Abstract
Immunoaffinity purification of polyribosomes (polysomes) from crude leaf extracts of Arabidopsis (Arabidopsis thaliana) was achieved with transgenic genotypes that overexpress a translational fusion of a ribosomal protein (RP) with a His6-FLAG dual epitope tag. In plants with a cauliflower mosaic virus 35S:HF-RPL18 transgene immunopurification with anti-FLAG agarose beads yielded 60-Svedberg ribosomal subunits, intact 80-Svedberg monosomes and polysomes. Sucrose density gradient fractionation of the purified complexes demonstrated that the distribution of polysome size was similar in crude cell extracts and the purified complexes. The immunopurified complexes included putative cytosolic RPs of Arabidopsis and ribosome-associated proteins, as well as full-length transcripts of high and low abundance. Whole-genome profiling using long DNA oligonucleotide-based microarrays provided a high level of reproducibility between polysomal mRNA samples immunopurified from two independent biological replicates (r approximately 0.90). Comparison of immunopurified and total cellular RNA samples revealed that for most of the genes, the mRNAs were associated with the epitope-tagged polysomal complexes, with an average relative level of association of 62.06% ± 4.39%. The results demonstrate that the immunopurification of polysomes can be a valuable tool for the quantification of mRNAs present in translation complexes in plant cells. This technology can be extended to evaluation of mRNA populations at the cell- or tissue-specific level by regulation of the tagged RP with distinct promoters.
Eukaryotic gene expression is regulated by multiple mechanisms. Nuclear events include transcription and posttranscriptional processing of pre-mRNAs, such as splicing, 5′ capping, and 3′ polyadenylation, as well as mRNA decay and transport of the mRNA from the nucleus to the cytoplasm. Gene expression is further controlled by the cytosolic mechanisms of mRNA decay and translation, protein targeting, and degradation. DNA microarrays are routinely used to measure the steady-state abundance of mRNAs under different developmental or physiological conditions. However, the steady-state level of an mRNA reflects its synthesis and decay and may not be well correlated with the level of the encoded protein. Gygi et al. (1999), for example, explored the relationship between mRNA (transcriptome) and protein (proteome) expression levels for a number of genes in Saccharomyces cerevisiae at the mid-log growth phase. This study showed a 30-fold variation in the abundance of transcripts that encode proteins with comparable levels, and a 20-fold variation in the abundance of proteins of mRNAs with comparable abundance (copies per cell). Both differential mRNA translation and protein degradation are thought to be responsible for the discrepancies between mRNA and protein levels observed in plant cells (for review, see Gutierrez et al., 1999; Hellmann and Estelle, 2002; Kawaguchi and Bailey-Serres, 2002).
Protein synthesis is primarily regulated at the initiation of translation (Preiss and Hentze, 2003). Actively translated mRNAs are associated with multiple ribosomes in large polyribosome (polysome) complexes, whereas other mRNAs can remain as ribonucleoprotein (RNP) complexes or in association with one or a few ribosomes. Therefore, the translational status of an mRNA can be evaluated by monitoring its association with polyribosomes. This has been routinely achieved by fractionation of polysomes of different size by differential centrifugation through Suc density gradients and monitoring mRNA levels in individual fractions (Tzamarias et al., 1989; Kawaguchi and Bailey-Serres, 2002). High-throughput technologies, such as DNA microarrays, have extended the analysis of translation control to the genomic scale (Zong et al., 1999; Kuhn et al., 2001; Ju et al., 2003; Preiss et al., 2003). DNA microarrays were used to evaluate the proportion of individual mRNA species from Arabidopsis (Arabidopsis thaliana) leaves associated with polysomes under nonstress and mild dehydration stress conditions (Kawaguchi et al., 2004; Kawaguchi and Bailey-Serres, 2005). Under nonstress conditions, the amount of each mRNA in polysomes ranged from less than 25% to over 95%. Dehydration promoted a reduction in the average proportion of mRNA in polysomes by 10%, with 71% of the individual mRNAs displaying a significant reduction polysome association. However, 60% of dehydration-inducible mRNAs displayed no significant reduction in polysome levels, supporting the notion that stress-induced changes in mRNA abundance may not necessarily reflect alteration in protein synthesis.
The affinity purification of epitope-tagged proteins combined with proteomics and DNA microarray technologies constitutes a powerful tool to identify components of RNA protein complexes and characterize posttranscriptional and translational regulation of mRNAs. Tenenbaum et al. (2002) described a high-throughput method termed ribonomics to identify mRNA subsets contained in RNP complexes. Human mRNAs associated with RNA-binding proteins, such as HuB, poly(A)-binding protein (PABP), and eukaryotic initiator factor 4E, were differentially coimmunoprecipitated from P19 carcinoma cells and identified by cDNA arrays (Tenenbaum et al., 2000). Similarly, a FLAG-tagged PABP was expressed using the myo-3 promoter to examine mRNAs that accumulate specifically in muscle cells of Caenorhabditis elegans at the first larval stage (Roy et al., 2002). Epitope tagging and affinity purification was also applied to study ribosome biogenesis and nuclear export in S. cerevisiae. Preribosomal particles were copurified with tandem-affinity purification-tagged versions of Nop7p, a yeast nucleolar protein, and Nug1p, a nuclear GTPase, allowing the identification of a significant number of proteins involved in the biogenesis of the 60 Svedberg (S) ribosomal subunit (Bassler et al., 2001; Harnpicharnchai et al., 2001). Another report described a one-step method for purification of yeast 80S ribosomes using an epitope-tagged ribosomal protein (RP) L25, the yeast ortholog of mammalian and plant RPL23a (Inada et al., 2002). Despite the copurification of ribosome-associated proteins and intact mRNAs, large polysomes were missing from the affinity-purified material. These examples demonstrate that epitope tagging provides an approach to examine mRNAs and proteins contained in RNA-protein complexes in fungi and metazoa. Rohila et al. (2004) recently described an improved tandem-affinity purification tag for purification of plant heterocomplexes, which proved successful for isolation of proteins that interact with a hybrid glucocorticoid receptor in Agrobacterium-infiltrated Nicotiana benthamiana leaves.
We present here an efficient method for affinity purification of Arabidopsis polysomal complexes based on the expression of an epitope-tagged RP from the large ribosomal subunit. The 60S subunit and intact 80S ribosomes, as well as large polysomes, were specifically immunopurified from crude leaf extracts. In addition, more than 50% of cytosolic RPs as well as a number of ribosome-associated non-RPs were detected in the immunopurified complex, as identified by mass spectrometry (MS). The integrity of rRNAs and mRNAs associated with immunopurified ribosomes was verified. Finally, we demonstrate the conversion of these mRNAs into fluorescent-labeled targets and their hybridization to microarrays for global characterization of polysomal mRNA expression profiles in Arabidopsis. This technological development provides a manner to isolate plant polysomes without differential centrifugation and constitutes a valuable tool for a genome scale evaluation of mRNAs present in translational complexes in plant cells.
RESULTS
Expression of Epitope-Tagged 60S RPs in Arabidopsis
A prerequisite for use of an epitope-tagged RP to affinity purify ribosomes is that the tagged-polypeptide terminus resides on an exposed surface of the ribosome. Therefore, we searched for RPs located on the solvent side of the small and large subunits. Since there is no high-resolution information on the structure of plant 80S ribosomes, but there is strong evolutionary conservation of RPs across kingdoms, we based the selection of candidate RPs on the x-ray crystallographic data available for the 50S ribosomal subunit of the archaebacterium Haloarcula marismortui and the cryo-electron microscopy map of the yeast 80S ribosome (Verschoor et al., 1996; Ban et al., 2000; Spahn et al., 2001). The selection of RPL23a was based on the report that a tagged version of the orthologous yeast protein, RPL25, facilitated purification of yeast ribosomes (Inada et al., 2002). RPL7, RPL12, and RPL18, located on the solvent side of the 60S subunit, were also selected as candidates, and the polypeptide terminus to be tagged was determined from available biochemical data (Jeeninga et al., 1996; Kumar et al., 1999; Park et al., 2001). The His6-FLAG (HF) dual tag was chosen based on the prediction that a small tag (about 2 kD) would not affect the assembly or function of ribosomes. RPL7, RPL12, and RPL18 were tagged at the amino (N) terminus with an HF, comprising six His residues followed by a spacer of three Gly residues, a FLAG epitope (eight amino acids), and a second spacer of seven Gly residues. The spacer of noncharged amino acids was included between the tag and the coding region of the RP to provide flexibility and facilitate the exposure of the tag on the solvent side of the ribosome. RPL23a was tagged at the carboxyl (C) terminus by the dual tag designed in the reverse orientation. The expression of the tagged RP genes was driven by the cauliflower mosaic virus (CaMV) 35S promoter and the tobacco mosaic virus (TMV) omega (Ω) 5′ untranslated leader in transgenic Arabidopsis.
