Abstract
Parkinson's disease is a neurodegenerative disorder clinically characterized by bradykinesia, rest tremor, rigidity, and postural and gait disturbances, which are frequently observed in older people. It also shows non‐motor symptoms, such as depression, anxiety, cognitive impairment and dementia. The number of patients is gradually increasing worldwide. Aging is a risk factor for the onset of Parkinson's disease, and various physiological effects of aging influence its progression. Frailty is a geriatric syndrome in which the reversible and vulnerable status between robustness and disability is affected by various physiological stressors with aging. Frailty consists of physical, psychological and social aspects. Furthermore, sarcopenia, a syndrome characterized by the loss of muscle mass, strength and function, is also significantly associated with frailty. To maintain the quality of life of older people, frailty, including sarcopenia, should be quickly and appropriately managed. Polypharmacy is an important factor causing the progression of frailty in geriatric syndrome. Although Parkinson's disease and frailty have similar symptoms, and are considered to affect each other, the clinical features and mechanisms of both largely remain unclear. Nevertheless, little literature on the relationship between frailty and Parkinson's disease is currently available. This narrative review aims to clarify the relationships between Parkinson's disease and frailty, not only on the physical, but also on the mental, cognitive, and social aspects and issues regarding polypharmacy in Parkinson's disease explored by previous studies. Geriatr Gerontol Int 2022; 22: 259–270.
Keywords: frailty, geriatric medicine, Parkinson's disease, polypharmacy, sarcopenia
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by bradykinesia, in combination with rest tremor or rigidity. 1 As the disease progresses, gait disturbance, postural instability and motor fluctuations, such as the wearing‐off phenomenon, shorter medication effect and dyskinesia, also occur. Since levodopa, a basic medication for PD, was first introduced, 2 the rate of mortality as a result of PD has continued to decrease significantly. 3
The world is currently facing an aging society. According to a Japanese government report, the global population of people aged >65 years currently exceeds 60 million, as compared with 13 million in 1950. 4 Additionally, the ratio of the population of people aged >65 years in developed countries was 17.6%, as compared with 7.7% in 1950. 4 Older people usually have various comorbidities, such as hypertension, diabetes mellitus, osteoporosis and neurological disorders. Of these, PD is one of the most frequent neurodegenerative disorders among older people. Between 1990 and 2015, the prevalence of PD increased by 117.8% 5 ; therefore, it is referred to as “Parkinson's disease pandemic”, as the number of patients is expected to keep increasing. 6
Aging is a risk factor for the onset of PD; therefore, patients with PD might have various physical, mental and social disabilities. Meanwhile, frailty is a current geriatric issue defined as a reversible condition between robustness and disability, focusing not only on physical status, but also on mental and social status. The relationship between PD and frailty largely remains unclear, and only a few studies on this relationship are currently available; thus, we aimed to clarify the relationships between PD and frailty in the physical, cognitive, mental, social and geriatric aspects of PD.
Concept of PD and frailty
PD was first described by a British doctor, James Parkinson, in 1817, and named by a French neurologist, Jean‐Martin Charcot. 7 Over 200 years, many clinical and basic studies have been carried out. As mentioned in the previous chapter, PD presents with various clinical symptoms. Furthermore, before the onset of motor symptoms, constipation, rapid eye movement, behavioral sleep disorder, depression and anxiety are frequently observed as non‐motor symptoms. 8 Furthermore, as the disease severity advances, orthostatic hypotension and cognitive impairment were observed as comorbidities. Importantly, cognitive impairment and dementia are highly prevalent in advanced PD, found in approximately 60% of patients with PD after 10 years and 80% after 20 years of disease, respectively. 9
The Lewy body, consisting of α‐synuclein, is a characteristic pathological entity of PD, and is distributed in the substantia nigra, locus coeruleus and dorsal vagal nucleus. Although the pathological progression of the Lewy body largely remains unclear, it is proposed that its deposition is initiated in the dorsal vagal nucleus, and spreads to the brainstem and cerebral cortex, 10 while it is also initiated in the olfactory bulb and spreads to the central nervous system. 11 Approximately 50–70% of dopaminergic neurons are lost at the onset of motor symptoms. 12 However, as histological changes also occur with aging, physiological and homeostatic dysfunctions would influence the onset of PD, isolated from aging. 13 A previous epidemiological study showed that the prevalence of PD increased with age, and it was 10‐fold in people their aged in their 80s compared with those aged in their 50s 14 ; that is, aging is a risk factor for the onset of PD. 15
Frailty has been introduced as a concept of geriatric syndrome regarding the preservation of the physically and mentally robust status of older generations. The term “frailty” is still not clearly defined among gerontological experts; however, the currently agreed definition is “a multidimensional syndrome characterized by decreased reserve and diminished resistance to stressors”. 16 The assessment of frailty is generally expressed as two main models: the phenotype model 17 and the cumulative deficit model. 18 The phenotype model is mainly based on how many clinical features are matched on the five physical frailty criteria: (i) slow gait speed; (ii) weight loss; (iii) muscle strength; (iv) fatigue; and (v) physical activities. 16 Of these five basic evaluation domains, zero is regarded as robust, one or two as pre‐frailty and three or more as frailty. The cumulative deficit model is a proportion calculated using the frailty index, which consists of various evaluation domains, including cognition, emotional status and physical functioning.
Focusing on physical frailty, the international clinical guideline was published in 2019. 19 The occurrence of comorbidities is regarded as a risk factor for frailty. Furthermore, in addition to physical frailty, cognitive and social frailty, which entirely influence each other, are also important components of gerontological medicine. Additionally, oral frailty is significant for older people, and oral hygiene is significantly associated with systemic disease and healthcare. Sarcopenia is a clinical syndrome characterized by progressive muscle weakness, decreased skeletal muscle mass and decreased physical function, causing physical disability, low quality of life and mortality, which are strictly related to frailty. 20 , 21
Integrated management for frailty, including sarcopenia, is essential for older people, and geriatric aspects should always be kept in mind by medical providers. Of these, older people generally have comorbidities; thus, various medications are prescribed to manage them. However, they sometimes influence each other interdependently and are harmful to the patients. Importantly, polypharmacy is also associated with the progression of frailty in older populations.
Multidimensional effects, such as oxidative stress, mitochondrial dysfunction, homeostatic dysfunction and endocrine dysfunction, will occur during the progression of frailty, and physiological changes are thought to be shared between frailty and PD through aging.
Relationships between PD and frailty
Relationship between PD, physical frailty and sarcopenia
The relationship between PD and frailty has been gradually documented for two decades. However, few studies are, even in the present day, available about them. Furthermore, most studies are focused on the physical aspects, probably because PD has a similar syndrome to physical frailty. The previous studies on the relationship between physical frailty and PD that we know of are presented in Table 1. In summary, the prevalence of frailty in PD ranges from 3.4% to 84%, 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 and as high as 10%, even in prodromal PD. 35 These variable ranges of the prevalence of frailty were derived from the study setting, participants' demographics, populations and frailty assessment methods. Fried's phenotype criteria were used to assess frailty in most studies. 22 , 23 , 24 , 25 , 29 , 33 , 35 , 36 Several studies have used the frailty index. 29 , 36 Two studies used the clinical frailty scale to assess frailty. 30 , 32 Phenotype criteria are frequently used for the assessment of frailty due to their simplicity of use, unlike the frailty index that is thought to be a complicated technique for use in daily clinical practice. 34 The prevalence of frailty was higher in the frailty index measurement than in the phenotype model in previous studies. Furthermore, most of the studies were cross‐sectional studies, and only one pathological study was carried out using the prospective cohort study design. 27 Additionally, a recent study used claim‐based information to analyze the relationship between frailty and PD, as a different prospect from previous studies. 37 Frailty was generally associated with older age of patients, longer disease duration, advanced disease severity as determined by the Hoehn–Yahr stage or the Unified Parkinson's Disease Rating Scale (UPDRS) and high levodopa equivalent dose. 23 , 24 , 32 , 33 Furthermore, female patients with PD tended to experience frailty more frequently.
Table 1.
