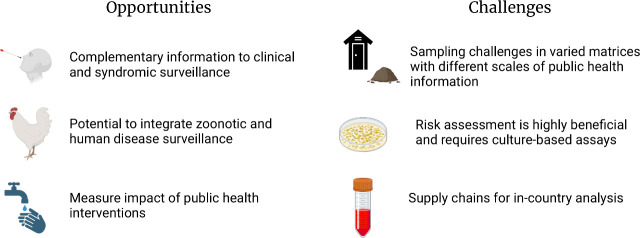
Although testing wastewater for public health surveillance has recently received significant attention in the United States, researchers and public health professionals have long realized the tremendous promise of environmental surveillance (ES), defined as tracking pathogens of interest in fecally contaminated surface waters, soil, or fomites, in low-resource settings for public health action.1,2 The power of ES in low-resource settings is most clearly illustrated with the over 30 years of well-established and standardized polio surveillance. As part of the Global Polio Eradication Initiative, the World Health Organization (WHO) has accredited 146 laboratories within the Global Polio Lab Network (GPLN). These facilities across 92 countries process over 8,000 wastewater or fecally contaminated surface water samples per year, in addition to over 220,000 stool samples annually.3 The data generated from the GPLN are closely linked to clear public health action related to targeted vaccination campaigns. The investment and adoption of ES for polio eradication efforts serves as a use-case for other targets of interest. To explore the current state of the field, the National Science Foundation-funded Research Coordination Network (RCN) on Wastewater Based Epidemiology for SARS-CoV-2 and Emerging Public Health Threats held a workshop in December 2023 to discuss the opportunities and challenges for ES in low-resource settings (Figure 1). Discussions during the workshop highlighted knowledge gaps, best-practices, target selection, academic/government/private partnerships and overall public health action and sustainability of these programs.
Figure 1.
Opportunities and challenges identified by workshop participants.
Among the potential opportunities associated with ES in low-resource settings is the opportunity to provide complementary ES data to clinical surveillance information. Low-resource settings can include populations with frequent clinical surveillance by local governmental public health infrastructure and nonprofits, in some instances with support from international development and public health organizations. For example, the Kenya Medical Research Institute (KEMRI), through support from various entities including the Kenya office of the U.S. Centers for Disease Control and Prevention, hosts a robust clinical surveillance system. Since 2006, KEMRI has been conducting population-based infectious disease (PBIDS) surveillance, including health facilities and household components, in two sites.4 Further, all countries in the WHO African Region have adopted the Integrated Disease Surveillance and Response framework (IDSR).5 This example of robust clinical information is important to validate ES, such as determining whether sampling schemes are representative of pathogens that are in circulation, how these systems could then be used in other geographies, or serve as more cost-effective longitudinal monitoring of infectious disease trends. Advancing ES in low-resource settings provides an opportunity to build robust and complementary environmental and clinical surveillance data sets that can contribute to a more complete picture of community health and guide public health action.
Another opportunity for ES activities is the potential to support the implementation of One Health approaches that can aid in identifying new pathogens of concern or emerging antimicrobial resistance mechanisms, particularly as zoonotic and human disease surveillance may be integrated via ES.6 ES for the identification of new or emerging pathogens could leverage available clinical surveillance data. For instance, using 10 years of hospital-based syndromic surveillance data and three years of ES at live-bird markets, researchers identified distinct seasonal characteristics of human influenza and avian influenza, with human influenza exhibiting seasonality whereas avian influenza circulated year-round and exhibited weak seasonality.7 These and similar efforts can be used to identify emerging viruses and better develop quantitative risk assessments. Lastly, there is an opportunity to evaluate the effects of public health interventions, such as mass vaccination campaigns, and infrastructure improvements8 that are expected to reduce the spread of infectious diseases.