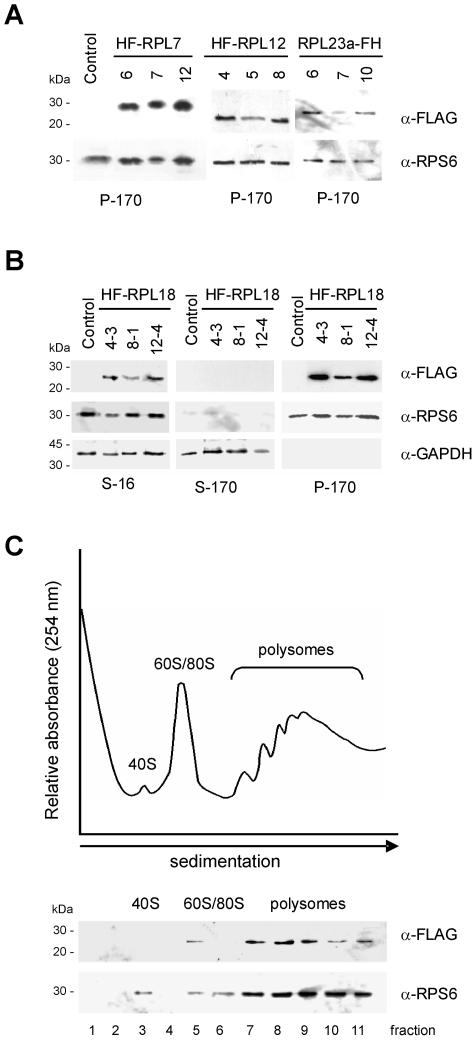
The expression of each tagged RP and its association with ribosomes was evaluated in mature rosette leaves from lines that were transformed with the empty T-DNA vector (control) or with the T-DNA vectors carrying a tagged RP. A crude extract (S-16) was prepared, and ribosome pellet (P-170) and postribosomal supernatant (S-170) fractions were obtained by ultracentrifugation through a 1.6 m Suc cushion. Fractions were analyzed by western blot with an anti-FLAG antibody. Figure 1A shows the expression and association with the ribosomal pellet fraction (P-170) of HF-RPL7, HF-RPL12, and RPL23a-FH detected in three independent T1 lines. A more detailed inspection of HF-RPL18 accumulation in the three fractions (S-16, S-170, and P-170) of T2 35S:HF-RPL18 lines is shown in Figure 1B. A polypeptide with an electrophoretic mobility of 24 kD, which corresponds to the theoretical molecular mass of HF-RPL18, was detected in crude extracts. HF-RPL18 was also detected in ribosomes (P-170), but not in the postribosomal supernatant (S-170), indicating that HF-RPL18 is efficiently incorporated into ribosomes. A polyclonal antiserum against the small subunit protein S6 (RPS6) of maize (Zea mays; Williams et al., 2003) was used as a marker of the ribosomal fraction, and a polyclonal antiserum against cytosolic glyceraldehyde-3-P dehydrogenase (GAPDH) of Arabidopsis (Wang et al., 1997) was used to evaluate contamination with soluble cytosolic proteins. Translational activity of the HF-RPL18-tagged ribosomes was evaluated by fractionation of leaf ribosomes (P-170) through a 20% to 60% (w/v) Suc density gradient and immunodetection of HF-RPL18 and RPS6 in individual fractions. HF-RPL18 was present in fractions that contained the 60S subunit, monosomes, and small-to-large polysome, but absent in the free-mRNA/mRNP and 40S fractions (Fig. 1C). This result further confirmed that HF-RPL18 was efficiently incorporated into the 60S subunit and moreover that ribosomes that contained HF-RPL18 were present in polysomal complexes.
Figure 1.
Epitope-tagged RPs are associated with translating ribosomes. A, Ribosomes were pelleted by centrifugation from detergent-treated cell extracts of control (transformed with empty HF T-DNA vector) and transgenic lines of Arabidopsis. Proteins (30 μg per lane) were separated by 15% (w/v) SDS-PAGE, transferred onto nitrocellulose membranes, and the epitope-tagged RPs were immunodetected with the anti-FLAG-horseradish-peroxidase-conjugated monoclonal antibody (α-FLAG). Identical membranes were used for immunodetection of RPS6, a component of the small ribosomal subunit (α-RPS6). The migration of molecular mass markers is indicated on the left. B, Crude extracts (S-16), postribosomal supernatants (S-170), and ribosome pellet fractions (P-170) were prepared from control and 35S:HF-RPL18 Arabidopsis lines and analyzed as described for A with α-FLAG, α-RPS6, and an antiserum against a soluble cytosolic GAPDH (α-GAPDH). C, The ribosomal pellet fraction (P-170) from 35S:HF-RPL18 plants was fractionated by ultracentrifugation through 20% to 60% (w/v) Suc density gradients, and the UV absorbance (254 nm) profile was recorded. The 11 gradient fractions were analyzed by immunoblot analysis with α-FLAG and α-RPS6. The position of the ribosomal subunits (40S, 60S), monosomes (80S), and polysomes are indicated.
Affinity Purification of Arabidopsis Ribosomes
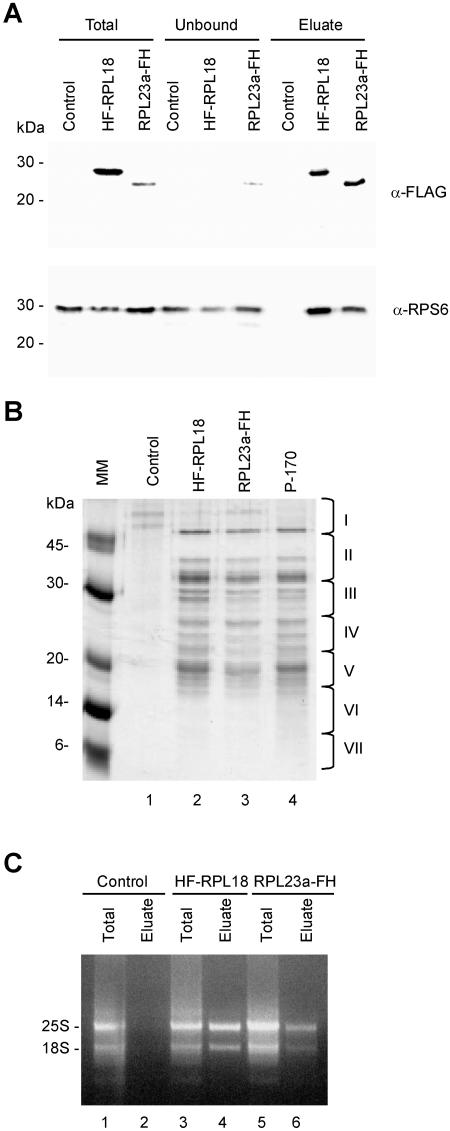
In a first attempt to affinity purify intact ribosomes, nickel-nitrilotriacetic acid agarose magnetic agarose beads were used to bind the His6-tagged proteins RPL12, RPL18, and RPL23a. Although the His6 tag was detected in ribosomes by western-blot analysis, we were unable to affinity purify the tagged RPs or protein complexes from whole-leaf extracts by use of this method under conditions that maintained polysomes and permitted purification of a His6-tagged protein (His6-eukaryotic initiator factor 4E) produced in Escherichia coli (data not shown). By contrast, the FLAG epitope allowed the affinity purification of ribosomal complexes by immunopurification with anti-FLAG agarose-conjugated beads. Whole-leaf extracts from control, 35S:HF-RPL18, and 35S:RPL23a-FH lines were incubated with affinity resin under conditions that maintain polysome integrity. After exhaustive washes, bound proteins were eluted with the [FLAG]3 peptide and analyzed by SDS-PAGE and Coomassie Blue staining or immunoblot detection (Fig. 2, A and B). A single band corresponding to the epitope-tagged RP, HF-RPL18, or RPL23a-FH, was detected in the crude leaf extracts and in the eluate from the immunopurifications (Fig. 2A, top). A proportion of RPL23a-FH protein was also found in the unbound fraction of the cell lysate, indicating the affinity purification was less efficient for this protein than for HF-RPL18 (Fig. 2A, top). Immunoblotting with anti-RPS6 revealed that the 40S subunit was also present in the eluted material from 35S:HF-RPL18 and 35S:RPL23a-FH lines, demonstrating that intact 80S ribosomes can be recovered by a single-step affinity purification (Fig. 2A, bottom). The purification was dependent upon the presence of the tagged RP, as indicated by absence of RPS6 in the immunopurified material from control lines (Fig. 2A, bottom). RPS6 was also detected in the unbound fraction. This was not unexpected since 40S ribosomal subunits not coupled with the 60S subunit would not be purified; however, it is also unlikely that all ribosomes contained the tagged RP (see “Discussion”). Immunoprecipitation reactions were also conducted with lines that expressed HF-RPL7 or HF-RPL12. However, these experiments resulted in a poor yield of the tagged RP and RPS6 in the eluate fraction (data not shown).
