Abstract
The Gag polyprotein of human immunodeficiency virus type 1 (HIV-1) organizes the assembly of nascent virions at the plasma membrane of infected cells. Here we demonstrate that a population of Gag is present in distinct raft-like membrane microdomains that we have termed “barges.” Barges have a higher density than standard rafts, most likely due to the presence of oligomeric Gag-Gag assembly complexes. The regions of the Gag protein responsible for barge targeting were mapped by examining the flotation behavior of wild-type and mutant proteins on Optiprep density gradients. N-myristoylation of Gag was necessary for association with barges. Removal of the NC and p6 domains shifted much of the Gag from barges into typical raft fractions. These data are consistent with a model in which multimerization of myristoylated Gag proteins drives association of Gag oligomers into raft-like barges. The functional significance of barge association was revealed by several lines of evidence. First, Gag isolated from virus-like particles was almost entirely localized in barges. Moreover, a comparison of wild-type Gag with Fyn(10)Gag, a chimeric protein containing the N-terminal sequence of Fyn, revealed that Fyn(10)Gag exhibited increased affinity for barges and a two- to fourfold increase in particle production. These results imply that association of Gag with raft-like barge membrane microdomains plays an important role in the HIV-1 assembly process.
The Gag protein of human immunodeficiency virus type 1 (HIV-1) is capable of directing the formation of virus-like particles (VLPs) in host cells (16, 20). These particles have morphology similar to that of immature virions. Cells expressing Gag are thus a useful system for monitoring viral assembly. Gag proteins bind to the plasma membrane by virtue of a membrane binding motif (the M domain) located within the N-terminal 31 amino acids of the protein (13, 20, 22, 59, 68). This motif consists of a covalently bound fatty acid, myristate, and a cluster of basic amino acid residues. Binding energy is provided by the myristate-plus-basic-residue motif via hydrophobic and electrostatic interactions, respectively (38, 68).
Immature virions and VLPs contain ∼1,200 to 1,500 Gag protein molecules (28, 29). Multimerization of Gag molecules begins at early stages of assembly and drives the formation of particles (60). The Gag protein contains several binding interfaces that promote Gag-Gag interactions. A trimer interface is located in the MA domain, and at least two dimer interfaces are found in CA (6, 17, 24, 29, 35, 37). The p2 spacer peptide is required for high-order multimerization and assembly (1, 27, 36). The interaction domain in NC has been shown to promote membrane binding, most likely via cooperative effects (13, 51). The RNA-binding zinc fingers within NC may also support Gag-Gag interactions indirectly, by recruiting an RNA thread upon which individual Gag molecules are strung (10, 11). The p6 domain contains determinants of particle size which may reflect an interaction motif in this domain as well (18).
Recent studies from this laboratory have established that, at steady state, Gag proteins are predominantly localized to the plasma membrane (23, 60). Examination of Gag-expressing cells by confocal imaging revealed the presence of Gag assembly complexes in a punctate staining pattern, suggesting that HIV-1 assembly occurs in discrete regions or subdomains of the plasma membrane. We hypothesized that Gag might be localizing to rafts, small subdomains within the plasma membrane that are highly enriched in sphingomyelin, glycosphingolipids, and cholesterol (7, 8, 43, 57, 58). Raft lipids exist in a liquid ordered phase that is segregated from phospholipid-rich domains (45). The unique lipid composition of the rafts serves to recruit proteins that are modified with saturated fatty acids, such as myristate and palmitate. For example, Src family kinases and G alpha subunits are enriched in rafts, and dual fatty acylation has been shown to be necessary and sufficient for raft association of these proteins (46, 47, 55, 56, 61). In addition, proteins modified with glycophosphatidylinositol anchors are bound to rafts on the exoplasmic leaflet of the plasma membrane (9, 47). Several transmembrane proteins have also shown to be localized in rafts. The length of the membrane-spanning domains of intrinsic membrane proteins may determine affinity for specific lipid phases, with longer transmembrane helices packing more efficiently into rafts by virtue of their increased thickness (7).
The principal biochemical tool used to study rafts is extraction with cold, nonionic detergent, such as Triton X-100 (TX-100) or NP-40 (9, 25, 31). Raft lipids are uniquely resistant to such treatment. Raft-associated proteins, by virtue of their continued association with raft lipids, exhibit a buoyant density that allows them to be separated from nonraft membrane proteins and from soluble proteins on density gradients.
Several studies have proposed a role for rafts in the HIV-1 life cycle. The virion envelope possesses a lipid profile quite similar to that of rafts, namely, a marked enrichment in sphingomyelin and cholesterol with respect to the plasma membrane of host cells, as well as a fluidity suggestive of a lipid ordered phase (3, 4, 21). Removal of cholesterol, an essential component of raft domains, renders virions noninfectious (14). In addition, the two major proteins present in the viral envelope, Gag and Env, contain bound fatty acids that might direct them into raft domains during early stages of assembly (62, 66). It was recently shown that palmitoylation of Env is necessary for its localization to rafts, as determined by detergent extraction and density gradient centrifugation (50). Moreover, a subpopulation of Gag was detected in raft-containing fractions of density gradients (40). In this study, we show that HIV-1 Gag is localized to distinct raft-like membrane microdomains that we have termed “barges.” We demonstrate that the myristoylated M domain is necessary but not sufficient for barge targeting and that multimerization of Gag proteins regulates barge association. In addition, these experiments reveal a physiological significance for barge localization by demonstrating that a Gag mutant that is more effectively targeted to barges exhibits increased VLP production.
MATERIALS AND METHODS
Antibodies and reagents.
Rabbit anti-p24CA antiserum or anti-p24CA monoclonal antibody (ABI, Columbia, Md.)was used to detect Gag. Rabbit anti-Fyn antiserum was used for immunoprecipitation of Fyn proteins. Sheep anti-gp120 antiserum was obtained from the National Institute of Health AIDS Research and Reference Reagent Program (Rockville, Md.). The following antibodies were purchased as indicated: anti-Fyn and anti-Lck monoclonal antibodies from Transduction Labs, Lexington, Ky.; anti-caveolin-1 polyclonal antibody and anti-CD45 monoclonal antibody from Santa Cruz Biotechnology, Santa Cruz, Calif.; anti-green fluorescent protein (GFP) monoclonal antibody from Roche, Indianapolis, Ind.; and anti-Na+-K+ ATPase (α3 subunit) from Biomol, Plymouth Meeting, Pa.. Optiprep was purchased from Gibco BRL Life Technologies (Grand Island, N.Y.). TX-100 was purchased from Fisher Biotech (Fair Lawn, N.J.). Methyl-β-cyclodextrin (mβCD), cholera toxin B subunit-peroxidase conjugate, and cytochalasin D were purchased from Sigma (St. Louis, Mo.). DNase-free RNase was purchased from Boehringer Mannheim (Indianapolis, Ind.). [1,2-3H(N)]-cholesterol ([3H]cholesterol) with a specific activity of ∼50 Ci/mmol was purchased from NEN Life Science Products (Boston, Mass.).
DNA plasmids.
Pr55Gag was expressed either from the noninfectious proviral vector pHXB2ΔBalID25S (68) or from the Rev-independent vector pCMV5 Gag in the absence of other HIV-1 gene products (60). Construction of pCMV5 Fyn, pCMV5 G2AFyn, and pCMV5 G2AFynKRas was described previously (2, 61). pCMV5 GagΔp15 was generated by the insertion of a stop codon following the p2 spacer peptide of Gag (Marc Tritel, unpublished results). pCMV5 Fyn(10)Gag, pCMV5 FynGagΔp6, and pCMV5 FynGagΔp15 were made by replacing the first 10 codons of Gag with those of the human Fyn protein followed by the insertion of a stop codon following the p1 spacer peptide (for Δp6) or the p2 spacer peptide (for Δp15) (Wouter van't Hof, unpublished results). Construction of pVALO Gag31eGFP was performed as described previously (69) with the exception that the enhanced GFP (eGFP) coding sequence was used (Tritel, unpublished).
