Abstract
Human astrocytes can be infected with human immunodeficiency virus type 1 (HIV-1) in vitro and in vivo, but, in contrast to T lymphocytes and macrophages, virus expression is inefficient. To investigate the HIV-1 life cycle in human fetal astrocytes, we infected cells with HIV-1 pseudotyped with envelope glycoproteins of either amphotropic murine leukemia virus or vesicular stomatitis virus. Infection by both pseudotypes was productive and long lasting and reached a peak of 68% infected cells and 1.7 μg of viral p24 per ml of culture supernatant 7 days after virus inoculation and then continued with gradually declining levels of virus expression through 7 weeks of follow-up. This contrasted with less than 0.1% HIV-1 antigen-positive cells and 400 pg of extracellular p24 per ml at the peak of astrocyte infection with native HIV-1. Cell viability and growth kinetics were similar in infected and control cells. Northern blot analysis revealed the presence of major HIV-1 RNA species of 9, 4, and 2 kb in astrocytes exposed to pseudotyped (but not wild-type) HIV-1 at 2, 14, and 28 days after infection. Consistent with productive infection, the 9- and 4-kb viral transcripts in astrocytes infected by pseudotyped HIV-1 were as abundant as the 2-kb mRNA during 4 weeks of follow-up, and both structural and regulatory viral proteins were detected in infected cells by immunoblotting or cell staining. The progeny virus released by these cells was infectious. These results indicate that the major barrier to HIV-1 infection of primary astrocytes is at virus entry and that astrocytes have no intrinsic intracellular restriction to efficient HIV-1 replication.
The central nervous system (CNS) is a major target for human immunodeficiency virus type 1 (HIV-1) infection. HIV-1 enters the CNS early after systemic infection and persists there for life (20, 26, 30). About 30% of AIDS patients develop prominent cognitive, motor, and behavioral manifestations known as HIV-1 encephalopathy, AIDS dementia complex, or HIV-associated dementia (45, 48, 49, 60). HIV-1 is considered the principal etiologic agent of this disorder (22, 39, 45), but the mechanism of HIV-1-induced neuropathogenesis is unknown. The virus can be found in the brain in infiltrating macrophages, microglial cells, astrocytes, oligodendrocytes, and brain endothelial cells but only infrequently in neurons (53, 57, 67, 68, 73), indicating that the observed neuronal damage cannot be directly attributed to HIV-1 replication in these cells. Macrophages and microglial cells are considered to be the primary source of HIV-1 replication within the CNS, and HIV-1 strains isolated from the CNS generally exhibit R5 or macrophage-tropic characteristics, indicating that such strains predominate in the brain (14, 31, 34, 40, 57, 67, 68, 73). Productive infection of macrophages and microglial cells is believed to contribute to neuropathogenesis through secretion of viral (gp120, Tat, and Nef) and cellular (cytokines, chemokines, and nitric oxide) neurotoxic products (reviewed in references 35 and 41). However, HIV-1 infection of these cells may not be sufficient for the development of neuropathology. Studies in a macaque model of simian immunodeficiency virus (SIV) encephalitis indicate, for example, that there is no direct correlation between macrophage tropism and neuroinvasiveness or neurovirulence of SIV (43, 66). Recent studies have shown that neurons express CXCR4 (23, 29) and that HIV-1 strains which utilize CXCR4 for entry can induce signal transduction and apoptosis in neurons (75) specifically through these receptors (52, 75). We have shown that some primary HIV-1 isolates obtained from vitreous from AIDS patients with cytomegalovirus retinitis can infect astrocytes and T lymphocytes but not macrophages (11). Thus, multiple HIV-1 strains and brain cell types infected with the virus may contribute to HIV-1-mediated neuropathogenesis.
Recently, attention has been directed toward the potential role of astrocytes in HIV-1 neuropathogenesis (9, 19). Astrocytes are critical for brain homeostasis and for responses to pathogens and brain injury (8, 15, 19, 71), and defects in astrocyte functions may lead to neurodegeneration (54). Astrocytes also are a major target for HIV-1 infection in the brain. Depending on methodology of HIV-1 detection, stage of brain disease, and brain region analyzed, reports have shown that anywhere from 0 to 20% of astrocytes may carry the HIV-1 genome in vivo (5, 10, 25, 51, 53, 57, 67, 68), with larger-scale surveys indicating a frequency of up to 1% (67). Considering that the number of astrocytes in the brain ranges between 4 × 1011 and 2 × 1012 cells (55), the total number of HIV-1-infected astrocytes in vivo may be substantial.
We are interested in the course of HIV-1 infection of astrocytes and potential effects of that infection on astrocyte function. In contrast to productive and cytopathic infection in T cells and macrophages (reviewed in reference 39), HIV-1 infection of astrocytes is inefficient, of low productivity, and generally noncytopathic (11, 13, 21, 24, 36, 47, 65, 69, 70). We and others have estimated that only about 1% of human fetal astrocytes express virus at the peak of infection (7, 28). On this basis, there is general agreement that infection or transfection of astrocytes produces a transient burst of viral replication, which diminishes to low levels of virus expression or latency (7, 11, 56, 58, 59, 69, 70). The reasons behind this limited infection are not well understood, but they could include inefficient virus entry, intracellular restrictions to virus replication, or a combination of the two. Intracellular restrictions are suggested by findings showing that infected astrocytes contain mostly viral regulatory proteins and transcripts coding for these products but only low levels of viral structural proteins (57, 67, 69, 70). Studies indicate that this restriction may result from limited expression of HIV-1 RNA encoding the major structural proteins (69), possibly due to inefficient Rev function (42, 50). Inefficient HIV-1 production in certain glioma cell lines has also been correlated with defects in processing of HIV-1 gp160 envelope glycoprotein (58) and inefficient translation of HIV-1 structural proteins (24). Block at HIV-1 entry into astrocytes is implied by the absence of surface CD4 expression by the cells (13). In some but not all glial cell lines (17, 27), stable expression of CD4 permits high-level, productive infection by HIV-1 (59, 61, 72, 74). Transient transfection of HIV-1 DNA into astrocytes or cocultivation with HIV-1-infected cells also permits more efficient virus replication than does exposure to cell-free virus (7, 21, 47, 65, 69), and some glioma cells stably transfected with HIV-1 DNA replicate virus at high levels (21, 59). Together, these studies suggest that virus entry is a major limiting step of HIV-1 infection in astrocytes.
