Abstract
ABSTRACT
Background
D-dimer is the only biomarker currently recommended in guidelines for the diagnosis of acute aortic syndrome (AAS). We undertook a systematic review to determine whether any alternative biomarkers could be useful in AAS diagnosis.
Methods
We searched electronic databases (including MEDLINE, EMBASE and the Cochrane Library) from inception to February 2024. Diagnostic studies were eligible if they examined biomarkers other than D-dimer for diagnosing AAS compared with a reference standard test in people presenting to the ED with symptoms of AAS. Case-control studies were identified but excluded due to high risk of bias. Selection of studies, data extraction and risk of bias assessments using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool were undertaken independently by at least two reviewers. We used narrative synthesis to summarise the findings.
Results
We identified 2017 citations, included 13 cohort studies (n=76–999), and excluded 38 case-control studies. Methodological quality was variable, with most included studies having unclear or high risk of bias and applicability concerns in at least one item of the QUADAS‐2 tool. Only two studies reported biomarkers with sensitivity and specificity comparable to D-dimer (ie, >90% and >50%, respectively). Wang et al reported 99.1% sensitivity and 84.9% specificity for soluble ST2; however, these findings conflicted with estimates of 58% sensitivity and 70.8% specificity reported in another study. Chun and Siu reported 95.6% sensitivity and 56.1% specificity for neutrophil count, but this has not been confirmed elsewhere.
Conclusion
There are many potential alternative biomarkers for AAS but few have been evaluated in more than one study, study designs are often weak and reported biomarker accuracy is modest or inconsistent between studies. Alternative biomarkers to D-dimer are not ready for routine clinical use.
PROSPERO registration number
CRD42022252121.https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022252121
Keywords: systematic review, arterial, cardiovascular system
WHAT IS ALREADY KNOWN ON THIS TOPIC
D-dimer has some diagnostic value in the assessment of suspected acute aortic syndrome (AAS), but the role of other potential biomarkers is unclear.
WHAT THIS STUDY ADDS
Our systematic review showed that the evidence for other biomarkers is weak and estimates of accuracy are generally modest or inconsistent.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Diagnostic biomarkers for AAS, other than D-dimer, are not ready for routine clinical use.
Large cohort studies are required to evaluate multiple biomarkers in an appropriate population with suspected AAS.
Introduction
Acute aortic syndrome (AAS) is a life-threatening emergency condition affecting the thoracic aorta that includes acute aortic dissection (AAD), intramural haematoma and penetrating ulcer. CT angiography (CTA) scanning of the aorta has high sensitivity and specificity for diagnosing AAS but incurs significant costs and the risks of ionising radiation.
Biomarkers could be used to select patients with suspected AAS for CTA. The pathophysiology of AAS allows researchers to select and investigate various biomarkers. In aortic dissection, intima rupture allows circulating blood to enter the media of the aorta, forming both true and false lumens. This results in an initial inflammatory response, followed by infiltration of inflammatory cells and subsequent vascular smooth muscle cell apoptosis, ultimately leading to aortic media degradation. This process potentiates aortic dilatation, aneurysm formation, progression to dissection and can lead to rupture.1 Recognising this condition early is paramount to avoid significant morbidity and mortality. Biomarkers can therefore be divided according to the process with which they are associated: clotting, inflammatory response, lipid metabolism, cardiac myocyte damage, vascular extracellular matrix damage and other protein metabolism.2 Biomarkers may also reflect the consequent effects of organ hypoperfusion. Such biomarkers would therefore play more of a role in severity of sequelae as opposed to identification of AAS.
D-dimer is the most extensively studied biomarker for AAS. The most recent meta-analysis included 18 studies with 7978 patients and reported pooled sensitivity of 96.5% (95% credible interval (CrI) 94.8% to 98%) and specificity of 56.2% (95% CrI 48.3% to 63.9%) for D-dimer above the diagnostic threshold of 500 ng/mL.3 4 D-dimer sensitivity can be improved by using it alongside a clinical probability score, such as the aortic dissection detection risk score (ADD-RS), which uses clinical features to estimate clinical risk on a score from 0 to 3. The combination of ADD-RS ≥1 and D-dimer >500 ng/mL in a recent meta-analysis of six studies demonstrated pooled sensitivity of 93.1% (95% CrI 87.1% to 96.3%) and specificity 67.1% (95% CrI 54.4% to 77.7%) when diagnosing AAS.5 These findings suggest a potential role for D-dimer, alone or alongside clinical probability scoring, to select patients for CTA, and suggest that alternative biomarkers with superior accuracy to D-dimer could have an important role in clinical practice.
A number of studies have evaluated biomarkers for AAS other than D-dimer. However, these have not yet been systematically examined to identify the most promising candidates for future research or to determine whether any have the potential to improve on the accuracy of D-dimer. We aimed to systematically review biomarkers other than D-dimer to determine their accuracy for diagnosing AAS.
Methods
A systematic review was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.6 7 This review was part of a larger National Institute for Health and Care Research-funded (January to December 2023) Aortic Syndrome Evidence Synthesis (ASES) project on diagnostic strategies for suspected AAS and was registered on the International Prospective Register of Systematic Reviews database (CRD42022252121).8
Eligibility criteria
Prospective or retrospective studies reporting diagnostic accuracy metrics were eligible if they examined any biomarkers (other than D-dimer) for diagnosing AAS compared with a reference standard test (eg, a definitive imaging modality such as CTA, ECG-gated CTA, echocardiography, and magnetic resonance angiography or confirmed/excluded by operation and autopsy). The study population of interest in our review consisted of people (any age) presenting to the ED with symptoms of AAS, including those with new-onset chest, back or abdominal pain, syncope or symptoms related to perfusion deficit. Studies including people with AAS following major trauma or as incidental findings were excluded. We initially planned to include all study designs in the review but only include cohort studies in any meta-analysis. After undertaking initial searches, we amended the protocol to exclude case-control designs from the review due to the potential for high bias resulting in inaccurate estimates and lack of representativeness of test accuracy in a clinical setting.9 10
Data sources and searches
Potentially relevant studies were identified through searches of several electronic databases including MEDLINE (OvidSP from 1946 to February 2024), EMBASE (OvidSP from 1974 to February 2024), and the Cochrane Library (https://www.cochranelibrary.com from inception to February 2024) by an experienced information specialist (MC), who is a member of the research team. The search strategy used free text and thesaurus terms and combined synonyms relating to the topic of interest (eg, AAS and diagnostic strategies) with diagnostic testing terms (adapted Scottish Intercollegiate Guidelines Network filter for identifying diagnostic studies). Searches were supplemented by hand-searching the reference lists of all relevant studies (including existing systematic reviews); forward citation searching of relevant articles; contacting key experts in the field and undertaking targeted searches of the World Wide Web using the Google search engine. No date or language restrictions were applied on any database. Further details on the search strategy can be found in online supplemental appendix S1.
