Abstract
Plantacyanins belong to the phytocyanin family of blue copper proteins. In the Arabidopsis (Arabidopsis thaliana) genome, only one gene encodes plantacyanin. The T-DNA-tagged mutant is a knockdown mutant that shows no visible phenotype. We used both promoter-β-glucuronidase transgenic plants and immunolocalization to show that Arabidopsis plantacyanin is expressed most highly in the inflorescence and, specifically, in the transmitting tract of the pistil. Protein levels show a steep gradient in expression from the stigma into the style and ovary. Overexpression plants were generated using cauliflower mosaic virus 35S, and protein levels in the pistil were examined as well as the pollination process. Seed set in these plants is highly reduced mainly due to a lack of anther dehiscence, which is caused by degeneration of the endothecium. Callose deposits occur on the pollen walls in plants that overexpress plantacyanin, and a small percentage of these pollen grains germinate in the closed anthers. When wild-type pollen was used on the overexpression stigma, seed set was still decreased compared to the control pollinations. We detected an increase in plantacyanin levels in the overexpression pistil, including the transmitting tract. Guidance of the wild-type pollen tube on the overexpression stigma is disrupted as evidenced by the growth behavior of pollen tubes after they penetrate the papillar cell. Normally, pollen tubes travel down the papilla cell and into the style. Wild-type pollen tubes on the overexpression stigma made numerous turns around the papilla cell before growing toward the style. In some rare cases, pollen tubes circled up the papilla cell away from the style and were arrested there. We propose that when plantacyanin levels in the stigma are increased, pollen tube guidance into the style is disrupted.
Pollination is a crucial step in the life cycle of flowering plants. The pistil, composed of the stigma, style, and ovary, is the female receptive organ in pollination through which the pollen tube travels to deliver the sperm cells to the egg. The stigma, as the entry into the pistil's specialized transmitting tract tissue, provides a receptive surface for compatible pollen to adhere, hydrate, germinate, and grow. Crucifers, like Arabidopsis (Arabidopsis thaliana), have dry stigmas, which do not secrete much extracellular matrix (ECM) for the pollen tube to encounter on their surface. The pollen tube must penetrate into the cell wall space of the papilla cell to enter the transmitting tract ECM. From there, the pollen tube tracks the secretory ECM to the micropyle of the ovule for fertilization. By contrast, in lily (Lilium longiflorum), pollen tubes land on a stigma with copious secretions and enter an open style to grow on an epidermal, secretory ECM, never penetrating stigma or stylar tissues. In these two extreme cases, though, pollen tubes are in contact with the ECM of the pistil transmitting tract from the stigma to the ovule for fertilization (Lord and Russell, 2002). For this reason, pollen tube chemotropic molecules have been sought from the ECM of pistil tissues. So far, a few such pistil ECM molecules have been implicated in pollen tube growth and directional guidance (Wu et al., 1995; Wolters-Arts et al., 1998; Kim et al., 2003; Palanivelu et al., 2003; Márton et al., 2005). Our knowledge of the proteins involved in regulation of pollen activities in the stigma, however, is very limited, except for those involved in recognition events in self-incompatibility, such as S-locus receptor kinase and S-locus glycoproteins in Brassica (Dixit and Nasrallah, 2001; Takayama et al., 2001).
Plantacyanins are ECM proteins of unknown function. They belong to the ancient, plant-specific phytocyanins, a subfamily of blue copper proteins (Ryden and Hunt, 1993). So far, most of the research on plantacyanins has been on their biochemical characteristics (for review, see Aikazyan and Nalbandyan, 1981; Sakurai, 1986; Nersissian et al., 1985, 1991; Nersissian and Nalbandyan, 1988), with much attention drawn to their spectroscopic and redox properties (Nersissian et al., 1998). Lily chemocyanin was the first functionally characterized plantacyanin (Kim et al., 2003). Chemocyanin and stigma/stylar Cys-rich adhesion, another stigma protein, together can form a gradient in vitro that attracts lily pollen tubes. Recent microarray data suggest that plantacyanins may also be stress-related proteins and be involved in plant defense responses (Kreps et al., 2002; Provart et al., 2003; Hampton et al., 2004). Other data also suggest that plantacyanins function as signaling molecules because they were found to be nodule specific in legumes (Fedorova et al., 2002) and to bind to tobacco (Nicotiana tabacum) S-RNase, which is involved in self-pollen rejection (Cruz-Garcia et al., 2005). Arabidopsis plantacyanin was recently predicted to be one of the targets of microRNAs (miRNAs), miR408 (Sunkar and Zhu, 2004). This finding is very interesting because some of the miRNA targets in plants are transcription factors involved in different aspects of plant development (Llave et al., 2002; Rhoades et al., 2002; Aukerman and Sakai, 2003; Chen, 2004).
The Arabidopsis genome contains a single plantacyanin gene (protein ID At2g02850). It shows 51.9% identity and 86.8% similarity to lily chemocyanin at the amino acid level (Kim et al., 2003). Here, we studied the expression and localization of Arabidopsis plantacyanin, focusing on the tissues of the pistil and anther. We also characterized the phenotype of Arabidopsis transgenic plantacyanin overexpression plants (OXPs), especially their defects in pollination. Our data suggest a role for Arabidopsis plantacyanin both in anther development and in pollination in Arabidopsis.
RESULTS
Determination of the Arabidopsis plantacyanin Expression Pattern
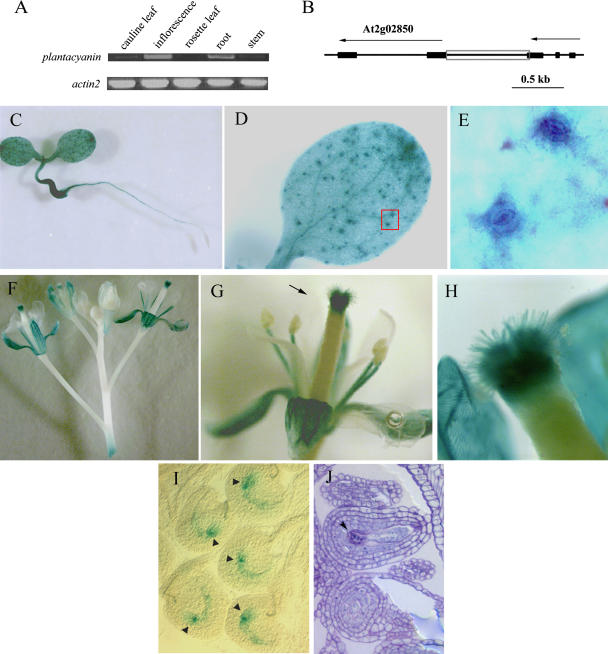
The expression patterns of Arabidopsis plantacyanin were first determined by reverse transcription (RT)-PCR. Gene-specific primers were utilized to amplify plantacyanin from the first-strand cDNA, which was reverse transcribed from total RNAs from tissues of a mature plant (6 weeks old). The highest level was found in Arabidopsis inflorescences, and moderate levels were found in the roots (Fig. 1A). Cauline leaves and stems had low levels of plantacyanin transcription and so exhibited a very low amplification in RT-PCR. We barely see an amplification using rosette leaves. Repeated (3×) RT-PCR experiments demonstrated similar expression patterns of plantacyanin in mature Arabidopsis plants.