Cell culture and transfections.
COS-1 cells (American Type Culture Collection, Manassas, Va.) were maintained in 10% fetal bovine serum in Dulbecco modified Eagle medium (DMEM) and passaged every 2 to 3 days. For transfections, COS-1 cells were seeded to approximately a 25% density and transfected with 3 to 5 μg of plasmid using Lipofectamine 2000 (Gibco BRL Life Technologies). Jurkat T cells (a kind gift from Leonard Freedman, Memorial Sloan-Kettering Cancer Center, New York, N.Y.) were maintained in 10% fetal bovine serum in RPMI medium and passaged every 2 days. For transfections, Jurkat cells were seeded to 2 × 105/ml and electroporated the following day. On the day after electroporation, the cells were activated with 50 ng of phorbol 12-myristate 13-acetate per ml and 2 μg of phytohemagglutinin (Sigma, St. Louis, Mo.) per ml.
Flotation experiments.
Five-fraction flotation gradients were performed as a modified version of a published protocol (33). Essentially, one day posttransfection COS-1 cells were split 1:2 and plated on 10-cm tissue culture dishes. The following day, the plates were cooled on ice, washed once with ice cold 10 mM Tris (pH 7.4)–100 mM NaCl–1 mM EDTA (STE) and harvested in STE with a rubber policeman. Cells were pelleted in a clinical centrifuge at 500 × g, and the wash buffer was removed by aspiration (Jurkat cells were harvested directly by centrifugation, followed by an STE wash). The cell pellet was resuspended in 300 μl of TNET buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.5% TX-100) containing a protease inhibitor cocktail. Cells were then extracted on ice for 20 min, Dounce homogenized, and adjusted to 35% Optiprep. One-third of the cell extract (240 μl) was placed at the bottom of an SW55 centrifuge tube and overlaid with 3.5 ml of 30% Optiprep in TNET followed by 200 μl of TNET. Following centrifugation at 170,000 × g at 4°C for 4 h, five 800-μl fractions were collected from the top of the gradient. The fractions were adjusted to 1× RIPA buffer (150 mM NaCl, 1.0% NP-40, 0.5% Na deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM Tris [pH 8.0]) and clarified by centrifugation for 10 min at 4°C at 14,000 rpm in an Eppendorf centrifuge. Aliquots of each clarified fraction were precipitated with 20% trichloroacetic acid and proteins were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) followed by Western blotting.
Eight-fraction gradients were performed in a similar fashion with the following modifications. Following Dounce homogenization, the cell extracts were adjusted to 50% Optiprep, after which 600 μl was overlaid with 1 ml each of 40, 30, and 20% Optiprep in TNET and finally with 400 μl of 10% Optiprep in TNET. After centrifugation, eight 500-μl fractions were collected and analyzed as described above. For flotations performed in the absence of detergent, the method was essentially the same, with the exception that TX-100 was omitted from all buffers.
For myristoylation inhibition experiments, cells were treated with 2-hydroxymyristate (2-OH-myr) 24 h after transfection as described previously (65). Briefly, cells were washed with serum-free DMEM and incubated overnight in 100 μM 2-OH-myr in DMEM containing 2% dialyzed fetal bovine serum and 0.5% defatted bovine serum albumin. For some experiments, 25 μg of cytochalasin D per ml in dimethyl sulfoxide was added to cells 1 h prior to detergent extraction to disrupt the actin cytoskeleton. Dimethyl sulfoxide alone was used as a control. Five hundred nanograms of RNase was added to the TNET buffer during some extractions to degrade RNA within the extract.
For cholesterol depletion experiments, the cell culture medium was replaced with serum-free DMEM plus 10 mg of mβCD/ml 1 h prior to the experiment.
Quantitation of Western blots.
For visualization of antibody-reactive bands on Western blots, horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, N.J.) were used according to the manufacturer's instructions. The blots were exposed to BioMax MR film (Eastman Kodak, Rochester, N.Y.), and films were scanned with an Epson Expression 636 scanner (Epson, Long Beach, Calif.). Quantitations of band densities were made using MacBAS software version 2.0 (Fuji Photo Film Co.; Kohshin Graphic Systems Inc.). Photos obtained within the linear range of the film were used for quantitation. When necessary, fractions with large amounts of protein were diluted before loading on gels and quantitations were adjusted accordingly.
Detection of GM1 and cholesterol.
For some experiments, cells were incubated with cholera toxin B subunit-peroxidase conjugate according to a previously described protocol (39) to label GM1. The labeled cells were subjected to flotation analysis, and 10-μl aliquots from each fraction were analyzed by dot blotting and enhanced chemiluminescence. For cholesterol-labeling experiments, cells were treated with [3H]cholesterol using a published method (53). Following Optiprep flotation, fractions were collected directly into scintillation vials and counted.
Preparation of VLPs.
VLPs were isolated essentially as described previously (15). Briefly, about 24 h posttransfection, COS-1 cells were fed with fresh complete medium. After another 24 h, the medium was collected and centrifuged at 1,000 × g for 20 min at 4°C. The clarified medium was layered on top of a 20% (wt/vol) sucrose cushion in phosphate-buffered saline and centrifuged for 2 h at 145,000 × g in an SW40 rotor. VLPs were recovered from the bottom of the tube. For earlier time points, the medium was collected 2 or 4 h prior to the experiment. To measure transfected protein expression, cells were harvested in RIPA buffer and analyzed by Western blotting as described above. To obtain sufficient amounts of VLPs for flotation experiments, six plates of transfected COS-1 cells were allowed to produce particles for 48 h. The supernatant was collected, clarified, and centrifuged for 2 h at 141,000 × g in an SW28 rotor.
RESULTS
Raft fractionation of Pr55Gag in Jurkat and COS-1 cells.
