Abstract
Objectives
B-cell depletion time after rituximab (RTX) treatment is prolonged in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) compared with other autoimmune diseases. We investigated central and peripheral B-cell development to identify the causes for the defect in B-cell reconstitution after RTX therapy.
Methods
We recruited 91 patients with AAV and performed deep phenotyping of the peripheral and bone marrow B-cell compartment by spectral flow and mass cytometry. B-cell development was studied by in vitro modelling and the role of BAFF receptor by quantitative PCR, western blot analysis and in vitro assays.
Results
Treatment-naïve patients with AAV showed low transitional B-cell numbers, suggesting impaired B-lymphopoiesis. We analysed bone marrow of treatment-naïve and RTX-treated patients with AAV and found reduced B-lymphoid precursors. In vitro modelling of B-lymphopoiesis from AAV haematopoietic stem cells showed intact, but slower and reduced immature B-cell development. In a subgroup of patients, after RTX treatment, the presence of transitional B cells did not translate in replenishment of naïve B cells, suggesting an impairment in peripheral B-cell maturation. We found low BAFF-receptor expression on B cells of RTX-treated patients with AAV, resulting in reduced survival in response to BAFF in vitro.
Conclusions
Prolonged depletion of B cells in patients with AAV after RTX therapy indicates a B-cell defect that is unmasked by RTX treatment. Our data indicate that impaired bone marrow B-lymphopoiesis results in a delayed recovery of peripheral B cells that may be further aggravated by a survival defect of B cells. Our findings contribute to the understanding of AAV pathogenesis and may have clinical implications regarding RTX retreatment schedules and immunomonitoring after RTX therapy.
Keywords: vasculitis, rituximab, B-lymphocytes
WHAT IS ALREADY KNOWN ON THIS TOPIC
B-cell depletion with rituximab is an established and effective treatment in antineutrophil cytoplasmic antibody-associated vasculitis (AAV).
Differently from other autoimmune diseases, B-cell depletion results in a defect or delay in B-cell reconstitution in a proportion of patients with AAV.
The underlying cause for this observation is unknown.
As a consequence, the risk of hypogammaglobulinemia is increased in patients with AAV after B-cell depletion.
WHAT THIS STUDY ADDS
We discovered defective bone marrow B-lymphopoiesis with reduced B-cell output in AAV, present already before rituximab treatment.
Reduced commitment into the B-cell lineage is not due to an intrinsic haematopoietic stem cell defect, but likely to a non-permissive bone marrow niche in AAV.
Low BAFF-receptor expression on B cells exiting the bone marrow may contribute to reduced peripheral B-cell survival and maturation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our observations may impact rituximab retreatment schedules and prompt close immune-monitoring with assessment of B-cell numbers and immunoglobulin concentrations after B-cell depleting therapy in patients with AAV.
Introduction
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) comprises granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic GPA (EGPA). The efficacy of B-cell depleting therapy with rituximab (RTX) for both remission induction and maintenance implicates B cells in AAV pathogenesis.1,3 After RTX treatment, relapses are associated either with B-cell reconstitution or with increase in ANCA titres.3,5 After RTX administration in other conditions, B-cell repopulation initiates at 6 months.6 Hence, EULAR recommendation7 and the MAINRITSAN trial,2 advise 6-monthly RTX infusions as maintenance therapy for GPA and MPA. In a relevant proportion of patients with AAV though, B-cell reconstitution after a single RTX administration is delayed and severe B lymphopenia and hypogammaglobulinemia are observed for up to 5 years.68,12 Hypogammaglobulinemia represents a risk factor for clinically relevant infections in patients with primary immunodeficiencies.13 In patients with secondary immunodeficiency after B-cell depletion this is less clear, likely due to differences in study design, observation time, hypogammaglobulinemia definition and concomitant immunosuppressive therapies.14,16 The pathophysiological basis of prolonged B-cell depletion in AAV is currently unclear.
B-lymphocytes are generated in the bone marrow (BM) from haematopoietic stem cells (HSCs), through a tightly regulated process that results in immature B cells17 exiting the BM as transitional B cells to further mature in periphery. Transitional B cells are dependent on BAFF-receptor (BAFF-R) signalling to mature into naïve B cells.18 Hence, the peripheral B-cell pool is regulated by output from the BM as well as survival and maturation in the periphery. In AAV, a reduction of transitional B cells has been reported, suggesting reduced BM output.8 We hypothesised that B-cell depleting therapy unmasks a defect in B-cell development, as both lower BM output and reduced survival of peripheral B cells could contribute to delayed B-cell repopulation. Hence, we analysed the peripheral B-cell compartment and BM B-cell development ex vivo and in vitro in patients with AAV before and after RTX treatment.
Results
Description of the AAV cohort
We included 91 patients with AAV (median age 60.78 years, IQR 51.14–71.15, 39.56% female) and 58 healthy donors (HDs, median age 59.9 years, IQR 49.75–67.2, 37.93% female). Sixty-two (68%) patients were diagnosed with GPA, 19 (21%) with EGPA and 10 (11%) with MPA. ANCA was detectable in 88% of patients, with specificity against proteinase-3 being more frequent (64%) than against myeloperoxidase (22%). 86 patients had generalised disease with pulmonary manifestations (77%), ear-nose-throat (75%), renal (54%) and peripheral neurological manifestations (45%). Patients’ characteristics are detailed in table 1.
Table 1. Patient characteristics.
| Patient characteristics | AAV (n=91) | HD (n=58) |
| Sex, female/male, n (%) | 36 (39.56)/55 (60.44) | 22 (37.93)/36 (62.07) |
| Age at analysis, median years (IQR) | 60.78 (51.14–71.15) | 59.9 (49.75–67.2) |
| ANCA specificity, n (%)* | ||
| PR3 | 58 (63.74) | |
| MPO | 20 (21.98) | |
| PR3 and MPO | 2 (2.2) | |
| No ANCA | 13 (14.29) | |
| Elevated CRP, n (%)† | 37 (41.57) | |
| Elevated CRP (mg/L), median (IQR) | 14.2 (6.93–23.8) | |
| CRP <5 mg/L, n (%)† | 52 (58.43) | |
| Diagnosis | ||
| GPA, n (%) | 62 (68.13) | |
| MPA, n (%) | 10 (10.99) | |
| EGPA, n (%) | 19 (20.88) | |
| Disease form, n | ||
| Localised | 5 | |
| Generalised | 86 | |
| Clinical manifestations, n (%) | ||
| Renal | 49 (53.85) | |
| Pulmonary | 70 (76.92) | |
| ENT | 68 (74.73) | |
| Skin | 20 (21.98) | |
| CNS | 16 (17.58) | |
| PNS | 41 (45.05) | |
| Cardiac | 11 (12.09) | |
| Creatinine (mg/dL), median (IQR) at time of analysis | 0.99 (0.85–1.27) | |
| eGFR (mL/min/1.73 m2), median (IQR) at time of analysis | 73 (49.25–87.75) | |
| Duration of disease, median months (IQR) | 70 (26.5–123) |
At diagnosis.
CRP values were not available at the time of analysis for two patientsOf patients no CRP values were available at time of analysis.
ANCA, antineutrophil cytoplasmic antibody; CNS, central nervous system; CRP, C reactive protein; EGPA, eosinophilic GPA; ENT, ear, nose, throat; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PNS, peripheral nervous systemPR3, proteinase 3
Treatment characteristics
Of the 91 patients with AAV included into the study, 77 had been treated with RTX as induction therapy and 14 were newly diagnosed. Of those 14, 4 had received no immunosuppressive treatment at all, and 10/14 short-term glucocorticoid (GC) treatment (median sum dose: 35 mg/day; IQR 0.63–93.75 mg/day; median treatment duration 3 days; IQR 0–5 days). The median RTX sum dosage was 2.67 g (IQR 2–4) and median cyclophosphamide (CYC) sum dose at the time of analysis was 6 g (IQR 0–13). The median time between last RTX administration and analysis of the B-cell compartment was 17 months (IQR 11–28.5 months). RTX treatment was followed by conventional maintenance therapy in most patients. At the time of B-cell compartment analysis, 62 patients received maintenance therapy with a conventional, synthetic immunosuppressant, mostly azathioprine (AZA) (n=19) or methotrexate (MTX) (n=20). The median prednisone equivalent dose at time of sampling was 5 mg/day.
As for patients included into BM analyses, one patient was treatment-naïve for GCs and immunosuppressants, two patients were treatment-naïve for immunosuppressants and had short-term GCs (median duration of 1 day; 0.5–3 days IQR). Three patients had received CYC (median sum dose of 1 g; 1–2.25 g IQR) and one patient was treated with leflunomide (LEF). One patient had received RTX induction 44 months before analysis and LEF maintenance therapy for 40 months at time of analysis. Indication for BM puncture was workup for hypereosinophilia (n=3), fever, weight loss and unclear inflammatory state (n=3) and abnormalities in the blood count (n=1). For further treatment details see table 2.
