Abstract
PURPOSE:
Myopia is a refractive error that impairs visual function and leads to visual blurring. This study aims to investigate the effect of violet light (VL) on controlling myopia, specifically in terms of axial length (AL), spherical equivalent refraction (SER), and visual acuity (VA).
METHODS:
A systematic review was conducted to compare VL and single-vision spectacles (SVSs) for treating childhood myopia. The search terms used were “Myopia” and “Violet Light.” Extensive searches were carried out in the PubMed, Embase, and Cochrane databases. The mean differences were evaluated. The effects of the therapy were examined. Publication bias was assessed with a funnel plot and further investigated through sensitivity analysis. Meta-analysis was performed using Bayesian statistics with Jeffery’s Amazing Statistical Package.
RESULTS:
The meta-analysis included 126 myopic children: 64 in the VL group and 62 in the SVS group. The pooled effect size for AL shortening was evaluated as 0.659 ± 0.184, with a 95% credible interval of 0.299–1.023. The pooled effect size for SER decrease was estimated as 0.669 ± 0.188, with a 95% credible interval of 0.303–1.036. Likewise, for VA in Log-MAR, after intervention (VL and SVS), the values were 0.082 ± 0.171 with a credible interval of 0.262–0.423. Publication bias was assessed with a funnel plot, which revealed no bias. Impact sizes for the fixed effect model were determined due to the similarity in study population, geography, type of intervention, and study design.
CONCLUSION:
VL transmission glasses play a significant role in controlling myopia among children, resulting in axial shortening, reduction of SER, and improvement in VA. However, further investigation is required to examine the long-term rebound effect.
Keywords: Axial length, spherical equivalent refraction, violet light, visual acuity
INTRODUCTION
Myopia, also known as short-sightedness, is a prevalent eye condition globally, particularly affecting children, making it difficult for them to see distant objects clearly.[1,2] High school students in several regions of East and Southeast Asia have a high prevalence of myopia, ranging from 80% to 90%.[3,4,5,6] High myopia is considered when the spherical equivalent refraction (SER) is more than −6.00Ds.[7] Progressive myopia, a disorder, in which the axial length (AL) elongates, can worsen myopia and raise the risk of permanent vision-threatening disorders such as glaucoma, myopic macular degeneration, and retinal detachment. Controlling AL progression and myopia can lessen these permanent eye-threatening disorders.[8,9,10,11] According to the Tajimi research, a domestic epidemiological study, high myopia was shown to be the main cause of WHO-defined blindness and to account for 20% of all myopia cases.[12] Moreover, 20% of the risk of severe myopia-related blindness is claimed to be reduced by one diopter of myopia suppression.[13,14]
The gold standard treatment for myopia progression, myopia management techniques, reduces both AL and myopia progression while delaying axial elongation.[15] Among these are orthokeratology, low-dosage atropine, contact lenses, and multifocal spectacle lenses.[16] However, these techniques can be risky and intrusive. For example, long-term use of low-dose atropine is linked to numerous ocular and systemic problems;[17] multifocal spectacle lenses are less effective; contact lenses and orthokeratology can cause keratitis and corneal problems.[18]
Outdoor lighting conditions contain predominantly shorter wavelengths ranging from 360 nm to 400 nm.[19] The findings that violet light (VL) stopped the development of myopia in mice,[20] chicks,[19] and people (adults[21] and children[19,22]) are promising. Retinal expression of Neuropsin (OPN5), a unique opsin sensitive to VL, is necessary for VL to prevent experimental myopia in mice. While much research has been conducted to ascertain why outdoor activities are so effective in avoiding myopia, some of them have focused on a particular light wavelength that is observed in outside environments. The contemporary myopia environment is defined by UV-blocking components, such as windows and spectacles.[23] The strongest wavelength among those that have been demonstrated in previous studies to be able to lower myopia is VL (VL: 360-400 nm).[15,19,21,22,24,25,26,27] Typical eyeglasses not only do not penetrate the ultraviolet but also block out VL.[23]
Consequently, the primary objective of this systematic review and meta-analysis was to conduct a comprehensive assessment of the efficacy of VL transmitting glasses in controlling the progression of myopia in pediatric patients. In addition, this study aims to provide more reliable evidence to support its potential clinical implementation. This study employed a meta-analysis methodology to thoroughly examine and evaluate existing material using modern Bayesian statistics. This approach allows for a robust evaluation of the true effect of VL-transmitting glasses on AL, SER, and visual acuity (VA). Through this analysis, additional confirmation on the efficacy of VL-transmitting glasses treatment as a novel approach to managing myopia is provided. The findings of this research have the potential to offer enhanced scientific support for the practical implementation of VL-transmitting glasses. Furthermore, they can serve as a valuable resource for the continued advancement and refinement of VL transmitting glasses.