Previous studies associated with Parkinson's disease and physical frailty
| First author | Year published | Study designs | Participants (n) | Frailty evaluation method | Prevalence of frailty, n (%) | Mean age at evaluation, years (SD) | Mean PD disease duration, years (SD) | PD evaluation method (motor) | PD evaluation method (non‐motor) |
|---|---|---|---|---|---|---|---|---|---|
| Ahmed | 2008 | Cross‐sectional | PD: 50 | Phenotype model | Frailty: 16 (32.7%) | 70.8 ± 9.2 | ー | UPDRS | ー |
| Non‐frail: 33 (67.3%) | |||||||||
| Roland et al. (Ref. 23) | 2012 | Cross‐sectional | 29 | Phenotype model | Frailty: 1 (3.4%) | 66.4 ± 8.5 | 7.2 ± 4.6 | H‐Y | PDQ‐39 |
| Pre‐frailty: 19 (65.5%) | |||||||||
| Non‐frailty: 9 (31%) | |||||||||
| Roland et al. (Ref. 25) | 2012 | Cross‐sectional | 17 | Phenotype model | Frailty: 5 (29.4%) | Frailty: 65 ± 11.2 | Frailty: 4.6 ± 1.8 | H‐Y | ー |
| Pre‐frailty: 65 ± 9.4 | |||||||||
| Pre‐frailty: 11.1 ± 7.9 | |||||||||
| Pre‐frailty: 8 (47.1%) | |||||||||
| Non‐frailty: 2.8 ± 2.2 | |||||||||
| Non‐frailty: 68 ± 2.6 | |||||||||
| Non‐frailty: 4 (23.5%) | |||||||||
|
Roland et al. (Ref. 24) |
2012 | Cross‐sectional |
PD:15 Control: 15 |
Phenotype model |
Frailty: 4 (26.7%) Pre‐frailty: 7 (46.7%) Non‐frailty: 4 (26.7%) |
Frailty: 63 ± 11 Pre‐frailty: 65 ± 10 Non‐frailty: 69 ± 1 |
ー | ー | ー |
|
Roland et al. (Ref. 26) |
2013 | Cross‐sectional | 13 | Phenotype model |
Frailty: 3 (23.1%) Pre‐frailty: 6 (46.2%) Non‐frailty: 4 (30.8%) |
Frailty: 67 ± 9 Pre‐frailty: 66 ± 9 Non‐frailty: 69 ± 1 |
Frailty: 5.7 ± 1.2 Pre‐frailty: 10.7 ± 7.9 Non‐frailty: 3.3 ± 2.3 |
H‐Y | ー |
| Buchman | 2013 | Prospective |
Lewy body pathology: 159 (20.1%) Nigral neuronal loss: 106 (13.4%) (All participants: 791) |
Phenotype model | ー | ー | ー | ー | ー |
| Liotta | 2016 | Cross‐sectional |
PD: 18 (All participants: 1331) |
FGE | ー | ー | ー | ー | ー |
| Torsney | 2018 | Cross‐sectional | PD: 393 | CSF | Frailty: 330 (84%) | PD: 82.8 | ー | CCI | Prevalence of depression, dementia or new cognitive impairment |
| Peball | 2018 | Cross‐sectional |
PD: 104 (Control: 434) |
CSHA CSF |
Frailty: 37 (35.6%) | PD: 73.8 ± 5.2 | 12.0 ± 7.9 |
H‐Y MDS‐UPDRS Part I–IV ADL |
PDQ‐39 |
| Tan | 2018 | Cross‐sectional |
PD: 93 (Control: 78) |
Phenotype model Frailty index |
PD Fried's criteria: (27.9%) Frailty index: (69.2) |
PD: 66.0 ± 8.5 | 8.5 ± 5.6 |
H‐Y MDS‐UPDRS Part I–IV |
ー |
| Rennne | 2018 | Cross‐sectional | PD: 6 (all participants: 241) | TFI | ー | PD: 79.3 | ー | ー | ー |
| Lin | 2019 | Cross‐sectional | 76 | Phenotype model | 29 (38%) |
PD with frailty: 2.74 (2.82) PD without frailty: 2.12 (2.70) |
H‐Y, UPDRS Part I–III, S&E ADL |
Attention function, Executive function Memory function Visuospatial function |
|
| Ntanasi | 2020 | Cross‐sectional |
PD: 34 Prodromal PD: 49 Non‐PD: 1682 |
Phenotype model Frailty index |
Phenotype criteria PD: 5 (14.7%) Prodromal PD: 5 (10.2%) Non‐PD: 65 (3.9%) Frailty index PD: 26 (76.5%) Prodromal PD: 33 (67.3%) Non‐PD: 332 (19.7%) |
PD: 76 (73–79) Prodromal PD: 76 (73–79) Non‐PD: 73 (69–77) |
ー | ー | ー |
| Smith | 2021 | Cross‐sectional | PD: 120 | Phenotype model | 31.2 (26%) | 70.2 ± 8.0 | MDS‐UPDRS Part I–IV | MoCAGDS | |
| Abraham | 2021 | Cross‐sectional | PD: 62786 | CFI | 34 708 (55.3%) | ー | ー | ー | ー |
Abbreviations: ADL, activities of daily living; CCI, Charlson Comorbidity Index; CFI, Claims‐based Frailty Index; CFS, Clinical Frailty Scale; CSHA, Canadian Scale of Health and Aging; FGE, Functional Geriatric Evaluation; GDS, Geriatric Depression Scale; H‐Y, Hoehn–Yahr stage; MDS‐UPDRS, Movement Disorders Society‐Unified Parkinson's Disease Rating Scale; MoCA, Montreal Cognitive Assessment; PD, Parkinson's disease; PDQ‐39, Parkinson's Disease Questionnaire‐39; SD, standard deviation; S&E, Schwab and England Activities of Daily Living Scale; UPDRS, Unified Parkinson's Disease Rating Scale.
The prevalence of sarcopenia in PD ranges from 6.6 to 55.8%. 29 , 32 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 This wide range of results depends on the characteristics of the participants and the evaluation method. The relationship between sarcopenia and PD is presented in Table 2. In previous studies, sarcopenia was mostly evaluated by the European Working Group on Sarcopenia in Older People method, 20 , 38 , 39 , 40 , 42 , 44 , 46 and the validated version was used in an Asian study. 29 Vetrano et al. compared three different evaluation methods for sarcopenia in PD, resulting in the highest agreement in European Working Group on Sarcopenia in Older People and the International Working Group for Sarcopenia. 40 , 47 Among studies related to sarcopenia and clinical manifestations of PD, relationships between disease severity by Hoehn–Yahr stage, UPDRS, frequency of falls and sarcopenia were observed. 32 , 42 , 45 Additionally, in a report, the prevalence of sarcopenia in female PD patients was lower compared to male patients. 46 A recent meta‐analysis showed that the fall incidence was higher in sarcopenic PD patients than in non‐sarcopenia patients, whereas sex differences were not found in the study. 41 Considering that the relationship between sarcopenia and PD influences non‐motor aspects, depression and cognitive impairment are both related to sarcopenia, 45 whereas other studies did not show the relationships between sarcopenia and non‐motor aspects of PD. 32 , 39
Table 2.