Table 2. Treatment characteristics.
| Treatment characteristics | |
| Cyclophosphamide sum dose (g) at time of analysis, median (IQR) | 6 (0–13) |
| Prednisone equivalent (mg/day) at time of analysis, median (IQR) | 5 (0–5) |
| DMARD at time of analysis, n; dose, median (IQR) | |
| Azathioprine (mg/day) | 19; 100 (100–150) |
| Methotrexate (mg/week) | 20; 15 (14.38–15) |
| Leflunomide (mg/day) | 11; 20 (20–20) |
| Mycophenolate mofetil (mg/day) | 5; 720 (720–1000) |
| Other | 1 |
| No DMARD | 14 |
| RTX prior to analysis, n (%) | 77 (84.66) |
| Median time from last RTX infusion to analysis; median months (IQR) | 17 (11–28.5) |
| Cumulative RTX dose (g), median (IQR) | 2.67 (2–4) |
DMARD, disease-modifying antirheumatic drug; RTX, rituximab
Disturbed peripheral B-cell maturation and prolonged B-cell depletion in patients with AAV
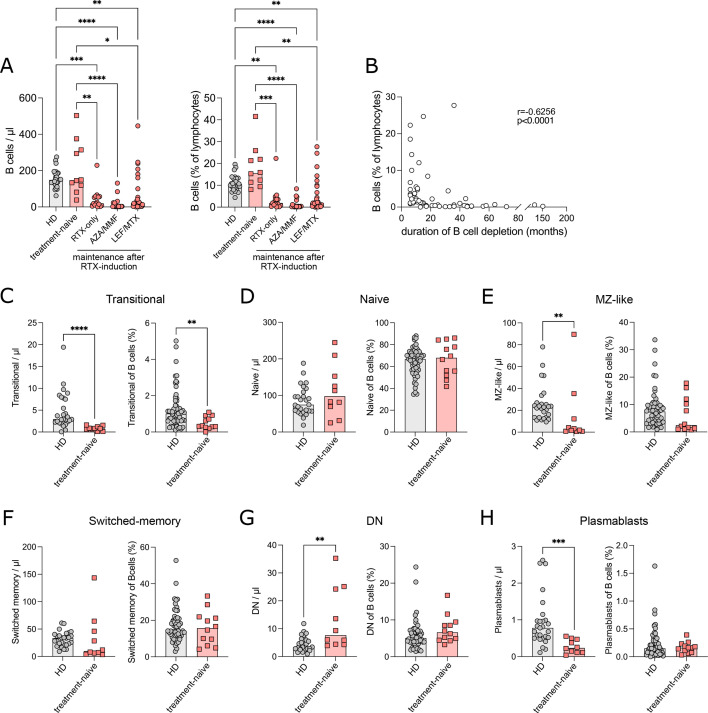
The peripheral B-cell compartment was analysed in 88/91 patients, as for 3/91 patients, material was not available. In 14/88 patients, B cells were undetectable or sparsely detected even after B-cell enrichment using negative magnetic separation. These patients were excluded from further analysis. In these 14 patients, time from RTX treatment to sampling was 11 months (IQR 8.25–16.5 months). Of the remaining 74 patients, 12 were treatment-naïve and 62 had previously been treated with RTX. In treatment-naïve patients with AAV, B-cell numbers were similar to HDs. The majority of RTX-treated patients with AAV had significantly lower B-cell numbers and frequencies compared with HDs (figure 1A). To account for the effect of maintenance immunosuppressive therapies, we grouped together patients treated with RTX monotherapy (RTX-only), AZA or mycophenolate mofetil (MMF), and with LEF or MTX (figure 1A). Patients were analysed after a median of 17 months (IQR 11–28.5 months) after RTX treatment (table 2). The median time of B-cell depletion (B cells <5 cells/µL) in RTX-treated patients was 17 months (IQR 9–36). We used the interval between RTX applications as time of depletion for 14 patients in which RTX was re-administered, despite persistent B-cell depletion. In six patients, B cells were still depleted at the last available time-point of follow-up. In these patients, time between RTX administration and last time-point of follow-up was used as time of depletion. In 15 patients, the duration of depletion was between 12 and 24 months, while in another 16 patients, it exceeded 24 months (figure 1B). Time of depletion correlated with the cumulative dose of RTX (p=0.0146; online supplemental figure S1A) and with a decrease in serum IgG, IgA and IgM concentrations (p<0.05 each, online supplemental figure S1B). After remission induction with RTX, maintenance therapy influenced B-cell depletion time that was significantly longer with AZA or MMF compared with RTX as monotherapy (p=0.0130) or LEF or MTX (p=0.0021) (online supplemental figure S1C). Within the RTX-treated patients, there was no difference between B-cell numbers between GPA, MPA and EGPA (online supplemental figure S1D). Previous CYC treatment did not influence B-cell numbers nor the duration of B-cell depletion (online supplemental figure S1E). In contrast to a previous report,12 delayed B-cell reconstitution did not correlate with impaired renal function (table 1, online supplemental figure S1F, G). Differences in the composition of our cohort, including, among other factors, less severely impaired renal function, may be the cause.12 In treatment-naïve patients, we analysed the distribution of B-cell subpopulations by spectral flow cytometry (figure 1C–H, online supplemental figure S2A, online supplemental table S1). Transitional B cells were significantly decreased in both counts and frequencies compared with HDs (figure 1C). Transitional B cells mature into naïve B cells that were normal in absolute counts and relative frequencies (figure 1D). Naïve B cells can develop into either marginal zone (MZ) B cells or into the extrafollicular and germinal centre pathway.19 MZ-like B cells were reduced in absolute counts in patients with AAV (figure 1E). Germinal centre activation gives rise to conventional class-switched memory cells, which were normal in frequency and absolute counts (figure 1F). The extrafollicular response includes IgD and CD27 negative B cells, called double negative (DN).20 DN B cells were increased in absolute counts in the patients (figure 1G). Plasmablasts can be the product of germinal centre and extrafollicular pathways and were reduced in absolute counts in patients (figure 1H). The distribution of B-cell subpopulations was similar in MPA/GPA and in EGPA patients (online supplemental figure S2B). Distribution of immunoglobulin subclasses in class-switched memory B cells was similar to controls, except for an increase in IgG4 (online supplemental figure S2C).
Figure 1. Disturbed peripheral B-cell maturation and prolonged B-cell depletion in patients with antineutrophil cytoplasmic antibody-associated vasculitis (AAV). Peripheral B-cell populations of patients with AAV and healthy donors (HD) were analysed by spectral flow cytometry. (A) Absolute count of B cells/µL blood and frequency (%) of B cells in lymphocytes in HD and AAV treatment groups. (B) Correlation of the frequency (%) of B cells with the duration (months) of B-cell depletion after RTX treatment. (C–H) Absolute count/µL and frequency within total B cells of transitional B cells (IgM++CD38++) (C), naïve B cells (IgD+CD27−) (D), marginal zone-like B cells (MZ, IgD+CD27+) (E), switched memory B cells (IgD−CD27+) (F), double negative B cells (DN, IgD−CD27−) (G) and plasmablasts (CD38++CD27++) (H) in HD and treatment-naïve patients. Data were analysed using Kruskal-Wallis test, corrected for multiple comparisons by Dunn’s multiple comparison tests (A) or Mann-Whitney U test (C–H) and depicted as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Correlation analysis was performed by non-parametric Spearman’s correlation.