We reported previously that HTB148 glioma cells which were stably transfected to express surface CD4 are susceptible to efficient HIV-1 infection (72). In the present work, we applied a slightly different strategy to investigate the life cycle of HIV-1 in primary human astrocytes. Rather than confer expression of CD4 on primary astrocytes, which was technically difficult, we extended HIV-1 tropism by pseudotyping, that is, endowing native HIV-1 with heterologous envelope glycoproteins that bind commonly expressed cellular receptors. Vesicular stomatitis virus (VSV) G protein mediates entry via an endocytic pathway (44), and amphotropic murine leukemia virus (MLV) glycoprotein mediates virus-cell membrane fusion, mimicking the entry of HIV-1 (46). HIV-1 pseudotyped with either VSV or MLV envelope glycoproteins efficiently infects CD4-negative cells (2, 3, 6, 64). Here, HIV-1 NL4-3 was pseudotyped with VSV or amphotropic MLV envelopes by cotransfection of intact NL4-3 DNA and either a VSV or MLV envelope expression vector, yielding VSV/NL4-3 and MLV/NL4-3, respectively. We compared infection of primary human fetal astrocytes by native NL4-3 to infection by pseudotyped HIV-1. We found that in contrast to native NL4-3, both VSV/NL4-3 and MLV/NL4-3 were able to productively infect the majority of astrocytes, permitting quantitative analysis of the viral life cycle in these cells by Western and Northern blotting and immunofluorescent staining of cells with anti-HIV sera. Our results suggest that primary astrocytes pose no fundamental intracellular block to HIV-1 replication. These results introduce a model system of efficient HIV-1 infection of primary astrocytes for studies of the course of the HIV-1 life cycle in these cells.
MATERIALS AND METHODS
Cells.
Fetal astrocytes were isolated from second-trimester (gestational age, 16–19 weeks) human fetal brains obtained from elective abortions in full compliance with National Institutes of Health (NIH) guidelines, as previously described (11, 75). Highly homogenous preparations of astrocytes were obtained using high-density culture conditions in the absence of growth factors in F12 Dulbecco's modified Eagle's medium (GIBCO-BRL, Gaithersburg, Md.) containing 10% fetal bovine serum, penicillin, streptomycin, and gentamicin (11, 75). Subsequently, the cells were maintained in this medium at 2 × 104 to 5 × 104 cells/cm2 and subcultured weekly up to six times. For each experiment, a single batch of astrocytes of the same gestational age and passage was used. Cultures were regularly monitored for expression of the astrocytic marker glial fibrillary acidic protein (GFAP) and either HAM56 or CD68 to identify cells of monocyte/macrophage lineage. Only cultures that contained ≥99% GFAP-positive cells and rare or no detectable HAM56- or CD68-positive cells were used in our experiments (75).
HIV-1 molecular clones, envelope expression vectors, and generation of pseudotyped HIV-1.
The HIV-1 molecular clones used were NL4-3, which expresses all known HIV-1 proteins (1), and NL-P1, an NL4-3 derivative carrying the marker gene human placental alkaline phosphatase (PLAP) inserted next to Nef (18). The VSV G expression vector pL-VSV-G was obtained from M. Emerman; it contains a VSV G insert in the pcDNA expression vector modified by replacing the cytomegalovirus promoter with the HIV-1 long terminal repeat (6). The amphotropic MLV envelope expression vector SV-A-MLV-Env was obtained from D. Littman; envelope expression in this vector is driven by the MLV long terminal repeat (38). High-titer virus stocks were produced in 293T human embryonic kidney cells transfected with the respective DNA by calcium phosphate precipitation (4). To generate pseudotyped virus, 1.5 × 106 293T cells cultured in 10-cm plates were cotransfected with 10 μg of HIV-1 clone DNA and 15 μg of VSV or MLV envelope expression plasmid DNA, a ratio of DNAs found to yield the highest HIV-1 infectious titers in our hands. For native HIV-1 production, 1.5 × 106 293T cells were transfected with 15 μg of NL4-3 or NL-P1 DNA. 293T culture supernatants were harvested 72 h after transfection, filtered through a 0.45-μm-pore-size Millipore filter, and stored at −80°C until use. Cell-free viral stock was tested for HIV-1 p24 core antigen content by enzyme-linked immunosorbent assay (ELISA) using the HIV-1 Ag kit as specified by the manufacturer (Coulter, Hialeah, Fla) and for titers of infectious virus by multinuclear activation of a β-galactosidase indicator (MAGI) assay (33). Culture supernatants contained 1 to 2 μg of viral p24 protein per ml and 1 × 106 to 2 × 106 infectious units (IU) per ml. In our hands, a multiplicity of infection of 1 for CD4-positive T cells is equivalent to approximately 1 pg of viral p24 per cell (21, 59).
Cell infection and analysis of HIV-1 expression by p24 ELISA and IF.
Confluent cultures of human fetal astrocytes were infected with native or pseudotyped HIV-1 at 1 pg of p24 per cell overnight and washed five times with Hanks balanced salt solution (GIBCO-BRL) before being returned to culture. At the indicated times after infection, culture supernatants were tested for the levels of HIV-1 p24 antigen by p24 ELISA and cells were removed, spotted on glass slides, fixed in acetone, and stained with AIDS patient serum to detect HIV-1 antigens by indirect immunofluorescence (IF). Astrocytes infected with NL-P1 virus were also stained for PLAP by IF using mouse anti-alkaline phosphatase antibody (Serotec, Raleigh, N.C.). GFAP-positive astrocytes were detected by IF staining using rabbit anti-GFAP (DAKO Corp., Carpinteria, Calif.). All secondary antibodies were conjugated with fluorescein isothiocyanate, positive cells were visualized under an Olympus BH-2 fluorescence microscope, and at least 200 cells were counted. HIV-1 antigen and GFAP staining were also performed on cells cultured on coverslips, plated, and infected under the same conditions as in large-scale cultures. The results were similar to those obtained with cells removed by trypsinization from large-scale cultures.
HIV-1 protein analysis by immunoblotting.
Cells were counted and lysed in a buffer containing 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 1% sodium deoxycholate, 5 mM iodoacetamide, and 0.2 U of phenylmethylsulfonyl fluoride per ml, and cell lysates corresponding to equivalent numbers of cells were resolved by SDS-polyacrylamide gel electrophoresis on 4 to 15% polyacrylamide ready gels (Bio-Rad, Hercules, Calif.) and transferred onto a 0.2-μm-pore-size Trans-Blot nitrocellulose membrane (Bio-Rad). The membranes were incubated in 5% (wt/vol) skim milk in T-PBS (0.1% polyoxyethyline-sorbitan monolaurate in phosphate-buffered saline [PBS]) and then stained with indicated primary antibodies followed by horseradish peroxidase-conjugated second antibody. Protein bands were visualized on X-ray film after a luminescence reaction using the ECL kit (Amersham, Arlington Heights, IU.). Samples were standardized by their α-tubulin content prior to final evaluations.
Viral RNA analysis by Northern blot hybridization.