Study selection
All titles were examined for inclusion by one reviewer (ME) and any citations that clearly did not meet the inclusion criteria (eg, non-human, unrelated to AAS) were excluded. All abstracts and full-text articles were then examined independently by two reviewers (ME and AP). Any disagreements in the selection process were resolved through discussion or if necessary, arbitration by a third reviewer (SG) and included by consensus.
Data extraction and quality assessment
Data relating to study design, methodological quality and outcomes were extracted by one reviewer (JW) into a standardised data extraction form and independently checked for accuracy by a second (AP). Any discrepancies were resolved through discussion to achieve agreement. Where differences were unresolved, a third reviewer’s opinion was sought (SG). Where multiple publications of the same study were identified, data were extracted and reported as a single study.
The methodological quality of each included study was assessed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool.11 This instrument evaluates four key domains: patient selection, index test, reference standard, flow and timing. Each domain is assessed in terms of risk of bias and concerns regarding the applicability of the study results (first three domains only). The subdomains about risk of bias include a number of signaling questions to help guide the overall judgement about whether a study is at high, low or an unclear (in the event of insufficient data in the publication to answer the corresponding question) risk of bias.
Data synthesis and analysis
We were unable to perform meta-analysis due to the limited number of studies per biomarker and variable reporting of items. As a result, a narrative synthesis approach was undertaken, with data being summarised in tables with accompanying narrative summaries that included a description of the included variables, statistical methods and performance measures (eg, sensitivity, specificity).12 13 All analyses were conducted using Microsoft Excel 2010 (Microsoft, Redmond, Washington, USA).
Patient and public involvement
Two members of the Aortic Dissection Charitable Trust (https://aorticdissectioncharitabletrust.org/) joined the ASES project management team and helped to develop the study proposal. SG presented the findings of this review to a webinar of Aortic Dissection Charitable Trust members and sought their feedback on interpretation of the results.
Results
Study flow
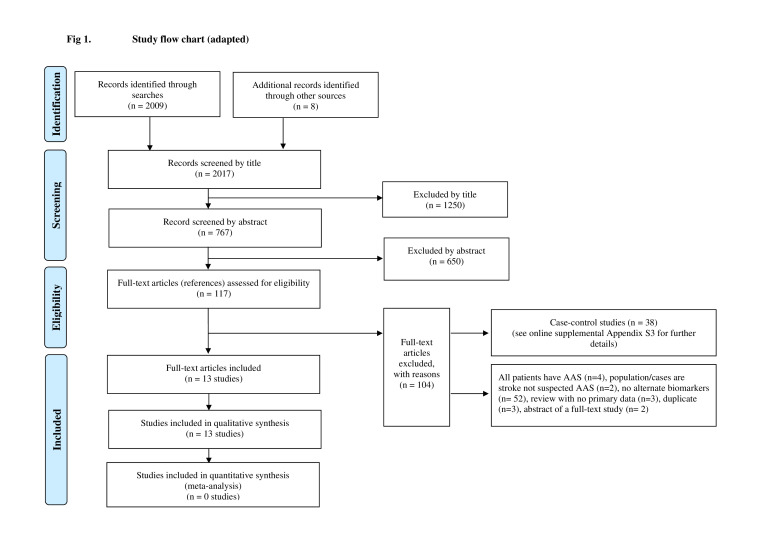
Figure 1 summarises the process of identifying and selecting relevant literature. Of the 2017 citations identified, 13 studies investigating 17 index tests met the inclusion criteria.14,26 The majority of the articles were excluded primarily on the basis of an inappropriate target population (patients with AAS or not suspected AAS), investigating intervention not an alternative biomarker or an unsuitable publication type (ie, reviews or abstract of full-text studies). A full list of excluded studies with reasons for exclusion can be found in online supplemental appendix S2. More specifically, 38 case-control studies were excluded due to the high potential for bias with this design.9 10 Four case-control studies reported comparisons with unselected controls with suspected AAS,27,29 whereas 34 case-control studies reported comparisons with healthy controls or controls with other diagnoses.30,63 A summary of the design and patient characteristics of the 38 excluded case-control studies can be found in online supplemental appendix S3. These studies evaluated a wide variety of biomarkers using a variety of different control groups. These studies may identify biomarkers for future research but do not provide reliable estimates of accuracy to inform clinical practice.
Figure 1. Study flow chart (adapted with permission from reference 7). AAS, acute aortic syndrome.
Study and patient characteristics
The design and patient characteristics of the 13 included studies are summarised in table 1. Sample size ranged from n=76–999, with prevalence of AAS ranging from 1% to 51.2%. A team of researchers in Italy undertook five of the studies, evaluating the following biomarkers in consecutive cohorts: matrix metalloproteinases (MMP) 8 and 9, lactate dehydrogenase (LDH), white blood cell (WBC) count, platelet count, fibrinogen, copeptin and soluble suppression of tumourigenicity-2 (sST2). The other studies were undertaken in China (five studies), Germany, Japan and Canada, evaluating the following biomarkers: troponin, α-smooth muscle actin (α-SMA), smooth muscle myosin heavy chain (smMHC), soluble elastin fragments (sELAF) in serum, polycystin-1 (PC1), acidic and basic calponin at 6 and 24 hours after presentation, sST2, neutrophil-to-lymphocyte ratio, neutrophil count and leucocyte count.