With this vast potential for improving human health, it is important to consider that ES in low-resource settings still has many unique challenges that need to be overcome for successful and widespread implementation. One notable challenge relates to developing both methods and representative sampling regimes for diverse matrices, which can include latrines,8 onsite sanitation systems, fecally contaminated drainage systems/informal sewage networks,9 soil that is impacted by fecal material,10 fomites,11 or air.12 The majority of sanitation in low- and middle-income countries is provided via systems that require fecal sludge management (e.g., community pit latrines).13 In these cases, there may be high confidence in the population contributing to a sample, but a lack of methods for determining the scale of sampling needed (number of latrines) or the temporal resolution that will yield the highest value information for public health action, especially relative to the cost of sampling and analysis. Furthermore, latrines may not frequently be emptied, making the temporal scale of the sample more uncertain. For other matrices (e.g., open sewage networks, soil) there is uncertainty about the contributing population which is compounded with an unknown rate of target decay in the environment, complicating site selection and data interpretation. Open sewers will be more affected by precipitation, and it is often unclear the direction or source of flows, obfuscating any potential estimates of populations contributing to a given sample collection point and complicating quantitative analysis. This challenge can be overcome,9 but requires significant investment in time and resources to map these changing sewer networks.
In some cases, there may be limited laboratory capacity for conducting ES analysis in-country, and a significant challenge is the cost and lead times associated with obtaining supplies in-country. ES programs would benefit from developing and sharing best practices to ensure long-term sustainability of surveillance activities. Another consideration, when establishing ES, is method selection. Laboratory capacity in some countries may not include molecular testing, but rather have the resources and interest to sustain culture methods. If possible, ES based on molecular measurements would ideally be accompanied by an analysis of pathogen viability for culturable organisms or measurements of antimicrobial resistance since these analyses would help with interpretation of molecular data and further public health action.14
Given the challenges and opportunities described above, close partnerships between researchers and public health practitioners or other governmental entities can help advance knowledge on the spread of infectious diseases and improving health outcomes. Partnerships between local governmental organizations and local researchers with relevant expertise may be beneficial. For instance, the icddr,b, based in Dhaka, Bangladesh has extensive research expertise on infectious diseases affecting Bangladesh and South Asia (e.g., refs (15−17)). Even with strong in-country research collaborations, public health officials may have limited capacity to engage with researchers because of competing priorities and finite resources.18 A clear and compelling public health action is needed to show that the benefits outweigh the costs of surveillance activities. Continual exchange between researchers and public health officials can lead to new target identification and can help researchers identify localities of greatest interest for ES. Dissemination of results of surveillance activities needs to occur very thoughtfully to eliminate stigma and in partnership with public health practitioners to ensure that the results of ES activities are accessible and interpretable by the community and in line with data reporting standards.19
The widespread interest in wastewater surveillance that emerged during the pandemic has presented an opportunity to increase investment in ES globally. The potential benefits of this line of research are vast, but capitalizing on these benefits requires thoughtful partnership and exchange to promote rooted collaborations that help mitigate global disease burdens.20,21
Acknowledgments
We thank Brian Kaplan and Venkata Raghava Mohan who participated in the workshop for their contributions to the discussions who are not included as authors. The workshop that prompted this work was supported by the National Science Foundation, grant 2202361. Figure 1 was made in BioRender.
Biography

Jeseth Delgado Vela is an Assistant Professor in the Department of Civil and Environmental Engineering at Duke University. Her work focuses on leveraging environmental biotechnology to improve urban water infrastructure. She is a recipient of an NSF CAREER Award and the Ford Foundation Dissertation Award, and she was named an Early Career Research Fellow by the Gulf Research Program in 2021.
Author Present Address
† Department of Civil and Environmental Engineering, University of Southern California Los Angeles, CA 90007, USA
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors declare no competing financial interest.
References
- Chigwechokha P.; Street R.; Holm R. H. Advancing the Use of Fecal Sludge for Timelier and Better-Quality Epidemiological Data in Low- and Middle-Income Countries for Pandemic Prevention. Environ. Sci. Technol. 2023, 57 (46), 17665–17666. 10.1021/acs.est.2c07788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard F. G. B.; Ban R.; Barr D. B.; Brown J.; Cannon J.; Colford J. M. Jr.; Eisenberg J. N. S.; Ercumen A.; Petach H.; Freeman M. C.; Levy K.; Luby S. P.; Moe C.; Pickering A. J.; Sarnat J. A.; Stewart J.; Thomas E.; Taniuchi M.; Clasen T. Measuring Environmental Exposure to Enteric Pathogens in Low-Income Settings: Review and Recommendations of an Interdisciplinary Working Group. Environ. Sci. Technol. 2020, 54 (19), 11673–11691. 10.1021/acs.est.0c02421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Polio Laboratory Network - GPEI. https://polioeradication.org/polio-today/polio-now/surveillance-indicators/the-global-polio-laboratory-network-gpln/ (accessed 2024–03–06).