Total cellular RNA was isolated with TRIzol (GIBCO-BRL) as specified by the manufacturer, samples were standardized by their optical density at 262 nm, and 20 μg of RNA per lane was resolved by electrophoresis through an agarose-formaldehyde gel (1% agarose, 2.2 M formaldehyde, 10% [vol/vol] 10× MOPS [0.4 M MOPS, pH7; 0.1 M sodium acetate, 0.01 M EDTA]) in 1× MOPS (morpholinepropanesulfonic acid) running buffer using a standard procedure (4). The gels were denatured in 0.05 M NaOH–1.5 M NaCl for 30 min, neutralized in 0.5 M Tris-Cl (pH7.4) to 1.5M NaCl for 20 min, and washed in 20× SSC (1× SSC in 0.15 M NaCl plus 0.015 M sodium citrate) for 45 min, and RNA was blotted onto a 0.45-μm-pore-size NYTRAN SPC membrane (Schleicher & Schuell, Keene, N.H.) and hybridized with an 8.9-kb SacI proviral DNA fragment derived from NL4-3 (58) labeled with [α-32P]dCTP using the RadPrime DNA Labeling System (GIBCO-BRL). Hybridization was carried out overnight at 42°C in a buffer containing 6× SSC, 5× Denhardt's reagent, 0.5% SDS, 100 μg of salmon sperm DNA per ml, and 50% formaldehyde; the membranes were then washed under stringent conditions and analyzed by autoradiography.
Other analytical procedures and reagents.
Cell viability was determined by trypan blue exclusion counting. The biological activity of progeny virus made in astrocytes was determined by testing virus infectivity in the MAGI assay (33). The following reagents used were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH: hybridoma cells producing monoclonal anti-Gag HIV-1 p24 (obtained from Bruce Chesebro and Hardy Chen) (183-H12-5C; 1513) (14), mouse monoclonal anti HIV-1 V3 (obtained from Jon Laman) (IIIB-V3-13; 1727) (37), and monoclonal anti-Nef antiserum (obtained from Ronald Swanstrom) (HIV-1 Nef antiserum; 2949) (62). The monoclonal α-tubulin antibody was purchased from Sigma (St. Louis, Mo.).
RESULTS
HIV-1 pseudotyped with VSV-G or MLV envelope causes lasting and highly productive infection in primary astrocytes.
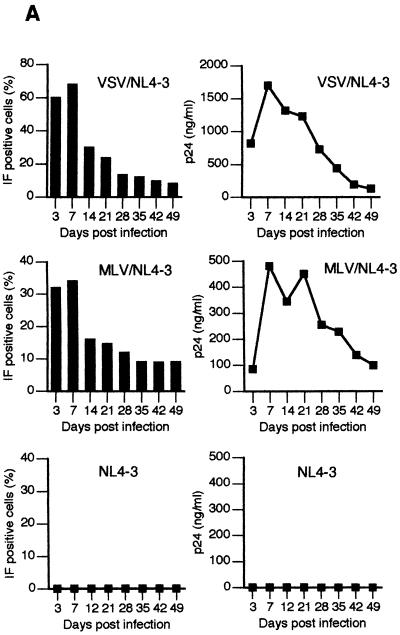
Infection of primary human astrocytes with native HIV-1 in vitro is inefficient, with fewer than 1% of cells expressing viral antigens and virus production being low (7, 28, 70). We first tested whether exposure of astrocytes to HIV-1 pseudotyped with VSV G or MLV envelope, which bypasses the natural mode of HIV-1 entry into these cells, can increase the susceptibility of astrocytes to HIV-1 infection and replication. Astrocytes were isolated from second-trimester human fetal brains and cultured under conditions that minimize the growth of nonastrocytic brain cells, as previously described (7, 11, 75). The cultures were regularly evaluated by IF staining for expression of GFAP, an astrocytic marker, and HAM56 or CD68, to detect macrophages and microglial cells. Cells found to contain ≥99% GFAP-positive cells and no detectable HAM56 and CD68 signals were used in further experiments. Staining of a representative culture for GFAP is shown in Fig. 1A. Astrocytes were exposed overnight to native NL4-3 or NL4-3 pseudotyped with MLV (MLV/NL4-3) or VSV-G (VSV/NL4-3) envelope glycoproteins at 1 pg of viral p24 per cell and tested at the indicated time intervals for expression of cell-associated HIV-1 antigens by IF staining (Fig. 1C and D) and for levels of viral p24 in culture supernatants by p24 ELISA (Fig. 2A). In parallel, cultures were monitored for cell growth kinetics and viability (Fig. 2B). As determined by IF staining, expression of cell-associated HIV-1 antigens peaked on day 7, with 68 and 34% of cells positive after VSV/NL4-3 and MLV/NL4-3 infection, respectively (Fig. 2A). Subsequently, virus expression declined at a similar rate in both VSV/NL4-3- and MLV/NL4-3-infected cultures, but, remarkably, viral proteins were still detected by IF in 8 to 9% of cells 7 weeks after infection (Fig. 2A). Figures 1C and D show examples of IF staining of HIV-1-infected astrocytes from this experiment. Consistent with IF results, parallel measurements of HIV-1 p24 antigen levels in culture supernatants revealed a peak of p24 production on day 7 at 1.7 μg of p24/ml for VSV/NL4-3-infected and 0.48 μg of p24/ml for MLV/NL4-3-infected astrocytes, followed by a gradual decline to approximately 100 ng/ml by 7 weeks after infection (Fig. 2A). As measured by IF staining and p24 antigen production, infection of astrocytes with VSV/NL4-3 was more efficient than infection with MLV/NL4-3 at the same dose (Fig. 2). The reasons for this difference are not clear, but similar results were noted in studies using other target cells (3). Infection of astrocytes with pseudotyped HIV-1 was virus dose dependent in a linear fashion as measured by IF and p24 antigen production (data not shown). In contrast to astrocytes infected with pseudotyped HIV-1 and consistent with previous studies (7, 70), only limited infection was observed in cells exposed to native NL4-3, with few astrocytes staining positive for HIV-1 antigens by IF and less than 400 pg of p24/ml being detectable in culture supernatants at the peak of infection (Fig. 2A). Similar results were obtained in more than 20 experiments using different batches of astrocytes and HIV-1. We conclude that use of HIV-1 pseudotyped with viral envelopes of a broad cellular tropism permits a rapid, highly productive, and long-lasting infection of the majority of astrocytes in culture.
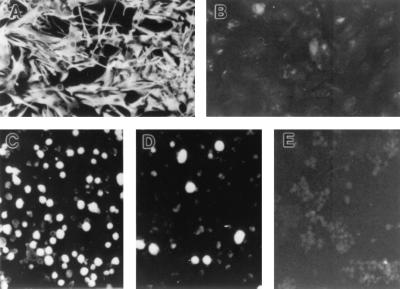
FIG. 1.
GFAP expression by human fetal astrocytes and IF detection of HIV-1 antigens in infected astrocytes. (A and B) Uninfected human fetal astrocytes (16 weeks of gestational age, third passage) were grown on glass chamber slides and stained with anti-GFAP antibody (A) or irrelevant control antibody (B). (C to E) VSV/NL4-3-infected astrocytes from the experiment described in the legend to Fig. 2 were harvested, fixed, and stained for HIV-1 antigens by IF 7 days (C) and 28 days (D) after infection; cells harvested 7 days after infection, stained with an irrelevant serum, are also shown (E). Photographs were taken using an Olympus BH-2 fluorescence microscope. Magnification, ×100.