Previous studies associated with Parkinson's disease and sarcopenia
| Author | Year published | Study designs | Participants (n) | Sarcopenia evaluation method | Prevalence of sarcopenia, n (%) | Mean age at evaluation, years (SD) | Mean PD disease duration, years (SD) | PD evaluation method (motor) | PD evaluation method (non‐motor) |
|---|---|---|---|---|---|---|---|---|---|
| Barichella | 2016 | Cross‐sectional |
PD: 235 Other Parkinsonism: 129 |
EWGSOP | 14 (6.0%) | ー | ー |
H‐Y UPDRS Part II–III |
MMSE Dysphagia SDQ score |
| Drey | 2017 | Prospective cohort | PD: 255 | EWGSOP | 38% | 64.9 ± 5.9 | — | MDS‐UPDRS Part III |
BDI RBDSQ Olfactory test |
| Vetrano | 2018 | Cross‐sectional | 210 |
FNIH EWGSOP IWG |
FNIH sarcopenia: 40.7 (male)/27.5 (female) Severe sarcopenia: 20.0 (Male)/11.3 (female) EWGSOP Sarcopenia: 28.8 (male)/17.5 (female) Severe sarcopenia: 16.8 (male)/18.8 (female) IWG sarcopenia: 35.4 (male)/32.5 (female) |
Male: 73.3 ± 7.4 Female: 74.4 ± 6.6 |
Male: 3.2 (1.1–7.0) Female: 4.0 (1.5–6.7) |
UPDRS, ADL | MMSE |
| Pabell | 2018 | Cross‐sectional |
PD: 104 (Control: 434) |
SARC‐F | Sarcopenia: 58 (55.8) |
PD: 73.8 ± 5.2 (Control: 75.3 ± 7.3) |
12.0 ± 7.9 |
H‐Y MDS‐UPDRS Part I–IV ADL |
PDQ‐39 |
| Yazar | 2018 | Cross‐sectional |
PD: 166 (Control: 249) |
EWGSOP | ー |
PD: Female: 71.57 ± 5.2 Male: 72.76 ± 4.43 |
ー | UPDRS | ー |
| Tan | 2018 | Cross‐sectional |
PD: 93 (Control: 78) |
Sarcopenia: AWGS |
PD Sarcopenia: (17.2) Control (Sarcopenia: (10.3)) |
PD: 66.0 ± 8.5 (Control: 62.4 ± 8.4) |
8.5 ± 5.6 |
H‐Y, MDS‐UPDRS Part I–IV |
ー |
| Lee | 2019 | Cross‐sectional |
PD: 52 (Control: 19) |
ASMMI | 21 (40.4%) |
PD with sarcopenia: 63.7 ± 11.6 PD without sarcopenia: 60.3 ± 9.8 (Control: 60.3 ± 7.6) |
PD with sarcopenia: 1.9 ± 2.0 PD without sarcopenia: 2.4 ± 2.4 |
UPDRS Part I–III, H‐Y, S&E | MMSE |
| Wang | 2019 | Cross‐sectional |
PD: 25 Control: 20 |
CHS‐PCF IPAQ‐SF |
N/A |
PD: 63.6 ± 5.5 (Control: 63.0 ± 4.1) |
1.70 ± 2.15 | H‐Y, UPDRS Part I–III, S&E ADL | ー |
| Ozer | 2020 | Cross‐sectional |
PD: 70 Control: 85 |
EWGSOP | 22 (31.4%) | PD: 68.3 ± 5.9 | PD: 6.0 ± 5.1 | ADL, IADL | |
| Krenovsky | 2020 | Cross‐sectional |
PD: 53 Atypical PS: 21 Control: 30 |
EWGSOP |
PD: 4 (7.5%) Atypical PS: 6 (28.6) |
PD: 70 ± 10.1 Atypical PS: 70.3 ± 9.7 Control: 70.8 ± 2.0 |
PD: 61 (16.4) Atypical PS: 103 (25.8) * months |
H‐Y | ー |
| Lima | 2020 | Cross‐sectional | 218 | EWGSOP | PD: 103 (47.4%) | 70.4 (59.9–76.8) | 9.4 ± 6.9 |
H‐Y S&E ADL |
PDQ‐39 GDS |
Abbreviations: ADL, activities of daily living; ASMMI, Appendicular Skeletal Muscle Mass Index; AWGS, Asian Working Group for Sarcopenia; BDI, Beck Depression Inventory; Dysphagia SDQ score, Dysphagia Swallowing Disturbance Questionnaire score; EWGSOP, European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health; GDS, Geriatric Depression Scale; H‐Y, Hoehn–Yahr stage; IADL, instrumental activities of daily living; IWGS, International Working Group on Sarcopenia; MDS‐UPDRS, Movement Disorders Society‐Unified Parkinson's Disease Rating Scale; MMSE, Mini‐Mental State Examination; PD, Parkinson's disease; PDQ‐39, Parkinson's Disease Questionnaire‐39; PS, Parkinson syndrome; RBDSQ, Rapid Eye Movement Behavioral Disorders Screening Questionnaire; S&E, Schwab and England Activities of Daily; SARC‐F, Strength, Assistance in walking, Rise from a chair, Climb stairs, Falls history questionnaire; SD, standard deviation; UPDRS, Unified Parkinson's Disease Rating Scale.
As aforementioned, Fried's phenotype criteria, an evaluation method for frailty, and symptoms of PD both share the same clinical syndrome. All Fried's phenotype criteria, weight loss, exhaustion, low gait speed and decreased physical activity, are also well‐recognized symptoms of PD. Furthermore, decreased grip strength was also observed in patients with PD. Thus, it is important to consider the overlap between the over‐diagnosis of frailty and PD.
All components of Fried's criteria can be found in patients with PD. First, weight loss is mainly caused by energy expenditure imbalance. 48 , 49 In the early stages of the disease, non‐motor symptoms, such as gastrointestinal dysfunction, dysphagia and depression, are associated with food intake. 48 , 49 Furthermore, rigidity, rest tremor and levodopa‐induced dyskinesia can consume energy. 48 , 49 Additionally, medication‐associated weight loss might occur due to levodopa intake, resulting in increased growth hormone secretion. 50 In previous studies, weight loss in PD was physiologically found to be caused by fat loss, which is different from the weight loss found in frailty associated with sarcopenia, which is attributed to muscle mass loss. 49 However, sarcopenia is also observed in patients with PD. As it is PD‐associated, frailty and sarcopenia‐associated weight loss simultaneously occur in these patients. Therefore, it is difficult to distinguish sarcopenia from PD‐associated weight loss. Pharmacological and non‐pharmacological interventions, such as appropriate treatment for PD, including dopaminergic therapy for motor symptoms and dysphagia, and nutrition support, should be correctly carried out on the patients.
Exhaustion, also known as fatigue, is a common symptom in PD, resulting in 50% of patients experiencing it in a recent meta‐analysis. 51 The assessment of fatigue mainly entails the use of the Movement Disorders Society‐UPDRS Part I, 52 included in the non‐motor symptom chapter or questionnaire. 53 Although the pathological entity of fatigue remains unclear, it was hypothesized that Lewy bodies spread from the olfactory bulb to the limbic area, associated with anxiety and pain, independently progressed from nigrostriatal deficits. 54 In the Movement Disorders Society report on the treatment for non‐motor symptoms, rasagiline is considered potentially useful; however, little literature is available on the treatment for fatigue; thus, further evidence is required to establish a management strategy. 55
Slow gait is more frequent in patients with PD than in older people, adjusting for age as a confounder. According to previous studies, gait speed in PD is negatively correlated with age, disease severity, and Timed Up and Go test time. 56 , 57 , 58 Furthermore, cognitive impairment, depression and anxiety were negatively correlated with gait speed. 56 Previous experience of falls and fear of falls influences gait speed. 56 Gait speed is considered to be the effect of dopaminergic system deficits that cause bradykinesia and body imbalance. However, Bohnen et al. showed that cholinergic denervation, in addition to dopaminergic system failure, also contributed to slow gait speed. 59 This evidence might be consistent with the finding that cholinergic medication using rivastigmine improves gait impairment in PD patients. 60 Decreased gait speed can be treated by levodopa administration, and physiological therapy might also be useful.
During the current coronavirus disease 2019 (COVID‐19) pandemic, to make matters worse, the pandemic has promoted sedentary behavior and decreased physical activities. 61 , 62 , 63 Thus, medical providers should pay attention to these low physical activities in patients with PD and manage them for as long as possible.
Little is known about the relationship between muscle strength and PD. Roberts et al. showed that grip strength was negatively correlated with motor severity in PD. 64 Furthermore, they reported that medications improved the muscle strength of PD, which hypothesized that central nervous dysfunction, including dopaminergic system failure, might contribute to muscle weakness. 64 At this point, muscle weakness derived from physical frailty and sarcopenia is different from nigrostriatal deficits in PD, possibly resulting in the combination of physical frailty and muscle weakness derived from PD.
