Abstract
The Friend spleen focus-forming virus (SFFV) encodes a unique envelope glycoprotein, gp55, which allows erythroid cells to proliferate and differentiate in the absence of erythropoietin (Epo). SFFV gp55 has been shown to interact with the Epo receptor complex, causing constitutive activation of various signal-transducing molecules. When injected into adult mice, SFFV induces a rapid erythroleukemia, with susceptibility being determined by the host gene Fv-2, which was recently shown to be identical to the gene encoding the receptor tyrosine kinase Stk/Ron. Susceptible, but not resistant, mice encode not only full-length Stk but also a truncated form of the kinase, sf-Stk, which may mediate the biological effects of SFFV infection. To determine whether expression of SFFV gp55 leads to the activation of sf-Stk, we expressed sf-Stk, with or without SFFV gp55, in hematopoietic cells expressing the Epo receptor. Our data indicate that sf-Stk interacts with SFFV gp55 as well as gp55P, the biologically active form of the viral glycoprotein, forming disulfide-linked complexes. This covalent interaction, as well as noncovalent interactions with SFFV gp55, results in constitutive tyrosine phosphorylation of sf-Stk and its association with multiple tyrosine-phosphorylated signal-transducing molecules. In contrast, neither Epo stimulation in the absence of SFFV gp55 expression nor expression of a mutant of SFFV that cannot interact with sf-Stk was able to induce tyrosine phosphorylation of sf-Stk or its association with any signal-transducing molecules. Covalent interaction of sf-Stk with SFFV gp55 and constitutive tyrosine phosphorylation of sf-Stk can also be detected in an erythroleukemia cell line derived from an SFFV-infected mouse. Our results suggest that SFFV gp55 may mediate its biological effects in vivo by interacting with and activating a truncated form of the receptor tyrosine kinase Stk.
The Friend spleen focus-forming virus (SFFV) causes an acute erythroleukemia in susceptible strains of mice (for a review, see reference 37). SFFV encodes a unique envelope glycoprotein, gp55, that associates specifically with the erythropoietin receptor (EpoR) at the cell surface (4, 10, 21, 45), allowing erythroid cells to proliferate in the absence of erythropoietin (Epo), the normal regulator of erythropoiesis. Epo stimulation of the EpoR activates a number of signal transduction pathways, including the Jak-Stat, the Ras/Raf-1/mitogen-activated protein kinase (MAPK), and the phosphatidylinositol 3-kinase (PI3-kinase) pathways (for a review, see reference 46). Using the Epo-dependent erythroleukemia cell line HCD-57, we have previously demonstrated that infection with SFFV, which abrogates the Epo dependence of these cells (36), constitutively activates Stat DNA-binding activity (30); Ras (27); Raf-1, MEK, and MAPK (26); PI 3-kinase and Akt kinase (29); and protein kinase C (27).
Although interaction of the SFFV envelope glycoprotein with the EpoR complex is essential for inducing the biological effects of the virus, other factors must also be involved, since not all strains of mice are susceptible to SFFV-induced erythroleukemia. A number of polymorphic mouse genes have been identified that affect susceptibility to Friend SFFV-induced erythroleukemia. Most of these host genes interfere with viral entry or integration, or they control immune responses against the virus-infected cells (for a review, see reference 7). However, the Fv-2 gene (22), which is located on mouse chromosome 9, acts at the level of the erythroid target cell for the virus (2, 3, 9, 13, 39). Mice carrying at least one copy of the Fv-2s allele are susceptible to SFFV-induced erythroleukemia, and their erythroid cells will form Epo-independent erythroid bursts when infected in vitro with SFFV. In contrast, mice homozygous for the Fv-2r allele fail to develop SFFV-induced erythroleukemia, and SFFV infection of erythroid cells from these mice does not result in the formation of Epo-independent erythroid bursts. Thus, erythroid cells from Fv-2-resistant mice appear to lack a component necessary for mediating the biological effects of SFFV infection. Although earlier studies suggested that there were differences between Fv-2-susceptible and -resistant erythroid cells in cell cycling (42), it was unclear how this affected the ability of SFFV to alter the growth of these cells. Recently, however, it was shown that the Fv-2 gene is identical to the gene encoding the Met-related tyrosine kinase Stk/Ron (33). Mice that carry the Fv-2s allele express both a full-length Stk and a truncated form of the kinase, sf-Stk, which is expressed from an alternate promoter. sf-Stk lacks almost the entire extracellular domain but retains the transmembrane and tyrosine kinase domains. In contrast to Fv-2s mice, mice that are homozygous for the Fv-2r allele express only full-length Stk due to a 3-nucleotide deletion in the alternate promoter. Transfer of bone marrow cells expressing sf-Stk to Fv-2rr mice confers susceptibility to SFFV-induced erythroleukemia, while targeted disruption of the Stk gene in Fv-2s mice results in resistance to disease (33). Thus, expression of sf-Stk in erythroid cells appears to play a critical role in determining the biological consequences of SFFV infection in the mouse. We, therefore, carried out studies to determine if SFFV gp55 interacts with sf-Stk and whether this interaction results in the activation of this truncated kinase and the downstream activation of signal transduction pathways.
MATERIALS AND METHODS
Plasmids.
The gp55-encoding sequence from SFFVA-FC, which exerts effects identical to SFFVp (5), was PCR amplified and cloned into the PCR-Script plasmid (Stratagene, La Jolla, Calif.). The BamHI-NotI fragment was inserted into the pMX retroviral vector (kindly provided by T. Kitamura, University of Tokyo, Tokyo, Japan) to create pMX-A-FC. To achieve high levels of expression of the sf-Stk protein that can be followed by fluorescence-activated cell sorting (FACS), we generated a bicistronic retroviral vector, pMX-IRES-EGFP, by inserting the encephalomyocarditis virus internal ribosome entry sequence (IRES) in front of the gene encoding enhanced green fluorescent protein (EGFP). sf-Stk was amplified on an Stk cDNA template (kindly provided by A. Danilkovich, National Cancer Institute, Frederick, Md., with permission of T. Suda) by PCR amplification with the following primer pairs: 5′-TGTGGCAGACTGTGTGACTGTG-3′ and 5′-CTAGTGCCTGTGGCCTACTCAGGGC-3′. The PCR product was cloned into the pCR-Blunt II-TOPO vector (Invitrogen, Carlsbad, Calif.), and the BamHI-NotI fragment of sf-Stk was inserted in front of the IRES sequence in the pMX-IRES-EGFP vector.
Cell lines.
The erythroleukemia cell lines DS19 and HCD-57 were maintained as previously described (36). BaF3-EpoR cells, which are BaF3 cells engineered to express the murine EpoR (BER28C kindly provided by H. Amanuma, RIKEN, Tsukuba, Japan) (50), were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 50 μM 2-mercaptoethanol, and 2 U of Epo/ml. BaF3-EpoR cells expressing SFFV gp55 or sf-Stk were obtained by infecting these cells with supernatants from BOSC 23 cells (32) (American Type Culture Collection, Manassas, Va.) that had been transfected 48 to 72 h earlier using Lipofectamine (Gibco/BRL, Gaithersburg, Md.) with 3 μg of plasmid DNA encoding SFFV gp55 or sf-Stk. Expression of SFFV gp55 or sf-Stk in the BOSC 23 cells was verified by Western blot analysis using an anti-SFFV gp55 monoclonal antibody (7C10) (47) or an anti-Stk polyclonal antibody. BaF3-EpoR cells infected with the SFFV gp55-expressing virus (pMX-A-FC) were grown in medium without Epo for selection of factor-independent cells. For establishing BaF3-EpoR cells expressing the mutant SFFV BB6, BaF3-EpoR cells were first infected with a mixture of SFFV BB6 clone13 (25) (kindly provided by R. Geib, Terre Haute, Id.) and Friend murine leukemia virus (MuLV) helper virus and then maintained in medium without Epo for selection of factor-independent growth. Cells infected with the sf-Stk-expressing virus (pMX-SF-STK-IRES-EGFP) were sorted by FACS to select those cells expressing high levels of EGFP. This technique allowed the isolation of cells stably expressing high levels of the sf-Stk protein.