FIG. 2.
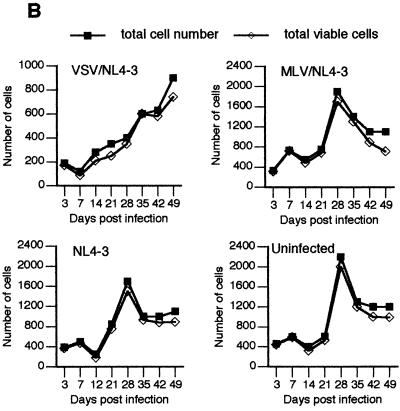
Expression of HIV-1 antigens, viability, and growth kinetics in fetal astrocytes infected with native and pseudotyped HIV-1. (A) Astrocyte cultures were infected with VSV/NL4-3, MLV/NL4-3, or NL4-3 as indicated and monitored for HIV-1-specific antigens expression by IF staining with AIDS sera and fluorescein isothiocyarate-conjugated second antibody (left panel) or by production of p24 in cell supernatants (right panel). The proportion of IF-positive cells was determined by counting at least 200 cells each in three different fields under ×20 magnification, using an Olympus BH-2 fluorescence microscope. (B) At the indicated times after infection, total cell numbers and total viable-cell numbers per system (×1,000) were determined as described in Materials and Methods.
The level of HIV-1 expression in astrocytes infected with VSV/NL4-3 or MLV/NL4-3 (Fig. 1 and 2) was similar to and often higher than the level we usually observed in HIV-1-infected peripheral blood lymphocytes (PBL) and macrophages (16, 63). Normally, HIV-1 replication in PBL is cytopathic while infected macrophages can produce large quantities of virus for extended periods without cytolysis (for a review, see: reference 39). To determine whether astrocytes survive productive HIV-1 infection, we tested cell growth kinetics and viability in parallel with virus expression measurements in the experiment summarized in Fig. 2. These results are shown in Fig. 2B. In all systems, i.e., uninfected cells, NL4-3-infected cells that produce minimal amounts of virus, and cells productively infected with VSV/NL4-3 or MLV/NL4-3, astrocytes proliferated and remained fully viable throughout 7 weeks of follow-up (Fig. 2B). The total number of astrocytes infected with VSV/NL4-3 was smaller at the end of the experiment than the numbers of control or other infected cells (Fig. 2B), possibly because of initial cell damage from membrane fusion caused by the VSV-G protein (6). A similar cell proliferation rate in all culture systems over a prolonged period indicates that HIV-1 infection of astrocytes, whether of low productivity (with native NL4-3) or of high productivity (with pseudotyped NL4-3), is largely noncytolytic.
Astrocytes infected with pseudotyped HIV-1 efficiently transcribe viral mRNA and persistently express viral structural proteins.
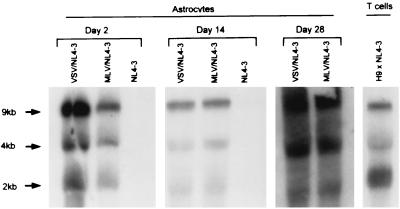
Previous studies of HIV-1 expression in primary astrocytes and glioma cell lines produced conflicting results, with some authors suggesting restricted expression of viral RNA or proteins (24, 50, 69) and others showing no intrinsic intracellular restrictions to efficient viral replication (7, 58, 74). Efficient infection of astrocytes with pseudotyped HIV-1 (Fig. 1 and 2) permitted us to evaluate HIV-1 RNA transcription and protein expression in these cells by standard biochemical techniques of Northern blot hybridization and immunoblotting, respectively (see Fig. 3 and 4). To determine the steady-state levels of the major HIV-1 mRNA species relative to each other at different times after infection, astrocytes were infected with VSV/NL4-3, MLV/NL4-3, or NL4-3 as described above and total cellular RNA was isolated and subjected to Northern blot analysis with an HIV-1-specific probe on days 2, 14, and 28 after infection (Fig. 3). As expected because of low virus production, viral RNA was undetectable by this method in astrocytes infected with native NL4-3 (Fig. 3). In contrast, the three major HIV-1 mRNA species of 9, 4, and 2 kb were clearly detectable in astrocytes productively infected with VSV/NL4-3 or MLV/NL4-3 at all the time points tested, including 4 weeks after infection (Fig. 3). The 9-kb RNA was a predominant viral RNA species 2 and 14 days after infection and was present at about a 1:1 ratio with respect to the 4-kb HIV-1 RNA at 4 weeks after infection (Fig. 3). The 2-kb viral RNA was the least abundant species at all three time points. The consistent presence of 9-kb viral RNA in pseudotyped HIV-1-infected astrocytes correlated well with efficient virus production over the same period, as indicated by p24 levels in culture supernatants (Fig. 2). T cells productively infected by HIV-1 and tested as positive control also contained the three major HIV-1 RNA species (Fig. 3). Similar results were obtained in three independent experiments. We conclude that in our model system of HIV-1 infection of primary astrocytes, there is no selective defect in steady-state expression of the 9- and 4-kb HIV-1 mRNA or, conversely, that the 2-kb viral mRNA does not predominate over the course of infection.
FIG. 3.
Northern blot analysis of HIV-1 RNA expression in astrocytes infected with pseudotyped HIV-1. Astrocytes were infected with VSV/NL4-3, MLV/NL4-3, or NL4-3 and analyzed for HIV-1 RNA by Northern blotting 2, 14, and 28 days after infection as described in Materials and Methods. A total of 20 μg of total-cell RNA was loaded per lane. Samples from day 2 and 14 were exposed for 24 h, and samples from day 28 were exposed for 14 days. NL4-3-infected H9 cells were analyzed in parallel as positive controls; the samples were exposed for 24 h.
FIG. 4.
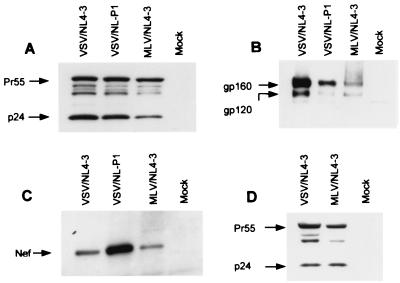
Western blot analysis of Gag, gp120, and Nef proteins by infected astrocytes. Astrocytes were infected with HIV-1 as indicated and tested for HIV-1 proteins by immunoblotting as described in Materials and Methods. All systems were normalized first by measurement of the α-tubulin content. (A and D) Gag levels 7 and 35 days after infection, respectively. (B and C) gp120 and Nef protein levels 7 days after infection. Panel D contains four times more protein per lane than does panel A. Mock indicates uninfected astrocytes, and infected and uninfected PBL are shown for comparison. The results are representative of three experiments.
