Abstract
Polar auxin transport has been implicated in the induction of vascular tissue and in the definition of vein positions. Leaves treated with chemical inhibitors of polar auxin transport exhibited vascular phenotypes that include increased vein thickness and vascularization. We describe a recessive mutant, thickvein (tkv), which develops thicker veins in leaves and in inflorescence stems. The increased vein thickness is attributable to an increased number of vascular cells. Mutant plants have smaller leaves and shorter inflorescence stems, and this reduction in organ size and height is accompanied by an increase in organ vascularization, which appears to be attributable to an increase in the recruitment of cells into veins. Furthermore, although floral development is normal, auxin transport in the inflorescence stem is significantly reduced in the mutant, suggesting that the defect in auxin transport is responsible for the vascular phenotypes. In the primary root, the veins appear morphologically normal, but root growth in the tkv mutant is hypersensitive to exogenous cytokinin. The tkv mutation was found to reside in the ACL5 gene, which encodes a spermine synthase and whose expression is specific to provascular cells. We propose that ACL5/TKV is involved in vein definition (defining the boundaries between veins and nonvein regions) and in polar auxin transport, and that polyamines are involved in this process.
The formation of the venation pattern in leaves is ideal for examining signaling pathways that recognize and respond to spatial and temporal information, since the pattern is two-dimensional and heritable, and arises during the intercalary growth of the leaf primordium (for review, see Nelson and Dengler, 1997). Although the molecular mechanisms underlying the patterning of leaf venation are not known, the role of the phytohormone auxin in vascular differentiation (e.g. Esau, 1965; Sachs, 1981) and, more specifically, the role of polar auxin transport in procambial (vascular precursor) strand formation and differentiation have been well documented (e.g. Lomax et al., 1995; Gälweiler et al., 1998; Mattsson et al., 1999; Sieburth, 1999). Auxin is thought to be transported in a primarily basipetal manner throughout the plant body from primary sources of auxin synthesis such as young developing leaves (e.g. Lomax et al., 1995), although recent studies have shown that the shoot apical meristem and very young leaf primordia are auxin sinks to which auxin is acropetally transported (Reinhardt et al., 2000; Avsian-Kretchmer et al., 2002). The basipetal transport of auxin from the leaf margin is thought to direct the venation pattern in the leaf (Mattsson et al., 1999; Sieburth, 1999; Aloni, 2001; Avsian-Kretchmer et al., 2002). When this transport is inhibited either chemically (Goto et al., 1991; Okada et al., 1991; Gälweiler et al., 1998; Mattsson et al., 1999; Sieburth, 1999) or genetically by knocking out the PIN FORMED-1 (PIN1) gene, which encodes a component of the auxin efflux carrier (Gälweiler et al., 1998; Müller et al., 1998; Mattsson et al., 1999; Steinmann et al., 1999), the leaves develop increased proliferation of veins near the leaf margin, suggesting that the leaf margin is a major source of auxin within the developing leaf.
A number of leaf venation pattern mutants have been characterized in Arabidopsis (Arabidopsis thaliana), many of which exhibit disruptions in the continuity of the vascular pattern, producing isolated, short stretches of veins. A subset of those also have been shown to have reduced polar auxin transport in the inflorescence stem (e.g. lop1, mp, sfc, and axr6; Carland and McHale, 1996; Przemeck et al., 1996; Deyholos et al., 2000; Hobbie et al., 2000). Conversely, many auxin response mutants have reported vascular defects in the leaf. For example, the bdl mutant has a phenotype similar but weaker than mp and axr6 mutants (Hamann et al., 1999).
Not surprisingly, many of the leaf venation pattern mutants with auxin defects contain mutations in genes whose products are involved in auxin response or polar auxin transport. For example, the MP gene encodes a transcriptional regulator of the auxin response factor family (ARF5; Hardtke and Berleth, 1998). The BDL gene encodes a member of the Aux/indole-3-acetic acid (IAA) family of transcription factors that has been shown to bind to MP and inhibit its activity in yeast (IAA12; Hamann et al., 2002). The AXR6 gene encodes a cullin/CDC53-like subunit of the SCFTIR1 ubiquiting ligase complex, which has been implicated in auxin-dependent degradation of Aux/IAA proteins (Gray et al., 2001; Hobbie et al., 2002). The VAN7/GN/EMB30 gene encodes a guanine nucleotide exchange factor protein involved in the polarized localization of PIN1 (Steinmann et al., 1999; Koizumi et al., 2000).
Still, the characterization of other non-auxin-related leaf venation pattern mutants has implicated other hormones/signaling pathways in vascular patterning. For example, the CVP1 gene encodes STEROL METHYLTRANSFERASE-2, an enzyme in the sterol biosynthetic pathway, suggesting that sterols may play a specific role in vascular patterning or a general role in membrane organization that is essential for the axialization of procambial cells (Carland et al., 2002). A related gene, ORC, encodes STEROL METHYLTRANSFERASE-1, which is required for the correct subcellular localization of the putative auxin efflux carriers PIN1 and PIN3 (Willemsen et al., 2003). The VEP1/AWI31 gene encodes a polypeptide similar to animal proteins that contain death domains and are involved in apoptosis (Jun et al., 2002). The PLS gene encodes a short polypeptide with no significant homology with known proteins and is required for correct auxin-cytokinin homeostasis (Casson et al., 2002).
Here, we identified a recessive Arabidopsis mutant, thickvein (tkv), which develops thicker veins in leaves and in inflorescence stems. The increased vein thickness is attributable to an increased number of both xylem and phloem cells. Mutant plants also have smaller adult leaves and shorter inflorescence stems. The reduction in organ size and height is accompanied by an increase in organ vascularization, which appears to be attributable to an increase in the recruitment of cells into veins. Moreover, the tkv mutant exhibits reduced auxin transport in the inflorescence stem, reinforcing the notion that auxin transport is responsible for vein definition. In the primary root, the veins appear morphologically normal, but root growth in the tkv mutant is hypersensitive to exogenous cytokinin. The tkv mutation was found to reside in the ACL5 gene, which encodes a putative spermine synthase and is expressed during vascular development in provascular cells (uncommitted meristematic cells with the potential to become vascular cells) and in procambial cells (meristematic precursors to vein cells).