Figure 2.
Immunopurification of Arabidopsis 80S ribosomes from crude leaf extracts by use of epitope-tagged RPL18 and RPL23a. Leaf proteins from control (transformed with the empty HF T-DNA vector), 35S:HF-RPL18, or 35S:RPL23a-FH transgenic lines were extracted in PEB, and approximately 300 OD260 units from each sample were incubated with anti-FLAG agarose beads for isolation of protein complexes that contained the HF epitope-tagged RP. A, Total, unbound, or immunopurified (Eluate) proteins were separated by 15% (w/v) SDS-PAGE, transferred onto a nitrocellulose membrane, and immunodetected with α-FLAG and α-RPS6 as described in Figure 1. Molecular mass markers are indicated on the left. B, Proteins (30 μg per lane) that were coaffinity-purified with HF-RPL18 (lane 2) or with RPL23a-FH (lane 3) and RPs prepared by conventional ultracentrifugation (P-170, lane 4) were visualized by Coomassie Blue staining. Lane 1 represents a proportional volume of the sample immunopurified from control plants. Proteins copurified with HF-RPL18 (lane 2) were identified by mass spectrometric analyses (see also Supplemental Table I). The position of the seven gel sections analyzed by MS is indicated on the right. C, RNA was extracted from leaf crude extracts (Total) or the immunopurified material (Eluate) of control, 35S:HF-RPL18, and 35S:RPL23a-FH transgenic lines by guanidine-HCl/ethanol precipitation. Two micrograms of total RNA from control, 35S:HF-RPL18, and 35S:RPL23a-FH lines (lanes 1, 3, and 5) and eluted RNA from 35S:HF-RPL18 and 35S:RPL23a-FH lines (lanes 4 and 6) were resolved in a 1.2% (w/v) agarose gel containing 10 μg mL−1 of ethidium bromide. Lane 2 is a proportional volume of the eluted sample from control plants. The 25S and 18S rRNAs are indicated.
To confirm that 80S ribosomes were copurified with HF-RPL18 and RPL23a-FH, the polypeptide composition of the immunopurified ribosomes was compared with that of ribosomes conventionally purified by differential ultracentrifugation. Figure 2B shows that polypeptides present in the immunoprecipitation eluate from leaf extracts of 35:HF-RPL18 and 35S:RPL23a-FH plants (Fig. 2B, lanes 2 and 3) were similar in electrophoretic mobility and stoichiometric intensity to those detected in the conventionally purified ribosomes (Fig. 2B, lane 4). The specificity of the immunopurification was supported by the limited detection of polypeptides in the eluted material from plants carrying the empty T-DNA vector (control; Fig. 2B, lane 1). Immunopurification of intact ribosomes was further confirmed by the presence of 25S and 18S rRNAs in the eluted material from 35:HF-RPL18 and 35S:RPL23a-FH plants, but not from control plants (Fig. 2C).
Since the immunopurification of ribosomes seemed to be more efficient for HF-RPL18 than RPL23a-FH (Fig. 2A), a line with a single-copy insertion of 35S:HF-RPL18 (line 12-4) was chosen for further analysis. This line showed the predicted 3:1 ratio of kanamycin resistance for a single-copy T-DNA insertion in the T2 generation. Southern-blot analysis using a probe corresponding to 3′-untranslated region of the Agrobacterium tumefaciens octopine synthase gene (ocs) confirmed integration of the transgene at a single genomic location (data not shown).
The development of transgenic and control (transformed with the empty vector) lines were compared with wild-type plants. No alteration in morphology or development was observed in the transgenic lines, as judged from the number, size, and shape of rosette and cauline leaves, stem length, flowering-time, number of flowers, and silique size, spacing, and number (data not shown).
Identification of Proteins Associated with HF-RPL18-Tagged Ribosomes by MS
To confirm the immunopurification of 80S ribosomes, polypeptides present in the eluted material from control and 35S:HF-RPL18 lines were analyzed by MS. The lane of the SDS-PAGE that contained the immunopurified proteins was divided into seven sections and subjected to in-gel trypsin digestion (Fig. 2B, sections I–VII). The resulting peptides were separated by liquid chromatography (LC) and identified by electro-spray ionization quadrupole tandem MS (MS/MS). This analysis detected more than 50% of the cytosolic RPs of Arabidopsis identified by more thorough genomic and proteomic analyses (Barakat et al., 2001; Chang et al., 2005), including 13 (40%) from the 40S and 30 (62.5%) from the 60S subunit, respectively, in the ribosomes copurified with HF-RPL18 (Supplemental Table I). No proteins with significant scores were identified in the immunoprecipitated sample purified from control plants. In addition, a number of non-RPs associated with immunopurified ribosomes were identified. These include a protein with homology to the human receptor for activated protein C-kinase and yeast Asc1p (At1g18080; Ron et al., 1994; Hoffmann et al., 1999); two RNA-binding proteins, a pumilio/puf RNA binding protein (At3g20250) and an RNA-binding domain-related protein (At4g28990); and two proteins involved in tRNA processing, a tRNA-splicing endonuclease-positive effector-related protein (At1g16800) and a tRNA 2′ phosphotransferase (At2g45330).
Analysis of the Integrity of Immunopurified Polysomes and Associated mRNAs
To address whether HF-RPL18 allows isolation of polysomes of all sizes, ribosomes purified simultaneously from the same leaf tissue by conventional ultracentrifugation (P-170) or by immunopurification (eluate) were fractionated in 20% to 60% (w/v) Suc density gradients and evaluated. The UV (254 nm) absorbance profiles and size distribution of immunopurified ribosomes was similar to that of the P-170 ribosome pellet (Fig. 3). There was no evidence of significant dissociation of ribosomes and polysomal complexes during the preparation of either ribosome samples. Large ribosomal subunits, monosomes and polysomes, from disome to large polysomes (>5 ribosomes per mRNA), were recovered from the immunopurified material (Fig. 3B). However, the 40S subunit was not resolved in this sample. In addition, the proportion of polysomes relative to monosomes and ribosomal subunits was higher in the eluate than in the P-170 ribosome pellet (Fig. 3, A and B). These differences could be due to prevalence of the 60S subunit in the immunoprecipitate and minimal dissociation of polysomes during the more rapid immunopurification procedure.
Figure 3.
Sedimentation characteristics of ultracentrifugation-concentrated and immunopurified ribosomes on Suc density gradients. Leaf ribosomes were isolated by ultracentrifugation through a 1.6 m Suc cushion or by affinity purification with anti-FLAG agarose beads. Ribosomes (approximately 15 OD260 units) were fractionated by ultracentrifugation through 20% to 60% (w/v) Suc density gradients, and the UV absorbance (254 nm) profile was recorded. A, Absorbance profile of ribosomes purified by conventional ultracentrifugation (P-170). B, Absorbance profile of ribosomes copurified with HF-RPL18 (Eluate). The positions of the 60S ribosomal subunit, 80S monosomes, and polysomes are indicated.
To test whether the immunopurification of polysomes yielded intact mRNAs, RNA-blot analysis was performed with RNAs extracted from a crude cell lysate that was prepared with polysome extraction buffer (PEB; total) and from the immunopurified material (eluate) of control and 35S:HF-RPL18 plants. The RNA was separated in a denaturing agarose gel and transferred onto a nylon membrane. After hybridization with a PABP2 gene probe, a 2.5-kb transcript was detected in total RNA from control and 35S:HF-RPL18 plants, and in the immunopurified RNA from 35S:HF-RPL18 plants (Fig. 4A). This result revealed that intact large transcripts copurified with HF-RPL18, demonstrating that the integrity of the mRNAs was not compromised during the immunopurification. Reverse transcription (RT)-PCR amplification was conducted to test whether high- and low-abundance mRNAs can be isolated by this methodology. Transcripts predominantly detected in polysomes (>65%) in nonstressed Arabidopsis leaves (Kawaguchi et al., 2004), such as those encoding RPS9, PABP2, WRKY75, and ACT2, were detected by nonquantitative RT-PCR in total and the immunopurified RNA samples of 35S:HF-RPL18 plants (Fig. 4B). No DNA was detected in the immunopurified material as evaluated by a PCR reaction without RT (data not shown). These results strongly support the conclusion that intact high- (e.g. ACT2) and low- (e.g. WRKY75) abundance mRNAs were present in the immunopurified material obtained from leaves of 35S:HF-RPL18 plants.
