Abstract
Tumor metastasis is one of the most significant biological characteristics of malignant tumors. Advances in nanotechnology provide a new direction for tackling malignant tumors. Unlike previous studies that focused on the use of nanomaterials to eliminate tumors and avoid metastasis, this work focuses on summarizing the mechanisms by which some nanomaterials promote tumor metastasis in some cases. In this Review, we summarized recent research about various nanomaterials promoting tumor metastasis. The mechanisms of nanomaterials in this process have been highlighted, including the induction of epithelial–mesenchymal transition in tumor cells by nanomaterials, the interaction of nanomaterials with the vasculature, and the induction of inflammation by nanomaterials. Elucidating the mechanism of nanomaterials promoting tumor metastasis is beneficial to guide the development and application of safe and efficient nanomaterials, which can effectively reduce the incidence of tumor metastasis in the future.
Keywords: Nanomaterials, Tumor metastasis, EMT, Vasculature, Inflammation
1. Introduction
1.1. Mechanisms and Influencing Factors of Tumor Metastasis
Tumor metastasis refers to the process by which cancer cells spread from the primary site to the secondary site, which is one of the most significant biological characteristics of malignant tumors.1 Due to the systemic and drug resistance of disseminated tumor cells, despite the rapid development of tumor treatment methods, the treatment of metastatic tumors is still a major challenge, and more than 90% of malignant tumor patients die of tumor metastasis.2,3 Tumors can metastasize through a variety of routes, such as transcoelomic spread, lymphatic spread, hematogenous spread, and canalicular spread.1 We will elucidate the process of tumor metastasis by describing hematogenous metastasis. It is worth noting that tumor metastasis is not an efficient process, and most of the cancer cells entering the systemic circulation are eliminated.4 Whether a primary tumor with metastatic potential can metastasize depends on many influencing factors. (1) Cells within tumors are morphologically, biochemically, and genetically heterogeneous, and such differences may arise from genetic, epigenetic, positional, or temporal variations.5,6 The epigenetic variations refer to no change in the DNA sequence of a tumor cell but a heritable change in the function of a gene. For example, Gameiro and Struhl found that nutrient deprivation can affect the translation process of mRNA in breast cancer cells through mTOR and eIF2 α signaling, increasing cell migration and thus enhancing the malignant phenotype of breast cancer cells.7 (2) The metastasis cascade refers to the process of tumor metastasis in distant organs, which includes local invasion, intravasation, survival in circulation, arrest at a distant organ site, extravasation, micrometastasis formation, metastatic colonization, etc.3,8 Influencing factors that can play a role in the tumor metastasis cascade largely affect tumor metastasis. For example, there are two phenotypic plasticity processes that can profoundly affect tumor metastasis. The first is dynamic phenotypic changes that occur on metastasis-initiating cells (MICs).9 For example, dissociation of epithelial structures can induce phenotypic plasticity in MICs, thereby obtaining L1CAM-dependent growth reinitiation capacity, exhibiting the phenotype of regenerative progenitors, and driving distant tumor metastasis.10 Another phenotypic plasticity process associated with metastasis is the epithelial–mesenchymal transition (EMT). Some tumor cells can reduce adhesion and increase invasiveness through EMT, which helps tumor cells detach from the primary site, and then, this part of the tumor cells can grow at a secondary site through the reverse process of mesenchymal–epithelial transition (MET).11 The vasculature not only provides nutrients for tumors but also is a way for tumors to metastasize to distant organs. The permeability of the vasculature affects the invasion process of the tumor to a large extent. A highly permeable vasculature will lead to an increase of tumor cells entering the circulation and increase the possibility of distant metastasis.12 Inflammation can also produce a series of effects that promote tumor metastasis, mainly including inducing EMT of tumor cells,13 participating in the remodeling of tumor stroma,14 inducing angiogenesis, and increasing the colonization of tumor cells in target organs.15 In addition, there are many influencing factors that can affect tumor metastasis, as shown in Table 1, such as metastasis-promoting genes, senescent cells, polymorphic microorganisms, tumor-associated macrophages, etc.
Table 1. Some Factors That Promote Tumor Metastasis.
| factors | source/cause | cell lines | cancer type | mechanism | form | ref |
|---|---|---|---|---|---|---|
| Metastasis-promoting genes | Capn4 | 5-8F | Nasopharyngeal carcinoma | Activating the PI3K/AKT/Snail/claudin-11 axis | Invasion and metastasis | (37) |
| CNE-2 | ||||||
| Circular RNA hsa_circ_ 0023404 | RL95–2 | Endometrial cancer | Regulating miR-217/MARK1 axis | Invasion and metastasis | (38) | |
| KLE | ||||||
| TATA-binding protein-associated factor-1 | H460 | Nonsmall cell lung cancer | Activating TGFβ1 | Invasion and metastasis | (39) | |
| H1299 | ||||||
| A549 | ||||||
| H1975 | ||||||
| Nonmutational epigenetic reprogramming | FOXA1 knockdown | MCF-7 | Breast cancer | Inducing local demethylation | Metastasis | (40) |
| Nutrient deprivation | MCF10A | Breast cancer | Inhibiting mRNA translation through mTOR and eIF2α signaling | Metastasis | (7) | |
| NFATc1 promoter hypermethylation | PANC-1 | Pancreatic cancer | ALDH1A3 transcription | Metastasis | (41) | |
| MiaPaCa-2 | ||||||
| Senescent cells | Therapy-induced senescence | MSTO211 | Mesothelioma | EMT induction and chemoresistance | Invasion | (42) |
| HNCI-H28 | ||||||
| NCIH2052 | ||||||
| NCI-H2452 | ||||||
| pten-loss-induced cellular senescence; therapy-induced senescence | DU145 | Prostate cancer | Chemoresistance and immunosuppression | Metastasis | (43) | |
| Oncogene-induced senescence | HTH83 | Thyroid cancer | Tissue invasion support and anoikis resistance | Invasion and metastasis | (44) | |
| Polymorphic microorganism | Gram-negative bacteria | HepG2 | Hepatic cholangiocarcinoma | Suppressing antitumor immunity in the liver | Invasion | (45) |
| Hep3B | ||||||
| Huh7 | ||||||
| Streptococcus and Veillonella | A549 | Lung cancer | Activating the ERK and PI3K pathways in airway epithelial cells | Metastasis | (46) | |
| Hypoxia | Hypoxia inducible factor-1 | MHCC97L | Hepatocellular carcinoma | Immunosuppression | Invasion | (47) |
| Hepa1-6 | ||||||
| Hypoxia inducible factor-1 | HCT116 | Colon carcinoma | Basement membrane disruption | Invasion | (48) | |
| Tumor-associated macrophages | Colony-stimulating factor-1 | MCF-7 | Breast cancer | Chemoresistance and angiogenesis | Invasion | (49) |
| PDGF and Rho GTPases | EGI-1 | Cholangio carcinoma | Inducing angiogenesis and remodeling of the extracellular matrix | Migration | (50) | |
| TFK-1 | ||||||
| HuCCT-1 | ||||||
| C–C motif chemokine 18 | MCF-7 | Breast cancer | Enhancing the adhesion of cancer cells to extracellular matrix | Invasion | (51) | |
| BT-474 | ||||||
| MDA-MB-231 | ||||||
| Cancer-associated fibroblasts | C–C motif chemokine 2 | HEK293T | Oral squamous cell carcinoma | Enhancing endogenous reactive oxygen species production in cells | Migration | (52) |
| p53-deficient fibroblasts | PC3 | Prostate carcinoma | Increased expression of the chemokine SDF-1 | Metastasis | (53) | |
| POSTN | HeyA8 | Ovarian carcinoma | Activating the PI3K/Akt pathway and inducing the EMT | Migration and invasion | (54) | |
| HO8910 | ||||||
| A2780 | ||||||
| MRC-5 |
1.2. The Application of Nanomaterials in Tumor Therapy
Although, in addition to traditional treatment methods such as surgery, chemotherapy, and radiotherapy, a large number of new technologies have emerged such as immunotherapy, targeted therapy, gene therapy, etc., the survival rate of patients with advanced malignant tumors is still low.16 On the one hand, this is related to the high malignancy of the patient’s systemic metastatic tumors; on the other hand, it is related to the increased drug resistance of tumor cells and the accumulation of systemic toxicity of therapeutic drugs during long-term treatment. For example, chemotherapy drugs cannot kill tumor cells while simultaneously avoiding damage to other normal tissues, which greatly limits the use of current treatment methods.17 Nanotechnology, which has gradually emerged in the past two decades, has provided new ideas for tumor treatment. People began to try to combine various nanomaterials with traditional technologies and discovered the huge potential of nanomaterials in tumor prevention, detection, diagnosis, imaging, and treatment.18−20 Nanomaterials are any organic, inorganic, or organometallic materials that possess unique chemical, physical, and/or electrical properties that are typically observed at the nanometer scale.21 Nanomaterials have good biocompatibility and unique optical, thermodynamic, magnetic, and mechanical properties.22,23 First of all, nanomaterials can be directly applied to tumor therapy as drugs. Due to their own antigenicity and cytotoxicity, some nanomaterials can kill tumor cells with the help of the immune system or directly.24,25 New therapies based on the special properties of nanomaterials have also emerged, such as photothermal and photodynamic therapies as well as magnetic nanoparticle hyperthermia.26 Nanomaterials can also act as drug carriers or additives to enhance the effect of tumor treatment, such as liposomal drug delivery, radiotherapy sensitization, radiofrequency ablation (RFA), etc.27−29 The role of some nonmetallic nanomaterials in tumor treatment has also been gradually discovered, such as C60, carbon nanotubes (CNTs), graphene, chitosan nanoparticles, etc., which shows great potential in the field of tumor therapy.30 It is worthy of recognition that nanomaterials have developed very rapidly in the field of tumor treatment in recent years, providing many new ideas and methods for tumor treatment.31 However, most studies are only in the laboratory stage, and the clinical transformation is seriously insufficient. Currently, only liposomal drug delivery and magnetic iron oxide nanoparticle-based magnetic fluid hyperthermia are approved for clinical application in antitumor nanodrugs. The reason for this problem is, on the one hand, the oversimplification and overemphasis of the models of physiology and cancer biology on the passive delivery process of nanoparticles. On the other hand, due to the limitations of experimental techniques, most studies on nanomedicines are conducted on immune deficient mouse models bearing crossspecies tissue grafts, which cannot simulate the complex interactions of nanoparticles with host biology and immune function.28,32 Moreover, the physicochemical properties of nanomaterials determine whether they can be used for tumor therapy or to promote tumor metastasis. Li et al. found that titanium dioxide nanoparticles (Nano-TiO2) can inhibit the EMT of the A549 cell line by blocking the transforming growth factor-β (TGF-β) signaling pathway,33 while Leong et al. found that Nano-TiO2 can promote the EMT of the SW480 cell line by activating the TGF-β and Wnt signaling pathways.34 The size of Nano-TiO2 used by Li et al. was 50 nm, while the size of Nano-TiO2 used by Leong et al. was 21 nm. Therefore, the nanomaterials’ size may be a factor that determines their role. In addition, the surface modification of the nanomaterials also determines their role. For example, Zhou et al. used chitosan modified graphene oxide (GO) to inhibit the progression of melanoma,35 while Liu et al. found that unmodified GO can induce EMT in lung cancer cells and increase the invasive ability of them.36 Such completely different results further illustrate the complex mechanism of the interaction between nanomaterials and cells, tissues, and organisms, so it is difficult to promote the clinical transformation of nano-antitumor drugs only by relying on a single angle of research. In addition, the development of nanotechnology in recent years has greatly increased human exposure to nanomaterials, which further enhances the importance of nanotoxicology. Therefore, we sorted out the related studies on the promotion of tumor metastasis by nanomaterials in recent years and classified them from the perspective of EMT, vascular system interaction, and promotion of inflammation as shown in Figure 1. It aims to provide guidance for the formulation of future research plans and analysis of experimental results, promote the development and clinical transformation of efficient and safe nanomedicines, and reduce the incidence of tumor metastasis.