RESULTS
tkv Mutant Exhibits Increased Vein Thickness and Organ Vascularization
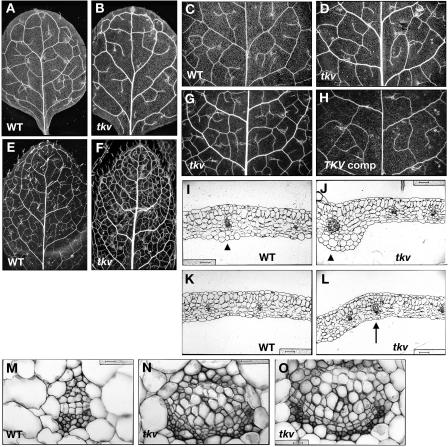
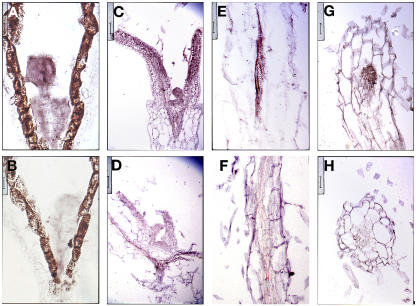
The tkv mutant was first identified from a mutant screen by its increased thickness of major veins in juvenile rosette leaves (Fig. 1, A and B). This phenotype was more conspicuous in adult rosette leaves, which were also smaller than those of wild type and had more veins per square area (Fig. 1, E and F). Transverse sections of tkv juvenile leaves confirmed that both the midvein (Fig. 1J) and secondary veins (Fig. 1L) were thicker than those of the wild type (Fig. 1, I and K, respectively), and indicated that the increased vein thickness was attributable to an increased number of both xylem and phloem cells as well as an increased number of procambial cells in between (Fig. 1, N and O). Cell size for all the leaf cell types appears normal at least cross-sectionally (Fig. 1, J and L; data not shown). Since in normal leaf vascular development the number of procambial cells increases by longitudinal divisions in the procambial cells themselves and by addition of cells from surrounding regions (Esau, 1965), it is very likely that the increase in vascular cell numbers found in the tkv leaf veins is due to an increase in outside cell recruitment and/or cell divisions within the procambium.
Figure 1.
Increased vein thickness and vascularization in tkv leaves. A to H, Cleared leaves of wild type (A, C, and E), tkv (B, D, F, and G), and fully restored tkv containing wild-type copy of the ACL5 transgene (H) were viewed under dark-field illumination to visualize the venation pattern. A and B, First pair of juvenile leaves. C, D, G and H, Second pair of juvenile leaves. E and F, Adult leaves. All leaves are in same magnification, and leaves used for comparison are developmentally equivalent (have similar number of secondary veins that form prominent arches that begin and end at the midvein) and representative of numerous samples. I to O, Transverse sections through the midvein (I, J, and M–O) and lateral veins (K and L) of fully expanded juvenile leaves. I, K, and M, Wild type. J, L, N, and O, tkv. Arrowheads indicate the midvein, and arrow indicates a secondary vein. In wild type, aside from the midvein, higher-order veins are indistinguishable by vein thickness. Scale bars in I to L = 50 μm, and scale bars in M to O = 16 μm.
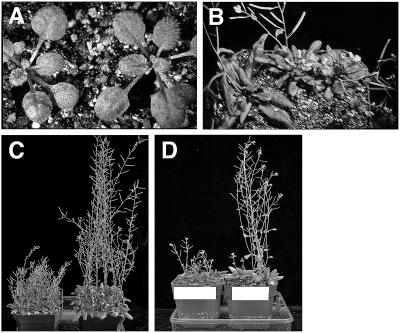
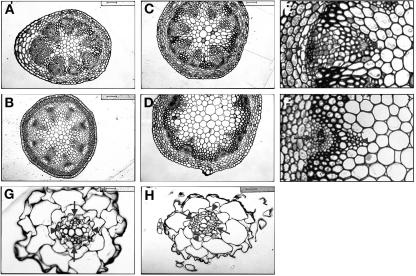
On a gross level, the tkv mutant resembled wild type up to the adult phase of vegetative development (Fig. 2A), where it exhibited a size reduction in its adult leaves (Fig. 2B). Since normal adult vegetative development involves an increase in the extent of leaf venation, which is reflected in overall leaf size, the size reduction and dense venation found in tkv adult leaves further substantiates the idea that cell recruitment into veins is increased. Inflorescence stems in the tkv mutant were similarly affected. There was severe defect in the length of stem internodes and a reduction in the number of stem nodes (Fig. 2, B and C). Furthermore, transverse sections of the most apical and basal parts of the inflorescence stem indicated a slight increase in xylem and phloem cell numbers and a large increase in the cambial-like cells between the phloem and xylem (Fig. 3E). This increase in vascular cell number is analogous to what was found in leaves, and appears to be partially at the expense of pith cell numbers. Primary root growth in the mutant was normal (data not shown), and transverse sections through the root elongation zone indicated that vascular cell numbers were also normal (Fig. 3H).
Figure 2.
Shortened inflorescence stems of tkv mutant. A, Two-week-old wild-type (left) and tkv (right) plants. B, Top view of 4-week-old wild-type (left) and tkv (two on right) plants. C, Fully grown tkv (left) and wild-type (right) plants. D, Fully grown tkv plants (left) and tkv plants containing the wild-type ACL5 transgene (right).
Figure 3.