RTX treatment unveils a block in B-cell maturation of patients with AAV at the transitional B-cell stage
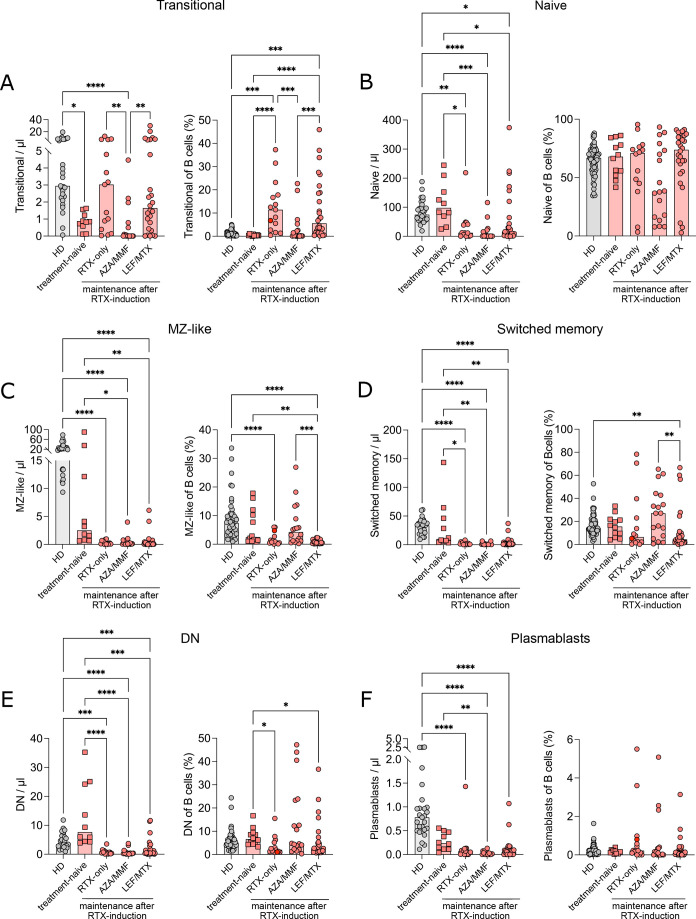
Transitional B cells are the earliest B cells reappearing in peripheral blood after RTX treatment.21 Transitional B cells were significantly decreased (figure 2A) and duration of B-cell depletion was significantly longer (online supplemental figure S1C) in patients with AAV treated with AZA or MMF for maintenance therapy after remission induction with RTX. In the RTX-only and LEF/MTX maintenance-treatment groups, transitional B cells were higher than in AZA-treated or MMF-treated patients (figure 2A) and similar to controls in absolute counts (figure 2A), even though, during reconstitution higher transitional B-cell numbers compared with HD are expected.21 Despite the presence of transitional B cells, later maturation stages of B cells in these two groups were reduced (figure 2B–F). While there was no significant difference in the frequency of naïve B cells between the AAV groups and HDs (figure 2B), the proportion of MZ-like and switched memory B cells within total B cells was increased in the AZA/MMF group. Conversely, there was a decrease in the MZ-like, switched memory and DN B-cell frequencies in patients with RTX-only or with LEF/MTX (figure 2C–E). There was no difference in plasmablast frequency between groups (figure 2F). The distribution of B-cell subpopulations was similar in treated MPA/GPA and in EGPA patients (online supplemental figure S3A). Within the few class-switched memory cells detectable, we observed a skewed distribution of the IgG subclasses (online supplemental figure S3B), with a reduced frequency of IgG1+ and IgG4+ switched memory B cells in RTX-only and LEF/MTX-treated groups. The study of activation markers (online supplemental figure S4) revealed a normal expression of the co-stimulatory molecule CD86 in B cells of treatment-naïve patients, that increased after RTX treatment (online supplemental figure S4A). The frequency of CD95+ (FAS) cells within naïve and switched memory B cells was similar to HDs in treatment-naïve patients and increased in all RTX-treated groups (online supplemental figure S4B). In summary, deep phenotyping of peripheral B cells in treatment-naïve patients revealed low transitional B cells, suggesting a dysregulation in BM B-cell development. Maintenance treatment with AZA or MMF resulted in reduced BM output with low transitional cells, while low counts of naïve B cells despite the presence of transitional B cells in RTX-only and RTX followed by LEF or MTX treatment suggest an additional defect in peripheral B-cell development.
Figure 2. RTX treatment unveils a block in B-cell maturation at transitional B-cell stage in patients with antineutrophil cytoplasmic antibody-associated vasculitis (AAV). Spectral flow cytometric analysis of peripheral B-cell populations of patients with AAV and HD. (A–F) Absolute count/µL blood and frequency (%) within total B cells of transitional B cells (IgM++CD38++) (A), naïve B cells (IgD+CD27−) (B), marginal zone-like B cells (MZ, IgD+CD27+) (C), switched memory B cells (IgD−CD27+) (D), double negative B cells (DN, IgD−CD27−) (E) and plasmablasts (CD38++CD27++) (F) in HD and patients with AAV grouped according to treatment. Data were analysed using Kruskal-Wallis test, corrected for multiple comparisons by Dunn’s multiple comparison tests and depicted as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Block in early B-cell development in AAV bone marrow
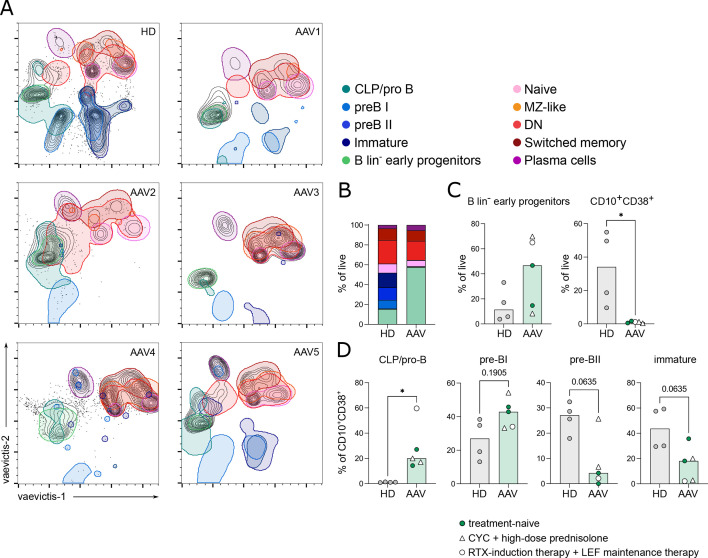
We studied the BM aspirates of seven patients (GPA, n=3; EGPA, n=3; MPA, n=1). In 5/7 BM, we dissected B-cell developmental stages from HSCs to immature/transitional B cells by mass cytometry by time of flight (CyTOF) and compared it with four HD BM (online supplemental figure S5A, online supplemental table S2). Using vaevictis dimensionality reduction,22 we observed cell clusters that could be attributed to distinct stages of B-cell development (figure 3A) as identified by manual gating23 (online supplemental figure S5A). All clusters were present in HD BM aspirates. On the contrary, major clusters representing the early stages of B-lymphopoiesis (common lymphoid progenitors (CLP), pro-B, pre-BI, pre-BII and immature B cells) were strongly reduced or absent in AAV BMs (figure 3A and B). The frequency of lineage negative HSCs and early progenitors was tendentially increased in AAV BM compared with HDs (figure 3C). B-lymphoid precursors, defined by the expression of CD10+ and CD38+ (online supplemental figure S5A), were significantly decreased in patients with AAV (figure 3C). In six AAV BM, flow cytometry analysis confirmed the significantly lower frequency of CD10+CD38+ B-lymphoid precursors and normal frequency of CD34+ HSCs (online supplemental figure S5B,C, online supplemental table S3). These data pointed to a block in specification into the B-cell lineage in patients with AAV. Within the CD10+CD38+ population, we further analysed individual developmental stages. CLP/pro-B cells were significantly increased, and pre-BI cells tended to be elevated compared with HD BMs (figure 3D). In contrast, pre-BII and immature B cells were tendentially decreased (p=0.0635, each, figure 3D). Flow cytometry analysis confirmed the increase in CLP/pro-B (online supplemental figure S5C), and demonstrated significantly reduced pre-B cell frequencies compared with HDs (online supplemental figure S5C). Our flow cytometry analysis did not allow the separation between the pre-BI and pre-BII cell stages. In summary, these data indicate a defective commitment of HSCs into the B-cell lineage resulting in strongly reduced B-lymphoid precursors (CD10+CD38+). Additionally, the development of B-lymphoid precursors is partially blocked at the pro-B/pre-BI cell stage in AAV BMs.
Figure 3. Impaired B-lymphopoiesis in bone marrow (BM) of patients with antineutrophil cytoplasmic antibody-associated vasculitis (AAV). Ex vivo BM aspirates of AAV and healthy donors (HD) were analysed by mass cytometry by time of flight. (A) Vaevictis dimensionality reduction projections of one HD and five patients with AAV with outlined regions of subpopulations. Defined regions show 95% probability contouring from indicated subpopulations. (B) Distribution of subpopulations as frequency of live (cPARP-/cleaved caspase 3−) cells of analysed BM samples. (C) Frequency of B lineage- (B lin-) early progenitor cells and CD10+CD38+ lymphoid precursor cells in live (cPARP−cleaved caspase 3−) cells. (D) Frequency of common lymphoid progenitors (CLP)/pro-B cells, pre-BI, pre-BII and immature B cells within CD10+CD38+ lymphoid precursor cells. (C, D) Filled circles: treatment-naïve except for short-term glucocorticoids; empty circle: RTX-induction therapy+LEF maintenance therapy; empty triangle: CYC+high-dose prednisolone. Data were analysed using Mann-Whitney U test and depicted as *p<0.05.