Even in healthy older people without neurodegenerative disorders, subtle parkinsonism, which does not meet the diagnostic criteria for PD, is occasionally observed. This phenomenon is generally called the mild parkinsonian sign (MPS). 65 The MPS shows similar clinical features with PD, such as slowness, rigidity, gait and balance deficits, and tremor. Of these, tremors are relatively less frequently observed in MPS. 65 Although the method of evaluation of MPS is still to be defined, it entails at least one UPDRS item for a rating of one or higher, 66 two or more MPSs, or one sign of moderate UPDRS score. 67 The prevalence of MPS in older people varies according to the evaluation method among the 15–95% of people who expressed it. 65
As for older people, comorbidities, such as orthopedic and other medical problems, might contribute to MPS, which is isolated from nigrostriatal neurodegeneration. However, MPS is thought to cause neuropathological alterations in Lewy body disease and vascular pathology. Furthermore, older people with MPS have a higher prevalence of hyposmia and rapid eye movement sleep disorder than normal controls. 68 Thus, MPS is a risk factor for the development of dementia, PD and cerebral vascular disease.
Bradykinesia and slow gait speed are common symptoms in older people, and these are also found in frail people as mentioned before; thus, it is possible to misdiagnose frail people with MPS. 69 The mechanism of MPS largely remains unclear; however, the clinical syndrome of MPS should be considered when older people are examined.
PD and physical frailty share similar syndromes; therefore, medical caregivers should pay attention to the elements of frailty potentially observed in PD and, by contrast, frailty symptoms are probably derived from PD itself. Furthermore, although the mechanisms underlying the progression of PD and frailty have remained unclear for the most part, there have been several hypotheses underlying these two relationships, which are biophysiological concordance, such as inflammation, oxidative stress, and mitochondrial and endocrine dysfunctions. These are generally found during aging as homeostatic involvements.
Relationship between PD and psychological frailty
Cognitive impairment and mental illness are significant complications, as well as physical frailty, in patients with PD. Cognitive impairment and dementia have been reported to occur in 60% and 80% of patients with PD 10 and 20 years, respectively, after disease onset. 9 Furthermore, dementia is a risk factor for poor prognosis. 70
Cognitive frailty has recently been thought to be associated with physical frailty. Ma et al. described the concept of these relationships, in which there were two proposed subtypes of cognitive frailty – reversible and potentially reversible cognitive frailty – causes higher mortality in older people. 71 , 72 Like physical frailty, it is hypothesized that cognitive frailty could occur as a result of oxidative stress, mitochondrial dysfunction and homeostatic dysfunction with aging. 73 Buchman et al. described the occurrence of frailty as a potential risk factor for Alzheimer's disease. 74 These relationships might be similar to the biophysiology of PD; however, at present, little literature is available on the relationship between cognitive frailty and physical frailty in PD. Lin et al. reported that executive dysfunction is an independent risk factor for the development of physical frailty in patients with PD. 33 In a recent neuroimaging study, lateral occipital gray matter cortex volume reduction was associated with cognitive impairment and physical frailty in PD, 75 which was distinct from the previous volumetric analysis of cognitive impairment in PD, 76 , 77 according to Chen et al. Furthermore, in previous studies that focused on physical frailty in PD, several showed the relationships between physical status and depression or cognitive impairment; however, some did not. These different results might have been caused by the number of participants and evaluation method. There is a large amount of evidence for the treatment of cognitive impairment in PD. Generally, cholinesterase inhibitors are often used to treat dementia or cognitive impairment. 55
Depression and anxiety are common clinical manifestations of PD. These are frequently observed during the early stages of the disease. 8 Although we have reported the impact of COVID‐19 on the mental status of patients with amyotrophic lateral sclerosis, it has also been impacting patients with PD during the pandemic. 78 Several reports have described the impact of COVID‐19 on PD, in which mental instabilities have worsened motor symptoms. 61 , 79 Older people with frailty, depression and anxiety are commonly observed. 80 , 81 , 82 PD patients are likely to experience depression and anxiety, as well as healthy older people. Treatments for depression and anxiety are generally based on medication and psychological interventions. According to a recent Movement Disorders Society report, several medications are recommended for clinical use. Of these, pramipexole, a dopamine agonist, has antidepressant effects; in contrast, other dopaminergic and non‐dopaminergic drugs for PD show little evidence of antidepressant effects at present. 55 , 83 Apart from basic anti‐parkinsonian treatment, tricyclic antidepressants and selective serotonin/noradrenaline reuptake inhibitors are usually used for the treatment. 55 Non‐pharmacological approaches, such as repetitive transcranial magnetic stimulation and cognitive‐behavioral therapy, might be useful. However, few methods are available for the treatment of anxiety. 55
Cognitive impairment, dementia and mental instability caused by depression and anxiety are common comorbidities in patients with PD. Furthermore, these cognitive and mental disorders are also found in frailty. Furthermore, they might be influenced by physical frailty and motor symptoms in PD; thus, psychological aspects should be considered to be as important as in the physical aspects of frailty and PD.
Relationship between PD and social frailty
Older people might be isolated from society and relationships with other people because of child independence, retirement from work and spousal bereavement. This social isolation might be the possible cause of the worsening physical and mental state in older people. In the literature review, older people in social isolation tend to have depression and cardiovascular disease risk factors, which reflects the social aspects of frailty; however, unified definitions and evaluation methods have not yet been established. 84 , 85 According to Brunt et al.'s conceptual model of social frailty, it consists of various social and general resources, social behaviors and activities, and self‐management abilities affecting social fulfillment of basic social needs that cause subjective well‐being. 86
Meanwhile, a recent report has shown that social isolation might worsen the symptom severity of PD. 87 Furthermore, during the COVID‐19 pandemic, older people and patients with PD have been forced to be isolated from society. 88 , 89 In addition to patients with PD, caregiver burden should be considered during this severe era. Previous studies on caregivers of patients with PD showed that they felt the burden, especially those with neuropsychiatric symptoms, 90 and just like those of patients with Alzheimer's disease. 91 Considering this difficult pandemic era, Subramanian recommended virtual video conference support with various experts for PD patients to maintain their quality of life. 92 Hence, patients must be treated using a multidisciplinary approach.
Relationship between PD and oral frailty
Oral frailty has recently been recognized. In Japan, the promotion of 80–20 by the Japanese Ministry of Health, Labor and Welfare and the Japan Dental Association – preserving 20 teeth by the age of 80 years – has for decades been a nationwide healthcare achievement. The proportion of older people between the ages of 75 and 84 was 51.2% in 2016, and the number of achievers has been increasing gradually (Ministry of Health Labor and Welfare: Survey of Dental Diseases. 2016. https://www.mhlw.go.jp/toukei/list/dl/62-28-02.pdf). Tooth loss is significantly associated with physical health and cognitive decline in older people, as well as with frailty, which is not the case for PD. 93 According to Hanaoka and Kashihara, there was no significant difference in the incidence of dental caries between patients with PD and controls. 94 Additionally, the frequency of dental caries has an association with cognitive decline and disease severity. 94 A recent nationwide cohort study in Taiwan and South Korea showed that oral hygiene was associated with the onset of PD. 95 , 96 Although the mechanism remains unclear, one hypothesis is the association of systemic inflammation, which is consistent with an increased prevalence myocardial infarction and ischemic stroke in patients with periodontal disease. 97 , 98 Although few studies on the relationship between oral healthcare and patients with PD are available, oral hygiene must be considered important from the point of view of oral frailty.
Tooth loss is also associated with difficulties in swallowing, 99 , 100 and both were associated with mortality in a previous study. 101 Swallowing disturbance is common in PD, and this causes patients with PD to develop aspiration pneumonia, even ending in mortality. 102 , 103 Dysphasia is likely to be observed in the advanced stage of PD; however, it could be objectively found even in the early stages of the disease. 104 In a previous meta‐analysis, 35–82% of patients with PD experienced dysphagia. 105 Aspiration pneumonia is a relevant comorbidity in PD, and a previous study showed that 70% of the associated mortality was due to aspiration pneumonia,103 and the incidence has increased, although the mortality tended to decrease for decades. 106 The prevalence of aspiration pneumonia in PD differed from the study demographics: 2.4% in‐hospital patients, 107 3.8–4.9% on the nationwide survey 106 and the mortality of PD was higher than that of controls. 108 According to a previous study, patients with dementia having Lewy bodies tended to have a higher prevalence of aspiration pneumonia than those with PD, and older and demented patients with PD frequently had aspiration pneumonia. 109 It still remained to be elucidated that levodopa has an impact on dysphagia, as Sutto et al. described. 110
Furthermore, patients with PD frequently experienced xerostomia in a previous study. 111 As mentioned before, patients with PD have a potential risk of oral frailty because of oral hygiene‐related comorbidities. Additionally, patients with PD tend to be less conscious of oral health care because of various entities, such as motor disability, cognitive impairment or dementia and socioeconomic situations. 112 , 113
Oral frailty is associated with sarcopenia; thus, preserving oral hygiene is essential, although the management of oral healthcare in patients with PD is still challenging, and few studies are currently available. Oral rehabilitation is a crucial factor in oral frailty. It improves the quality of life and oral function of older people. 114 , 115 To prevent oral frailty, medical and dental care providers should collaborate with each other. 116 This applies to patients with PD, and it should be kept in mind that oral hygiene is significantly associated with sarcopenia and frailty.