Generation of polyclonal antibodies against Stk.
An antiserum against Stk was raised in rabbits by immunization with a keyhole limpet hemocyanin (KLH)-conjugated synthetic polypeptide corresponding to the C-terminal 16 amino acids (RSTSKPRPLSEPPLPT; amino acids 1363 to 1378) of the Stk protein.
Protein analysis.
To prepare cell lysates, cells were washed and resuspended in lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM Na3 VO4, and aprotinin and leupeptin at 1 μg/ml each). For some experiments, cells were starved in RPMI 1640 plus 0.5% bovine serum albumin for 7 h and either stimulated for 15 min with 10 U of Epo/ml or left unstimulated before being washed and resuspended in lysis buffer. Cell extracts were subjected to immunoprecipitation as described previously (29) with either anti-Stk (see above), an anti-phosphotyrosine antibody (4G10) cross-linked to protein A-agarose (Upstate Biotechnology, Lake Placid, N.Y.), anti-EpoR (SC-697, from Santa Cruz Biotechnology, Santa Cruz, Calif.), anti-Shc (S14630 or S68020, from Transduction Laboratories, Lexington, Ky.), anti-SHIP (SC-1964, from Santa Cruz) or anti-Cbl (SC-170, from Santa Cruz). Western blot analysis was conducted using anti-phosphotyrosine (4G10; Upstate Biotechnology), an anti-gp55 monoclonal antibody (7C10) (47), or the antibodies listed above. An anti-PU.1 antibody (SC-352, from Santa Cruz) was used as a control. Immunoprecipitated proteins were separated by electrophoresis on 8% or 10% Tris-glycine minigels (Invitrogen) under reducing conditions (with 3.52 × 10−2 M 2-mercaptoethanol) or nonreducing conditions (without 2-mercaptoethanol). Separated proteins were then transferred electrophoretically to nitrocellulose filters for Western blotting with specific antisera and visualization using enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, N.J.).
RT-PCR analysis.
To examine Stk expression, we obtained total RNA from DS19, HCD-57, and BaF3-EpoR cells using RNA STAT-60 (Tel-Test, Inc., Friendswood, Tex.). Reverse transcription and PCR analysis (RT-PCR analysis) were performed with the Titan One Tube RT-PCR System (Boehringer Mannheim Corp., Indianapolis, Id.). PCR primers for Stk were 5′-CAGCAGTGGACAGCCTGTTCA-3′ and 5′-ATGCCTTCCACTCGGAAGTGC-3′. sf-Stk-specific primers were 5′-TCTGGCTGATCCTTCTGTCTG-3′ and 5′-GCAGCAGTGGGACACTTGTCC-3′ (33). PCR primers for β-actin were obtained from Stratagene.
RESULTS
Expression of full-length and truncated Stk in EpoR-expressing hematopoietic cell lines.
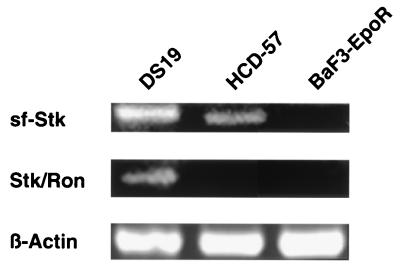
It has previously been demonstrated that infection with SFFV induces factor independence in the Epo-dependent erythroleukemia cell line HCD-57 as well as in BaF3 and other interleukin-3 (IL-3)-dependent hematopoietic cell lines engineered to express the EpoR (19, 20, 36). In order to determine whether these cells express full-length Stk (Stk/Ron) and/or sf-Stk, we performed RT-PCR using specific primer pairs. As shown in Fig. 1, both full-length and truncated Stk can be detected in DS19 cells, an erythroleukemia cell line derived from an SFFV-infected mouse, consistent with the findings of an earlier study (16). HCD-57 cells were also shown to express sf-Stk, although full-length Stk could not be detected in these cells. In contrast, BaF3-EpoR cells failed to express either full-length Stk or sf-Stk. This finding was confirmed by Southern blot analysis using an Stk probe (data not shown). Since BaF3-EpoR cells fail to express either full-length Stk or sf-Stk, we used this cell line to express these proteins and study their potential interaction with SFFV gp55.
FIG. 1.
Expression of full-length and truncated Stk in EpoR-expressing cell lines. Total RNA was obtained from DS19, an erythroleukemia cell line derived from an SFFV-infected mouse; HCD-57, an Epo-dependent mouse erythroleukemia cell line; and BaF3-EpoR, an IL-3-dependent pro-B-cell line engineered to express the EpoR. RT-PCR using specific primer pairs was then carried out to examine expression of full-length Stk (Stk/Ron) or truncated Stk (sf-Stk).
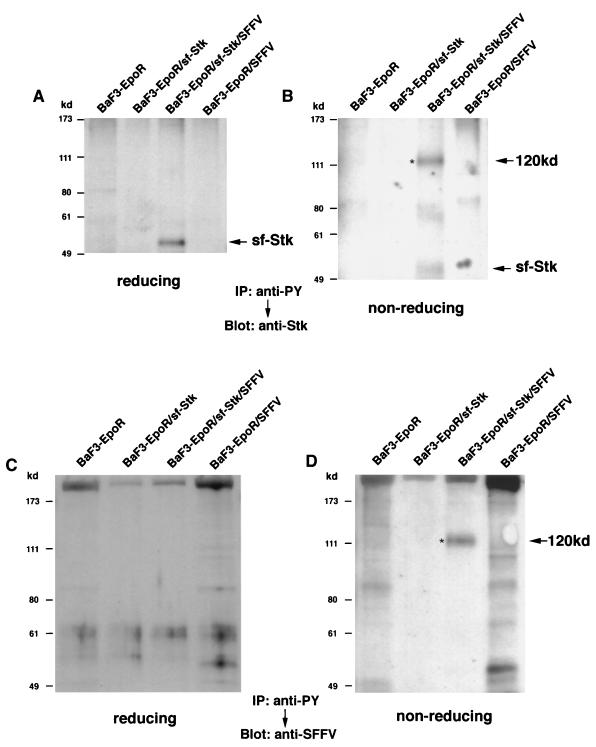
Analysis under reducing and nonreducing conditions of BaF3-EpoR cells engineered to express sf-Stk and/or SFFV gp55.