Table 1. Study characteristics of the 13 included cohort studies.
| Study | Country | Cohort(n with AAS/N in cohort) | Selection process | Biomarkers evaluated | Method of measurement | Reference standard |
| Chun and Siu14 | China | 198/534 | Consecutive patients | Neutrophil count | Automated analyser | CTA |
| Giachino et al15 | Italy | 52/126 | Consecutive patients | Plasma MMP-8 and MMP-9 | ELISA | Chest and abdominal CT with contrast |
| Lian et al16 | China | 49/179 | Consecutive patients | Acidic calponin | ELISA | CTA |
| Meng et al17 | Canada | 2/201 | Convenience sampling | Troponin | NR | CTA |
| Morello et al18 | Italy | 201/999 | Convenience sampling | LDH | Plasma LDH assay | CTA, TEE |
| Morello et al19 | Italy | 110/891 | Consecutive patients | WBC count, platelet count and fibrinogen | Assayed with automatic counter | CTA |
| Morello et al20 | Italy | 104/313 | Convenience sampling | Copeptin | BRAHMS KRYPTOR automated method | CTA; if unavailable, 14-day clinical follow-up |
| Morello et al21 | Italy | 88/297 | Not reported | sST2 | ELISA | CTA, TEE; if unavailable, 30-day clinical follow-up |
| Peng et al22 | China | 35/76 | Not reported | α-SMA, smMHC, sELAF in serum, PC1 | ELISA | CTA |
| Suzuki et al23 | Japan | 59/217 | Convenience sampling | Calponin (acidic and basic) | Sandwich-type enzyme immunoassay | Confirmed on imaging (type not specified) |
| von Kodolitsch et al24 | Germany | 128/250 | Consecutive patients | Leucocyte count | NR | CTA, MRI, TEE, digital angiography, autopsy |
| Wang et al25 | China | 114/333 | Not reported | sST2 and troponin | sST2—ELISA; troponin—NR | CT |
| Zhang et al26 | China | 323/697 | Consecutive patients | Neutrophil-to-lymphocyte ratio | Automated haematology analyser | Blinded clinical review of imaging |
CTA, CT angiography; LDH, lactate dehydrogenase; MMP, matrix metalloproteinase; NR, not reportedPC1, polycystin-1; sELAF, soluble elastin fragments; smMHC, smooth muscle myosin heavy chain; sST2, soluble suppression of tumourigenicity-2; TEE, trans-oesophageal echocardiography; WBC, white blood cellα-SMA, α-smooth muscle actin
Risk of bias and applicability assessment
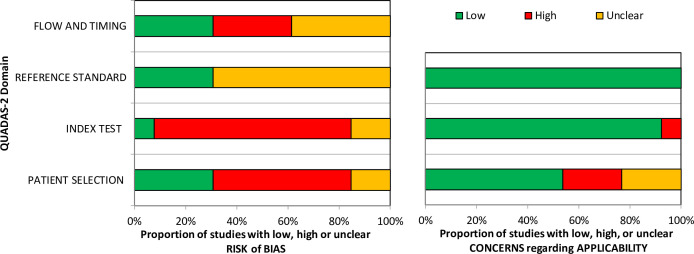
The overall methodological quality of the 13 included studies is summarised in table 2 and figure 21517,26 and in the online supplemental appendix S4. The methodological quality of the included studies was variable, with most studies having unclear or high risk of bias and applicability concerns in at least one item of the QUADAS‐2 tool. The following sections attributed a high level of bias: patient selection, primarily due to the use of convenience sampling; index test, due to the absence of prespecified threshold values and flow and timing, principally because patients received different reference standards (imaging or follow-up). The reference standard item attributed an unclear level of bias due to a lack of clarity as to whether the reference standard results were interpreted without knowledge of the index test.
Table 2. QUADAS-2 quality assessment summary—review authors’ judgements.
| Study | Risk of bias | Applicability concerns | |||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Chun and Siu14 | High | Unclear | Unclear | Unclear | Low | Low | Low |
| Giachino et al15 | Low | High | Unclear | Unclear | Low | Low | Low |
| Lian et al16 | Low | High | Unclear | Unclear | Low | Low | Low |
| Meng et al17 | High | High | Unclear | Unclear | High | High | Low |
| Morello et al18 | High | Low | Low | Low | Unclear | Low | Low |
| Morello et al19 | Low | High | Low | Low | Low | Low | Low |
| Morello et al20 | High | High | Low | High | Low | Low | Low |
| Morello et al21 | Unclear | High | Low | High | Low | Low | Low |
| Peng et al22 | High | High | Unclear | Low | High | Low | Low |
| Suzuki et al23 | High | High | Unclear | Unclear | Unclear | Low | Low |
| von Kodolitsch et al24 | Unclear | Unclear | Unclear | High | Unclear | Low | Low |
| Wang et al25 | High | High | Unclear | Low | High | Low | Low |
| Zhang et al26 | Low | High | Unclear | High | Low | Low | Low |
QUADAS-2Quality Assessment of Diagnostic Accuracy Studies 2
Figure 2. Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) assessment summary graph—review authors’ judgements.
Diagnostic performance of alternate biomarkers
The accuracy results (sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios and area under the receiver operating characteristic (AUROC) of the 13 included studies are summarised in table 3 and online supplemental appendix S5. The AUROC for the biomarkers was generally modest, with high sensitivity with tight CIs achieved only when a threshold was used that resulted in low specificity. In general, the alternative biomarkers did not achieve the sensitivity and sensitivity of D-dimer reported in recent meta-analysis.3 There were two exceptions. Wang et al25 reported 99.1% sensitivity and 84.9% specificity for soluble ST2, but this differed markedly from the sensitivity of 58% and specificity of 70.8% reported by Morello et al.21 Chun and Siu14 reported 95.6% sensitivity and 56.1% specificity for neutrophil count, but this has not been confirmed by other studies. Accuracy improved in the event the biomarkers were combined with D-dimer but was not clearly superior to D-dimer alone.
