Abstract
Aim
The risk of developing infectious diarrhea among elderly residents at Japanese geriatric intermediate care facilities is unclear. We investigated the incidence rate and risk factors of norovirus‐related diarrhea at such facilities.
Methods
This prospective cohort study followed 1727 residents from November 2018 to April 2020 at 10 geriatric intermediate care facilities in Osaka, Japan regarding the occurrence of diarrhea. Resident data were collected from their medical records using structured forms at two to three of the following three time points: at recruitment, if they developed diarrhea, and when they left the facility. Residents who developed diarrhea were tested using rapid diagnostic tests for norovirus. Cox proportional hazard model was employed to hazard ratios (HRs) with 95% confidence intervals (CIs) to estimate the risk factors for norovirus‐related diarrhea.
Results
During the study period, 74 residents developed diarrhea, 13 of whom were norovirus positive. The incidence rate of norovirus‐related diarrhea was 10.11 per 1000 person‐years (95% CI: 4.61–15.61). In terms of risk factors, people with care‐needs level 3 were at a higher risk for developing norovirus‐related diarrhea (adjusted HR [aHR] = 7.35, 95% CI: 1.45–37.30). Residents with hypertension (aHR = 3.41, 95% CI: 1.05–11.04) or stroke (aHR = 8.84, 95% CI: 2.46–31.83), and those who walked with canes (aHR = 16.68, 95% CI: 1.35–206.52) also had a significantly higher risk for norovirus‐related diarrhea.
Conclusions
Throughout the study period, the incidence of development of diarrhea was low. Care‐needs level 3, stroke, hypertension and use of a cane were identified as risk factors for norovirus‐related diarrhea in Japanese geriatric intermediate care facilities. Geriatr Gerontol Int 2023; 23: 179–187.
Keywords: cohort study, diarrhea, intermediate care facility, Japan, norovirus
The study aimed to investigate the incidence rate and risk factors of norovirus‐related diarrhea at Japanese geriatric intermediate care facilities. The incidence rate of norovirus‐related diarrhea was 10.11 per 1000 person‐years, and people with care‐needs level 3, stroke, hypertension and use of a cane were identified as risk factors for norovirus‐related diarrhea.

Introduction
Japan has a super‐aging society, where the number of persons ≥65 years has been increasing, with this age group accounting for 29% of the population in 2020. 1 , 2 Accordingly, those who need residential care services have also been increasing. To address this situation, long‐term care insurance has been launched in Japan since 2000. 3
Geriatric intermediate care facilities, which provide facility‐based care services, have special features, including residents receiving medical care from a full‐time doctor, in addition to nursing care and rehabilitation for their return to home. At these facilities, however, two to four residents usually live in the same room, which increases their risk of contracting infectious diseases and might lead to cluster outbreaks. 4 , 5 , 6 Norovirus is the leading cause of outbreaks of acute viral gastroenteritis, 7 with the attack rate of norovirus outbreaks of between 3% and 45% at long‐term care facilities. 8 Moreover, facility residents often have many underlying diseases and poor physical functions, 9 and once infected, they are likely to develop serious conditions, including dehydration, pulmonary aspiration of vomitus and even death. 10 , 11 A previous review showed a case fatality rate among the institutionalized elderly of 0.3%–1.6%. 8 Another study reported that when hospitalized for norovirus gastroenteritis, the norovirus‐related mortality among patients living in long‐term care facilities was about three‐fold higher than in patients living at home. 12 However, the incidence of norovirus‐related diarrhea among institutionalized elderly residents in Japan is limited, because national surveillance on infectious diarrheas is based on reports from pediatric sentinel sites, and because subjects who develop diarrhea are not always tested for pathogens. 12 , 13 Thus, it is hard to estimate the actual morbidity and mortality of norovirus‐related diarrhea among institutionalized elderly residents.
This study aimed to estimate the incidence and risk factors for norovirus‐related diarrhea among residents at Japanese geriatric intermediate care facilities. In this study, we actively performed rapid diagnostic tests for norovirus in all subjects with diarrhea, to diagnose norovirus‐related diarrhea accurately. In addition, we also tested diarrhea subjects for Clostridioides difficile (C. difficile), since C. difficile is also a potential pathogen in these facility residents.
Methods
Study design and participants
This prospective cohort study was conducted at 10 geriatric intermediate care facilities that belong to the Osaka Association of Geriatric Health Service Facilities. All residents at the participating facilities during November 1, 2018 and April 30, 2020 were enrolled, except for those who received “short stay” residential services for less than 1 month, or who were ostomate. These facilities cater to residents aged ≥65 years, or those aged 40–64 years with specified diseases, including dementia and cerebrovascular disorders, under the long‐term care insurance law in Japan. 4
Data collection
At the time of enrollment (i.e., November 1, 2018 for already institutionalized residents, or the date of admission for newly admitted residents), we asked the staff to complete a structured form about the following characteristics of the study subjects based on their medical records: sex, age, underlying diseases (i.e. dementia, hypertension, stroke, heart disease, diabetes, malignancy, kidney disease, thyroid disease, chronic lung disease and liver disease), care‐needs level, activities of daily living (ADL) grade, level of cognitive dysfunction, number of roommates, history of hospitalization in the previous year, tube feeding, pad usage, independence in walking (“independent,” “using a cane,” “using a walking frame” and “impossible”), and prescription drugs (i.e., anticancer drugs, steroids, non‐steroidal anti‐inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), laxatives and probiotics).