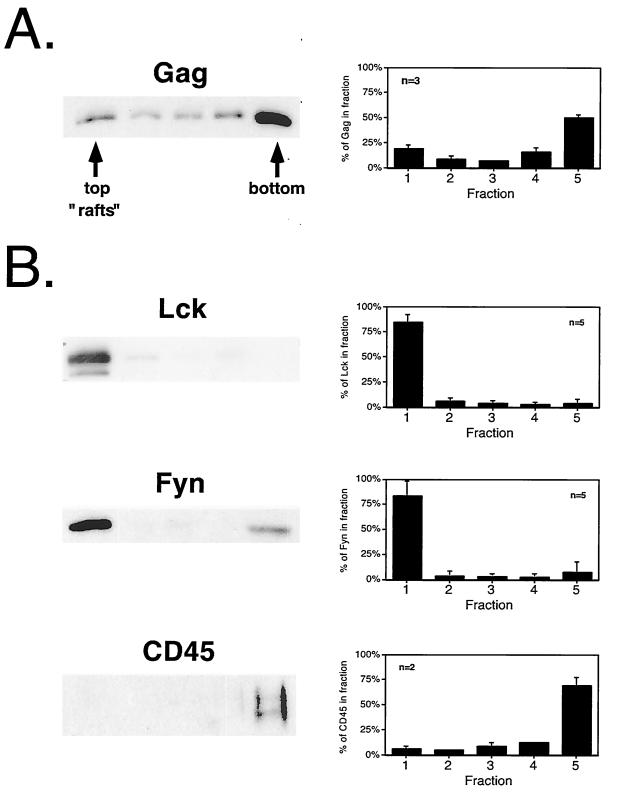
In light of recent evidence suggesting a role for lipid rafts in budding of HIV-1 (40, 50), we designed experiments to analyze the structural requirements for HIV-1 Pr55Gag localization to raft microdomains. Lipid raft microdomains are resistant to solubilization at 4°C with nonionic detergents. Raft-associated proteins exhibit a relatively buoyant density after detergent treatment due to continued association with raft-derived lipids. These protein-lipid complexes can be resolved from soluble and detergent-sensitive cellular factors by flotation on density gradients. We initially chose the Jurkat T-cell leukemia cell line for our experiments because of its relevance for HIV-1 biology and because of previous evidence for an enrichment of Src family kinases in rafts in Jurkat cells (26). Pr55Gag was expressed in Jurkat T cells after electroporation with the noninfectious proviral vector pHXB2ΔBalID25S. At 48 h after electroporation, the cells were harvested and extracted on ice in buffer containing 0.5% TX-100. After flotation on Optiprep step gradients, five fractions were collected such that the top fraction (fraction 1) contained the 0%–30% Optiprep interface, to which rafts float. The distribution of Gag as well as endogenous T-cell proteins is depicted in Fig. 1. Western blot analysis of gradients fractions revealed that ∼19% of the total Pr55Gag was recovered from the raft fraction. In contrast, 75 to 80% of the endogenous Src family kinases Lck and Fyn was observed to be raft associated. Thus, the flotation profile of Pr55Gag was not indicative of a large degree of raft targeting and, in fact, more closely resembled that of the negative control CD45, an endogenous plasma membrane protein that has been shown to be excluded from rafts (26, 48).
FIG. 1.
Raft fractionation of Pr55Gag and endogenous cellular proteins in Jurkat cells. Jurkat cells were transfected with the proviral vector pHXB2ΔBalID25S and harvested 48 h posttransfection. Following extraction on ice with 0.5% TX-100, the lysates were subjected to flotation on Optiprep gradients as described in Materials and Methods. Five fractions were collected, trichloroacetic acid precipitated, and analyzed by SDS-PAGE followed by Western blotting. (A) Detection of Pr55Gag with rabbit anti-p24CA serum. A representative gel is shown. The top of the gradient (fraction 1) contains the raft fraction. At right is the quantitation of several independent experiments. Data are means and standard deviations. (B) Endogenous Jurkat proteins from the same fractions were detected using monoclonal antibodies to the indicated proteins.
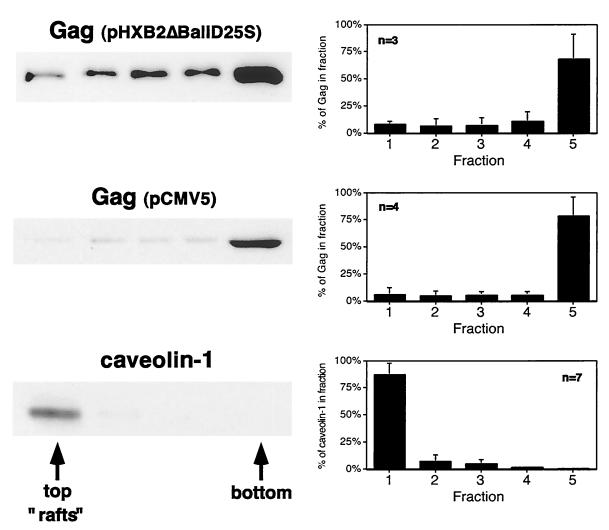
The raft flotation experiments were repeated in COS-1 cells transfected with Gag-expressing plasmids. We used caveolin-1 as an endogenous protein marker for lipid rafts. Caveolins are present in caveolae, flask-shaped invaginations of the plasma membrane which are a subset of lipid rafts (5, 54). As depicted in Fig. 2, the vast majority of caveolin-1 partitioned to the raft fraction. In contrast, only ∼6 to 8% of Pr55Gag was recovered from the raft fraction. Similar results were obtained when Gag was expressed from the plasmid pCMV5 Gag, in the absence of other HIV-1 gene products, or from pHXB2ΔBalID25S (which expresses other viral gene products, including Env). It is not known why less Gag fractionates with rafts in COS-1 cells than in Jurkat cells. However, similar cell type-specific differences in raft localization have also been observed for the Src family kinase Fyn (65).
FIG. 2.
Raft fractionation of Pr55Gag in COS-1 cells. COS-1 cells were transfected with pHXB2ΔBalID25S or pCMV5 Gag and subjected to extraction on ice with 0.5% TX-100 and flotation on five-fraction Optiprep gradients. Representative Western blots are shown on the left. Pr55Gag was detected with rabbit anti-p24CA serum. Caveolin-1 was detected with anti-caveolin-1 polyclonal antibody. Quantitations of several independent experiments are depicted graphically on the right.
Addition of a myristoylated, palmitoylated sequence from Fyn to the N terminus of Gag increases raft localization.
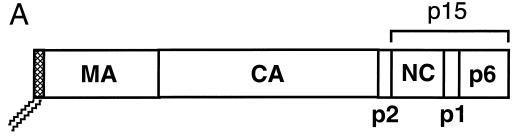
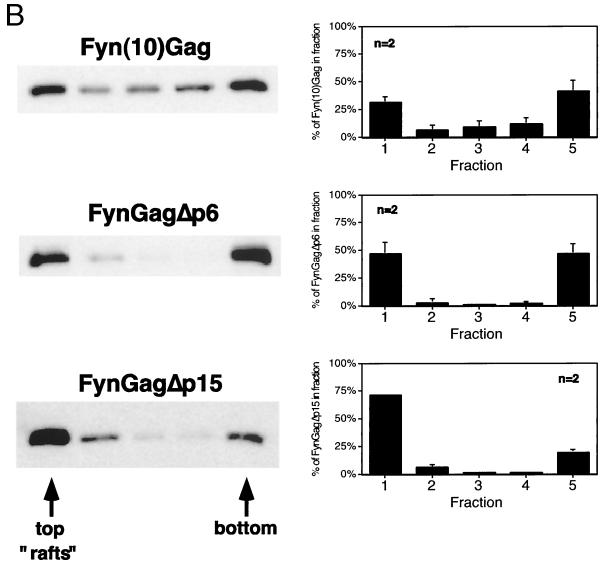
To increase the efficiency of targeting Gag to rafts, we designed the plasmid pCMV5 Fyn(10)Gag, which encodes a chimeric protein in which the first 10 amino acids of the human Fyn protein replace those of Gag (Fig. 3A). Fyn(10)Gag contains an N-terminal sequence that is both myristoylated and palmitoylated (56), thereby providing a raft-targeting motif to the chimeric protein. COS-1 cells were transfected with pCMV5 Fyn(10)Gag and subjected to the extraction and flotation protocol described above. As depicted in Fig. 3B, ∼31% of Fyn(10)Gag was found in the raft fraction of the Optiprep gradients, compared to ∼6% of wild-type Gag (Fig. 2). Thus, attachment of a dually fatty acylated raft-targeting motif to Gag increased the amount of chimeric Gag protein that was recovered in rafts.
FIG. 3.