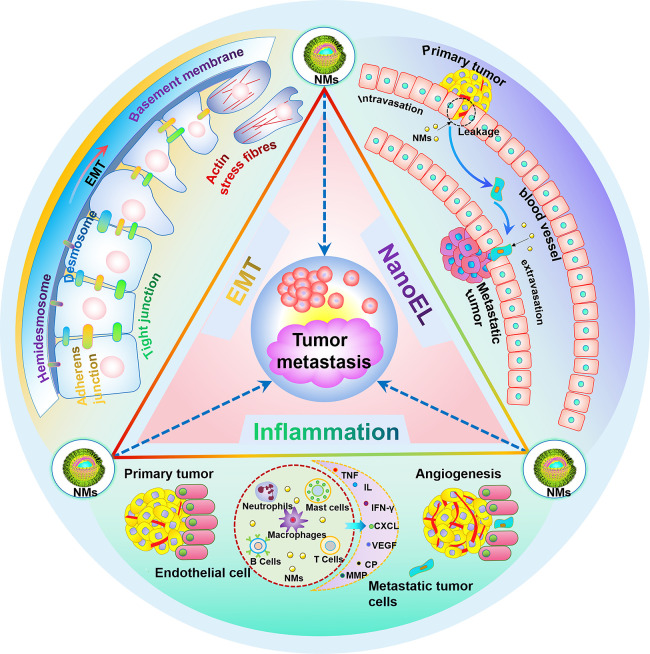
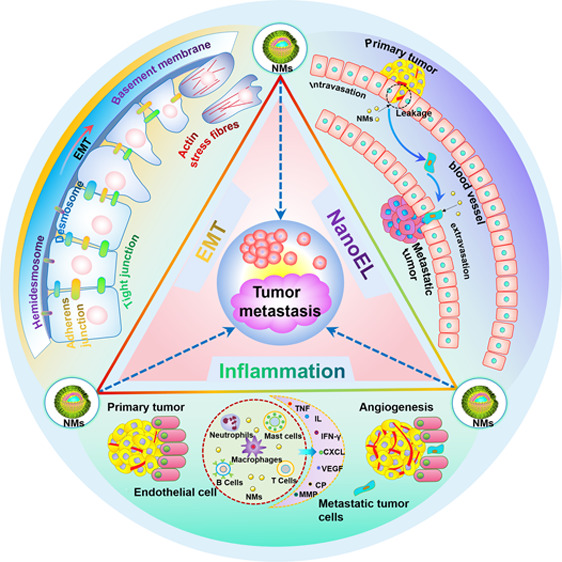
Figure 1.
Schematic illustration of the mechanism of nanomaterials promoting tumor metastasis, mainly including EMT, vasculature disruption, and inflammation.
2. Various Mechanisms Involved in Nanomaterials Promoting Tumor Metastasis
2.1. Inducing EMT in Tumor Cells by Nanomaterials
EMT is a dynamic, often reversible cellular process in which epithelial cells are downregulated with epithelial features and able to acquire a mesenchymal phenotype and behavior. This process is manifested through a loss of epithelial cell apical-basal polarity, regulation of the cytoskeleton, and reduced cell–cell adhesive properties. Ultimately epithelial cells can acquire mesenchymal properties individually or collectively and increase the motility and invasive capacity. Oncology-related EMT is the most popular field of EMT-related research in recent years.55 After EMT, the morphology of tumor cells changes from polygonal to spindle, and the adhesion between cells and cell polarity decrease significantly. The motility, dissemination ability, and resistance to apoptosis of tumor cells increase, and finally, the ability to invade and metastasize tumors is significantly improved. It is worth noting that not all metastases are necessarily related to EMT. Some cancer cells may be able to migrate locally without activating EMT. However, whether primary cancer cells can have distant metastases without activating EMT remains to be further studied. It can be considered that the malignant progression of all types of cancer is related to the activation of EMT. The current view is that the morphological and functional changes in tumor cells during EMT are mainly due to changes in gene expression, most of which are driven by EMT transcription factors (EMT-TFs). Typically, the activation of EMT is mainly directly or indirectly activated by one or several core EMT-TFs, including the zinc finger E-box binding homeobox factors ZEB1 and ZEB2, SNAIL (also known as SNAI1), SLUG (also known as SNAI2), and the basic helix–loop–helix factors TWIST1.56 In addition, studies have shown that the amounts of other EMT-TFs, numerous microRNAs, and long noncoding RNAs also play important roles in cellular EMT.55 The process of EMT-TFs regulating the expression of genes related to the epithelial state and mesenchymal state of tumor cells is an epigenetic process that does not depend on changes in the DNA sequence of tumor cells; therefore, the occurrence of EMT cannot be determined by sequencing cancer cell genomes. It has been argued that the occurrence of EMT can be judged by comprehensive analysis of changes in cellular properties and molecular markers, such as changes in cell morphology, activation of EMT-TFs, and changes in the expression of different proteins (such as E-cadherin, certain cytokeratin, N-cadherin, vimentin, fibronectin, and β1 and β3 integrin).57 When tumor cells are exposed to specific signals in the tumor microenvironment (TME), the corresponding signaling pathways are activated to generate EMT-TFs, inducing tumor cells to develop EMT. Common signaling pathways include the transforming growth factor-β (TGF-β) pathway, WNT signaling pathways, the NOTCH pathway, etc. These signaling pathways function not only alone but also crosstalk between each other, forming a complex signal network. For example, when the Notch signaling pathway of small intestinal stem cells is inhibited, the suppressed Wnt signaling pathway will be activated, resulting in misexpression of presecretory genes.58 As shown in Table 2, in recent years, more and more research has been conducted on the effect of nanomaterials on EMT of tumor cells. These studies focus on the inhibitory effect of nanomaterials on tumor EMT, and there are few studies on the promotion of tumor metastasis by nanomaterials. In order to promote the development of this field, we summarized the nanomaterials that can induce EMT in tumor cells and promote tumor metastasis from the perspective of activation of different signaling pathways.
Table 2. Some Nanoparticles That Promote or Inhibit the EMT of Tumor Cells.
| nanoparticles | combined treatments | cell lines | cancer types | EMT markers | inhibit/promote EMT | ref |
|---|---|---|---|---|---|---|
| Gold nanoparticles | None | PANC-1 | Pancreatic cancer | E-Cadherin↑ | Inhibit | (59) |
| AsPC-1 | N-Cadherin↓ | |||||
| HPAF II | Vimentin↓ | |||||
| Gold nanoparticles | Cold plasma | T98G | Glioblastoma | E-Cadherin↑ | Inhibit | (60) |
| A459 | Lung cancer | N-Cadherin↓ | ||||
| Slug↓ | ||||||
| ZEB1↓ | ||||||
| Gold nanoparticles | Dexamethasone (DSH) thiol derivative | B16F10 | Murine melanoma | E-Cadherin↑ | Inhibit | (61) |
| Withaferin (WFA) | Vimentin↓ | |||||
| Nano-TiO2 | None | A549 | Lung cancer | E-Cadherin↑ | Inhibit | (33) |
| N-Cadherin↓ | ||||||
| Smad2/3↓ | ||||||
| Nano-TiO2 | None | SW480 | Colon epithelial adenocarcinoma | E-Cadherin↓ | Promote | (34) |
| Vimentin↑ | ||||||
| Slugs↑ | ||||||
| Twist↑ | ||||||
| Snail↑ | ||||||
| α-SMA↑ | ||||||
| ERK↑ | ||||||
| β-Catenin↑ | ||||||
| TCF4↑ | ||||||
| Pi-Smad3↑ | ||||||
| ZnO nanostructures | None | T98G | Glioblastoma | N-Cadherin↓ | Inhibit | (62) |
| SNU-80 | Thyroid cancer | ZEB1↓ | ||||
| H-460 | Lung cancer | |||||
| Silver nanoparticles | Gallic acid | A549 | Lung cancer | Vimentin↓ | Inhibit | (63) |
| N-Cadherin↓ | ||||||
| Snail1↓ | ||||||
| E-Cadherin↑ | ||||||
| Graphene oxide | None | PC3, A549, HepG2 | Lung cancer | E-Cadherin↓ | Promote | (36) |
| N-Cadherin↑ | ||||||
| CD109↓ | ||||||
| Vimentin↑ | ||||||
| Slugs↑ | ||||||
| Smad2/3↑ | ||||||
| TGFβ R1↑ | ||||||
| Nano-SiO2 | None | SW480 | Colon epithelial adenocarcinoma | CDH1↓ | Promote | (34) |
| ACTA2↑ | ||||||
| Vimentin↑ | ||||||
| Nanohydroxyapatite | None | SW480 | Colon epithelial adenocarcinoma | CDH1↓ | Promote | (34) |
| ACTA2↑ | ||||||
| Vimentin↑ | ||||||
| Zinc arsenite | Arsenic trioxide | Hep3b | Liver cancer | E-Cadherin↑ | Inhibit | (64) |
| HepG2 | Vimentin↓ | |||||
| Bel7402 | Slug↓ | |||||
| MHCC97L | ||||||
| Liposome | 188Re | FaDu | Head and neck squamous cell carcinoma | E-Cadherin↑ | Inhibit | (65) |
| SAS | N-Cadherin↓ | |||||
| TWIST1/2↓ | ||||||
| Vimentin↓ | ||||||
| ZEB1↓ | ||||||
| Slugs↓ | ||||||
| Polymeric micelles | Salinomycin | A549 | Lung cancer | Vimentin↓ | Inhibit | (66) |
| Exosome | None | PANC-1 | Pancreatic cancer | E-Cadherin↓ | Promote | (67) |
| BxPC-3 | N-Cadherin↑ | |||||
| Vimentin↑ | ||||||
| MMP7↑ | ||||||
| Exosome | None | MHCC-97H | Hepatocellular carcinoma | E-Cadherin↓ | Promote | (68) |
| N-Cadherin↑ | ||||||
| Huh7 | β-Catenin↑ | |||||
| Snail↑ | ||||||
| ECO lipid carrier | β3 integrin siRNA | MDA-MB-231 | Triple negative breast cancer | PAI-1↓ | Inhibit | (69) |
| N-Cadherin↓ | ||||||
| E-Cadherin↑ | ||||||
| CK19↑ | ||||||
| Gelatin nanoparticles | AXL siRNA | H820 | Nonsmall cell lung cancer | MMP9↓ | Inhibit | (70) |
| MMP2↓ | ||||||
| H1975 | Vimentin↓ | |||||
| N-Cadherin↓ |
The TGF-β family mainly includes TGF-βs, activins, bone morphogenetic proteins, and growth and differentiation factors.71 They play an important role in normal physiological activities of the human body, such as controlling cell proliferation and differentiation, regulating cell metabolism, promoting or protecting against cell death, promoting extracellular matrix protein expression, and so on. In normal tissues, the TGF-β signaling pathway dynamically regulates the homeostasis of the body’s internal environment and has a certain degree of inhibitory effect on the early stage of tumor formation, such as inhibiting cell proliferation, promoting apoptosis and autophagy, inhibiting inflammation, and blocking angiogenesis.72,73 However, with further tumor progression, the inhibitory effect of the TGF-β signaling pathway on tumors is weakened, and it may show the opposite effect compared to normal tissue, which can induce tumor EMT and promote tumor invasion and metastasis, promoting tumor progression to a certain extent. This is mainly due to changes in the TME during tumor development and the disruption or mutation of regulators of TGF-β signaling. The TGF-β signaling pathway is a double-edged sword, where it can not only inhibit tumor growth but also promote tumor progression under different circumstances. The activation of the TGF-β signaling pathway is one of the important reasons for the production of EMT-TFs to promote EMT, which has been widely studied. This process is mediated by receptors and downstream intracellular effectors, mainly Smad proteins (Smads). The TGF-β signaling pathway is further divided into a classical pathway with Smads participation and a nonclassical pathway without Smads participation (non-Smad signaling pathways). As shown in Figure 2A, in the classical pathway involving Smads, TGF-βs bind and interact with transforming growth factor-β receptor type 2 (TGF-βR2). TGF-βR2 can activate transforming growth factor-β receptor type 1 (TGF-βR1), and then, TGF-βR1 recruits and phosphorylates Smad2 and Smad3. Phosphorylated Smad2 and Smad3 can combine with Smad4 to form these trimeric SMAD complexes, which will then be transferred to the nucleus as transcription factors to regulate various activities, including cellular EMT.74 With further research, our understanding of the canonical pathway of TGF is also expanded. Recently, the team led by Hill found that TGF-β can also induce the phosphorylation of Smad1 and Smad5 and that the combined signaling of this pathway and the Smad2/3 pathway is indispensable for TGF-β to induce a complete EMT process.750 In addition to the canonical pathway, activated receptors can also conduct cell signaling through other pathways; these pathways are called non-Smad signaling pathways, and the common ones are the mitogen-activated protein kinase (MAPK) pathways, including the extracellular signal regulated kinases (Erks), c-Jun amino terminal kinase (JNK), and p38 MAPK as well as IkB kinase, phosphatidylinositol-3 kinase (PI3K), Akt, Rho family GTPases, etc. These pathways can be activated directly through ligand-occupied receptors and can modulate downstream cellular responses independently or by affecting the Smad pathway.75 Studies have shown that nanomaterials can activate the TGF-β signaling pathway with or without the involvement of Smad in tumor cells in different ways, thereby inducing EMT in tumor cells and ultimately promoting tumor metastasis.