Figure 4.
Analysis of mRNAs associated with immunopurified ribosomes. A, Approximately 8 μg of total or immunopurified (Eluate) RNA was resolved in a formaldehyde agarose gel and transferred onto a nylon membrane. In the control sample a volume proportional to HF-RPL18 eluate sample was loaded. The membrane was hybridized to [32P]-labeled PABP2 and exposed to x-ray film. The size (kilobase) of the PABP2 transcript is indicated on the left. B, Total or immunopurified (Eluate) RNA (50 ng) isolated from control and 35S:HF-RPL18 transgenic lines were subjected to one-step RT-PCR amplification with gene-specific primers for RPS6, PABP, WRKY75, and ACT2. Twenty-five cycles of amplification were used for RPS6, PABP, and ACT2, and 30 cycles were used for WRKY75. The PCR products were separated in a 2% (w/v) agarose gel containing 10 μg mL−1 of ethidium bromide. The size (basepair) of each product is indicated.
DNA Microarray Analysis of Total and Immunoaffinity-Purified Polysome-Associated mRNAs
To expand to a genomic scale, the characterization of mRNAs associated with epitope-tagged polysomes, total cellular and immunopurified RNA samples isolated from Arabidopsis leaf tissue were amplified, converted into fluorescence targets, and hybridized to full-genome Arabidopsis long-oligonucleotide microarrays (see “Materials and Methods”). To determine the reproducibility of the immunopurification procedure, microarray hybridizations were performed with two biological replicates and dye-swap technical replicates for each sample. The reproducibility measurements between technical (dye-swap) and biological replicates were calculated by linear regression analysis of the log2 intensity values for each sample and observed to be highly correlated (Table I). Examples of the scatter plots and linear regression analysis that compares total as well as immunopurified RNA samples extracted from two independent experiments are presented in Supplemental Figure 1. This result indicated that the isolation of mRNAs was highly reproducible in independent immunopurification experiments.
Table I.
Technical and biological reproducibility measurements comparing total cellular and immunopurified polysomal RNA
Correlation coefficients (r) as reproducibility measurements obtained by linear regression analysis of log2 intensity values comparing technical and biological replicates. The values include both biological variation and slide-to-slide variation.
| RNA Sample | Rep No. 1-Cy3 | Rep No. 1-Cy5 | Rep No. 2-Cy3 | Rep No. 2-Cy5 |
|---|---|---|---|---|
| Total Cellular RNA | ||||
| Rep no. 1-Cy3 | 1 | 0.857 | 0.874 | 0.876 |
| Rep no. 1-Cy5 | 1 | 0.870 | 0.957 | |
| Rep no. 2-Cy3 | 1 | 0.905 | ||
| Rep no. 2-Cy5 | 1 | |||
| Immunopurified RNA | ||||
| Rep no. 1-Cy3 | 1 | 0.802 | 0.865 | 0.765 |
| Rep no. 1-Cy5 | 1 | 0.882 | 0.752 | |
| Rep no. 2-Cy3 | 1 | 0.836 | ||
| Rep no. 2-Cy5 | 1 |
The relative percentage of each mRNA species associated with epitope-tagged polysomes was determined for genes classified as present in both total and immunopurified polysomal RNA samples (n = 23,471). The gene frequency distribution of the percentage of each mRNA in polysomes is presented in Figure 5, and the data for individual genes is provided in Supplemental Table III. The percentages of mRNA associated with the epitope-tagged ribosomes for each gene are provided in Supplemental Table II. Over 99% (n = 23,365) of the gene transcripts showed a relative level in tagged polysomes of 35% to 85%, with an average and sd of 62.06% ± 4.39%. This suggests that for most of the detected transcripts, the majority of the mRNA molecules are associated with at least one epitope-tagged ribosome in leaves of plants grown under standard conditions.
Figure 5.
Frequency distribution of mRNA levels in polysomal complexes. The relative percentage of each mRNA found in the immunopurified polysomal sample was determined for 23,471 genes as described in “Material and Methods,” and used to bin the number of expressed genes (frequency) at 5% intervals. The average value and sd of percentage of association with immunopurified polysomes (62.06% ± 4.39%) is indicated.
DISCUSSION
Polysomes are a complex RNP component of the gene expression machinery in prokaryotic and eukaryotic organisms. This report describes a method for affinity purification of ribosomes from Arabidopsis leaves that is based on the use of an epitope-tagged RP from the large ribosomal subunit. The 60S subunit, 80S monosomes, and large polysomes, as well as intact polysome-associated mRNAs, were successfully copurified from crude leaf extracts of a transgenic line that expresses a HF epitope-tagged RPL18. The 35S:HF-RPL18 line also allowed for efficient purification of polysome-associated mRNAs from 10-d-old Arabidopsis seedlings (M.E. Zanetti and J. Bailey-Serres, unpublished data). Preribosomal particles and small polysomes were previously isolated from yeast by use of affinity purification techniques (Bassler et al., 2001; Harnpicharnchai et al., 2001; Inada et al., 2002; Schafer et al., 2003). By contrast, HF-RPL18 described here allowed for immunopurification of complexes that included small and large polysomes.
RPs (RPL7, RPL12, RPL18, and RPL23a) predicted to reside on an exposed surface of the large subunit were engineered with a dual-HF epitope tag with the goal of affinity purification of polysomal complexes. The use of nickel-nitrilotriacetic acid agarose magnetic agarose beads proved unsuccessful for the affinity purification of ribosomes, presumably because the interaction between the chelating His residues and the Ni2+ ions was not sufficient to capture high molecular mass complexes such as ribosomes (approximately 3.2 × 106 D; Chang et al., 2005). On the other hand, immunopurification with anti-FLAG agarose beads yielded ribosomes in transgenic lines that express HF-RPL18 or RPL23a-FH under the regulation of the CaMV 35S promoter (Fig. 2). Although lines were obtained that incorporated HF-RPL7 and HF-RPL12 into ribosomes, they did not allow for immunopurification of ribosomes. This may indicate that the N terminus of these proteins is not located on the surface of the 60S ribosomal subunit. The immunopurification of ribosomes proved more efficient for HF-RPLl8 than RPL23a-FH, since a proportion of RPL23a-FH remained in the unbound fraction after immunopurification (Fig. 2A). RPL23a is the Arabidopsis ortholog of yeast Rpl25. In their pioneering demonstration of affinity purification of yeast ribosomes by use of Rpl25-FH, Inada et al. (2002) recovered 60S subunit, 80S monosomes, and small polysomes, but failed to affinity select large polysomes. The authors attributed this failure to an inability of large polysomes to enter the matrix of the affinity resin. This work showed that ribosomes with HF-RPL18 form large polysomal complexes (Fig. 1C), which are recovered after immunopurification (Fig. 3B). The difference in the purification efficiency with RPL23a-HF and HF-RPL18 may be attributable to the location of these proteins on the ribosome. RPL18 resides on the top of the backside of the 60S subunit, whereas RPL23a is positioned on the bottom of the backside of this subunit (Ban et al., 2000). In this context, RPL23a-FH detected in the unbound fraction (Fig. 2A) would likely represent RPL23a-FH present in large polysomal complexes that is not sufficiently exposed to allow immunopurification. The results also revealed that a portion of cellular RPS6 remains in the unbound fraction of the immunopurification sample in 35S:HF-RPL18 and 35S:RPL23a-FH lines (Fig. 2A). This RPS6 could be that of 40S subunits not coupled with the 60S subunit or ribosomes that lack the epitope-tagged RP. The latter is not unexpected due to the presence of two and three endogenous RPL23a and RPL18 genes, respectively (Barakat et al., 2001). It is reasonable to assume that some proportion of the ribosomes in leaf cells may possess the product of the native RP gene. Additionally, the level of the endogenous RP gene product would be expected to reflect cell type distinctions in the expression of the endogenous RP and CaMV 35S promoters.
The immunopurification of tagged ribosomes was coupled with MS to en mass identify RPs and ribosome-associated proteins present in the affinity-purified sample. Sixty-two percent of the predicted 60S RPs and 40% of the predicted 40S subunit RPs of Arabidopsis cytosolic ribosomes (Barakat et al., 2001) were identified by LC/MS/MS (Supplemental Table I). A more comprehensive proteomic study of Arabidopsis ribosomes confirmed the presence of 74 RPs, including 30 and 44 RPs from the small and large subunits, respectively (Chang et al., 2005). In addition to the RPs, a number of non-RPs were identified associated in the immunopurified complex. One of these non-RPs, receptor for activated protein C kinase, was present at similar stoichiometry as RPs in the 40S subunit and polysomes isolated by conventional ultracentrifugation from cultured Arabidopsis cells (Chang et al., 2005) and in ribosomes affinity purified from yeast (Inada et al., 2002). This protein has recently been recognized as a scaffold for regulatory factor and RNA-binding proteins involved in translational regulation (Nilsson et al., 2004). The non-RPs identified by LC/MS/MS in the immunopurified complexes also included RNA-binding proteins and tRNA-processing proteins.