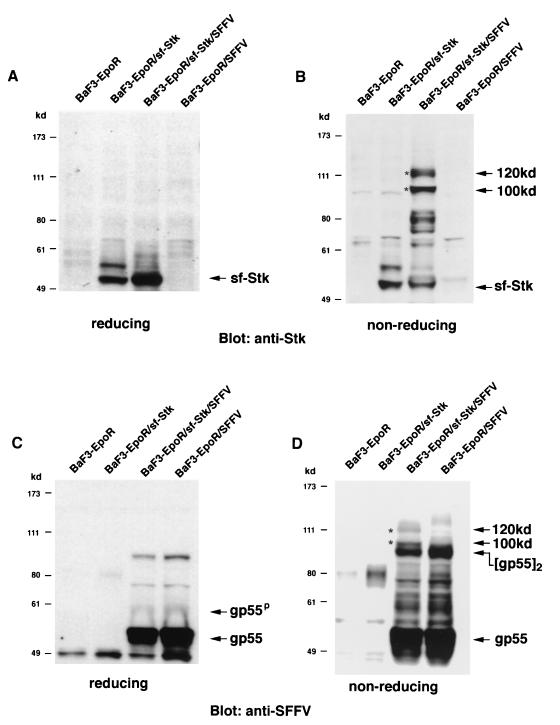
To determine whether SFFV gp55 interacts with sf-Stk, we engineered BaF3-EpoR cells to stably express sf-Stk in the absence or presence of SFFV gp55. Expression of sf-Stk did not change the growth requirements of BaF3-EpoR cells, which require Epo or IL-3 to proliferate, or those of BaF3-EpoR cells expressing SFFV gp55, which grow in the absence of Epo. As shown in Fig. 2A, when cell lysates from BaF3-EpoR cells expressing sf-Stk and from BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 were electrophoresed under reducing conditions and then immunoblotted with an antiserum against the C-terminal region of Stk, sf-Stk migrated as a 52-kDa protein. In contrast, when the proteins were separated under nonreducing conditions, an anti-Stk antiserum also detected 100- and 120-kDa proteins, but only in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 (Fig. 2B). Densitometer scans of this lane of Fig 2B indicate that the majority (72%) of the sf-Stk detected in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 is present in these high-molecular-weight complexes, which disappeared under reducing conditions (Fig. 2A).
FIG. 2.
Analysis of BaF3-EpoR cells engineered to express sf-Stk and SFFV gp55. Cell lysates from BaF3-EpoR cells, BaF3-EpoR cells expressing sf-Stk, BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, and BaF3-EpoR cells expressing SFFV gp55 were separated by electrophoresis under reducing (A and C) or nonreducing (B and D) conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with an antiserum against the C-terminal region of Stk (A and B) or against 7C10, a monoclonal antibody specific for SFFV gp55 (C and D). Asterisks indicate 100- and 120-kDa complexes.
Cell lysates separated under reducing and nonreducing conditions were also immunoblotted with a monoclonal antibody that specifically detects SFFV gp55. Under reducing conditions (Fig. 2C), the unprocessed monomer of gp55 was detected in BaF3-EpoR cells expressing SFFV gp55 and in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. In addition, the processed plasma membrane form of SFFV gp55 (gp55P) was detected in these cells. Under nonreducing conditions (Fig. 2D), a band corresponding to the disulfide-bonded form of gp55, [gp55]2, was detected in SFFV gp55-expressing cells (right two lanes). Interestingly, the SFFV gp55-specific antibody also detected 100- and 120-kDa proteins, but only in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 (Fig. 2D), suggesting that they may be heterodimers of SFFV gp55 and sf-Stk. Densitometer scans of this lane of Fig. 2D indicate that approximately 20% of the SFFV gp55 was present in 100- and 120-kDa complexes, which disappeared under reducing conditions (Fig. 2C). When the blot was stripped and reprobed with an anti-Stk antiserum, the same 100- and 120-kDa proteins were detected (data not shown). In contrast to the results obtained by coexpressing SFFV gp55 and truncated Stk, coexpression of SFFV gp55 and full-length Stk did not result in formation of a complex between the two proteins (data not shown).
Interaction between SFFV gp55 and sf-Stk.
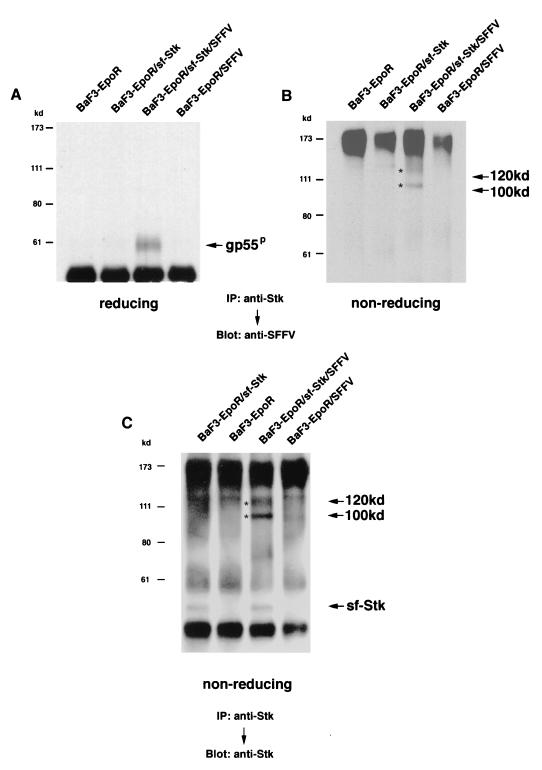
In order to determine whether or not SFFV gp55 and sf-Stk physically interact, cell lysates from BaF3-EpoR cells expressing both proteins were immunoprecipitated with an anti-Stk antiserum and then immunoblotted with the anti-SFFV gp55 monoclonal antibody 7C10. As shown in Fig. 3A, when the proteins are separated under reducing conditions, a band corresponding to SFFV gp55P was detected only in the anti-Stk immunoprecipitate from the BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. A shorter exposure showed that the unprocessed form of gp55, which migrates with the heavy chain band of immunoglobulin, was also precipitated from these cells with the anti-Stk antiserum (data not shown). Under nonreducing conditions (Fig. 3B), 100- and 120-kDa proteins which specifically react with the SFFV gp55 monoclonal antibody were detected in the anti-Stk immunoprecipitate from BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. The 100- and 120-kDa proteins disappeared under reducing conditions (Fig. 3A). Cell lysates were also immunoprecipitated with an anti-Stk antiserum and after electrophoresis under nonreducing conditions, they were immunoblotted with the anti-Stk antiserum. As shown in Fig. 3C, in addition to a 52-kDa sf-Stk protein, bands corresponding to 100- and 120-kDa complexes were also detected by the anti-Stk antiserum in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. Densitometer scans indicated that 96% of the sf-Stk precipitated from these cells was present in high-molecular-weight complexes. These complexes disappeared under reducing conditions (data not shown). Blotting with normal rabbit serum or an antiserum against an irrelevant protein (rabbit anti-PU.1) failed to detect 100- or 120-kDa proteins in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 (data not shown).
FIG. 3.
Interaction between SFFV gp55 and sf-Stk. Cell lysates from BaF3-EpoR cells, BaF3-EpoR cells expressing sf-Stk, BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, and BaF3-EpoR cells expressing SFFV gp55 were immunoprecipitated (IP) with an antiserum against the C-terminal region of Stk and then separated by electrophoresis under reducing (A) or nonreducing (B and C) conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with an antiserum against 7C10, a monoclonal antibody specific for SFFV gp55 (A and B), or with an anti-Stk antiserum (C). Asterisks indicate 100- and 120-kDa complexes.
Association of sf-Stk with the EpoR.