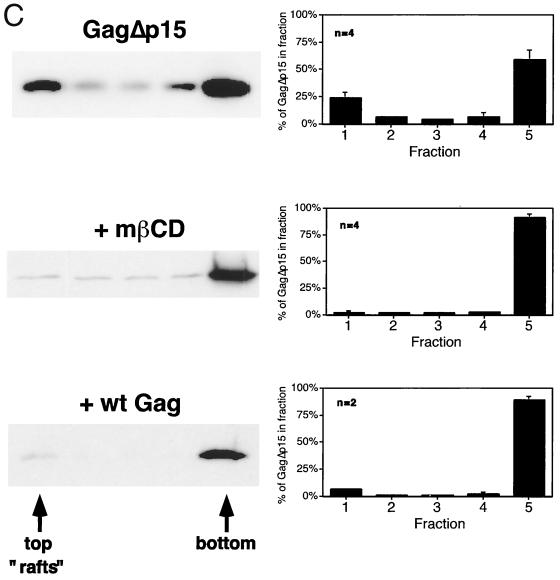
Gag-Gag interactions interfere with the isolation of Gag proteins from low-buoyant-density raft fractions. (A) Diagram of the Fyn-Gag chimeric proteins. The hatched box represents the coding sequence derived from Fyn (squiggles represent posttranslational modification of this sequence with myristate and palmitate). Open boxes designate the boundaries of the domains of Pr55Gag. Also shown are the p6 domain and p15 region, which are deleted from FynGagΔp6 and FynGagΔp15, respectively. (B) COS-1 cells were transfected with the indicated constructs and subjected to raft flotation. Representative Western blots are shown on the left, and quantitations are on the right. (C) COS-1 cells were transfected with pCMV5 GagΔp15 or cotransfected with both pCMV5 Gag and pCMV5 GagΔp15 (+ wt Gag). Where indicated, cells were treated with 10 mg of mβCD/ml prior to the experiment. Cell extracts were processed according to the five-fraction flotation protocol and analyzed by Western blotting. Representative blots are on the left, and quantitations of the percentage of GagΔp15 in each fraction are on the right. The distribution of wild-type Gag was essentially unchanged from that seen in single-transfection experiments (data not shown). Approximately equal amounts of wild-type Gag and Gag mutant proteins were expressed.
Removal of Gag multimerization domains increases recovery of Gag proteins from raft fractions.
We suspected that high-density protein-protein and/or protein-RNA complexes formed by multimerized Gag proteins might interfere with the ability of full-length Gag proteins to float up through the 30% Optiprep layer of the gradients. To test this possibility, COS-1 cells were transfected with truncated versions of Fyn(10)Gag that lack portions of the Gag sequence implicated in Gag-Gag multimerization (Fig. 3A). As depicted in Fig. 3B, FynGagΔp6, a construct that lacks the p6 domain, was enriched in rafts (∼47%) compared to Fyn(10)Gag. Further removal of the NC, p1, and p6 domains resulted in a protein (FynGagΔp15) that partitioned primarily into rafts (∼72%). We conclude that Gag-Gag multimerization masks the ability of Fyn(10)Gag to float up through raft flotation gradients.
We next addressed whether Gag multimerization influenced the fractionation behavior of wild-type Gag. To this end, we designed GagΔp15, which contains the wild-type Gag sequence with NC, p1, and p6 deleted. As depicted in Fig. 3C, ∼24% of GagΔp15 partitioned to rafts when expressed in COS-1 cells, approximately a fourfold increase over full-length Gag. To test whether GagΔp15 flotation was dependent on raft integrity, cells were treated with 10 mg mβCD/ml, which depletes cellular membranes of cholesterol. This treatment completely removed GagΔp15 from fraction 1, indicating that flotation of this protein correlates with its presence in membrane rafts. It is important to consider that removal of the NC domain affects not only Gag multimerization but also RNA recruitment and binding to the cytoskeleton (10, 16, 30, 52). However, addition of RNase to the extraction buffer did not increase the amount of Gag protein detected in the raft fraction (data not shown). Moreover, pretreatment of cells with cytochalasin D, which disrupts the actin cytoskeleton, did not affect the amount of Gag found in the raft fraction (data not shown). These results suggest that Gag-Gag multimerization is the principal factor that accounts for the high density of full-length Gag-containing rafts. Increased density due to Gag multimerization prevents Gag from floating up to the 0%–30% interface, where rafts fractionate in the five-fraction Optiprep gradients. Removal of some of the multimerization determinants within the protein increases the amount of Gag that floats to the 0%–30% Optiprep interface, revealing an intrinsic raft association not otherwise detected.
If Gag-Gag multimerization is responsible for the flotation behavior of Gag on density gradients, it should be possible to alter the flotation characteristics of Gag truncation mutants by formation of hetero-oligomers with wild-type Gag. Truncated Gag proteins containing the MA and CA domains can be incorporated into full-length Gag-containing VLPs (12, 41). Coexpression of Gag and GagΔp15 would thus be expected to cause the recruitment of GagΔp15 molecules to higher-density assembly sites. To test this concept, Gag and GagΔp15 were coexpressed in COS-1 cells and the five-fraction gradient protocol was performed. Figure 3C shows an anti-p24CA Western blot from a representative experiment as well as the average quantitations from several experiments. When coexpressed with full-length Gag, GagΔp15 was effectively removed from the raft fraction, shifting to the higher-density fractions. The distribution of full-length Gag was essentially the same as when it was expressed in the absence of GagΔp15.
The myristoylated M domain of Gag is necessary but not sufficient for raft localization.
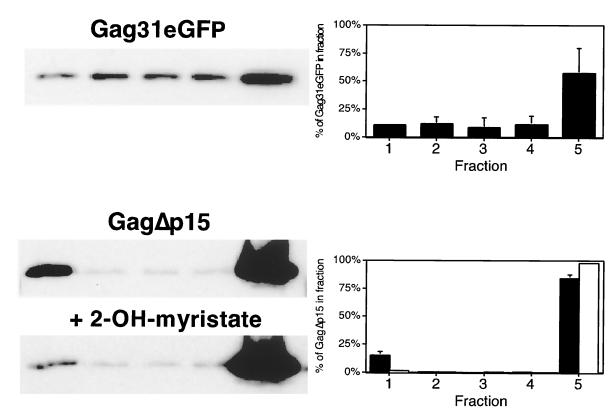
We next assessed the contribution of the membrane-binding M domain to raft localization. Flotation experiments were performed on lysates from COS-1 cells transfected with Gag31eGFP, a chimeric protein in which the first 31 amino acids of Gag, which comprise the M domain, have been fused to the N terminus of eGFP. The Gag31 sequence includes the N-myristoylation site as well as the basic residue cluster involved in membrane binding. As shown in Fig. 4, Gag31eGFP was not concentrated in the raft fraction but rather appeared at the bottom of the gradient. To determine the role of N-myristoylation alone, cells expressing GagΔp15 were pretreated with 2-OH-myr to inhibit myristoylation. As shown in Fig. 4, treatment with the inhibitor effectively removed GagΔp15 from the raft fraction. Taken together, these results suggest that raft localization of Gag requires a myristoylated N terminus and that the myristate-plus-basic-residue motif is necessary but not sufficient for such targeting.
FIG. 4.
The myristoylated M domain of Gag is necessary but not sufficient for raft partitioning of Gag proteins. (Top) Cells were transfected with pVALO Gag31eGFP and subjected to raft fractionation. (Bottom) Cells were transfected with pCMV 5 GagΔp15 and treated on the following day for 12 h with 100 μM 2-OH-myr. Treated (open bars) and untreated (filled bars) control cells were then subjected to the five-fraction extraction and flotation protocol to detect raft-associated protein.