Polypharmacy in patients with PD
Polypharmacy is an issue usually encountered in older people; however, there is an obvious definition for it. 117 According to the World Health Organization's statement in 2019, it is generally recognized as the use of five or more ordinal drugs. Importantly, the appropriate management of multiple drugs is required. 118 According to an epidemiological study in Japan, older people who took six or more medications had a higher risk for adverse drug reactions at admission. 119 Furthermore, patients with more than five medications had a high risk of falls. 120
As many medications for PD have been developed over decades, various pharmacotherapeutics, such as levodopa, dopamine agonists, monoamine oxidase‐B inhibitors, adjunctive drugs to levodopa‐like catechol‐O‐methyltransferase inhibitors, zonisamide and istradefylline, are usually used as tailor‐made therapies for each PD patient. 121 Furthermore, device‐aided therapy, such as deep brain stimulation and levodopa‐carbidopa continuous infusion gel (LCIG), are considered when oral medications are not sufficient to manage PD symptoms. Recently, transdermal medication has been introduced to achieve continuous dopaminergic stimulation. Although it was recently hypothesized that intermittent dopaminergic stimulation can cause the onset of motor complications, transdermal medication is thought to be consistent with continuous dopaminergic stimulation. Additionally, as the disease progresses, various comorbidities, such as cognitive impairment, depression and orthostatic hypotension, are frequently found in patients with PD. These comorbidities are different from dopaminergic neuron failure; thus, acetylcholinesterase inhibitors, antidepressants and other noradrenergic drugs are required in addition to anti‐parkinsonian drugs. 55
A recent prescription data‐based analysis in Japan showed that daily dose frequencies and the number of tablets of anti‐parkinsonian drugs both increased with disease duration. 122 These results might be attributed to the lower medication adherence among patients with PD. However, increasing the number of anti‐parkinsonian drugs, including adjunctive medications, is generally inevitable for advanced stage PD patients to maintain their quality of life. Due to the pathophysiological complexity of PD and comorbidities, polypharmacy is common among PD patients. 123 These results were consistent with those of community‐dwelling older adults. 124 In a cross‐sectional study, patients in the PD with polypharmacy group tended to have lower cognitive impairment than those in the PD with non‐polypharmacy group. 125 It is still unclear whether medication reduction contributes to the improvement of cognitive impairment among PD patients; however, a previous study showed that cognitive improvement was observed in patients receiving LCIG therapy. 126 Additionally, LCIG therapy contributes to the reduction of medication efficiently among patients with advanced PD in a large‐scale cross‐sectional study. 127 Nevertheless, polypharmacy has a possible influence on physical aspects, such as falls and frailty, among older people. 128 However, just like with cognitive effects, little evidence is available on the relationship between physical conditions and polypharmacy among people with PD. In older people, polypharmacy is significantly associated with falls; however, it is unclear whether this applies to patients with PD. Generally, as PD advances, the daily dose and frequency of anti‐parkinsonian medications gradually increase, as mentioned earlier. Furthermore, in addition to anti‐parkinsonian medications, pharmacotherapy for various comorbidities is usually required. Thus, medical care providers should devise a treatment approach for patients with PD. For instance, a once‐daily transdermal patch is a resolution, as well as the implementation of continuous dopaminergic stimulation . At a more advanced stage of PD, device‐aided therapy, such as LCIG, will probably have a positive impact in terms of polypharmacy and medication adherence. Furthermore, multidisciplinary interventions are required to improve polypharmacy and drug adherence in patients with PD. 123 The concept of relationships between frailty and PD is shown in Figure 1.
Figure 1.
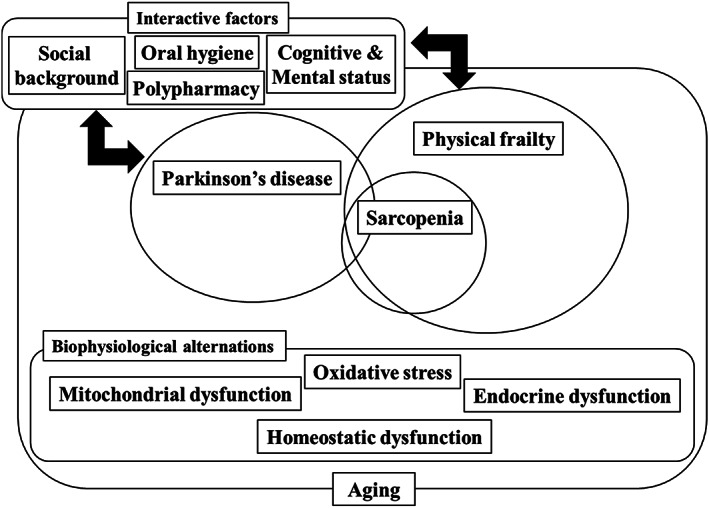
Conceptual diagram. With aging, various physiological alternations occur in individuals. Aging is a risk factor of the onset of Parkinson's disease (PD) and frailty. Sarcopenia is a component of frailty. Biophysiological alternations with increasing age exert an influence on both PD and physical frailty. Furthermore, various interactive factors, such as cognitive and mental status, social background, oral hygiene, and polypharmacy, might also influence both PD and frailty. PD and physical frailty have similar symptoms.
How to manage frailty in patients with PD
How we manage frailty remains to be elucidated. To our knowledge, although scarce literature is available for the interventional study between frailty and PD, a Japanese study using a robotic neurorehabilitation method on frail PD and non‐PD patients showed significant improvement in motor abilities. 129 Rehabilitation and exercise, therefore, could be beneficial for the prevention of frail progression in PD patients at present; however, further studies are required to establish the evidence. Nutritional support, and mental and social interventions, are simultaneously required. 130 , 131 Furthermore, medical providers should pay attention to the oral hygiene of patients with PD. In summary, multidisciplinary care is required to prevent the progression of frailty in patients with PD.
Conclusions
We mentioned various relationships and aspects between frailty and PD through geriatrics prospects in the literature. To our knowledge, this is the first review article that focuses on the relationship between frailty and PD and related geriatric issues beyond the physical aspects.
What is more important for neurologists, gerontologists and general practitioners, and all people who take care of patients with PD in daily clinical practice, is to find the vulnerability quickly and prevent frailty. Furthermore, although management strategies for PD with frailty or sarcopenia have still not been established, the prevalence of these geriatric syndromes should be quickly recognized by medical care providers, and managed appropriately in terms of nutrition, rehabilitation, social support and typical medications for PD.
In conclusion, all those who take care of the patients with PD have to keep in mind that there are potentially more cases of frailty hidden among patients with PD than expected, and care for patients with PD should focus not only on the physical aspects, but also on the mental, social and pharmacotherapeutic aspects.
Disclosure statement
The authors declare no conflict of interest.
Acknowledgements
None.