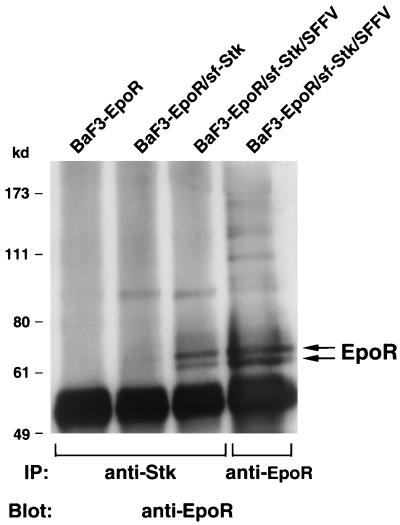
It has previously been demonstrated that SFFV gp55 interacts with the EpoR (4, 10, 20, 49). In order to determine if sf-Stk also interacts with the EpoR, we carried out Western blot analysis using an EpoR antiserum. Cell lysates were immunoprecipitated with either anti-EpoR or anti-Stk antisera and then immunoblotted with an anti-EpoR antibody. As shown in Fig. 4, the EpoR antibody (far-right lane) precipitates 62- and 64-kDa EpoR proteins in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. An antiserum against Stk precipitated a small amount of EpoR proteins from BaF3-EpoR cells expressing only sf-Stk, but it precipitated a large amount of EpoR proteins, as much as the anti-EpoR antiserum did, from BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. Thus, sf-Stk clearly associates, either directly or indirectly, with the EpoR in cells coexpressing SFFV gp55. Unlike the association between sf-Stk and SFFV gp55, the association between sf-Stk and the EpoR does not appear to be covalent, since we failed to detect a high-molecular weight complex containing the EpoR when lysates were analyzed under nonreducing conditions (data not shown).
FIG. 4.
Association of sf-Stk with the EpoR. Cell lysates from BaF3-EpoR cells, BaF3-EpoR cells expressing sf-Stk, and BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 were immunoprecipitated (IP) with an antiserum against the C-terminal region of Stk or an antiserum against the EpoR (lane 4) and then separated by electrophoresis under reducing conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with an antiserum against the EpoR.
Tyrosine phosphorylation of sf-Stk.
In order to determine if sf-Stk was tyrosine phosphorylated in the BaF3-EpoR cells in which it was expressed, proteins were precipitated from cell lysates with an anti-phosphotyrosine antibody cross-linked to protein A-agarose (4G10), separated by electrophoresis under reducing or nonreducing conditions, electrophoretically transferred to filters, and then immunoblotted with an anti-Stk antiserum. Under reducing conditions (Fig. 5A), tyrosine-phosphorylated sf-Stk was detected in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, but not in cells expressing only sf-Stk. The molecular size of tyrosine phosphorylated sf-Stk is slightly higher (by approximately 3 kDa) than that of unphosphorylated sf-Stk. Under nonreducing conditions (Fig. 5B), a 120-kDa tyrosine-phosphorylated protein was detected with the anti-Stk antiserum only in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. This high-molecular-weight tyrosine-phosphorylated protein disappeared under reducing conditions (Fig. 5A). Although most of the tyrosine-phosphorylated sf-Stk was present in a high-molecular-weight complex (72%, as determined by densitometer scans of the third lane of Fig. 5B), we could also detect a 55-kDa tyrosine-phosphorylated protein corresponding to uncomplexed sf-Stk. A 120-kDa tyrosine-phosphorylated protein could also be detected in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 when the blots were reacted with a monoclonal antibody specific to SFFV gp55 (Fig. 5D). This tyrosine-phosphorylated 120-kDa protein disappeared under reducing conditions (Fig. 5C), and could not be detected with a control antiserum (rabbit anti-PU.1).
FIG. 5.
Tyrosine phosphorylation of sf-Stk. Cell lysates from BaF3-EpoR cells, BaF3-EpoR cells expressing sf-Stk, BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, and BaF3-EpoR cells expressing SFFV gp55 were immunoprecipitated (IP) with the anti-phosphotyrosine antibody 4G10 (anti-PY) and then separated by electrophoresis under reducing (A and C) or nonreducing (B and D) conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with an antiserum against the C-terminal region of Stk (A and B) or 7C10, a monoclonal antibody specific for SFFV gp55 (C and D). Asterisks indicate the 120-kDa tyrosine-phosphorylated complex.
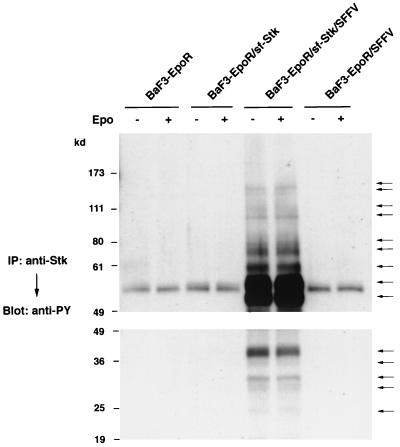
Sf-Stk associates with multiple tyrosine-phosphorylated proteins only in cells coexpressing SFFV gp55.
In order to determine if tyrosine-phosphorylated sf-Stk associates with specific substrates in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, the cells were starved and then either left unstimulated or stimulated with Epo for 15 min. Cell lysates were then immunoprecipitated with an anti-Stk antiserum and immunoblotted with an anti-phosphotyrosine antibody. As shown in Fig. 6, tyrosine-phosphorylated sf-Stk constitutively associates with multiple tyrosine-phosphorylated proteins only in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55. In contrast, we could detect tyrosine-phosphorylated proteins in all of these cells after Epo stimulation using an antiserum to the EpoR (data not shown). Anti-Stk-associated bands included those with molecular masses of 150, 145, 110, 100, 74, 68, 58, 53, 49, 39, 34, 31, 28, and 24 kDa. Stimulation of these cells with Epo did not change the density and number of tyrosine-phosphorylated bands precipitated with the anti-Stk antiserum. Moreover, Epo stimulation of BaF3-EpoR cells expressing sf-Stk in the absence of SFFV gp55 did not result in the tyrosine phosphorylation of sf-Stk or its association with any tyrosine-phosphorylated proteins (Fig. 6).
FIG. 6.
Association of tyrosine-phosphorylated sf-Stk with other tyrosine-phosphorylated proteins. BaF3-EpoR cells, BaF3-EpoR cells expressing sf-Stk, BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, and BaF3-EpoR cells expressing SFFV gp55 were left unstimulated or stimulated with Epo for 15 min. Cell lysates were then immunoprecipitated (IP) with an antiserum against the C-terminal region of Stk and separated by electrophoresis under reducing conditions on 8% (upper panel) and 10% (lower panel) polyacrylamide gels. After transfer to nitrocellulose filters, the proteins were immunoblotted with the anti-phosphotyrosine antiserum 4G10 (anti-PY). Tyrosine-phosphorylated proteins immunoprecipitated with the anti-Stk antiserum are indicated by arrows.
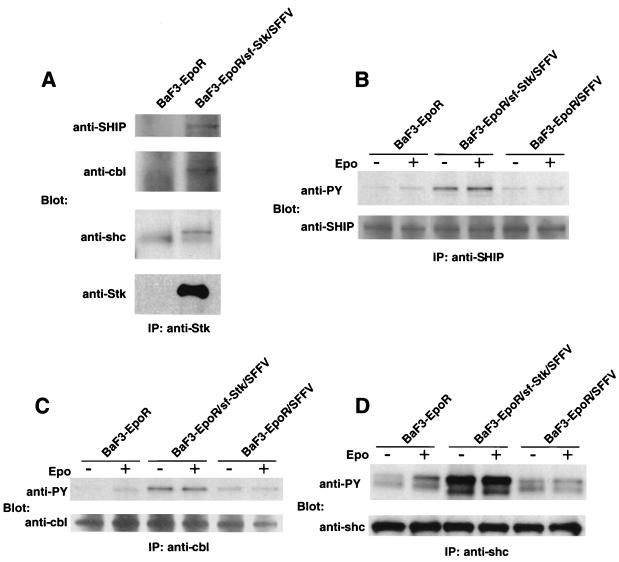
Phosphorylated sf-Stk forms complexes with known signaling molecules.