Isolation of Gag protein-laden barges on eight-fraction gradients.
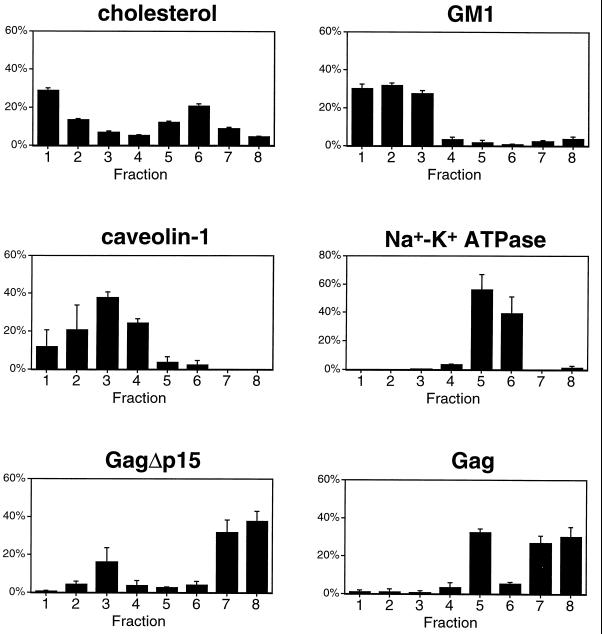
If wild-type Gag complexes are truly associated with raft components, it should be possible to isolate these complexes by adjusting the density of the centrifugation medium used for flotation. Herein, we refer to plasma membrane rafts that are dense with Gag protein complexes as “barges.” To distinguish between Gag-containing barges and other rafts, the gradients were modified to contain 50, 40, 30, 20, and 10% Optiprep steps. Eight fractions were collected from these gradients such that fraction 1 contained the 10%–20% interface, fraction 3 contained the 20%–30% interface, fraction 5 contained the 30%–40% interface, and fraction 7 contained the 40%–50% interface. Barges are expected to have greater density than 30% Optiprep due to their inability to float through this layer in our previous experiments. Detergent-sensitive proteins are expected to be found in fractions 7 and 8. Figure 5 shows the results of experiments conducted in transfected COS-1 cells. After extraction in 0.5% TX-100, the cell extracts were floated through the modified gradients. Eight fractions were collected and analyzed for the presence of various protein and lipid markers. As depicted in Fig. 5, cholesterol and GM1, lipid markers for rafts, were primarily distributed in fractions 1 to 3. Caveolin-1 also fractionated in the lighter fractions of the gradient (fractions 2 and 3), as did GagΔp15, a raft-associated Gag mutant. We conclude that fraction 3 represents the raft fraction. In contrast, Gag (expressed from pCMV5) exhibited a biphasic distribution. Most of the Gag protein was found at the bottom of the gradient (fractions 7 and 8). In addition, a population that reproducibly floated to fraction 5 was evident. Fraction 5 thus represents the barge fraction that can be separated from the traditional raft fraction by virtue of its increased density. The distribution pattern of Gag in the barge fraction was also distinct from that of other proteins. Na+-K+ ATPase, a marker for bulk plasma membrane, migrated towards the denser regions of the gradient in both fractions 5 and 6. As a marker for cytosolic proteins we used G2AFyn, a nonmyristoylated mutant of Fyn. G2AFyn, exhibited a broad distribution primarily located towards the bottom of the gradient (fractions 6 to 8) (data not shown). The only protein that we have observed to be enriched in fraction 5 of the eight-fraction gradients is Gag, supporting the designation of this fraction as Gag-laden barges.
FIG. 5.
Separation of barges from rafts on eight-fraction Optiprep gradients. COS-1 cells were transfected with pCMV5 Gag or pCMV5 GagΔp15 or were mock transfected. After extraction on ice in 0.5% TX-100, cell extracts were subjected to the TX-100 eight-fraction Optiprep flotation protocol described in Materials and Methods. The lipid markers cholesterol and GM1 were detected as described in Materials and Methods (these lipids showed no significant difference in fractionation when isolated from mock-transfected [this figure] or Gag-transfected [data not shown] cells). Gag proteins were detected by Western blotting with rabbit anti-p24CA. Endogenous caveolin-1 was detected with anti-caveolin-1 polyclonal antibody, and endogenous Na+-K+ ATPase was detected using anti-Na+-K+ ATPase α3 subunit monoclonal antibody. The data for cholesterol and GM1 are means of four experiments; those for protein markers are means of two to four experiments.
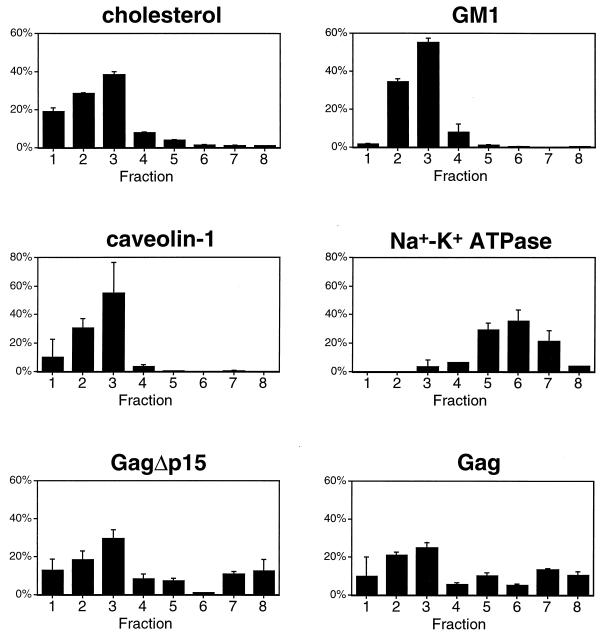
To further examine the relationship between rafts and barges, we compared the fractionation behavior of Gag and the above markers in the absence of detergent. This technique allows the separation of rafts and other light membranes from bulk plasma membrane and cytosolic proteins (44). In addition, any differences in detergent insolubility among proteins do not influence this analysis, as it is performed under detergent-free conditions. The results of eight-step gradient fractionations performed in the absence of detergent are illustrated in Fig. 6. Both full-length Gag and GagΔp15 primarily fractionated in the light membrane fractions, along with the raft markers cholesterol, GM1, and caveolin. Bulk plasma membrane, represented by Na+-K+ ATPase, was clearly separated from the rafts. Thus, in the absence of detergent, Gag localizes to light membrane fractions which include rafts and raft-like membrane microdomains.
FIG. 6.
Characterization of eight-fraction Optiprep gradients in the absence of detergent. These experiments were conducted as described in the legend to Fig. 5, with the exception that no detergent was present in the extraction buffers or the gradients. Note the altered distributions of cholesterol and Gag. Data for cholesterol and GM1 are means of four experiments, and those for protein markers are means of two experiments.
Barge localization increases VLP production.