- Herman-Roloff A.; Aman R.; Samandari T.; Kasera K.; Emukule G. O.; Amoth P.; Chen T.-H.; Kisivuli J.; Weyenga H.; Hunsperger E.; Onyango C.; Juma B.; Munyua P.; Wako D.; Akelo V.; Kimanga D.; Ndegwa L.; Mohamed A. A.; Okello P.; Kariuki S.; Cock K. M. D.; Bulterys M.; Adapting Longstanding Public Health Collaborations between Government of Kenya and CDC Kenya in Response to the COVID-19 Pandemic, 2020–2021. Emerg. Infect. Dis. 2022, 28 (13), S159. 10.3201/eid2813.211550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihekweazu C.; Agogo E. Africa’s Response to COVID-19. BMC Med. 2020, 18 (1), 151. 10.1186/s12916-020-01622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifels M.; Khalilur Rahman O.; Sam I.-C.; Cheng D.; Chua F. J. D.; Nainani D.; Kim S. Y.; Ng W. J.; Kwok W. C.; Sirikanchana K.; Wuertz S.; Thompson J.; Chan Y. F. The One Health Perspective to Improve Environmental Surveillance of Zoonotic Viruses: Lessons from COVID-19 and Outlook Beyond. ISME Commun. 2022, 2 (1), 107. 10.1038/s43705-022-00191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry I.; Rahman M.; Flora M. S.; Shirin T.; Alamgir A. S. M.; Khan M. H.; Anwar R.; Lisa M.; Chowdhury F.; Islam M. A.; Osmani M. G.; Dunkle S.; Brum E.; Greer A. L.; Morris S. K.; Mangtani P.; Fisman D. N. Seasonality of Influenza and Coseasonality with Avian Influenza in Bangladesh, 2010–19: A Retrospective, Time-Series Analysis. Lancet Glob. Health 2022, 10 (8), e1150–e1158. 10.1016/S2214-109X(22)00212-1. [DOI] [PubMed] [Google Scholar]
- Holcomb D. A.; Knee J.; Capone D.; Sumner T.; Adriano Z.; Nalá R.; Cumming O.; Brown J.; Stewart J. R. Impacts of an Urban Sanitation Intervention on Fecal Indicators and the Prevalence of Human Fecal Contamination in Mozambique. Environ. Sci. Technol. 2021, 55 (17), 11667–11679. 10.1021/acs.est.1c01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski McQuade E. T.; Blake I. M.; Brennhofer S. A.; Islam M. O.; Sony S. S. S.; Rahman T.; Bhuiyan M. H.; Resha S. K.; Wettstone E. G.; Hughlett L.; Reagan C.; Elwood S. E.; Mira Y.; Mahmud A. S.; Hosan K.; Hoque M. R.; Alam M. M.; Rahman M.; Shirin T.; Haque R.; Taniuchi M. Real-Time Sewage Surveillance for SARS-CoV-2 in Dhaka, Bangladesh versus Clinical COVID-19 Surveillance: A Longitudinal Environmental Surveillance Study (December, 2019-December, 2021). Lancet Microbe 2023, 4 (6), e442–e451. 10.1016/S2666-5247(23)00010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaum L.; Njenga S. M.; Kihara J.; Boehm A. B.; Davis J.; Null C.; Pickering A. J. Soil-Transmitted Helminth Eggs Are Present in Soil at Multiple Locations within Households in Rural Kenya. PLoS One 2016, 11 (6), e0157780. 10.1371/journal.pone.0157780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujcic J.; Ram P. K.; Hussain F.; Unicomb L.; Gope P. S.; Abedin J.; Mahmud Z. H.; Sirajul Islam M.; Luby S. P. Toys and toilets: cross-sectional study using children’s toys to evaluate environmental faecal contamination in rural Bangladeshi households with different sanitation facilities and practices. Trop. Med. Int. Health 2014, 19 (5), 528–536. 10.1111/tmi.12292. [DOI] [PubMed] [Google Scholar]
