Abstract
The herpes simplex virus 1 (HSV-1) infected cell proteins 0 and 4 (ICP0 and ICP4) are multifunctional proteins extensively posttranscriptionally processed by both cellular and viral enzymes. We examined by two-dimensional separations the posttranslational forms of ICP0 and ICP4 in HEp-2 cells and in human embryonic lung (HEL) fibroblasts infected with wild-type virus, mutant R325, lacking the sequences encoding the US1.5 protein and the overlapping carboxyl-terminal domain of ICP22, or R7914, in which the aspartic acid 199 of ICP0 was replaced by alanine. We report the following (i) Both ICP0 and ICP4 were sequentially posttranslationally modified at least until 12 h after infection. In HEL fibroblasts, the processing of ICP0 shifted from A+B forms at 4 h to D+G forms at 8 h and finally to G, E, and F forms at 12 h. The ICP4 progression was from the A′ form noted at 2 h to B′ and C′ forms noted at 4 h to the additional D′ and E′ forms noted at 12 h. The progression tended to be toward more highly charged forms of the proteins. (ii) Although the overall patterns were similar, the mobility of proteins made in HEp-2 cells differed from those made in HEL fibroblasts. (iii) The processing of ICP0 forms E and F was blocked in HEL fibroblasts infected with R325 or with wild-type virus and treated with roscovitine, a specific inhibitor of cell cycle-dependent kinases cdc2, cdk2, and cdk5. R325-infected HEp-2 cells lacked the D′ form of ICP4, and roscovitine blocked the appearance of the most highly charged E′ form of ICP4. (iv) A characteristic of ICP0 is that it is translocated into the cytoplasm of HEL fibroblasts between 5 and 9 h after infection. Addition of MG132 to the cultures late in infection resulted in rapid relocation of cytoplasmic ICP0 back into the nucleus. Exposure of HEL fibroblasts to MG132 late in infection resulted in the disappearance of the highly charged ICP0 G isoform. The G form of ICP0 was also absent in cells infected with R7914 mutant. In cells infected with this mutant, ICP0 is not translocated to the cytoplasm. (v) Last, cdc2 was active in infected cells, and this activity was inhibited by roscovitine. In contrast, the activity of cdk2 exhibited by immunoprecipitated protein was reduced and resistant to roscovitine and may represent a contaminating kinase activity. We conclude from these results that the ICP0 G isoform is the cytoplasmic form, that it may be phosphorylated by cdc2, consistent with evidence published earlier (S. J., Advani, R. R. Weichselbaum, and B. Roizman, Proc. Natl. Acad. Sci. USA 96:10996–11001, 2000), and that the processing is reversed upon relocation of the G isoform from the cytoplasm into the nucleus. The processing of ICP4 is also affected by R325 and roscovitine. The latter result suggests that ICP4 may also be a substrate of cdc2 late in infection. Last, additional modifications are superimposed by cell-type-specific enzymes.
Herpes simplex virus 1 (HSV-1) gene expression is sequentially ordered in a cascade fashion (33). The α genes are the first set of genes to be transcribed. The products, the infected cell polypeptide 0 (ICP0), ICP4, ICP22/US 1.5, ICP27, and ICP47, play a prominent role in regulating viral replication and the environment of the infected cell to ensure an orderly expression of viral genes and evasion of cellular responses to infection. To attain these objectives, the α proteins with the possible exception of ICP47 express multiple functions. Related to the multifunctionality of these proteins is the extensive posttranslational processing to which they are subjected throughout the replicative cycle of the virus. The posttranslational processing includes poly(ADP-ribosyl)ation (ICP4), nucleotidylylation by casein kinase II (ICP0, ICP4, ICP22, and ICP27), and phosphorylation by both viral and cellular kinases (4, 5, 25, 26, 31, 41–43, 45). While earlier reports have focused on the viral kinases (US3 and UL13) and certain cellular kinases (protein kinases A and C and casein kinase II) and their role in modifications of HSV-1 α proteins, recent reports have suggested a possible involvement of JNK1 and of the cyclin-dependent kinase cdc2 in the regulation of viral gene expression (2, 21). The focus of this report is on posttranslational modifications of two α proteins, ICP4 and ICP0.
ICP4, a DNA-binding nuclear phosphoprotein, is the major regulatory protein encoded by HSV-1. The effect of its many and not fully characterized functions is to regulate viral gene expression both positively and negatively. Negative regulation is achieved by binding to high-affinity response elements situated at the transcription initiation sites (18, 33), whereas positive regulation of transcription is associated with low-affinity, nonconsensus sites scattered throughout the genome (23, 24). The protein contains consensus phosphorylation sites for cellular protein kinases A and C and casein kinase II (42, 43). The state of phosphorylation of ICP4 has been reported to differentially regulate its ability to bind to HSV-1 viral promoters of different kinetic gene classes (28). Whereas unphosphorylated ICP4 can retain its ability to bind to α promoter elements, phosphorylation of ICP4 is needed to bind to promoter elements of β and γ genes.
Early in infection, ICP0 localizes to the nucleus. In some cells, and particularly in primary human embryonic lung (HEL) fibroblasts, ICP0 is translocated to the cytoplasm between 5 and 9 h after infection (14, 40). The apparent phenotype of ICP0 is that of a promiscuous transactivator. Biochemical studies indicate that ICP0 interacts with several viral and cellular proteins (14–16, 39, 44). Consistent with the presence of a ring finger structure, ICP0 is involved in the ubiquitin-proteasomal degradation pathway (8–11, 29). In addition to the posttranslational modifications described above, ICP0 is also phosphorylated by both US3 and UL13 viral protein kinases (26, 31).
Both ICP0 and ICP4 form multiple bands on electrophoresis in denaturing polyacrylamide gels. In early studies, each of these proteins has been shown to form multiple spots on two-dimensional separations (1). In order to relate the posttranslational modifications of these two proteins to stages of viral replication and, in the case of ICP0, to the translocation from nucleus to the cytoplasm, we have used two-dimensional gel electrophoresis to discern specific forms of ICP0 and ICP4 that accumulate during a 12-h time course of infection. Two-dimensional gel electrophoresis analysis allows resolution of differentially charged protein isoforms that migrate at similar apparent molecular weights on one-dimensional separations in denaturing polyacrylamide gels. Relevant to these studies are the following observations.