To identify some of the HIV-1 structural and regulatory proteins expressed in astrocytes, cells were infected with pseudotyped NL4-3 or NL-P1, harvested at the peak of infection (7 days) and during the decline of viral replication (35 days), and analyzed by Western blotting for the presence of the HIV-1 regulatory protein Nef and viral structural proteins Env and Gag (Fig. 4). NL-P1 is a reporter HIV-1 which expresses human PLAP (18) and which is used in the kinetics studies described below (see Fig. 5). HIV-1 Gag was detected using a monoclonal anti-p24 antibody that recognizes both mature p24 and Gag p55 polyprotein; the anti-V3 antibody used detects gp160 and gp120 envelope glycoproteins but not gp41. The samples were standardized by measurement of their α-tubulin content. All the proteins tested were readily detected in astrocytes 7 days after infection with pseudotyped HIV-1 (Fig. 4A to C). As expected, no viral proteins could be detected in NL4-3-infected astrocytes by this method (data not shown). Consistent with the less efficient infection of astrocytes by MLV/NL4-3 than by VSV/NL4-3 (Fig. 2), the protein signals in the MLV/NL4-3 lanes in Fig. 4 were weaker than in other systems. The stronger Nef protein signal in the VSV/NL-P1 lane in Fig. 4 compared to corresponding bands in VSV/NL4-3 and MLV/NL4-3 lanes was probably due to overexpression of Nef by NL-P1, in which Nef (as well as PLAP) is expressed under the control of a picornavirus element, the internal ribosome entry site, allowing cap-independent initiation of translation (12, 18). Infected cells tested with an anti-Env antibody contained both the HIV-1 envelope precursor glycopolyprotein gp160 and the processed envelope protein gp120 (Fig. 4B). We also detected the envelope glycoprotein gp41 by using an anti-gp41 antibody (data not shown). These results indicate that primary astrocytes express the cellular enzymes required for gp160 processing into component envelope proteins. Notably, the Gag p55 polyprotein and mature p24 core protein were also readily detected 35 days after infection (Fig. 4D), indicating that in our system, the observed gradual decline in HIV-1 production in astrocytes over time can not be attributed to a shutoff of Gag polyprotein processing. Similar results were obtained in three independent experiments. The results of Western blot analysis (Fig. 4) are consistent with those of IF and extracellular p24 assays (Fig. 2), which also indicated long-term productive HIV-1 infection in astrocytes. We conclude that in our system, human primary astrocytes permit the expression of HIV-1 structural proteins for an extended period after infection.
FIG. 5.
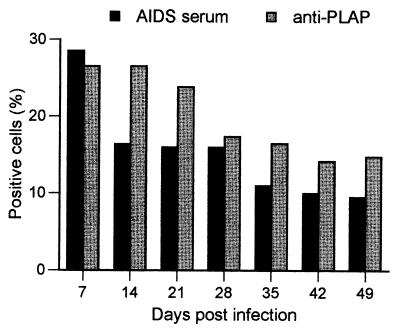
Expression of HIV-1 PLAP marker after pseudotyped HIV-1 infection. Astrocytes were infected with MLV/NL-P1 and evaluated at the indicated times by IF staining with AIDS sera or anti-PLAP antibody as described in Materials and Methods. Each time point represents counts of 200 cells each in three different fields.
Previous studies using primary astrocytes transfected with HIV-1 DNA in vitro indicated that an initial productive phase of viral replication in that system was followed by a more restrictive phase characterized by predominant expression of doubly spliced 2-kb transcripts encoding viral Nef, Tat, and Rev (69). We have not seen such preferential expression (Fig. 3), but in our system virus production also declines over time (Fig. 2). In the study whose results are shown in Fig. 5, we used MLV/NL-P1 as a reporter virus to investigate whether the proportion of infected astrocytes expressing viral structural proteins (synthesized from 9- and 4-kb RNAs) declines at a similar rate to the proportion of cells expressing a 2-kb viral RNA product, here represented by the PLAP marker protein expressed from a doubly spliced Nef mRNA (12). Astrocytes were infected with MLV/NL-P1 and tested at the indicated times for expression of HIV-1 antigens and PLAP by IF staining (Fig. 5). HIV-1 antigens were detected using AIDS serum that recognizes mostly viral structural proteins (data not shown), and PLAP was detected with anti-PLAP antibody. Overall, the kinetics of expression of HIV-1 proteins and PLAP in MLV/NL-P1-infected astrocytes followed the pattern described above for VSV/NL4-3 and MLV/NL4-3 (Fig. 2): the infection peaked on day 7 with about 30% positive cells for both HIV-1 proteins and PLAP, and the overall proportions of IF-positive cells declined gradually to lower but detectable levels throughout the 7 weeks of follow-up (Fig. 5). The ratio of HIV-1-positive cells to PLAP-positive cells declined from 1 on day 7 to 0.7 on day 14 but remained constant at this level thereafter. We found that 10% of cells were still positive for HIV-1 antigens and 15% were positive for PLAP 49 days after infection (Fig. 5). Thus, within the limits of this experiment, infected astrocytes did not selectively decrease the expression of structural (late) HIV-1 proteins detected by AIDS serum or upregulate the expression of regulatory (early) viral proteins, here indicated by the surrogate marker PLAP. These results confirm our earlier data (Fig. 2 to 4) showing that a significant proportion of HIV-1-infected astrocytes continues to express structural HIV-1 products for several weeks after primary infection, indicating that these cells exhibit no intracellular restrictions to HIV-1 replication.
Astrocytes infected with pseudotyped HIV-1 produce infectious virus.
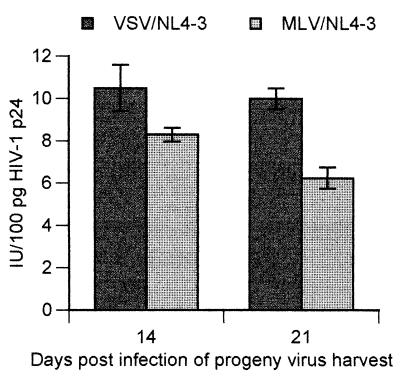
To determine whether progeny virions made by astrocytes infected with pseudotyped HIV-1 are infectious, supernatants were collected on days 14 and 21 after VSV/NL4-3 or MLV/NL4-3 infection of astrocytes, standardized for p24 content, and were subjected to titer determination for infectious HIV-1 by the MAGI assay (Fig. 6). Progeny virions collected at both time points had 6 to 10 IU per 100 pg of p24 of culture supernatant (Fig. 6). Thus, astrocytes producing HIV-1 can serve as a source of infectious progeny virus.
FIG. 6.
HIV-1-infected astrocytes produce infectious progeny virus. Astrocytes were infected with VSV/NL4-3 or MLV/NL4-3 as described in the text, and culture supernatants were collected 14 and 21 days after infection, filtered through 0.45-μm-pore-size filters, and tested for the presence of infectious virus in the MAGI assay. Values represent means and standard deviations from three different experiments.