RNA-profiling experiments at the genomic scale revealed a high reproducibility (r approximately 0.95) in quantitation of mRNA levels from independent replicate samples of total RNA extracted from Arabidopsis leaf tissue. Importantly, a high level of reproducibility (r approximately 0.90) was also observed between polysomal RNA samples independently immunopurified from two biological replicates (Table I). Thus, mRNAs associated with the epitope-tagged polysomal complexes were consistently purified by this method, providing an approach for RNA profiling in Arabidopsis. The analysis of the proportion of each mRNA species associated with polysomes (Fig. 5) revealed that most of the mRNA species are associated to some degree with the epitope-tagged polysomal complexes in Arabidopsis leaves. The average value of percentage of association with polysomes was estimated in 62% for Arabidopsis leaf mRNAs, which is close to the 71% association of mRNAs with polysomes described for S. cerevisiae cells (Arava et al., 2003). The 62% average of association with polysomes calculated for affinity-purified polysomal mRNAs was lower than the 82% calculated by analysis of nonpolysomal and polysomal (disome to large polysomes) mRNAs in nonstressed Arabidopsis leaves (Kawaguchi et al., 2004). This could be due to a number of reasons including variation in growing conditions (i.e. photoperiod, leaf age), ribosome complexes examined, microarray platforms used, and statistical treatments.
The method described here provides a means to isolate ribosomes for gene expression studies or structure and function analyses. Advantages of this methodology include isolation of complexes from crude cell extracts in about 4 h without need of ultracentrifugation steps and avoidance of contamination by large protein or RNP complexes that cosediment with ribosomes. It is envisioned that this technology will allow immunopurification of polysomes and associated mRNAs from specific cell types by expression of the tagged RP with a cell type-specific promoter. Hundreds of different cell types can be identified within the complex tissues and organs of higher eukaryotes, which exhibit differential gene expression patterns (Galbraith, 2003). Methods such as in situ hybridization and reporter genes have permitted analysis of mRNA-specific expression patterns of individual genes in a range of plant species (Meyerowitz, 1987; Jefferson, 1989; Chiu et al., 1996). However, these methods do not provide genome scale analysis of cell-specific gene expression. Recent technological developments in the isolation of protoplasts that express promoter-green fluorescent protein constructs, laser micro-dissection of plant organs, and aspiration of individual cells have been used to evaluate cell-specific gene expression at a more global level (Birnbaum et al., 2003; Jones and Grierson, 2003; Kerk et al., 2003). A drawback of these methods is the potential perturbation of steady-state mRNA levels caused by disruption of cell-cell interactions, osmotic stress during protoplast production, damage associated with laser microdissection, or wounding occasioned during cell aspiration. This method may provide a valuable tool to capture polysome-associated mRNA subsets in a cell type-specific manner from intact complex tissues or organs with a minimal perturbation of global gene expression. Furthermore, as demonstrated in Arabidopsis leaves under nonstress and mild-dehydration conditions, differential mRNA translation contributes to gene regulation in plant cells (Kawaguchi et al., 2004). Therefore, comparative RNA-profiling experiments that quantify polysomal mRNA levels may more accurately reveal regulation of biological significance. Other applications of this technology can be envisioned, including analysis of polysome-associated alternatively spliced mRNAs, microRNAs (miRNAs), and noncoding mRNAs.
Finally, biochemical and structural aspects about plant ribosomes can also be addressed by use of immunopurified ribosomes. Arabidopsis RPs are encoded by members of small gene families (Barakat et al., 2001), which are differentially regulated in a cell or tissue type-specific manner, leading to the possibility of ribosome heterogeneity. In maize kernel tissues, ribosome heterogeneity was observed at the tissue and subcellular level due to variation in the accumulation of two distinct forms of the acidic phosphorylated RPP2 (Szick-Miranda and Bailey-Serres, 2001). Proteomic characterization of Arabidopsis ribosomes provided evidence of posttranslational modification of a number of RPs (Chang et al., 2005). In addition, plant ribosomes display heterogeneity in the phosphorylation of multiple C-terminal residues of RPS6 (Turck et al., 1998; Williams et al., 2003; Chang et al., 2005). Immunopurification of ribosomes from specific cell types or possessing specific RPs can contribute to the elucidation of cell-specific gene expression, translational regulation, and the functional significance of ribosome heterogeneity during plant development.
MATERIALS AND METHODS
Vector Construction
Expression cassettes are diagrammed in Supplemental Figure 2. To generate a cassette for N terminus-tagged proteins, two complementary oligonucleotides, HFG1 (5′-CATGGGACATCACCATCATCACCATGGTGGAGGTGATTATAAGGATGATGATGATAAGGGAGGTGGTGGAGGAGGTGGATCCATCTAT-3′) and HFG2 (5′-CTAGATAGATGGATCCACCTCCTCCACCACCTCCCTTATCATCATCATCCTTATAATGACCTCCACCATCGTGATGATGGTGATGTCC-3′), that encode an HF epitope, followed by a spacer of seven Gly residues, were annealed. The 5′ overhang of the NcoI restriction site in the 5′-primer end and a 5′ overhang of the XbaI restriction site in the 3′-primer end are shown in italics, and an internal BamHI restriction site 5 bp from the XbaI site is underlined. The double-stranded oligonucleotide was cloned into the NcoI and XbaI sites of the SLJ4D4 plasmid (Jones et al., 1992) to create the SLJ-HF plasmid. An EcoRI-HindIII fragment of SLJ-HF plasmid containing the 1,343-bp CaMV 35S promoter, the 66-bp TMV Ω leader, the FH epitope, and the ocs 3′-flanking region was cloned into the EcoRI and HindIII sites of the binary vector pZPZ111 (Hajdukiewicz et al., 1994) to create p35S:HF. The coding regions of AtRPL7B (At2g01250), AtRPL12A (At2g37190), and AtRPL18B (At3g05590) were amplified by PCR using cDNA and the following pairs of primers: 5′-CCGGATCCGTTGAGTCAAAGGTTGTAGTTCC-3′ and 5′-GCTCTAGACTAATTCATCCTCCTGATAAGCTC-3′ for AtRPL7, 5′-CATCTGATCACCGCCAAAGTTGGATCCG-3′ and 5′-GCTCTAGATCAGTTCTCAGGAATCTCAAC-3′ for AtRPL12, 5′-CGGGATCCGGTATTGATCTGATCGCCGGAG-3′ and 5′-GCTCTAGATTAAACCTTGAATCCACGACTC-3′ for AtRPL18B. The BamHI or BclI and XbaI restriction sites are underlined. The PCR fragments were cloned between the BamHI and XbaI site of p35S:HF to create p35:HF-RPL7, p35S:HF-RPL12, and p35S:HF-RPL18.
To generate a cassette for C terminus-tagged proteins, two complementary oligonucleotides, GFH1 (5′-CATGGGAATCGGATCCGGAGGTGGAGGTGGAGATTATAAGGATGATGATGATAAGGGTGGAGGTCATCACCATCATCACCATTAAT-3′) and GFH2 (5′-CTAGATTAATGGTGATGATGGTGATGACCTCCACCCTTATCATCATCATCCTTATAATCTCCACCTCCACCTCCGGATCCGATTCC-3′) that encode a spacer of seven Gly residues and a FLAG-His6 (FH) epitope were annealed. The 5′ overhang of the NcoI restriction site in the 5′-primer end and 5′ overhang of the XbaI restriction site in the 3′-primer end are shown in italics, and an internal BamHI restriction site 5 bp from the NcoI site is underlined. The double-stranded oligonucleotide was cloned into the NcoI and XbaI sites of the SLJ4D4 plasmid to create the SLJ-FH plasmid. The open reading frame of AtRPL23aA (At2g39460) was amplified by PCR using cDNA and the following combination of primers: 5′-CATCCCATGGGATCTCCGGCTAAAGTTGATACTACC-3′ and 5′-CGCGGATCCGATGATGCCGATCTTGTTAGCAAC-3′. The NcoI and BamHI restriction sites are underlined. The fragment was cloned between the NcoI and BamHI sites of the SLJ-FH vector. An EcoRI-HindIII fragment from SLJ-FH plasmid that contained the CaMV 35S promoter, the TMV Ω 5′ untranslated leader, the AtRPL23aA open reading frame, the FH epitope tag, and the ocs 3′-flanking region was cloned into the EcoRI and HindIII sites of the binary vector pZPZ111 to create the p35S:RPL23a-FH. The expression cassettes of all constructs were sequenced on both strands. The binary T-DNA vectors were electroporated into Agrobacterium tumefaciens (strain LBA4404).