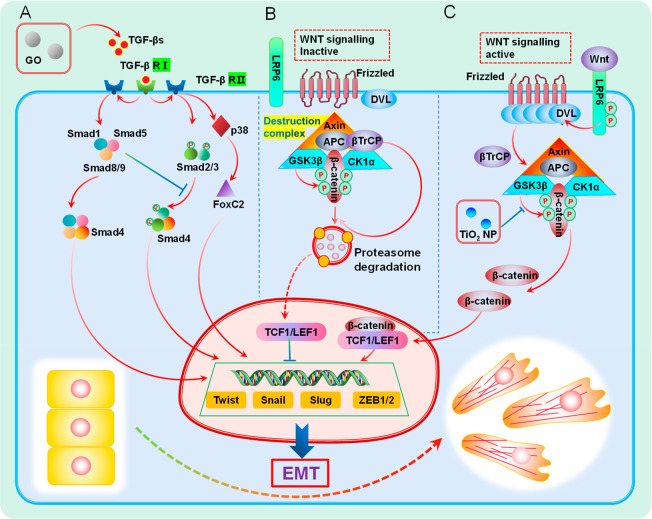
Figure 2.
Schematic illustration of the EMT signaling pathway induced by nanomaterials. (A) TGF-β signaling pathway. Activated by GO by increasing the concentration of TGF-βR. (B) Wnt signaling inactive. β-Catenin is phosphorylated by the construction complex and then ubiquitinated by β-TrCP200 and sent to proteasome for degradation. (C) Wnt signaling active. Nano-TiO2 inhibits the phosphorylation of β-catenin by GSK3β, which stabilizes and accumulates β-catenin, thereby activating the Wnt signaling pathway.
In 2019, a research team led by Liu found that a low dose of GO could interact with cancer cells to increase the concentration of TGF-β receptor (TGF-βR), thus activating the classical pathway of TGF-β signaling pathway, inducing tumor cells to produce EMT, and finally promoting tumor metastasis.36 The team demonstrated the increase in the TGF-β receptor concentration mainly by detecting an increase in the level of TGF-βR1 and a decrease in the level of CD109 in tumor cells after GO treatment. CD109 is a coreceptor of TGF-β and a partner of TGF-βR, which has been shown to negatively regulate TGF-βR by promoting the internalization and degradation of TGF-βR, so the decrease of CD109 concentration can further confirm the increase of TGF-βR concentration.76 For the investigation of signaling pathways through which GO promoted tumor metastasis, the team detected the content of phosphorylated Smad2/3 (P-Smad2/3), Smad2, and Smad3 and found that P-Smad2/3 in GO-treated tumor cells increased over time, while the levels of Smad2 and Smad3 were relatively stable, which to some extent proved the opening of the canonical pathway of the TGF-β signaling pathway. However, this study did not expand on the demonstration of different pathways in the TGF-β signaling pathway and did not conduct further experiments to exclude noncanonical pathways, such as detecting markers associated with activation of noncanonical pathways. We believe that there may also be activation of noncanonical pathways in this process, as some research teams have indeed found that nanomaterials can induce EMT in tumor cells through some nonclassical pathways. In 2018, Leong et al. studied the effect of Nano-TiO2 on the EMT process of intestinal epithelial cancer cells and found that Nano-TiO2 could induce the continuous production of reactive oxygen species (ROS) in colorectal cancer cells in a dose-dependent manner and activate the noncanonical pathway of the TGF-β signaling pathway: the JNK and p38 MAPK signaling pathways, thereby upregulating the expression of Slug and Twist transcription factors, inducing EMT in cancer cells, and enhancing the invasiveness and migration ability of cancer cells.34 In the team’s experiments, it was found that the content of P-Smad3 in the Nano-TiO2-treated group did not increase, indicating that Nano-TiO2 did not activate the classical TGF-β signaling pathway. Subsequently, the activation of p38 and ERK pathways existed in the Nano-TiO2 treated group by detection of phosphorylation events that were detected on ERK at two residues, Thr 202 and Tyr 204, and on residue Tyr 182 of p38.
Wnts are a class of secreted glycoproteins that act by autocrine or paracrine pathways, which were cloned in mouse breast cancer cells by Nusse and Varmus in 1982.77 If they are stimulated by the corresponding external environment or the key proteins in the signal pathway are abnormally mutated, abnormal activation of the Wnt signal pathway can occur and induce tumor EMT, thus promoting tumor invasion and metastasis. For example, the team led by Jacks found that the activation of the Wnt signaling pathway promoted the proliferation and metastasis of lung adenocarcinoma.78 The Wnt signaling pathway is mainly operated through the binding of 19 distinct WNT ligands to the Frizzled family of cell surface receptors. Currently, three different Wnt signaling pathways have been found, namely, the canonical WNT signaling pathway, the noncanonical WNT–calcium pathway, and planar cell polarity pathway. The canonical WNT signaling pathway is one of the key signaling pathways to activate EMT in tumor cells and has been extensively studied. After activation of this pathway, a series of signaling events are triggered, leading to the nuclear translocation of β-catenin, resulting in the production of nuclear β-catenin, which can act as a transcriptional cofactor to induce the expression of genes related to cellular EMT.57,79 As shown in Figure 2B,C, the mechanism can be divided into two states of the signaling pathway for elaboration. First, (1) Wnt signaling is inactive, where in this state, loss of the Wnt ligand results in the phosphorylation of β-catenin by the construction complex consisting of the scaffold protein Axin, APC, and the kinases GSK3β and casein kinase (CK1α), in which GSK3β plays a major role. β-Catenin is then ubiquitinated by β-TrCP200 and targeted for proteasomal degradation. In the absence of the nuclear translocation of β-catenin, a repressive complex containing T-cell factor (TCF) or lymphoid enhancer factor (LEF) and transducing-like enhancer protein (TLE/Groucho) recruits HDACs to repress target genes. Second, (2) Wnt signaling is active when Wnt ligands, such as Wnt3a and Wnt1, bind to Frizzied (Fzd) receptors and LRP coreceptors, and the classical pathway will be activated. LPR receptors are phosphorylated by CK1α and GSK3β, resulting in the aggregation and activation of Dishevelled (Dvl) proteins at the plasma membrane. The Dvl polymers can lead to the inactivation of the construction complex through sequestration in multivesicular bodies, etc., resulting in the stabilization and accumulation of β-catenin, thereby promoting the nuclear translocation of β-catenin and producing nuclear β-catenin. In the nucleus, β-catenin forms an active complex with LEF and TCF proteins by displacing TLE/Groucho complexes and recruiting histone modifying coactivators such as CBP/p300, BRG1, BCL9, and Pygo.80 This transcriptional switch leads to a change of multiple cellular processes.81 In addition to the classical pathway, two other nonclassical pathways have also been shown to promote tumor EMT. For example, it has been reported that the high expression of the Wnt receptor Fzd2 and its ligands Wnt5a/b in metastatic liver, lung, colon, and breast cancer cell lines is closely related to the EMT process of tumor cells. Blocking Fzd2 with specific antibodies can effectively inhibit the process of tumor EMT and reduce tumor metastasis.82 In addition, in a study on prostate cancer, ABI1 loss was found to unblock FYN-STAT3 downstream of noncanonical WNT signaling, thereby activating this signaling pathway and inducing EMT in tumor cells.83
When studying the effect of Nano-TiO2 on the EMT process of intestinal epithelial cancer cells, Leong et al. not only observed the activation of the TGF-β signaling pathway without Smad participation but also analyzed the Wnt1 mRNA level under cellular Nano-TiO2 exposure. After that, a series of experiments were carried out to explore the content and location of β-catenin and its binding partner TCF4 in tumor cells.34 The expression level of Wnt1 mRNA in the Nano-TiO2 treatment group increased 4-fold, and the content of β-catenin increased by 1.5 times and was mainly located in the cytoplasm and nucleus, rather than concentrated on the cell membrane as in the control group. In addition, the increase of TCF4 content and the combination of β-catenin and TCF4 were observed, which revealed that the Wnt signal pathway was also involved in the process of tumor cell EMT induced by Nano-TiO2. In this experiment, it is suggested that the activation of the Wnt signal pathway may be due to the inhibition of GSK3 β-phosphorylation of β-catenin by Nano-TiO2, which changes the Wnt signal pathway from an inactive state to active state. This study found that there is crosstalk between the TGF-β signal pathway and Wnt signal pathway, which coincides with the previous results that TGF-β/MAPK pathways can inhibit GSK3 β activity against β-catenin, Slug, and Twist.84,85 At present, there are few studies on tumor cell EMT induced by nanomaterials by activating the Wnt signaling pathway, and the Wnt signaling pathway has many potential targets of nanomaterials. Hence, the exploration of effects of nanomaterials on different aspects of the Wnt signaling pathway in tumor cells could be a potential subsequent research interest.