Ebina J, Ebihara S, Kano O. Similarities, differences and overlaps between frailty and Parkinson's disease. Geriatr. Gerontol. Int. 2022;22:259–270. 10.1111/ggi.14362
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Postuma RB, Berg D, Stern M et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015; 30: 1591–1601. [DOI] [PubMed] [Google Scholar]
- 2. Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord 2015; 30: 4–18. [DOI] [PubMed] [Google Scholar]
- 3. Rajput AH, Uitti RJ, Rajput AH, Offord KP. Timely levodopa (LD) administration prolongs survival in Parkinson's disease. Parkinsonism Relat Disord 1997; 3: 159–165. [DOI] [PubMed] [Google Scholar]
- 4. Annual Report on the Ageing Society 2020, Cabinet Office, Government of Japan, [Internet]. 2020;6–8. Available from: https://www8.cao.go.jp/kourei/whitepaper/w-2020/zenbun/pdf/1s1s_02.pdf
- 5. Feigin VL, Abajobir AA, Abate KH et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16: 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorsey ER, Sherer T, Okun MS, Bloemd BR. The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 2018; 8: S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jost WH, Reichmann H. “An essay on the shaking palsy” 200 years old. J Neural Transm 2017; 124: 899–900. [DOI] [PubMed] [Google Scholar]
- 8. Kalia LV, Lang AE. Parkinson's disease. Lancet 2015; 386: 896–912. [DOI] [PubMed] [Google Scholar]
- 9. Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney Multicenter Study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008; 23: 837–844. [DOI] [PubMed] [Google Scholar]
- 10. Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003; 24: 197–211. [DOI] [PubMed] [Google Scholar]
- 11. Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual‐hit hypothesis. Neuropathol Appl Neurobiol 2007; 33: 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 2010; 67: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stark AK, Pakkenberg B. Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res 2004; 318: 81–92. [DOI] [PubMed] [Google Scholar]
- 14. Pringsheim T, Jette N, Frolkis A, Steeves TDL. The prevalence of Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2014; 29: 1583–1590. [DOI] [PubMed] [Google Scholar]
- 15. Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson's disease: evidence from studies of non‐human primates. Nat Rev Neurosci 2011; 12: 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodríguez‐Mañas L, Féart C, Mann G et al. Searching for an operational definition of frailty: a Delphi method based consensus statement. The frailty operative definition‐consensus conference project. J Gerontol A Biol Sci Med Sci 2013; 68: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: 146–157. [DOI] [PubMed] [Google Scholar]
- 18. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–727. [DOI] [PubMed] [Google Scholar]
- 19. Dent E, Morley JE, Cruz‐Jentoft AJ et al. Physical frailty: ICFSR international clinical practice guidelines for identification and management. J Nutr Heal Aging 2019; 23: 771–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cruz‐Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xue QL, Bandeen‐Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the women's health and aging study II. J Gerontol A Biol Sci Med Sci 2008; 63: 984–990. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed NN, Sherman SJ, VanWyck D. Frailty in Parkinson's disease and its clinical implications. Parkinsosnism Relat Disord 2008; 14: 334–337. [DOI] [PubMed] [Google Scholar]
- 23. Roland KP, Jkobi JM, Hones GRPC. Quality of life as a determinant of frailty phenotype in community‐dwelling persons with Parkinson's disease. J Am Geriatr Soc 2012; 60: 590–592. [DOI] [PubMed] [Google Scholar]
- 24. Roland KP, Cornett KMD, Theou O, Jakobi JM, Jones GR. Physical activity across frailty phenotypes in females with Parkinson's disease. J Aging Res 2012; 2012: 468156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roland KP, Cornett KMD, Theou O, Jakobi JM, Jones GR. Concurrence of frailty and Parkinson's disease. J Frailty Aging 2012; 1: 123–127. [DOI] [PubMed] [Google Scholar]
- 26. Roland KP, Jones GR, Jakobi JM. Daily electromyography in females with Parkinson's disease: a potential indicator of frailty. Arch Gerontol Geriatr 2014; 58: 80–87. [DOI] [PubMed] [Google Scholar]
- 27. Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology 2013; 80: 2055–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liotta G, O'Caoimh R, Gilardi F et al. Assessment of frailty in community‐dwelling older adults residents in the Lazio region (Italy): a model to plan regional community‐based services. Arch Gerontol Geriatr 2017; 68: 1–7. [DOI] [PubMed] [Google Scholar]
- 29. Tan AH, Hew YC, Lim SY et al. Altered body composition, sarcopenia, frailty, and their clinico‐biological correlates, in Parkinson's disease. Parkinsonism Relat Disord 2018; 56: 58–64. [DOI] [PubMed] [Google Scholar]
- 30. Torsney KM, Romero‐Ortuno R. The clinical frailty scale predicts inpatient mortality in older hospitalised patients with idiopathic Parkinson's disease. J R Coll Physicians Edinb 2018; 48: 103–107. [DOI] [PubMed] [Google Scholar]
- 31. Renne I, Gobbens RJJ. Effects of frailty and chronic diseases on quality of life in dutch community‐dwelling older adults: a cross‐sectional study. Clin Interv Aging 2018; 13: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peball M, Mahlknecht P, Werkmann M et al. Prevalence and associated factors of sarcopenia and frailty in Parkinson's disease: a cross‐sectional study. Gerontology 2019; 65: 216–228. [DOI] [PubMed] [Google Scholar]
- 33. Lin WC, Huang YC, Leong CP et al. Associations between cognitive functions and physical frailty in patients with Parkinson's disease. Front Aging Neurosci 2019; 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith N, Brennan L, Gaunt DM, Ben‐Shlomo Y, Henderson E. Frailty in Parkinson's disease: a systematic review. J Parkinsons Dis 2019; 9: 517–524. [DOI] [PubMed] [Google Scholar]
- 35. Ntanasi E, Maraki M, Yannakoulia M et al. Frailty and prodromal Parkinson's disease: results from the HELIAD study. J Gerontol A Biol Sci Med Sci 2021; 76: 622–629. [DOI] [PubMed] [Google Scholar]
- 36. Smith N, Gaunt DM, Whone A, Ben‐Shlomo Y, Henderson EJ. The association between frailty and parkinson's disease in the ReSPOnD trial. Can Geriatr J 2021; 24: 22–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abraham DS, Pham Nguyen TP, Willis AW. Claims‐based frailty and outcomes: applying an aging measure to older adults with Parkinson's disease. Mov Disord 2021; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barichella M, Pinelli G, Iorio L et al. Sarcopenia and dynapenia in patients with Parkinsonism. J Am Med Dir Assoc 2016; 17: 640–646. [DOI] [PubMed] [Google Scholar]
- 39. Drey M, Hasmann SE, Krenovsky JP et al. Associations between early markers of Parkinson's disease and sarcopenia. Front Aging Neurosci 2017; 9: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vetrano DL, Pisciotta MS, Laudisio A et al. Sarcopenia in Parkinson disease: comparison of different criteria and association with disease severity. J Am Med Dir Assoc 2018; 19: 523–527. [DOI] [PubMed] [Google Scholar]
- 41. Cai Y, Feng F, Wei Q, Jiang Z, Ou R, Shang H. Sarcopenia in patients with Parkinson's disease: a systematic review and meta‐analysis. Front Neurol 2021; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yazar T, Yazar HO, Zayimoğlu EÇS. Incidence of sarcopenia and dynapenia according to stage in patients with idiopathic Parkinson's disease. Neurol Sci 2019; 40: 625. [DOI] [PubMed] [Google Scholar]
- 43. Lee CY, Chen HL, Chen PC et al. Correlation between executive network integrity and sarcopenia in patients with Parkinson's disease. Int J Environ Res Pub Heal 2019; 16: 4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krenovsky JP, Bötzel K, Ceballos‐Baumann A et al. Interrelation between sarcopenia and the number of motor neurons in patients with parkinsonian syndromes. Gerontology 2020; 66: 409–415. [DOI] [PubMed] [Google Scholar]
- 45. Lima DP, de Almeida SB, de Carvalho BJ et al. Clinical correlates of sarcopenia and falls in Parkinson's disease. PLoS One 2020; 15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ozer FF, Akın S, Gultekin M, Zararsız GE. Sarcopenia, dynapenia, and body composition in Parkinson's disease: are they good predictors of disability? A case–control study. Neurol Sci 2020; 41: 313–320. [DOI] [PubMed] [Google Scholar]