Arabidopsis Growth Conditions and Agrobacterium-Mediated Transformation
Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) plants were grown in a growth chamber at 22°C under long-day photoperiod (16 h light, 200 μE m−2 s−1). Six-week-old plants were transformed by the floral-dip method (Clough and Bent, 1998). Seeds were selected on Murashige and Skoog (Sigma, St. Louis) agar plates containing 50 μg mL−1 of kanamycin for 7 d. Kanamycin-resistant seedlings were transferred to soil for propagation. Rosette leaves were harvested from 5- to 6-week-old wild-type or transgenic plants, pulverized in liquid N2, and stored at −80°C.
Isolation of Arabidopsis Ribosomes
Ribosomes were isolated from Arabidopsis leaves according to Williams et al. (2003) with minor modifications. Briefly, frozen leaf tissue was homogenized in 2 mL per milliliter of tissue of PEB (200 mm Tris-HCl, pH 9.0, 200 mm KCl, 25 mm EGTA, 36 mm MgCl2, 5 mm dithiothreitol (DTT), 50 μg mL−1 cycloheximide, 50 μg mL−1 chloramphenicol, 0.5 mg mL−1 heparin, 1% (v/v) Triton X-100, 1% (v/v) Tween 20, 1% (w/v) Brij-35, 1% (v/v) Igepal CA-630, 2% (v/v) polyoxyethylene, and 1% (w/v) deoxycholic acid). All procedures were carried out at 4°C. After clarification by centrifugation at 16,000g for 10 min, 1.5 mL of extract was loaded onto 3.5 mL-1.6 m Suc cushion and ultracentrifuged at 170,000g for 18 h at 4°C in a 70Ti rotor (Beckman, Fullerton, CA). Pellets were resuspended into 150 μL of Stahelin A buffer (20 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 1 mm sodium molybdate, and 1 mm DTT). Protein concentration was determined by Bradford assay using ovoalbumin as the standard according to the manufacturer's instructions (United States Biochemical, Cleveland).
Immunopurification of Ribosomes
Frozen, pulverized leaf tissue was homogenized with 2 mL of PEB per milliliter of pulverized tissue. Homogenates were clarified by centrifugation at 16,000g for 10 min, and approximately 300 OD600 units of the supernatant were incubated with 100 μL of EZ-View anti-FLAG agarose beads (Sigma) at 4°C for 2 h with gentle shaking. For Suc gradient fractionation of polysomes, approximately 1,000 OD600 units of supernatant and 400 μL of anti-FLAG agarose beads were used. The unbound fraction was recovered, and the beads were washed four times for 5 min with 20 mL per milliliter of agarose beads of wash buffer (200 mm Tris-HCl, pH 9.0, 200 mm KCl, 25 mm EGTA, 36 mm MgCl2, 5 mm DTT, 50 μg mL−1 cycloheximide, and 50 μg mL−1 chloramphenicol). Elution was performed by incubation of the agarose beads with 250 μL of wash buffer that also contained 50 units mL−1 of RNase inhibitor (Promega, Madison, WI) and 200 μg mL−1 of [FLAG]3 peptide (Sigma) at 4°C for 30 min. Eluted material was fractionated onto Suc density gradients, subjected to RNA extraction, or stored at −80°C prior to analyses by SDS-PAGE.
Suc Density Gradient Fractionation of Polysomes
For fractionation of total polysomes, ribosomes were isolated as follows: approximately 5 mL of frozen, pulverized tissue were homogenized with 15 mL of PEB (see above). The crude extract was clarified by centrifugation at 16,000g for 15 min at 4°C in a JA-20 rotor (Beckman) and the supernatant loaded onto an 8-mL-1.6 m Suc cushion and centrifuged for 18 h at 170,000g as described above. Pellets were resuspended in 700 μL of buffer R (200 mm Tris-HCl, pH 9.0, 200 mm KCl, 25 mm EGTA, 36 mm MgCl2, 5 mm DTT, 50 μg mL−1 cycloheximide, and 50 μg mL−1 chloramphenicol) and incubated at 4°C for 1 h.
Ribosomes isolated by conventional ultracentrifugation or by immunopurification were fractionated through 20% to 60% (v/v) Suc density gradients as described by Kawaguchi et al. (2004). For immunoblotting, fractions were precipitated by addition of two volumes of 100% (v/v) ethanol, incubation for 6 h at 4°C, centrifugation at 16,000g for 15 min, and resuspension in 20 μL of 1× SDS-loading buffer.
SDS-PAGE and Western Blots
Proteins (30 μg per lane) were separated on 15% (w/v) SDS-PAGE and stained with Coomassie Blue R250 or subjected to immunoblot analyses as described previously (Williams et al., 2003). Immunodetection of protein was performed with anti-FLAG-horseradish-peroxidase-conjugated monoclonal antibody (1:500; Sigma) with a polyclonal antiserum against maize (Zea mays) RPS6 (1:5,000; Williams et al., 2003) or with an antiserum against Arabidopsis cytosolic GAPDH (1:10,000; kindly provided by Ming-Che Shih, Iowa State University). Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000; Bio-Rad, Hercules, CA) was used as a secondary antibody and visualization was by use of enhanced chemiluminescence system according to manufacturer's instructions (Amersham, Piscataway, NJ) and exposure to x-ray film.
MS and Protein Identification
The 1D-SDS-PAGE lane with proteins immunopurified from 35S:HF-RPL18 plants was divided in seven sections as follows: I, >45 kD; II, 35 to 45 kD; III, 25 to 35 kD; IV, 20 to 25 kD; V, 17 to 25 kD; VI, 11 to 17 kD; and VII, 6 to 11 kD. Individual sections were subjected to in-gel trypsin digestion as previously described (Williams et al., 2003). MS was performed by use of a nano-spray electro-spray ionization quadrupole time-of-flight MS coupled with an LC system (Waters, Milford, MA; Keck Proteomics Laboratory, Biological Mass Spectrometry Facility, Institute for Integrative Genome Biology at University of California, Riverside). For LC analysis, a dC18 column (Waters) was used. After LC/MS/MS analysis, a peak list file containing the mass value of the parental peaks and fragmented (y or b) ions was generated by the ProteinLynx algorithm (Waters). Protein identification was performed against National Center for Biotechnology Information nonredundant database by use of the Mascot MS/MS Ion Search algorithm (http://www.matrixscience.com/cgi/search; Perkins et al., 1999) by setting parental peak mass tolerance (observed mass [in D] − theoretical mass)/theoretical mass) at 350 ppm and MS/MS tolerance at 0.3 D. The maximum allowable number of missed trypsin cleavage sites was set at one. The validation of protein identifications was further confirmed by manual inspection of the mass spectrum and Mascot score.
RNA Isolation and Analysis
RNA was purified from crude extracts or immunopurified complexes by guanidine-HCl precipitation followed by cleanup using RNeasy columns (Qiagen, Valencia, CA) as described by Kawaguchi et al. (2004). RNA was resolved in a 1.2% (w/v) agarose gel that contained 10 μg mL−1 ethidium bromide and visualized under UV light. For RNA-blot analysis, the RNA (8 μg per lane) was resolved in a 13% (v/v) formaldehyde 1.3% (w/v) agarose gel and transferred onto a nylon membrane according to standard procedures (Sambrook et al., 1989). The membrane was hybridized with a random-primed [α-32P]dCTP-labeled DNA probe corresponding to the full-length coding sequence of the PABP2 (At4g34110) transcript. Hybridization was as previously described (Fennoy and Bailey-Serres, 1995). RT-PCR reactions were performed with 50 ng of total or immunopurified RNA using a SuperScript One-step RT-PCR reaction kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For amplification RPS6B (At5g15200), primer pairs were as described by Kawaguchi et al. (2004). For the other transcripts, the following pairs of primers were used: 5′-AATGCTACTCCAGAGCAACAGAGG-3′and 5′-GCTCTAGAGGGAGAGACAAAAGCCAAAGATC-3′ for PABP2 (At4g34110); 5′-GTCGTTGTATGCTCCTTTTTT-3′ and 5′-CATTTGAGTGAGAATATGCTC-3′ for WRKY75 (At5g13080); and 5′-TGGTTGGTATGGGTCAGAAA-3′ and 5′-GGATGCAAGGATTGATCCTC-3′ for ACT2 (At5g09810). RT-PCR reactions were performed as follows: 45 min at 42°C (RT reaction), 2 min at 94°C (first cycle); 1 min at 94°C, 1 min at 54°C for RPS6 and ACT2, and at 52°C for PABP and WRKY75, 1 min at 72°C (25 cycles for RPS6, ACT2, and PABP, and 30 cycles for WRKY75); and 10 min at 72°C (final cycle). PCR products were separated on a 2% (w/v) agarose gel containing 10 μg mL−1 of ethidium bromide and visualized under UV light.