2.2. Interacting with Vasculature by Nanomaterials
Regardless of the route, such as intravenous injection, skin absorption, inhalation, or ingestion, most nanomaterials entering the human body will eventually enter the vasculature and interact with the body. The interaction between nanomaterials and vasculature can have an extensive and comprehensive impact on the body, which will not only affect the effect of nanodrugs, which could make drugs with good performance in vitro difficult to perform in vivo, but also give rise to certain toxic effects on the body, affecting a variety of normal physiological functions, of which the more serious is to accelerate the progress of the tumor and promote tumor invasion and metastasis. Tumor metastasis is a very complex process, and current research has not been able to clearly explain the full extent of the mechanism of tumor metastasis.86 However, the vasculature has a very close relationship with tumor metastasis, being involved in all aspects of tumor metastasis. Endothelial integrity and molecular characteristics, the number of microvessels, etc. will have a great impact on tumor metastasis.12 Endothelium is a single-layer structure composed of endothelial cells, mainly distributed in the inner cellular lining of the blood vessels (arteries, veins, and capillaries) and lymphatic system. It plays a very important role in controlling the entry and exit of substances into and out of the vascular system, regulating blood fluidity, platelet aggregation, and vascular tension, and participating in immune response and inflammatory response. Endothelium is also an important metabolic and endocrine organ that can participate in various physiological processes of the body. Solutes and cells must pass through the endothelium to enter or leave the vasculature. The barrier function of endothelial cells is the prerequisite for the body to perform various life activities. There are abundant cell connections between endothelial cells, and they are important for the endothelium to play a barrier function and control the entry and exit of substances. It is currently believed that tight junctions and adhesive junctions are mainly involved in the maintenance and regulation of the endothelial barrier function. Although studies have shown that gap junctions are involved in the formation of endothelial barrier function in some cases, the specific mechanism still needs further research, and there is some controversy; there is no current mainstream understanding.87 Tight junctions form close focal contacts in the plasma membrane of adjacent cells in the form of hemifusions. Tight junctions are the meshwork of fibrils composed of rows of transmembrane particles, which are considered as the diffusion barriers, mainly including claudins protein, occluding protein, tricellulin protein, MARVEL domain-containing protein 3, etc.88,89 Tight junctions also contain an electron-dense junctional plaque composed of cytosolic proteins, mainly including zonula occludens 1 (ZO-1) and adapter proteins such as ZO-2 and ZO-3, the membrane-associated guanylate kinase inverted proteins, etc.90,91 Adhesion junctions are mainly composed of vascular endothelial cells cadherin (VE-cadherin) and β-catenin, p120-catenin, and γ-catenin, etc. VE-cadherin, as the main component, can form Ca2+-dependent and homologous interactions between adjacent endothelial cells, which play an important role in maintaining the endothelial barrier.87 Under physiological conditions, endothelial cells mainly rely on mechanical and humoral factors to maintain endothelial barrier function and avoid the loss of body fluids and solutes.92 However, a series of pathological processes, such as inflammation, tumor, infection, trauma, etc. will lead to the weakening of endothelial barrier function and the increase of vascular permeability, resulting in a series of harmful effects.93 Due to the special TME and growth characteristics of the tumor site, the shape of blood vessels formed in the tumor site is often irregular, and the gaps between vascular endothelium are large. The tumor itself can also activate a series of complex processes to destroy the endothelial barrier and induce vascular endothelial leakage. For example, tumor cells can destroy the VE-cadherin-β-catenin complex by secreting vascular endothelial growth factor (VEGF) or TGFβ1, thus destroying the connection between endothelial cells.94,95 The combined action of a variety of factors can cause more tumor cells to enter the vascular system through the endothelial barrier to prepare the cells for the next step of metastasis. Tumor cells pass through the endothelium in different ways under different conditions but generally similar to the way white blood cells pass through the endothelium and are summarized as two ways: transcellular and paracellular.96 The transcellular pathway means that tumor cells pass through the endothelial barrier directly through endothelial cells. There are relatively few examples of supporting this pathway, and it is not considered to be the main way for tumor cells to pass through the endothelium.97 It is the current mainstream understanding that tumor cells pass through the endothelium through the paracellular pathway in vivo, which is related to the destruction of endothelial cell junction.98 It has been found that some nanomaterials can directly or indirectly destroy the connection of endothelial cells, reducing the barrier effect of endothelial cells and inducing endothelial leakage. Although endothelial leakage is generally thought to result only in the weakening of the endothelium’s control over solute entry and exit with the participation of nanomaterials, micron-scale gaps will form sufficiently for cells to pass through.99 Therefore, when this effect occurs uncontrollably in the tumor site and the potential metastatic target site of the tumor, as shown in Figure 3, it may cause more tumor cells to enter the vascular system through the endothelial barrier and enter the target site from the vasculature. To a large extent, it will promote the metastasis of the tumor with serious potential harm. We summarize the nanomaterials that have been found to break the endothelial barrier, induce endothelial leakage, and promote tumor metastasis. The aim is to avoid the potential harm of nanomaterials to tumor metastasis and to play a greater role in targeted transportation and disease treatment through a reasonable application.
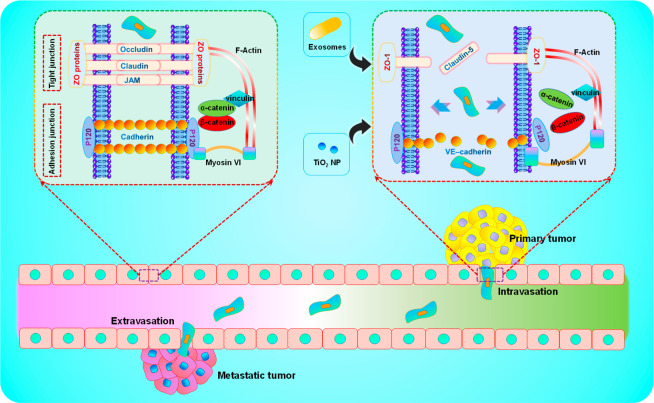
Figure 3.
Schematic illustration of the interaction between nanomaterials and the vasculature. Exosomes and Nano-TiO2 affect cellular junctions between endothelial cells, thereby increasing endothelial leakage and promoting tumor metastasis.
In 2013, a team led by Leong found that Nano-TiO2 could bind to adhesion through physical interaction in situ, leading to phosphorylation of the intracellular domain of VE-cadherin at its Y658 and Y731 residues. As a result, the interaction between VE-cadherin, β-catenin, and p120-catenins is lost. Actin remodeling is activated, resulting in a widening of the intercellular space and increased endothelial leakage. Ultimately, more secondary metastases to the lungs of B16F10 mouse melanoma cells were observed in the mouse models. In this study, nanomaterial-induced endothelial leakiness (NanoEL) is defined for the first time, that is, endothelial leakage caused by nanomaterials, which creates a precedent for endothelial leakage induced by nanomaterials.100 In subsequent studies, it has been found that nanomaterials such as Ag, SiO2, Au, and ZnO2 have similar effects to Nano-TiO2, and its mechanism is mainly focused on the remodeling of cytoskeleton and the destruction of VE-cadherin, which is mainly determined by the diameter, charge, and density of nanoparticles.101−104 More recently, a study by Peng et al. also found that the injection of nanoparticles such as Nano-TiO2, silica, and gold in animal models will induce NanoEL, which will significantly accelerate the infiltration and extravasation of breast cancer cells and promote tumor metastasis. The mechanism is mainly due to the disruption of the VE-cadherin–VE-cadherin homophilic interactions at the Adherin junctions by nanoparticles.105 In addition, some nanomaterials can induce NanoEL through indirect action, which refers to endothelial leakage caused by secondary events generated by the interaction of nanomaterials with endothelial or endothelial cells. For example, nanodiamonds can cause an increase in intracellular ROS and Ca2+, resulting in loss of cell junctions and remodeling of the cytoskeleton leading to NanoEL.106 Other examples of NanoEL caused by the indirect action of nanomaterials are discussed in this excellent review.99 In these studies on NanoEL caused by the indirect effect of nanomaterials, the promoting effect on tumor metastasis was not clearly pointed out. However, the endothelial space caused by NanoEL can allow tumor cells to pass through, so this indirectly caused NanoEL has a certain potential to promote tumor metastasis, which is worthy of further study. In addition, in the field of exosomes that has emerged in recent years, some teams have also found that exosomes can destroy the integrity of the vascular endothelium to promote tumor metastasis. For example, Lin et al. found that exosomes derived from HeLa cells can trigger endoplasmic reticulum stress in endothelial cells and reduce tight junction-related proteins, such as ZO-1 and CLDN5, to disrupt the integrity of vascular endothelium and ultimately promote tumor metastasis.107 In a similar study, Cen et al. also found that the expression of VE-cadherin and ZO-1 in human umbilical vein endothelial cells treated with exocrine secreted by metastatic breast cancer cells decreased significantly, thus increasing the number of cancer cells migrating across the endothelial cell layer and promoting tumor metastasis to a certain extent.108 Nanoplastics have also been reported to be exogenous substances that induce endothelial leakage. With more production and use, the probability of nanoplastics entering the body and interacting with blood vessels has been greatly increased and may have a certain role in tumor metastasis. Song, Ke and co-workers have found that the nanoplastic forms of anionic polystyrene and poly(methyl methacrylate) can destroy the vascular endothelial cadherin junctions in a dose-dependent manner. This is the first time that nanoplastics have been shown to induce NanoEL.109 Although the related research on tumor metastasis has not been carried out, this study suggests that nanoplastics have a certain potential in promoting tumor metastasis, and further research is needed.
2.3. Inducing Systemic Inflammation of TME by Nanomaterials
At present, inflammation has been regarded as one of the two enabling characteristics to acquire a series of abilities in the process of tumor progression and has received more attention in recent years.110 In this context, inflammation refers both to tumor-promoting immune cell infiltration (that is, most tumors are infiltrated by a variety of cells of the immune system, including subtle infiltrations, which can only be detected with specific antibodies) and to general inflammation, which can be detected using standard histochemical staining techniques.111 With continuous improvement in understanding of inflammation, there is a growing belief that inflammation is not only a failed attempt by the body to eradicate tumors but also a double-edged sword that may promote tumor progress. The promoting effect of inflammation on tumors is mainly based on the role of tumor-promoting inflammatory cells, including macrophage subtypes, mast cells, and neutrophils, as well as T and B lymphocytes and so on.110 These inflammatory cells can secrete a series of signaling molecules such as epidermal growth factor, VEGF, fibroblast growth factor-2 (FGF2), chemokines, and cytokines that amplify the inflammatory state, etc. They can also produce proangiogenic or proinvasive matrix-degrading enzymes, including matrix metalloproteinases (MMPs) and other matrix metalloproteinases, cysteine cathepsin proteases, and heparinase. Under the action of various secretions, inflammation can finally show the promotion of tumor progression, such as inducing tumor angiogenesis, promoting cancer cell proliferation, promoting cancer cell invasion and metastasis, etc.110 In addition, it has been found that inflammatory cells can release chemicals and notably reactive oxygen species, which can induce genetic evolution toward states of heightened malignancy of nearby cancer cells.112 In general, with the continuous improvement of people’s understanding, the infiltrating cells of the immune system in the tumor site are more accepted as one of the components of the TME, and these infiltrating cells have certain antitumor functions. However, they can also promote tumor progress, including tumor metastasis, and play a very important role in various tumor activities. In recent years, with the continuous development of nanoscience, the potential hazards of nanomaterials have been continuously discovered. Studies have shown that nanomaterials can not only activate or enhance the role of various tumor-promoting inflammatory cells in the TME but also cause systemic inflammation, leading to immune dysfunction. As shown in Figure 4, these effects of nanomaterials can lead to the continuous acceleration of tumor progression, such as promoting tumor angiogenesis and ultimately tumor invasion and metastasis, which has very serious consequences and deserves attention.
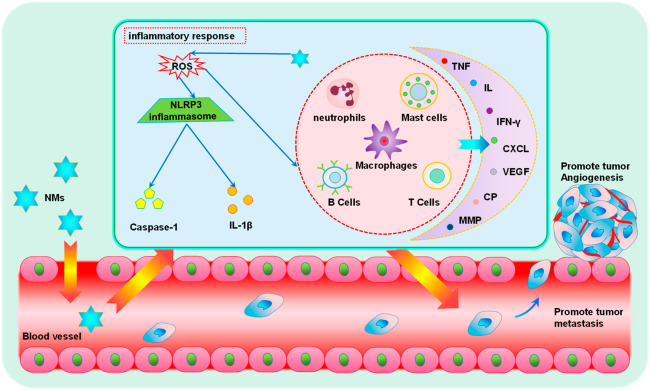
Figure 4.
Schematic illustration of nanomaterial-induced inflammation and promotion of tumor metastasis. The role of various pro-tumor inflammatory cells in the TME is activated or enhanced by nanomaterials, which can also cause systemic inflammation and ultimately promote tumor angiogenesis and tumor metastasis.