(i) Cdc2 kinase activity increases in HSV-1-infected cell lysates between 8 to 12 h after infection (2) even though its partners, cyclins A an B, are degraded and virtually undetectable at that time after infection. Cdc2 is a proline-directed serine/threonine kinase normally active during the G2/M phase of the cell cycle, and its consensus phosphorylation site has been defined as (S/T)PX(K/H/R) (17, 20). This consensus phosphorylation site is present in many HSV-1-encoded proteins (3). Of particular interest are the α proteins ICP0 and ICP4, which contain multiple potential cdc2 kinase phosphorylation sites. In HSV-1-infected cells treated with roscovitine, an inhibitor of cdc2, cdk2, and cdk5, viral replication and transcription are inhibited (13, 34–36).
(ii) The activity of cdc2 is regulated by both ICP22 and UL13 (2). Inhibition of cellular cdc2 kinase by overexpression of a dominant negative form results in diminished accumulation of US11 (a γ2 protein) (3). ICP22 and UL13 expression are essential for wild-type-level expression of γ2 genes (27, 32).
(iii) ICP0 binds to and stabilizes cyclin D3. A single amino acid substitution (D199A) abolishes this function of ICP0. In addition, ICP0 of the D199A substitution mutant (R7914) is not translocated from the nucleus to the cytoplasm, and the mutant virus exhibits reduced replication in quiescent HEL fibroblasts and reduced neuroinvasiveness in mice infected at a site peripheral to the central nervous system (39). Published evidence suggests that the translocation of ICP0 to the cytoplasmic compartment of HEL fibroblasts correlates with binding of cyclin D3 and involvement of proteasomal components. The latter association is based on the observation that proteasomal degradation inhibitor MG132 administered late in infection causes the relocation of ICP0 from the cytoplasm to the nucleus (19).
The central hypotheses tested in this study are that the posttranslational modifications of ICP4 and ICP0 are sequential and that they are associated with specific locations of the proteins within cellular compartments. We report that this is indeed the case.
MATERIALS AND METHODS
Cells and viruses.
HEp-2 cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium supplemented with 10% serum. HEL fibroblasts were initially obtained from Aviron (Mountain View, Calif.) and maintained in 10% newborn calf serum. HSV-1(F) is the prototype wild-type HSV-1 strain used in our laboratory (7). R325, lacking the carboxyl-terminal domain of ICP22, and R7914, in which the aspartic acid 199 of ICP0 was replaced by alanine, have been previously described (30, 39).
Cell Infection.
HEp-2 cells were harvested and reseeded on 25-cm2 flasks. Cells were allowed to adhere for 1 h, after which unattached cells were aspirated. The adhered cells were exposed to 2 × 107 PFU of appropriate virus in 1 ml of 199V (mixture 199 supplemented with 1% calf serum) on a rotary shaker at 37°C. After 2 h, the inoculum was replaced with 5 ml of fresh Dulbecco's Modified Eagle's medium supplemented with 10% calf serum. Flasks were incubated at 37°C until the cells were harvested. HEL fibroblasts grown to 100% confluency in 150-cm2 flasks were maintained for 1 week prior to infection. They were exposed to virus in 199V for 2 h and then maintained in the spent growth medium. The proteasome inhibitor, MG132, was added to infected HEL fibroblast cultures at 8 h after infection at a final concentration of 0.5 mg/ml (Calbiochem).
Two-dimensional gel electrophoresis.
Cells were harvested for two-dimensional electrophoresis at time points indicated in Results as follows. The medium was aspirated from flasks, and the cells were rinsed with phosphate-buffered saline, scraped into 5 ml of phosphate-buffered saline, pelleted by centrifugation, and suspended in 80 μl of lysis solution (8 M urea, 4% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, 40 mM Tris base). The extract was kept on ice for 1 h and sonicated, and the insoluble material was pelleted by centrifugation. The soluble fraction was transferred to a new tube, 170 μl of rehydration stock solution (8 M urea, 2% CHAPS, 20 mM dithiothreitol [DTT], bromophenol blue) was added to the sample for a total volume of 250 μl, and 0.5% of pH 3 to 10 IPG buffer (Amersham-Pharmacia Biotech) was added. First-dimension isoelectric focusing was done in an IGPhor electrophoresis unit (Amersham-Pharmacia Biotech). Immobilized pH 3 to 10 linear gradient strips (13 cm in length) were rehydrated with the sample solution for 12 h and then subjected to the following procedures: 500 V for 1 h, 1,000 V for 1 h, and 8,000 V for 2 h, for a total of 17,500 V-h. The immobilized strips were then equilibrated in 10 ml of sodium dodecyl sulfate (SDS) buffer (50 mM Tris [pH 8.8], 6 M urea, 30% glycerol, 2% SDS, 70 mM DTT, bromophenol blue) for 15 min. The immobilized strips were then overlaid onto 6% bisacrylamide gels and sealed with 0.5% agarose in SDS-gel running buffer containing bromophenol blue. Molecular weight markers were placed adjacent to the pH 10 side of the immobilized strips. Gels were subjected to 20 mA for the first hour followed by 30 mA. The electrophoretically separated proteins in the second dimension were then transferred to a nitrocellulose sheet and probed with antibodies to ICP0 and ICP4 as described elsewhere (1).
32P labeling and roscovitine treatment.
HEp-2 cells were seeded and infected as above. At 5 h after infection, the cells were incubated in phosphate-depleted Eagle's minimal essential medium (EMEM) containing dimethyl sulfoxide (DMSO) or 100 μM roscovitine (Calbiotech) in DMSO. The cells were labeled from 6 to 10 h after infection with 200 μCi (per 25-cm2 flask) of 32P-orthophosphate (Amersham) in EMEM (1% serum) containing DMSO or roscovitine and DMSO and harvested as above for two-dimensional gel electrophoresis. After transfer to nitrocellulose, membranes were analyzed with the aid of a Molecular Dynamics Storm 860 PhosphorImager and autoradiography and then immunoblotted with antibodies to ICP0 and ICP4.