- 47. Fielding RA, Vellas B, Evans WJ et al. Sarcopenia:an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kashihara K. Weight loss in Parkinson's disease. J Neurol 2006; 253: 38–41. [DOI] [PubMed] [Google Scholar]
- 49. Ma K, Xiong N, Shen Y et al. Weight loss and malnutrition in patients with Parkinson's disease: current knowledge and future prospects. Front Aging Neurosci 2018; 10: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hanew K, Utsumi A. The role of endogenous GHRH in arginine‐, insulin‐, clonidine‐ and L‐dopa‐induced GH release in normal subjects. Eur J Endocrinol 2002; 146: 197–202. [DOI] [PubMed] [Google Scholar]
- 51. Siciliano M, Trojano L, Santangelo G, De Micco R, Tedeschi G, Tessitore A. Fatigue in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2018; 33: 1712–1723. [DOI] [PubMed] [Google Scholar]
- 52. Goetz CG, Tilley BC, Shaftman SR et al. Movement disorder society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23: 2129–2170. [DOI] [PubMed] [Google Scholar]
- 53. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 1995; 4: 241–248. [DOI] [PubMed] [Google Scholar]
- 54. Marras C, Chaudhuri KR. Nonmotor features of Parkinson's disease subtypes. Mov Disord 2016; 31: 1095–1102. [DOI] [PubMed] [Google Scholar]
- 55. Seppi K, Ray Chaudhuri K, Coelho M et al. Update on treatments for nonmotor symptoms of Parkinson's disease—an evidence‐based medicine review. Mov Disord 2019; 34: 180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paker N, Bugdayci D, Goksenoglu G, Demircioğlu DT, Kesiktas N, Ince N. Gait speed and related factors in Parkinson's disease. J Phys Ther Sci 2015; 27: 3675–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mirelman A, Bonato P, Camicioli R et al. Gait impairments in Parkinson's disease. Lancet Neurol 2019; 18: 697–708. [DOI] [PubMed] [Google Scholar]
- 58. Atrsaei A, Corrà MF, Dadashi F et al. Gait speed in clinical and daily living assessments in Parkinson's disease patients: performance versus capacity. NPJ Parkinsons Dis 2021; 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bohnen NI, Frey KA, Studenski S et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 2013; 81: 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Henderson EJ, Lord SR, Brodie MA et al. Rivastigmine for gait stability in patients with Parkinson's disease (ReSPonD): a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol 2016; 15: 249–258. [DOI] [PubMed] [Google Scholar]
- 61. Van Der Heide A, Meinders MJ, Bloem BR, Helmich RC. The impact of the COVID‐19 pandemic on psychological distress, physical activity, and symptom severity in Parkinson's disease. J Parkinsons Dis 2020; 10: 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sainz‐Amo R, Baena‐Álvarez B, Pareés I et al. COVID‐19 in Parkinson's disease: what holds the key? J Neurol 2020; 268: 2666–2670. 10.1007/s00415-020-10272-0 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Helmich RC, Bloem BR. The impact of the COVID‐19 pandemic on Parkinson's disease: hidden sorrows and emerging opportunities. J Parkinsons Dis 2020; 10: 351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roberts HC, Syddall HE, Butchart JW, Stack EL, Cooper C, Sayer AA. The association of grip strength with severity and duration of Parkinson's: a cross‐sectional study. Neurorehab Neural Re 2015; 29: 889–896. [DOI] [PubMed] [Google Scholar]
- 65. Louis ED, Bennett DA. Mild parkinsonian signs: an overview of an emerging concept. Mov Disord 2007; 22: 1681–1688. [DOI] [PubMed] [Google Scholar]
- 66. Louis ED, Luchsinger JA, Tang MX, Mayeux R. Parkinsonian signs in older people: prevalence and associations with smoking and coffee. Neurology 2003; 61: 24–28. [DOI] [PubMed] [Google Scholar]
- 67. Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community‐based study: risk of incident dementia. Arch Neurol 2004; 61: 1273–1276. [DOI] [PubMed] [Google Scholar]
- 68. Lerche S, Brockmann K, Wurster I et al. Reasons for mild parkinsonian signs – which constellation may indicate neurodegeneration? Parkinsonism Relat Disord 2015; 21: 126–130. [DOI] [PubMed] [Google Scholar]
- 69. Baloh RW, Ying SH, Jacobson KM. A longitudinal study of gait and balance dysfunction in normal older people. Arch Neurol 2003; 60: 835–839. [DOI] [PubMed] [Google Scholar]
- 70. Forsaa EB, Larsen JP, Wentzel‐Larsen T, Alves G. What predicts mortality in Parkinson disease? A prospective population‐based long‐term study. Neurology 2010; 75: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 71. Ruan Q, Yu Z, Chen M, Bao Z, Li J, He W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev 2015; 20: 1–10. [DOI] [PubMed] [Google Scholar]
- 72. Ma L, Chan P. Understanding the physiological links between physical frailty and cognitive decline. Aging Dis 2020; 11: 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment – a review of the evidence and causal mechanisms. Ageing Res Rev 2013; 12: 840–851. [DOI] [PubMed] [Google Scholar]
- 74. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer's disease and cognitive decline in the elderly. Psychosom Med 2007; 69: 483–489. [DOI] [PubMed] [Google Scholar]
- 75. Chen YS, Chen HL, Lu CH et al. Reduced lateral occipital gray matter volume is associated with physical frailty and cognitive impairment in Parkinson's disease. Eur Radiol 2019; 29: 2659–2668. [DOI] [PubMed] [Google Scholar]
- 76. Luft AR. Patterns of age‐related shrinkage in cerebellum and brainstem observed in vivo using three‐dimensional MRI volumetry. Cereb Cortex 1999; 9: 712–721. [DOI] [PubMed] [Google Scholar]
- 77. Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain 2013; 136: 696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yanagihashi M, Sugisawa T, Fuchimoto M et al. Contradictory responses to the COVID‐19 pandemic in amyotrophic lateral sclerosis patients and their families and caregivers in Japan. Intern Med 2021; 60: 1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shalash A, Roushdy T, Essam M et al. Mental health, physical activity, and quality of life in Parkinson's disease during COVID‐19 pandemic. Mov Disord 2020; 35: 1097–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Buigues C, Padilla‐Sánchez C, Fernández Garrido J, Navarro‐Martínez R, Ruiz‐Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Heal 2015; 19: 762–772. [DOI] [PubMed] [Google Scholar]
- 81. Vaughan L, Corbin AL, Goveas JS. Depression and frailty in later life: a systematic review. Clin Interv Aging 2015; 10: 1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tavares DM d S, Faria PM, Pegorari MS, Ferreira PC d S, Nascimento JS, Marchiori GF. Frailty syndrome in association with depressive symptoms and functional disability among hospitalized elderly. Issues Ment Health Nurs 2018; 39: 433–438. [DOI] [PubMed] [Google Scholar]
- 83. Kano O, Ikeda K, Cridebring D, Takazawa T, Yoshii Y, Iwasaki Y. Neurobiology of depression and anxiety in Parkinson’ s disease. Parkinsons Dis 2011; 2011: 143547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Courtin E, Knapp M. Social isolation, loneliness and health in old age: a scoping review. Heal Soc Care Commun 2017; 25: 799–812. [DOI] [PubMed] [Google Scholar]
- 85. Xia N, Li H. Loneliness, social isolation, and cardiovascular health. Antioxidants Redox Signal 2018; 28: 837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bunt S, Steverink N, Olthof J, van der Schans CP, Hobbelen JSM. Social frailty in older adults: a scoping review. Eur J Ageing 2017; 14: 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Subramanian I, Farahnik J, Mischley LK. Synergy of pandemics‐social isolation is associated with worsened Parkinson severity and quality of life. NPJ Parkinsons Dis 2020; 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Papa SM, Brundin P, Fung VSC et al. Impact of the COVID‐19 pandemic on Parkinson's disease and movement disorders. Mov Disord 2020; 35: 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stoessl AJ, Bhatia KP, Merello M. Movement disorders in the world of COVID‐19. Mov Disord 2020; 35: 709–710. [DOI] [PubMed] [Google Scholar]
- 90. Chahine LM, Feldman R, Althouse A et al. Contribution of neuropsychiatric symptoms in Parkinson's disease to different domains of caregiver burden. J Neurol 2021; 268: 2961–2972. 10.1007/s00415-021-10443-7 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sugimoto T, Ono R, Kimura A et al. Physical frailty correlates with behavioral and psychological symptoms of dementia and caregiver burden in Alzheimer's disease. J Clin Psychiatry 2018; 79: e1–e7. [DOI] [PubMed] [Google Scholar]