Figure 1.
Expression patterns of Arabidopsis plantacyanin revealed by RT-PCR and transgenic lines expressing GUS driven by the Arabidopsis plantacyanin promoter region. A, Equal amounts of total RNA (1.2 μg) extracted from different plant organs were used as the starting material for RT-PCR. Thirty cycles were carried out for PCR amplification. Actin2 was amplified as a control. B, The plantacyanin promoter region (821 bp; gray box) was amplified by PCR for the plantacyanin promoter-GUS construct. Exons are represented by black boxes and introns by lines. Arrows indicate the orientation of the gene. The Arabidopsis plantacyanin protein ID is At2g02850. C to E, GUS expression in 10-d-old seedlings grown on Murashige and Skoog medium. GUS expression was detected in the whole seedling, except the root apical meristem (C). E, Enlarged view of D, red box showing guard cells. F to H, GUS expression was detected in the reproductive tissues. In addition to sepals and stamen filaments, GUS signals were strongly expressed in the stigma/style, starting from flower stage 11 (when stigma papillae begin to elongate; data not shown) and showing the highest level at stage 13 (G; when pollination occurs). H, Enlarged view of the style shown in G (arrow). No GUS signal was found in mature anther and pollen. I and J, GUS signal also appeared in the mature embryo sac. Arrows are at the chalazal end of the embryo sac. A toluidine blue-stained section (J) shows a longitudinal view of the mature embryo sac.
To further characterize the Arabidopsis plantacyanin expression pattern, we generated transgenic plants harboring the β-d-glucuronidase (GUS) gene driven by the plantacyanin promoter. There is only a short DNA fragment (about 800 bp) between the Arabidopsis plantacyanin (At2g02850) gene and the upstream gene (Fig. 1B). This piece of DNA was used as the promoter region of plantacyanin to drive GUS expression. In 10-d-old seedlings, GUS signals were detected in almost all tissues (cotyledons, hypocotyls, and vascular bundles of roots), except in the root apical meristem (Fig. 1C). GUS expression was not only found in mesophyll cells and vascular bundles (Fig. 1D), but also was prevalent in guard cells (Fig. 1E). plantacyanin promoter activity was detected in mature rosette and cauline leaves (data not shown), but at a very low level, consistent with the RT-PCR result.
We carefully examined the GUS signals in the inflorescences of the transgenic plants (Fig. 1F). Overall, inflorescences showed strong plantacyanin promoter activity as revealed by RT-PCR. GUS expression was found in the pistil, with the strongest level in the style (Fig. 1G). We also saw GUS expression in stigmatic papilla cells starting at flower stage 11 (stages as defined by Smyth et al., 1990) when these cells initiate. GUS signals reached the highest level in papilla cells and the style at flower stage 13, when pollination occurs (Fig. 1H). Pollen deposited on the stigma did not show GUS expression. We have also seen GUS expression exhibited in the mature embryo sacs of ovules before and after fertilization (Fig. 1I). A higher level of GUS expression was found in the chalazal end of the embryo sac (Fig. 1I, arrowheads). GUS expression was detected as well in the cell layer right beneath the seed coat (data not shown). Besides the pistil, sepals and stamen filaments but not petals also showed GUS staining (Fig. 1, F and G). No GUS signal was detected in the anthers or the pollen at flower stage 12 to 13 (Fig. 1G), although very weak GUS expression in anther tissues was detected at an early stage (9–10) when the tapetum is still present (data not shown).
Plantacyanin Localizes to the Transmitting Tissues in the Arabidopsis Pistil
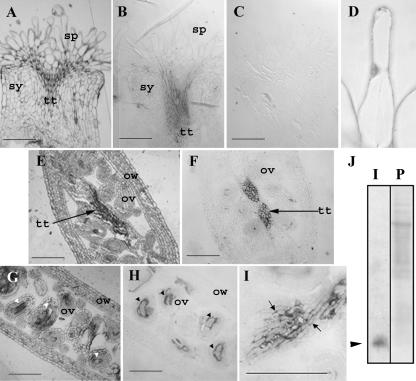
In order to investigate the subcellular expression patterns of plantacyanin in Arabidopsis flowers, we carried out an immunohistochemical analysis on wild-type tissues of the pistil. Tissues were fixed and embedded in LR White (Fig. 2, A, E, and G, are sections stained with toluidine blue after immunolocalization; Fig. 2, B–D, F, H, and I, are immunolocalizations, with C being a preimmune control). The polyclonal antibodies against Escherichia coli-expressed plantacyanin specifically recognized plantacyanin on a protein blot with total cell proteins extracted from wild-type inflorescences (Fig. 2J). A plantacyanin gradient was detected in the stigma and style, with a lower level in the stigma (Fig. 2, B and D) and a much higher level in the stylar (Fig. 2B) and ovary (Fig. 2F) transmitting tract tissues. Preimmune serum gave low to no background labeling (Fig. 2C). Plantacyanin levels were high as well in the mature embryo sac (Fig. 2H). It is evident, at a higher magnification, that plantacyanin is a cell wall protein secreted into the ECM of the transmitting tract tissues (Fig. 2I).
Figure 2.
Plantacyanin is localized to the wild-type Arabidopsis stigma and to the transmitting tract tissues of the style and ovary. Toluidine blue-stained sections after immunolocalization (A, E, G) show the tissue structures of the Arabidopsis pistil. Immunolocalizations (B–D, F, H, and I), using an antibody against recombinant plantacyanin made in E. coli, show that the transmitting tract and the embryo sacs produce plantacyanin protein. No signal was found when the preimmune serum was incubated with the section (C). The expression of Arabidopsis plantacyanin in the stigmatic papilla cell was detected at a very low level on the LR White-embedded sections (fixation with FAA; B), and at a slightly higher level using glutaraldehyde fixation (D). Expression in the stylar transmitting tract is high (B). Ovary transmitting tract tissues (the septum; F) and the embryo sacs show strong localization as well (arrowheads in G and H, longitudinal sections at flower stage 12). An enlarged image of the septum transmitting tract cells (I) shows the extracellular localization of plantacyanin (arrows). The antibody used for immunolocalization recognized a single plantacyanin band (arrowhead in J) on an immunoblot analysis (labeled as I in J) of total cell proteins extracted from Arabidopsis inflorescences; the blot was stained with Ponceau S (labeled as P in J) before immunoblotting. sp, Stigmatic papilla cell; sy, style; tt, transmitting track; ov, ovule; ow, ovary wall. Bars = 100 μm.