In vitro kinase assays.
HEp-2 cells were mock or HSV-1(F) infected as above. Five hours postinfection, roscovitine (10 or 100 μM) or an equivalent volume of vehicle (DMSO) was added to the cells. Cells were harvested 12 h after infection in high-salt lysis buffer (20 mM Tris [pH 8.0], 1 mM EDTA, 0.5% NP-40, 400 mM NaCl, 2 mM DTT, 0.1 mM sodium orthovanadate, 10 mM NaF 100 μg each of phenylmethylsulfonyl fluoride and tolylsulfonyl phenylalanyl chloromethyl ketone per ml, 2 μg each of aprotonin and leupeptin per ml). Equivalent protein concentrations of cell lysates were initially precleared with preimmune sera followed by the addition of 50 μl of a 50% protein A slurry (Sigma). Cdk2 and cdc2 were then immunoprecipitated with their specific antibodies and collected by addition of 20 μl of a 50% protein A slurry. The immunoprecipitates were then washed twice in high-salt lysis buffer, twice in low-salt lysis buffer (20 mM Tris [pH 8.0], 1 mM EDTA, 0.5% NP-40, 1 mM NaCl, 2 mM DTT), and twice in incomplete kinase buffer (50 mM Tris [pH 7.4], 10 mM MgCl2, 5 mM DTT). In vitro kinase reactions were carried out using histone H1 (Boehringer Mannheim) as the substrate. Forty microliters of complete kinase buffer (50 mM Tris [pH 7.4], 10 mM MgCl2, 5 mM DTT, 10 μM ATP, 20 μCi of [γ-32P]ATP, 2 μg of histone H1) was added to immunoprecipitated cdk2 or cdc2. The samples were reacted at 30°C for 20 min, and the reaction was terminated by the addition of SDS-gel loading buffer (2% SDS, 5% β-mercaptoethanol, 50 mM Tris [pH 6.8], 2.75% sucrose) and heated to 95°C for 5 min. The samples were resolved by polyacrylamide gel electrophoresis, transferred to a nitrocellulose sheet, and analyzed by autoradiography. Quantification of 32P phosphorylation of the histone H1 was done with the aid of a PhosphorImager (Storm 860; Molecular Dynamics).
RESULTS
ICP0 and ICP4 are progressively modified over the course of HSV-1 infection.
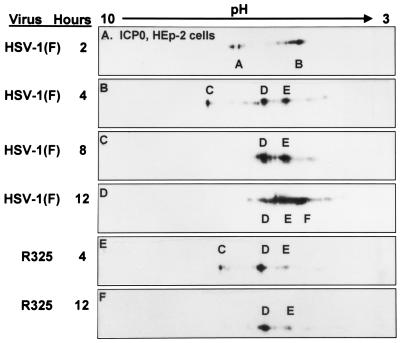
The purpose of these experiments was to determine the sequence of accumulation of modified forms of ICP0 and ICP4 in the course of HSV-1 infection. Infected cells were harvested at 2, 4, 8, and 12 h after infection for HEp-2 cells and at 4, 8, and 12 h after infection of HEL fibroblasts. The cell lysates were then subjected to two-dimensional electrophoresis as described in Materials and Methods. The two-dimensionally separated polypeptides were reacted with antibodies to ICP0 and ICP4. Over the course of the first 12 h of infection, both ICP0 and ICP4 were continuously posttranslationally modified. Figure 1 shows the forms of ICP0 that were detected in HEp-2 cells. At 2 h after infection, two sets of species, designated A and B, were present. At 4 h after infection, A and B were replaced by three new species, C, D, and E. By 8 h after infection, species C disappeared, whereas species D and E accumulated in higher quantities. Finally at 12 h after infection, a reduction in species D occurred and there was a trend toward more negatively charged forms (E and F).
FIG. 1.
Immunoblots of ICP0 in two-dimensionally separated HSV-1- or R325-infected HEp-2 cell lysates. Infected cells were harvested at the indicated times postinfection. Time zero was defined as the time the viral inoculum was added to the cells. Lysates were first separated by charge on linear pH 3 to 10 gradients and then separated by size on 6% bisacrylamide gels as described in Materials and Methods. Blots were reacted with a monoclonal antibody to ICP0. The major isoforms that accumulated are given letter designations.
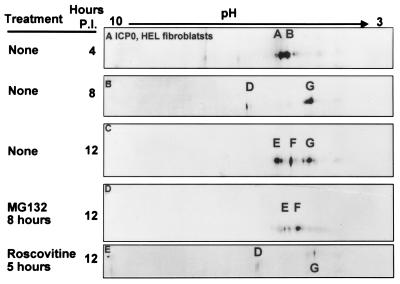
The patterns of posttranslational modifications of ICP0 processing in infected HEL fibroblasts are shown in Fig. 2. At 4 h after HSV-1 infection, the prominent species of ICP0 present were designated A and B. By 8 h after infection, these forms were replaced by a faster, less negatively charged D form and a more abundant, slower-migrating, more negatively charged G species of ICP0. By 12 h after infection, the three major species of ICP0 were E and F in addition to the G form. The C isoform of ICP0 was not detected in lysates of infected HEL fibroblasts, suggesting that the accumulation of this isoform is cell type dependent.
FIG. 2.
Immunoblots of ICP0 in two-dimensionally separated HSV-1-infected HEL fibroblast cell lysates. HSV-1-infected cells were harvested at 4, 8, and 12 h postinfection (P.I.). In addition cells were also treated with MG132 or roscovitine. MG132 was given to HSV-1-infected cells 8 h after infection and harvested at 12 h. Roscovitine was added to HSV-1-infected cells at 5 h after infection, and cells were harvested at 12 h postinfection. Immunoblots were reacted with ICPO monoclonal antibody.