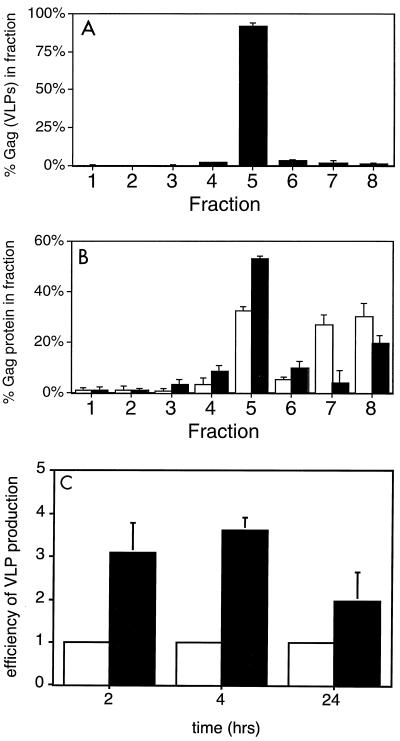
The presence of cellular Pr55Gag in barges suggests a potential role for these membrane microdomains in promoting assembly and/or budding. To test this hypothesis, we examined the fractionation behavior of Gag derived from VLPs. As depicted in Fig. 7A, nearly all of the VLP-derived Gag fractionated in fraction 5, the barge-containing region of the gradient. This suggests that VLP Gag is derived from barges.
FIG. 7.
Attachment of the myristoylated, palmitoylated N terminus of Fyn to Gag increases localization to barges and VLP production. (A) VLPs were isolated from COS-1 cells transfected with pCMV5 Gag. After resuspension of the VLP pellet in cold TNET and extraction on ice, the lysate was fractionated using the eight-fraction Optiprep flotation protocol. Note that TX-100-extracted VLPs float to fraction 5, the barge fraction. (B) COS-1 cells were transfected with pCMV5 Gag (open bars) or with pCMV5 Fyn(10)Gag (filled bars). At 48 h posttransfection, the cells were subjected to the eight-fraction detergent gradient described above. (C) COS-1 cells were transfected with pCMV5 Gag or with pCMV5 Fyn(10)Gag. At 2, 4, or 24 h prior to each experiment the medium was replaced with fresh DMEM plus 10% fetal bovine serum. At the indicated times, the medium was removed from the plates and VLPs were isolated. The cells were harvested in ice-cold RIPA buffer. Gag protein in VLP and cell fractions was analyzed by SDS-PAGE followed by Western blotting. VLP production was quantitated by dividing the amount of Gag in VLPs by the amount of Gag in the cells. This number was set to 1 for each time point (open bars). VLP production by Fyn(10)Gag (filled bars) was determined as discussed above and directly compared to that of Gag. Error bars indicate standard deviations. n = 2 (2 and 4 h) and 4 (24 h).
We next tested the role of barges in VLP production, by comparing wild-type Gag with Fyn(10)Gag. As depicted in Fig. 7B, ∼53% of Fyn(10)Gag was localized to the barge fraction, compared with ∼32% of wild-type Gag.
Because Fyn(10)Gag is more efficiently targeted to barges than wild-type Gag, we examined whether Fyn(10)Gag exhibited an enhanced ability to produce VLPs. COS-1 cells were transfected with vectors encoding either wild-type Gag or Fyn(10)Gag. Two days later, the medium was replaced and VLPs were isolated after 2, 4, or 24 h. The amount of Gag or Fyn(10)Gag present in the VLPs and the cells was quantitated by Western blotting. VLP production was quantitated by dividing the amount of Gag in VLPs by the amount of Gag in the cell lysate; this number was set to 1 at each time point. VLP production by Fyn(10)Gag was then directly compared to that of Gag at each time point. As depicted in Fig. 7C, the efficiency of VLP production by Fyn(10) Gag was approximately two- to fourfold greater than that of wild-type Gag. This increased efficiency was more pronounced at the earlier time points, suggesting that more efficient barge targeting leads to enhanced kinetics of assembly and/or budding.
DISCUSSION
In this study, we examined the partitioning of the HIV-1 Gag protein to raft membrane microdomains. Raft proteins are defined biochemically by their ability to float through density gradients following extraction in cold nonionic detergent (7, 8). In contrast to Src family kinases and caveolin, which are highly enriched in raft fractions of the density gradients, very little HIV-1 Gag was present in the raft fractions of lysates from either Jurkat T cells or COS-1 cells. A similar finding was obtained by others (40), who observed that p17MA was broadly distributed throughout a density gradient, with only a small proportion of the total p17MA protein located in the classically defined raft fractions. Here we provide a biochemical explanation for these findings by showing that Gag-Gag multimerization increases the density of Gag-containing rafts, thereby hindering the isolation of these membrane complexes in standard density gradients.
Localization of multimerized Gag to barges.
The Gag protein contains several regions that contribute to Gag-Gag multimerization, a property essential for the assembly process. Dense patches of membrane-bound Gag protein are evident at the plasma membranes of HIV-1-infected cells using transmission electron microscopy (19). Since the procedure for isolating rafts relies on the buoyant density of proteins that remain associated with detergent-resistant lipid membranes, the protein-dense patches may be too dense to float through the 30% Optiprep layer in the gradients. Here we show that removal of C-terminal assembly domains that have been shown to promote Gag-Gag interactions and the formation of dense VLPs (10, 18, 27, 36, 41, 51) increases the amount of Gag protein that floats to the raft fraction (Fig. 3 and 4). These data indicate that at least 25% of the truncated Gag protein is present in rafts at steady state. Since some degree of multimerization is still possible for these truncated proteins, it is likely that even more GagΔp15 is located in the rafts than is detected on the gradients.
Several lines of evidence support the hypothesis that a substantial amount of Gag is localized in raft-like fractions that we have termed “barges.” Adjustment of the density of the Optiprep gradients allowed recovery of a population of Gag that floated to a fraction of intermediate density between the traditional raft fraction and the bottom of the gradient. This barge fraction was enriched in full-length Gag protein but not the truncation mutant GagΔp15 or caveolin-1. However, when coexpressed with full-length Gag, GagΔp15 shifted down in the gradient towards the barge fraction (Fig. 3C and data not shown), a consequence of incorporation into Gag-Gag oligomeric assembly complexes. We propose that the barge fraction has an increased density due to the presence of high-valence Gag protein multimers and thus should be considered a high-density raft. This increased density becomes apparent only upon treatment with detergent, either because the microdomain in which Gag is present is more sensitive to extraction with TX-100 than standard rafts or because partial extraction of raft lipids is sufficient to alter the protein/lipid ratio such that Gag-containing rafts are separable from non-Gag-containing rafts.
The N terminus of HIV-1 Gag contains a myristate-plus-basic-residue motif that is necessary for plasma membrane localization. However, this sequence was not sufficient to target chimeric proteins (Gag31eGFP) to rafts. The presence of the myristoyl moiety is necessary for raft localization, as nonmyristoylated GagΔp15 was excluded from the rafts. It is therefore likely that membrane localization, although necessary, is not sufficient to target Gag to rafts or barges. Taken together, the data presented in this paper suggest a model in which multimerization of Gag generates a complex with multiple saturated fatty acids (i.e., myristate) inserted into the plasma membrane. Such a complex should exhibit a high affinity for binding to the liquid ordered lipid environment of a raft. This model suggests that Gag monomers, containing only a single myristate, would have a weaker affinity for rafts, resulting in exclusion from this fraction. This is supported by the finding that Src, which also is modified by a single myristate moiety, is relatively excluded from rafts compared to other dually fatty acylated Src family members, such as Fyn and Lck (56).
The existence of multiple, heterogeneous lipid microdomains within the plasma membrane of mammalian cells is becoming apparent in light of a number of recent papers. For example, H-Ras is present in light density membranes that also contain raft markers (44). Treatment with low concentrations of TX-100 renders H-Ras soluble, unlike the other raft components. The protein prominin has been shown to be present in cholesterol-enriched microdomains that are sensitive to TX-100 but not to Lubrol WX, another nonionic detergent (49). In addition, porcine lung membranes have been shown to contain two distinct TX-100-resistant fractions of differential densities (42). Interestingly, although both fractions contain approximately equal concentrations of cholesterol and sphingomyelin, only the lower-density fraction contains significant amounts of glycosphingolipids, including presumably GM1. The glycosphingolipid-containing fraction has a greater resistance to detergent extraction and otherwise resembles what we have defined as genuine rafts.