DISCUSSION
Astrocytes are infected by HIV-1 in vivo (57, 67, 68), and it has been important to devise experimental systems to investigate the course and consequences of their infection. Using HIV-1 capable of entry into such CD4-negative cells through pseudotyping with VSV or MLV envelope proteins, we have found that primary human fetal astrocytes are permissive to highly productive HIV-1 infection. At the peak of infection, up to 70% of astrocytes expressed HIV-1 antigens and viral p24 production reached 1.7 μg/ml of culture supernatant (Fig. 1 and 2). Virus expression then gradually declined, but 5 to 10% of the cells still expressed virus antigens and secreted viral p24 at 0.1 μg/ml 7 weeks after infection, indicating persistent infection in a substantial minority of cells. As observed in many previous studies of astrocytes and other CD4-negative cells (7, 69), infection by native NL4-3 was 3 orders of magnitude less productive (Fig. 2). Similar to productively infected T cells (32), pseudotyped HIV-1-infected astrocytes expressed the major HIV-1 RNA species at equivalent levels, synthesized viral structural and regulatory proteins, and released infectious progeny virions. We conclude that the primary restriction to HIV-1 infection of astrocytes is at virus entry and that once this block is surmounted, astrocytes replicate HIV-1 efficiently for weeks before downregulating virus expression.
Transfection of viral DNA has been a method of choice to circumvent inefficient HIV-1 entry into primary astrocytes. Using this method, other investigators, as well as ourselves, have shown that astrocytes can transiently express HIV-1 structural proteins (7, 21, 69). The results varied depending on the extent and period of viral production, ranging from 5 to 20 days and from 200 to 50,000 pg of viral p24 antigen per ml of culture medium (7, 69). Different methods of transfection resulting in different levels of DNA uptake may account for the ranges observed. Infection by pseudotyped HIV-1 also results in transient viral expression; however, its period is on the order of weeks and its extent is 1 to 2 orders of magnitude higher than by transfection. The form of viral DNA introduced into cells by these different approaches is likely to contribute to the differences in the extent of viral expression. Transfected HIV-1 DNA is embedded in plasmid DNA and lacks the viral proteins which mediate nuclear entry and integration, and most of it is degraded in the cytoplasm. In contrast, both VSV G protein and MLV envelope protein mediate efficient uptake of retroviral nucleocapsid into cells, initiating a conventional retroviral life cycle (2, 3, 64). Use of pseudotyped HIV-1 enabled us to investigate the basic parameters of HIV-1 infection in astrocytes. We found that viral doses comparable to a multiplicity of infection of 1 in T lymphocytes i.e., about 1 pg of p24 per cell, when pseudotyped, were sufficient for infection of 50 to 70% of astrocytes, indicating that after virus entry, T lymphocytes and astrocytes are similarly susceptible to HIV-1 replication. The susceptibility of astrocytes to productive HIV-1 infection was highly reproducible. In multiple trials using different virus stocks, cells from more than 20 donors secreted microgram levels of the structural protein p24 after infection by NL4-3 pseudotyped with VSV or MLV envelope proteins. Consistent with p24 secretion, infected astrocytes also produced infectious progeny virus, indicating that HIV-1 can complete its life cycle in astrocytes, including all posttranslational processing and assembly events. Indeed, rescue of infectious HIV-1 from astrocytes was first reported in 1987 (13). These findings raise the possibility that astrocytes in the brain which carry HIV-1 may serve as a reservoir of infectious virus.
Using efficient infection, we revisited some of the basic questions regarding HIV-1 replication in astrocytes. Given previous reports that doubly spliced transcripts were preferentially expressed in astrocytes following HIV-1 DNA transfection (69) and analogous findings of Nef but not envelope proteins in astrocytes from HIV-1-infected brains (57), one goal of this work was to investigate the relative expression of HIV-1 structural and regulatory products by infected astrocytes. Northern blot analysis of viral RNA 1 week after pseudotype HIV-1 infection of astrocytes revealed expression of the three major HIV-1 transcripts at similar levels; 2 or 3 weeks after infection, the 9-kb genomic transcript was somewhat more abundant than the singly or doubly spliced transcripts, but all three were detectable by Northern blotting. To confirm that transcripts for structural and regulatory genes were similarly expressed, exported from the nucleus, and translated by astrocytes in the present system, we evaluated HIV-1 protein production by Western blotting and IF staining. Env, Gag, and Nef were detectable by immunoblotting 1 week after infection, and Gag was also detectable 5 weeks after infection, consistent with the continued secretion of large amounts of p24 core antigen. Similar findings were obtained using IF staining of the marker protein, PLAP expressed from a doubly spliced mRNA like Nef (12, 18) or HIV-1 structural proteins. The numbers of cells expressing structural proteins or PLAP were similar, reaching a peak of about 30% of cells and declining at 7 weeks to 10 and 15%, respectively. Our findings indicate that HIV-1 infection of primary astrocytes results in a very high peak of coordinated expression of viral structural and regulatory genes followed by a decline in parallel of the major viral products over several weeks. Conversely, we have seen no evidence in our system for preferential expression of the 2-kb HIV-1 RNA and Nef protein observed in other systems (57, 69).
Another approach to investigate astrocyte susceptibility to HIV-1 infection in culture employed transformed astrocytic cell lines transiently or stably transfected with HIV-1 DNA. Several studies found specific and well-defined blocks to virus replication, including abnormal HIV-1 RNA transcription (69), block of Rev function (42, 50), or inefficient translation of some viral mRNA species (24). However, using different glioblastoma cell lines, other investigators, including ourselves, found that once appropriate receptors for virus entry were expressed, the cells were highly susceptible to HIV-1 infection (61, 72, 74). In another study, we established chronically infected U251-MG glioblastoma cell lines by transfection of HIV-1 DNA and drug selection (21). Like the system described here, these cells also downregulated HIV-1 expression over time but with a coordinated decline in viral products and no clear evidence of abnormal HIV-1 RNA transcription or translation or of blockage of Rev function (59, 72). At this point it is not clear which of these different styles of virus replication reflects the course of HIV-1 infection of astrocytes in the brain.
The consensus of many studies of brains from HIV-1-infected persons is that astrocytes carry viral DNA at frequencies approximating 1%. It is a very interesting question to ask what route of entry was used by HIV-1 to establish infection in these CD4-negative cells, but the virus seemed to be able to enter astrocytes and synthesize viral DNA. In culture, transmission of HIV-1 to astrocytes by cell contact is more efficient than exposure to cell-free virus (47), and this may play a role in the brain. A different critical question is the extent to which this HIV-1 DNA is expressed in astrocytes in the brain. The consensus is that the majority of HIV-1-infected astrocytes do not express viral RNA, at least at levels easily detected in productively infected macrophages or microglia, although there are reports of HIV-1 RNA and structural proteins in astrocytes in the brain (25, 53). We suggest that the system described here is one reasonable approach to investigate the regulation of viral DNA expression by astrocytes. We have shown that cells initially synthesize the major HIV-1 products, including infectious progeny virus, but that after a time this expression is attenuated. Our system offers the possibility of investigating the mechanism of this attenuation by ensuring that the majority of astrocytes in culture are infected, express viral products, and, later, coordinately downregulate this expression.