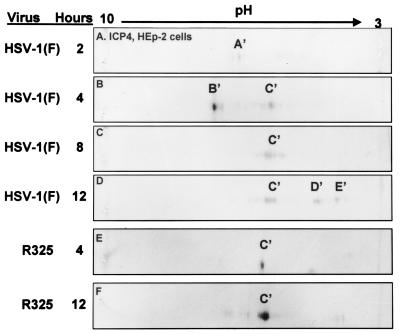
Figure 3 shows the pattern of ICP4 forms that accumulate over the first 12 h after infection in HEp-2 cells. At 2 h after infection, we identified a species designated A′. At 4 h after infection, ICP4 was present as a major species (B′) with a less negative charge and a more highly negatively charged, C′ species. By 8 h after infection, the majority of ICP4 migrated around C′. Finally, at 12 h after infection, as was the case for ICP0, the accumulating ICP4 formed two new, more highly negatively charged species designated D′ and E′ in addition to the preexisting C′ species.
FIG. 3.
Immunoblots of ICP4 in two-dimensionally separated HSV-1-+ and R325-infected HEp-2 cell lysates. Immunoblots were prepared and reacted with the anti-ICP4 monoclonal antibody as described in the legend to Fig. 1.
Phosphorylation of ICP0 and ICP4 is sensitive to roscovitine.
Roscovitine has been reported to be a relatively specific inhibitor of certain cyclin-dependent kinases (cdk2, cdk5, and cdc2) (22). In addition, roscovitine was reported to adversely affect HSV-1 transcription and overall replication (13, 34–36). Elsewhere, this laboratory reported that cdc2 kinase activity is upregulated in HSV-1-infected cells between 8 and 12 h after infection (2). Also, ICP0 and ICP4 both contain multiple consensus phosphorylation sites for cdc2, and a glutathione S-transferase fusion protein encoding ICP0 exon II was phosphorylated by cdc2 kinase (3).
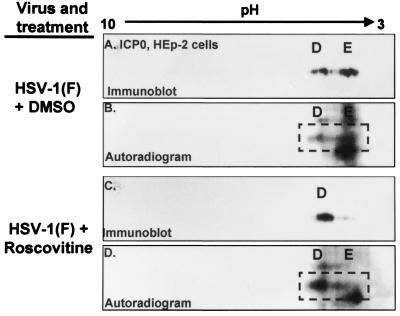
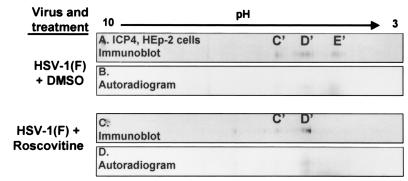
To determine whether roscovitine blocked the accumulation of any of the isoforms of ICP0 or ICP4, HEp-2 cells were infected with HSV-1(F). At 5 h after infection, the cells were treated with 100 μM roscovitine or an equivalent volume of DMSO (0.1%) in phosphate-depleted EMEM. At 6 h after infection, the medium was supplemented with 32P-orthophosphate, and the exposure to roscovitine or DMSO was continued until 10 h after infection. Cells were then harvested and lysed, and whole-cell lysates were subjected to two-dimensional gel electrophoresis. Autoradiograms and immunoblots for ICP0 and ICP4 were done. The results are shown in Fig. 4 and 5.
FIG. 4.
Immunoblots (A and C) and corresponding autoradiograms (B and D) of ICPO in two-dimensionally separated 32P-orthophosphate-labeled HSV-1-infected HEp-2 cell lysates to determine roscovitine-sensitive ICP0 phosphorylation. HSV-1-infected HEp-2 cells were treated with roscovitine or an equivalent volume of DMSO at 5 h after infection in phosphate-free medium. Infected cells were labeled with 32P-orthophosphate from 6 to 10 h postinfection in the presence of roscovitine. Cells were harvested at 10 h postinfection, and whole-cell lysates were subjected to two-dimensional electrophoresis. Membranes were developed by autoradiography and immunoblotted with an ICP0 antibody. Isoform designations are identical to those shown in Fig. 1.
FIG. 5.
Immunoblots (A and C) and corresponding autoradiograms (B and D) of ICP4 in two-dimensionally separated 32P-orthophosphate-labeled HSV-1-infected HEp-2 cell lysates to determine roscovitine-sensitive ICP4 phosphorylation. HSV-1-infected HEp-2 cells were treated with roscovitine or an equivalent volume of DMSO at 5 h after infection in phosphate-free medium. Infected cells were labeled with 32P-orthophosphate from 6 to 10 h after infection in the presence of roscovitine. Cells were harvested at 10 h postinfection, and whole-cell lysates were subjected to two-dimensional electrophoresis. Membranes were developed by autoradiography and immunoblotted with an ICP4 antibody. Isoform designations are idential to those shown in Fig. 3.
At 10 h after infection, the lysates of cells exposed to DMSO contained two isoforms of ICP0 corresponding to isoforms D and E of Fig. 1 that were readily apparent by their reactivity with the anti-ICP0 antibody (Fig. 1 and 4A). These forms corresponded to phosphoforms as determined by autoradiography of the same blot (Fig. 4B). Exposure to roscovitine resulted in a decreased accumulation of the more negatively charged E species of ICP0 and greater accumulation of the less negatively charged D isoform (Fig. 4A and C). The results of autoradiography of the two-dimensionally separated polypeptides from roscovitine-treated infected cell lysate yielded concordant decreased accumulation of the labeled E species compared to that of DMSO-treated controls (Fig. 4B and D).
The same blots were also probed with antibody against ICP4. At 10 h after infection, three species of ICP4 corresponding to the C′, D′, and E′ of Fig. 3 reacted with the anti-ICP4 antibody (Fig. 3D and 5A). All three forms were labeled with 32P-orthophosphate (Fig. 5B). Roscovitine had a similar effect on the accumulation of specific species of ICP4 phosphoproteins. Roscovitine treatment resulted in the loss of the most negatively charged ICP4 species (E′) (Fig. 5C). In lysates of roscovitine-treated cells, there was a greater accumulation of less negatively charged phosphorylated species (C′ and D′). We conclude from this experiment that roscovitine blocked the accumulation of specific, highly negatively charged species of ICP0 (isoform E) and ICP4 (isoform E′) in HEp-2 cells infected with wild-type virus. The forms blocked by roscovitine were those that accumulated in significant amounts at or after 8 h after infection.