Table 3. Summary of accuracy results (sensitivity, specificity and AUROC) along with their respective 95% CIs and cut-off values where provided for the biomarkers investigated in the 13 included cohort studies.
| Study | Biomarker | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | AUROC | 95% CI | Cut-off* |
| Chun and Siu14 | Neutrophil count (2–8 hours after symptom onset) | 94.8 | 84.7 to 98.6 | 59.4 | 50 to 68.4 | NR | NR | ≥6.2×109/L |
| Neutrophil count (8–24 hours) | 96.9 | 82 to 99.8 | 45 | 29.6 to 61.3 | NR | NR | ||
| Neutrophil count (2–24 hours) | 95.6 | 88.4 to 98.6 | 56.1 | 47.9 to 63.9 | NR | NR | ||
| Neutrophil count (2–24 hours) and ADD-RS ≤1) | 94.6 | 84.2 to 98.6 | 52.3 | 42.1 to 61.9 | NR | NR | ||
| Giachino et al15 | MMP-8 | 100 | 93.2 to 100 | 9.5 | 3.9 to 18.5 | 0.75 | NR | 3.6 ng/mL |
| MMP-9 | 96.2 | 86.8 to 99.5 | 16.2 | 8.7 to 26.6 | 0.7 | NR | 20 ng/mL | |
| D-dimer | 97.6 | 87.4 to 99.9 | 32.8 | 21.3 to 46 | 0.87 | 0.8 to 0.94 | 500 ng/mL | |
| Log2 D-dimer and MMP-8 | 100 | 91.6 to 100 | 13.1 | 5.8 to 24.2 | 0.89 | 0.82 to 0.95 | >0.77 | |
| Lian et al16 | Acidic calponin | 77.6 | NR | 87.7 | NR | 0.889 | NR | 6.96 ng/mL |
| Acidic calponin+ascending aortic root dilation | 83.7 | NR | 89.2 | NR | 0.927 | NR | Calponin 6.96 ng/mL and diameter >40 mm | |
| Meng et al17 | Troponin | 16.7 | NR | 76.7 | NR | NR | NR | >0.04 µg/mL (old), ≥30 µg/L (new) |
| D-dimer | 100 | NR | 51.3 | NR | NR | NR | >500 ng/mL | |
| Morello et al18 | LDH | 44 | 37 to 51 | 73 | 69 to 76 | 0.61 | 0.57 to 0.66 | 450 U/L |
| Morello et al19 | WBC count | 67.3 | 57.7 to 75.9 | 59 | 55.5 to 62.5 | 0.69 | 0.63 to 0.74 | >9×103/μL |
| Platelet count | 68.2 | 58.6 to 76.7 | 56.2 | 52.7 to 59.7 | 0.64 | 0.58 to 0.69 | >200×103/μL | |
| Fibrinogen | 50.9 | 41.2 to 60.2 | 63.6 | 60.2 to 67 | 0.62 | 0.55 to 0.68 | <350 mg/dL | |
| ≥1 alteration(s) | 95.5 | 89.7 to 98.5 | 18.3 | 15.7 to 21.2 | NR | NR | N/A | |
| Morello et al20 | Copeptin | 78.8 | 70.1 to 85.6 | 74.6 | 68.3 to 80.1 | 0.81 (AAS) 0.83 (AAD) | 0.75 to 0.86 (AAS) 0.77 to 0.88 (AAD) | 14 pmol/L |
| D-dimer | 95.2 | 88.3 to 98.1 | 65.4 | 58.5 to 71.8 | 0.92 | 0.89 to 0.96 | ≥500 ng/mL | |
| Copeptin+D-dimer | 95.2 | 88.3 to 98.1 | 46.6 | 39.7 to 53.7 | 0.92 | 0.88 to 0.95 | D-dimer <500 ng/mL and copeptin <10 pmol/L | |
| Morello et al21 | sST2 | 58 | 47 to 68.4 | 70.8 | 64.1 to 76.9 | 0.675 | 0.61 to 0.736 | 39.8 ng/mL |
| 0.717 (when low pretest risk) | 0.655 to 0.772 | |||||||
| D-dimer | 95.8 | 88.1 to 99.1 | 30.7 | 19.6 to 43.7 | 0.842 | 0.753 to 0.908 | 500 ng/mL | |
| Peng et al22 | α-SMA | 54.29 | NR | 90.24 | NR | 0.62 | 0.49 to 0.76 | 49.62 ng/mL |
| smMHC | 68.57 | NR | 90.24 | NR | 0.81 | 0.71 to 0.91 | 2.11 ng/mL | |
| sELAF | 82.86 | NR | 68.29 | NR | 0.82 | 0.73 to 0.91 | 97.07 ng/mL | |
| PC1 | 85.71 | NR | 75.61 | NR | 0.9 | 0.83 to 0.96 | 357.33 pg/mL | |
| 1 variable positive | 100 | NR | 53.66 | NR | NR | NR | N/A | |
| 2 variables positive | 94.29 | NR | 85.37 | NR | 0.95 | 0.87 to 0.99 | N/A | |
| 3 variables positive | 77.14 | NR | 95.12 | NR | NR | NR | N/A | |
| All positive | 51.43 | NR | 100 | NR | NR | NR | N/A | |
| D-dimer | 80 | NR | 90.21 | NR | 0.93 | 0.87 to 0.98 | >2110 ng/mL | |
| Suzuki et al23 | Acidic calponin (initial 6 hours) | 50 | NR | 87 | NR | 0.63 | NR | 2.8 ng/L |
| Acidic calponin (initial 24 hours) | 58 | NR | 72 | NR | 0.63 | NR | 2.3 ng/L | |
| Basic calponin (initial 6 hours) | 63 | NR | 73 | NR | 0.67 | NR | 159 ng/L | |
| Basic calponin (initial 24 hours) | 50 | NR | 66 | NR | 0.58 | NR | 139 ng/L | |
| von Kodolitsch et al24 | Leucocyte count | 25.8 | NR | 77.9 | NR | NR | NR | ≥15×109/L |
| Wang et al25 | Soluble ST2 | 99.1 | NR | 84.9 | NR | 0.97 | 0.95 to 0.98 | 34.6 ng/mL |
| Troponin | NR | NR | NR | NR | 0.5 | 0.44 to 0.56 | NR | |
| D-dimer | 93.9 | NR | 78.5 | NR | 0.91 | 0.88 to 0.94 | 323 ng/mL | |
| Zhang et al26 | Neutrophil-to-lymphocyte ratio | 76 | 71 to 81 | 79 | 74 to 83 | 0.845 | 0.816 to 0.871 | NR |
| D-dimer | 74 | 69 to 79 | 76 | 72 to 80 | 0.822 | 0.792 to 0.850 | NR |
Morello et al18 and Chun and Siu 202314 used the standard laboratory cut-offs; Giachino et al 2013,15 Morello et al 2018,20 Morello et al 2020,21 Peng et al 2015,22 Wang et al 201825 and Lian et al 202316 all used accuracy data to determine the optimal cut-off and von Kodolitsch et al 200024 and Zhang et al 202326 did not record how the cut-off was reached.