In vitro modelling of early B-cell development reveals reduced and delayed immature B-cell development in AAV
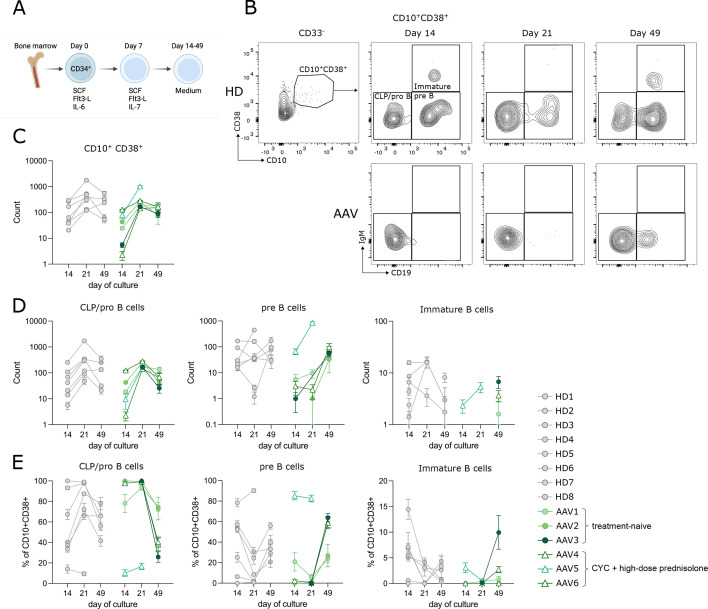
As B-lymphoid precursors were strikingly reduced in the ex vivo BM samples of patients with AAV, we wanted to investigate the capacity of HSCs to undergo B-cell development in vitro. We used a feeder-free in vitro model of early human B-cell development.24 25 Here, magnetically isolated CD34+ cells were expanded with stem cell factor (SCF), Flt3-L and first interleukin (IL)-6, substituted by IL-7 to favour lymphoid commitment.24 25 From day 14 on, cells were cultivated in cytokine-free medium to allow B-cell differentiation (figure 4A). BM-derived CD34+ cells encompass HSCs, CLP/pro-B (CD10+CD38+CD19−IgM−) and pre-BI (CD10+CD38+CD19+IgM−) cells. As previously shown,25 in HDs we observed a first wave of development into immature B cells (CD10+CD38+CD19+IgM+) from pro-B and pre-B at day 14 of culture (figure 4B–D), and a second wave at day 49, derived from HSCs development into B cells (figure 4B–D). Using this model, we studied BM-derived CD34+ cells from patients with AAV (n=7, treatment-naïve n=3). The CD34+ cells from one AAV-BM failed to expand and develop beyond day 7 and were not further analysed. In the remaining six patients, independent of treatment, the CD10+CD38+ B-lymphoid precursors developed less efficiently compared with HDs, as demonstrated by lower cellularity of the culture (figure 4C, day 14 counts). Accordingly, absolute numbers of further stages of B-cell development were lower compared with HDs (figure 4D). Looking at the dynamic of development (figure 4E), patients with AAV showed high frequency of CLP/pro-B cells at day 14 and day 21, and delayed appearance of pre-B cells at day 49 of culture. In contrast to HDs, in patients with AAV we did not observe the appearance of immature B cells at day 14. In both, HD and AAV, we observed immature B cells formation at day 49 (figure 4D,E). Hence, in vitro development of BM-derived progenitor cells revealed that CD34+ HSCs in AAV retain their intrinsic ability to develop into immature B cells. Together with the low/absent B-lymphoid precursors observed ex vivo, and the low transitional B cell number in treatment-naïve patients, this points to a non-permissive environment to B-cell development in patients with AAV, independent of RTX treatment. In addition, early B-cell development is less efficient and delayed, resulting in an overall lower number of B-cell precursors. In these culture conditions, myeloid cells developed comparably to controls in all patients (online supplemental figure S5D).
Figure 4. Delayed but intact B-cell development in vitro of antineutrophil cytoplasmic antibody-associated vasculitis (AAV) bone marrow (BM). (A) Experimental setup of in vitro development of BM-derived CD34+ cells to immature B cells. Isolated CD34+cells from the BM of HD (n=8) and patients with AAV (n=6) were cultured as described24 25 and analysed by flow cytometry every 7 days. (B) Representative plots show the development of CD10+CD38+ lymphoid precursors to IgM+CD19+ immature B cells over 49 days in HD (upper row) and AAV (lower row). (C–E) Development of subpopulations over time. Absolute counts of CD10+CD38+ lymphoid precursors (C), common lymphoid progenitors (CLP)/pro-B cells, pre-B cells and immature B cells (D) as well as frequency of CLP/pro-B cells, pre-B cells and immature B cells in CD10+CD38+ (E). (C–E) Filled circles: treatment-naïve except for short-term glucocorticoids; empty triangle: CYC+high-dose prednisolone. Mean of 3–5 technical replicates per donor±SEM is shown.
Low BAFF-R expression by enhanced BAFF-R processing contributes to impaired survival in AAV B cells after RTX treatment
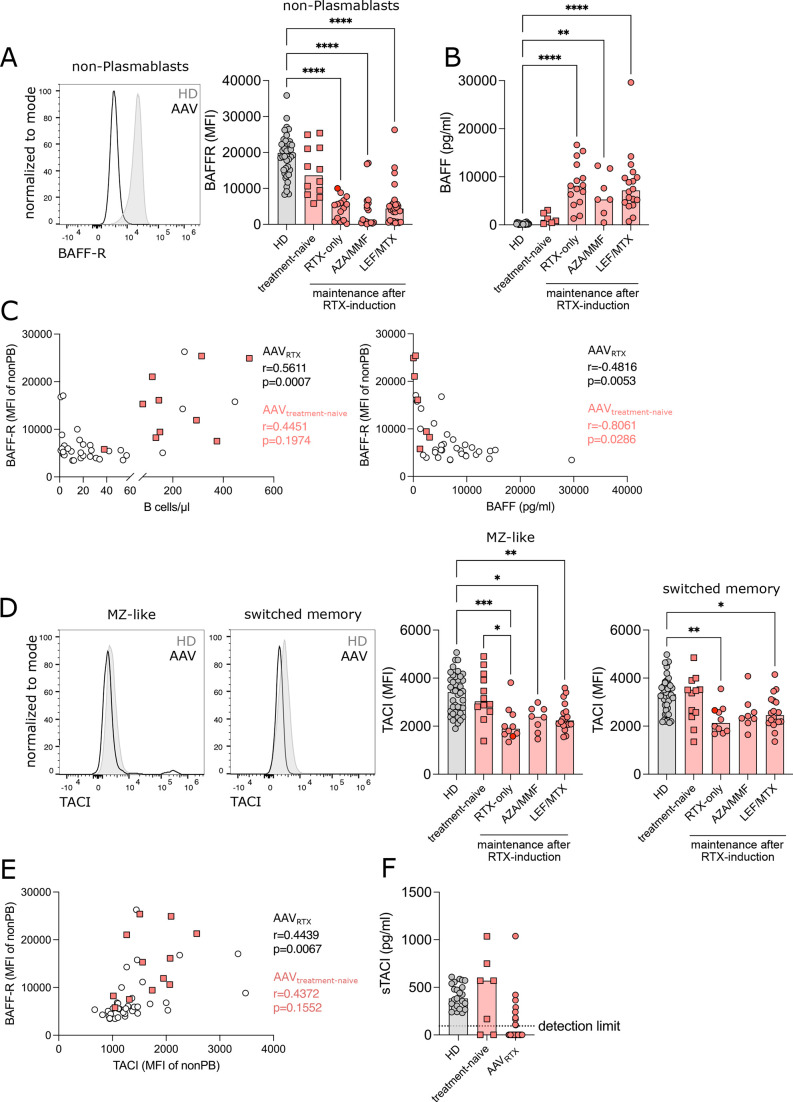
In addition to a defective B-lymphopoiesis, impaired survival and maturation of peripheral B cells could also contribute to a delayed B-cell reconstitution after RTX treatment. BAFF-R mediates peripheral B-cell survival and supports the maturation of transitional B cells.26 BAFF-R expression was lower in treatment-naïve patients with AAV compared with HDs and significantly reduced in all RTX-treated groups in peripheral B-cell subpopulations (figure 5A, online supplemental figure S6A,B). BAFF-R expression remained low also after acidic-elution of ligand BAFF from the receptor (online supplemental figure S6C), excluding reduced detection of the receptor because of ligand-binding. As expected,27 BAFF serum levels of RTX-treated patients with AAV were significantly increased compared with treatment-naïve patients with AAV and HDs (figure 5B). In line with its pro-survival function, BAFF-R expression positively correlated with the absolute numbers of B cells in RTX-treated patients and inversely correlated with BAFF serum levels in treatment-naïve and RTX-treated patients (figure 5C). Transmembrane activator and CAML interactor (TACI) is another BAFF-binding receptor.26 TACI expression was normal in treatment-naïve AAV, but decreased in all RTX-treated groups in switched memory and MZ-like B cells (figure 5D) and correlated positively with BAFF-R expression in RTX-treated patients with AAV (figure 5E). The decrease in TACI expression did not correspond to increased soluble TACI in the serum of patients with AAV (figure 5F).