Specific forms of ICP0 in HEL fibroblasts were also sensitive to roscovitine treatment (Fig. 2B, C, and E). In this instance roscovitine blocked the accumulation of the E and F species, which normally accumulated between 8 and 12 h after infection. Instead, roscovitine-treated HEL fibroblasts accumulated the less negatively charged D isoform of ICP0 at 12 h after infection that was present in wild-type virus-infected cells at 8 h after infection. The pattern of ICP0 accumulation at 12 h after 7 h of exposure to roscovitine was similar to that observed at 8 h after infection, suggesting that roscovitine blocked further processing of ICP0 in HEL fibroblasts so that forms E and F of ICP0 could not accumulate.
Exposure of HSV-1-infected cells to roscovitine inhibits cdc2 but not the background activity associated with immunoprecipitated cdk2 kinase.
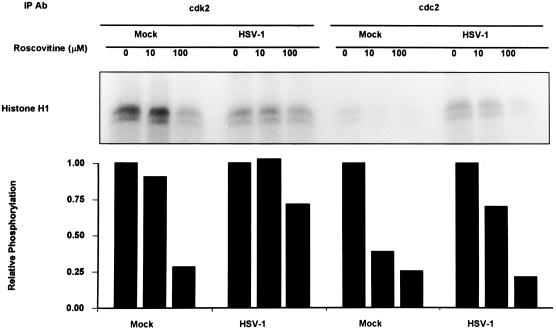
As stated above, roscovitine is a relatively specific inhibitor of cyclin-dependent kinases cdc2, cdk2, and cdk5 (22). To determine if cdk2 or cdc2 is a target of roscovitine in HSV-1-infected cells, the following experiment was done. Mock- or HSV-1-infected cells were exposed to either roscovitine (10 and 100 μM) or DMSO at 5 h after infection. Cdk2 and cdc2 kinase activities were then measured at 12 h after infection. The results are shown in Fig. 6. Mock-infected cells had elevated levels of cdk2 kinase activity, with significantly lower levels of cdc2 activity. In mock-infected cells, a dose response was seen upon addition of roscovitine when histone H1 was used as a substrate. At 100 μM concentrations of roscovitine, both cdk2 and cdc2 resulted in a 75% reduction in phosphorylation of histone H1. In HSV-1-infected cells, cdk2 was not activated. Addition of up to 100 μM roscovitine resulted in only a 25% reduction in histone H1 phosphorylation by cdk2-immunoprecipitated samples from HSV-1-infected lysates. Thus, the level of histone H1 phosphorylation by immunoprecipitated cdk2 from lysates of mock-infected cells treated with 100 μM roscovitine was comparable to cdk2 activity in HSV-1-infected cell lysates with no roscovitine. These results argue that cdk2 activity is shut down in HSV-1 infected cells and that the phosphorylation of histone H1 observed in HSV-1 lysates is background phosphorylation, consistent with the results previously reported for cdk2 kinase activity in HSV-1 infected cells (6, 40). In contrast, immunoprecipitated cdc2 kinase activity is greater in HSV-1-infected cell lysates than in mock-infected cell lysates, as has been previously reported (2). cdc2 kinase activity from HSV-1-infected cell lysates shows a roscovitine dose-dependent response. At 100 μM roscovitine, the cdc2 kinase activity from HSV-1-infected cell lysates was reduced by 80% compared to 0 μM roscovitine.
FIG. 6.
Autoradiograms of histone H1 phosphorylated by in vitro kinase assays using immunoprecipitated cdk2 or cdc2 from HSV-1- or mock-infected cells. HEp-2 cells were HSV-1 or mock infected; 5 h after infection, the medium was replaced with medium containing 0, 10, or 100 μM roscovitine. Cells were harvested 12 h after infection, and cdk2 or cdc2 was immunoprecipitated (IP) with antibodie (Ab) as described in Materials and Methods. Kinase assays were done using histone H1 as the substrate. Phosphorylated histone H1 was quantitated by a PhosphorImager. Relative reduction in the phosphorylation of histone H1 due to roscovitine was determined relative to untreated samples.
We conclude from these studies that roscovitine at the dose used (100 μM) targets cdc2 kinase in HSV-1-infected cells. The effect on cdk2 is inapparent since this cyclin-dependent kinase is either inactive or rendered insusceptible to inhibition by roscovitine.
Involvement of α22/US1.5 in the accumulation of specific forms of ICP0 and ICP4.
Earlier studies have shown that in cells infected with the R325 mutant lacking the carboxyl-terminal domain of ICP22 and also the domain encoding the overlapping US1.5 open reading frame, the expression of γ2 genes was reduced (30, 37). Activation of cdc2 in HSV-1-infected cells is also dependent on ICP22, and overexpression of a dominant negative form of cdc2 resulted in the decreased accumulation of US11, a γ2 protein (2, 3, 38). To examine the effects of α22 deletion virus on ICP0 and ICP4 processing, two-dimensional electrophoresis was done on lysates of HEp-2 cells harvested at 4 and 12 h after infection with R325 deletion mutant. The results shown in Fig. 1 and 3 were as follows.
(i) As illustrated in Fig. 1, the electrophoretic mobility of ICP0 in lysates of R325 mutant-infected HEp-2 cells harvested at 4 h after infection was similar to that of ICP0 harvested from wild-type virus-infected cells harvested at the same time after infection. ICP0 present in lysates of cells harvested 12 h after infection with R325 mutant consisted primarily of the D isoform and a small amount of the E isoform. Absent were the highly negatively charged F species present in lysates of wild-type virus-infected cells. The electrophoretic mobility of ICP0 accumulated in R325 virus-infected cells at 12 h after infection corresponded to that of ICP0 in wild-type virus-infected cells at 8 h after infection.
(ii) As illustrated in Fig. 3, ICP4 present in HEp-2 cells harvested 4 h after infection with R325 mutant was primarily of the C′ isoform, although we also detected trace amounts of the B′ isoform. In contrast to ICP4 harvested from wild-type virus-infected cells, the B′ species was absent (Fig. 3B and E). The posttranslational modification of ICP4 was arrested at this point since the protein present in cells harvested at 12 h after infection with R325 mutant formed primarily the C′ species (Fig. 3E and F). Absent were the highly negatively charged D′ and E′ species which accumulated in cells harvested at 12 h after infection with wild-type virus (Fig. 3D).