Genetic Approaches to Dissect the Function of Arabidopsis Plantacyanin
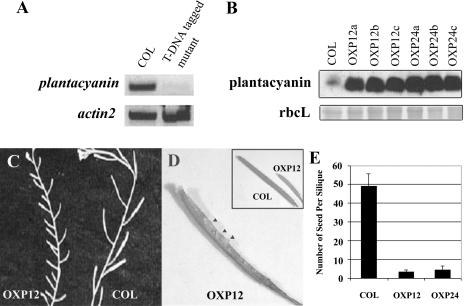
To dissect the function of Arabidopsis plantacyanin, we obtained a T-DNA-tagged mutant line (SALK_091945) from the Arabidopsis Biological Resource Center (ABRC), with the T-DNA integrated into the intron region of the gene. Unfortunately, it proved to be a knockdown line based on the RT-PCR result (Fig. 3A), and we did not see any visible phenotype under normal growth conditions. Wild-type pollen on the mutant pistil did not exhibit the aberrant pollen tube growth we observed in the OXPs (see below). Lack of a phenotype was most likely due to residual plantacyanin expression in these plants. Immunolocalizations detected plantacyanin protein in mutant pistils, but without a detectable difference from wild type (data not shown). Thus, we resorted to the overexpression approach to determine the function of plantacyanin in reproduction.
Figure 3.
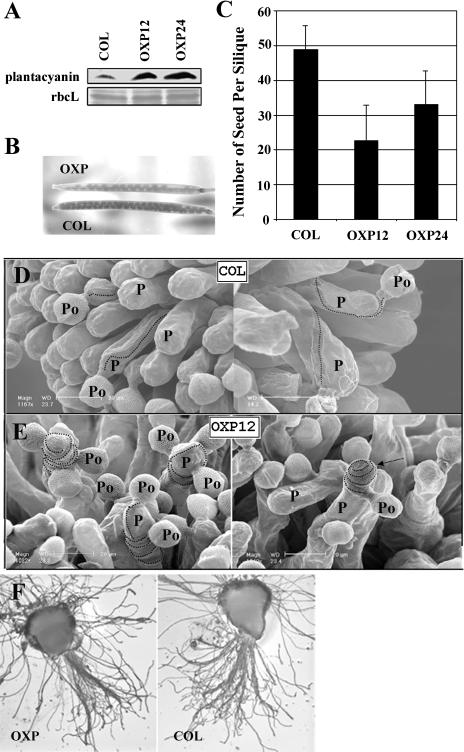
The T-DNA-tagged mutant is a knockdown mutant and does not show an obvious phenotype, but overexpression of plantacyanin in Arabidopsis results in reduced seed set. A, RT-PCR analysis revealed that the T-DNA-tagged mutant, with a T-DNA fragment inserted in the intron region of the Arabidopsis plantacyanin gene, is not a null allele. One microgram of total RNA extracted from inflorescences was used for RT-PCR and amplified for 30 cycles. Actin2 was amplified as a loading control. B, Plantacyanin expression levels were much higher in inflorescences of Arabidopsis OXPs (OXP12a–c and OXP24a–c; homozygous T2 plants) than in the wild type (COL). Rubisco large subunit was stained with Ponceau S as a protein-loading control. C, Siliques on the 6-week-old plants are shorter in OXP12 (T1 generation) compared to the wild type (COL). Arrowheads point to the very few seeds formed in a silique of OXP12. E, Number of seeds formed per silique in the overexpression transgenic lines showed a dramatic decrease compared with the wild type (COL). At least five siliques were randomly picked and opened for seed counts from each line. The values are mean ± sd.
Overexpression Lines Show Lowered Seed Set Due to Defects in Both Anther Development and Pistil Function
In order to create Arabidopsis plantacyanin OXPs, we used a cauliflower mosaic virus (CaMV) 35S promoter to drive protein expression in the plant. Inflorescences of homozygous transgenic lines (T2) contain higher levels of plantacyanin, compared to wild type, on an immunoblot detected with antibodies against E. coli-expressed plantacyanin (Fig. 3B). Many fewer seeds formed in these Arabidopsis plantacyanin overexpressor siliques (Fig. 3C), which are shorter than the wild type as a result (Fig. 3D, inset). Seed formation was dramatically decreased in OXP lines (T1) when siliques were randomly picked for seed counts (Fig. 3E). In the T1 generation, 33% of OXPs (23 out of 70 lines) displayed a lower seed set.
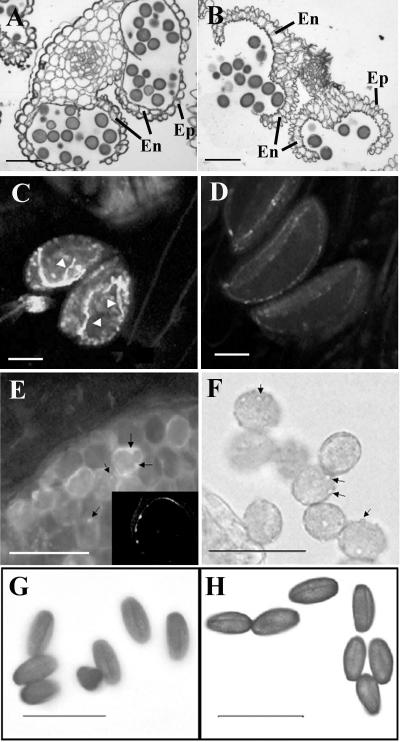
The overexpression anthers from the most severe lines, such as OXP12 and OXP24, showed defects in anther dehiscence. Toluidine blue-stained sections of the mature indehiscent anthers showed a malformed or absent endothecium, which could contribute to the lack of dehiscence (Fig. 4, A and B). The endothecium and tapetum initiated normally at an earlier stage (data not shown), but the endothecium had degenerated by stage 14 in the overexpression anthers (Fig. 4A). At this stage, the endothecium is active in anther dehiscence in the wild type (Fig. 4B). In many overexpression anthers, we observed a low number of pollen grains (≤5%) germinating precociously inside the closed anthers at flower stage 12 (Fig. 4C), corresponding to stage 13 of anther development. This abnormality was never seen in the wild-type anthers at the same stage (Fig. 4D). Pollen grains from the overexpression anthers had a bumpy surface (Fig. 4F) compared with the relatively smooth surface of a wild-type pollen grain. When stained with decolorized aniline blue, the bumps were revealed as depositions of callose (Fig. 4E). The callose staining of wild-type pollen grains was negligible. A confocal single optical section, while scanning a pollen grain inside the overexpression anther, reveals that the abnormal callose deposition was on the pollen grain wall and not inside the cell (Fig. 4E, arrows and inset). The irregularities of pollen in growth and callose wall formation were detected only at late stages of overexpression anther development (after flower stage 12). Pollen in the overexpression anthers dehydrated normally, as compared to the wild-type pollen (Fig. 4, G and H), and it resulted in full seed set when used to hand pollinate a wild-type pistil (data not shown).
Figure 4.