Increased cambium in inflorescence stems of tkv mutant. A to F, Transverse sections through the apical end of the inflorescence stem (just below the youngest silique; A and B) and the basal end of the inflorescence stem (C, D, E, and F). A, C, E, tkv. B, D, F, Wild type. E and F are enlargements of a vascular bundle in C and D, respectively. Opaque cells inside of the phloem are cambial-like cells and possibly include xylem parenchyma cells. Arrowheads point to xylem, and arrows point to phloem. Scale bars in A, B, C, D, G, and H = 50 μm, and scale bars in E and F = 25 μm. G and H, Transverse sections through the primary root of week-old seedlings in the root elongation zone, where root hairs emerge. G, Wild type. H, tkv. Arrowheads point to xylem axes, and arrows point to phloem cells. Scale bars = 16 μm.
tkv Has Reduced Auxin Transport in the Inflorescence Stem
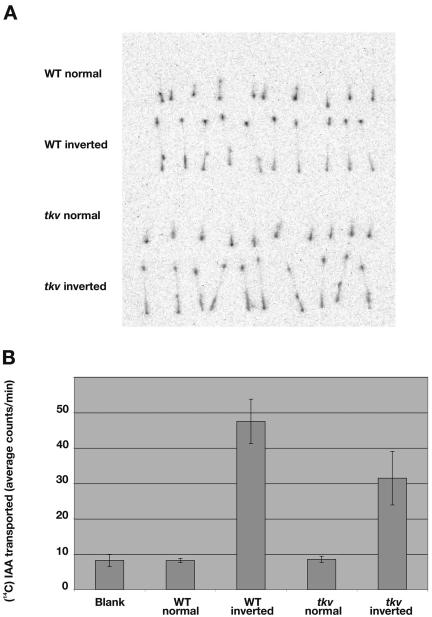
The increased vein thickness and vascularization in tkv leaves are reminiscent of the vascular phenotypes found in leaves treated with chemical inhibitors of polar auxin transport (Mattsson et al., 1999; Sieburth, 1999). To test if the cellular basis of the tkv vascular phenotype is due to a reduced transport of auxin, we measured the basipetal transport of 14C-IAA in excised inflorescence stems of both wild-type and tkv mutant plants. We found polar auxin transport in tkv stems to be 66.2% of that in wild type (Fig. 4B). Furthermore, the distribution of transported 14C-IAA in the tkv stem segment resembled that of wild type (Fig. 4A), indicating that shortened internodes did not interfere with auxin transport. Unlike wild-type plants treated with polar auxin transport inhibitors (Okada et al., 1991) and other characterized auxin transport mutants exhibiting female sterility and/or floral bud deficiencies, e.g. lop1 (22.7% of wild-type auxin transport; Carland and McHale, 1996), weak alleles of mp (31.6%; Przemeck et al., 1996), pin1 (50%; Okada et al., 1991; Bennett et al., 1995), and pid mutant (74%; Bennett et al., 1995), tkv plants are fully fertile and produce normal flowers. The production of fertile, fully formed flowers suggests that IAA content and auxin response in the tkv inflorescence apex are sufficient for organ formation, since exogenous application of IAA on pin1 and pid inflorescence apices can induce organ formation (Reinhardt et al., 2000, 2003).
Figure 4.
Reduced polar auxin transport in inflorescence stems of tkv mutant. A, The basipetal (inverted orientation) and acropetal (normal orientation) transport of 14C-IAA in excised inflorescence stem segments were visualized using the Fujix image analyzer. B, Average cpm of 14C-IAA transported to the distal end of the stem segments were calculated from 80 samples of each genotype from four separate experiments. Error bars indicate sd.
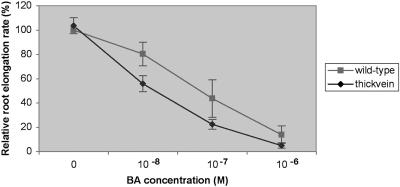
This auxin-related result encouraged us to test the tkv mutant for a number of hormonal responses by measuring primary root growth in media containing exogenous amounts of auxin (IAA and 2,4-dichlorophenoxyacetic acid), cytokinin (6-benzylaminopurine), epi-brassinolide, gibberellin, or abscisic acid. Only exogenous cytokinin produced an altered hormone response in the tkv mutant, severely reducing root growth relative to that of wild type (Fig. 5). This cytokinin hypersensitivity is intriguing because of the normal vascular morphology of the tkv root.
Figure 5.
Root growth in tkv mutant is hypersensitive to exogenous cytokinin. Relative root elongation rates of wild-type (squares) and tkv mutant (diamonds) 10-d-old seedlings. Mean values for 100% root elongation were determined on Murashige and Skoog medium containing no 6-benzylaminopurine (BA). Error bars indicate sd.
tkv Mutation Resides in ACL5 Gene, Which Encodes a Spermine Synthase
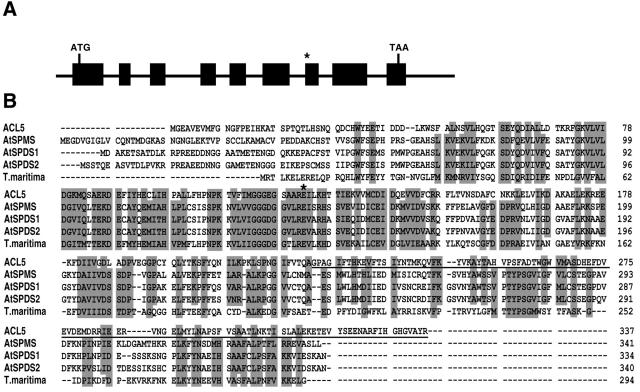
A map-based cloning strategy was used to isolate the gene. Genetic analysis of F2 progeny indicated that the tkv mutant phenotype segregated as a single recessive locus. The tkv mutation was mapped initially between simple sequence length polymorphic markers nga106 and nga139 on the top of chromosome 5, and then finely mapped to a region spanned by bacterial artificial chromosomes F7K24 and T20D1 that contained nine predicted genes. One of the genes, ACAULIS5 (ACL5), encodes a spermine synthase involved in polyamine biosynthesis, and null mutations in the gene resulted in a severe defect in the elongation of the stem internode (Hanzawa et al., 2000). Since the tkv mutant has a similar internodal elongation defect, the ACL5 gene in the mutant was selected for sequencing, and was found to contain a base pair deletion at the start of exon 7 (Fig. 6A). The resulting frameshift in the mRNA would encode a premature stop codon after 19 amino acids (Fig. 6B). The ACL5 gene consists of nine exons that span a 1.9-kb genomic region (Fig. 6A). Introduction of the ACL5 promoter-driven wild-type ACL5 transgene into the tkv mutant fully complemented the mutant phenotype with respect to both the thick veins and the short internodes for five independent transgenic lines (Figs. 1, C and F, and 2D, respectively).