In HEL fibroblasts, R325 infection resulted in the accumulation of species of ICP0 that resembled those present in infected cells treated with roscovitine (Fig. 2E and 7B). At 12 h after infection, the G isoform of ICP0 was present in lysates of both wild-type virus- and R325 mutant-infected HEL fibroblasts (Fig. 7). However, form E was not observed in R325-infected cell lysates; instead, a less negatively charged, D species was present. This species was also present in lysates of cells infected with wild-type virus and treated with roscovitine (Fig. 2E).
FIG. 7.
Immunoblots of ICP0 in two-dimensionally separated lysates of HSV-1- or R325-infected HEL fibroblasts. Cells were harvested at 12 h after infection and separated by two-dimensional electrophoresis. Immunoblots were reacted with ICP0 monoclonal antibody. Isoform designations are the same as in Fig. 2.
The pattern of accumulation of the ICP0 isoforms in cells infected with R7914 was similar to that of HEL fibroblasts infected with wild-type virus and treated with MG132.
Studies reported elsewhere have shown that in HSV-1(F)-infected HEL fibroblasts, ICP0 localized in the nucleus early in infection and in the cytoplasmic compartment at late times after infection (14, 40). Treatment of HSV-1-infected HEL fibroblasts late in infection with the proteasome inhibitor MG132 resulted in the relocation of ICP0 to the nucleus (19). The goal of this set of studies was to determine if differential subcellular localization of ICP0 was in part dependent on its posttranslational modifications. To this end, two experiments were done. HEL fibroblasts were infected with R7914 or HSV-1 and then treated with MG132.
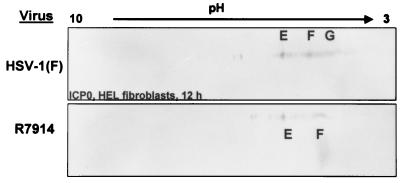
HEL fibroblasts infected with HSV-1 or R7914 were harvested 12 h after infection. Two-dimensional gel electrophoresis revealed that whereas isoforms E and F were found in lysates from both HSV-1- and R7914-infected cells, the highly negatively charged species (G) was present only in HSV-1-infected cell lysates (Fig. 8). Treatment of HSV-1-infected HEL fibroblasts with MG132 at 8 h after infection also resulted in the loss of species G (Fig. 2D). MG132 had no appreciable effect on the accumulation of E and F forms of ICP0. These results suggest that the G species may represent cytoplasmic forms of ICP0 whereas the E and F species represent nuclear species of ICP0.
FIG. 8.
Immunoblots of ICP0 in two-dimensionally separated lysates of HSV-1 or R7914 (alanine substituted for aspartic acid of ICP0)-infected HEL fibroblasts. The cells were harvested at 12 h after infection, lysed, and subjected to two-dimensional electrophoresis. Immunoblots were reacted with anti ICP0 antibody. Isoform designations are the same as in Fig. 2.
DISCUSSION
The fundamental hypothesis underlying these studies is that in the case of multifunctional HSV-1 proteins, posttranslational modifications determine the specific function performed by the protein at the specific time or cellular compartment in which it is localized. ICP4 and ICP0, the two α regulatory proteins selected for this study, are posttranslationally modified by multiple viral and cellular enzymes and play a role in viral replication throughout the viral replicative cycle. In this report we show that consistent with this hypothesis, both ICP4 and ICP0 are sequentially modified throughout at least 12 h after infection and that at least some of the modifications reflect the action of specific enzymes or correlate with localization in specific cellular compartments. The salient features of the data are schematically depicted in Fig. 9 and may be summarized as follows.
FIG. 9.
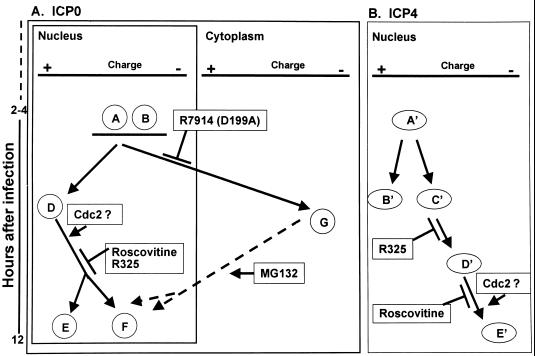
Schematic representation of ICP0 and ICP4 isoforms observed after two-dimensional separation of lysates of HSV-1-infected HEL fibroblasts. (A) Absence of specific isoforms from lysates of HEL fibroblasts infected with HSV-1 mutants or with wild-type virus and treated with roscovitine. Isoforms E and F failed to accumulate in lysates of R325-infected or roscovitine-treated, HSV-1-infected cells. These isoforms are putative nuclear forms, and their processing is dependent on cdc2 kinase. HEL fibroblasts infected with R7914 or wild-type virus and treated with MG132 failed to accumulate the G isoform, and this isoform appears to be associated with ICP0 translocated into the cytoplasm. (B) Isoforms D′ and E′ of ICP4 failed to accumulate in HEL fibroblasts infected with R325 mutant. The most negatively charged, E′ isoform of ICP4 was not detected in significant amounts in cells infected with wild-type virus and treated with roscovitine. The accumulation of the ICP4 isoform E′ may also be dependent on cdc2 kinase.
(i) Over the course of infection, ICP0 and ICP4 are sequentially processed. The general trend was the accumulation of more negatively charged forms of both ICP0 and ICP4 as infection proceeded. This was observed in both infected HEp-2 cells and HEL fibroblasts. Earlier reports indicated that hyperphosphorylated forms of ICP4 are necessary to bind to promoter elements on β and γ genes (28). Increasing accumulation of negatively charged forms of ICP0 and ICP4 is consistent with the requirements for post-α gene expression and with the evidence that ICP4 is required throughout viral replication.