- Osman M.; Ibrahim H.; Yousef F.; Elnasr A. A.; Saeed Y.; Hameed A. A. A Study on Microbiological Contamination on Air Quality in Hospitals in Egypt. Indoor Built Environ. 2018, 27 (7), 953–968. 10.1177/1420326X17698193. [DOI] [Google Scholar]
- Berendes D. M.; Sumner T. A.; Brown J. M. Safely Managed Sanitation for All Means Fecal Sludge Management for At Least 1.8 Billion People in Low and Middle Income Countries. Environ. Sci. Technol. 2017, 51 (5), 3074–3083. 10.1021/acs.est.6b06019. [DOI] [PubMed] [Google Scholar]
- Haas C. N. Quantitative Microbial Risk Assessment and Molecular Biology: Paths to Integration. Environ. Sci. Technol. 2020, 54 (14), 8539–8546. 10.1021/acs.est.0c00664. [DOI] [PubMed] [Google Scholar]
- Ud-Din A. I. M. S.; Wahid S. U. H.; Latif H. A.; Shahnaij M.; Akter M.; Azmi I. J.; Hasan T. N.; Ahmed D.; Hossain M. A.; Faruque A. S. G.; Faruque S. M.; Talukder K. A. Changing Trends in the Prevalence of Shigella Species: Emergence of Multi-Drug Resistant Shigella Sonnei Biotype g in Bangladesh. PLoS One 2013, 8 (12), e82601. 10.1371/journal.pone.0082601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen S.; Luby S. P.; Brooks W. A.; Homaira N.; Mamun A. A.; Bhuiyan M. U.; Rahman M.; Ahmed D.; Abedin J.; Rahman M.; Alamgir A. S. M.; Fry A. M.; Streatfield P. K.; Rahman A.; Bresee J.; Widdowson M.-A.; Azziz-Baumgartner E. Population-Based Incidence of Severe Acute Respiratory Virus Infections among Children Aged < 5 Years in Rural Bangladesh, June-October 2010. PLoS One 2014, 9 (2), e89978. 10.1371/journal.pone.0089978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. U.; Gurley E. S.; Gerloff N.; Rahman M. Z.; Simpson N.; Rahman M.; Haider N.; Chowdhury S.; Balish A.; Zaman R. U.; Nasreen S.; Chandra Das B.; Azziz-Baumgartner E.; Sturm-Ramirez K.; Davis C. T.; Donis R. O.; Luby S. P. Avian Influenza Surveillance in Domestic Waterfowl and Environment of Live Bird Markets in Bangladesh, 2007–2012. Sci. Rep. 2018, 8 (1), 9396. 10.1038/s41598-018-27515-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease 2019. The Lancet. https://www.thelancet.com/infographics-do/gbd-2019 (accessed 2024–03–06).
- McClary-Gutierrez J. S.; Aanderud Z. T.; Al-faliti M.; Duvallet C.; Gonzalez R.; Guzman J.; Holm R. H.; Jahne M. A.; Kantor R. S.; Katsivelis P.; Kuhn K. G.; Langan L. M.; Mansfeldt C.; McLellan S. L.; Mendoza Grijalva L. M.; Murnane K. S.; Naughton C. C.; Packman A. I.; Paraskevopoulos S.; Radniecki T. S.; Roman F. A.; Shrestha A.; Stadler L. B.; Steele J. A.; Swalla B. M.; Vikesland P.; Wartell B.; Wilusz C. J.; Wong J. C. C.; Boehm A. B.; Halden R. U.; Bibby K.; Delgado Vela J. Standardizing Data Reporting in the Research Community to Enhance the Utility of Open Data for SARS-CoV-2 Wastewater Surveillance. Environ. Sci. Water Res. Technol. 2021, 7 (9), 1545–1551. 10.1039/D1EW00235J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan-Hernandez L.; Boehm A. B.; Mihelcic J. R. Parachute Environmental Science and Engineering. Environ. Sci. Technol. 2020, 54 (23), 14773–14774. 10.1021/acs.est.0c07462. [DOI] [PubMed] [Google Scholar]
- Yozwiak N. L.; Happi C. T.; Grant D. S.; Schieffelin J. S.; Garry R. F.; Sabeti P. C.; Andersen K. G. Roots, Not Parachutes: Research Collaborations Combat Outbreaks. Cell 2016, 166 (1), 5–8. 10.1016/j.cell.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]