Figure 6.
ACL5 encodes a spermine synthase involved in polyamine biosynthesis. A, Genomic structure of the ACL5 gene. Exons are indicated by black boxes. Asterisk indicates the missing nucleotide in tkv allele. B, Alignment of the deduced amino acid sequences of ACL5, AtSPDS1, AtSPDS2, AtSPMS, and Thermotoga maritima spermidine synthase (for which a crystal structure was solved [Korolev et al., 2002]). Amino acids affected by the tkv mutation are underlined, and the Glu residue mutated in acl5-1 allele is marked with an asterisk.
ACL5 Expression Is Provascular/Procambial-Specific
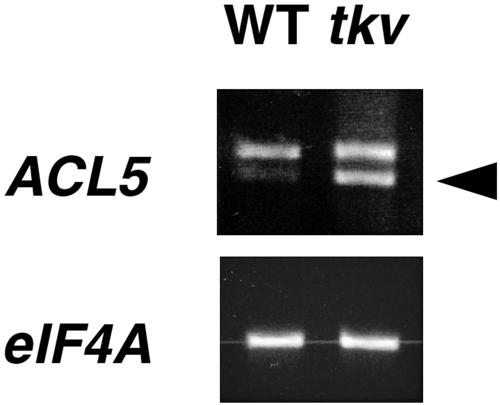
Hanzawa et al. (2000) had shown that ACL5 expression is up-regulated in the acl5-1 mutant (lesion shown in Fig. 6B), suggesting that ACL5 expression may be under negative-feedback control. The expression of the ACL5 gene was examined in wild-type and tkv plants by reverse transcription (RT)-PCR and was found to be similarly up-regulated in tkv mutant plants (Fig. 7).
Figure 7.
ACL5 mRNA expression analyses. Total RNA from wild-type and tkv inflorescences were reverse transcribed and PCR amplified using primers to the 3′ end of ACL5 cDNA. RT-PCR products were run on an ethidium bromide-stained gel. PCR-amplified eIF4A was the loading control. Arrowhead marks spliced transcript.
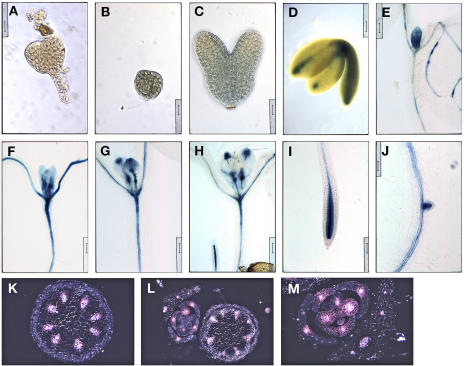
The expression pattern of ACL5 was visualized by RNA in situ hybridizations as well as histochemical staining of plants containing a transgenic copy of the ACL5 promoter driving the β-glucuronidase (GUS) reporter gene. ACL5 expression was found throughout early globular-staged embryos and persisted throughout embryogenesis until bent cotyledon-staged embryos where the expression domain was delimited to procambial cells (Figs. 8A and 9, B–D). The procambial-specific expression pattern continues during primary root development (Figs. 8, E and G, 9I) and early leaf development after an initially broad expression domain in leaf primordia (Figs. 8C and 9, E–H). This expression pattern holds true during inflorescence development. Transverse sections through GUS-stained inflorescence stems revealed GUS expression throughout the vascular bundle of the stem as well as the procambial strands of axillary buds (Fig. 9, K–M). Recently, an independent group performed digital in situ hybridization and conventional RNA in situ hybridization of ACL5 on primary roots and found its expression to be specific to and abundant in procambial cells (Birnbaum et al., 2003).
Figure 8.
ACL5 expression is associated with provascular/procambial cells. A to H, Sections were hybridized with sense (B, D, F, H) and antisense (A, C, E, G) DIG-labeled ACL5 RNA probe. A and B, Longitudinal sections through globular-staged embryos. Scale bars = 12.5 μm. C and D, Longitudinal sections through leaf primordia of week-old seedlings. Scale bars = 50 μm. E and F, Longitudinal sections through root of week-old seedlings. Scale bars = 25 μm. Note the DIG-labeled vasculature. G and H, Transverse sections through root of week-old seedlings. Scale bars = 25 μm.
Figure 9.
ACL5 promoter activity is associated with provascular/procambial cells. A, Nontransgenic globular-staged embryo was stained for GUS activity. Note the absence of GUS stain. Scale bar = 16 μm. B to M, Wild-type plants containing a transgenic copy of the ACL5 promoter-driven GUS reporter gene were stained for GUS activity and viewed under bright-field illumination unless noted otherwise. B, Globular-staged embryo. Scale bar = 16 μm. C, Torpedo-staged embryo. Staining is weak and diffuse. Scale bar = 16 μm. D, Bent cotyledon-staged embryo. Scale bar = 62.5 μm. E to J, Cleared GUS-stained week-old seedlings were arranged in developmental order. Note that GUS stain gradually localizes to and around PC cells. Scale bars = 100 μm. K to M, Transverse sections through inflorescence stems and axillary buds were viewed under dark-field illumination to visualize GUS staining, which appears pink. Note that GUS staining is localized to developing vasculature. Scale bars = 50, 100, and 50 μm, respectively.