Arabidopsis OXPs show defects in anther dehiscence and pollen irregularities. Toluidine blue-stained cross-sections revealed the anther structure (stage 14 of anther development; Sanders et al. [1999]) of Arabidopsis OXP (A) and wild type (B). No visible differences between overexpression and wild-type anthers were detected in early stages of anther development (data not shown). At stage 14, the plantacyanin overexpression anthers (A) are indehiscent compared to the wild type (B), which are dehiscent at this stage. The endothecium of the overexpression anthers is degenerated by stage 14 (A), while it is intact and has functioned in dehiscence in the wild type (B). C, Precocious pollen germination (arrowheads on pollen tubes) occurred at a low level in many of the nondehiscent overexpression anthers (visualized by decolorized aniline blue staining and fluorescence imaging). D, Using the same method, pollen grains were barely visible in wild-type anthers, and no precocious germination was evident. E, Pollen of overexpression anthers showed callose depositions on the pollen wall (arrows, viewed by decolorized aniline blue under fluorescence imaging). E, Inset, Single confocal optical section of an enlarged pollen grain showing the callose is on the wall and not inside the cell. F, When viewed under the light microscope, hydrated pollen grains from the overexpression lines exhibited bumps on their surface (arrows), while the surface of wild-type pollen was smooth (data not shown). Pollen grains, hand-dissected from overexpression anthers, show normal dehydration and are not detectably different from wild-type pollen (H). En, Endothecium; Ep, epidermis. Bars = 50 μm.
Pollen Tubes Lose Directional Growth on the Plantacyanin Overexpression Stigma But Eventually Penetrate the Style and Enter the Ovary
We further analyzed plantacyanin levels in pistils of the overexpression lines in stage 13 flowers, when pollination occurs. We found more plantacyanin protein in these overexpression pistils compared to the wild type (Fig. 5A). The overexpression pistils pollinated with wild-type pollen produced about one-half the number of seeds as the wild-type control (Fig. 5, B and C).
Figure 5.
Plantacyanin overexpression lines were used to examine the effect of increased levels of Arabidopsis plantacyanin in the stigma on pollination with wild-type (COL) pollen. Plantacyanin protein levels in the pistils (A) at flower stage 12 to 13 of OXPs (homozygous T2 generation) are much higher than those of the wild type (COL), as revealed by protein blots. The protein-loading control used was Ponceau S staining of Rubisco large subunit. B and C, Overexpression pistils pollinated with wild-type pollen produce siliques with fewer seeds than the wild type (COL). Number of T2 homozygous pistils hand pollinated after emasculation of flowers are Col (n = 5), OXP12 (n = 21), and OXP24 (n = 23). Values are mean ± sd. D, SEM images of wild-type pollen on wild-type stigma (two images). Dotted lines trace the path of the pollen tube after it penetrates the papilla cell wall. P, Papilla cell, Po, pollen grain. D, Wild-type pollen on overexpression stigmas showed aberrant tube growth after penetration of the papilla cell wall. Pollen tubes make many turns around the papilla cell in the overexpression stigmas (OXP12; left). One pollen tube shown (OXP12; right) grew away from the style and ended up at the papilla cell tip (arrow). In a semi-in vivo analysis (F), the overexpression stigma (left) and the wild-type stigma (right) were pollinated with wild-type pollen and cultured on an Arabidopsis pollen growth medium. Pollen tubes that penetrate the stigma/style were quantified. No significant difference in number was found between the transgenic and control samples. Bars = 20 μm.
Pollen tube growth behavior on the stigma was visualized using scanning electron microscopy (SEM) images. We manually applied wild-type pollen on pistils from emasculated flowers of both the wild type (control) and OXP. In wild-type pistils, pollen tubes penetrate the papilla cell and normally show fewer than one encircling pattern inside the papilla cell wall before growing down to the base of the cell and into the style (Table I; Fig. 5D). On the overexpression stigmas, nearly one-half of the wild-type pollen tubes exhibited aberrant growth (Table I; Fig. 5E, left), making many turns in the papilla cell wall before growing into the style. In some severe cases (seen in about 10 out of 600 samples), pollen tubes grew around the papilla cell several times and away from the style and arrested growth at the tip of the cell (Fig. 5E, right).
Table I.
The path of pollen tube growth on stigmas of wild type and OXPs
| <1a | >1a | −b | No. of Pollen Tubes Examined in Two Experiments | |
|---|---|---|---|---|
| N | ||||
| Wild type | 86.03% ± 6.39% | 10.41% ± 7.07% | 3.58% ± 0.67% | N1 = 74 |
| N2 = 292 | ||||
| OXP | 58.34% ± 0.58% | 31.38% ± 0.18% | 10.29% ± 0.40% | N1 = 160 |
| N2 = 511 |
If a wild-type pollen tube path showed less than one encircling of the papilla cell after penetration, it was scored as <1. If the pollen tube encircled the papilla cell at least once, it was scored >1.
The growth direction of a pollen tube was away from the style.
To determine whether the lowered seed set in the crosses was due to failure of the wild-type pollen to penetrate the style, we did hand pollinations on overexpression and wild-type pistils, removed the stigma and style, and placed them in vitro on agar growth medium. No differences in numbers of pollen tubes emerging from the style were detected in the two samples (Fig. 5F).
Overexpression Pistils Show Elevated Levels of Plantacyanin
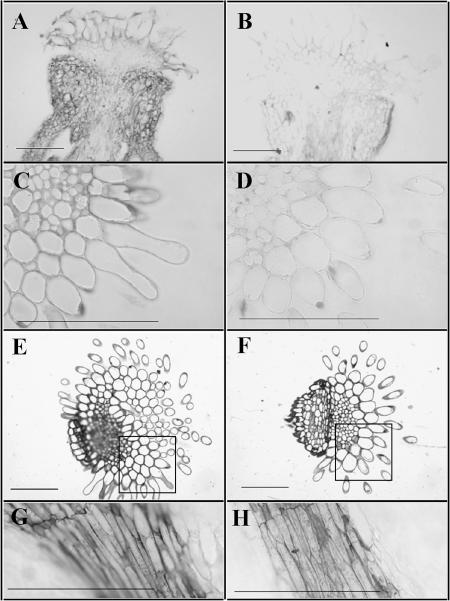
We used several methods of fixation (glutaraldehyde, formaldehyde-acetic acid [FAA]), embedding (LR White, paraffin), and sectioning (3- and 8-μm-thick sections) to detect plantacyanin in the pistils of plants using antibodies to E. coli-expressed plantacyanin (Fig. 6). Overexpression results in plantacyanin localization throughout the pistil (Fig. 6A) and higher levels in the transmitting tract tissues of the stigma (Fig. 6, A–D) and a slightly higher level in the style (Fig. 6, A, B, G, and H).
Figure 6.
Plantacyanin protein levels are increased in Arabidopsis plantacyanin overexpression pistils. Overexpression pistils are on left, wild-type on right. Immunohistochemical analysis on FAA-fixed, paraffin-embedded tissues (cut at 8 μm) shows that plantacyanin levels are elevated throughout the overexpression pistil (A) compared to that of the wild type (B). Glutaraldehyde-fixed, LR White-embedded tissues (cut at 3 μm) show that, in the stigma (C and D) and the style (G and H), there is a modest increase in plantacyanin levels in the transmitting tract of the overexpressor compared to that of the wild type. E and F, Toluidine blue-stained sections of the stigma/style. The squared areas are comparable to those seen in C and D. Bars = 100 μm.