In 2019, Zhu et al. conducted a study on the effects of long-term multiwalled carbon nanotube (MWCNT) exposure in the lungs on breast cancer.113 In this study, they found that long-term exposure to MWCNTs in the lungs will lead to chronic inflammation in the lungs, leading to changes in gene expression, which help shape the TME and systemic environment suitable for breast cancer metastasis and ultimately promote the colonization and growth of breast cancer cells from in situ to the lungs. The main mechanisms consist of: (1) increased angiogenesis, mainly manifested as CNT exposure in the lungs leading to increased levels of pro-angiogenic and pro-transfer factors in mouse lung tissue, serum, and/or breast cancer cells, including VEGFA, basic fibroblast growth factor, and cyclooxygenase-2 (COX-2), and (2) the formation of a premetastatic niche and metastatic niche, mainly manifested by the increased expression of COX-2, chemokine (C-X-C motif) ligand 2, S100 calcium-binding protein A9, MMP9, and Fibronectin 1 in CNT-exposed lung tissue, which may establish a TME favorable for colonization and metastasis. NOD-like receptor protein 3 (NLRP3) inflammasome is a multiprotein platform composed of NLRP3 and caspase-1. Its activation is closely related to the formation of ROS. After activation, the secretion of interleukin-1β will increase, thus causing a series of inflammatory reactions.114 Fu et al. found that PEI-CyD and 25 kDa polyethylenimine-based polymeric nanoparticles can activate the immune response in vivo and promote the phagocytosis of macrophages and the secretion of proinflammatory cytokines. It can also promote the production of ROS and the activation of NLRP3 inflammasome in the liver and ultimately promote the metastasis of breast cancer in the liver and lung, which may be related to the immune imbalance of the body as a whole.115 Soenen et al. obtained similar results in a study of aluminum oxide nanomaterial, where it can activate NLRP3 inflammasome to promote the release of inflammatory factors and ultimately promote distant metastasis of the tumor.116
3. Conclusion, Discussion, and Future Perspective
This Review first summarizes several nanomaterials that promote tumor metastasis by inducing EMT of tumor cells, focusing on describing the mechanism of nanomaterials affecting the properties of tumor cells themselves. Second, it summarizes how nanomaterials promote tumor metastasis from the perspective of the interaction between nanomaterials and vasculature, focusing on describing the impact of nanomaterials on the tumor metastasis process. We propose that nanomaterials may play a promoting role in the tumor metastasis process by disrupting the connection between the vascular endothelium at the primary tumor site and potential metastasis sites. Finally, the mechanism by which different nanomaterials cause inflammation in the body and promote tumor metastasis is summarized, and the impact of nanomaterials on the TME is described from a relatively holistic perspective. Through comparison, it can be found that these three perspectives, respectively, represent the three parts of tumor metastasis, namely, the tumor itself, the tumor metastasis process, and the driving factors of tumor metastasis. The properties of tumor cells themselves play a decisive role in tumor metastasis, and the driving factors that affect the process of tumor metastasis and change tumor metastasis can inhibit or promote tumor metastasis. Therefore, we believe that nanomaterials can have a greater impact on tumor metastasis by inducing the EMT in tumor cells. With the continuous enrichment of research, in addition to EMT, vasculature, and inflammation, the specific content of these three perspectives will have a more comprehensive development. The study of the mechanism of nanomaterials promoting tumor metastasis is helpful to arouse people’s attention to the biosafety of nanomaterials and avoid nano exposure. In addition, it is conducive to the design and production of safe and efficient nanomaterials, which are of great significance to the development of nanotechnology.
Due to the obvious advantages in improving performance, reducing cost, and providing convenience, nanomaterials have been widely used in various fields of daily life. For example, Nano-TiO2 is used in sunscreens and paints, and iron oxide nanoparticles are used in groundwater treatment. Silica nanoparticles are used in the electronics industry; graphene is used as electrodes, and so on.117 The massive global use of nanomaterials has greatly increased the human body’s exposure to nanoparticles. Research shows that the skin can prevent micrometer-sized particles from entering the body; however, it is unable to do so at the nanoscale. This results in nanoparticles inevitably entering the body’s circulation system when using skin care products, sunscreen, and other products, which will have some potential effects.118 The respiratory tract is another route for nano exposure. Some nanoparticles can enter the body through the respiratory tract, such as asbestos, graphene, etc. Some of them can be cleared through the beating of lung cilia and the phagocytosis of macrophages, but some will be deposited in the lungs and enter the circulatory system, causing various harmful effects on the body.119 Nanoparticles can also enter the body through the digestive system. In daily life, food and water intake are essential, and it is highly likely that large amounts of nanoparticles will be ingested during this process. For example, it is reported that the detection rate of microplastics in human feces exceeds 95.8% and the content is as high as 138.9 items/g.120 Based on the characteristics of the digestive tract itself, the possibility of nanoparticles being absorbed into the circulatory system is obviously higher than the above two pathways, and they are more likely to have an impact on human health.117 The increasing exposure of nanomaterials continues to drive the development of nanotoxicology, and more nanomaterials have been shown to have varying degrees of negative effects on the body, such as neurotoxicity, vascular toxicity, liver toxicity, etc.121,122 In the face of nano exposure, we can remove nanomaterials based on their own properties such as charge and diameter, and we can also intervene in the process of promoting tumor metastasis by nanomaterials from a mechanism perspective, such as adjusting the inflammatory state of the body and developing specific inhibitors for different links of nanomaterials promoting tumor metastasis.
Although some progress has been made in the mechanisms by which nanomaterials promote tumor metastasis, there are still many issues that need to be further investigated. There is a complex network of EMT-related signaling pathways in tumor cells; thus, can nanomaterials induce EMT in tumor cells by activating signaling pathways other than TGF-β and Wnt signaling pathways? Through the optimization design of nanomaterials, can the currently observed ability to promote tumor metastasis be transformed into a positive effect of tumor inhibition? Recent research advances have shown that EMT is not a binary process, but it occurs in a progressive manner, which allows cells undergoing EMT to exhibit varying degrees of epithelial and mesenchymal states, an intermediate state known as partial, incomplete, or hybrid EMT states.123 Cells in different intermediate states can express different levels of epithelial and mesenchymal markers and can show morphological, transcriptional, and epigenetic characteristics between epithelial and mesenchymal cells.124 At present, many studies have shown that there are different degrees of the EMT intermediate state in all kinds of tumor cells, showing different functional characteristics, which can affect tumor cell proliferation, reproduction, plasticity, invasion, and metastasis and play a very important role in tumor progression.125 For example, in pancreatic tumors with KrasG12D/p53cKO gene alteration, hybrid EMT cancer cells have a stronger proliferation ability than mesenchymal cells.126 Different from judging whether EMT occurs in tumor cells only by the loss of epithelial marker E-cadherin and the increased expression of mesenchymal marker vimentin, the recognition of distinct EMT transition states is mainly through cell surface markers and single-cell RNA-sequencing. It is worth noting that recent studies have found that tumor cells have the highest metastatic potential when they are in a hybrid EMT state. Some studies have also shown that tumor cells in the hybrid EMT state have higher proliferation potential and are more clonogenic.125 In addition, the hybrid EMT phenotype has a stronger ability to enter the blood circulation and can further increase metastasis.124 The hybrid EMT state is the result of the coordination of tumor cells in increasing the ability of invasion and colonization so that tumor cells can enter the most suitable state of metastasis. Therefore, intervening and preventing tumor cells from entering the hybrid EMT state is an excellent way to avoid tumor metastasis and cause serious harm. Due to their unique properties, nanomaterials have broad application prospects in regulating the EMT process of tumor cells. Therefore, we propose that, on the one hand, nanomaterials can block the process of tumor EMT and make it stay in the early EMT stage with less invasiveness but, on the other hand, nanomaterials can also further induce tumor EMT to enter the late EMT stage with poor colonization ability. For example, overexpression of the paired-related homeobox transcription factor (Prrx1 TF) can induce tumor cell EMT derived from renal epithelial cells to make it more invasive. However, it is only when PRRXTF is silenced that the tumor cells revert to an epithelial phenotype and gain the ability to colonize at a distance. This study suggests that, if EMT inducers are used correctly, the inhibitory effect of them on tumors can be achieved to some extent.127 At present, nanodrugs have been developed to inhibit tumor cell EMT. For example, Wang et al. developed an etoposide loaded layered double hydroxide nanocomposite, which can significantly reduce the stemness of tumorigenic glioma stem cells (GSC) and reverse its EMT process, allowing it to restore the epithelial cell morphology, thereby overcoming drug resistance and inhibiting GSC metastasis.128 However, there is no related research on using nanomaterials to make tumor cells stay in the late EMT state to inhibit tumor metastasis.129 Therefore, based on summarized studies on the induction of tumor cell EMT by nanomaterials to promote tumor metastasis, we propose that it may be possible to further design nanomaterials to fix tumor cells in a late EMT state and inhibit tumor metastasis, turning the promotion effect of nanomaterials on tumor metastasis into an inhibitory effect and further expanding the application scope of nanomaterials in the field of tumor therapy.
In the field of nanomedicine, the abnormal vascular system formed by the tumor derived enhanced permeability and retention (EPR) effect in solid tumors shows a leaky characteristic, which is the mainly regarded method for many powerful nano-anticancer drugs to enter the tumor through the vasculature of the tumor site. Although the EPR effect has been used as the “gold standard” for the design of most cancer-targeted drugs, there are still many controversies and deficiencies. A study by Chan et al. showed that up to 97% of nanoparticles are not passively transported through the leaky endothelium into the tumor site by paracellular transport but are transported by an active process through endothelial cells. This calls into question the true role of the EPR effect in nanodrug delivery.130 In addition, the EPR effect has also been found to have problems such as low delivery efficiency, restrictions by the nature of the tumor itself, poor controllability, susceptibility to TME, and insufficient clinical translation of drugs.131 Although NanoEL can promote tumor metastasis to a certain extent, this endothelial leakage has a very broad application prospect in solving the problems of the EPR effect and improving the targeting of nanodrugs. Through the reasonable design of nanomaterials in terms of size, surface charge, density, and exposure time, as well as the continuous understanding of its mechanism and results, the realization of controllable and adjustable NanoEL has gradually become a reality, which provides the possibility to further improve the delivery efficiency of nanomaterials and clinical transformation. In the process of NanoEL’s clinical transformation, it is of great significance to avoid its promotion effect on tumor metastasis, which requires more rigorous animal experiments and the development of reasonable delivery strategies.
Acknowledgments
We acknowledge the funding provided by National Natural Science Foundation of China (22104073), Natural Science Foundation of Shandong Province (ZR2021QB119, 2022HWYQ-079), and the Youth Innovation Science and Technology Program of Shandong Provincial Universities (2021KJ100).
The authors declare no competing financial interest.