In order to identify tyrosine-phosphorylated proteins detected in Fig. 6 that are associated with sf-Stk in SFFV gp55-expressing cells, we carried out a Western blot analysis on anti-Stk immunoprecipitates using antibodies specific to signal-transducing molecules of similar molecular weights. As shown in Fig. 7A, SHIP (150 kDa), Cbl (110 kDa), and Shc (53 kDa) were coimmunoprecipitated with an anti-Stk antiserum in BaF3-EpoR cells expressing both SFFV gp55 and sf-Stk. To determine the tyrosine phosphorylation level of these proteins in cells expressing SFFV gp55 or coexpressing sf-Stk and SFFV gp55, lysates from cells left unstimulated or stimulated with Epo were immunoprecipitated with anti-SHIP, anti-Cbl, or anti-Shc antisera and then immunoblotted with an anti-phosphotyrosine antibody. As shown in Fig. 7B to D, SHIP, Cbl, and Shc were tyrosine phosphorylated in BaF3-EpoR cells in response to Epo (leftmost two lanes) but were constitutively phosphorylated in BaF3-EpoR cells expressing SFFV gp55 in the presence (middle two lanes) or absence (rightmost two lanes) of sf-Stk. However, the levels of these tyrosine-phosphorylated proteins were always appreciably higher in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55.
FIG. 7.
Association of phosphorylated sf-Stk with known signal-transducing molecules and their tyrosine phosphorylation. (A) Lysates from BaF3-EpoR cells and BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 were immunoprecipitated (IP) with an antiserum to the C-terminal region of Stk and separated by electrophoresis under reducing conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with antisera to SHIP, Cbl, Shc, or Stk. (B to D) BaF3-EpoR cells, BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, and BaF3-EpoR cells expressing SFFV gp55 were left unstimulated or stimulated with Epo for 15 min. Lysates were immunoprecipitated (IP) with an antiserum against SHIP (B), Cbl (C), or Shc (D) and then separated by electrophoresis under reducing conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with the anti-phosphotyrosine antiserum 4G10 (anti-PY) (B to D) or an antiserum to SHIP (B), Cbl (C), or Shc (D).
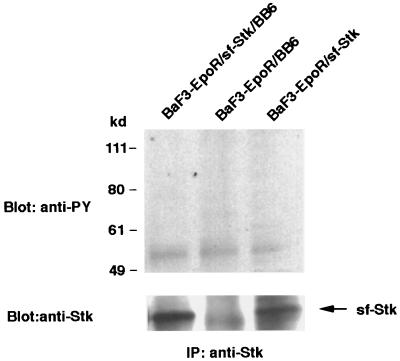
A mutant SFFV, BB6, does not induce the tyrosine phosphorylation of sf-Stk.
It was previously shown that a variant of SFFV, BB6, encodes an envelope glycoprotein, gp42, that is deleted in a cysteine-containing region that could be involved in the interaction of SFFV gp55 with sf-Stk (19, 25). To test whether the BB6 virus can induce the tyrosine phosphorylation of sf-Stk, we established BaF3-EpoR cells expressing sf-Stk with and without BB6 virus. Lysates from these cells were immunoprecipitated with an anti-Stk antiserum and then immunoblotted with an anti-phosphotyrosine antibody (Fig. 8). In contrast to BaF3-EpoR cells coexpressing sf-Stk and wild-type SFFV (see Fig. 6), we failed to detect the tyrosine phosphorylation of sf-Stk or any tyrosine-phosphorylated proteins associated with sf-Stk in cells coexpressing sf-Stk and BB6 virus (Fig. 8, left lane). Consistent with this result, we could not detect an interaction between the BB6 envelope protein and sf-Stk when cell lysates were immunoprecipitated with an anti-Stk antiserum and then immunoblotted with an antiserum that detects both wild-type SFFV gp55 and the envelope protein of BB6 virus (data not shown).
FIG. 8.
The mutant SFFV BB6 does not induce the tyrosine phosphorylation of sf-Stk. Cell lystates from BaF3-EpoR cells expressing sf-Stk, BaF3-EpoR cells expressing sf-Stk and then infected with the BB6 virus, and BaF3-EpoR cells infected with the BB6 virus were immunoprecipitated (IP) with an antiserum to the C-terminal region of Stk and separated by electrophoresis under reducing conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with the anti-phosphotyrosine antiserum 4G10 (anti-PY) or the anti-Stk antiserum.
Sf-Stk associates with SFFV gp55 in an erythroleukemia cell line derived from an SFFV-infected mouse.
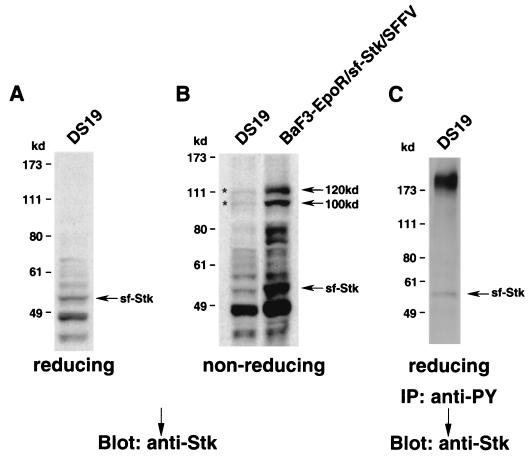
In addition to studying BaF3-EpoR cells engineered to express sf-Stk and SFFV gp55, we also examined DS19, an erythroleukemia cell line derived from an SFFV-infected mouse (Fig. 9). These cells naturally express both SFFV gp55 (35) and sf-Stk (Fig. 1 and 9A). As in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, the sf-Stk in DS19 cells migrates as 100- and 120-kDa bands under nonreducing conditions (Fig. 9B, left lane). The same bands are detected using an antiserum against SFFV gp55 (data not shown), indicating that sf-Stk and SFFV gp55 covalently interact in these erythroleukemia cells. When the level of sf-Stk in DS19 cells was reduced by inducing the cells to differentiate with chemicals (16), the level of SFFV gp55–sf-Stk dimers was also greatly reduced (data not shown). As previously shown with BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, the sf-Stk in DS19 cells is constitutively tyrosine phosphorylated (Fig. 9C).
FIG. 9.
Association of sf-Stk and SFFV gp55 in an SFFV-induced erythroleukemia cell line. (A and B) A cell lysate prepared from DS19 cells, an erythroleukemia cell line derived from an SFFV-infected mouse, was separated by electrophoresis under reducing (A) or nonreducing (B) conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with an antiserum against the C-terminal region of Stk. BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55 are shown in panel B for comparison. Asterisks indicate 100- and 120-kDa complexes. (C) A lysate from DS19 cells was immunoprecipitated (IP) with the anti-phosphotyrosine antiserum 4G10 (anti-PY) and then separated by electrophoresis under reducing conditions. After transfer to nitrocellulose filters, the proteins were immunoblotted with an antiserum against the C-terminal region of Stk in order to detect the phosphorylated form of sf-Stk.