DISCUSSION
TKV Is Involved in Vein Definition
In plants, vein formation in the leaf is closely linked to cell proliferation in the leaf since much of the minor venation is formed between older veins during the intercalary, extension growth of the leaf blade (Esau, 1965). Many Arabidopsis leaf venation mutants with reduced venation have also reduced leaf size. They include mutants lop1 (Carland and McHale, 1996), cvp1 (Carland et al., 1999), scf (Deyholos et al., 2000), van7 (Koizumi et al., 2000), mp (Przemeck et al., 1996), axr6 (Hobbie et al., 2000), and vep1 (Jun et al., 2002). By contrast, the tkv mutant has more venation per leaf surface area in adult leaves and more vascular cell files per vein despite the smaller adult leaves and shorter internodes. The first four rosette leaves are considered juvenile leaves and were indistinguishable in size between tkv mutant and wild type. Subsequent rosette leaves are considered adult leaves and arise during the switching of the shoot meristem from vegetative phase to reproductive phase. Although these leaves were smaller sized in the tkv mutant, leaf cell size appeared normal at least cross-sectionally. Similar observations were made with the acl5 mutant, whose reduced adult leaf size was attributed mainly to reduced cell elongation in the petioles after flowering (Hanzawa et al., 1997). The increased venation in the tkv mutant appears to be at the expense of a cell population normally recruited to mesophyll cell development. Alternatively, it may also arise from increased cell divisions within the procambium. If that is the case, these increased divisions have a suppressive effect on the proliferation of surrounding cells, for instance, by siphoning off resources (hormones, nutrients, etc.) necessary for proliferation. Whether the tkv mutation solely affects cell recruitment into veins, or the balance between vein formation and cell proliferation in the surrounding cells, or both, it does appear that TKV is involved in a mechanism that defines the boundaries between veins and nonvein regions. This mechanism may be analogous to the lateral inhibition mechanism involving Notch signaling to establish the correct vein thickness in Drosophila wings (de Celis et al., 1997). We propose that vein definition is achieved through the polar auxin transport with its built-in lateral inhibitory effect on auxin transport capacities of surrounding cells and inductive effect on procambium formation and differentiation on transporting cells (see “canalization of signal flow hypothesis”; Sachs, 1981), and that the tkv mutant documents this link.
Finally, because auxin transport is hypothesized to be involved in the induction of procambial cells with their characteristic narrow elongate shape, there may be a causal connection between rate of auxin transport and extent of cell elongation along the longitudinal axis. If that is the case, then the reduced inflorescence stems in the tkv mutant can be attributed to a reduced rate of auxin transport through procambial cells and xylem parenchyma cells.
TKV Is Involved in Polar Auxin Transport
Like the tkv mutant, four other Arabidopsis mutants have been reported to display reduced auxin transport in stem segments (in order of increasing severity: pid, pin1, mp, and lop1; Okada et al., 1991; Bennett et al., 1995; Carland and McHale, 1996; Przemeck et al., 1996). However, unlike these mutants, the tkv mutant produced fully fertile and well-formed flowers despite the fact that tkv mutant stems displayed auxin transport capacities between those of pid and pin1 mutants. This indicates that IAA content and auxin response in the tkv inflorescence apex are sufficient for organ formation, since exogenous application of IAA on pin1 and pid inflorescence apices can induce organ formation (Reinhardt et al., 2000, 2003).
Similarly in the tkv root, auxin response and vascular formation appeared normal, which would suggest that TKV gene activity is dispensable in roots except for the fact that the roots exhibited cytokinin hypersensitivity. In addition to the well-documented role of polar auxin transport in vascular induction and procambium differentiation, cytokinin also has been implicated in vascular development, functioning in a complex and sometimes synergistic relationship with auxin (for review, see Lyndon, 1990; Klee and Lanahan, 1995). TKV's provascular gene expression pattern localizes this cytokinin hypersensitivity to the vasculature of the root. Because of the nature of the TKV gene product, this defect is likely to be secondary. Furthermore, the otherwise normal morphology suggests the existence in the root of additional gene functions overlapping with that of TKV.
Unlike lop1 and mp mutants, which have reduced vascular systems (Carland and McHale, 1996; Przemeck et al., 1996), the tkv mutant has increased formation of xylem, phloem, and procambium, as well as increased vascularization in leaves, all of which correspond to vascular features induced by auxin transport inhibitors such as 1-N-naphthylphtalamic acid (NPA; Mattsson et al., 1999; Sieburth, 1999). The reduced auxin transport found in mp and lop1 mutant stem segments is likely to result from vascular discontinuity and/or reduced number of developing vascular strands, rather than from impaired auxin transport capacities of individual cells. In contrast to lop1, mp, pin1, and tkv mutants, the pid mutant (which exhibited the least reduction in auxin transport and only in stems that had ceased elongating) exhibited very mild vascular defects that appeared only in flowers, suggesting that the regulation of vascular development and/or auxin transport is not the primary task of PID (Christensen et al., 2000; Benjamins et al., 2001).
Despite the superficial similarity between pid mutant inflorescences to those of pin1, the vascular defects found in pin1 mutant leaves are similar to but weaker than those found in the leaves of NPA-treated plants (Mattsson et al., 1999). Furthermore, the PIN1 gene encodes a putative membrane protein that is located at the basal end of auxin transport-competent cells, indicating that the PIN1 gene product is part of the general auxin efflux mechanism that is inhibited by the auxin transport inhibitor NPA (Lomax et al., 1995; Gälweiler et al., 1998). However, the vascular features found in tkv mutant leaves correspond more so in detail and in severity to those found in NPA-treated plants. Furthermore, this correspondence extends to inflorescence stems, whereas pin1 mutant stems have only increased xylem formation (Gälweiler et al., 1998). Because PIN1 is a member of a large gene family, it is likely that there are related gene products with partially overlapping functions that can complement the loss of pin1 function in the root and in the stem and thereby account for the imperfect correspondence between tkv and pin1 mutant stems and between NPA-treated leaves and pin1 mutant leaves, respectively. All of this suggests that TKV's role in polar auxin transport may be in the general efflux mechanism that is sensitive to NPA inhibition.