METHODS
In accordance with the PRISMA 2020 statement, this review adhered to all the recommended procedures for systematic reviews and meta-analyses.[28] The systematic review and meta-analysis were registered in PROSPERO with the Registration ID: CRD42024552140 [Figure 1].
Figure 1.
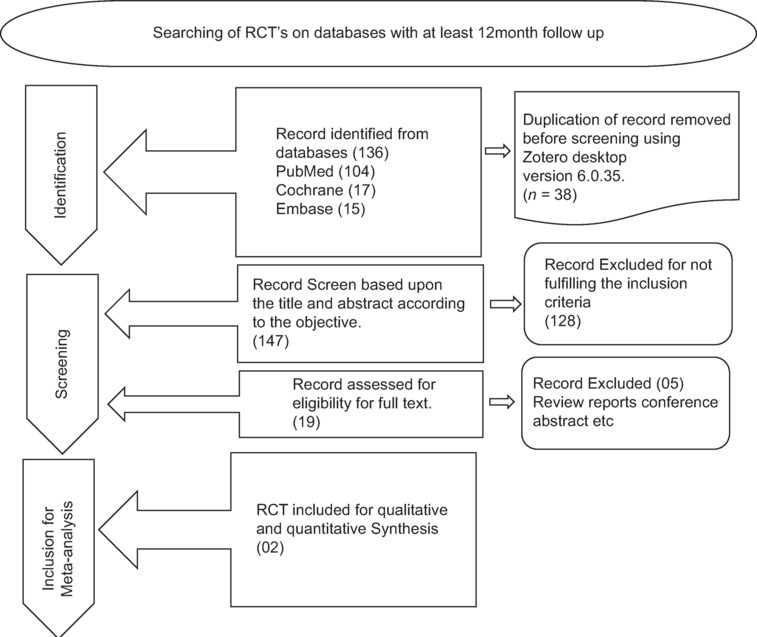
PRISMA paper selection a flow chart. The PRISMA flowchart providing information about the studies that were ultimately included in the meta-analysis as well as the search approach
This systematic review was undertaken after submitting a proposal to the supervisor and co-supervisor at Lincoln University. Upon receiving approval, the project was initiated as part of the partial fulfillment of a Doctor of Philosophy degree in Health Sciences, specializing in Vision Sciences (Optometry).
Search methodology
A comprehensive review of the existing literature was undertaken to investigate the long-term effectiveness of violet transmission glasses as a therapeutic intervention for myopia in individuals aged 18 years or less. The PICO question was formulated as follows: Can children with myopia (P) who undergo VL therapy with single-vision spectacles (SVSs) (I) observe a decreased progression of myopia without any adverse or significant negative consequences, compared to children who only wear SVSs?
A search strategy was adopted based on the above PICO framework. Searches were conducted on PubMed, Embase, and Cochrane databases. The bibliography list of the included studies was also inspected for additional relevant studies not included in the initial search.
Eligibility criteria and study selection
The following criteria were used to select articles for inclusion in the study.
Randomized Control Trials (RCTs)
Studies that included subjects with myopia and a cycloplegic SER of 0.50 D or above, ranging in age from 3 to 18 years
Studies that compared the efficacy of SVSs worn alone with those worn in conjunction with VL
Studies with a minimum follow-up duration of 6 months that reported the intended outcomes, which included AL, SER, and VA.
Exclusion criteria
The following types of studies were excluded from this review:
Studies that included animals
In vitro research
Quasi-experimental studies
Case reports and case series
Articles written in languages other than English.
Duplicate records were automatically eliminated.