ADD-RS, aortic dissection detection risk score; LDH, lactate dehydrogenase; MMP, matrix metalloproteinase; N/Anot availableNR, not reportedPC1, polycystin-1; sELAF, soluble elastin fragments; smMHC, smooth muscle myosin heavy chain; sST2, soluble suppression of tumourigenicity-2; WBC, white blood cellα-SMA, α-smooth muscle actin
Discussion
Summary of results
This systematic review has shown that biomarkers for AAS, other than D-dimer, currently have insufficient evidence of acceptable accuracy to support routine clinical use. We identified numerous studies evaluating many different biomarkers, but the quality and heterogeneity of the studies limited the conclusions we could draw. The estimates of sensitivity, specificity and AUROC from the cohort studies generally suggested poor diagnostic accuracy for AAS and thus these biomarkers have no current role in clinical practice.
We are aware of one additional review of alternative biomarkers for AAS.64 Chen et al concluded that microRNA biomarkers may have better specificity than D-dimer but current studies are insufficient. Their review included case-control studies, which are known to overestimate diagnostic accuracy,10 and undertook limited quality assessment of included studies, so any conclusions should be interpreted with caution.
Interpretation of results
Soluble ST2 is primarily found in inflammatory processes and T-cell-mediated immune responses. The studies of soluble ST2 produced conflicting results, with Wang et al25 reporting diagnostic accuracy superior to that of D-dimer but Morello et al21 reporting modest accuracy. These differences could be attributed to differences in ethnicity and age across the two study populations. Further studies are required to determine accuracy in an appropriate cohort.
MMPs are key to aortic remodelling and part of the family of extracellular matrix markers. The study of Giachino et al15 suggested modest accuracy for AAS, but the combination of D-dimer and MMP-8 improved the sensitivity of D-dimer at the expense of specificity. Morello et al20 reported that copeptin, a biomarker released by the neurohypophysis in response to stress, provided suboptimal diagnostic accuracy for AAS. Suzuki et al23 reported that calponin, an analogue of cardiac troponin and released during muscle fibre apoptosis, had modest diagnostic accuracy for AAS. This was replicated by Lian et al,16 who demonstrated that including ascending aortic root dilation of >40 mm significantly increased the accuracy of calponin.
Peng et al22 focused on two distinct groups of biomarkers: smooth muscle biomarkers (α-SMA, smMHC and PC1) and extracellular matrix markers (sELAF). smMHC has been found to be released from damaged aortic medial muscle cells during aortic dissection, while PC1 plays a key role in stability and integrity of aortic vessel walls. sELAF is released on rupture of elastic vascular wall fibres. In isolation, none of these markers offered superior performance to D-dimer, but the combination of three of the biomarkers alongside D-dimer could offer better accuracy than D-dimer alone. This requires validation in a new cohort.
Some of the biomarkers evaluated for AAS are already used in routine clinical assessment for other conditions. Morello et al19 showed that WBC count, platelet count and fibrinogen are not accurate biomarkers for AAS but the modest diagnostic information they provide could be used in pretest probability assessment, while the study of von Kodolitsch et al24 suggested that leucocyte count provided no useful diagnostic information. Similarly, LDH was found to have poor diagnostic accuracy for AAS.18 The study of Zhang et al26 suggested that the neutrophil-to-lymphocyte ratio may have similar or superior accuracy to D-dimer in the diagnosis of AAS.26 This finding requires replication in other studies. Two studies of troponin showed no diagnostic value for AAS.17 25 Chun and Siu14 reported similar accuracy data to that of D-dimer, although these findings will require validation in further studies.
Strengths and limitations of the systematic review
We used established and robust methods to ensure our review was comprehensive and involved objective assessment of study quality. However, it may have some limitations. Indexing and reporting of diagnostic studies may be suboptimal, especially if biomarker analysis is a secondary study objective, so we may have missed some potentially relevant data. The primary studies had important limitations, with insufficient numbers of cohort studies evaluating any individual biomarker to support meta-analysis and potential biases affecting patient selection and reference standard adjudication. There was also a lack of implementation studies, threshold rationale and economic data, thus making it difficult to draw conclusions regarding the practicality and affordability of clinical implementation of these alternative biomarkers. Furthermore, we noted that a number of our selected cohort studies reported high AAS prevalence, compared with an unselected population with possible AAS, such as reported in the Diagnosis of Acute Aortic Syndrome in the Emergency Department (DAShED) study.65 This likely represents selection of patients who received a definitive imaging reference standard, which limits the applicability of findings to the unselected general population.