RNA Quality and Quantity Assessment, Amplification, CyDye Labeling, Microarray Hybridization, and Data Extraction
RNA was quantified by use of a NanoDrop ND-1000 UV-Vis Spectrophotometer according to the manufacturer's instructions (Nanodrop Technology, Wilmington, DE). RNA quality was assessed using an Agilent 2100 Bioanalyzer with either RNA 6000 Nano or Pico Assay reagent kits (Agilent Technology, Palo Alto, CA). The RNA 6000 ladder was purchased from Ambion (Austin, TX).
For RNA samples of amounts less than 1 μg, linear in vitro RNA amplification was conducted using a modification of the Eberwine method (van Gelder et al., 1990) to produce sufficient signals for slide-based oligonucleotide microarray hybridization and to provide more reliability by generating stable cDNA targets rather than cRNA. First-strand cDNA synthesis was conducted by priming the RNA sample (20–1,000 ng) with an anchored T7-(dT)24V primer (100 ng) in a thermal cycler at 70°C for 10 min, chilling the RNA primer mixture on ice for 5 min, and initiation of the RT reaction was by addition of the appropriate amount of first-strand buffer, 1 mm DTT, 1 mm dNTPs, 40 units of RNaseOu,t and 200 units of reverse transcriptase Superscript II (Invitrogen) in a final volume of 20 μL and incubated for 90 min at 42°C according to the procedure recommended by the manufacturer. Second-strand cDNA synthesis was performed at 16°C for 3 h after addition of 40 units of Escherichia coli DNA polymerase I, 10 units of E. coli DNA ligase, 2 units of E. coli RNase H (2 units) in a final volume of 150 μL as previously described (Baugh et al., 2001; Zhao et al., 2002). Double-strand (ds) cDNAs were polished by addition of 20 units T4 DNA polymerase (Invitrogen) and incubation at 16°C for 15 min. ds-cDNAs were purified using the QIAquick PCR Purification kit (Qiagen) and eluted with a 1:10 dilution of the elution buffer supplied with the kit. The eluted ds-cDNAs were transferred into a PCR tube, dried, and resuspended in 8 μL RNase free water. The first cycle of RNA amplification was conducted at 37°C for 5 h in a thermal cycler with heating lid on, using the MEGAscript T7 kit according to the supplied protocol (Ambion). cRNA was purified with RNeasy Mini kit columns (Qiagen). A portion (10%) of the amplified cRNA was used for the quantity and quality assessments as described above, and the remainder was transferred into PCR reaction tubes for volume reduction in a Savant SpeedVac (Ramsey, Minnesota). The second-cycle cDNA synthesis was completed using the same steps as described for the first-cycle cDNA synthesis except that T7-(dN)9 primer (100 pmol) was employed in the RNA priming to reverse the strand orientation of the amplified RNAs. The second-cycle RNA (sense) amplification was an exact repetition of the first-cycle cRNA (anti-sense) amplification. However, the resultant amplified RNA is in the same strand orientation as the initial mRNA. The amplified sense-strand RNA was converted into Cy3- or Cy5-labeled cDNA targets through RT with Superscript II reverse transcriptase as previously described (Deyholos and Galbraith, 2001).
Arabidopsis long oligonucleotide microarrays, which contain 29,110 oligonucleotide probes of Operon Arabidopsis V3.0 AROS whole-genome oligonucleotide set, plus the probes for printing quality control and expression detection monitoring, were fabricated in-house as described previously (Galbraith et al., 2004). The Arabidopsis oligonucleotide set was designed and synthesized based on the ATH1 release 5.0 of The Institute for Genomic Research Arabidopsis genome annotation database (http://www.tigr.org/tdb/e2k1/ath1) and release 4.0 of the miRNA Registry at the Sanger Institute (http://www.sanger.ac.uk/Software/Rfam/mirna/index.shtml). The set represents 26,173 protein-coding genes and 28,964 transcripts.
Slide rehydration was performed over 50°C water, followed by snap drying on 65°C heating block for 5 s; this process was repeated three times. Slides were UV-cross-linked at 120 mJ, washed in 1% (w/v) SDS for 5 min at room temperature, then in 100% (v/v) ethanol for 30 s, and spin dried by centrifugation at 1,000g for 2 min. Hybridization was performed in 2× SSC, 0.08% (w/v) SDS, 6% (v/v) liquid block (Amersham) at 55°C for 12 h. Slides were washed at 55°C once with 2× SSC containing 0.5% (w/v) SDS, once with 0.5× SSC, and once with 0.05× SSC for 5 min each. The washed slides were scanned using an Axon GenePix 4200AL scanner (Axon, Union City, CA). Cy5- and Cy3-signals extracted from the hybridization images were converted into numerical data using GenePix 6.0 (Axon).
Experimental Design and Statistical Analyses
A pairwise design was used for microarray analyses. Immunopurified RNA was paired with the total cellular RNA from the same biological sample, with dye reversal arranged to partition out any dye effects. Two biological replicates were employed for the comparison of immunopurified RNA versus total cellular RNA samples. The element calls (expressed genes) were determined using the proprietary algorithm in Axon's GenePix Pro 6.0 with customer input parameters. The called elements were also inspected visually in GenePix Pro to adjust the parameters and ensure the accuracy of the element call. In this experiment, an equal quantity of total cellular RNA and immunopurified RNA was used for amplification and target labeling. The immunopurified RNA equivalency in total RNA was calculated based on the peak height ratio of 25S rRNA of the equal amount of immunopurified ribosomal RNA versus total RNA. One microgram of total RNA was estimated approximate 0.73 μg of immunopurified RNA equivalency.
For the calculation of ribosome loading (Fig. 5), the average fold changes (log2 value of differential gene expression) of immunopurified RNA versus total RNA were estimated using two-stage mixed-linear models after log2 transformation and normalization (Wolfinger et al., 2001). The first stage (normalization model) was employed primarily to eliminate dye effects. The residuals from the normalization, after subtraction of the fitted value for the dye effect from the measured value, were used to compare polysomal and total RNA. SAS package version 9.0, running on the SunOS platform (SAS Institute, Cary, NC) was used to program the macrocode for the analyses. The extreme negative and positive values were used as the endpoints of the ribosomal-loading scale: the lowest endpoint defined as 0% and the highest endpoint as 100% transcript presence in polysomal complexes. The lowest endpoint of the scale was adjusted accordingly (approximately 1.37) based on the equivalency of immunopurified RNA in total RNA. The adjusted scale was used to bin the expressed genes at 5% intervals.
Microarray experiments were described following minimum information about a microarray experiment guidelines (Brazma et al., 2001). The microarray platform has been deposited at Gene Expression Omnibus data repository available at the National Center for Biotechnology Information Web site (http://www.ncbi.nlm.nih.gov/geo/) under accession number GPL1787 (arrays version AT v3 01.01.Z). Microarray data set has been deposited at the same repository under accession numbers GSM38610, GSM38605, GSM38607, and GSM38608. Vectors and transgenic lines are available upon request.
Supplementary Material
Acknowledgments
We are grateful to Changqing Zhang for handling the microarray data deposit. We also thank Dr. Ming-Che Shih for kindly providing the antibody against Arabidopsis cytosolic GAPDH, Dr. Songqin Pan for assistance in the mass-spectrometry analysis, and Joanna Werner-Fraczek for technical assistance.
This work was supported by the National Science Foundation Plant Genome Research Program (grant no. DBI 0211857 to J.B.-S. and D.W.G.).
The online version of this article contains Web-only data.