References
- Ganesh K.; Massagué J. Targeting metastatic cancer. Nat. Med. 2021, 27 (1), 34–44. 10.1038/s41591-020-01195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhikara B.; Parang K. Global Cancer Statistics 2022: The Trends Projection Analysis. Chem. Biol. Lett. 2023, 10, 451. [Google Scholar]
- Valastyan S.; Weinberg R. A. Tumor Metastasis: Molecular Insights and Evolving Paradigms. Cell 2011, 147 (2), 275–292. 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.; Kang Y. Cancer fitness genes: emerging therapeutic targets for metastasis. Trends Cancer 2023, 9 (1), 69–82. 10.1016/j.trecan.2022.08.007. [DOI] [PubMed] [Google Scholar]
- Welch D. R. Tumor Heterogeneity—A ‘Contemporary Concept’ Founded on Historical Insights and Predictions. Cancer Res. 2016, 76 (1), 4–6. 10.1158/0008-5472.CAN-15-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W.; Amin R.; Deasy S.; Ha N.-H.; Wakefield L. Genetic insights into the morass of metastatic heterogeneity. Nat. Rev. Cancer 2018, 18 (4), 211–223. 10.1038/nrc.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro P. A.; Struhl K. Nutrient Deprivation Elicits a Transcriptional and Translational Inflammatory Response Coupled to Decreased Protein Synthesis. Cell Rep. 2018, 24 (6), 1415–1424. 10.1016/j.celrep.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D. R.; Hurst D. R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79 (12), 3011–3027. 10.1158/0008-5472.CAN-19-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E.; Clevers H. Cancer stem cells revisited. Nat. Med. 2017, 23 (10), 1124–1134. 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- Ganesh K.; Basnet H.; Kaygusuz Y.; Laughney A. M.; He L.; Sharma R.; O’Rourke K. P.; Reuter V. P.; Huang Y.-H.; Turkekul M.; Er E. E.; Masilionis I.; Manova-Todorova K.; Weiser M. R.; Saltz L. B.; Garcia-Aguilar J.; Koche R.; Lowe S. W.; Pe’er D.; Shia J.; Massagué J. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 2020, 1 (1), 28–45. 10.1038/s43018-019-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; Xia L.; Huang P.; Wang Z.; Guo Q.; Huang C.; Leng W.; Qin S. Heterogeneity and plasticity of epithelial–mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms. Cell Prolif. 2023, 56 (6), e13423 10.1111/cpr.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B. M.; Yang J.; Cai B.; Fan J.; Zhang L.; Zeng M. Reinforcing endothelial junctions prevents microvessel permeability increase and tumor cell adhesion in microvessels in vivo. Sci. Rep. 2015, 5 (1), 15697. 10.1038/srep15697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu A.; Di Modugno F.; Manic G.; Nisticò P. Deciphering the loop of epithelial-mesenchymal transition, inflammatory cytokines and cancer immunoediting. Cytokine Growth Factor Rev. 2017, 36, 67–77. 10.1016/j.cytogfr.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Shang S.; Ji X.; Zhang L.; Chen J.; Li C.; Shi R.; Xiang W.; Kang X.; Zhang D.; Yang F.; Dai R.; Chen P.; Chen S.; Chen Y.; Li Y.; Miao H. Macrophage ABHD5 Suppresses NFκB-Dependent Matrix Metalloproteinase Expression and Cancer Metastasis. Cancer Res. 2019, 79 (21), 5513–5526. 10.1158/0008-5472.CAN-19-1059. [DOI] [PubMed] [Google Scholar]
- Albrengues J.; Shields M. A.; Ng D.; Park C. G.; Ambrico A.; Poindexter M. E.; Upadhyay P.; Uyeminami D. L.; Pommier A.; Küttner V.; Bružas E.; Maiorino L.; Bautista C.; Carmona E. M.; Gimotty P. A.; Fearon D. T.; Chang K.; Lyons S. K.; Pinkerton K. E.; Trotman L. C.; Goldberg M. S.; Yeh J. T. H.; Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 2018, 361 (6409), eaao4227 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Fan C. Q.; Dong H.; Wang S. M.; Yang X. C.; Yang S. M. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017, 12, 1815–1825. 10.2147/IJN.S127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brianna; Lee S. H. Chemotherapy: how to reduce its adverse effects while maintaining the potency?. Med. Oncol. 2023, 40 (3), 88. 10.1007/s12032-023-01954-6. [DOI] [PubMed] [Google Scholar]
- Pourmadadi M.; Mahdi Eshaghi M.; Ostovar S.; Mohammadi Z.; Sharma R. K.; Paiva-Santos A. C.; Rahmani E.; Rahdar A.; Pandey S. Innovative nanomaterials for cancer diagnosis, imaging, and therapy: Drug delivery applications. J. Drug Delivery Sci. Technol. 2023, 82, 104357 10.1016/j.jddst.2023.104357. [DOI] [Google Scholar]
- Xie S.; Fei X.; Wang J.; Zhu Y.-C.; Liu J.; Du X.; Liu X.; Dong L.; Zhu Y.; Pan J.; Dong B.; Sha J.; Luo Y.; Sun W.; Xue W. Engineering the MoS2/MXene Heterostructure for Precise and Noninvasive Diagnosis of Prostate Cancer with Clinical Specimens. Adv. Sci. 2023, 10 (15), 2206494 10.1002/advs.202206494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Yu T.; Li X.; Lei Y.; Li J.; Wang X.; Peng P.; Ni D.; Wang X.; Luo Y. Second near-infrared photothermal-amplified immunotherapy using photoactivatable composite nanostimulators. J. Nanobiotechnol. 2021, 19 (1), 433. 10.1186/s12951-021-01197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochella M. F.; Mogk D. W.; Ranville J.; Allen I. C.; Luther G. W.; Marr L. C.; McGrail B. P.; Murayama M.; Qafoku N. P.; Rosso K. M.; Sahai N.; Schroeder P. A.; Vikesland P.; Westerhoff P.; Yang Y. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363 (6434), eaau8299 10.1126/science.aau8299. [DOI] [PubMed] [Google Scholar]
- Yu X.; Rong J.; Zhan Z.; Liu Z.; Liu J. Effects of grain size and thermodynamic energy on the lattice parameters of metallic nanomaterials. Mater. Des. 2015, 83, 159–163. 10.1016/j.matdes.2015.06.019. [DOI] [Google Scholar]
- Oladipo A. O.; Lebelo S. L.; Msagati T. A. M. Nanocarrier design–function relationship: The prodigious role of properties in regulating biocompatibility for drug delivery applications. Chem.-Biol. Interact. 2023, 377, 110466 10.1016/j.cbi.2023.110466. [DOI] [PubMed] [Google Scholar]
- Xu X.; Xu S.; Wan J.; Wang D.; Pang X.; Gao Y.; Ni N.; Chen D.; Sun X. Disturbing cytoskeleton by engineered nanomaterials for enhanced cancer therapeutics. Bioact. Mater. 2023, 29, 50–71. 10.1016/j.bioactmat.2023.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Ge H.; Ma Y.; Song L.; Ma Y.; Tian G.; Wang L.; Meng Q.; Sun X. Engineered anti-cancer nanomedicine for synergistic ferroptosis-immunotherapy. Chem. Eng. J. 2023, 455, 140688 10.1016/j.cej.2022.140688. [DOI] [Google Scholar]
- Dong L.; Li W.; Luo Y.; Liu C.; Li K.; Chen Y.; Hong G. Engineering Molybdenum-Assisted Tellurium Nanosonosensitizers for Enhanced Sonodynamic Tumor Nanotherapy. Adv. Funct. Mater. 2023, 33 (31), 2302541 10.1002/adfm.202302541. [DOI] [Google Scholar]
- Wang J.; Gong J.; Wei Z. Strategies for Liposome Drug Delivery Systems to Improve Tumor Treatment Efficacy. AAPS PharmSciTech 2022, 23 (1), 27 10.1208/s12249-021-02179-4. [DOI] [PubMed] [Google Scholar]
- Soetaert F.; Korangath P.; Serantes D.; Fiering S.; Ivkov R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Delivery Rev. 2020, 163–164, 65–83. 10.1016/j.addr.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xu X.; Liu H.; Ni N.; Liu S.; Gong Y.; Ma G.; Song L.; Meng Q.; Fan Q.; Sun X. CCR2-overexpressing biomimetic carrier-free nanoplatform for enhanced cascade ferroptosis tumor therapy. Acta Biomater. 2023, 166, 604–614. 10.1016/j.actbio.2023.05.006. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Fan C.-Q.; Dong H.; Wang S.-M.; Yang X.-C.; Yang S.-M. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017, 12, 1815–1825. 10.2147/IJN.S127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Liu A.; Liu S.; Ma Y.; Zhang X.; Zhang M.; Zhao J.; Sun S.; Sun X. Application of molecular dynamics simulation in self-assembled cancer nanomedicine. Biomater. Res. 2023, 27 (1), 39. 10.1186/s40824-023-00386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia M. A.; Shurin M.; Shvedova A. A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. 10.1016/j.taap.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Song L.; Hu X.; Liu C.; Shi J.; Wang H.; Zhan L.; Song H. Inhibition of Epithelial–Mesenchymal Transition and Tissue Regeneration by Waterborne Titanium Dioxide Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10 (4), 3449–3458. 10.1021/acsami.7b18986. [DOI] [PubMed] [Google Scholar]
- Setyawati M. I.; Sevencan C.; Bay B. H.; Xie J.; Zhang Y.; Demokritou P.; Leong D. T. Nano-TiO2 Drives Epithelial–Mesenchymal Transition in Intestinal Epithelial Cancer Cells. Small 2018, 14 (30), 1800922 10.1002/smll.201800922. [DOI] [PubMed] [Google Scholar]
- Liu C.; Xie H.; Yu J.; Chen X.; Tang S.; Sun L.; Chen X.; Peng D.; Zhang X.; Zhou J. A targeted therapy for melanoma by graphene oxide composite with microRNA carrier. Drug Des., Dev. Ther. 2018, 12, 3095–3106. 10.2147/DDDT.S160088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.; Li B.; Xu M.; Liu R.; Xia T.; Zhang Z.; Xu Y.; Liu S. Graphene Oxide Promotes Cancer Metastasis through Associating with Plasma Membrane To Promote TGF-β Signaling-Dependent Epithelial–Mesenchymal Transition. ACS Nano 2020, 14 (1), 818–827. 10.1021/acsnano.9b07891. [DOI] [PubMed] [Google Scholar]
- Yang F.; Wang F.; Gao Z.-S.; Quang G.-Q.; Hu H.-B.; Zheng M. Capn4 regulates Snail to promote the epithelial–mesenchymal transition of nasopharyngeal carcinoma by mediating the transcriptional activity of claudin-11. Kaohsiung J. Med. Sci. 2023, 39 (2), 134–144. 10.1002/kjm2.12614. [DOI] [PubMed] [Google Scholar]
- Lin K.-b.; Fan F.-h.; Cai M.-q.; Yu Y.; Fu C.-l.; Ding L.-y.; Sun Y.-d.; Sun J.-w.; Shi Y.-w.; Dong Z.-f.; Yuan M.-J.; Li S.; Wang Y.-p.; Chen K.-k.; Zhu J.-n.; Guo X.-w.; Zhang X.; Zhao Y.-w.; Li J.-b.; Huang D. Systemic immune inflammation index and system inflammation response index are potential biomarkers of atrial fibrillation among the patients presenting with ischemic stroke. Eur. J. Med.Res. 2022, 27 (1), 106. 10.1186/s40001-022-00733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Li R.; Zhang B.; Cui X. TAF1 promotes NSCLC cell epithelial-mesenchymal transition by transcriptionally activating TGFβ1. Biochem. Biophys. Res. Commun. 2022, 636, 113–118. 10.1016/j.bbrc.2022.10.099. [DOI] [PubMed] [Google Scholar]
- Lemma R. B.; Fleischer T.; Martinsen E.; Ledsaak M.; Kristensen V.; Eskeland R.; Gabrielsen O. S.; Mathelier A. Pioneer transcription factors are associated with the modulation of DNA methylation patterns across cancers. Epigenet. Chromatin 2022, 15 (1), 13 10.1186/s13072-022-00444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Kröller L.; Miao B.; Boekhoff H.; Bauer A. S.; Büchler M. W.; Hackert T.; Giese N. A.; Taipale J.; Hoheisel J. D. Promoter Hypermethylation Promotes the Binding of Transcription Factor NFATc1, Triggering Oncogenic Gene Activation in Pancreatic Cancer. Cancers. 2021, 13 (18), 4569. 10.3390/cancers13184569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino C.; Mori F.; Cambria A.; Diamantini A.; Germoni S.; Alessandrini G.; Borsellino G.; Galati R.; Battistini L.; Blandino R.; Facciolo F.; Citro G.; Strano S.; Muti P.; Blandino G.; Cioce M. SASP mediates chemoresistance and tumor-initiating-activity of mesothelioma cells. Oncogene 2012, 31 (26), 3148–3163. 10.1038/onc.2011.485. [DOI] [PubMed] [Google Scholar]