Care‐needs level, ADL grade and level of cognitive dysfunction are variables that indicate the level of assistance that residents require. These variables are determined based on Japanese local government criteria using a national standardized procedure when patients seek long‐term care. 3 Care‐needs level, which allows for admission to geriatric intermediate care facilities, consists of five levels. 3 People with care‐needs level 1 are the least dependent, and people with care‐needs level 5 are bedridden, cannot communicate fully, and cannot eat by themselves. ADL grade was evaluated using the “Independence Criteria of the Daily Life of the Impaired Elderly.” 14 These criteria have five ranks as follows: independent, almost independent (rank J), requires assistance when outside (rank A), wheelchair mobility (rank B), and bedridden (rank C). 14 , 15 Level of cognitive dysfunction was evaluated using the criteria for “Rating of dementia.” 15 These criteria consist of six ranks: independent, presence of some cognitive disorder but almost independent (rank I), independent with supervision (rank II), requires some nursing care (rank III), requires nursing care all day (rank IV) and requires medical care (rank M). 5 , 15
Follow‐up survey and outcome definitions
All residents were followed up through their medical records from enrollment until April 30, 2020, developing diarrhea, or leaving the facility, whichever came first.
Diarrhea was defined as two or more unformed or watery stools in 24 h. Residents who regularly took laxatives or suppositories were regarded as developing diarrhea if they had a greater frequency of defecation than usual. All residents with diarrhea received rapid diagnostic tests for norovirus (Immunocatch Norovirus Plus [Immunocatch]; Eiken Chemical, Tokyo, Japan) and C. difficile (GE‐test immunochromato‐CD GDH/TOX mini [GE‐test]; Nissui, Tokyo, Japan). The sensitivity and specificity of these kits are 100% and 93% (Immunocatch), 16 and 98.1% and 98.2% (GE‐test), 17 respectively, when compared with immunochromatography. Patients with positive results on these tests were regarded as having norovirus‐related diarrhea or C. difficile‐related diarrhea, respectively. In general, the guideline defines diarrhea as more than two unformed stools in 24 h. 18 However, since many residents suffer from constipation, we were concerned to overlook the diarrhea occurrence when using the definition of this guideline. We therefore applied the broader criteria to grasp the diarrhea occurrence as much as possible and to employ the diagnostic tests of norovirus and C. difficile thoroughly for all diarrhea cases. Such active testing also enabled distinguishing between a laxative‐effective condition and diseased diarrhea in patients on laxatives.
If residents left the facility during the study period, the facility staff provided information on the date of discharge and the reason for leaving (return home, transfer to other type of geriatric care facility, hospitalization, or death) using a structured questionnaire.
Statistical analysis
The incidence rate of diarrhea was calculated per 10 000 person‐days as the number of residents with diarrhea divided by the total follow‐up period of all participants. For residents with repeated admissions to and discharges from the facility during the study period, person‐days at risk were counted only for the period for which they were institutionalized. When presenting the results, the incidence rate using 10 000 person‐days was converted to 1000 person‐years. The incidence rate of norovirus‐related diarrhea was calculated by the same procedure as that for diarrhea.
The following characteristics were selected as possible predictive variables to assess their association with diarrhea: sex, age, care‐needs level, ADL grade, level of cognitive dysfunction, underlying diseases, prescription drugs, number of roommates, history of hospitalization in the previous year, tube feeding, pad usage and independence in walking. According to the Japanese long‐term care insurance system and previous research, age was divided into three categories: <65 years, 65–84 years and ≥85 years. 5 , 9
The Cox proportional hazard model was used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) to estimate the risk factors for diarrhea. Since care‐needs levels are determined based on the patient's ADL grade and level of cognitive dysfunction, a strong association is expected between them. 5 Therefore, care‐needs level was considered as the first priority in the variable selection. The multivariate model included sex, age and care‐needs level as adjustment variables. In consideration of diarrhea outbreaks, an analysis focusing on the facility where residents developed norovirus‐positive diarrhea was also conducted. All analyses used SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Ethics statement
According to the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects, since the study utilized only their pre‐existing data, subjects were notified about conduct of this study by displaying a poster, and patient consent for participation was obtained using an opt out method. 19 If their stool samples were tested, the patients' verbal consent was obtained prior to the testing.
This protocol was approved by the Ethics Committee of Osaka City University Graduate School of Medicine (no. 4162; date of approval: October 25, 2018), and was performed in accordance with the Declaration of Helsinki.
Results
Among 1734 residents initially included in the study, seven residents with missing data of predictive variables were excluded, and the data of 1727 residents were analyzed. Their baseline characteristics are shown in Table 1. One‐third of the residents were male, and the median age was 86 years. Dementia was the most common underlying disease (61%), followed by hypertension (51%) and stroke (33%). Residents with care‐needs level 4 constituted about 28% of all residents. More than half the residents had a history of hospitalization in the previous year, and tube feeding was required by 80 residents (5%). Twenty percent of the residents needed supportive items when walking, such as a cane or walking frame. Regarding prescription drugs, 951 residents (55%) took laxatives, and 563 residents (33%) took PPIs.
Table 1.