References
- Arava Y, Wang Y, Storey JD, Liu CL, Brown PO, Herschlag D (2003) Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 100: 3889–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289: 905–920 [DOI] [PubMed] [Google Scholar]
- Barakat A, Szick-Miranda K, Chang IF, Guyot R, Blanc G, Cooke R, Delseny M, Bailey-Serres J (2001) The organization of cytoplamic ribosomal protein genes in the Arabidopsis genome. Plant Physiol 127: 398–415 [PMC free article] [PubMed] [Google Scholar]
- Bassler J, Grandi P, Gadal O, Lessmann T, Petfalski E, Tollervey D, Lechner J, Hurt E (2001) Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol Cell 8: 517–529 [DOI] [PubMed] [Google Scholar]
- Baugh LR, Hill AA, Brown EL, Hunter CP (2001) Quantitative analysis of mRNA amplification by in vitro transcription. Nucleic Acids Res 29: E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN (2003) A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, et al (2001) Minimum information about a microarray experiment (MIAME): toward standards for microarray data. Nat Genet 29: 365–371 [DOI] [PubMed] [Google Scholar]
- Chang IF, Szick-Miranda K, Pan S, Bailey-Serres J (2005) Proteomic characterization of evolutionarily conserved and variable proteins of Arabidopsis cytosolic ribosomes. Plant Physiol 137: 848–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6: 325–330 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Deyholos MK, Galbraith DW (2001) High-density DNA microarrays for gene expression analysis. Cytometry 43: 229–238 [PubMed] [Google Scholar]
- Fennoy SL, Bailey-Serres J (1995) Posttranscriptional regulation of gene expression in oxygen-deprived roots of maize. Plant J 7: 287–295 [DOI] [PubMed] [Google Scholar]
- Galbraith DW (2003) Global analysis of cell type-specific gene expression. Comp Funct Genom 4: 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Elumalai R, Gong FC (2004) Integrative flow cytometric and microarray approaches for use in transcriptional profiling. Methods Mol Biol 263: 259–280 [DOI] [PubMed] [Google Scholar]
- Gutierrez RA, MacIntosh GC, Green PJ (1999) Current perspectives on mRNA stability in plants: multiples levels and mechanisms of control. Trends Plant Sci 4: 429–438 [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R (1999) Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19: 1720–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Harnpicharnchai P, Jakovljevic J, Horsey E, Miles T, Roman J, Rout M, Meagher D, Imai B, Guo Y, Brame C, et al (2001) Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol Cell 8: 505–515 [DOI] [PubMed] [Google Scholar]
- Hellmann H, Estelle M (2002) Plant development: regulation by protein degradation. Science 297: 793–797 [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Mosch HU, Sattlegger E, Barthelmess IB, Hinnebusch A, Braus GH (1999) The WD protein Cpc2p is required for repression of Gcn4 protein activity in yeast in the absence of amino-acid starvation. Mol Microbiol 31: 807–822 [DOI] [PubMed] [Google Scholar]
- Inada T, Winstall E, Tarun SZ, Yates JR, Schieltz D, Sachs AB (2002) One step affinity purification of yeast ribosome and its associated proteins and mRNAs. RNA 8: 948–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeeninga RE, Venema J, Raue HA (1996) Rat RPL23a ribosomal protein efficiently competes with Saccharomyces cerevisiae L25 homologue for assembly into 60 S subunit. J Mol Biol 263: 648–656 [DOI] [PubMed] [Google Scholar]
- Jefferson RA (1989) The GUS reporter gene system. Nature 342: 837–838 [DOI] [PubMed] [Google Scholar]
- Jones JDG, Shlumukov L, Carland F, English J, Scofield S, Bishop G, Harrison K (1992) Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Res 1: 285–297 [DOI] [PubMed] [Google Scholar]
- Jones MA, Grierson CS (2003) A simple method for obtaining cell-specific cDNA from small numbers of growing root-hair cells in Arabidopsis thaliana. J Exp Bot 54: 1373–1378 [DOI] [PubMed] [Google Scholar]
- Ju J, Huang C, Minskoff SA, Mayotte JE, Taillon BE, Simons JF (2003) Simultaneous gene expression analysis of steady-state and actively translated mRNA populations from osteosarcoma MG-63 cells in response to IL-1 via an open expression analysis platform. Nucleic Acids Res 31: 5157–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Bailey-Serres J (2002) Regulation of translational initiation in plants. Curr Opin Plant Biol 5: 460–465 [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Bailey-Serres J (2005) mRNA sequence features responsible for translational regulation in Arabidopsis. Nucleic Acids Res 33: 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Girke T, Bray EA, Bailey-Serres J (2004) Differential mRNA translation contributes to gene regulation under non-stress and dehydration stress conditions in Arabidopsis thaliana. Plant J 38: 823–839 [DOI] [PubMed] [Google Scholar]
- Kerk NM, Ceserani T, Tausta SL, Sussex IM, Nelson TM (2003) Laser capture microdissection of cells from plant tissues. Plant Physiol 132: 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KM, DeRissi JL, Brown PO, Sarnow P (2001) Global and specific translational regulation in the genomic response of Saccharomyces cerevisiae to a rapid transfer from a fermentable to a non fermentable carbon source. Mol Cell Biol 21: 916–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KU, Srivastava SP, Kaufman RJ (1999) Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol Cell Biol 19: 1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz EM (1987) In situ hybridization to RNA in plant tissue. Plant Mol Biol Rep 5: 242–250 [Google Scholar]
- Nilsson J, Sengupta J, Frank J, Nissen P (2004) Regulation of eukaryotic translation by the RACK1 protein: a platform for signaling molecules on the ribosome. EMBO Rep 5: 1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Himmelbach A, Browning KS, Hohn T, Ryabova LA (2001) A viral reinitiation factor interacts with the host translational machinery. Cell 106: 723–733 [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence data based using mass spectrometry data. Electrophoresis 20: 3551–3567 [DOI] [PubMed] [Google Scholar]
- Preiss T, Baron-Benhamou J, Ansorge W, Hentze M (2003) Homodirectional changes in transcriptome and mRNA translation induced by rapamycin and heat shock. Nat Struct Biol 10: 1039–1047 [DOI] [PubMed] [Google Scholar]
- Preiss T, Hentze M (2003) Starting the protein synthesis machine: eukaryotic translation initiation. Bioessays 10: 1201–1211 [DOI] [PubMed] [Google Scholar]
- Rohila JS, Chen M, Cerny R, Fromm ME (2004) Improved tandem affinity purification tag and methods for isolation of protein heterocomplexes from plants. Plant J 38: 172–181 [DOI] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D (1994) Cloning of an intracellular receptor for protein kinase C: a homolog of the β subunit of G proteins. Proc Natl Acad Sci USA 91: 839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy PJ, Stuart JM, Lund J, Kim SK (2002) Chromosomal clustering of muscle-expressed gene in Caenorhabditis elegans. Nature 418: 975–979 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritish F, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schafer T, Strauss D, Petfalski E, Tollervey D, Hurt E (2003) The path form nucleolar 90S to cytoplasmic 40S pre-ribosomes. EMBO J 22: 1370–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G, Frank J (2001) Structure of the 80S ribosome from Saccharomyces cerevisiae tRNA-ribosome and subunit-subunit interactions. Cell 107: 373–386 [DOI] [PubMed] [Google Scholar]
- Szick-Miranda K, Bailey-Serres J (2001) Regulated heterogeneity in 12-kDa P-protein phosphorylation and composition of ribosomes in maize (Zea mays L.). J Biol Chem 276: 10921–10928 [DOI] [PubMed] [Google Scholar]
- Tenenbaum SA, Carson CC, Lager PJ, Keene JD (2000) Identifying subsets in messenger ribonucleoprotein complexes by cDNA arrays. Proc Natl Acad Sci USA 97: 14085–14090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum SA, Lager PJ, Carson CC, Keene JD (2002) Ribonomics: identifying subset in mRNP complexes using antibodies to RNA-binding proteins and genomics array. Methods 26: 191–198 [DOI] [PubMed] [Google Scholar]
- Turck F, Kozma SC, Thomas G, Nagy F (1998) A heat-sensitive Arabidopsis thaliana kinase substitutes for human p70s6k function in vivo. Mol Cell Biol 18: 2038–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamarias D, Roussou I, Thireos G (1989) Coupling of GCN4 mRNA translational activation with decreased rates of polypeptide chain initiation. Cell 57: 947–954 [DOI] [PubMed] [Google Scholar]
- van Gelder RN, von Zastrow ME, Yool A, Dement WC, Barchas JD, Eberwine JH (1990) Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc Natl Acad Sci USA 87: 1663–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor A, Srivastava S, Grassucci R, Frank J (1996) Native 3D structure of eukaryotic 80S ribosome: morphological homology with the E. coli 70S ribosome. J Cell Biol 133: 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Guo L, Sjolund R, Shih MC (1997) Immunolocalization of GAPDH in Arabidopsis thaliana. Protoplasma 198: 155–162 [Google Scholar]
- Williams AJ, Werner-Fraczek J, Chang IF, Bailey-Serres J (2003) Regulated phosphorylation of 40S ribosomal protein S6 in root tips of maize. Plant Physiol 132: 2086–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS (2001) Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol 8: 625–637 [DOI] [PubMed] [Google Scholar]
- Zhao H, Hastie T, Whitfield ML, Borresen-Dale AL, Jeffrey SS (2002) Optimization and evaluation of T7 based RNA linear amplification protocols for cDNA microarray analysis. BMC Genomics 3: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Q, Schummer M, Hood L, Morris DR (1999) Messenger mRNA translation state: the second dimension of high-throughput expression screening. Proc Natl Acad Sci USA 96: 10632–10636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.