Figure 5. Expression of BAFF-receptor (BAFF-R) and TACI is reduced in recirculating B cells after rituximab (RTX) treatment. (A) Representative flow cytometry plot of BAFF-R expression (left) and bar graph of median fluorescence intensity (MFI) of BAFF-R (right) in non-plasmablast B cells. (B) BAFF concentration (pg/mL) was measured in the serum of healthy donors (HD) and patients with antineutrophil cytoplasmic antibody-associated vasculitis (AAV) using LEGENDplex assay. (C) Correlation of BAFF-R MFI in non-plasmablast B cells with absolute count of B cells/µL blood (left) and BAFF concentration in serum (right). (D) Representative flow cytometry plot of TACI expression (left) and bar graph of MFI of TACI (right) in marginal zone-like (MZ-like) and switched memory B cells. (E) Correlation of BAFF-R MFI with TACI MFI. (F) Soluble TACI (sTACI) concentration (pg/mL) was measured in the serum of HD and patients with AAV by ELISA. Data were analysed using Kruskal-Wallis test, corrected for multiple comparisons by Dunn’s multiple comparison tests and depicted as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Correlation analysis was performed by non-parametric Spearman’s correlation.
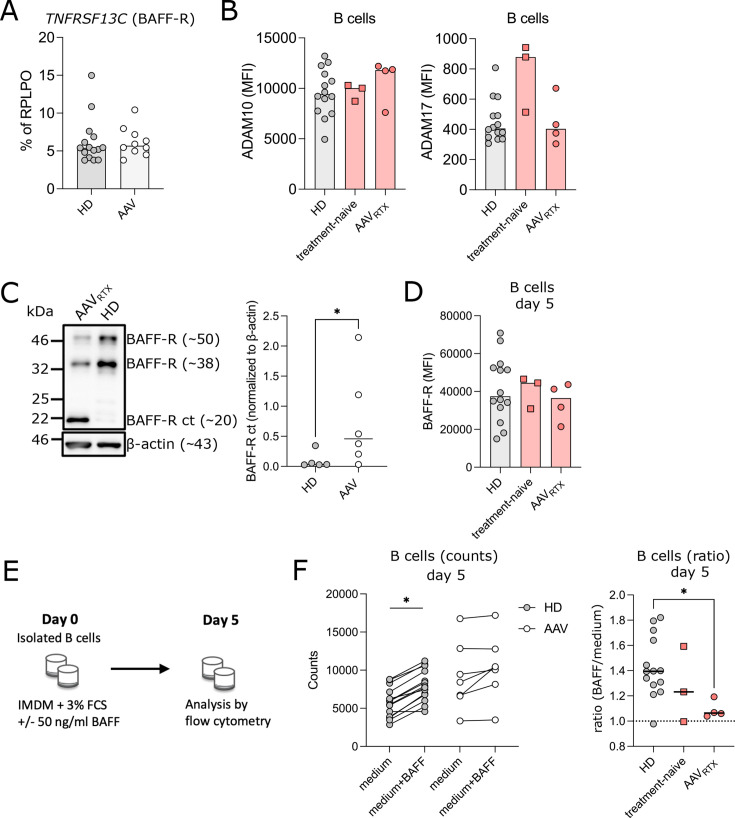
Low BAFF-R expression was not linked to reduced mRNA expression in AAV (figure 6A). BAFF-R is processed upon ligand binding by metalloproteases ADAM10 and ADAM17.28 We observed similar expression of ADAM10 in patients with AAV and controls (figure 6B, online supplemental table S4). ADAM17 expression was increased in two patients only (figure 6B). After shedding of the extracellular domain of BAFF-R, the C-terminal part (BAFF-R CT) remains in the cell membrane.28 Hence, BAFF-R processing can be analysed by detecting BAFF-R CT by western blot analysis. We analysed B cells of RTX-treated patients with AAV (n=6) with low BAFF-R expression and found increased BAFF-R CT expression (figure 6C), indicating that low surface BAFF-R expression may derive from increased BAFF-R shedding. BAFF is needed for shedding of BAFF-R.28 Indeed, incubation of AAV B cells in BAFF-free medium, resulted in re-expression of BAFF-R at similar levels to HDs (figure 6D). To study the functional impact of low BAFF-R expression on survival, isolated B cells were cultivated in the presence or absence of BAFF (figure 6E). In contrast to HDs, addition of BAFF did not improve survival of B cells isolated from RTX-treated patients with AAV, and only minimally in treatment-naïve patients with AAV (figure 6F).
Figure 6. Low BAFF-receptor (BAFF-R) expression by enhanced BAFF-R processing contributes to impaired B-cell survival in antineutrophil cytoplasmic antibody-associated vasculitis (AAV). (A) TNFRSF13C mRNA expression relative to RPLPO was determined by quantitative PCR. (B) ADAM10 and ADAM17 expression was analysed as median fluorescence intensity (MFI) in B cells. (C) Western blot analysis of ex vivo isolated B cells of BAFF-R c-terminal fragment (BAFF-R ct) in patients with AAV compared with HD. Representative western blot analysis (left) and quantification of BAFF-R ct in relation to β-actin (right). (D) Isolated B cells were cultured in medium+3% fetal calf serum (FCS) for 5 days. BAFF-R expression in B cells was analysed as MFI. (E) Experimental setup of performed survival assay. Isolated B cells were cultured as indicated in medium+3% FCS in absence or presence of 50 ng/mL BAFF-60mer. Cells were analysed by flow cytometry. (F) Survival benefit of BAFF addition to the culture was analysed. Counts of B cells on day 5 of both culture conditions were compared (left). Ratio of BAFF/medium treated B cells (counts) on day 5 (right). Data were analysed using Kruskal-Wallis test, corrected for multiple comparisons by Dunn’s multiple comparison tests or Mann-Whitney U test and depicted as *p<0.05.
Discussion
RTX treatment results in prolonged B-cell depletion in a substantial proportion of patients with AAV,9 10 independently of the retreatment schedule. Here, we addressed the underlying cause by exploring the central and peripheral B-cell compartment in patients with AAV before and after RTX treatment. We discovered a defect in B-lymphopoiesis in the BM that preceded RTX treatment and resulted in low transitional B cells in treatment-naïve patients with AAV. The modelling of early B-cell development revealed residual ability of AAV HSCs to develop into B cells in vitro, pointing to a non-permissive BM environment, associated with an insufficient generation of mature B cells. Additionally, BAFF-R expression on peripheral B cells of patients with AAV is reduced after RTX treatment, reducing survival of these cells in response to BAFF.
The peripheral B-cell pool is replenished starting with transitional B cells emerging from the BM.21 In our cohort, transitional B cells were decreased in treatment-naïve patients, in line with previous reports.8 29 Low transitional B cells suggest either an impaired central B-cell development or a defect of immature B cells in exiting the BM. BM analysis showed the almost complete absence of B-cell progenitors in patients with AAV. Defects in B-lymphopoiesis have been described in patients with agammaglobulinemia with mutations in lineage-determining transcription factors or components of the B-cell receptor signalling pathway.17 Differently from agammaglobulinemia, in our patients B-lymphoid precursors were almost absent in BM. As mature B-cell stages were detectable in AAV BM and periphery, B-cell development can occur in patients with AAV, even though inefficiently, as shown by low transitional B cell numbers, and by a partial block in B-lymphopoiesis. Disturbed B-cell development becomes clinically apparent by a stressor, such as the autoimmune disease itself or the B-cell depleting treatment. Impaired B-lymphopoiesis can result from intrinsic B cell defects, and from a ‘hostile’, non-permissive BM environment, as observed in patients with common variable immunodeficiency (CVID). HSCs of patients with CVID develop into immature B cells in vitro, while a block in early B-cell development is observed in ex vivo BM aspirates.25 We found a similar scenario in patients with AAV, where AAV CD34+ progenitors were able to produce immature B cells in vitro, although at much lower speed but not in vivo. The HSC niche is a tightly regulated environment and positioning of B cells in microniches, which provide the developing B cells with signals like SCF, IL-7 and CXCL12, is essential for progression through the developmental stages.30 31 Modulation of Flt3 signalling controls proliferation of multipotent progenitors and CLPs as well as further development.17 Indeed, altered Flt3L was reported in AAV serum,32 pointing to a potential involvement of this signalling pathway. In addition, chronic inflammation can redirect or exhaust lymphopoiesis because of unfavourable cytokine environment,33 34 steroids35 36 or sex hormones.37 The effects are usually temporary and normal lymphopoiesis can be then restored. In AAV, we observed a persistent (up to 12.5 years) defect in B-cell reconstitution after RTX treatment, suggesting persistent changes in the BM environment and/or in HSCs that hinders development. Further studies will be required to identify these primary factors. In treatment-naïve AAV, low transitional B cells did not translate into low naïve B cell numbers. This can be explained by the different half-life between transitional (3 days) and naïve B cells (3 months).38 39 Hence, a reduction in BM output is rapidly manifested by low transitionals, while the naïve B-cell compartment will change much later in time. Therefore, manifestation in the mature B-cell compartment will require a stressor such as RTX treatment.