DISCUSSION
The development of erythroleukemia in mice by Friend SFFV requires expression of the viral envelope glycoprotein at the surfaces of erythroid cells as well as expression of the Fv-2s gene. Previous studies have indicated that the SFFV envelope glycoprotein, gp55, interacts with the EpoR complex at the cell surface, causing constitutive activation of signal transduction pathways and Epo-independent erythroid hyperplasia (4, 10, 21, 26, 27, 29, 30). The product of the Fv-2s gene was unknown until recently, when it was shown that this gene encodes both a full-length and a truncated version of the receptor tyrosine kinase Stk (33). Since resistant mice carry an allele of the Fv-2 gene that encodes only full-length Stk, truncated Stk appears to be required for the induction of SFFV-induced disease. The purpose of this study was to gain a better understanding of how SFFV gp55 and sf-Stk may cooperate to induce erythroleukemia. Our studies demonstrate that SFFV gp55 can covalently interact with sf-Stk, leading to its constitutive activation and the subsequent tyrosine phosphorylation of signal-transducing molecules.
For these studies, we expressed sf-Stk in hematopoietic cells expressing the EpoR and then examined the cells before and after introduction of SFFV gp55. Our results indicate that SFFV gp55 constitutively binds to sf-Stk, forming disulfide-linked heterodimers of 100 and 120 kDa. Previous studies have shown that the envelope glycoprotein of SFFV is synthesized as a 55-kDa glycoprotein, most of which remains in the endoplasmic reticulum as a monomer containing high-mannose type oligosaccharide chains. However, a small proportion of gp55 is modified by the addition of O-linked and complex N-linked oligosaccharide chains and processed as a disulfide-bonded dimer to the cell surface as gp55P (35, 38, 40), the biologically relevant form of the viral envelope glycoprotein that is transported to the cell surface. Our data suggest that both gp55 and gp55P interact with sf-Stk, forming heterodimers of 100 and 120 kDa, respectively. Consistent with this, the level of SFFV gp55 homodimers is decreased in BaF3-EpoR cells expressing both SFFV gp55 and sf-Stk. The majority of the sf-Stk expressed in cells coexpressing sf-Stk and SFFV gp55 is present in heterodimers with the SFFV envelope proteins, and the interaction of sf-Stk with SFFV gp55P results in the constitutive tyrosine phosphorylation of sf-Stk. We failed to detect any activation of sf-Stk in the absence of SFFV gp55 expression. Although most of the tyrosine-phosphorylated sf-Stk is disulfide linked with SFFV gp55P, some of the phosphorylated kinase is not. Thus, activation of sf-Stk by SFFV gp55P can result from both covalent and noncovalent interactions between the two proteins. Like BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55, erythroleukemia cell lines derived from SFFV-infected mice also express heterodimers of SFFV gp55 and sf-Stk and show constitutive tyrosine phosphorylation of sf-Stk.
Further studies using BaF3-EpoR cells indicated that SFFV-activated sf-Stk associates with multiple tyrosine-phosphorylated proteins, including SHIP, Cbl, and Shc, each of which has been shown to be associated with activated Met family kinases (11, 18, 41). Interaction of SFFV gp55 with the EpoR in the absence of sf-Stk also leads to the tyrosine phosphorylation of these signal-transducing molecules, but the level of expression is greatly amplified in BaF3-EpoR cells expressing both SFFV gp55 and sf-Stk. We previously reported that infection of the erythroid cell line HCD-57 with SFFV results in the constitutive tyrosine phosphorylation of Shc and SHIP (27, 29), but this study is the first demonstration that SFFV infection leads to the constitutive phosphorylation of Cbl. We are currently carrying out studies to characterize the other tyrosine-phosphorylated proteins associated with sf-Stk in SFFV gp55-expressing cells, which may include Gab proteins, PI 3-kinase, and Grb2, each of which has been shown to be constitutively activated by SFFV infection (27, 29) and to interact with activated Met family kinases (18, 28). It will be important to determine whether sf-Stk kinase activity is required for the tyrosine phosphorylation of signal-transducing molecules associated with sf-Stk in BaF3-EpoR cells coexpressing sf-Stk and SFFV gp55.
We could also detect a strong physical, but noncovalent, interaction between sf-Stk and the EpoR in BaF3 cells coexpressing SFFV gp55. However, this interaction was weak in cells lacking SFFV gp55 and did not result in detectable tyrosine phosphorylation of sf-Stk or its association with tyrosine-phosphorylated signal-transducing molecules. This suggests that SFFV gp55 may stabilize the interaction of sf-Stk with the EpoR or that SFFV gp55 brings sf-Stk to the cell surface, where it can interact with the EpoR complex.
The interaction between SFFV gp55 and sf-Stk is a covalent linkage mediated by disulfide bonds. gp55 contains 12 cysteine residues, 8 in the polytropic N-terminal domain and 4 in the ecotropic C-terminal domain (1, 6, 48). The eight cysteine residues in the polytropic domain have been shown to be involved in intrachain disulfide bonds within the envelope glycoproteins of MuLVs (23, 24), suggesting that they would not be available for dimer formation. This suggests that cysteines in the ecotropic C-terminal domain of SFFV gp55 at positions 306, 309, 337, and 338 may be involved in the interaction with sf-Stk. The mutant SFFV BB6 (25), which we show fails to interact with sf-Stk, contains a deletion that includes two of these cysteines, C337 and C338. Thus, the covalent linkage between SFFV gp55 and sf-Stk may be occurring between the cysteines at positions 337 and 338 in SFFV gp55 and two of the four cysteine residues present in the extracellular domain of sf-Stk, the only cysteines retained in the truncated protein (16). Studies are in progress to specifically mutate the cysteine residues in both SFFV gp55 and sf-Stk in order to determine their importance to the formation of heterodimers of the two molecules.
Although sf-Stk appears to be essential for the development of Epo-independent erythroid bursts after in vitro infection with SFFV (3, 14) and for the development of SFFV-induced erythroleukemia (22), it was surprising to find that it is not required for the induction of Epo independence by SFFV gp55 in BaF3-EpoR cells. This suggests that BaF3-EpoR cells, which are pro-B cells (31), express another tyrosine kinase that can be activated by SFFV gp55. Such a kinase may not be expressed in primary erythroid cells, which require expression of sf-Stk to be rendered Epo independent by SFFV infection. Alternatively, induction of Epo independence by SFFV in BaF3-EpoR cells may require a lower threshold of induction of signal transduction pathways than that required to sustain the Epo-independent proliferation of primary erythroid cells infected with SFFV, which may also require the activation of sf-Stk-activated pathways. Consistent with this idea are data obtained using the mutant SFFV BB6. We showed in this study that the envelope gene of BB6 SFFV does not interact with sf-Stk, most likely due to the deletion of a cysteine-containing region in its envelope glycoprotein (25). BB6 SFFV is very efficient, often better than wild-type SFFV, in inducing factor independence after infection of BaF3-EpoR cells (15) or HCD-57 cells (our unpublished data). However, BB6 SFFV is a poor inducer of Epo-independent erythroid bursts in vitro (S. Ruscetti, unpublished data) and is weakly pathogenic when injected into either Fv-2-susceptible or -resistant strains of mice (25). Thus, the role of sf-Stk in mediating the biological effects of SFFV may be clear only when erythroid precursors are infected in vitro or after mice are injected with the virus. It is not known whether SFFV-activated sf-Stk generates signals that are distinct from those activated by the interaction of Epo or SFFV gp55 with the EpoR or whether it serves to raise the level of common signal-transducing molecules above a threshold needed to sustain a biological effect in primary erythroid cells.