DISCUSSION
Plantacyanin Localization in Tissues of the Pistil and the Effects of Overexpression on Pollination
Chemotropism is defined as directional growth determined by a chemical gradient. The chemical can be an attractant or a repellent (Lush, 1999). γ-Aminobutyric acid (GABA) is proposed to be an agent of pollen tube guidance in Arabidopsis by its maintenance of a concentration gradient along the pollen tube path in the pistil. Perturbation of the GABA gradient in vivo is correlated with disrupted pollen tube guidance, but in an in vitro assay a gradient of GABA has no chemotropic effect on pollen tube growth (Palanivelu et al., 2003). Lily pollen tube directional growth occurs up a gradient of chemocyanin and stigma-stylar Cys-rich adhesin in an in vitro assay (Kim et al., 2003). A gradient of these two proteins is hypothesized to exist in the lily stigma. Here, using polyclonal antibodies specifically recognizing Arabidopsis plantacyanin, we show that this small, secreted protein, which is closely related to chemocyanin, forms a concentration gradient along the pollen tube growth path, with a lower level in the stigma papilla cell wall and a higher level in the transmitting tract ECM of the style. We also show that increasing the levels of plantacyanin in the pistil, which may perturb this gradient, disrupts pollen tube guidance on the stigma.
Using a strong promoter, CaMV 35S, we increased the expression level of Arabidopsis plantacyanin in Arabidopsis pistils. We used antibody localization to detect increased levels of plantacyanin in the transmitting tract of the pistil. We then utilized SEM to visualize the wild-type pollen tube activities on the overexpression pistils. After the pollen grains were manually deposited on the overexpression stigmas, they hydrated and germinated normally, as on the wild-type stigma, and penetrated the papilla cell wall growing underneath the stigmatic cuticle. At this stage, a striking irregularity of pollen tube growth was observed; many pollen tubes appeared to lose the ability to grow directly into the style. They made many turns around the papilla cell before penetrating from the stigma to the style. In some rare cases, pollen tubes totally lost their direction, turning away from the style and growing to the tip of the papilla cell where they arrested. By contrast, the control pollinations showed little of this behavior. In other guidance systems, when cells are presented with a uniform distribution of a chemoattractant, they show random growth or movement (Caterina and Devreotes, 1991) much as we see for these pollen tubes on the overexpression stigma. Arabidopsis plantacyanin displays 86.8% similarity (amino acid level) to lily chemocyanin (Kim et al., 2003). Recombinant Arabidopsis plantacyanin is active in the lily in vitro pollen tube guidance assay (data not shown). To firmly establish a role for plantacyanin in Arabidopsis pollen tube guidance, an in vitro assay for Arabidopsis pollen must be developed.
Our data have shown a dramatic increase of the plantacyanin expression level in overexpression pistils, but the increase in expression in the transmitting tract tissues is less dramatic, comparing the overexpression and the wild type. Plantacyanin is a putative target of one of the miRNAs in Arabidopsis (Sunkar and Zhu, 2004). Indeed, the GUS expression is high in the stigma, but protein levels there are low. This discrepancy may be explained by regulation of plantacyanin protein levels by its miRNA in the pistil.
Effects of Plantacyanin Overexpression on the Anther and Pollen
Expression of Arabidopsis plantacyanin was not detected in mature anthers using the plantacyanin promoter-GUS transgenic plants, nor was it seen in pollen, which is consistent with pollen transcriptome data (Becker et al., 2003; Honys and Twell, 2003). A very low level of expression in the tapetum and endothecium was revealed by promoter-GUS and with immunolocalization in the anthers of a wild-type flower (data not shown). When we created the Arabidopsis plantacyanin OXPs using the CaMV 35S promoter, we obtained transgenic plants with very high levels of plantacyanin in the inflorescence, including the anther tissues (confirmed by immunolocalization; data not shown). The major effect of overexpression in the anther is a disruption of endothecium development, which explains the lack of dehiscence in the anthers (Steiner-Lange et al., 2003).
Pollen development in the overexpression anthers does not seem to be much affected, based on the fact that it desiccates normally and gives rise to full seed set when deposited on the wild-type pistil. But we did find irregular callose formation on the pollen intine (the inner layer of the pollen wall) in the mature anthers of OXPs and a low level of precociously germinated pollen. Pollen germination in the anther has been documented in many cleistogamous species where anther proximity to the stigma is sufficient to allow normal pollination and seed set in these closed flowers (Lord, 1979; Anderson, 1980; Mayers and Lord, 1984). An Arabidopsis male gametophytic mutant, raring-to-go, was identified for its precocious pollen germination in the anthers (Johnson and McCormick, 2001). Pollen of raring-to-go plants does not desiccate completely and so germinates when it is mature during the later stages of pollen development in the anther. Pollen of Arabidopsis OXPs does desiccate normally, and it is not in proximity with the stigma since it is in anthers that never dehisce. The pollen may interpret the presence of plantacyanin in mature anthers on overexpression plants as a stigmatic signal and respond by germinating. An in vitro assay using purified plantacyanin protein on wild-type anthers will be used to test this hypothesis.
Plantacyanin, a Member of an Ancient Family of Blue Copper Proteins
One of the most striking characteristics of plantacyanins is that they are copper-binding proteins (Nersissian et al., 1998). Four amino acids form the copper-binding site, two His, one Cys, and one Met, Gln, or Leu. Arabidopsis plantacyanin, with one Met at the fourth ligand site, as in spinach and cucumber plantacyanins, is believed to display a high redox potential (Nersissian et al., 1998). Because of the special redox chemistry of copper, plantacyanins, with their copper exposed on the surface, as predicted by three-dimensional structure analysis (Guss et al., 1996; Einsle et al., 2000), may readily participate in reactive oxygen species (ROS) production, including hydroxyl radicals (Halliwell and Gutteridge, 1984). If Arabidopsis plantacyanin binds copper and is capable of producing ROS, the three apparently disparate phenotypes we observed in the OXPs can be explained. These are the disruption of endothecium development, callose deposition on the pollen grain wall, and perturbed pollen tube guidance in the stigma. ROS may be implicated in all three events.
Degeneration of the endothecium in the Arabidopsis plantacyanin overexpression anthers may be due to plantacyanin-induced precocious programmed cell death (PCD). PCD is a normal event in endothecium development (van Doorn and Woltering, 2005). ROS, especially H2O2, involvement in PCD in plants has been extensively studied (Pennell and Lamb, 1997; Gechev and Hille, 2005).