- Toso A.; Revandkar A.; Di Mitri D.; Guccini I.; Proietti M.; Sarti M.; Pinton S.; Zhang J.; Kalathur M.; Civenni G.; Jarrossay D.; Montani E.; Marini C.; Garcia-Escudero R.; Scanziani E.; Grassi F.; Pandolfi P. P.; Catapano C. V.; Alimonti A. Enhancing Chemotherapy Efficacy in Pten-Deficient Prostate Tumors by Activating the Senescence-Associated Antitumor Immunity. Cell Rep. 2014, 9 (1), 75–89. 10.1016/j.celrep.2014.08.044. [DOI] [PubMed] [Google Scholar]
- Kim Y. H.; Choi Y. W.; Lee J.; Soh E. Y.; Kim J.-H.; Park T. J. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat. Commun. 2017, 8 (1), 15208. 10.1038/ncomms15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Ma C.; Duan Y.; Heinrich B.; Rosato U.; Diggs L. P.; Ma L.; Roy S.; Fu Q.; Brown Z. J.; Wabitsch S.; Thovarai V.; Fu J.; Feng D.; Ruf B.; Cui L. L.; Subramanyam V.; Frank K. M.; Wang S.; Kleiner D. E.; Ritz T.; Rupp C.; Gao B.; Longerich T.; Kroemer A.; Wang X. W.; Ruchirawat M.; Korangy F.; Schnabl B.; Trinchieri G.; Greten T. F. Gut Microbiome Directs Hepatocytes to Recruit MDSCs and Promote Cholangiocarcinoma. Cancer Discov 2021, 11 (5), 1248–1267. 10.1158/2159-8290.CD-20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.; Cheng J.; Zhang J.; Zhou F.; He X.; Shi Y.; Tao Y. The role of respiratory microbiota in lung cancer. Int. J. Biol. Sci. 2021, 17 (13), 3646. 10.7150/ijbs.51376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu D. K.-C.; Tse A. P.-W.; Xu I. M.-J.; Di Cui J.; Lai R. K.-H.; Li L. L.; Koh H.-Y.; Tsang F. H.-C.; Wei L. L.; Wong C.-M.; Ng I. O.-L.; Wong C. C.-L. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017, 8 (1), 517. 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachary B.; Berg-Dixon S.; Kelly B.; Agani F.; Feldser D.; Ferreira G.; Iyer N.; LaRusch J.; Pak B.; Taghavi P.; Semenza G. L. Regulation of Colon Carcinoma Cell Invasion by Hypoxia-Inducible Factor 11. Cancer Res. 2003, 63 (5), 1138–1143. [PubMed] [Google Scholar]
- Paulus P.; Stanley E. R.; Schäfer R.; Abraham D.; Aharinejad S. Colony-Stimulating Factor-1 Antibody Reverses Chemoresistance in Human MCF-7 Breast Cancer Xenografts. Cancer Res. 2006, 66 (8), 4349–4356. 10.1158/0008-5472.CAN-05-3523. [DOI] [PubMed] [Google Scholar]
- Cadamuro M.; Nardo G.; Indraccolo S.; Dall’Olmo L.; Sambado L.; Moserle L.; Franceschet I.; Colledan M.; Massani M.; Stecca T.; Bassi N.; Morton S.; Spirli C.; Fiorotto R.; Fabris L.; Strazzabosco M. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013, 58 (3), 1042–1053. 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Yao Y.; Gong C.; Yu F.; Su S.; Chen J.; Liu B.; Deng H.; Wang F.; Lin L.; Yao H.; Su F.; Anderson K. S.; Liu Q.; Ewen M. E.; Yao X.; Song E. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer cell 2011, 19 (4), 541–55. 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Xu Q.; Wu Y.; Li J.; Tang D.; Han L.; Fan Q. A CCL2/ROS autoregulation loop is critical for cancer-associated fibroblasts-enhanced tumor growth of oral squamous cell carcinoma. Carcinogenesis 2014, 35 (6), 1362–70. 10.1093/carcin/bgu046. [DOI] [PubMed] [Google Scholar]
- Addadi Y.; Moskovits N.; Granot D.; Lozano G.; Carmi Y.; Apte R. N.; Neeman M.; Oren M. p53 Status in Stromal Fibroblasts Modulates Tumor Growth in an SDF1-Dependent Manner. Cancer Res. 2010, 70 (23), 9650–9658. 10.1158/0008-5472.CAN-10-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue H.; Li W.; Chen R.; Wang J.; Lu X.; Li J. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecol. Oncol. 2021, 160 (2), 530–538. 10.1016/j.ygyno.2020.11.026. [DOI] [PubMed] [Google Scholar]
- Yang J.; Antin P.; Berx G.; Blanpain C.; Brabletz T.; Bronner M.; Campbell K.; Cano A.; Casanova J.; Christofori G.; Dedhar S.; Derynck R.; Ford H. L.; Fuxe J.; Garcia de Herreros A.; Goodall G. J.; Hadjantonakis A.-K.; Huang R. Y. J.; Kalcheim C.; Kalluri R.; Kang Y.; Khew-Goodall Y.; Levine H.; Liu J.; Longmore G. D.; Mani S. A.; Massague J.; Mayor R.; McClay D.; Mostov K. E.; Newgreen D. F.; Nieto M. A.; Puisieux A.; Runyan R.; Savagner P.; Stanger B.; Stemmler M. P.; Takahashi Y.; Takeichi M.; Theveneau E.; Thiery J. P.; Thompson E. W.; Weinberg R. A.; Williams E. D.; Xing J.; Zhou B. P.; Sheng G. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21 (6), 341–352. 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongre A.; Weinberg R. A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20 (2), 69–84. 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- Lamouille S.; Xu J.; Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15 (3), 178–196. 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H.; Biehs B.; Chiu C.; Siebel C. W.; Wu Y.; Costa M.; de Sauvage F. J.; Klein O. D. Opposing Activities of Notch and Wnt Signaling Regulate Intestinal Stem Cells and Gut Homeostasis. Cell Rep. 2015, 11 (1), 33–42. 10.1016/j.celrep.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai Y.; Zhang Y.; Xiong X.; Das S.; Bhattacharya R.; Mukherjee P. Gold Nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell stress 2019, 3 (8), 267–279. 10.15698/cst2019.08.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N. K.; Kaushik N.; Yoo K. C.; Uddin N.; Kim J. S.; Lee S. J.; Choi E. H. Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signaling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials 2016, 87, 118–130. 10.1016/j.biomaterials.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Agarwalla P.; Mukherjee S.; Sreedhar B.; Banerjee R. Glucocorticoid receptor-mediated delivery of nano gold–withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomedicine 2016, 11 (19), 2529–2546. 10.2217/nnm-2016-0224. [DOI] [PubMed] [Google Scholar]
- Wahab R.; Kaushik N.; Khan F.; Kaushik N. K.; Choi E. H.; Musarrat J.; Al-Khedhairy A. A. Self-Styled ZnO Nanostructures Promotes the Cancer Cell Damage and Supresses the Epithelial Phenotype of Glioblastoma. Sci. Rep. 2016, 6 (1), 19950. 10.1038/srep19950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil Gowda S. N.; Rajasowmiya S.; Vadivel V.; Banu Devi S.; Celestin Jerald A.; Marimuthu S.; Devipriya N. Gallic acid-coated sliver nanoparticle alters the expression of radiation-induced epithelial-mesenchymal transition in non-small lung cancer cells. Toxicol. In Vitro 2018, 52, 170–177. 10.1016/j.tiv.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Zhou B.; Luo H.; Mao J.; Huang Y.; Zhang K.; Mei C.; Yan Y.; Jin H.; Gao J.; Su Z.; Pang P.; Li D.; Shan H. ZnAs@SiO(2) nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling. Theranostics 2019, 9 (15), 4391–4408. 10.7150/thno.32462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-Y.; Chen C.-C.; Lin L.-T.; Chang C.-H.; Chen L.-C.; Wang H.-E.; Lee T.-W.; Lee Y.-J. PEGylated liposome-encapsulated rhenium-188 radiopharmaceutical inhibits proliferation and epithelial–mesenchymal transition of human head and neck cancer cells in vivo with repeated therapy. Cell Death Discovery 2018, 4 (1), 100. 10.1038/s41420-018-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa C.; Gouveia L. F.; Kreutzer B.; Silva-Lima B.; Maphasa R. E.; Dube A.; Videira M. Polymeric Micellar Formulation Enhances Antimicrobial and Anticancer Properties of Salinomycin. Pharm. Res. 2019, 36 (6), 83. 10.1007/s11095-019-2615-6. [DOI] [PubMed] [Google Scholar]
- Wang X.; Luo G.; Zhang K.; Cao J.; Huang C.; Jiang T.; Liu B.; Su L.; Qiu Z. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018, 78 (16), 4586–4598. 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- Yang B.; Feng X.; Liu H.; Tong R.; Wu J.; Li C.; Yu H.; Chen Y.; Cheng Q.; Chen J.; Cai X.; Wu W.; Lu Y.; Hu J.; Liang K.; Lv Z.; Wu J.; Zheng S. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene 2020, 39 (42), 6529–6543. 10.1038/s41388-020-01450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvani J. G.; Gujrati M. D.; Mack M. A.; Schiemann W. P.; Lu Z.-R. Silencing β3 Integrin by Targeted ECO/siRNA Nanoparticles Inhibits EMT and Metastasis of Triple-Negative Breast Cancer. Cancer Res. 2015, 75 (11), 2316–2325. 10.1158/0008-5472.CAN-14-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh D.; Zambre A.; Mukherjee S.; Ghoshdastidar S.; Jiang Y.; Joshi T.; Upendran A.; Kannan R. Silencing AXL by covalent siRNA-gelatin-antibody nanoconjugate inactivates mTOR/EMT pathway and stimulates p53 for TKI sensitization in NSCLC. Nanomedicine: Nanotechnology, Biol. Med. 2019, 20, 102007 10.1016/j.nano.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Morikawa M.; Derynck R.; Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harbor Perspect. Biol. 2016, 8 (5), a021873. 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L.; Robin T. P.; Ford H. L. Molecular Pathways: Targeting the TGF-β Pathway for Cancer Therapy. Clin. Cancer Res. 2012, 18 (17), 4514–4521. 10.1158/1078-0432.CCR-11-3224. [DOI] [PubMed] [Google Scholar]
- Kim B.-G.; Malek E.; Choi S. H.; Ignatz-Hoover J. J.; Driscoll J. J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 2021, 14 (1), 55. 10.1186/s13045-021-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.; Lamouille S.; Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009, 19 (2), 156–172. 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A.; Vizán P.; Das D.; Chakravarty P.; Vogt J.; Rogers K. W; Müller P.; Hinck A. P; Sapkota G. P; Hill C. S TGF-β uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. elife 2018, 7, e31756. 10.7554/eLife.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harbor Perspect. Biol. 2017, 9 (2), a022129. 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mii S.; Enomoto A.; Shiraki Y.; Taki T.; Murakumo Y.; Takahashi M. CD109: a multifunctional GPI-anchored protein with key roles in tumor progression and physiological homeostasis. Pathol. Int. 2019, 69 (5), 249–259. 10.1111/pin.12798. [DOI] [PubMed] [Google Scholar]
- Nusse R.; Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982, 31 (1), 99–109. 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Tammela T.; Sanchez-Rivera F. J.; Cetinbas N. M.; Wu K.; Joshi N. S.; Helenius K.; Park Y.; Azimi R.; Kerper N. R.; Wesselhoeft R. A.; Gu X.; Schmidt L.; Cornwall-Brady M.; Yilmaz Ö. H.; Xue W.; Katajisto P.; Bhutkar A.; Jacks T. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 2017, 545 (7654), 355–359. 10.1038/nature22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127 (3), 469–480. 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Lien W. H.; Fuchs E. Wnt some lose some: transcriptional governance of stem cells by Wnt/β-catenin signaling. Genes Dev. 2014, 28 (14), 1517–32. 10.1101/gad.244772.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan T.; Rindtorff N.; Boutros M. Wnt signaling in cancer. Oncogene 2017, 36 (11), 1461–1473. 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral T. S.; Chan M.; Peshkin L.; Sorger P. K.; Kirschner M. W.; MacBeath G. A Noncanonical Frizzled2 Pathway Regulates Epithelial-Mesenchymal Transition and Metastasis. Cell 2014, 159 (4), 844–856. 10.1016/j.cell.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath D.; Li X.; Mondragon C.; Post D.; Chen M.; White J. R.; Hryniewicz-Jankowska A.; Caza T.; Kuznetsov V. A.; Hehnly H.; Jamaspishvili T.; Berman D. M.; Zhang F.; Kung S. H. Y.; Fazli L.; Gleave M. E.; Bratslavsky G.; Pandolfi P. P.; Kotula L. Abi1 loss drives prostate tumorigenesis through activation of EMT and non-canonical WNT signaling. Cell Commun. Signaling 2019, 17 (1), 120 10.1186/s12964-019-0410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. H.; Wang W. L.; Chen C. Y.; Chang Y. L.; Wu Y. Y.; Wang Y. T.; Wang S. P.; Nesvizhskii A. I.; Chen Y. J.; Hong T. M.; Yang P. C. GSK3β controls epithelial–mesenchymal transition and tumor metastasis by CHIP-mediated degradation of Slug. Oncogene 2014, 33 (24), 3172–3182. 10.1038/onc.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q.; Xia W.; Liu J. C.; Yang J. Y.; Lee D. F.; Xia J.; Bartholomeusz G.; Li Y.; Pan Y.; Li Z.; Bargou R. C.; Qin J.; Lai C. C.; Tsai F. J.; Tsai C. H.; Hung M. C. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol. Cell 2005, 19 (2), 159–70. 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Tee J. K.; Yip L. X.; Tan E. S.; Santitewagun S.; Prasath A.; Ke P. C.; Ho H. K.; Leong D. T. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48 (21), 5381–5407. 10.1039/C9CS00309F. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L.; Dejana E.; McDonald D. M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends in Molecular Medicine 2021, 27 (4), 314–331. 10.1016/j.molmed.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude P.; Goodenough D. A. Fracture faces of zonulae occludentes from ″tight″ and ″leaky″ epithelia. J. Cell Biol. 1973, 58 (2), 390–400. 10.1083/jcb.58.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A.; Mukherjee T. M.; Williams A. W. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma 1969, 67 (2), 165–84. 10.1007/BF01248737. [DOI] [PubMed] [Google Scholar]