Although SFFV gp55 can form a covalent complex with truncated Stk, it does not interact with full-length Stk. Full-length Stk, which is the receptor for macrophage-stimulating protein (MSP) (12, 43, 44), is synthesized as a precursor and cleaved to α- and β-chains that are disulfide linked at the cell surface (34, 43). Thus, the α-chain of Stk may compete with SFFV gp55 for binding the β-chain of Stk. Alternatively, engagement of the Stk receptor by its ligand, MSP, which is present in the FCS used to grow BaF3-EpoR cells (17), may alter the conformation of the receptor so that it can no longer bind SFFV gp55. Since sf-Stk does not encode an α-chain and cannot bind MSP, either of these possibilities may account for the lack of interaction between full-length Stk and SFFV gp55. In contrast to BaF3-EpoR cells, whose growth is not altered by expression of full-length Stk, we observed that overexpression of full-length Stk in the mouse erythroleukemia cell line DS19 or in the Epo-dependent erythroid cell line HCD-57 leads to the death of these cells by apoptosis. This is consistent with data from a previous report using a mouse erythroleukemia cell line (18) and indicates that the activation of sf-Stk by SFFV results in different biological consequences compared with activation of full-length Stk by MSP.
The role of sf-Stk in normal erythropoiesis is unknown. Stk was first isolated from murine hematopoietic stem cells (16), and both the full-length and short form can be detected in mouse erythroleukemia cell lines (16) as well as in primary fetal liver cells (33). In both cases, sf-Stk is the major expressed form of the gene. Stk does not appear to be essential for erythropoiesis, since Stk-deficient mice which have normal erythropoiesis can be generated (8), although the mice may be slower to respond to erythropoietic stress (33). Furthermore, Fv-2rr mice, which do not express sf-Stk, have no obvious defects in erythropoiesis, although their erythroid cells may progress more slowly through the cell cycle than those of their Fv-2s counterparts (42). Thus, sf-Stk may be activated in erythroid cells only under conditions that require rapid red blood cell replenishment, such as during hemorrhage or when oxygen levels are low. As shown in this study, sf-Stk can be activated by interacting with the envelope glycoprotein of SFFV, and this appears to contribute to the development of erythroleukemia. Studies are in progress to determine if sf-Stk is also activated in response to erythropoietic stress, perhaps by endogenous retroviral envelope proteins.
ACKNOWLEDGMENTS
We thank Karen Cannon and Angelo Spadaccini for valuable assistance in the preparation of this report. We also thank Toshio Kitamura, Toshio Suda, and Hiroshi Amanuma for generously providing reagents and Alla Danilkovich, Edward Leonard, and Michiaki Masuda for helpful discussions.
REFERENCES
- 1.Amanuma H, Katori A, Obata M, Sagata N, Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci USA. 1983;80:3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behringer R R, Dewey M J. Cellular site and mode of Fv-2 gene action. Cell. 1985;40:441–447. doi: 10.1016/0092-8674(85)90158-8. [DOI] [PubMed] [Google Scholar]
- 3.Bondurant M C, Koury M J, Krantz S B. The Fv-2 gene controls induction of erythroid burst formation by Friend virus infection in vitro: studies of growth regulators and viral replication. J Gen Virol. 1985;66:83–93. doi: 10.1099/0022-1317-66-1-83. [DOI] [PubMed] [Google Scholar]
- 4.Casadevall N, Lacombe C, Muller O, Gisselbrecht S, Mayeux P. Multimeric structure of the membrane erythropoietin receptor of murine erythroleukemia cells (Friend cells) J Biol Chem. 1991;266:16015–16020. [PubMed] [Google Scholar]
- 5.Chung S, Wolff L, Ruscetti S K. Transmembrane domain of the envelope gene of a polycythemia-inducing retrovirus determines erythropoietin-independent growth. Proc Natl Acad Sci USA. 1989;86:7957–7960. doi: 10.1073/pnas.86.20.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark S P, Mak T W. Complete nucleotide sequences of an infectious clone of Friend spleen focus-forming provirus: gp55 is an envelope fusion glycoprotein. Proc Natl Acad Sci USA. 1983;80:5037–5041. doi: 10.1073/pnas.80.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffin J M, Hughes S H, Varmus H E. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1997. [PubMed] [Google Scholar]
- 8.Correll P H, Iwama A, Tondat S, Mayrhofer G, Suda T, Bernstein A. Deregulated inflammatory response in mice lacking the STK/RON receptor tyrosine kinase. Genes Function. 1997;1:69–83. doi: 10.1046/j.1365-4624.1997.00009.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans L H, Duesberg P H, Scolnick E M. Replication of spleen focus-forming virus in fibroblasts from C57BL mice that are genetically resistant to spleen focus formation. Virology. 1980;101:534–539. doi: 10.1016/0042-6822(80)90469-9. [DOI] [PubMed] [Google Scholar]
- 10.Ferro F E, Jr, Kozak S L, Hoatlin M E, Kabat D. Cell surface site for mitogenic interaction of erythropoietin receptors with the membrane glycoprotein encoded by Friend erythroleukemia virus. J Biol Chem. 1993;268:5741–5747. [PubMed] [Google Scholar]
- 11.Garcia-Guzman M, Larsen E, Vuori K. The proto-oncogene c-Cbl is a positive regulator of Met-induced MAP kinase activation: a role for the adaptor protein Crk. Oncogene. 2000;19:4058–4065. doi: 10.1038/sj.onc.1203750. [DOI] [PubMed] [Google Scholar]
- 12.Gaudino G, Follenzi A, Naldini L, Collesi C, Santoro M, Gallo K A, Godowski P J, Comoglio P M. Ron is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J. 1994;13:3524–3532. doi: 10.1002/j.1460-2075.1994.tb06659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geib R W, Anand R, Lilly F. Characterization of cell lines derived from enlarged spleens induced in C57BL/6 mice by the variant BSB strain of Friend erythroleukemia virus. Virus Res. 1987;8:327–333. doi: 10.1016/0168-1702(87)90040-2. [DOI] [PubMed] [Google Scholar]
- 14.Hankins W D, Luna J. Influence of Fv-1 and Fv-2 gene systems on in vitro erythroid transformation by Friend leukemia virus. In: Yohn D, Blakeslee J, editors. Advances in comparative leukemia research. Amsterdam, The Netherlands: Elsevier/North-Holland; 1981. pp. 107–111. [Google Scholar]
- 15.Hoatlin M E, Ferro F E, Jr, Geib R W, Fox M T, Kozak S L, Kabat D. Deletions in one domain of the Friend virus-encoded membrane glycoprotein overcome host range restrictions for erythroleukemia. J Virol. 1995;69:856–863. doi: 10.1128/jvi.69.2.856-863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwama A, Okano K, Sudo T, Matsuda Y, Suda T. Molecular cloning of a novel receptor tyrosine kinase gene, STK, derived from enriched hematopoietic stem cells. Blood. 1994;83:3160–3169. [PubMed] [Google Scholar]
- 17.Iwama A, Wang M-H, Yamaguchi N, Ohno N, Okano K, Sudo T, Takeya M, Gervais F, Morissette C, Leonard E J, Suda T. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86:3394–3403. [PubMed] [Google Scholar]
- 18.Iwama A, Yamaguchi N, Suda T. STK/RON receptor tyrosine kinase mediates both apoptotic and growth signals via the multifunctional docking site conserved among the HGF receptor family. EMBO J. 1996;15:5866–5875. [PMC free article] [PubMed] [Google Scholar]