ACKNOWLEDGMENTS
We thank G. Benstman for technical assistance; H. Gendelman, L. Sharer, and G. Trillo-Pazos for their comments; and I. M. Totillo for her help with the manuscript. We also gratefully acknowledge the following colleagues for donation of reagents: M. Martin for the NL4-3 proviral clone, K. Collins for the NL-P1 clone, M. Emerman for the VSV G vector, D. Littman for the MLV-envelope vector, B. Chesebro and H. Chen for hybridoma cells producing anti-p24 antibody, J. Laman for anti-HIV-2 V3 antibody, and R. Swanstrom for anti-Nef antibody.
This work was supported by NIH grants to D.J.V., M.C., and M.J.P.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akkina K R, Walton R M, Chen M L, Li Q, Planelles V, Chen I S Y. High-efficiency gene transfer into CD34+ cells with human immunodeficiency virus type 1-based retroviral vector pseudotyped with vesicular stomatitis virus envelope glycoprotein G. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel M F, Brent R, Kingston E R, Moore D D, Seidman G J, Smith A J, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 5.Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner J P, Tawadros R, Pomerantz R J. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky D J. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J Neurovirol. 1999;5:115–124. doi: 10.3109/13550289909021993. [DOI] [PubMed] [Google Scholar]
- 8.Benveniste E N. Inflammatory cytokines within the central nervous system: sources, function, and mechanisms of action. Am J Physiol. 1992;263:C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 9.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 10.Brew B J, Rosenblum M, Cronin M, Price R W. AIDS dementia complex and HIV-1 brain infection: clinical-virologic correlations. Ann Neurol. 1995;38:563–570. doi: 10.1002/ana.410380404. [DOI] [PubMed] [Google Scholar]
- 11.Canki M, Potash M J, Bentsman G, Chao W, Flynn T, Heinemann M, Gelbard H, Volsky D J. Isolation and long-term culture of primary ocular human immunodeficiency virus type 1 isolates in primary astrocytes. J Neurovirol. 1997;3:10–15. doi: 10.3109/13550289709015788. [DOI] [PubMed] [Google Scholar]
- 12.Chen B K, Gandhi R T, Baltimore D. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol. 1996;70:6044–6053. doi: 10.1128/jvi.70.9.6044-6053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng-Mayer C, Rutka J E, Rosenblum M L, McHugh T, Stites D P, Levy J A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci USA. 1987;84:3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesebro B, Wehrly K, Nishio J, Peterman S. Macrophage-tropic human immunodeficiency virus isolate from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:65407–66554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi D W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury I H, Chao W, Potash M J, Sova P, Gendelman H E, Volsky D J. vif-negative human immunodeficiency virus type 1 persistently replicates in primary macrophages, producing attenuated progeny virus. J Virol. 1996;70:5336–5345. doi: 10.1128/jvi.70.8.5336-5345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clapham P R, Blanc D, Weiss R A. Specific cell surface requirements for the infection of CD4-positive cells by human immunodeficiency virus types 1 and 2 and by simian immunodeficiency virus. Virology. 1991;181:703–715. doi: 10.1016/0042-6822(91)90904-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 19.Conant K, Major E O. Astrocytes as mediators of CNS injury in AIDS. In: Gendelman H E, Lipton A S, Epstein L, Swindells S, editors. The neurology of AIDS. New York, N.Y: Chapman & Hall; 1998. [Google Scholar]
- 20.Davis L E, Hjelle B L, Miller V E, Palmer D L, Llewellyn A L, Merlin T L, Young S A, Mills R G, Wachsman W, Wiley C A. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 21.Dewhurst S, Sakai K, Bresser J, Stevenson M, Evinger-Hodges M J, Volsky D J. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987;61:3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein L G, Gendelman H E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenic mechanisms. Ann Neurol. 1993;33:429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- 23.Gabuzda D, Wang J. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J Neurovirol. 2000;6:S24–S32. [PubMed] [Google Scholar]
- 24.Gorry P R, Howard J L, Churchill M J, Anderson J L, Cunningham A, Adrian D, McPhee D A, Purcell D F J. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73:352–361. doi: 10.1128/jvi.73.1.352-361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosztony G, Artigas J, Lamperth L, Webster H. Human immunodeficiency virus (HIV) distribution in HIV encephalitis: study of 19 cases with combined use of in situ hybridization and immunocytochemistry. J Neuropathol Exp Neurol. 1994;53:521–534. doi: 10.1097/00005072-199409000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Griffin D E. HIV infection of the brain: viruses, cytokines, and immune regulatory factors associated with dementia. In: Gendelman H E, Lipton A S, Epstein L, Swindells S, editors. The neurology of AIDS. New York, N.Y: Chapman & Hall; 1998. [Google Scholar]
- 27.Harrington R D, Geballe A P. Cofactor requirement for human immunodeficiency virus type 1 entry into CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay R C, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 29.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 30.Hollander H, Levy J A. Neurologic abnormalities and recovery of human immunodeficiency virus from cerebrospinal fluid. Ann Intern Med. 1987;106:692–695. doi: 10.7326/0003-4819-106-5-692. [DOI] [PubMed] [Google Scholar]
- 31.Katsuhiro K, Lyman D W, Weidenheim M K, Dickson W D. HIV-1 antigen in subacute AIDS encephalitis. Am J Pathol. 1990;136:1085–1092. [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Byrn R, Groopman J, Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989;63:3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated b-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koening S, Gendelman H, Orenstein M J, Canto M C, Pezeshkpour G H, Yungbluth G H, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 35.Kolson D L, Pomerantz R J. AIDS dementia and HIV-1 induced neurotoxicity: possible pathogenic associations and mechanisms. J Biomed Sci. 1996;3:389–414. doi: 10.1007/BF02258044. [DOI] [PubMed] [Google Scholar]