Role of Polyamines in Polar Auxin Transport
Numerous studies have implicated polar auxin transport in the induction of vascular tissue and in the definition of vein positions. The increased thickness of veins and vascularization of leaves found in the tkv mutant can be phenocopied in wild type by the chemical treatment of auxin transport inhibitors (Mattsson et al., 1999; Sieburth, 1999). Similarly, the mutant also exhibits a reduction of auxin transport through its short inflorescence stem (with its thick vascular bundles). The presence of fertile and normal-looking flowers in the mutant suggests that auxin response is not generally affected in the shoot apex and that the auxin transport defect is specific and may be primarily responsible for the vascular phenotypes. The nature of the TKV gene product suggests that the relationship between polar auxin transport and vein definition may involve spermine. The tkv mutation resides in the ACL5 gene, which was previously found to encode a putative spermine synthase. Although the in vivo function of ACL5 is still unclear, ACL5 expression is auxin inducible (Hanzawa et al., 2000) and provascular/procambial specific (this work), further implicating ACL5 enzyme activity in the polar transport of auxin through veins.
Auxin is not the only growth factor that is actively transported. Polyamines, which are small, evolutionarily ancient polycationic amines that are ubiquitously present in bacteria, animals, and plants, are necessary for normal cell growth and development (Cohen, 1998) and have been implicated in many cellular processes such as cell proliferation, transcription, translation, and chromatin structure (Marton and Morris, 1987; Cohen, 1998; Pollard et al., 1999). In animal systems, polyamines have been shown to move between cells via specific transporters in an energy-dependent manner (Seiler and Dezeure, 1990). In Arabidopsis, biochemical and physiological data suggest that polyamines can be transported through xylem and phloem (Caffaro et al., 1993; Antognoni et al., 1998). Because the knockout of ACL5 function had no effect on the endogenous levels of free and conjugated polyamines in Arabidopsis (Imai et al., 2004), it remains to be seen whether the active transport (and not the levels) of polyamines (more specifically, spermine) is involved in vein definition and/or auxin transport.
Spermine is one of three plant polyamines (spermidine and putrescine are the other two), and in plants, polyamines, chiefly spermidine, were found to modulate the activity of KAT1-like inward-rectifying K+ channels and thus to regulate the closure of guard cells during stress (Liu et al., 2000). Still, the specifics regarding the developmental function of polyamines remain a mystery. Most plants form putrescine directly from Orn and indirectly from Arg. Spermidine is formed from putrescine and spermine from spermidine by successive addition of aminopropyl groups by spermidine and spermine synthases, respectively. Arabidopsis contains two spermidine synthase genes (AtSPDS1 and AtSPDS2) and two spermine synthase genes (ACL5 and AtSPMS), and all are likely to encode cytoplasmic enzymes (Hanzawa et al., 2002). Of the four, ACL5 is the most dissimilar (Hanzawa et al., 2002), suggesting its unique genetic origin. Recently, a polyamine metabolon (a complex consisting of successive enzymes) has been isolated in Arabidopsis, and it contained AtSPDS1, AtSPDS2, and AtSPMS, but not ACL5 (Panicot et al., 2002). All three enzymes found in the metabolon directly interacted with one another in yeast and in vitro, but no interaction was found in a direct two-hybrid test with ACL5 (Panicot et al., 2002), suggesting that ACL5 may have a divergent function or role in development. Although ACL5 has been shown to function as a spermine synthase in Escherichia coli (Hanzawa et al., 2000), the knockout of ACL5 function had no effect on the endogenous levels of free and conjugated polyamines in Arabidopsis (Imai et al., 2004), suggesting that ACL5 may have a very specific or altogether different in vivo function.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ectoypes Columbia (Col-O) and Landsberg carrying the erecta mutation (Ler) were used for comparison with mutant plants and for crosses. Seeds were grown under constant white light (approximately 300 μE m−2 s−1) either on 0.75% agar media consisting of Murashige and Skoog (1962) basal salts, Haughn and Somerville (1986) nutrient solution, 0.5 g/L MES, and 10 g/L Suc, or on soil (3:1 mix of Metro-Mix 200 to vermiculite; Scotts, Marysville, OH).
Mutant Isolation
A visual screen for venation pattern mutants was performed on diepoxybutane-mutagenized M2 Arabidopsis (ecotype Col-O) seeds. Briefly, hydrated seeds were incubated in 18 mm diepoxybutane (Sigma, St. Louis) for 4 h and washed extensively before planting (M1 generation). M2 seeds, the progeny of self-fertilized M1 plants, were pooled from every 10 M1 lines and screened about 2 weeks after germination. One to two rosette leaves from each M2 plant were fixed in 3:1 ethanol to acetic acid, dehydrated in an ethanol series, cleared in Hemo-De (Fisher-Scientific, Loughborough, Leicestershire, UK), mounted on slides with 2:1 Permount (Fisher-Scientific) to xylene, and viewed under dark-field optics for alterations in the venation pattern. Putative venation pattern mutants were confirmed by a secondary screen and backcrossed twice to wild-type Col-O plants for further phenotypic characterization.
Histochemical Localization of GUS Activity and Histological Analyses
Plant tissues were stained for GUS activity overnight at 37°C in GUS buffer, 20% methanol, and 0.5 mg/mL X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronidase) as described by Malamy and Benfey (1997). For observation of whole mounts, both stained and unstained tissue were fixed, cleared, and mounted on slides as described above. Specimens were examined with a Zeiss Axiophot microscope (Oberkochen, Germany). Tissues used for RNA in situ hybridizations were fixed overnight at 4°C in formaldehyde-acetic acid (3.7% formaldehyde, 50% ethanol, and 5% acetic acid). For histological analysis, fixed dehydrated specimens were embedded in Spurr's media (EM Sciences, Ft. Washington, PA) or in Paraplast Plus (Fisher-Scientific). Plastic sections (2 μm) were cut on a Sorvall MT2-B ultramicrotome (Newtown, CT) with glass knives and were stained briefly with 1% toluidine blue. Paraffin sections (8 μm) were cut on a model 820 microtome (Arthur H. Thomas, Philadelphia).