MZ B cell survival and maturation is spleen-dependent and BAFF-R-dependent.18 26 40 We found low MZ-B cells in AAV. On one side, functional hyposplenism because of micro-infarction has been reported in AAV,41 42 on the other side, we observed a tendentially low BAFF-R expression in untreated patients with AAV B cells, and a significantly lower BAFF-R expression in RTX-treated patients with AAV. Hence, both hyposplenism and low BAFF-R expression may contribute to low MZ-B cells. While a defect in B-lymphopoiesis seems to be the major cause of defective peripheral B-cell replenishment, low BAFF-R expression in RTX-treated AAV may hinder peripheral B-cell development as immature B cells may inefficiently proceed to the mature B-cell stage. Reduced BAFF-R expression in repopulating B cells after RTX therapy has been observed in other autoimmune diseases with normal B-cell repopulation time, such as rheumatoid arthritis and thrombotic thrombocytopenic purpura.43,45 Thus, low BAFF-R could also be a feature of B-cell repopulation after RTX due to high BAFF environment. Thus, the impact of BAFF-R expression on the peripheral B-cell development defect in AAV has to be further examined.
Skewed BM development towards myelopoiesis has been described in AAV. Furthermore, clonal haematopoiesis of indeterminate potential has been associated with AAV and bias for the myeloid lineage.46 A myeloid skewing was not observed in our culture system in AAV HSCs compared with controls, suggesting that AAV HSC precursors are not intrinsically biased to develop more into myelopoiesis. Different immunosuppressive agents appear to impact the repopulation time after RTX-mediated depletion. AZA and MMF are associated with the longest B-cell repopulation time and with a striking reduction in transitional B cells. Indeed, it was reported that thiopurines can significantly reduce peripheral B cells and especially transitional B cells.47 Furthermore, MMF inhibits B-cell proliferation in vitro.48 Mycophenolic acid inhibits inosine monophosphate dehydrogenase, potentially stimulating toll-like receptor (TLR) signalling. It has been shown that stimulation of HSCs with TLR agonists leads to a differentiation along the myeloid lineage,49 50 and chronic exposure of expanding HSCs to TLR ligands leads to a defect in their repopulation.51 52 Whether this mechanism adds to a per se impaired repopulation capacity of the B-cell compartment in AAV and contributes to the prolonged B-cell repopulation, warrants further studies. CYC has been associated with B-cell depletion and reduced serum antibody levels.11 Overall, we estimate the influence of CYC on the results presented here to be rather small, as CYC did not lead to lower B-cell counts or different B-cell depletion times compared with patients who had not received CYC therapy.
Our observations are meaningful to the current discussion on the length of RTX-maintenance therapy. Data pointing towards a defect in BM B-cell development are important, as they call for caution with regard to long-term B-cell depletion, stimulate the discussion on biomarker-guided on-demand therapy after RTX administration, emphasise the need for regular immunoglobulin controls and indicate that the combination of RTX with subsequent administration of AZA or MMF can significantly prolong the B-cell depletion time.
Our study has several limitations. First, the analyses of BM were limited to seven patients. Second, having obtained BM ‘per indication’ might have introduced a selection bias, but as the results of the analyses of the peripheral B-cell compartment (eg, low transitional B cells) are perfectly in line with the findings of the BM analyses, we do not think, that indications for BM puncture did relevantly skew the results of our analyses. Third, the molecular mechanisms behind the BM niche defect and the alterations in peripheral B-cell maturation and survival in patients with AAV remain to be defined. Therefore, our observations prompt further studies analysing BM in a larger AAV cohort.
In conclusion, we found a profound dysregulation of the B-cell compartment in AAV with a disturbed central B-lymphopoiesis and almost absent B-lymphoid precursors in the BM. The reduced output from the BM impairs B-cell reconstitution after RTX treatment in AAV and stimulates the debate regarding RTX retreatment schedules.
Materials and methods
Patients’ characteristics and inclusion criteria
Patients included in this retrospective analysis were identified from the IR-Biobank and the FREEZE-biobank of the University Hospital Freiburg between 2012 and 2021. Clinical data were extracted from the database of the Department of Rheumatology and Clinical Immunology, University Hospital Freiburg. Inclusion into the study required a diagnosis of ANCA-associated vasculitis (GPA, MPA or EGPA) according to the ACR 1990 or the 2022 ACR/EULAR criteria.53,57 Patients were included into the analysis if they:
had received RTX treatment with a minimal subsequent follow-up observation period of at least 6 months or
had newly diagnosed AAV and were treatment-naïve (except for short-term GCs).
Patients undergoing plasmapheresis or substitution of immunoglobulins because of hypogammaglobulinemia were excluded from immunoglobulin analyses. In all patients, peripheral B-cell counts were measured regularly after RTX treatment. B-cell depletion time was calculated from RTX administration to B-cell repopulation, with B-cell repopulation defined as more than five B cells (CD19+ cells in the lymphocyte gate) per µL in peripheral blood.
Clinical and laboratory assessments
Routine laboratory parameters were extracted from the clinical information system of the University Hospital Freiburg. Ig serum concentrations were determined by nephelometry (Behring Nephelometer; normal ranges: IgG 7–16 g/L, IgM 0.4–2.3 g/L, IgA 0.7–4 g/L). ANCA staining patterns were assessed by indirect immunofluorescence. The specificity of ANCA for myeloperoxidase or proteinase was assessed by ELISA (Euroimmun) or by Alegria (Orgentec) with the cut-off of 5 U/mL for anti-MPO and anti-PR3. Lymphocyte and B-cell absolute counts and frequencies were analysed by Gallios flow cytometer (Beckmann Coulter, Krefeld, Germany).
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-blood by density gradient centrifugation and were cryopreserved until use. PBMCs were either stained directly or for patients that were expected to have very low frequency of B cells, magnetic B-cell enrichment using the EasySep Human B cell Isolation Kit (STEMCELL Technologies) following the manufacturer’s protocol was performed. Samples were stained using the antibodies listed in online supplemental table S1. To exclude bound BAFF preventing binding of the BAFF-R antibody, a representative number of patients with AAV and HD PBMCs were stained after acidic elution to remove bound BAFF from the receptor. For this, samples were incubated for a minute with citrate buffer (0.133 M citric acid+0.066 M Na2HPO4, pH 3.3) before washing and staining. Samples were acquired with the Cytek Aurora (Cytek) using SpectroFlo software (Cytek) and analysed by FlowJo. Absolute B-cell counts of patients with AAV were measured by routine diagnostics and extracted from the clinical information system. Absolute counts of subpopulations were calculated using the frequency of the respective population measured at Cytek Aurora. Absolute count of B cells and subpopulations of HDs: median of lymphocytes/µL (from nine HD measured by routine diagnostics) was used as standard reference to calculate individual absolute counts of B cells and subpopulations from the frequencies measured at Cytek Aurora.
Bone marrow analysis
AAV BM aspirates were obtained from n=7 consecutive patients. Patients were hospitalised because of active new onset (n=3) or relapsing (n=4) AAV. HD BM was collected from orthopaedic surgery or testing for exclusion of malignancy. Ex vivo BM aspirates were analysed by mass cytometry (CyTOF) (see ‘Mass cytometry, sample staining and acquisition’ section) or flow cytometry. Flow cytometric analysis was performed using a FACS Canto II (BD) or for some HD samples a Cytek Aurora (Cytek) with SpectroFlo software (Cytek) (for panels see online supplemental tables S2 and S3) and data were analysed by FlowJo.
Mass cytometry, sample staining and acquisition
Metal-tagged antibodies were either purchased (Standard BioTools) or conjugated in-house using Maxpar X8 Antibody Labelling Kit (Standard BioTools) according to manufacturer’s instructions. Antibodies were validated and titrated for the appropriate concentrations and are listed in online supplemental table S2. The samples were stained as described previously58 and according to the MaxPar Nuclear Antigen Staining with Fresh Fix (Standard BioTools) protocol as described by the manufacturer. Mass cytometry sample acquisition was performed on Helios instrument (Standard BioTools, CyTOF 6.7.1014 software) after preparation according to the manufacturer’s recommendation. For visualisation of the mass cytometry data, we used our deep learning-based dimensionality reduction technique using the vaevictis model,22 one of the autonomous modules integrated in the tviblindi tool.