The QUADAS-2 assessment of the reference standard did not differentiate between studies using AD and those using AAS as the reference standard. However, between 73% and 86% of cases of AAS were AD in the cohort studies that used AAS as the reference standard, so any differences between the studies using AD and AAS are likely to be modest and unlikely to impact our conclusions.
Implications for policy, practice and future research
More research is needed on biomarkers for AAS. However, the low incidence of AAS presentations in the ED creates a substantial barrier to conducting adequately powered studies with robust designs. The researchers who undertook the studies included in this review deserve credit for their efforts, particularly the Italian researchers who provided 5 of the 13 studies. Our review highlights the need for other research teams to evaluate new biomarkers for AAS so we can determine whether findings are reproduced elsewhere. Case-control studies can provide initial data to identify biomarkers that show an association with AAS but cohort studies are required to provide data to guide clinical practice. Studies should ideally record clinical risk assessment, such as the ADD-RS, and measure D-dimer, to determine the additional contribution to diagnostic assessment of novel biomarkers. An optimal future study might involve recording the ADD-RS and taking blood for measurement of D-dimer and multiple alternative biomarkers from a large prospective cohort of patients with suspected AAS. Analysis could then determine whether and how alternative biomarkers provide additional diagnostic information beyond that provided by the ADD-RS and D-dimer.
Conclusions
Currently available research is insufficient to recommend any biomarker as an alternative or in addition to D-dimer in the diagnostic assessment of AAS. Large cohort studies are required to evaluate multiple biomarkers in an appropriate population with suspected AAS.
supplementary material
Acknowledgements
The authors would like to thank all additional members of the core project group for NIHR151853 for their valuable input and commentary throughout the work. The authors are indebted to Joanne Hinde for assistance with logistics and administration. The authors would also like to thank Catherine Fowler, Valerie Lechene and other members of the Aortic Dissection Charitable Trust for their help with this project.
The views expressed in this paper are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Any errors are the responsibility of the authors. The funders had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript and in the decision to submit the manuscript for publication.
Footnotes
Funding: This study was funded by the UK National Institute for Health and Care Research (project number 151853).
Provenance and peer review: Not commissioned; externally peer reviewed.
Handling editor: Richard Body
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the 'Methods' section for further details.
Contributor Information
Joshua Wren, Email: j.wren@nhs.net.
Steve Goodacre, Email: s.goodacre@sheffield.ac.uk.
Abdullah Pandor, Email: a.pandor@sheffield.ac.uk.
Munira Essat, Email: m.essat@sheffield.ac.uk.
Mark Clowes, Email: m.clowes@sheffield.ac.uk.
Graham Cooper, Email: graham@tadct.org.
Robert Hinchliffe, Email: robert.hinchliffe@bristol.ac.uk.
Matthew J Reed, Email: mattreed@ed.ac.uk.
Steven Thomas, Email: steven.thomas1@nhs.net.
Sarah Wilson, Email: sarah.wilson2@nhs.net.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Wen D, Zhou X-L, Li J-J, et al. Biomarkers in aortic dissection. Clin Chim Acta. 2011;412:688–95. doi: 10.1016/j.cca.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 2.Zhao Y, Fu W, Wang L. Biomarkers in aortic dissection: diagnostic and prognostic value from clinical research. Chin Med J. 2023:10–97. doi: 10.1097/CM9.0000000000002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essat M, Goodacre S, Pandor A, et al. Diagnostic accuracy of D-dimer for acute aortic syndromes: systematic review and meta-analysis. Ann Emerg Med. 2024 doi: 10.1016/j.annemergmed.2024.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Yao J, Bai T, Yang B, et al. The diagnostic value of D-dimer in acute aortic dissection: a meta-analysis. J Cardiothorac Surg. 2021;16:343. doi: 10.1186/s13019-021-01726-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren S, Essat M, Pandor A, et al. Diagnostic accuracy of the aortic dissection detection risk score alone or with D-dimer for acute aortic syndromes: systematic review and meta-analysis. PLOS ONE. 2024;19:e0304401. doi: 10.1371/journal.pone.0304401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting Items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319:388–96. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 7.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 8.Goodacre S, Essat M, Thokala P, et al. UK: National Institute for Health and Care Research; 2023. Diagnostic strategies for suspected acute aortic syndrome (AAS): systematic review, meta-analysis, decision-analytic modelling, and value of information analysis. [Google Scholar]
- 9.Leeflang MMG. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin Microbiol Infect. 2014;20:105–13. doi: 10.1111/1469-0691.12474. [DOI] [PubMed] [Google Scholar]
- 10.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA . 1999;282:1061–6. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 11.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med . 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie JE, Brennan SE, Ryan RE, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2019. Summarizing study characteristics and preparing for synthesis; pp. 229–40. [Google Scholar]