(ii) We have previously shown that cdc2 kinase activity is enhanced in HSV-1-infected cells (2). A central question related to that observation is why cdc2 was activated so late in the replicative cycle. This question was solved to some extent in studies with an inactive, dominant negative mutant form of cdc2 (3, 38). Cells infected with wild-type virus and transfected with cdc2 dominant negative construct expressed representative α, β, and γ1 genes but failed to accumulate significant amounts of US11, a representative γ2 protein. Optimal expression of γ2 genes is in part dependent on ICP22 and UL13 (27, 32). A cascade can be envisioned involving UL13 and ICP22 activating cdc2, resulting in enhanced γ2 gene expression. The data in the present study add further weight to such a scenario. Forms of ICP0 and ICP4 that accumulated between 8 and 12 h after HSV-1 infection were sensitive to both roscovitine as well as infection by the recombinant R325, which lacks the domain encoding the US1.5 protein and the coterminal carboxyl-terminal domain of ICP22.
Roscovitine treatment of HSV-1-infected cells resulted in a loss of specific phospholabeled forms of both ICP0 and ICP4 (Fig. 4, 5, and 9). The forms of ICP0 and ICP4 phospholabeled in the presence of roscovitine may reflect the effects of other kinases that phosphorylate ICP0 and ICP4. Roscovitine is a relatively selective inhibitor of cdk2, cdc2, and cdk5 (22). The effects of roscovitine on HSV-1 replication and gene transcription have been previously reported (13, 34–36). The major effect of roscovitine in HSV-1-infected cells was on cdc2; the cdk2-associated activity, the other target of roscovitine, either represented background kinase activity not associated with this enzyme or was shielded from the inhibitory effects of roscovitine.
The absence of the carboxyl-terminal domain of ICP22 and the corresponding absence of US1.5 protein had a drastic effect on the posttranslational modification of ICP0 and ICP4. In contrast to wild-type virus-infected cells, cells infected with the R325 mutant virus accumulated at 12 h after infection the same forms of ICP0 and ICP4 as those that were present at 4 h after infection with either wild-type or mutant virus (Fig. 9). The missing forms were those that were more negatively charged and that were sensitive to roscovitine. These observations are consistent with the data showing that activation of cdc2 requires intact α22/US1.5 gene products. In light of the observation reported earlier that exon II of ICP0 is a substrate for cdc2, it is likely that ICP0 and ICP4 serve as bona fide in vivo substrates for cdc2. However, it appears that ICP22 may influence the activity of other kinases as well.
(iii) The results presented in this study indicate a correlation between the extent of posttranslational modifications of ICP0 and its localization in the infected cell. In HEL fibroblasts, ICP0 initially localizes to the nucleus (14, 40) and later, between 5 and 9 h after infection, is translocated to the cytoplasm. One mechanism for altering subcellular localization of proteins is through posttranslational processing (12). In lysates of HSV-1-infected HEL fibroblasts, a novel, highly negative charged form of ICP0 appeared between 4 and 8 h after infection (isoform G [Fig. 2]). Two lines of evidence support that this highly negatively charged form of ICP0 in HEL cells correlates with cytoplasmic localization. First, ICP0 encoded by the recombinant virus R7914 accumulates in the nucleus and is not translocated to the cytoplasm (40). In HEL fibroblasts infected with the R7914 mutant, the highly negatively charged G form did not accumulate (Fig. 8). Second, in cells infected with wild-type virus and treated with MG132 at late times after infection, ICP0 is translocated from the cytoplasm to the nucleus (again), the D form of ICP0 was not detected in HEL fibroblasts infected with HSV-1(F) and treated with MG132 late in infection (Fig. 2C and D). The latter observation is particularly significant since it suggests that the posttranslational modification associated with the G form of ICP0 is reversible and specifically associated with the cytoplasmic localization of the protein. Whereas the G form of ICP0 is cytoplasmic, the E and F isoforms may represent nuclear species.
The G form of ICP0 was present in cells infected with R325 and in cells infected with wild-type virus and exposed to inhibitory concentrations of roscovitine. These results indicate that neither ICP22/US1.5 protein or cdc2 is required for cytoplasmic localization of ICP0.
(iv) Finally, we observed differences in the electrophoretic mobility of ICP0 and ICP4 derived from HEp-2 cells or HEL fibroblasts infected with wild-type or mutant virus. A notable example of these differences is the absence of the C isoform of ICP0 in lysates of wild-type virus-infected HEL fibroblasts. The differences most likely reflect posttranslational modifications of the viral proteins effected by enzymes specific for each cell type. The significance of these observations stems from the observation that the level of accumulation of many HSV-1 proteins varies depending on the cell line. In this laboratory at least, several cell lines are screened to determine the level of accumulation of specific proteins. The significant aspect relevant to this study are deletion mutants in α22/US1.5. The recombinant virus R325, for example, replicates almost as well as wild-type virus in HEp-2 cells but at much reduced levels in primary human cell lines or in cell lines of rodent or rabbit derivation (37). Cell infected with this mutant underproduce a subset of γ2 proteins; the magnitude of the effect is cell type dependent (27, 37). It is conceivable that cell type specificity reflects the mixture of enzymes available for posttranslational modifications of ICP0 and ICP4 and that host enzymes can compensate, at least in part, for the absence of appropriate viral regulatory proteins.
ACKNOWLEDGMENTS
We thank Charles Van Sant for invaluable advice, Alice P. W. Poon for a careful reading of the manuscript, and Vladimir Mogilner for photographic services.
These studies were aided by Public Health Service grants CA87761, CA83939, CA71933, and CA78766 U from the National Cancer Institute. R.H. is a Howard Hughes Medical Institute predoctoral fellow.