Baseline characteristics of all the geriatric intermediate care facility residents
| Residents (N = 1727) | |
|---|---|
| Characteristics | n (%) |
| Sex | |
| Male | 536 (31) |
| Age (years) | |
| Median (range) | 86 (43–107) |
| <65 | 36 (2) |
| 65–84 | 705 (41) |
| ≥85 | 986 (57) |
| Underlying diseases | |
| Dementia | 1060 (61) |
| Hypertension | 887 (51) |
| Stroke | 562 (33) |
| Heart disease | 511 (30) |
| Diabetes | 358 (21) |
| Malignancy | 254 (15) |
| Kidney disease | 152 (9) |
| Thyroid disease | 82 (5) |
| Chronic lung disease | 76 (4) |
| Liver disease | 66 (4) |
| Care‐needs level | |
| Level 1 | 194 (11) |
| Level 2 | 328 (19) |
| Level 3 | 415 (24) |
| Level 4 | 483 (28) |
| Level 5 | 307 (18) |
| Grade of ADL | |
| Independent, Rank J | 39 (2) |
| Rank A | 530 (31) |
| Rank B | 964 (56) |
| Rank C | 194 (11) |
| Level of cognitive dysfunction | |
| Independent, Rank I | 238 (14) |
| Rank II | 808 (47) |
| Rank III | 590 (34) |
| Rank IV, Rank M | 91 (5) |
| Number of roommates | |
| 1 | 186 (11) |
| 2 | 114 (7) |
| 3 | 117 (7) |
| 4 | 1310 (76) |
| History of hospitalization in the previous year | |
| Yes | 1037 (60) |
| Tube feeding | |
| Yes | 80 (5) |
| Pad usage (night only or always) | |
| Yes | 1454 (84) |
| Independence in walking | |
| Independent | 187 (11) |
| Using a cane | 88 (5) |
| Using a walking frame | 251 (15) |
| Impossible | 1201 (70) |
| Prescription drugs | |
| Anticancer drugs | 6 (0.4) |
| Steroids | 67 (4) |
| NSAIDs | 134 (8) |
| PPIs | 563 (33) |
| Laxatives | 951 (55) |
| Probiotics | 162 (9) |
Abbreviations: ADL, activities of daily living; NSAIDs, non‐steroidal anti‐inflammatory drugs; PPIs, proton pump inhibitors.
Figure 1 indicates the distribution of residents who developed diarrhea by week. During the study period, 74 residents developed diarrhea (4.3%). The incidence rate of developing diarrhea was 59.03 per 1000 person‐years (95% CI: 45.58–72.48). Among these patients, 13 residents were positive for norovirus, all of which occurred only in December 2019 at a single facility. Other diarrhea cases were reported sporadically, and only one resident tested positive for C. difficile, although a retest was negative. Therefore, further analyses focused on norovirus‐related diarrhea as the study outcome. The incidence rate of developing norovirus‐related diarrhea was 10.11 (95% CI: 4.61–15.61) per 1000 person‐years.
Figure 1.
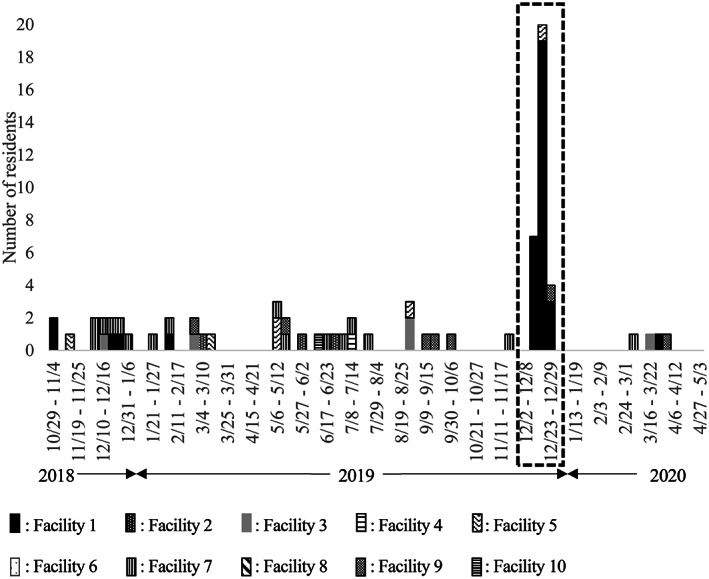
Distribution of residents who developed diarrhea by week and facility during the study period. During the study period (surrounded by the broken line), 13 residents were positive for norovirus. No residents were positive for norovirus at periods other than that indicated by the broken line.
Table 2 shows the relationship between background characteristics and the risk of developing norovirus‐related diarrhea. Sex and age did not have a significant relationship with the occurrence of norovirus‐related diarrhea. Compared with residents with care‐needs level 1 or 2, those with care‐needs level 3 had a significantly increased risk of developing norovirus‐related diarrhea (aHR = 7.35, 95% CI: 1.45–37.30).
Table 2.