The use of TX-100 allowed us to separate Gag-containing barges (fraction 5) from rafts (fractions 1 to 3). Fraction 5 also contains a portion of cellular cholesterol. The density of TX-100-extracted Gag was not perturbed by treatment of cells with up to 20 mg of mβCD/ml (data not shown), a condition which is known to disrupt rafts. This may indicate that barge integrity is not cholesterol dependent or that depletion of cholesterol from barge membranes which are held together by multimerized Gag does not significantly alter their already protein-dense nature. Alternatively, as suggested by studies of other raft-derived viral envelopes (53), Gag may protect cholesterol from extraction with mβCD. Fraction 5 also contained a significant amount of Na+-K+ ATPase, a nonraft protein known to be localized throughout the bulk plasma membrane. Until new methods are devised for the isolation of distinct membrane microdomains, the heterogeneity of the preparations will hamper any definitive analysis. Nonetheless, the evidence presented in this paper strongly suggests that HIV-1 Gag is localized to barges, protein-laden rafts whose increased density in the presence of detergent is due to the presence of viral assembly complexes (see below).
Functional significance of barge localization.
To address the potential functional significance of barge localization of HIV-1 Gag, we compared the VLP-forming ability of Gag with that of the Fyn(10)Gag chimera, which has a greater affinity for barges (Fig. 7B). The addition of two palmitates at Cys 3 and Cys 6 gives Fyn(10)Gag a high degree of hydrophobicity, even at low oligomerization states. We showed that Fyn(10)Gag produces VLPs at two- to fourfold efficiency compared to the wild type. This effect is more pronounced at earlier time points. The enhanced kinetics of budding may be due to a more rapid association of Fyn(10)Gag with rafts. The increase in VLP production by Fyn(10)Gag could be due to more efficient concentration of the protein because of its higher affinity for plasma membrane rafts or to a specific requirement of raft localization for Gag-directed VLP production. That there is a specific raft requirement is suggested by recent studies that show a role for rafts in the incorporation of Env into virions and in infectivity (50).
Virus budding from rafts: a common theme.
Several groups studying disparate enveloped viruses have reported a requirement for cholesterol and/or membrane rafts for budding and assembly. For example, influenza fowl plague virus glycoproteins are concentrated in lipid rafts, and their envelopes exhibit raft-like properties (53). In contrast, vesicular stomatitis virus glycoproteins are excluded from rafts and show very little susceptibility to cholesterol extraction with cyclodextrin (53). The palmitoylated hemagglutinin (HA) glycoproteins of influenza viruses are associated with rafts, and this targets HA, and hence viral assembly, to the apical membrane of polarized epithelial cells (57). The HA and NA glycoproteins are responsible for recruitment of the other viral components into rafts (67). In measles virus, the opposite arrangement occurs. Cytosolically synthesized matrix (M) and nucleocapsid (N) proteins associate with membrane rafts and recruit the viral glycoproteins to these sites (34, 63). Since the M and N proteins must be coexpressed in order for raft association to occur, it is likely that protein-protein or protein-RNA complexes rather than protein monomers are the minimal units which are targeted to rafts (63).
A model for assembly of HIV-1 in membrane microdomains is emerging. Both the Gag polyprotein and the Env glycoprotein are found in rafts (50; also this study). Similar to the M and N proteins of measles virus, HIV-1 Gag must form relatively high-order oligomers in order to be present in barges (this study). As Gag protein complexes form at the plasma membrane, the large number of saturated fatty acids (myristate) present in a small patch of the membrane may resemble the lipid microenvironment of a raft. Whether this attracts nearby raft lipids, forming a multimerization-driven barge microdomain, or whether the Gag protein complex subsequently translocates to a preformed lipid raft is not yet known.
That rafts are important for other stages of the HIV-1 life cycle is evidenced by the requirement for cholesterol in target membranes for viral infectivity (32). It is possible that the raft-like nature of HIV-1 envelopes promotes fusion with raft-like membranes or that the aggregation of receptors in membrane rafts is required for the efficient docking of newly infecting virions. It is interesting that the HIV-1 Nef protein is associated with rafts. Raft association is needed for Nef to interact with raft-associated T-cell signaling proteins, thereby rendering the cells hyperresponsive to interleukin 2 (64). The presence of at least three HIV-1 proteins, Gag, Env, and Nef, in rafts argues that these membrane microdomains play important roles in HIV-1 assembly and infectivity.
ACKNOWLEDGMENTS
We thank Luz Hermida-Matsumoto and Marc Tritel for critical reading of the manuscript, Raisa Louft-Nisenbaum for expert technical assistance, and Debra Alston for manuscript preparation. We also thank Luz Hermida-Matsumoto for coining the term “barges.”
This work was supported by NIH grants CA72309 and GM57966. O.W.L. is a Dorris J. Hutchison Graduate Fellow.
REFERENCES
- 1.Accola M A, Strack B, Gottlinger H G. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J Virol. 2000;74:5395–5402. doi: 10.1128/jvi.74.12.5395-5402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alland L, Peseckis S M, Atherton R E, Berthiaume L, Resh M D. Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J Biol Chem. 1994;269:16701–16705. [PubMed] [Google Scholar]
- 3.Aloia R C, Jensen F C, Curtain C C, Mobley P W, Gordon L M. Lipid composition and fluidity of the human immunodeficiency virus. Proc Natl Acad Sci USA. 1988;85:900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aloia R C, Tian H, Jensen F C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson R G. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 6.Berthet-Colominas C, Monaco S, Novelli A, Sibai G, Mallet F, Cusack S. Head-to-tail dimers and interdomain flexibility revealed by the crystal structure of HIV-1 capsid protein (p24) complexed with a monoclonal antibody Fab. EMBO J. 1999;18:1124–1136. doi: 10.1093/emboj/18.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown D A, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Brown D A, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 9.Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 10.Burniston M T, Cimarelli A, Colgan J, Curtis S P, Luban J. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J Virol. 1999;73:8527–8540. doi: 10.1128/jvi.73.10.8527-8540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell S, Vogt V M. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carriere C, Gay B, Chazal N, Morin N, Boulanger P. Sequence requirements for encapsidation of deletion mutants and chimeras of human immunodeficiency virus type 1 Gag precursor into retrovirus-like particles. J Virol. 1995;69:2366–2377. doi: 10.1128/jvi.69.4.2366-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craven R C, Parent L J. Dynamic interactions of the Gag polyprotein. Curr Top Microbiol Immunol. 1996;214:65–94. doi: 10.1007/978-3-642-80145-7_3. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov D S. Cell biology of virus entry. Cell. 2000;101:697–702. doi: 10.1016/s0092-8674(00)80882-x. [DOI] [PubMed] [Google Scholar]
- 15.Dorfman T, Bukovsky A, Ohagen A, Hoglund S, Gottlinger H G. Functional domains of the capsid protein of human immunodeficiency virus type 1. J Virol. 1994;68:8180–8187. doi: 10.1128/jvi.68.12.8180-8187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freed E O. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 17.Gamble T R, Yoo S, Vajdos F F, von Schwedler U K, Worthylake D K, Wang H, McCutcheon J P, Sundquist W I, Hill C P. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science. 1997;278:849–853. doi: 10.1126/science.278.5339.849. [DOI] [PubMed] [Google Scholar]
