in unruptured sidewall-type and terminal-type aneurysms. World Neurosurg. 2019;126:e753–7. doi: 10.1016/j.wneu.2019.02.145. [DOI] [PubMed] [Google Scholar]
- 23.Toccaceli G, Diana F, Cagnazzo F, et al. Microsurgical clipping compared with new and most advanced endovascular techniques in the treatment of unruptured middle cerebral artery aneurysms: a meta-analysis in the modern era. World Neurosurg. 2020;137:451–64. doi: 10.1016/j.wneu.2019.12.118. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz C, Aster H-C, Al-Schameri R, et al. Microsurgical clipping and endovascular treatment of middle cerebral artery aneurysms in an interdisciplinary treatment concept: comparison of long-term results. Interv Neuroradiol. 2018;24:608–14. doi: 10.1177/1591019918792231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson AK, Heiferman DM, Lopes DK. Stent-assisted embolization of 100 middle cerebral artery aneurysms. J Neurosurg. 2013;118:950–5. doi: 10.3171/2013.1.JNS121298. [DOI] [PubMed] [Google Scholar]
- 26.Choi HH, Cho YD, Yoo DH, et al. Stent-assisted coil embolization of anterior communicating artery aneurysms: safety, effectiveness, and risk factors for procedural complications or recanalization. J Neurointerv Surg. 2019;11:49–56. doi: 10.1136/neurintsurg-2018-013943. [DOI] [PubMed] [Google Scholar]
- 27.Cortese J, Caroff J, Girot J-B, et al. Impact of A1 asymmetry on the woven EndoBridge device in anterior communicating artery aneurysms. AJNR Am J Neuroradiol. 2021;42:1479–85. doi: 10.3174/ajnr.A7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleme S, Iosif C, Ponomarjova S, et al. Flow-diverting stents for intracranial bifurcation aneurysm treatment. Neurosurgery. 2014;75:623–31. doi: 10.1227/NEU.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 29.Cagnazzo F, Limbucci N, Nappini S, et al. Flow-diversion treatment of unruptured saccular anterior communicating artery aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2019;40:497–502. doi: 10.3174/ajnr.A5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogilvy CS, Natarajan SK, Jahshan S, et al. Stent-assisted coiling of paraclinoid aneurysms: risks and effectiveness. J Neurointerv Surg. 2011;3:14–20. doi: 10.1136/jnis.2010.002303. [DOI] [PubMed] [Google Scholar]
- 31.Colby GP, Paul AR, Radvany MG, et al. A single center comparison of coiling versus stent assisted coiling in 90 consecutive paraophthalmic region aneurysms. J Neurointerv Surg. 2012;4:116–20. doi: 10.1136/jnis.2011.004911. [DOI] [PubMed] [Google Scholar]
- 32.Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the PREMIER study 1 year results. J Neurointerv Surg. 2020;12:62–6. doi: 10.1136/neurintsurg-2019-015091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adeeb N, Griessenauer CJ, Foreman PM, et al. Comparison of stent-assisted coil embolization and the Pipeline embolization device for endovascular treatment of ophthalmic segment aneurysms: a multicenter cohort study. World Neurosurgery. 2017;105:206–12. doi: 10.1016/j.wneu.2017.05.104. [DOI] [PubMed] [Google Scholar]
- 34.Enriquez-Marulanda A, Salem MM, Ascanio LC, et al. No differences in effectiveness and safety between pipeline embolization device and stent-assisted coiling for the treatment of communicating segment internal carotid artery aneurysms. Neuroradiol J. 2019;32:344–52. doi: 10.1177/1971400919845368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Dong L, Liu J, et al. Pipeline embolization device versus Atlas stent assisted coiling for intracranial aneurysm treatment: a retrospective, propensity score matched study with a focus on midterm outcomes and hospital costs. J NeuroIntervent Surg. 2024;16:379–84. doi: 10.1136/jnis-2023-020173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.