Relationship between risk of development of norovirus‐related diarrhea and selected background characteristics
| Characteristics | N | Norovirus‐related diarrhea | Person‐years at risk | Incidence rate per 1000 person‐years | Facility where residents developed norovirus‐related diarrhea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Crude HR (95% CI) | Adjusted HR † (95% CI) | Crude HR (95% CI) | Adjusted HR † (95% CI) | ||||||||
| Sex | ||||||||||||
| Male | 536 | 2 (0.4) | 344.9 | 5.80 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Female | 1191 | 11 (0.9) | 940.8 | 11.69 | 1.83 | (0.40–8.27) | 1.55 | (0.33–7.16) | 1.12 | (0.25–5.10) | 1.20 | (0.26–5.56) |
| Age (years) | ||||||||||||
| <65 | 36 | 0 (0) | 21.8 | NA | NA | NA | NA | NA | ||||
| 65–84 | 705 | 3 (0.4) | 504.4 | 5.95 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| ≥85 | 986 | 10 (1.0) | 759.6 | 13.17 | 2.12 | (0.58–7.70) | 1.85 | (0.50–6.87) | 1.91 | (0.53–6.96) | 1.05 | (0.27–4.13) |
| Care‐needs level | ||||||||||||
| Level 1 | 194 | 0 (0) | 133.0 | NA | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Level 2 | 328 | 2 (0.6) | 250.6 | 7.98 | ||||||||
| Level 3 | 415 | 7 (1.7) | 308.0 | 22.72 | 4.30 | (0.89–20.71) | 4.49 | (0.93–21.63) | 7.26 | (1.49–35.44) | 7.35 | (1.45–37.30) |
| Level 4 | 483 | 4 (0.8) | 358.6 | 11.16 | 2.13 | (0.39–11.60) | 2.13 | (0.39–11.64) | 1.33 | (0.24–7.29) | 1.43 | (0.26–7.89) |
| Level 5 | 307 | 0 (0) | 235.6 | NA | NA | NA | NA | NA | ||||
| Grade of ADL ‡ | ||||||||||||
| Independent, Rank J | 39 | 0 (0) | 23.2 | NA | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Rank A | 530 | 3 (0.6) | 399.4 | 7.51 | ||||||||
| Rank B | 964 | 9 (0.9) | 713.4 | 12.62 | 1.74 | (0.47–6.42) | 1.82 | (0.49–6.77) | 1.68 | (0.45–6.21) | 1.78 | (0.47–6.77) |
| Rank C | 194 | 1 (0.5) | 149.8 | 6.68 | 0.87 | (0.09–8.33) | 1.69 | (0.17–17.22) | 0.83 | (0.09–8.05) | 1.27 | (0.11–14.87) |
| Level of cognitive dysfunction ‡ | ||||||||||||
| Independent, Rank I | 238 | 1 (0.4) | 154.7 | 6.46 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Rank II | 808 | 8 (1.0) | 596.1 | 13.42 | 2.03 | (0.25–16.25) | 1.86 | (0.23–14.93) | 0.54 | (0.07–4.48) | 0.50 | (0.06–4.39) |
| Rank III | 590 | 4 (0.7) | 459.2 | 8.71 | 1.26 | (0.14–11.29) | 1.09 | (0.12–9.96) | 0.44 | (0.05–4.18) | 0.43 | (0.04–4.36) |
| Rank IV, Rank M | 91 | 0 (0) | 75.7 | NA | NA | NA | NA | NA | ||||
Abbreviations: ADL, activities of daily living; CI, confidence interval; HR, hazard ratio; NA, not applicable.
Model includes sex, age and care‐needs level.
Due to the presence of competing variables, the model includes sex, age, grade of ADL and level of cognitive dysfunction.
The risk of norovirus‐related diarrhea according to underlying diseases and prescription drugs is summarized in Table 3. Residents with hypertension or stroke had a significantly greater risk of norovirus‐related diarrhea (aHR of hypertension = 3.41, 95% CI: 1.05–11.04, aHR of stroke = 8.84, 95% CI: 2.46–31.83). Underlying diseases, such as malignancy, kidney disease and liver disease did not show any relationship with norovirus‐related diarrhea. Regarding prescription drugs, nine residents with norovirus‐related diarrhea took laxatives (69%), although there was no relationship with norovirus‐related diarrhea.
Table 3.
Relationship between risk of development of norovirus‐related diarrhea and underlying diseases, and each prescription drugs
| Characteristics | N | Norovirus‐related diarrhea | Person‐years at risk | Incidence rate per 1000 person‐years | Facility where residents developed norovirus‐related diarrhea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Crude HR (95% CI) | Adjusted HR † (95% CI) | Crude HR (95% CI) | Adjusted HR † (95% CI) | |||||||||
| Underlying diseases | |||||||||||||
| Dementia | No | 667 | 5 (0.8) | 463.5 | 10.79 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 1060 | 8 (0.8) | 822.2 | 9.73 | 0.87 | (0.29–2.67) | 0.86 | (0.28–2.63) | 0.49 | (0.16–1.55) | 0.53 | (0.16–1.72) | |
| Hypertension | No | 840 | 5 (0.6) | 600.7 | 8.32 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 887 | 8 (0.9) | 685.0 | 11.68 | 1.39 | (0.46–4.26) | 1.35 | (0.44–4.13) | 2.52 | (0.82–7.74) | 3.41 | (1.05–11.04) | |
| Stroke | No | 1165 | 6 (0.5) | 862.8 | 6.95 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 562 | 7 (1.3) | 422.9 | 16.55 | 2.33 | (0.78–6.94) | 3.88 | (1.25–12.04) | 3.09 | (1.04–9.19) | 8.84 | (2.46–31.83) | |
| Heart disease | No | 1216 | 8 (0.7) | 904.4 | 8.85 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 511 | 5 (1.0) | 381.3 | 13.11 | 1.49 | (0.49–4.54) | 1.46 | (0.47–4.50) | 1.65 | (0.54–5.06) | 1.35 | (0.39–4.67) | |
| Diabetes | No | 1369 | 10 (0.7) | 1042.7 | 9.59 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 358 | 3 (0.8) | 243.1 | 12.34 | 1.35 | (0.37–4.90) | 1.53 | (0.41–5.65) | 1.75 | (0.48–6.41) | 3.75 | (0.77–18.31) | |
| Malignancy | No | 1473 | 13 (0.9) | 1112.4 | 11.69 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 254 | 0 (0) | 173.4 | NA | NA | NA | NA | NA | |||||