Modelling of early B-cell development in vitro
CD34+ cells were isolated from BM aspirates using the CD34 MicroBead Kit (Miltenyi Biotec) following manufacturer’s protocol. BM culture was performed as described previously.24 25 In brief, CD34+ cells were plated in 96-well plates at 105 cells/mL in Iscove’s medium containing insulin, transferrin, non-essential amino acids, glutamine, glutathione and 10% FCS. Culture medium was supplemented with stem cell factor (SCF), Flt3 ligand (Flt3L) and IL-6 (each 25 ng/mL; Immunotools). On day 7, cells were replated at 105 cells/mL and medium supplements were changed to IL-7 (20 ng/mL; Immunotools), SCF and Flt3L. From day 14 on, no cytokines were added to the culture medium that was changed twice a week. Flow cytometric analysis was performed every 7 days and cells were collected for RNA isolation if sufficient material was available.
Culture was analysed using CD34 PE-Cy7 (clone 581, BioLegend), CD10 FITC (clone HI10a BioLegend, W8E7, BD), CD38 PB (clone HIT2, Exbio), CD19 BV510 (clone HIB19, BioLegend) or CD19 APC-Cy7 (clone HIB19, BioLegend), CD33 PerCP-Cy5.5 (clone WM53, BioLegend), IgD PE (Southern Biotech) or IgD PE-Cy7 (clone IA6-2, BioLegend), IgM AF647 (Jackson Immuno Research). Dead cells were identified by zombie NIR Fixable Viability Kit (APC-Cy7, BioLegend) or zombie Aqua Fixable Viability Kit (AmCyan, BioLegend). Data were acquired using a FACS Canto II (BD) and analysed by FlowJo. For analysis of the frequency of CLP/pro B, preB and immature B cells within CD10+CD38+, a threshold of 10 cells within the CD10+CD38+ was set to include the sample into the analysis.
LEGENDplex and ELISA
Serum BAFF concentrations were quantified using LEGENDplex Human B Cell Panel (BioLegend) according to the manufacturer’s protocol. Serum ADAM10 (LifeSpan BioSciences), TACE/ADAM17 (DuoSet ELISA, R&D Systems), BAFF-R (Elabscience) and TACI/ TNFRSF13B (DuoSet ELISA, R&D Systems) concentrations were measured using commercially available ELISA kits according to the manufacturer’s protocol.
Quantitative PCR
Total RNA was isolated from isolated B cells using either the RNeasy Mini Kit (Qiagen) for >500 000 B cells or RNeasy Micro Kit (Qiagen), if <500 000 B cells were available. Kits were used according to the manufacturer’s protocol. RNA concentration was measured at the NanoDrop 2000c (ThermoScientific). For complementary DNA, RNA was reverse transcribed using random hexamer primers (Invitrogen) and Superscript III reverse transcriptase (Invitrogen). Quantitative PCR was carried out using a TaqMan 2x Gene Expression Master Mix (Applied Biosystems) and primers: BAFF-R (Hs00606874_g1, ThermoScientific), RPLPO (4333761F, ThermoScientific). The mRNA levels were standardised to the reference gene RPLPO and relative expression was calculated using the ΔCT method.
Western blot analysis
Western blot analysis was performed on ex vivo isolated B cells of patients with AAV and HDs using anti-BAFF-R CT (Enzo Life Sciences) and β-actin (clone C4, Santa Cruz Biotechnology). Chemiluminescence signal was acquired using a Fusion-FX7 (Vilber) and signal was quantified using Fusion Software. Signal of BAFF-R bands (BAFF-R Ct, 20 kDa; BAFF-R 50 kDa; BAFF-R 38 kDa) were normalised to β-actin.
Survival assay
B cells isolated in a total of 5×104 total isolated B cells from HD or patients with AAV were cultured in Iscove’s medium+3% FCS with or without addition of 50 ng/mL BAFF60mer (AdipoGen Life Sciences) for 5 days. Medium was changed on day 2. Cells were analysed on day 0 and day 5 by flow cytometry on a Cytek Aurora (Cytek) using SpectroFlo software (Cytek) and analysed by FlowJo (antibodies are listed in online supplemental table 4).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software V.10.1.1 as indicated in figure legends.
supplementary material
Acknowledgements
We thank the Immunology-Rheumatology Biobank (IR-Biobank) of the Department of Rheumatology and Clinical Immunology and the FREEZE-Biobank of the University Medical Center Freiburg for support in sample collection and storage.
Footnotes
Funding: This study was funded by the Heisenberg-Programm (to MR), the SFB1160 project B02 (to MR) and the TRR353 project A01 (to MR) of the German Research Foundation (DFG), the Grant 23-05561S from the Czech Science Foundation and the National Institute for Cancer Research (Programme EXCELES, ID Project No. LX22NPO5102) funded by the European Union—Next Generation EU (to TK).
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Josef S Smolen
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study was approved by the ethics committee of the Albert-Ludwigs University Freiburg (EC Freiburg 218/20). Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Jens Thiel, Email: jens.thiel@medunigraz.at.
Franziska M Schmidt, Email: franziska.schmidt@meduniwien.ac.at.
Raquel Lorenzetti, Email: raquel.lorenzetti@medunigraz.at.
Arianna Troilo, Email: arianna.troilo@gmail.com.
Iga Janowska, Email: iga.janowska@uniklinik-freiburg.de.
Lena Nießen, Email: lenaniessen@t-online.de.
Sophie Pfeiffer, Email: sophie.pfeiffer@uniklinik-freiburg.de.
Julian Staniek, Email: julian.staniek@uniklinik-freiburg.de.
Bruno Benassini, Email: bruno.benassini.orozco@uniklinik-freiburg.de.
Marei-Theresa Bott, Email: theresa.bott@uniklinik-freiburg.de.
Jakov Korzhenevich, Email: jakov.korzhenevich@meduniwien.ac.at.
Lukas Konstantinidis, Email: lukas.konstantinidis@uniklinik-freiburg.de.
Frank Burgbacher, Email: frank.burgbacher@uniklinik-freiburg.de.
Ann-Katrin Dufner, Email: Ann-Kathrin.Dufner@sbk-vs.de.
Natalie Frede, Email: natalie.frede@uniklinik-freiburg.de.
Reinhard E Voll, Email: reinhard.voll@uniklinik-freiburg.de.
Jan Stuchly, Email: jan.stuchly@lfmotol.cuni.cz.
Marina Bakardjieva, Email: marina.bakardjieva@lfmotol.cuni.cz.
Tomas Kalina, Email: tomas.kalina@lfmotol.cuni.cz.
Cristian Roberto Smulski, Email: cristian.smulski@gmail.com.
Nils Venhoff, Email: nils.venhoff@uniklinik-freiburg.de.
Marta Rizzi, Email: marta.rizzi@uniklinik-freiburg.de.
Data availability statement
Data are available on reasonable request.