Plantacyanins have been proposed to be involved in the oxidative burst that occurs in pathogen infection and in the cross-linking and insolubilization of cell wall materials, such as lignin and callose (Nersissian et al., 1998). Callose is the major component of the papilla, a physical barrier to impede microbial penetration during microbial attack in plants (Stone and Clarke, 1992). Callose formation in the pollen intine may be a result of the plantacyanin overexpression in the anther.
It is known that ROS regulates intracellular signaling pathways through the activation of Ca2+ channels on the plasma membrane in the root hair (Foreman et al., 2003) and guard cell (Pei et al., 2000). An intracellular calcium gradient at the pollen tube tip is required for pollen tube growth, and this high calcium concentration is hypothesized to control the direction of tube growth (Malhó et al., 2000), probably via the calmodulin- and cAMP-modulated secretory pathway (Rato et al., 2004). Calcium influx at a new growing site can be achieved by activation of calcium channels in the plasma membrane on the flanks of the pollen tube tip (Hepler et al., 2001). This is the proposed model for how a gradient of lily chemocyanin can cause directional pollen tube growth (Kim et al., 2003). The gradient of plantacyanin we detected in the Arabidopsis pistil may act in this manner to guide pollen tubes from the stigma into the style.
A direct connection between plantacyanin and ROS can be confirmed by testing the level of ROS in these Arabidopsis OXPs.
Summary and Perspectives
In this study, we provide data that support the hypothesis that Arabidopsis plantacyanin may function in pollination since it is a component of the transmitting tract ECM and its overexpression in the pistil disrupts pollen tube guidance from the stigma to the style. It appears to act as well in anther development because overexpression results in an indehiscent anther with a degenerated endothecium. This small, ECM protein is not only expressed in the vegetative and reproductive sporophytic tissues, but also in the female gametophyte. Plantacyanin is a putative target of one of the miRNAs in Arabidopsis and it appears to play several roles in the biology of this plant. Further study of this intriguing protein is warranted to better define its functions in Arabidopsis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col) plants were soil grown in a University of California, Riverside, growth room in Sunshine Mix No. 1 (SunGro, Bellevue, WA), supplemented with fertilizer and insecticide (Springer et al., 2000). Plants were grown at 22°C with a 16-h/8-h photoperiod with 200 μE m−2 s−1.
Constructs for Plant Transformation
The promoter region of Arabidopsis plantacyanin was amplified from genomic DNA with primers 5′-AGGAGCTCGAGAGTAAATGAGGATGAATTGAAG-3′ and 5′-ACGAATTCTATCGAGTTCTTTCAAGTCCAC-3′. The primers contain restriction sites SacI and EcoRI to be fused with the uidA gene and a 3′ octopine synthase transcription terminator and cloned into the binary T-DNA vector pCAMBIA3200. Plant transformants were selected with BASTA.
The construct for overexpression of Arabidopsis plantacyanin, OXP, was made by introducing the open reading frame of Arabidopsis plantacyanin into a binary vector pPS119 (Shuai et al., 2002) via BamHI and XbaI sites. The primers to amplify the Arabidopsis plantacyanin open reading frame were 5′-TGTCTAGATCAAACCGCGGTGACTG-3′ and 5′-ATGGATCCATGGCCAAGGGAAGAGG-3′. The expression of Arabidopsis plantacyanin was driven by the CaMV 35S promoter. The transgenic plants harboring the OXP construct are resistant to kanamycin.
Plants were transformed using Agrobacterium tumefaciens strain GV3101 with the floral-dip method (Clough and Bent, 1998). Transformants were selected on Murashige and Skoog (1962) medium containing 50 μm kanamycin (Sigma-Aldrich, St. Louis) or on soil by spraying with 1,000-fold dilution of Finale (AgrEvo Environmental Health, Montvale, NJ; BASTA). Spraying was initiated at 10 d after germination and was performed three times every 2 to 3 d.
RT-PCR Analysis
Total RNA was extracted from different tissues of mature Arabidopsis plants using the RNeasy mini kit (Qiagen, Chatsworth, CA). For RT-PCR analysis, cDNA was synthesized from 1.2 μg of total RNA in a 20-μL RT reaction, which contains an oligo(dT) primer, SuperScript II Rnase H− reverse transcriptase (20 units), and ribonuclease inhibitor (10 units; Invitrogen, Carlsbad, CA). One microliter of each cDNA sample was used as the template for PCR amplification. The primers flanking Arabidopsis plantacyanin's two exons were 5′-TGTCTAGATCAAACCGCGGTGACTG-3′ and 5′-ATGGATCCATGGCCAAGGGAAGAGG-3′. The amplifications were under the following conditions: denaturation at 94°C for 4 min, followed by 30 cycles of 30 s at 94°C, 30 s at 60°C, and 1 min at 72°C. Control reactions using the Actin2 gene-specific primers (An et al., 1996) were performed on the same cDNA samples.
Analysis of the T-DNA-Tagged Mutant
For isolating the homozygous plants of the T-DNA-tagged mutant line (SALK_091945), PCR amplifications were carried out using genomic DNAs extracted from 10-d-old seedlings. The primers flanking the Arabidopsis plantacyanin gene are the ones used in RT-PCR, and the T-DNA left-border primer LBb1 was described at http://signal.salk.edu/tdnaprimers.html.
The conditions for RT-PCR to estimate the transcript level of Arabidopsis plantacyanin were as described above, except that total RNAs were extracted from inflorescences of the homozygous T-DNA-tagged mutant.
Histochemical Localization of GUS
Treated seedlings or plant tissues were incubated at 37°C in a reaction buffer containing 10 mm EDTA, 100 mm sodium phosphate, pH 7.0, 0.5 mm potassium ferrocyanide, 0.5 mm potassium ferricyanide, and 0.1% Triton X-100 with 1 mm 5-bromo-4-chloro-β-d-glucuronide overnight. The samples were then transferred to 70% ethanol to remove chlorophyll and were viewed with the Leica MZ12 stereomicroscope.
Production of Recombinant Proteins and Antibodies
DNA fragments encoding Arabidopsis plantacyanin (At2g02850) mature protein (without the signal peptide) were amplified from the cDNA clones. The Arabidopsis plantacyanin cDNA clone U21110 was available at the ABRC (Columbus, OH). The PCR products were designed to have proper restriction sites, NcoI and EcoRI, for a multicloning site in a vector (pHIS8-3) to generate recombinant proteins fused with a His-8-tag. Protein expression was induced by the addition of IPTG (ICN Biomedicals, Irvine, CA) at a final concentration of 1 mm. Expressed protein was purified on a Ni-NTA affinity column (Qiagen) following the manufacturer's instructions. Before protein refolding, the purified protein (in 8 m urea) was incubated with the reducing reagent β-mercaptoethanol (final concentration, 10 mm) at room temperature for 1 h. A 10-fold (v/v) fast dilution was made to refold recombinant proteins by adding a refolding buffer (5 mm sodium phosphate buffer, 0.5 mm EDTA, 1 mm GSH, and 0.5 mm GSSG, pH 7.2; modified from Nersissian et al., 1996). The protein sample was kept on ice with stirring for 1 h. The refolded protein was finally dialyzed against double-distilled water at 4°C and quantified with the modified Lowry protein assay (Pierce Chemical, Rockford, IL). The polyclonal antibodies against Escherichia coli-expressed His plantacyanin were generated in rabbits at Cocalico Biologicals (Reamstown, PA).