| Kidney disease | No | 1575 | 13 (0.8) | 1163.0 | 11.18 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 152 | 0 (0) | 122.8 | NA | NA | NA | NA | NA | |||||
| Thyroid disease | No | 1645 | 11 (0.7) | 1220.2 | 9.02 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 82 | 2 (2.4) | 65.6 | 30.49 | 3.30 | (0.73–14.87) | 2.48 | (0.54–11.34) | 2.90 | (0.64–13.11) | 1.53 | (0.32–7.29) | |
| Chronic lung disease | No | 1651 | 12 (0.7) | 1237.4 | 9.70 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 76 | 1 (1.3) | 48.3 | 20.70 | 2.13 | (0.28–16.38) | 2.34 | (0.30–18.29) | 4.30 | (0.55–33.89) | 4.38 | (0.51–37.79) | |
| Liver disease | No | 1661 | 13 (0.8) | 1227.2 | 10.59 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 66 | 0 (0) | 58.5 | NA | NA | NA | NA | NA | |||||
| Prescription drugs | |||||||||||||
| Anticancer drugs | No | 1721 | 13 (0.8) | 1283.5 | 10.13 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 6 | 0 (0) | 2.3 | NA | NA | NA | NA | NA | |||||
| Steroids | No | 1660 | 13 (0.8) | 1233.0 | 10.54 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 67 | 0 (0) | 52.8 | NA | NA | NA | NA | NA | |||||
| NSAIDs | No | 1593 | 12 (0.8) | 1184.3 | 10.13 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 134 | 1 (0.8) | 101.5 | 9.85 | 0.95 | (0.12–7.31) | 0.91 | (0.12–7.11) | 1.71 | (0.22–13.30) | 1.24 | (0.15–10.00) | |
| PPIs | No | 1164 | 12 (1.0) | 909.5 | 13.19 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 563 | 1 (0.2) | 376.3 | 2.66 | 0.21 | (0.03–1.65) | 0.22 | (0.03–1.73) | 1.36 | (0.17–10.72) | 2.74 | (0.30–24.89) | |
| Laxatives | No | 776 | 4 (0.5) | 542.8 | 7.37 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 951 | 9 (1.0) | 742.9 | 12.11 | 1.56 | (0.48–5.06) | 1.65 | (0.51–5.38) | 1.37 | (0.42–4.46) | 1.07 | (0.31–3.67) | |
| Probiotics | No | 1565 | 12 (0.8) | 1178.2 | 10.19 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 162 | 1 (0.6) | 107.6 | 9.30 | 0.94 | (0.12–7.19) | 1.11 | (0.14–8.57) | 2.18 | (0.28–16.81) | 4.30 | (0.40–46.78) | |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable; NSAIDs, non‐steroidal anti‐inflammatory drugs; PPIs, proton pump inhibitors.
Model includes sex, age and care‐needs level.
Table 4 shows the association between the risk of developing norovirus‐related diarrhea and selected variables related to daily life. After adjustment for sex, age and care‐needs level, residents who used pads had a significantly lower risk of developing norovirus‐related diarrhea (aHR: 0.14, 95% CI: 0.05–0.45), although the association was not significant in the analysis of only subjects at the facility where residents developed norovirus‐related diarrhea. In contrast, residents who walked using a cane had a more than 10‐fold risk for norovirus‐related diarrhea, with statistical significance (aHR: 16.68, 95% CI: 1.35–206.52).
Table 4.
Relationship between risk of development of norovirus‐related diarrhea and each variable related to lifestyle behaviors
| Characteristics | N | Norovirus‐related diarrhea | Person‐years at risk | Incidence rate per 1000 person‐years | Facility where residents developed norovirus‐related diarrhea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Crude HR (95% CI) | Adjusted HR † (95% CI) | Crude HR (95% CI) | Adjusted HR † (95% CI) | ||||||||
| Number of roommates | ||||||||||||
| 1 | 186 | 0 (0) | 119.6 | NA | NA | NA | NA | NA | ||||
| 2 | 114 | 0 (0) | 75.7 | NA | NA | NA | NA | NA | ||||
| 3 | 117 | 3 (2.6) | 102.9 | 29.16 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 4 | 1310 | 10 (0.8) | 987.5 | 9.11 | 0.38 | (0.10–1.38) | 0.37 | (0.10–1.36) | 0.66 | (0.18–2.42) | 0.82 | (0.21–3.17) |
| History of hospitalization in the previous year | ||||||||||||
| No | 690 | 9 (1.3) | 616.5 | 14.60 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 1037 | 4 (0.4) | 669.2 | 5.98 | 0.46 | (0.14–1.49) | 0.48 | (0.15–1.58) | 1.14 | (0.34–3.85) | 1.20 | (0.34–4.23) |
| Tube feeding | ||||||||||||
| No | 1647 | 12 (0.7) | 1218.2 | 9.85 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yes | 80 | 1 (1.3) | 67.5 | 14.81 | 1.36 | (0.18–10.48) | 6.82 | (0.69–67.39) | 1.01 | (0.13–7.80) | 6.49 | (0.59–70.82) |
| Pad usage | ||||||||||||
| No | 273 | 7 (2.6) | 202.9 | 34.50 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Used (night only or always) | 1454 | 6 (0.4) | 1082.8 | 5.54 | 0.16 | (0.06–0.49) | 0.14 | (0.05–0.45) | 0.29 | (0.10–0.86) | 0.46 | (0.12–1.78) |
| Independence in walking | ||||||||||||
| Independent | 187 | 1 (0.5) | 145.4 | 6.88 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Using a cane | 88 | 2 (2.3) | 62.6 | 31.97 | 5.45 | (0.49–60.23) | 5.80 | (0.52–64.33) | 11.80 | (1.06–130.95) | 16.68 | (1.35–206.52) |
| Using a walking frame | 251 | 0 (0) | 191.7 | NA | NA | NA | NA | NA | ||||
| Impossible | 1201 | 10 (0.8) | 886.1 | 11.29 | 1.69 | (0.22–13.22) | 1.58 | (0.19–13.10) | 2.87 | (0.37–22.46) | 5.49 | (0.54–55.88) |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable.