- 36.Kunsch C, Hartle H T, Wigdahl B. Infection of human fetal dorsal root ganglion glial cells with human immunodeficiency virus type 1 involves an entry mechanism independent of the CD4 T4A epitope. J Virol. 1989;63:5054–5061. doi: 10.1128/jvi.63.12.5054-5061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laman J D, Schellekens M M, Abacioglu Y F, Lewis G K, Tersmette M, Fouchier R A M, Langedjik J P M, Claasen E, Boersma W J A. Variant-specific monoclonal and group-specific polyclonal human immunodeficiency virus type 1 neutralizing antibodies raised with synthetic peptides from the gp120 third variable domain. J Virol. 1992;66:1823–1831. doi: 10.1128/jvi.66.3.1823-1831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type 1 broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy J A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Kappes J, Conway J, Price R, Shaw G, Hahn B. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured brain tissue, identification of replication competent and defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipton S, Gendelman H E. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;233:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig E, Ceccherini-Silberstein F, van Empel J, Erfle V, Neumann M, Brack-Werner R. Diminished Rev-mediated stimulation of human immunodeficiency virus type 1 is a hallmark of human astrocytes. J Virol. 1999;73:8279–8289. doi: 10.1128/jvi.73.10.8279-8289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mankowski L J, Flaherty T M, Spelman P J, Hauer A D, Didier J P, Amedee M A, Murphy-Corb M, Kirstein M L, Munoz A, Clements E J, Zink C M. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulance. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matlin K S, Reggio H, Helenius A, Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982;156:609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- 45.McArthur J C, Grant I. HIV neurocognitive disorders. In: Gendelman H E, Lipton S A, Epstein L, Swindells S, editors. The neurology of AIDS. New York, N.Y: Chapman & Hall; 1998. pp. 499–523. [Google Scholar]
- 46.McClure M O, Sommerfelt M A, Marsh M, Weiss R A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990;71:767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- 47.Nath A, Hartloper V, Furer M, Fowke K R. Infection of human fetal astrocytes with HIV-1: viral tropism and the role of cell to cell contact in viral transmission. J Neuropath Exp Neurol. 1995;54:320–330. doi: 10.1097/00005072-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Navia B A, Cho E S, Petito C K, Price R W. The AIDS dementia complex. II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 49.Navia B A, Jordan B D, Price R W. The AIDS dementia complex. I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 50.Neumann M, Felber B K, Kleinschmidt A, Froese B, Erfle V, Pavlakis G N, Brack-Werner R. Restriction of hunan immunodeficiency virus type 1 production in a human astrocytoma cell line is associated with a cellular block in Rev function. J Virol. 1995;69:2159–2167. doi: 10.1128/jvi.69.4.2159-2167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuovo G J, Gallery F, MacConnell P, Braun A. In situ detection of polymerase chain reaction-amplified HIV-1 nucleic acid and tumor necrosis-alpha RNA in the central nervous system. Am J Pathol. 1994;144:659–666. [PMC free article] [PubMed] [Google Scholar]
- 52.Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol. 1999;73:897–906. doi: 10.1128/jvi.73.2.897-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasolo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Rothstein J D, Dykes-Hoberg M, Pardo C A, Bristol L A, Jin L, Kunci R W, Kanai Y, Hediger M A, Wang Y, Scielke J P, Welty D F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 55.Rutka J T, Murakami M, Dirks P B, Hubbard S L, Becker L E, Fukuyama K, Jung S, Tsugu A, Matsuzawa K. Role of glial filaments in cells and tumors of glial origin: a review. J Neurosurg. 1997;87:420–430. doi: 10.3171/jns.1997.87.3.0420. [DOI] [PubMed] [Google Scholar]
- 56.Sabri F, Tresoldi E, Di Stefano M, Polo S, Monaco M C, Verani A, Fiore R J, Lusso P, Major E, Chiodi F, Scarlatti G. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology. 1999;264:370–384. doi: 10.1006/viro.1999.9998. [DOI] [PubMed] [Google Scholar]
- 57.Saito Y, Sharer L R, Epstein L G, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich T A, Blumberg B M. Overexpression of Nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- 58.Shahabuddin M, Bentsman G, Volsky B, Rodriguez I, Volsky D J. A mechanism of restricted human immunodeficiency virus type 1 expression in human glial cells. J Virol. 1996;70:7992–8002. doi: 10.1128/jvi.70.11.7992-8002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shahabuddin M, Volsky B, Kim H, Sakai K, Volsky D J. Regulated expression of human immunodeficiency virus type 1 in human glial cells: induction of dormant virus. Pathobiology. 1992;60:195–205. doi: 10.1159/000163723. [DOI] [PubMed] [Google Scholar]
- 60.Sharer L R, Epstein L G, Cho E S, Joshi V V, Meyenhofer M F, Rankin L F, Petito C K. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986;17:271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu N, Soda Y, Kanbe K, Liu H Y, Jinno A, Kitamura T, Hoshino H. An orphan G protein-coupled receptor, GPR1, acts as a coreceptor to allow replication of human immunodeficiency virus type 1 and 2 in brain-derived cells. J Virol. 1999;73:5231–5239. doi: 10.1128/jvi.73.6.5231-5239.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shugars D C, Smith M S, Glueck D H, Nantermet P V, Seillier-Moiseiwitsch F, Swanstrom R. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J Virol. 1993;67:4639–4650. doi: 10.1128/jvi.67.8.4639-4650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Simm M, Shahabuddin M, Chao W, Allan J S, Volsky D J. Aberrant gag protein composition of a human immunodeficiency virus type 1 vif mutant produced in primary lymphocytes. J Virol. 1995;69:4582–4586. doi: 10.1128/jvi.69.7.4582-4586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spector D H, Wade D A, Wright V, Koval C, Jaquish D, Spector S A. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990;64:2298–2308. doi: 10.1128/jvi.64.5.2298-2308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srinivasan A, Dorsett D, York D, Bohan C, Anand R. Human immunodeficiency virus replication in human brain cells. Arch Virol. 1988;98:135–141. doi: 10.1007/BF01311031. [DOI] [PubMed] [Google Scholar]
- 66.Stephens E B, Liu Z Q, Zhu G W, Adany I, Joag S V, Foresman L, Berman N E J, Narayan O. Lymphocyte-tropic simian immunodeficiency virus causes persistent infection in the brains of rhesus monkeys. Virology. 1995;213:600–614. doi: 10.1006/viro.1995.0032. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi K, Wesselingh S L, Griffin D E, McArthur J C, Johnson R T, Glass J D. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 68.Tornatore C, Chandra R, Berger J R, Major E O. HIV-1 infection of subcortical astrocytes in the pediatric CNS. Neurology. 1994;44:481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- 69.Tornatore C, Meyers K, Atwood W, Conant K, Major E O. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tornatore C, Nath A, Amemiya K, Major E O. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verkhratsky A, Orkand R K, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:100–130. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 72.Volsky B, Sakai K, Reddy M M, Volsky D J. A system for the high efficiency replication of HIV-1 in neural cells and its application to anti-viral evaluation. Virology. 1992;186:303–308. doi: 10.1016/0042-6822(92)90086-5. [DOI] [PubMed] [Google Scholar]
- 73.Wiley C A, Schrier R D, Nelson J A, Lampert P W, Oldstone M B A. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, He T, Huang Y, Chen Z, Guo Y, Wu S, Kuntsman K J, Brown C R, Phair J P, Neumann A U, Ho D D, Wolinsky S M. Chemokine coreceptor usage by diverse primary isolates of human immunodeficiency virus type 1. J Virol. 1998;72:9307–9312. doi: 10.1128/jvi.72.11.9307-9312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng J, Ghoparde A, Niemann D, Cotter R L, Thylin M R, Epstein L, Swartz J M, Shepard R B, Liu X, Nukuna A, Gendelman H E. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 1999;73:8256–8267. doi: 10.1128/jvi.73.10.8256-8267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]