REFERENCES
- 1.Ackermann M, Braun D K, Pereira L, Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani S J, Brandimarti R, Weichselbaum R R, Roizman B. The disappearance of cyclins A and B and the increase in activity of the G2/M phase cellular kinase cdc2 in herpes simplex virus 1-infected cells requires the expression of α22/US1.5 and UL13 viral genes. J Virol. 2000;74:8–15. [PMC free article] [PubMed] [Google Scholar]
- 3.Advani S J, Weichselbaum R R, Roizman B. The role of cdc2 in the expression of herpes simplex virus genes. Proc Natl Acad Sci USA. 2000;97:10996–11001. doi: 10.1073/pnas.200375297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaho J A, Roizman B. ICP4, the major regulatory protein of herpes simplex virus, shares features common to GTP-binding proteins and is adenylated and guanylated. J Virol. 1991;65:3759–3769. doi: 10.1128/jvi.65.7.3759-3769.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaho J A, Mitchell C, Roizman B. Guanylylation and adenylylation of the alpha regulatory proteins of herpes simplex virus require a viral beta or gamma function. J Virol. 1993;67:3891–3900. doi: 10.1128/jvi.67.7.3891-3900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehmann G L, McLean T I, Bachenheimer S L. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology. 2001;267:335–349. doi: 10.1006/viro.1999.0147. [DOI] [PubMed] [Google Scholar]
- 7.Ejercito P, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 8.Everett R D, Maul G G. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett R D, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:566–577. doi: 10.1093/emboj/16.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett R D, Earnshaw W C, Findlay J, Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999;18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett R D. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J Virol. 2000;74:9994–10005. doi: 10.1128/jvi.74.21.9994-10005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hood J K, Silver P A. In or out? Regulating nuclear transport. Curr Opin Cell Biol. 1999;11:241–247. doi: 10.1016/s0955-0674(99)80032-5. [DOI] [PubMed] [Google Scholar]
- 13.Jordan R, Schang L, Schaffer P A. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J Virol. 1999;73:8843–8847. doi: 10.1128/jvi.73.10.8843-8847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 alpha regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Tanaka M, Yokoymama A, Matsuda G, Kato K, Kagawa H, Hirai K, Roizman B. Herpes simplex virus 1 alpha regulatory protein ICP0 functionally interacts with cellular transcription factor BMAL1. Proc Natl Acad Sci USA. 2001;98:1877–1882. doi: 10.1073/pnas.041592598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King R W, Jackson P K, Kirschner M W. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 18.Leopardi R, Michael N, Roizman B. Repression of the herpes simplex virus 1 alpha 4 gene by its gene product (ICP4) within the context of the viral genome is conditioned by the distance and stereoaxial alignment of the ICP4 DNA binding site relative to the TATA box. J Virol. 1995;69:3042–3048. doi: 10.1128/jvi.69.5.3042-3048.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez P, Van Sant C, Roizman B. Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J Virol. 2001;75:3832–3840. doi: 10.1128/JVI.75.8.3832-3840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin O, Meggio F, Draetta G, Pinna L A. The consensus sequences for cdc2 kinase and for casein kinase-2 are mutually incompatible. A study with peptides derived from the beta-subunit of casein kinase-2. FEBS Lett. 1992;301:111–114. doi: 10.1016/0014-5793(92)80221-2. [DOI] [PubMed] [Google Scholar]
- 21.McLean T I, Bachenheimer S L. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meijer L, Borgne A, Mulner O, Chong J P, Blow J J, Inagaki N, Inagaki M, Delcros J G, Moulinoux J P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 23.Michael N, Spector D, Mavromara-Nazos P, Kristie T M, Roizman B. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science. 1988;239:1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- 24.Michael N, Roizman B. Binding of the herpes simplex virus major regulatory protein to viral DNA. Proc Natl Acad Sci USA. 1989;86:9808–9812. doi: 10.1073/pnas.86.24.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell C A, Blaho J A, McCormick A L, Roizman B. The nucleotidylation of herpes simplex virus 1 regulatory protein α22 by human casein kinase II. J Biol Chem. 1997;272:25394–25400. doi: 10.1074/jbc.272.40.25394. [DOI] [PubMed] [Google Scholar]
- 26.Ogle W O, Ng T I, Carter K L, Roizman B. The UL13 protein kinase and the infected cell type are determinants of post-translational processing modifications of ICP0. Virology. 1997;235:406–413. doi: 10.1006/viro.1997.8710. [DOI] [PubMed] [Google Scholar]
- 27.Ogle W O, Roizman B. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J Virol. 1999;73:4305–4315. doi: 10.1128/jvi.73.5.4305-4315.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papavassiliou A G, Wilcox K W, Silverstein S J. The interaction of ICP4 with cell/infected-cell factors and its state of phosphorylation modulate differential recognition of leader sequences in herpes simplex virus DNA. EMBO J. 1991;10:397–406. doi: 10.1002/j.1460-2075.1991.tb07961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parkinson J, Lees-Miller S P, Everett R D. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J Virol. 1999;73:650–657. doi: 10.1128/jvi.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 31.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2296. [Google Scholar]
- 34.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schang L M, Rosenberg A, Schaffer P A. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schang L M, Rosenberg A, Schaffer P A. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J Virol. 2000;74:2107–2020. doi: 10.1128/jvi.74.5.2107-2120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant defective in the alpha 22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 39.Van Sant C, Kawaguchi Y, Roizman B. A single amino acid substitution in the cyclin D binding domain of the infected cell protein no. 0 abrogates the neuroinvasiveness of herpes simplex virus without affecting its ability to replicate. Proc Natl Acad Sci USA. 1999;96:8184–8189. doi: 10.1073/pnas.96.14.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Sant C, Lopez P, Advani S J, Roizman B. Role of cyclin D3 in the biology of herpes simplex virus ICP0. J Virol. 2001;75:1888–1898. doi: 10.1128/JVI.75.4.1888-1898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilcox K W, Kohn A, Sklyanaskaya E, Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980;33:167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia K, Knipe D M, DeLuca N A. Role of protein kinase A and the serine-rich region of herpes simplex virus type 1 ICP4 in viral replication. J Virol. 1996;70:1050–1060. doi: 10.1128/jvi.70.2.1050-1060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia K, DeLuca N A, Knipe D M. Analysis of the phosphorylation sites of herpes simplex virus type 1 ICP4. J Virol. 1996;70:1061–1071. doi: 10.1128/jvi.70.2.1061-1071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao F, Schaffer P A. Physical interaction between the herpes simplex virus type 1 immediate-early regulatory proteins ICP0 and ICP4. J Virol. 1994;68:8158–8168. doi: 10.1128/jvi.68.12.8158-8168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhi Y, Sandri-Goldin R M. Analysis of the phosphorylation sites of herpes simplex virus 1 regulatory protein ICP27. J Virol. 1999;73:3246–3257. doi: 10.1128/jvi.73.4.3246-3257.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]