Model includes sex, age and care‐needs level.
Discussion
The present cohort study at Japanese geriatric intermediate care facilities demonstrated that the incidence rate of norovirus‐related diarrhea is 10.11 per 1000 person‐years, and that four characteristics of residents are possible risk factors for norovirus‐related diarrhea: care‐needs level 3, stroke, hypertension and use of a cane for walking.
The incidence rate of norovirus‐related diarrhea in the present study subjects was much lower than that previously reported in a prospective cohort study at care homes in England, which found an incidence rate of 225.2 per 1000 person‐years. 20 Although the incidences vary according to the study season, location and population, the difference between studies can partly be explained by the following three reasons. First, epidemics of infectious gastroenteritis in the community were low during the study seasons, 21 which would have affected our results. At the end of the study period in 2020, in particular, due to the emergence of the global COVID‐19 pandemic, protective measures for infectious diseases at the facilities might have been more stringent. The second reason might have been derived from a notable feature of Japanese geriatric intermediate care facilities; since these facilities are managed by full‐time doctors and nurses, they might take more strict preventive measures against infectious diseases, including norovirus‐related diarrhea. In fact, a previous study indicated that Japanese geriatric intermediate care facilities pay more careful attention to dealing with healthcare facility‐related infections than Japanese intensive care homes. 22 These characteristics may also explain why there was no relationship between the number of roommates and the occurrence of diarrhea. Third, we conducted tests for norovirus in all patients with diarrhea, which might have led to early detection of the index case in the facility and earlier disease control.
Residents with care‐needs level 3 were found to have a higher risk for norovirus‐related diarrhea. Typically, patients with care‐needs level 3 can walk with supportive items and can communicate with others. 4 Compared with those with care‐needs level 4 or 5, they are likely to have a wider range of activities and come in contact with more people, leading to more opportunities for exposure to pathogens through contact, including norovirus.
The association between hypertension and norovirus‐related diarrhea might be explained by the immune condition of subjects with hypertension. 23 Patients with hypertension have sympathetic nerve activation and higher levels of noradrenaline secretion, which suppresses the activation of T cells and cytokine production in their spleen, 24 which might make patients more susceptible to developing infectious diseases.
In relation to the association between stroke and the risk of norovirus‐related diarrhea, a similar mechanism as with hypertensive patients might be involved, because the incidence of stroke is higher among patients with hypertension. In a previous study focusing on immune function in post‐stroke patients, two‐thirds of subjects had a history of hypertension. 25 In the present study as well, 56% of residents with stroke had hypertension. Besides, dysfunction of neutrophils and immunodepression mediated by deactivation of monocytes might predispose post‐stroke patients to infections. 25 , 26 Another possible reason for the causality of these associations could be that some patients with hypertension or stroke receive assistance in their ADLs, such as meal assistance. Although these associations were observed after controlling for care‐needs level, residual confounding due to each type of daily life assistance might have remained. Besides, as far as we know, the association between hypertension or stroke and infectious diarrhea is very limited. 27 , 28 This suggests that it would be prudent to have reservations about these positive relationships.
In terms of cane usage, a retrospective cohort study in Hong Kong nursing homes indicated that residents who are dependent on a wheelchair for mobility have an increased risk of developing norovirus gastroenteritis. 29 The authors suggested that indirectly touching surfaces contaminated with norovirus via the wheel of the wheelchair accounted for the higher risk, representing a fomite transmission pathway. 30 In the present study facilities, since the canes belonged to each resident and were not shared, the association between norovirus‐related diarrhea and cane usage appeared to reflect indirect contact via walls touched by residents with infectious diarrhea.
Our study is noteworthy for the following two reasons. First, this study was conducted over a period of 1.5 years, which included two epidemic seasons for infectious gastroenteritis. We confirmed that the incidence of norovirus‐related diarrhea in the study facilities was in parallel with epidemics in the community in both seasons. Second, in this prospective and active follow‐up survey, all residents who developed diarrhea were tested for norovirus using kits with high sensitivity and specificity, so that the actual incidence rate of norovirus‐related diarrhea could be calculated.