- 92. Subramanian I. Virtual Parkinson's disease support groups in the COVID‐19 era: social connection in the time of social distancing. Mov Disord Clin Pract 2020; 7: 739–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tsakos G, Watt RG, Rouxel PL, De Oliveira C, Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. J Am Geriatr Soc 2015; 63: 91–99. [DOI] [PubMed] [Google Scholar]
- 94. Hanaoka A, Kashihara K. Increased frequencies of caries, periodontal disease and tooth loss in patients with Parkinson's disease. J Clin Neurosci 2009; 16: 1279–1282. [DOI] [PubMed] [Google Scholar]
- 95. Chen CK, Huang JY, Wu YT, Chang YC. Dental scaling decreases the risk of Parkinson's disease: a nationwide population‐based nested case‐control study. Int J Environ Res Public Health 2018; 15: 1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Woo HG, Chang Y, Lee JS, Song TJ. Association of tooth loss with new‐onset Parkinson's disease: a nationwide population‐based cohort study. Parkinsons Dis 2020; 2020: 4760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shi Q, Zhang B, Huo N, Cai C, Liu H, Xu J. Association between myocardial infarction and periodontitis: a meta‐analysis of case‐control studies. Front Physiol 2016; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke 2003; 34: 47–52. [DOI] [PubMed] [Google Scholar]
- 99. Okamoto N, Morikawa M, Yanagi M et al. Association of tooth loss with development of swallowing problems in community‐dwelling independent elderly population: the Fujiwarakyo study. J Gerontol A Biol Sci Med Sci 2015; 70: 1548–1554. [DOI] [PubMed] [Google Scholar]
- 100. Saito M, Shimazaki Y, Fukai K et al. A multilevel analysis of the importance of oral health instructions for preventing tooth loss: the 8020 Promotion Foundation study of Japanese dental patients. BMC Oral Health 2020; 20: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Furuta M, Takeuchi K, Adachi M et al. Tooth loss, swallowing dysfunction and mortality in Japanese older adults receiving home care services. Geriatr Gerontol Int 2018; 18: 873–880. [DOI] [PubMed] [Google Scholar]
- 102. Cereda E, Cilia R, Klersy C et al. Swallowing disturbances in Parkinson's disease: a multivariate analysis of contributing factors. Parkinsonism Relat Disord 2014; 20: 1382–1387. [DOI] [PubMed] [Google Scholar]
- 103. Mehanna R, Jankovic J. Respiratory problems in neurologic movement disorders. Parkinsonism Relat Disord 2010; 16: 628–638. [DOI] [PubMed] [Google Scholar]
- 104. Müller J, Wenning GK, Verny M et al. Progression of dysarthria and dysphagia in postmortem‐confirmed Parkinsonian disorders. Arch Neurol 2001; 68: 259–264. [DOI] [PubMed] [Google Scholar]
- 105. Kalf JG, de Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson's disease: a meta‐analysis. Parkinsonism Relat Disord 2012; 18: 311–315. [DOI] [PubMed] [Google Scholar]
- 106. Akbar U, Dham B, He Y et al. Incidence and mortality trends of aspiration pneumonia in Parkinson's disease in the United States, 1979‐2010. Parkinsonism Relat Disord 2015; 21: 1082–1086. [DOI] [PubMed] [Google Scholar]
- 107. Martinez‐Ramirez D, Almeida L, Giugni JC et al. Rate of aspiration pneumonia in hospitalized Parkinson's disease patients: a cross‐sectional study. BMC Neurol 2015; 15: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Won JH, Byun SJ, Oh BM, Park SJ, Seo HG. Risk and mortality of aspiration pneumonia in Parkinson's disease: a nationwide database study. Sci Rep 2021; 11: 6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yamamoto T, Kobayashi Y, Murata M. Risk of pneumonia onset and discontinuation of oral intake following videofluorography in patients with Lewy body disease. Parkinsonism Relat Disord 2010; 16: 503–506. [DOI] [PubMed] [Google Scholar]
- 110. Sutton JP. Dysphagia in Parkinson's disease is responsive to levodopa. Parkinsonism Relat Disord 2013; 19: 282–284. [DOI] [PubMed] [Google Scholar]
- 111. Debowes SL, Tolle SL, Bruhn AM. Parkinson's disease: considerations for dental hygienists. Int J Dent Hyg 2013; 11: 15–21. [DOI] [PubMed] [Google Scholar]
- 112. Bakke M, Larsen SL, Lautrup C, Karlsborg M. Orofacial function and oral health in patients with Parkinson's disease. Eur J Oral Sci 2011; 119: 27–32. [DOI] [PubMed] [Google Scholar]
- 113. Müller T, Palluch R, Ackowski JJ. Caries and periodontal disease in patients with Parkinson's disease. Spec Care Dentist 2011; 31: 178–181. [DOI] [PubMed] [Google Scholar]
- 114. McKenna G, Allen PF, Hayes M, DaMata C, Moore C, Cronin M. Impact of oral rehabilitation on the quality of life of partially dentate elders in a randomized controlled clinical trial: 2 year follow‐up. PLoS One 2018; 13: e0203349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim HJ, Lee JY, Lee ES, Jung HJ, Ahn HJ, Il KB. Improvements in oral functions of elderly after simple oral exercise. Clin Interv Aging 2019; 14: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Shiraishi A, Wakabayashi H, Yoshimura Y. Oral management in rehabilitation medicine: oral frailty, oral sarcopenia, and hospital‐associated oral problems. J Nutr Heal Aging 2020; 24: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pazan F, Wehling M. Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatr Med 2021; 12: 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. World Health Organization . Medication safety in polypharmacy 2019;1–63. Available from: https://apps.who.int/iris/bitstream/handle/10665/325454/WHO‐UHC‐SDS‐2019.11‐eng.pdf?sequence=1&isAllowed=y
- 119. Kojima T, Akishita M, Kameyama Y et al. High risk of adverse drug reactions in elderly patients taking six or more drugs: analysis of inpatient database. Geriatr Gerontol Int 2012; 12: 761–762. [DOI] [PubMed] [Google Scholar]
- 120. Kojima T, Akishita M, Nakamura T et al. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr Gerontol Int 2012; 12: 425–430. [DOI] [PubMed] [Google Scholar]
- 121. Fox SH, Katzenschlager R, Lim SY et al. International Parkinson and movement disorder society evidence‐based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord 2018; 33: 1248–1266. [DOI] [PubMed] [Google Scholar]
- 122. Maeda T, Hattori A, Yamamoto J, Tsunekawa K, Ibe SKF. Prescribing patterns for anti‐parkinsonian drugs in Japan: prescription‐based database study. Brain Nerve 2021; 73: 273–281. [DOI] [PubMed] [Google Scholar]
- 123. McLean G, Hindle JV, Guthrie B, Mercer SW. Co‐morbidity and polypharmacy in Parkinson's disease: insights from a large Scottish primary care database. BMC Neurol 2017; 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Leelakanok N, D'Cunha RR. Association between polypharmacy and dementia – a systematic review and metaanalysis. Aging Ment Heal 2019; 23: 932–941. [DOI] [PubMed] [Google Scholar]
- 125. Ishii N, Mochizuki H, Sakai K, Ogawa G, Shiomi K, Nakazato M. Polypharmacy associated with cognitive decline in newly diagnosed Parkinson's disease: a cross‐sectional study. Dement Geriatr Cogn Dis Extra 2019; 9: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nyholm D, Nilsson Remahl AIM, Dizdar N et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 2005; 64: 216–223. [DOI] [PubMed] [Google Scholar]
- 127. Fasano A, Gurevich T, Jech R et al. Concomitant medication usage with levodopa‐carbidopa intestinal gel: results from the COSMOS study. Mov Disord 2021; 36(8): 1853–1862. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33908647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Fried TR, O'Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community‐dwelling older adults: a systematic review. J Am Geriatr Soc 2014; 62: 2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kotani N, Morishita T, Yatsugi A et al. Biofeedback core exercise using hybrid assistive limb for physical frailty patients with or without Parkinson's disease. Front Neurol 2020; 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tosserams A, de Vries NM, Bloem BR, Nonnekes J. Multidisciplinary care to optimize functional mobility in Parkinson disease. Clin Geriatr Med 2020; 36: 159–172. [DOI] [PubMed] [Google Scholar]
- 131. Qamar MA, Harington G, Trump S, Johnson J, Roberts F, Frost E. Multidisciplinary care in Parkinson's disease. Int Rev Neurobiol 2017; 132: 511–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