- Hamazaki Y.; Itoh M.; Sasaki H.; Furuse M.; Tsukita S. Multi-PDZ Domain Protein 1 (MUPP1) Is Concentrated at Tight Junctions through Its Possible Interaction with Claudin-1 and Junctional Adhesion Molecule *. J. Biol. Chem. 2002, 277 (1), 455–461. 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- Zihni C.; Mills C.; Matter K.; Balda M. S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17 (9), 564–580. 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- Wettschureck N.; Strilic B.; Offermanns S. Passing the Vascular Barrier: Endothelial Signaling Processes Controlling Extravasation. Physiol. Rev. 2019, 99 (3), 1467–1525. 10.1152/physrev.00037.2018. [DOI] [PubMed] [Google Scholar]
- Xu S.; Pang X.; Zhang X.; Lv Q.; Zhang M.; Wang J.; Ni N.; Sun X. Nanomaterials disrupting cell-cell junctions towards various diseases. Nano Res. 2023, 16 (5), 7053–7074. 10.1007/s12274-023-5455-y. [DOI] [Google Scholar]
- Hoeben A.; Landuyt B.; Highley M. S.; Wildiers H.; Van Oosterom A. T.; De Bruijn E. A. Vascular Endothelial Growth Factor and Angiogenesis. Pharmacol. Rev. 2004, 56 (4), 549. 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- Drabsch Y.; ten Dijke P. TGF-β Signaling in Breast Cancer Cell Invasion and Bone Metastasis. J. Mammary Gland Biol. Neoplasia 2011, 16 (2), 97–108. 10.1007/s10911-011-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond N.; d’Água B. B.; Ridley A. J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 2013, 13 (12), 858–870. 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- Carman C. V.; Springer T. A. Trans-cellular migration: cell–cell contacts get intimate. Curr. Opin. Cell Biol. 2008, 20 (5), 533–540. 10.1016/j.ceb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. Relevance of endothelial junctions in leukocyte extravasation and vascular permeability. Ann. N.Y. Acad. Sci. 2012, 1257 (1), 184–192. 10.1111/j.1749-6632.2012.06558.x. [DOI] [PubMed] [Google Scholar]
- Tee J. K.; Yip L. X.; Tan E. S.; Santitewagun S.; Prasath A.; Ke P. C.; Ho H. K.; Leong D. T. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48 (21), 5381–5407. 10.1039/C9CS00309F. [DOI] [PubMed] [Google Scholar]
- Setyawati M. I.; Tay C. Y.; Chia S. L.; Goh S. L.; Fang W.; Neo M. J.; Chong H. C.; Tan S. M.; Loo S. C. J.; Ng K. W.; Xie J. P.; Ong C. N.; Tan N. S.; Leong D. T. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE–cadherin. Nat. Commun. 2013, 4 (1), 1673. 10.1038/ncomms2655. [DOI] [PubMed] [Google Scholar]
- Setyawati M. I.; Tay C. Y.; Bay B. H.; Leong D. T. Gold Nanoparticles Induced Endothelial Leakiness Depends on Particle Size and Endothelial Cell Origin. ACS Nano 2017, 11 (5), 5020–5030. 10.1021/acsnano.7b01744. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhang L.; Peng F.; Shi X.; Leong D. T. Targeting Endothelial Cell Junctions with Negatively Charged Gold Nanoparticles. Chem. Mater. 2018, 30 (11), 3759–3767. 10.1021/acs.chemmater.8b00840. [DOI] [Google Scholar]
- Tay C. Y.; Setyawati M. I.; Leong D. T. Nanoparticle Density: A Critical Biophysical Regulator of Endothelial Permeability. ACS Nano 2017, 11 (3), 2764–2772. 10.1021/acsnano.6b07806. [DOI] [PubMed] [Google Scholar]
- Ni N.; Su Y.; Wei Y.; Ma Y.; Zhao L.; Sun X. Tuning Nanosiliceous Framework for Enhanced Cancer Theranostic Applications. Adv. Ther. 2021, 4 (4), 2000218 10.1002/adtp.202000218. [DOI] [Google Scholar]
- Peng F.; Setyawati M. I.; Tee J. K.; Ding X.; Wang J.; Nga M. E.; Ho H. K.; Leong D. T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14 (3), 279–286. 10.1038/s41565-018-0356-z. [DOI] [PubMed] [Google Scholar]
- Setyawati M. I.; Mochalin V. N.; Leong D. T. Tuning Endothelial Permeability with Functionalized Nanodiamonds. ACS Nano 2016, 10 (1), 1170–1181. 10.1021/acsnano.5b06487. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Zhang C.; Xiang P.; Shen J.; Sun W.; Yu H. Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells. J. Extracell. Vesicles 2020, 9 (1), 1722385 10.1080/20013078.2020.1722385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen J.; Feng L.; Ke H.; Bao L.; Li L. Z.; Tanaka Y.; Weng J.; Su L. Exosomal Thrombospondin-1 Disrupts the Integrity of Endothelial Intercellular Junctions to Facilitate Breast Cancer Cell Metastasis. Cancers. Cancers 2019, 11 (12), 1946. 10.3390/cancers11121946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.; Li Y.; Lee M.; Andrikopoulos N.; Lin S.; Chen C.; Leong D. T.; Ding F.; Song Y.; Ke P. C. Anionic nanoplastic exposure induces endothelial leakiness. Nat. Commun. 2022, 13 (1), 4757. 10.1038/s41467-022-32532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. Hallmarks of cancer: the next generation. Cell 2011, 144 (5), 646–74. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Pagès F.; Galon J.; Dieu-Nosjean M. C.; Tartour E.; Sautès-Fridman C.; Fridman W. H. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2010, 29 (8), 1093–1102. 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- Grivennikov S. I.; Greten F. R.; Karin M. Immunity, inflammation, and cancer. Cell 2010, 140 (6), 883–99. 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X.; Zhu Y.; Bai R.; Wu Z.; Qian W.; Yang L.; Cai R.; Yan H.; Li T.; Pandey V.; Liu Y.; Lobie P. E.; Chen C.; Zhu T. Long-term pulmonary exposure to multi-walled carbon nanotubes promotes breast cancer metastatic cascades. Nat. Nanotechnol. 2019, 14 (7), 719–727. 10.1038/s41565-019-0472-4. [DOI] [PubMed] [Google Scholar]
- Bauernfeind F.; Ablasser A.; Bartok E.; Kim S.; Schmid-Burgk J.; Cavlar T.; Hornung V. Inflammasomes: current understanding and open questions. Cell. Mol. Life Sci. 2011, 68 (5), 765–783. 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q.; Zhao F.; Guo F.; Wang C.; Fu Z. Polymeric Nanoparticles Induce NLRP3 Inflammasome Activation and Promote Breast Cancer Metastasis. Macromol. Biosci. 2017, 17 (12), 1700273 10.1002/mabi.201700273. [DOI] [PubMed] [Google Scholar]
- Manshian B. B.; Poelmans J.; Saini S.; Pokhrel S.; Grez J. J.; Himmelreich U.; Mädler L.; Soenen S. J. Nanoparticle-induced inflammation can increase tumor malignancy. Acta Biomater. 2018, 68, 99–112. 10.1016/j.actbio.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Ganguly P.; Breen A.; Pillai S. C. Toxicity of Nanomaterials: Exposure, Pathways, Assessment, and Recent Advances. ACS Biomater. Sci. Eng. 2018, 4 (7), 2237–2275. 10.1021/acsbiomaterials.8b00068. [DOI] [PubMed] [Google Scholar]
- Arora S.; Rajwade J. M.; Paknikar K. M. Nanotoxicology and in vitro studies: The need of the hour. Toxicol. Appl. Pharmacol. 2012, 258 (2), 151–165. 10.1016/j.taap.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Hartwig A.; van Thriel C. Risk Assessment of Nanomaterials Toxicity. Nanomaterials. 2023, 13 (9), 1512. 10.3390/nano13091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A.; Wang W.-X. Human Exposure to Microplastics and Its Associated Health Risks. Environ. Health 2023, 1 (3), 139–149. 10.1021/envhealth.3c00053. [DOI] [Google Scholar]
- Wu B.; Jiang M.; Liu X.; Huang C.; Gu Z.; Cao Y. Evaluation of toxicity of halloysite nanotubes and multi-walled carbon nanotubes to endothelial cells in vitro and blood vessels in vivo. Nanotoxicology 2020, 14 (8), 1017–1038. 10.1080/17435390.2020.1780642. [DOI] [PubMed] [Google Scholar]
- Ni N.; Zhang X.; Ma Y.; Yuan J.; Wang D.; Ma G.; Dong J.; Sun X. Biodegradable two-dimensional nanomaterials for cancer theranostics. Coord. Chem. Rev. 2022, 458, 214415 10.1016/j.ccr.2022.214415. [DOI] [Google Scholar]
- Nieto M. A.; Huang R. Y.-J.; Jackson R. A.; Thiery J. P. EMT: 2016. Cell 2016, 166 (1), 21–45. 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Pastushenko I.; Brisebarre A.; Sifrim A.; Fioramonti M.; Revenco T.; Boumahdi S.; Van Keymeulen A.; Brown D.; Moers V.; Lemaire S.; De Clercq S.; Minguijón E.; Balsat C.; Sokolow Y.; Dubois C.; De Cock F.; Scozzaro S.; Sopena F.; Lanas A.; D’Haene N.; Salmon I.; Marine J.-C.; Voet T.; Sotiropoulou P. A.; Blanpain C. Identification of the tumour transition states occurring during EMT. Nature 2018, 556 (7702), 463–468. 10.1038/s41586-018-0040-3. [DOI] [PubMed] [Google Scholar]
- Pastushenko I.; Blanpain C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29 (3), 212–226. 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Krebs A. M.; Mitschke J.; Lasierra Losada M.; Schmalhofer O.; Boerries M.; Busch H.; Boettcher M.; Mougiakakos D.; Reichardt W.; Bronsert P.; Brunton V. G.; Pilarsky C.; Winkler T. H.; Brabletz S.; Stemmler M. P.; Brabletz T. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 2017, 19 (5), 518–529. 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- Ocaña O. H.; Córcoles R.; Fabra Á.; Moreno-Bueno G.; Acloque H.; Vega S.; Barrallo-Gimeno A.; Cano A.; Nieto M. A. Metastatic Colonization Requires the Repression of the Epithelial-Mesenchymal Transition Inducer Prrx1. Cancer cell 2012, 22 (6), 709–724. 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Liang P.; He X.; Wu B.; Liu Q.; Xu Z.; Wu H.; Liu Z.; Qian Y.; Wang S.; Zhu R. Etoposide loaded layered double hydroxide nanoparticles reversing chemoresistance and eradicating human glioma stem cells in vitro and in vivo. Nanoscale 2018, 10 (27), 13106–13121. 10.1039/C8NR02708K. [DOI] [PubMed] [Google Scholar]
- Cordani M.; Strippoli R.; Somoza Á. Nanomaterials as Inhibitors of Epithelial Mesenchymal Transition in Cancer Treatment. Cancers 2020, 12 (1), 25. 10.3390/cancers12010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhwani S.; Syed A. M.; Ngai J.; Kingston B. R.; Maiorino L.; Rothschild J.; MacMillan P.; Zhang Y.; Rajesh N. U.; Hoang T.; Wu J. L. Y.; Wilhelm S.; Zilman A.; Gadde S.; Sulaiman A.; Ouyang B.; Lin Z.; Wang L.; Egeblad M.; Chan W. C. W. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19 (5), 566–575. 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- Ni N.; Wang W.; Sun Y.; Sun X.; Leong D. T. Inducible endothelial leakiness in nanotherapeutic applications. Biomaterials 2022, 287, 121640 10.1016/j.biomaterials.2022.121640. [DOI] [PubMed] [Google Scholar]