Polar Auxin Transport Assay
Polar auxin transport in inflorescence axes was measured using a modification of the method described by Okada et al. (1991). Two-centimeter-long stem segments from inflorescence axes of wild-type and tkv mutant plants between 1 and 2 weeks after bolting were taken from near the rosette to the first flower-bearing node. Segments were placed in either normal or inverted orientation in 1.75-mL tubes containing 30 μL of half-strength Murashige and Skoog (1962) basal medium containing 1.45 μm 14C-labeled IAA (Sigma), incubated in closed tubes at room temperature for 19 h, washed three times in IAA-free medium, arrayed between sheets of plastic wrapping, and exposed to a phosphorimaging plate for 12 h. Distribution of labeled IAA in the segments were visualized by a Fujix BAS2000 image analyzer (Fuji Photo Film, Tokyo). After exposure, 5-mm-long segments were cut from the upper end of the stem segments and incubated overnight in Opti-fluor scintillation fluid (Packard Bioscience, PerkinElmer, Shelton, CT). Radioactivity released by the upper stem segments were counted by a Beckman LS6000IC scintillation counter (Beckman Instruments, Munich).
Genetic Mapping and Plant Transformation Vector Construction
Plants homozygous for the tkv mutation were crossed to wild-type Ler plants to generate an F2 mapping population. DNA from 20 mutant plants were used with simple sequence length polymorphic markers (Bell and Ecker, 1993; Ponce et al., 1998) to map the chromosomal location of the tkv mutation, and DNA from approximately 500 mutant plants, respectively, were used with Cereon's insertion/deletion and single-nucleotide polymorphism markers (Jander et al., 2002) to map tkv to a region spanned by a single bacterial artificial chromosome.
To generate the complementation construct, 4.24 kb of ACL5 genomic sequence, which includes 2.10 kb of upstream sequence (minus the stop codon), was PCR-amplified and subcloned into HindIII/StuI sites of the PJIM19smGFP binary vector upstream of the smGFP gene. To generate the reporter construct, 2.13 kb of upstream sequence was PCR-amplified and subcloned into HindIII/BamH1 sites of the PBI101 binary vector upstream of the GUS reporter gene. Both contructs were sequenced for errors and introduced into tkv mutant and wild-type plants, respectively, via Agrobacterium-mediated floral dip method (Clough and Bent, 1998), and transformants were selected on agar media containing 50 mg/mL kanamycin.
Total RNA Isolation and RT-PCR
Total RNA was isolated from inflorescences including open flowers and 2-cm-long stems near the apex with TRIzol (Gibco-BRL, Gaithersburg, MD) according to manufacturer's instructions. Two micrograms of total RNA was reverse transcribed with 200 units of SuperscriptII (Invitrogen, Carlsbad, CA). The resulting cDNA:RNA hybrids were treated with 10 units of DNase I (Roche, Indianapolis) for 30 min at 37°C, purified on Qiaquick PCR column (Qiagen USA, Valencia, CA), and used as template to PCR-amplify ACL5 (40 cycles) and eIF4A (39 cycles). PCR conditions are as follows: 94°C for 15 s, 52°C for 15 s, and 72°C for 15 s. PCR products (350–400 bp) were electrophoresed in 1.5% agarose gel and visualized with a Gel Doc 2000 system (Bio-Rad, Hercules, CA).
RNA in Situ Hybridization
ACL5 cDNA (nucleotides 996–1,106 relative to ATG) was PCR amplified using primers containing engineered T3 and T7 RNA polymerase promoter sites and was used as template to generate dioxigenin (DIG)-labeled sense and antisense RNA probes. In situ hybridizations and labeling reactions were carried out as described by Jackson (1991) with some modifications, which were described by Clay and Nelson (2002).
Acknowledgments
Sequencing analysis was performed by the HHMI Biopolymer/Keck Foundation Biotechnology Resource Lab (Yale University). Thanks to Dr. James A. Sullivan for his generous gift of the PJIM19 vectors.
This work was supported by the National Science Foundation (grant nos. IBN–0110730 and IBN–0416731).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.055756.