- 18.Garnier L, Ratner L, Rovinski B, Cao S X, Wills J W. Particle size determinants in the human immunodeficiency virus type 1 Gag protein. J Virol. 1998;72:4667–4677. doi: 10.1128/jvi.72.6.4667-4677.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelderblom H R, Hausmann E H, Ozel M, Pauli G, Koch M A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987;156:171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 20.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 21.Gordon L M, Jensen F C, Curtain C C, Mobley P W, Aloia R C. Thermotropic lipid phase separation in the human immunodeficiency virus. Biochim Biophys Acta. 1988;943:331–342. doi: 10.1016/0005-2736(88)90565-2. [DOI] [PubMed] [Google Scholar]
- 22.Gottlinger H G, Sodroski J G, Haseltine W A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermida-Matsumoto L, Resh M D. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J Virol. 2000;74:8670–8679. doi: 10.1128/jvi.74.18.8670-8679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill C P, Worthylake D, Bancroft D P, Christensen A M, Sundquist W I. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc Natl Acad Sci USA. 1996;93:3099–3104. doi: 10.1073/pnas.93.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilangumaran S, Arni S, van Echten-Deckert G, Borisch B, Hoessli D C. Microdomain-dependent regulation of Lck and Fyn protein-tyrosine kinases in T lymphocyte plasma membranes. Mol Biol Cell. 1999;10:891–905. doi: 10.1091/mbc.10.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janes P W, Ley S C, Magee A I. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krausslich H G, Facke M, Heuser A M, Konvalinka J, Zentgraf H. The spacer peptide between human immunodeficiency virus capsid and nucleocapsid proteins is essential for ordered assembly and viral infectivity. J Virol. 1995;69:3407–3419. doi: 10.1128/jvi.69.6.3407-3419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Layne S P, Merges M J, Dembo M, Spouge J L, Conley S R, Moore J P, Raina J L, Renz H, Gelderblom H R, Nara P L. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Hill C P, Sundquist W I, Finch J T. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature. 2000;407:409–413. doi: 10.1038/35030177. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Dai R, Tian C J, Dawson L, Gorelick R, Yu X F. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J Virol. 1999;73:2901–2908. doi: 10.1128/jvi.73.4.2901-2908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.London E, Brown D A. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 32.Manes S, del Real G, Lacalle R A, Lucas P, Gomez-Mouton C, Sanchez-Palomino S, Delgado R, Alcami J, Mira E, Martinez-A C. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manes S, Mira E, Gomez-Mouton C, Lacalle R A, Keller P, Labrador J P, Martinez A C. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 1999;18:6211–6220. doi: 10.1093/emboj/18.22.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manie S N, Debreyne S, Vincent S, Gerlier D. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J Virol. 2000;74:305–311. doi: 10.1128/jvi.74.1.305-311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Momany C, Kovari L C, Prongay A J, Keller W, Gitti R K, Lee B M, Gorbalenya A E, Tong L, McClure J, Ehrlich L S, Summers M F, Carter C, Rossmann M G. Crystal structure of dimeric HIV-1 capsid protein. Nat Struct Biol. 1996;3:763–770. doi: 10.1038/nsb0996-763. [DOI] [PubMed] [Google Scholar]
- 36.Morikawa Y, Hockley D J, Nermut M V, Jones I M. Roles of matrix, p2, and N-terminal myristoylation in human immunodeficiency virus type 1 Gag assembly. J Virol. 2000;74:16–23. doi: 10.1128/jvi.74.1.16-23.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morikawa Y, Zhang W H, Hockley D J, Nermut M V, Jones I M. Detection of a trimeric human immunodeficiency virus type 1 Gag intermediate is dependent on sequences in the matrix protein, p17. J Virol. 1998;72:7659–7663. doi: 10.1128/jvi.72.9.7659-7663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray D, Hermida-Matsumoto L, Buser C A, Tsang J, Sigal C T, Ben-Tal N, Honig B, Resh M D, McLaughlin S. Electrostatics and the membrane association of Src: theory and experiment. Biochemistry. 1998;37:2145–2159. doi: 10.1021/bi972012b. [DOI] [PubMed] [Google Scholar]
- 39.Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem. 1997;272:6324–6331. doi: 10.1074/jbc.272.10.6324. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen D H, Hildreth J E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono A, Demirov D, Freed E O. Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding. J Virol. 2000;74:5142–5150. doi: 10.1128/jvi.74.11.5142-5150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkin E T, Turner A J, Hooper N M. Isolation and characterization of two distinct low-density, Triton-insoluble, complexes from porcine lung membranes. Biochem J. 1996;319:887–896. doi: 10.1042/bj3190887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pralle A, Keller P, Florin E L, Simons K, Horber J K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prior I A, Harding A, Yan J, Sluimer J, Parton R G, Hancock J F. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 45.Rietveld A, Simons K. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim Biophys Acta. 1998;1376:467–479. doi: 10.1016/s0304-4157(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 46.Robbins S M, Quintrell N A, Bishop J M. Myristoylation and differential palmitoylation of the HCK protein-tyrosine kinases govern their attachment to membranes and association with caveolae. Mol Cell Biol. 1995;15:3507–3515. doi: 10.1128/mcb.15.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodgers W, Crise B, Rose J K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodgers W, Rose J K. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roper K, Corbeil D, Huttner W B. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2:582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 50.Rousso I, Mixon M B, Chen B K, Kim P S. Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity. Proc Natl Acad Sci USA. 2000;97:13523–13525. doi: 10.1073/pnas.240459697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandefur S, Varthakavi V, Spearman P. The I domain is required for efficient plasma membrane binding of human immunodeficiency virus type 1 Pr55Gag. J Virol. 1998;72:2723–2732. doi: 10.1128/jvi.72.4.2723-2732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki H, Nakamura M, Ohno T, Matsuda Y, Yuda Y, Nonomura Y. Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc Natl Acad Sci USA. 1995;92:2026–2030. doi: 10.1073/pnas.92.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 54.Schnitzer J E, McIntosh D P, Dvorak A M, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 55.Shaul P W, Smart E J, Robinson L J, German Z, Yuhanna I S, Ying Y, Anderson R G, Michel T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 56.Shenoy-Scaria A M, Dietzen D J, Kwong J, Link D C, Lublin D M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 58.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 59.Spearman P, Wang J J, Vander Heyden N, Ratner L. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J Virol. 1994;68:3232–3242. doi: 10.1128/jvi.68.5.3232-3242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tritel M, Resh M D. Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates. J Virol. 2000;74:5845–5855. doi: 10.1128/jvi.74.13.5845-5855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van't Hof W, Resh M D. Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J Cell Biol. 1999;145:377–389. doi: 10.1083/jcb.145.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veronese F D, Copeland T D, Oroszlan S, Gallo R C, Sarngadharan M G. Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol. 1988;62:795–801. doi: 10.1128/jvi.62.3.795-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent S, Gerlier D, Manie S N. Measles virus assembly within membrane rafts. J Virol. 2000;74:9911–9915. doi: 10.1128/jvi.74.21.9911-9915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J K, Kiyokawa E, Verdin E, Trono D. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc Natl Acad Sci USA. 2000;97:394–399. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webb Y, Hermida-Matsumoto L, Resh M D. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 66.Yang C, Spies C P, Compans R W. The human and simian immunodeficiency virus envelope glycoprotein transmembrane subunits are palmitoylated. Proc Natl Acad Sci USA. 1995;92:9871–9875. doi: 10.1073/pnas.92.21.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Pekosz A, Lamb R A. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou W, Resh M D. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]