Immunoblot Analysis
Plant tissues (seedlings, inflorescences, or pistils) were ground in liquid nitrogen to a fine powder. Total proteins were prepared by vortexing a sample of tissue powder in phosphate-buffered saline (PBS; 0.14 m NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, and 8.1 mm Na2HPO4) and separated by 13% SDS-PAGE. Proteins were transferred onto nitrocellulose membrane (MSI) by electroblotting. The membranes were blocked with 5% nonfat milk and incubated with a 2,000-fold dilution of antiserum against His plantacyanin. The antibody was detected with a horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG; Bio-Rad, Boston) using the western-blot Chemiluminescence Reagent Plus (Bio-Rad).
Pollination: SEM
Stage 12 Arabidopsis flowers (Smyth et al., 1990) from both the wild-type and the overexpression lines were emasculated and pollinated with mature pollen (flower stage 13) from wild-type plants for 3 to 4 h. Pollinated pistils were fixed in 2.5% (v/v) glutaraldehyde (EM Sciences, Fort Washington, PA) in PBS at 4°C overnight. Pistils were rinsed with PBS and dehydrated through an ethanol series (70%, 75%, 80%, 85%, 90%, 95%, and 100%; v/v) at 4°C. Dehydrated tissue was critical-point dried in liquid carbon dioxide, mounted, and coated on SEM stubs. Samples were then observed under SEM (XL30-FEG; Philips, Eindhoven, The Netherlands) at an accelerating voltage of 15 kV. Images were processed with Adobe Photoshop 5.5 (Adobe Systems, Mountain View, CA).
Stigma/Style Semi-in Vivo Assay
Stage 12 to 13 flowers (nonpollinated) were emasculated and pollinated with wild-type pollen. The stigma/style was then cut off from the ovary using a razor blade and transferred to an 0.5% (v/v) agarose growth medium [containing 0.1% (v/v) boric acid, 25 mm CaCl2, 25 mm Ca(NO3)2, 10 mm MgSO4, and 18% (v/v) Suc, 1 m KOH added to adjust pH value to 6.5 to 7]. Samples were kept in a humid chamber overnight at room temperature. The samples were dried in a 60°C oven, stained with Coomassie Blue, and then observed under the light microscope. Images were documented with the Spot Insight camera (Diagnostic Instruments, Sterling Heights, MI) attached to a Leica (Deerfield, IL) MZ 12 stereomicroscope.
Light Microscopy
Plant tissues were fixed in FAA containing 37% (w/v) formaldehyde, glacial acetic acid, and 70% (v/v) ethanol (5:5:90; v/v/v), overnight at 4°C or in 2.5% glutaraldehyde/PBS overnight at 4°C (Lennon et al., 1998). Samples were then dehydrated through an ethanol series up to 100% ethanol. The ethanol was replaced gradually by the Technovit 7100 embedding solution (EMS, Hatfield, PA). After polymerization, 3-μm sections were cut and mounted on glass slides and stained with 0.5% toluidine blue for visualizing on a Nikon Microphot FXA microscope (Nikon, Garden City, NY).
Anthers from stage 13 flowers were squashed to release pollen and mounted in 50% (v/v) glycerol and viewed on a Nikon microscope. Pollen in Arabidopsis plantacyanin overexpression and wild-type anthers was released by hand dissection and viewed under a compound microscope.
Immunolocalization
Plant tissues were fixed with a FAA solution or a 2.5% glutaraldehyde/PBS solution and dehydrated as described above for light microscopy and then embedded in LR White (medium grade; EMS) and polymerized in a 56°C oven for 24 h. Three-micrometer sections were cut and mounted on Poly-l-Lys-coated slides (Fisher Scientific, Loughborough, Leicestershire, UK). Arabidopsis plantacyanin expression was detected by polyclonal antibodies (1:100 dilution) against E. coli-expressed plantacyanin (made by Cocalico Biologicals) and an alkaline phosphatase-conjugated secondary antibody (goat anti-rabbit IgG; 1:200 dilution; Bio-Rad).
Tissues for paraffin embedding were fixed first in 2.5% glutaraldehyde/PBS for 2 h and then transferred to FAA overnight. After serial dehydration with ethanol (30%, 50%, 70%, 95%, and 100%), tissues were soaked with a series of tert-butanol (50%, 70%, and 100%) and infiltrated with Paraplast X-TRA (Fisher Scientific). Sections (8 μm) were cut and mounted on Poly-l-Lys-coated slides. Sections were deparaffinized by soaking in CitriSolv (Fisher) for 10 min and rehydrated in an ethanol series (100%, 95%, 70%, 50%, and 30%). For immunohistochemistry, sections were incubated with the primary antibody (anti-His plantacyanin; 1:200) and an alkaline phosphatase-conjugated secondary antibody (goat anti-rabbit IgG; 1:500). The samples were visualized mounted in nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (Bio-Rad).
Fluorescence Microscopy
Anthers containing pollen were fixed in a solution of 75% (v/v) ethanol and 25% (v/v) acetic acid for 1 h and cleared overnight in 1 m NaOH at room temperature. After the samples were washed with double-distilled water for several changes, they were stained with decolorized aniline blue for callose (O'Brien and McCully, 1981) and observed on a Nikon Microphot FXA microscope (Nikon) using the UV-2B filter combination or visualized by a Leica TCS SP2/UV confocol microscope (Leica Microsystems, Heidelberg).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permission will be the responsibility of the requester.
Acknowledgments
We thank Dr. Zhenbiao Yang for his critical reading of the manuscript, Dr. Patricia Springer for providing the vectors constructed for promoter-GUS and gene overexpression, and Kimberly Tan for general lab assistance.
This work was supported by the National Science Foundation (grant nos. 0077886 and 0420445 to E.M.L.) and the Center for Plant Cell Biology at the University of California, Riverside (microscopy and imaging grant to J.D.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.063388.