References
- 1.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–80. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 3.Terrier B, Pagnoux C, Perrodeau É, et al. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis. 2018;77:1150–6. doi: 10.1136/annrheumdis-2017-212768. [DOI] [PubMed] [Google Scholar]
- 4.Fussner LA, Hummel AM, Schroeder DR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. 2016;68:1700–10. doi: 10.1002/art.39637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369:417–27. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roll P, Palanichamy A, Kneitz C, et al. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–86. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 7.Hellmich B, Sanchez-Alamo B, Schirmer JH, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. 2024;83:30–47. doi: 10.1136/ard-2022-223764. [DOI] [PubMed] [Google Scholar]
- 8.Thiel J, Salzer U, Hässler F, et al. B cell homeostasis is disturbed by immunosuppressive therapies in patients with ANCA-associated vasculitides. Autoimmunity. 2013;46:429–38. doi: 10.3109/08916934.2013.798652. [DOI] [PubMed] [Google Scholar]
- 9.Thiel J, Rizzi M, Engesser M, et al. B cell Repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther. 2017;19:101. doi: 10.1186/s13075-017-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venhoff N, Niessen L, Kreuzaler M, et al. Reconstitution of the peripheral B lymphocyte compartment in patients with ANCA-associated vasculitides treated with rituximab for relapsing or refractory disease. Autoimmunity. 2014;47:401–8. doi: 10.3109/08916934.2014.914174. [DOI] [PubMed] [Google Scholar]
- 11.Venhoff N, Effelsberg NM, Salzer U, et al. Impact of Rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS ONE. 2012;7:e37626. doi: 10.1371/journal.pone.0037626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mescia F, Salviani C, Tonoli M, et al. Sustained post-rituximab B-cell depletion is common in ANCA-associated vasculitis and is affected by sex and renal function. Nephrol Dial Transplant. 2024;39:683–93. doi: 10.1093/ndt/gfad197. [DOI] [PubMed] [Google Scholar]
- 13.Gathmann B, Mahlaoui N, Gérard L, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134:116–26. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 14.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tieu J, Smith RM, Gopaluni S, et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Front Immunol. 2021;12:671503. doi: 10.3389/fimmu.2021.671503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vollenhoven RF, Emery P, Bingham CO, III, et al. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Ann Rheum Dis. 2013;72:1496–502. doi: 10.1136/annrheumdis-2012-201956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korzhenevich J, Janowska I, van der Burg M, et al. Human and mouse early B cell development: so similar but so different. Immunol Lett. 2023;261:1–12. doi: 10.1016/j.imlet.2023.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Warnatz K, Salzer U, Rizzi M, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci USA. 2009;106:13945–50. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–91. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 20.Jenks SA, Cashman KS, Woodruff MC, et al. Extrafollicular responses in humans and SLE. Immunol Rev. 2019;288:136–48. doi: 10.1111/imr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anolik JH, Friedberg JW, Zheng B, et al. B cell reconstitution after Rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122:139–45. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Stuchly J, Novak D, Brdickova N, et al. Deconstructing complexity: a computational topology approach to trajectory inference in the human thymus with Tviblindi. Immunology. 2023 doi: 10.1101/2023.07.13.547329. Preprint. [DOI] [Google Scholar]
- 23.Bakardjieva M, Stuchlý J, Pelák O, et al. Tviblindi algorithm identifies branching developmental Trajectories of human B cell development. Immunology. 2023 doi: 10.1101/2024.01.11.575178. Preprint. [DOI] [PMC free article] [PubMed]
- 24.Kraus H, Kaiser S, Aumann K, et al. A feeder-free differentiation system identifies autonomously proliferating B cell precursors in human bone marrow. J Immunol. 2014;192:1044–54. doi: 10.4049/jimmunol.1301815. [DOI] [PubMed] [Google Scholar]
- 25.Troilo A, Wehr C, Janowska I, et al. Nonpermissive bone marrow environment impairs early B-cell development in common variable immunodeficiency. Blood. 2020;135:1452–7. doi: 10.1182/blood.2019003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smulski CR, Eibel H. BAFF and BAFF-receptor in B cell selection and survival. Front Immunol. 2018;9:2285. doi: 10.3389/fimmu.2018.02285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreuzaler M, Rauch M, Salzer U, et al. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188:497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 28.Smulski CR, Kury P, Seidel LM, et al. BAFF- and TACI-dependent processing of BAFFR by ADAM proteases regulates the survival of B cells. Cell Rep. 2017;18:2189–202. doi: 10.1016/j.celrep.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Elmér E, Smargianaki S, Pettersson Å, et al. Increased frequencies of switched memory B cells and plasmablasts in peripheral blood from patients with ANCA-associated vasculitis. J Immunol Res. 2020;2020:8209737. doi: 10.1155/2020/8209737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLean KC, Mandal M. It takes three receptors to raise a B cell. Trends Immunol. 2020;41:629–42. doi: 10.1016/j.it.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehentmeier S, Pereira JP. Cell circuits and niches controlling B cell development. Immunol Rev. 2019;289:142–57. doi: 10.1111/imr.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venhoff N, Thiel J, Voll R, et al. THU0341 FLT3 ligand concentrations are elevated in ANCA-associated vasculitides (AAV) and are influenced by immunosuppressive therapy. Annals of the Rheumatic Diseases. 2017;76:332 [Google Scholar]
- 33.Hirayama F, Clark SC, Ogawa M. Negative regulation of early B Lymphopoiesis by interleukin 3 And interleukin 1 alpha. Proc Natl Acad Sci U S A. 1994;91:469–73. doi: 10.1073/pnas.91.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy DE, Knight KL. Inhibition of B lymphopoiesis by adipocytes and IL-1-producing myeloid-derived suppressor cells. J Immunol. 2015;195:2666–74. doi: 10.4049/jimmunol.1500957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laakko T, Fraker P. Rapid changes in the lymphopoietic and granulopoietic compartments of the marrow caused by stress levels of corticosterone. Immunology. 2002;105:111–9. doi: 10.1046/j.1365-2567.2002.01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang S-E, Guo L, Tian J, et al. Autoimmune bone marrow environment severely inhibits B cell development by inducing extensive cell death and inhibiting proliferation. Autoimmunity. 2012;45:210–7. doi: 10.3109/08916934.2011.632455. [DOI] [PubMed] [Google Scholar]
- 37.Kincade PW, Medina KL, Smithson G. Sex hormones as negative regulators of lymphopoiesis. Immunol Rev. 1994;137:119–34. doi: 10.1111/j.1600-065x.1994.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 38.Rocha B, Penit C, Baron C, et al. Accumulation of bromodeoxyuridine-labeled cells in central and peripheral lymphoid organs: minimal estimates of production and turnover rates of mature lymphocytes. Eur J Immunol. 1990;20:1697–708. doi: 10.1002/eji.1830200812. [DOI] [PubMed] [Google Scholar]
- 39.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–48. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 41.Gercik O, Karasu S, Solmaz D, et al. Splenic infarction is not rare in granulomatosis with polyangiitis. Clin Rheumatol. 2020;39:1929–34. doi: 10.1007/s10067-020-04993-w. [DOI] [PubMed] [Google Scholar]
- 42.Ghinoi A, Pipitone N, Cavazza A, et al. Wegener granulomatosis with spleen infarction: case report and review of the literature. Semin Arthritis Rheum. 2008;37:328–33. doi: 10.1016/j.semarthrit.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Becerra E, De La Torre I, Leandro MJ, et al. B cell phenotypes in patients with rheumatoid arthritis relapsing after rituximab: expression of B cell-activating factor-binding receptors on B cell Subsets. Clin Exp Immunol. 2017;190:372–83. doi: 10.1111/cei.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becerra E, Scully MA, Leandro MJ, et al. Effect of rituximab on B cell phenotype and serum B cell-activating factor levels in patients with thrombotic thrombocytopenic purpura. Clin Exp Immunol. 2015;179:414–25. doi: 10.1111/cei.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de la Torre I, Moura RA, Leandro MJ, et al. B-cell-activating factor receptor expression on naive and memory B cells: relationship with relapse in patients with rheumatoid arthritis following B-cell depletion therapy. Ann Rheum Dis. 2010;69:2181–8. doi: 10.1136/ard.2010.131326. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez-Rodrigues F, Wells KV, Jones AI, et al. Clonal haematopoiesis across the age spectrum of vasculitis patients with Takayasu’s arteritis, ANCA-associated vasculitis and giant cell arteritis. Ann Rheum Dis. 2024;83:508–17. doi: 10.1136/ard-2023-224933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lord JD, Shows DM. Thiopurine use associated with reduced B and natural killer cells in inflammatory bowel disease. WJG. 2017;23:3240. doi: 10.3748/wjg.v23.i18.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Borstel A, Abdulahad WH, Dekkema G, et al. Mycophenolic acid and 6-mercaptopurine both inhibit B-cell proliferation in granulomatosis with polyangiitis patients, whereas only Mycophenolic acid inhibits B-cell IL-6 production. PLoS ONE. 2020;15:e0235743. doi: 10.1371/journal.pone.0235743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Megías J, Yáñez A, Moriano S, et al. Direct toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells. 2012;30:1486–95. doi: 10.1002/stem.1110. [DOI] [PubMed] [Google Scholar]
- 50.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–12. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aluri J, Cooper MA, Schuettpelz LG. Toll-like receptor signaling in the establishment and function of the immune system. Cells. 2021;10:1374. doi: 10.3390/cells10061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esplin BL, Shimazu T, Welner RS, et al. Chronic exposure to a TLR ligand Injures hematopoietic stem cells. J Immunol. 2011;186:5367–75. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 54.Masi AT, Hunder GG, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and Angiitis) Arthritis Rheum. 1990;33:1094–100. doi: 10.1002/art.1780330806. [DOI] [PubMed] [Google Scholar]
- 55.Grayson PC, Ponte C, Suppiah R, et al. American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81:309–14. doi: 10.1136/annrheumdis-2021-221794. [DOI] [PubMed] [Google Scholar]
- 56.Robson JC, Grayson PC, Ponte C, et al. American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Ann Rheum Dis. 2022;81:315–20. doi: 10.1136/annrheumdis-2021-221795. [DOI] [PubMed] [Google Scholar]
- 57.Suppiah R, Robson JC, Grayson PC, et al. American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis. 2022;81:321–6. doi: 10.1136/annrheumdis-2021-221796. [DOI] [PubMed] [Google Scholar]
- 58.Kuzilková D, Bugarin C, Rejlova K, et al. Either IL-7 activation of JAK-STAT or BEZ inhibition of Pi3K-AKT-mTOR pathways dominates the single-cell phosphosignature of ex vivo treated pediatric T-cell acute lymphoblastic leukemia cells. Haematologica. 2022;107:1293–310. doi: 10.3324/haematol.2021.278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.