References
- Aloni R (2001) Foliar and axial aspects of vascular differentiation: hypotheses and evidence. J Plant Growth Regul 20: 22–34 [Google Scholar]
- Antognoni F, Fornale S, Grimmer C, Komor E, Bagni N (1998) Long-distance translocation of polyamines in phloem and xylem of Ricinus communis L. plants. Planta 204: 520–527 [Google Scholar]
- Avsian-Kretchmer O, Cheng J-C, Chen L, Moctezuma E, Sung R (2002) Indole acetic acid distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny. Plant Physiol 130: 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR (1993) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137–144 [DOI] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R (2001) The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128: 4057–4067 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR (1995) Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J 8: 505–520 [Google Scholar]
- Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN (2003) A gene expression map of the Arabidopsis root. Science 302: 1956–1960 [DOI] [PubMed] [Google Scholar]
- Caffaro S, Scaramagli S, Antognoni F, Bagni N (1993) Polyamine content and translocation in soybean plants. J Plant Physiol 141: 563–568 [Google Scholar]
- Carland FM, Fujioka S, Takatsuto S, Yoshida S, Nelson T (2002) The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell 14: 2045–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, Berg BL, Fitzgerald JN, Jinamornphongs S, Nelson T, Keith B (1999) Genetic regulation of vascular tissue patterning in Arabidopsis. Plant Cell 11: 2123–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland FM, McHale NA (1996) LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development 122: 1811–1819 [DOI] [PubMed] [Google Scholar]
- Casson SA, Chilley PM, Evans IM, Souter MA, Lindsey K (2002) The Polaris gene of Arabidopsis encodes a predicted peptide required for correct root growth and leaf vascular patterning. Plant Cell 14: 1705–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D (2000) Regulation of auxin response by the protein kinase PINOID. Cell 100: 469–478 [DOI] [PubMed] [Google Scholar]
- Clay NK, Nelson T (2002) VH1, a provascular cell-specific receptor kinase that influences leaf cell patterns in Arabidopsis. Plant Cell 14: 2707–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cohen S (1998) A Guide to the Polyamines. Oxford University Press, Oxford
- de Celis JF, Bray S, Garcia-Bellido A (1997) Notch signalling regulates veinlet expression and established boundaries between veins and interveins in the Drosophila wing. Development 124: 1919–1928 [DOI] [PubMed] [Google Scholar]
- Deyholos MK, Cordner G, Beebe D, Sieburth LE (2000) The SCARFACE gene is required for cotyledon and leaf vein patterning. Development 127: 3205–3213 [DOI] [PubMed] [Google Scholar]
- Esau K (1965) Vascular Differentiation in Plants. Holt, Rinehart and Winston, New York
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Goto N, Katoh N, Kranz AR (1991) Morphogenesis of floral organs in Arabidopsis: predominant carpel formation of the pin-formed mutant. Jpn J Genet 66: 551–567 [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Hamann T, Benkova E, Baurle I, Kientz M, Jurgens G (2002) The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev 16: 1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jurgens G (1999) The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development 126: 1387–1395 [DOI] [PubMed] [Google Scholar]
- Hanzawa Y, Imai A, Michael AJ, Komeda Y, Takahashi T (2002) Characterization of the spermidine synthase-related gene family in Arabidopsis thaliana. FEBS Lett 527: 176–180 [DOI] [PubMed] [Google Scholar]
- Hanzawa Y, Takahashi T, Komeda Y (1997) ACL5: an Arabidopsis gene required for internodal elongation after flowering. Plant J 12: 863–874 [DOI] [PubMed] [Google Scholar]
- Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, Coupland G, Komeda Y (2000) ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J 19: 4248–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Yang LN, Bandyopadhyay A, Estelle M (2000) The axr6 mutants of Arabidopsis thaliana: a gene involved in auxin response and early development. Development 127: 23–32 [DOI] [PubMed] [Google Scholar]
- Hobbie LJ, Sherman S, Adelphi Arabidopsis Mapping Team, Hellmann H, Estelle M (2002) Molecular characterization of the AUXIN-RESISTANT6 gene. In 13th International Conference on Arabidopsis Research, June 28–July 2, 2002, Seville, Spain
- Imai A, Akiyama T, Kato T, Sato S, Tabata S, Yamamoto KT, Takahashi T (2004) Spermine is not essential for survival of Arabidopsis. FEBS Lett 556: 148–152 [DOI] [PubMed] [Google Scholar]
- Jackson D (1991) In-situ hybridisation in plants. In DJ Bowles, JSJ Gurr, M McPherson, eds, Molecular Plant Pathology: A Practical Approach. Oxford University Press, Oxford, pp 163–174
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL (2002) Arabidopsis map-based cloning in the post-genome era. Plant Physiol 129: 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JH, Ha CM, Nam HG (2002) Involvement of the VEP1 gene in vascular strand development in Arabidopsis thaliana. Plant Cell Physiol 43: 323–330 [DOI] [PubMed] [Google Scholar]
- Klee HJ, Lanahan MB (1995) Transgenic plants in hormone biology. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 340–353
- Koizumi K, Sugiyama M, Fukuda H (2000) A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network: calling the auxin signal flow canalization hypothesis into question. Development 127: 3197–3204 [DOI] [PubMed] [Google Scholar]
- Korolev S, Ikeguchi Y, Skarina T, Beasley S, Arrowsmith C, Edwards A, Joachimiak A, Pegg AE, Savchenko A (2002) The crystal structure of spermidine synthase with a multi-substrate adduct inhibitor. Nat Struct Biol 9: 27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Fu H, Bei Q, Luan S (2000) Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol 124: 1315–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH (1995) Auxin transport. In PJ Davies, ed, Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 509–530
- Lyndon RF (1990) Plant Development: The Cellular Basis. Unwin Hyman, Boston, pp 135–165
- Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Marton LJ, Morris DR (1987) Inhibition of polyamine biosynthesis. In PP McCann, AE Pegg, A Sjoerdsma, eds, Biological Significance and Basis for New Therapies. Academic Press, New York, pp 79–105
- Mattsson J, Sung ZR, Berleth T (1999) Responses of plant vascular systems to auxin transport inhibition. Development 126: 2979–2991 [DOI] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) Atpin2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17: 6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nelson T, Dengler N (1997) Leaf vascular pattern formation. Plant Cell 9: 1121–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueada J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicot M, Minguet EG, Ferrando A, Alcázar R, Blázquez MA, Carbonell J, Altabella T, Koncz C, Tiburcio AF (2002) A polyamine metabolon involving aminopropyl transferase complexes in Arabidopsis. Plant Cell 14: 2539–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KJ, Samuels ML, Crowley KA, Hansen JC, Peterson CL (1999) Functional interactions between GCN and polyamines: a new role for core histone acetylation. EMBO J 18: 5622–5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce MR, Quesada V, Micol JL (1998) Rapid discrimination of sequences flanking and within T-DNA insertions in the Arabidopsis genome. Plant J 14: 497–501 [DOI] [PubMed] [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung RZ, Berleth T (1996) Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200: 229–237 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce E-R, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426: 255–260 [DOI] [PubMed] [Google Scholar]
- Sachs T (1981) The control of patterned differentiation of vascular tissues. Adv Bot Res 9: 151–262 [Google Scholar]
- Seiler N, Dezeure F (1990) Polyamine transport in mammalian cells. Int J Biochem 22: 211–218 [DOI] [PubMed] [Google Scholar]
- Sieburth LE (1999) Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol 121: 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318 [DOI] [PubMed] [Google Scholar]
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B (2003) Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell 15: 612–625 [DOI] [PMC free article] [PubMed] [Google Scholar]