However, our study has certain limitations, which would suggest caution when interpreting the results. First, although we adjusted for possible confounders, the presence of residual confounding could not be denied. Due to the low incidence of norovirus‐related diarrhea, multivariate models included an extremely limited number of factors as confounders, such as sex, age and care‐needs level. Second, since all residents who developed norovirus‐related diarrhea lived in the same facility, the risk factors presented in the study might also have been affected by the characteristics of the residents in this facility. To minimize this effect, however, we separately evaluated the results of residents at a facility where those with norovirus‐related diarrhea lived. Third, since this study was conducted at a single prefecture in Japan, the results cannot be generalized. Therefore, future studies in other regions or other seasons are required to confirm the validity of the study results.
In conclusion, the present study provides evidence related to norovirus‐related diarrhea in older people who live at geriatric intermediate care facilities, who are not reported on in national surveillance systems and have rarely been focused on in previous studies. The incidence rate of norovirus‐related diarrhea among residents at Japanese geriatric intermediate care facilities during the study period was lower than expected. However, people with care‐needs level 3, hypertension, stroke and those who used a cane for walking had a significantly higher risk for developing norovirus‐related diarrhea. These results highlight the disease burden of norovirus‐related diarrhea in geriatric intermediate care facilities. To prevent a norovirus outbreak in such facilities, the staff should continue taking standard precautions on a daily basis, including hygiene control, particularly during nursing care of residents with high risk factors, and thoroughly disinfecting the handrails and door handles touched by residents using canes with alcohol. In addition to these basic precautions, when a resident develops suspected norovirus‐positive diarrhea or vomit, following the facilities' precautions, the handrails and door handles should also be disinfected with sodium hypochlorite as soon as possible, since disinfection with alcohol is known to be less effective for norovirus.
Disclosure statement
The authors declare no conflict of interest.
Author contributions
AS contributed to study design, statistical analysis, data interpretation and manuscript writing (Author Contribution Index [ACI]: 1.77). SO contributed to study concept, study design, data management and data interpretation (ACI: 1.54). WF, KI1, TK1 and KK1 contributed to study concept, study design and data interpretation (ACI: 1.21). MI, HK, SK and YH contributed to study concept and study design (ACI: 1). AD and HN1 contributed to study concept, study design and data collection (ACI: 1). KI2, TK2, KK2, KH, MF, YF, ES and HN2 contributed to data acquisition (ACI:0.79). All authors provided comments on the drafts and have read and approved the final manuscript.
Acknowledgements
This study was supported by a grant for Research on Emerging and Re‐emerging Infectious Diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labor and Welfare, Japan (H29‐SHINKOGYOSEI‐SHITEI‐003, R2‐SHINKOGYOSEI‐SHITEI‐003). The authors thank the nurses and staff of all the geriatric intermediate care facilities that participated in this study. The funding body had no role in the design of the study, collection, analysis and interpretation of data, or in writing the manuscript.
Suita A, Ohfuji S, Fukushima W, et al. Incidence and risk factors for norovirus‐related diarrhea in Japanese geriatric intermediate care facilities: A prospective cohort study. Geriatr. Gerontol. Int. 2023;23:179–187. 10.1111/ggi.14539
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
References
- 1. Muramatsu N, Akiyama H. Japan: Super‐aging society preparing for the future. Gerontologist 2011; 51: 425–432. 10.1093/geront/gnr067. [DOI] [PubMed] [Google Scholar]
- 2. Statistics Bureau of the Ministry of Internal Affairs and Communications, Japan . Population Estimates, Japan. 2020. [Cited February 5, 2021]. Available from the URL: https://www.stat.go.jp/data/jinsui/pdf/202010.pdf. (in Japanese).
- 3. Campbell JC, Ikegami N. Long‐term care insurance comes to Japan. Health Affairs 2000; 19: 26–39. 10.1377/hlthaff.19.3.26. [DOI] [PubMed] [Google Scholar]
- 4. Morita K, Ono S, Ishimaru M, Matsui H, Naruse T, Yasunaga H. Factors affecting discharge to home of geriatric intermediate care facility residents in Japan. J Am Geriatr Soc 2018; 66: 728–734. 10.1111/jgs.15295. [DOI] [PubMed] [Google Scholar]
- 5. Nakanishi M, Hattori K, Nakashima T, Sawamura K. Health care and personal care needs among residents in nursing homes, group homes, and congregate housing in Japan: why does transition occur, and where can the frail elderly establish a permanent residence? J Am Med Dir Assoc 2014; 15: e1–e6. 10.1016/j.jamda.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 6. Hamada S, Ohno Y, Kojima T, Ishii S, Okochi J, Akishita M. Prevalence of cytochrome P450‐mediated potential drug‐drug interactions in residents of intermediate care facilities for older adults in Japan. Geriatr Gerontol Int 2019; 19: 513–517. 10.1111/ggi.13652. [DOI] [PubMed] [Google Scholar]