- 13.Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–10. doi: 10.1136/bmj.39500.677199.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun H, Siu KM. A diagnostic and screening strategy with neutrophil counts in patients with suspected aortic dissection in a certain time window. World J Emerg Med. 2023;14:307–11. doi: 10.5847/wjem.j.1920-8642.2023.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giachino F, Loiacono M, Lucchiari M, et al. Rule out of acute aortic dissection with plasma matrix metalloproteinase 8 in the emergency department. Crit Care . 2013;17:R33. doi: 10.1186/cc12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lian R, Zhang T, Liu J, et al. Routine use of a pocket-sized handheld echoscopic device plus a biomarker by emergency medicine residents with an early screening algorithm for suspected Type A acute aortic syndrome. J Clin Med. 2023;12:1346. doi: 10.3390/jcm12041346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng J, Mellnick VM, Monteiro S, et al. Acute aortic syndrome: yield of computed tomography angiography in patients with acute chest pain. Can Assoc Radiol J. 2019;70:23–8. doi: 10.1016/j.carj.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Morello F, Ravetti A, Nazerian P, et al. Plasma lactate dehydrogenase levels predict mortality in acute aortic syndromes: a diagnostic accuracy and observational outcome study. Medicine (Balt) 2016;95:e2776. doi: 10.1097/MD.0000000000002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morello F, Cavalot G, Giachino F, et al. White blood cell and platelet count as adjuncts to standard clinical evaluation for risk assessment in patients at low probability of acute aortic syndrome. Eur Heart J Acute Cardiovasc Care. 2017;6:389–95. doi: 10.1177/2048872615600097. [DOI] [PubMed] [Google Scholar]
- 20.Morello F, Oddi M, Cavalot G, et al. Prospective diagnostic and prognostic study of copeptin in suspected acute aortic syndromes. Sci Rep. 2018;8:16713. doi: 10.1038/s41598-018-35016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morello F, Bartalucci A, Bironzo M, et al. Prospective diagnostic accuracy study of plasma soluble ST2 for diagnosis of acute aortic syndromes. Sci Rep. 2020;10:3103. doi: 10.1038/s41598-020-59884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng W, Peng Z, Chai X, et al. Potential biomarkers for early diagnosis of acute aortic dissection. Heart & Lung . 2015;44:205–8. doi: 10.1016/j.hrtlng.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Distante A, Zizza A, et al. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur Heart J. 2008;29:1439–45. doi: 10.1093/eurheartj/ehn162. [DOI] [PubMed] [Google Scholar]
- 24.von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977. doi: 10.1001/archinte.160.19.2977. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Tan X, Gao H, et al. Magnitude of soluble ST2 as a Novel Biomarker for acute aortic dissection. Circulation. 2018;137:259–69. doi: 10.1161/CIRCULATIONAHA.117.030469. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Yuan N, Guo J, et al. Comparisons of potential values of D-dimer and the neutrophil- to-lymphocyte ratio in patients with suspected acute aortic syndrome. Am J Emerg Med. 2023;69:44–51. doi: 10.1016/j.ajem.2023.03.059. [DOI] [PubMed] [Google Scholar]
- 27.Ohlmann P, Faure A, Morel O, et al. Diagnostic and prognostic value of circulating D-dimers in patients with acute aortic dissection. Crit Care Med. 2006;34:1358–64. doi: 10.1097/01.CCM.0000216686.72457.EC. [DOI] [PubMed] [Google Scholar]
- 28.Xiao Z, Xue Y, Yao C, et al. Acute aortic dissection biomarkers identified using isobaric tags for relative and absolute quantitation. Biomed Res Int. 2016;2016:6421451. doi: 10.1155/2016/6421451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohle R, Um J, Anjum O, et al. High risk clinical features for acute aortic dissection: a case-control study. Acad Emerg Med. 2018;25:378–87. doi: 10.1111/acem.13356. [DOI] [PubMed] [Google Scholar]
- 30.Cakir A, Payza U, Aksun S, et al. Validity of signal peptide-CUB-EGF domain-containing protein-1 (Scube-1) in the diagnosis of aortic dissection. Signa Vitae. 2021;17:112–6. doi: 10.22514/sv.2020.16.0043. [DOI] [Google Scholar]
- 31.Goliopoulou A, Oikonomou E, Antonopoulos A, et al. Expression of tissue microRNAs in ascending aortic aneurysms and dissections. Angiol Open Access. 2023;74:88–94. doi: 10.1177/00033197221098295. [DOI] [PubMed] [Google Scholar]
- 32.Hagiwara A, Sakamoto D, Sasaki R, et al. Diagnosis of acute aortic dissection using a fibrinolytic marker. Crit Care Med. 2010;12:A178. doi: 10.1177/20480040211047122. [DOI] [Google Scholar]
- 33.Jiang Y, Tang X, Wang Y, et al. Serum oxylipin profiles identify potential biomarkers in patients with acute aortic dissection. Metabolites. 2022;12:587. doi: 10.3390/metabo12070587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li T, Zhou Y, Li D, et al. The role of genome-scale leukocyte long noncoding RNA in identifying acute aortic dissection. Signa Vitae. 2022;18:101–10. doi: 10.22514/sv.2022.030. [DOI] [Google Scholar]
- 35.Lu P, Feng X, Li R, et al. A novel serum biomarker model to discriminate aortic dissection from coronary artery disease. Dis Markers. 2022;2022:9716424. doi: 10.1155/2022/9716424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinohara T, Suzuki K, Okada M, et al. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol. 2003;23:1839–44. doi: 10.1161/01.ATV.0000085016.02363.80. [DOI] [PubMed] [Google Scholar]
- 37.Song R, Xu N, Luo L, et al. Diagnostic value of aortic dissection risk score, coagulation function, and laboratory indexes in acute aortic dissection. Biomed Res Int. 2022;2022:7447230. doi: 10.1155/2022/7447230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki T, Katoh H, Watanabe M, et al. Novel biochemical diagnostic method for aortic dissection. Results of a prospective study using an immunoassay of smooth muscle myosin heavy chain. Circulation. 1996;93:1244–9. doi: 10.1161/01.cir.93.6.1244. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z, Wei M, Guo X, et al. Changes of serum D-dimer, NT-proBNP, and Troponin I levels in patients with acute aortic dissection and the clinical significance. Evid Based Complement Alternat Med. 2022;2022:1–5. doi: 10.1155/2022/8309505. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Zhao G, Zhao Y, Zhang H. Value of duration of chest pain, troponin, and D-dimer in differentiating acute high-risk chest pain patient. Acta Med Mediterr. 2020;36:1587–91. [Google Scholar]