References
- Aikazyan V, Nalbandyan RM (1981) Studies on plantacyanin. I. Distribution in the plant kingdom, subcellular localization and physicochemical properties. Biochim Biophys Acta 667: 421–432 [DOI] [PubMed] [Google Scholar]
- An YQ, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB (1996) Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J 10: 107–121 [DOI] [PubMed] [Google Scholar]
- Anderson WR (1980) Cryptic self-fertilization in the Malpighiaceae. Science 207: 892–893 [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JD, Boavida JC, Carneiro J, Haury M, Feijó JA (2003) Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiol 133: 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Devreotes PN (1991) Molecular insights into eukaryotic chemotaxis. FASEB J 5: 3078–3085 [PubMed] [Google Scholar]
- Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AJ (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cruz-Garcia F, Hancock CN, Kim D, McClure B (2005) Stylar glycoproteins bind to S-RNase in vitro. Plant J 42: 295–304 [DOI] [PubMed] [Google Scholar]
- Dixit R, Nasrallah JB (2001) Recognizing self in the self-incompatibility response. Plant Physiol 125: 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einsle O, Mehrabian Z, Nalbandyan R, Messerschmidt A (2000) Crystal structure of plantacyanin, a basic blue cupredoxin from spinach. J Biol Inorg Chem 5: 666–672 [DOI] [PubMed] [Google Scholar]
- Fedorova M, van de Mortel J, Matsumoto PA, Cho J, Town CD, Vanden-Bosch KA, Gantt JS, Vance CP (2002) Genome-wide identification of nodule-specific transcripts in the model legume Medicago truncatula. Plant Physiol 130: 519–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torresk MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Gechev TS, Hille J (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol 168: 17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss JM, Merritt EA, Phizackerley RP, Freeman HS (1996) The structure of a phytocyanin, the basic blue protein from cucumber, refined at 1.8 A resolution. J Mol Biol 262: 686–705 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and diseases. Biochem J 219: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton CR, Bowen HC, Broadley MR, Hammond JP, Mead A, Payne KA, Pritchard J, White PJ (2004) Cesium toxicity in Arabidopsis. Plant Physiol 136: 3824–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132: 640–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, McCormick S (2001) Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiol 126: 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Mollet JC, Dong J, Zhang K, Park SY, Lord EM (2003) Chemocyanin, a small basic protein from the lily stigma, induces pollen tube chemotropism. Proc Natl Acad Sci USA 100: 16125–16130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130: 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon KA, Roy S, Hepler PK, Lord EM (1998) The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sex Plant Reprod 11: 49–59 [Google Scholar]
- Llave C, Xie Z, Kasschau KD, Carrington JC (2002) Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297: 2053–2056 [DOI] [PubMed] [Google Scholar]
- Lord EM (1979) The development of cleistogamous and chasmogamous flowers in Lamium amplexicaule (Labiatae): an example of heteroblastic inflorescence development. Bot Gaz 140: 39–50 [Google Scholar]
- Lord EM, Russell SD (2002) The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18: 81–105 [DOI] [PubMed] [Google Scholar]
- Lush WM (1999) Whither chemotropism and pollen tube guidance? Trends Plant Sci 4: 413–418 [DOI] [PubMed] [Google Scholar]
- Malhó R, Camacho L, Moutinho A (2000) Signalling pathways in pollen tube growth and re-orientation. Ann Bot 85: 59–68 [Google Scholar]
- Márton ML, Cordts S, Broadhvest J, Dresselhaus T (2005) Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 308: 573–576 [DOI] [PubMed] [Google Scholar]
- Mayers AM, Lord EM (1984) Comparative flower development in the cleistogamous species Viola odorata: III. A histological study. Bot Gaz 145: 83–91 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Nersissian AM, Babayan MA, Sarkissian LK, Sarukhanian EG, Nalbandyan RM (1985) Studies on plantacyanin. II. NMR data, redox properties, reaction with nitrite and the formation of complex with plastocyanin. Biochim Biophys Acta 830: 195–205 [Google Scholar]
- Nersissian AM, Immoos C, Hill MG, Hart PJ, Williams G, Herrmann RG, Valentine JS (1998) Uclacyanins, stellacyanins, and plantacyanins are distinct subfamilies of phytocyanins: plant-specific mononuclear blue copper proteins. Protein Sci 7: 1915–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersissian AM, Mehrabian ZB, Nalbandyan RM, Hart PJ, Fraczkiewicz G, Czernuszewicz RS, Bender CJ, Peisach J, Herrmann RG, Valentine JS (1996) Cloning, expression, and spectroscopic characterization of Cucumis sativus stellacyanin in its nonglycosylated form. Protein Sci 5: 2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersissian AM, Melkonyan VZ, Nalbandyan RM (1991) Studies on plantacyanin. IV. Reconstitution with Cu-thionein, oxidation by cytochrome oxidase and autooxidation in the presence of cardiolipin. Biochim Biophys Acta 1076: 337–342 [DOI] [PubMed] [Google Scholar]
- Nersissian AM, Nalbandyan RM (1988) Studies on plantacyanin. III. Structural data obtained by CD and MCD methods and antigenic properties of the protein. Biochim Biophys Acta 957: 446–453 [Google Scholar]
- O'Brien TP, McCully ME (1981) The Study of Plant Structure: Principles and Selected Methods. Termarcarphi Pty, Melbourne, Australia
- Palanivelu R, Brass L, Edlund AF, Preuss D (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114: 47–59 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Pennell R, Lamb C (1997) Programmed cell death in plants. Plant Cell 9: 1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provart NJ, Gil P, Chen W, Han B, Chang HS, Wang X, Zhu T (2003) Gene expression phenotypes of Arabidopsis associated with sensitivity to low temperatures. Plant Physiol 132: 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rato C, Monteiro D, Hepler PK, Malho R (2004) Calmodulin activity and cAMP signalling modulate growth and apical secretion in pollen tubes. Plant J 38: 887–897 [DOI] [PubMed] [Google Scholar]
- Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002) Prediction of plant microRNA targets. Cell 110: 513–520 [DOI] [PubMed] [Google Scholar]
- Ryden JM, Hunt LT (1993) Evolution of protein complexity: the blue copper containing oxidases and related proteins. J Mol Evol 36: 41–56 [DOI] [PubMed] [Google Scholar]
- Sakurai T (1986) Preparation and spectroscopic studies of cobalt (I1)-substituted cucumber basic blue protein “plantacyanin.” Biochem Biophys Res Commun 139: 961–966 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Bui AQ, Weterings K, McIntire KN, Hsu YC, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther developmental defects in Arabidopsis thaliana male sterile mutants. Sex Plant Reprod 11: 297–322 [Google Scholar]
- Shuai B, Reynaga-Peña CG, Springer PS (2002) The Lateral Organ Boundaries gene defines a novel, plant-specific gene family. Plant Physiol 129: 747–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer PS, Holding DR, Groover A, Yordan C, Martienssen RA (2000) The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during the G (1) phase and is required maternally for early Arabidopsis development. Development 127: 1815–1822 [DOI] [PubMed] [Google Scholar]
- Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H (2003) Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J 34: 519–528 [DOI] [PubMed] [Google Scholar]
- Stone BA, Clarke AE (1992) Chemistry and Biology of (1→3)-β-d-Glucans. La Trobe University Press, Victoria, Australia
- Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16: 2001–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Shimosato H, Shiba H, Funato M, Che FS, Watanabe M, Iwano M, Isogai A (2001) Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413: 534–538 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Plant Sci 10: 117–122 [DOI] [PubMed] [Google Scholar]
- Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen-tube growth. Nature 392: 818–821 [DOI] [PubMed] [Google Scholar]
- Wu H, Wang H, Cheung AY (1995) A floral transmitting tissue specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 82: 383–393 [DOI] [PubMed] [Google Scholar]