- 7. Utsumi M, Makimoto K, Quroshi N, Ashida N. Types of infectious outbreaks and their impact in elderly care facilities: a review of the literature. Age Ageing 2010; 39: 299–305. 10.1093/ageing/afq029. [DOI] [PubMed] [Google Scholar]
- 8. Lindsay L, Wolter J, De Coster I, Van Damme P, Verstraeten T. A decade of norovirus disease risk among older adults in upper‐middle and high income countries: a systematic review. BMC Infect Dis 2015; 15: 425. 10.1186/s12879-015-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Šubelj M, Učakar V. An outbreak of acute gastroenteritis associated with group a rotavirus in long‐term care facility in Slovenia. Wien Klin Wochenschr 2015; 127: 415–420. 10.1007/s00508-014-0672-8. [DOI] [PubMed] [Google Scholar]
- 10. Rao K, Micic D, Chenoweth E et al. Poor functional status as a risk factor for severe Clostridium difficile infection in hospitalized older adults. J Am Geriatr Soc 2013; 61: 1738–1742. 10.1111/jgs.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect 2006; 12: 69–74. 10.1111/j.1469-0691.2005.01299.x. [DOI] [PubMed] [Google Scholar]
- 12. Ohfuji S, Kondo K, Ito K et al. Nationwide epidemiologic study of norovirus‐related hospitalization among Japanese older adults. BMC Infect Dis 2019; 19: 400. 10.1186/s12879-019-4007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato H, Senoh M, Honda H et al. Clostridioides (Clostridium) difficile infection burden in Japan: a multicenter prospective study. Anaerobe 2019; 60: 102011. 10.1016/j.anaerobe.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 14. Tokuhashi Y, Ajiro Y, Umezawa N. Outcomes of posterior fusion using pedicle screw fixation in patients >or=70 years with lumbar spinal canal stenosis. Orthopedics 2008; 31: 1096. [PubMed] [Google Scholar]
- 15. Ministry of Health, Labour and Welfare . Long‐term care insurance in Japan, Survey of institutions and establishments for long‐term care, 2010; Explanation of Main Terms. 2012. [Cited February 5, 2021]. Available from the URL: https://www.mhlw.go.jp/english/database/db-hss/dl/siel-2010-04.pdf. (in Japanese).
- 16. Package insert of IMMUNOCATCH® Norovirus Plus . 2nd ed. (in 2018). [Cited April 13, 2021]. Available from the URL: https://www.eiken.co.jp/POCT/products/noro_plus/pdf/noro_Plus_tenpu2.pdf. (in Japanese).
- 17. Package insert of GE‐test immunochromato‐CD GDH/TOX mini . 4th ed. (in 2018) and 5th ed. (in 2019). [Cited August 23, 2022] Available from the URL: https://www.info.pmda.go.jp/downfiles/ivd/PDF/530121_22800EZX00028000_A_01_08.pdf. (in Japanese).
- 18. McDonald LC, Gerding DN, Johnson S et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66: e1–e48. 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ministry of Health, Labour and Welfare . Ethical Guidelines for Medical and Health Research Involving Human Subjects. [Cited January 4, 2022]. Available from the URL: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf.
- 20. Inns T, Pulawska‐Czub A, Harris JP et al. Prospective cohort study to investigate the burden and transmission of acute gastroenteritis in care homes: epidemiological results. BMJ Open 2019; 9: e033239. 10.1136/bmjopen-2019-033239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osaka Institute of Public Health . The information of infectious gastroenteritis, Comparison the number of infectious gastroenteritis cases reported per sentinels in Osaka Prefecture during past 10 years; 2020. [Cited February 5, 2021]. Available from URL: http://www.iph.pref.osaka.jp/infection/noro/noro.html. (in Japanese).
- 22. Takushima H, Yamamoto K, Tokuzumi K, Moritsuka M. Infection control in long‐term care facilities of present situation‐results from an investigation of managers at long‐term care facilities. J Health Sci 2013; 10: 25–34 (in Japanese). [Google Scholar]
- 23. Case AJ, Zimmerman MC. Sympathetic‐mediated activation versus suppression of the immune system: consequences for hypertension. J Physiol 2016; 594: 527–536. 10.1113/jp271516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marvar PJ, Thabet SR, Guzik TJ et al. Central and peripheral mechanisms of T‐lymphocyte activation and vascular inflammation produced by angiotensin II‐induced hypertension. Circ Res 2010; 107: 263–270. 10.1161/circresaha.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haeusler KG, Schmidt WU, Föhring F et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis 2008; 25: 50–58. 10.1159/000111499. [DOI] [PubMed] [Google Scholar]
- 26. van Gemmeren T, Schuppner R, Grosse GM et al. Early post‐stroke infections are associated with an impaired function of neutrophil granulocytes. J Clin Med 2020; 9: 872. 10.3390/jcm9030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trifan A, Girleanu I, Stanciu C et al. Clostridium difficile infection in hospitalized octogenarian patients. Geriatr Gerontol Int 2018; 18: 315–320. 10.1111/ggi.13186. [DOI] [PubMed] [Google Scholar]
- 28. Cadena J, Thompson GR 3rd, Patterson JE et al. Clinical predictors and risk factors for relapsing Clostridium difficile infection. Am J Med Sci 2010; 339: 350–355. 10.1097/MAJ.0b013e3181d3cdaa. [DOI] [PubMed] [Google Scholar]
- 29. Lin H, Ng S, Chan S et al. Institutional risk factors for norovirus outbreaks in Hong Kong elderly homes: a retrospective cohort study. BMC Public Health 2011; 11: 297. 10.1186/1471-2458-11-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao S, Tang JW, Li Y. Airborne or fomite transmission for norovirus? A Case study revisited. Int J Environ Res Public Health 2017; 14: 1571. 10.3390/ijerph14121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.