- 41.Cheng N, Wang H, Zhang W, et al. Comparative proteomic investigation of plasma reveals novel potential biomarker groups for acute aortic dissection. Dis Markers. 2020;2020:4785068. doi: 10.1155/2020/4785068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong J, Bao J, Feng R, et al. Circulating microRNAs: a novel potential biomarker for diagnosing acute aortic dissection. Sci Rep. 2017;7:12784. doi: 10.1038/s41598-017-13104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong J, Duan X, Feng R, et al. Diagnostic implication of fibrin degradation products and D-dimer in aortic dissection. Sci Rep. 2017;7:43957. doi: 10.1038/srep43957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eggebrecht H, Naber CK, Bruch C, et al. Value of plasma fibrin D-dimers for detection of acute aortic dissection. J Am Coll Cardiol. 2004;44:804–9. doi: 10.1016/j.jacc.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 45.Forrer A, Schoenrath F, Torzewski M, et al. Novel blood biomarkers for a diagnostic workup of acute aortic dissection. Diagnostics (Basel) 2021;11:615. doi: 10.3390/diagnostics11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagiwara A, Shimbo T, Kimira A, et al. Using fibrin degradation products level to facilitate diagnostic evaluation of potential acute aortic dissection. J Thromb Thrombolysis. 2013;35:15–22. doi: 10.1007/s11239-012-0779-6. [DOI] [PubMed] [Google Scholar]
- 47.Han C, Liu Q, Li Y, et al. S100A1 as a potential biomarker for the diagnosis of patients with acute aortic dissection. J Int Med Res. 2021;49:3000605211004512. doi: 10.1177/03000605211004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazui H, Fukumoto H, Negoro N, et al. Simple and useful tests for discriminating between acute aortic dissection of the ascending aorta and acute myocardial infarction in the emergency setting. Circ J . 2005;69:677–82. doi: 10.1253/circj.69.677. [DOI] [PubMed] [Google Scholar]
- 49.König C, Lahm H, Dreßen M. Aggrecan: a new biomarker for acute thoracic aortic dissection. 50th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery (DGTHG); 2021. [DOI] [Google Scholar]
- 50.Li T, Jiang B, Li X, et al. Serum matrix metalloproteinase-9 is a valuable biomarker for identification of abdominal and thoracic aortic aneurysm: a case-control study. BMC Cardiovasc Disord. 2018;18:202. doi: 10.1186/s12872-018-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Zheng X, Su X, et al. Plasma resistin levels in patients with acute aortic dissection: a propensity score-matched observational case-control study. Med Sci Monit. 2018;24:6431–7. doi: 10.12659/MSM.909469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okazaki T, Yamamoto Y, Yoda K, et al. The ratio of D-dimer to brain natriuretic peptide may help to differentiate between cerebral infarction with and without acute aortic dissection. J Neurol Sci. 2014;340:133–8. doi: 10.1016/j.jns.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Pan X, Zhou Y, Yang G, et al. Lysophosphatidic acid may be a novel biomarker for early acute aortic dissection. Front Surg. 2021;8:789992. doi: 10.3389/fsurg.2021.789992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sbarouni E, Georgiadou P, Analitis A, et al. High neutrophil to lymphocyte ratio in type A acute aortic dissection facilitates diagnosis and predicts worse outcome. Expert Rev Mol Diagn. 2015;15:965–70. doi: 10.1586/14737159.2015.1042367. [DOI] [PubMed] [Google Scholar]
- 55.Sbarouni E, Georgiadou P, Kosmas E, et al. Platelet to lymphocyte ratio in acute aortic dissection. J Clin Lab Anal. 2018;32:e22447. doi: 10.1002/jcla.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sbarouni E, Georgiadou P, Marathias A, et al. D-dimer and BNP levels in acute aortic dissection. Int J Cardiol. 2007;122:170–2. doi: 10.1016/j.ijcard.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 57.Xu Z, Wang Q, Pan J, et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Sci Rep. 2017;7:13659. doi: 10.1038/s41598-017-13696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan S-M, Shi Y-H, Wang J-J, et al. Elevated plasma D-dimer and hypersensitive C-reactive protein levels may indicate aortic disorders. Rev Bras Cir Cardiovasc. 2011;26:573–81. doi: 10.5935/1678-9741.20110047. [DOI] [PubMed] [Google Scholar]
- 59.Zeng Q, Rong Y, Li D, et al. Identification of serum biomarker in acute aortic dissection by global and targeted metabolomics. Ann Vasc Surg. 2020;68:497–504. doi: 10.1016/j.avsg.2020.06.026. [DOI] [PubMed] [Google Scholar]
- 60.Fletcher A, Syed MBJ, Iskander Z, et al. Plasma desmosine as a biomarker in acute aortic syndrome. Eur Heart J. 2021;42 doi: 10.1093/eurheartj/ehab724.2011. [DOI] [Google Scholar]
- 61.Wagner A, Domanovits H, Holzer M, et al. Plasma endothelin in patients with acute aortic disease. Resuscitation. 2002;53:71–6. doi: 10.1016/s0300-9572(01)00502-0. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki T, Trimarchi S, Sawaki D, et al. Circulating transforming growth factor-beta levels in acute aortic dissection. J Am Coll Cardiol. 2011;58:775. doi: 10.1016/j.jacc.2010.01.079. [DOI] [PubMed] [Google Scholar]
- 63.Ziyu Z, Zi Y, Jialin Y. Value of D-dimer for detection of acute aortic dissection. Heart. 2012;98:E268. doi: 10.1136/heartjnl-2012-302920u.4. [DOI] [Google Scholar]
- 64.Chen H, Li Y, Li Z, et al. Diagnostic biomarkers and aortic dissection: a systematic review and meta-analysis. BMC Cardiovasc Disord . 2023;23:497. doi: 10.1186/s12872-023-03448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLatchie R, Reed MJ, Freeman N, et al. Diagnosis of Acute Aortic Syndrome in the Emergency Department (DAShED) study: an observational cohort study of people attending the emergency department with symptoms consistent with acute aortic syndrome. Emerg Med J . 2024;41:136–44. doi: 10.1136/emermed-2023-213266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.