- 19.Kozak S L, Hoatlin M E, Ferro F E, Jr, Majumdar M K, Geib R W, Fox M T, Kabat D. A Friend virus mutant that overcomes Fv-2rr host resistance encodes a small glycoprotein that dimerizes, is processed to cell surfaces, and specifically activates erythropoietin receptors. J Virol. 1993;67:2611–2620. doi: 10.1128/jvi.67.5.2611-2620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, D'Andrea A D, Lodish H F, Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990;343:762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- 21.Li J-P, Hu H-O, Niu Q-T, Fang C. Cell surface activation of the erythropoietin receptor by Friend spleen focus-forming virus gp55. J Virol. 1995;69:1714–1719. doi: 10.1128/jvi.69.3.1714-1719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response of Friend leukemia in mice. J Natl Cancer Inst. 1970;45:163–169. [PubMed] [Google Scholar]
- 23.Linder M, Linder D, Hahnen J, Schott H-H, Stirm S. Localization of the intrachain disulfide bonds of the envelope glycoprotein 71 from Friend murine leukemia virus. Eur J Biochem. 1992;203:65–73. doi: 10.1111/j.1432-1033.1992.tb19828.x. [DOI] [PubMed] [Google Scholar]
- 24.Linder M, Wenzel V, Linder D, Stirm S. Structural elements in glycoprotein 70 from polytropic Friend mink cell focus-inducing virus and glycoprotein 71 from ecotropic Friend murine leukemia virus, as defined by disulfide-bonding pattern and limited proteolysis. J Virol. 1994;68:5133–5141. doi: 10.1128/jvi.68.8.5133-5141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumdar M K, Cho C-L, Fox M T, Eckner K L, Kozak S, Kabat D, Geib R W. Mutations in the env gene of Friend spleen focus-forming virus overcome Fv-2r-mediated resistance to Friend virus-induced erythroleukemia. J Virol. 1992;66:3652–3660. doi: 10.1128/jvi.66.6.3652-3660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muszynski K W, Ohashi T, Hanson C, Ruscetti S K. Both the polycythemia- and anemia-inducing strains of Friend spleen focus-forming virus induce constitutive activation of the Raf-1/mitogen-activated protein kinase signal transduction pathway. J Virol. 1998;72:919–925. doi: 10.1128/jvi.72.2.919-925.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muszynski K W, Thompson D, Hanson C, Lyons R, Spadaccini A, Ruscetti S K. Growth factor-independent proliferation of erythroid cells infected with Friend spleen focus-forming virus is protein kinase C dependent but does not require Ras-GTP. J Virol. 2000;74:8444–8451. doi: 10.1128/jvi.74.18.8444-8451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen L, Holgado-Madruga M, Maroun C, Fixman E D, Kamikura D, Fournier T, Charest A, Tremblay M L, Wong A J, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 29.Nishigaki K, Hanson C, Ohashi T, Thompson D, Muszynski K, Ruscetti S. Erythroid cells rendered erythropoietin independent by infection with Friend spleen focus-forming virus show constitutive activation of phosphatidylinositol 3-kinase and Akt kinase: involvement of insulin receptor substrate-related adapter proteins. J Virol. 2000;74:3037–3045. doi: 10.1128/jvi.74.7.3037-3045.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohashi T, Masuda M, Ruscetti S K. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood. 1995;85:1454–1462. [PubMed] [Google Scholar]
- 31.Palacios R, Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 32.Pear W L, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persons D A, Paulson R F, Loyd M R, Herley M T, Bodner S M, Bernstein A, Correll P H, Ney P A. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- 34.Ronsin C, Muscatelli F, Matgei M G, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. [PubMed] [Google Scholar]
- 35.Ruscetti S, Linemeyer D, Feild J, Troxler D, Scolnick E M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979;30:787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruscetti S K, Janesch N J, Chakraborti A, Sawyer S T, Hankins W D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990;64:1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruscetti S K. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int J Biochem Cell Biol. 1999;31:1089–1109. doi: 10.1016/s1357-2725(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 38.Ruta M, Clarke S, Boswell B, Kabat D. Heterogeneous metabolism and subcellular localization of a potentially leukemogenic membrane glycoprotein encoded by Friend erythroleukemia virus. J Biol Chem. 1982;257:126–134. [PubMed] [Google Scholar]
- 39.Silver J, Teich N. Expression of resistance to Friend virus-stimulated erythropoiesis in bone marrow chimera containing Fv-2r and Fv-2s bone marrow. J Exp Med. 1981;154:126–137. doi: 10.1084/jem.154.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivas R V, Compans R W. Membrane association and defective transport of spleen focus-forming virus glycoproteins. J Biol Chem. 1983;258:14718–14724. [PubMed] [Google Scholar]
- 41.Stefan M, Koch A, Mancini A, Mohr A, Weidner K M, Niemann H, Tamura T. Src homology 2-containing inositol 5-phosphatase 1 binds to the multifunctional docking site of c-Met and potentiates hepatocyte growth factor-induced branching tubulogenesis. J Biol Chem. 2001;276:3017–3023. doi: 10.1074/jbc.M009333200. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki S, Axelrad A A. Fv-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980;19:225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- 43.Wang M-H, Ronsin C, Gesnel C, Coupey L, Skeel A, Leonard E J, Breathnach R. Identification of the ron gene product as the receptor for the human macrophage-stimulating protein. Science. 1994;266:117–119. doi: 10.1126/science.7939629. [DOI] [PubMed] [Google Scholar]
- 44.Wang M-H, Iwama A, Skeel A, Suda T, Leonard E J. The murine stk gene product, a transmembrane protein tyrosine kinase, is a receptor for macrophage-stimulating protein. Proc Natl Acad Sci USA. 1995;92:3933–3937. doi: 10.1073/pnas.92.9.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Kayman S C, Li J-P, Pinter A. Erythropoietin receptor (EpoR)-dependent mitogenicity of spleen focus-forming virus correlates with viral pathogenicity and processing of env protein but not with formation of gp52-EpoR complexes in the endoplasmic reticulum. J Virol. 1993;67:1322–1327. doi: 10.1128/jvi.67.3.1322-1327.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wojchowski D M, Gregory R C, Miller C P, Pandit A K, Pircher T J. Signal transduction in the erythropoietin receptor system. Exp Cell Res. 1999;253:143–156. doi: 10.1006/excr.1999.4673. [DOI] [PubMed] [Google Scholar]
- 47.Wolff L, Koller R, Ruscetti S. Monoclonal antibody to spleen focus-forming virus-encoded gp52 provides a probe for the amino-terminal region of retroviral envelope proteins that confers dual tropism and xenotropism. J Virol. 1982;43:472–481. doi: 10.1128/jvi.43.2.472-481.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolff L, Scolnick E, Ruscetti S. Envelope gene of the Friend spleen focus-forming virus: deletion and insertions in 3′ gp70/p15E-encoding region have resulted in unique features in the primary structure of its protein product. Proc Natl Acad Sci USA. 1983;80:4718–4722. doi: 10.1073/pnas.80.15.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimura A, D'Andrea A D, Lodish H F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci USA. 1990;87:4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yugawa T, Amanuma H. Impaired activation and binding of the erythropoietin receptor by a mutant gp55 of Friend spleen focus-forming virus, which has a cytoplasmic domain. Virology. 1998;246:232–240. doi: 10.1006/viro.1998.9214. [DOI] [PubMed] [Google Scholar]