Data extraction
An Excel spreadsheet was used by a team of writers to extract data from the included studies based on the following criteria: quantity, study methodology, and geographical location. These characteristics were directly extracted from the summary sheets (XYZ), (DEF), and (ABC). The most important parts of the research included the centers, intervention and control group samples, length of follow-up (at least 6 months), research groups (treatment and control), main inclusion criteria for children, explanation of the VL used, and study conclusion. After the extraction, ABC checked the final data set.
The first participant variables retrieved from the baseline sheets (DEF) and (XYZ) included the number of patients in each group, age, sex (male or female), AL (measured in millimeters), and SER. After this, ABC checked the final data set.
The subject’s AL (mm), SER, and VA were obtained using the result sheets (DEF) and (ABC).
Risk of bias assessments for eligible studies
To ensure that the studies were free of bias, they were evaluated using the Risk of Bias Assessment (ROB version 2) technique, which was developed by Cochrane. This tool utilizes five domains:
D1: Bias in the Randomization Process
D2: Bias Caused by Deviation from Intended Interventions
D3: Bias Due to Missing Outcome Data
D4: Bias in Measurement of the Outcome
D5: Bias in Selection of the Reported Result.
Figure 2a and b visually represent the bias across all these domains. Moreover, publication biases were assessed for the three main parameters, i.e., AL, SER, and VA with funnel plot revealed no significant publication biasness [Figure 3a-c].
Figure 2.
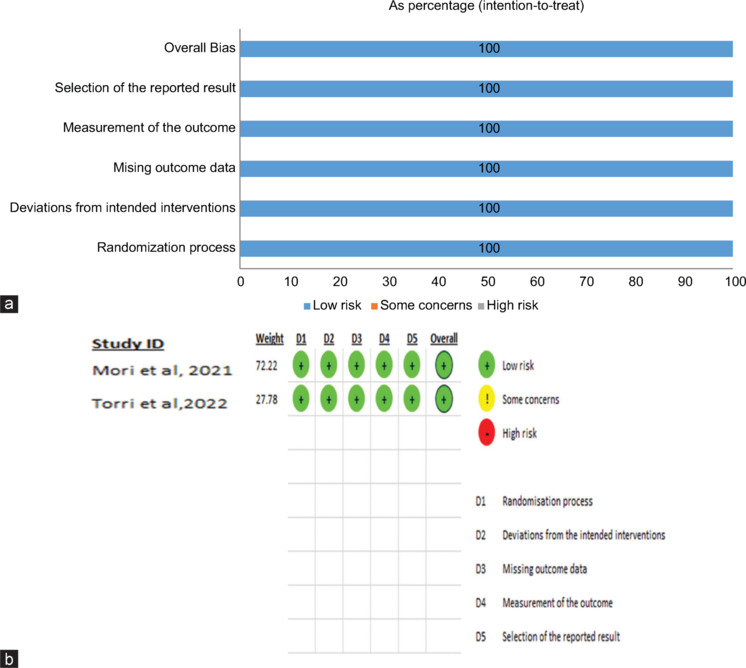
(a and b) Summary of the risk of bias estimate. The risk of bias evaluation for each trial is demonstrated through the utilization of the Cochrane risk of bias tool ROB 2. The green symbol represents a low risk of prejudice, the yellow symbol represents an unclear danger of bias, and the red symbol represents a high risk of bias
Figure 3.
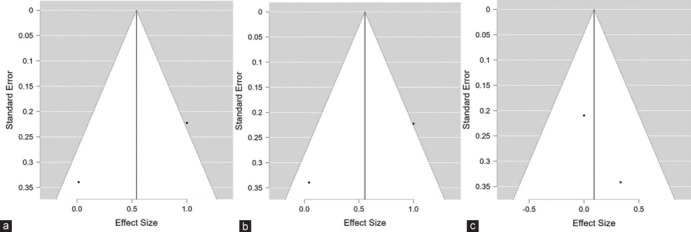
(a) Funnel plot for the assessment of publication Bias for estimation of effect size of violet light (VL) transmission glasses on axial length indicating no publication bias (b) Funnel plot for the assessment of publication Bias for estimation of effect size of VL transmission glasses on spherical equivalent refraction indicating no publication bias (c) Funnel plot for the assessment of publication bias for estimation of effect size of VL transmission glasses on Visual acuity indicating no publication bias
Data analysis
For data analysis, the mean and the standard deviation (SD) were used to provide the baseline values for each experiment for continuous variables [refer to Table 1]. For categorical variables, the number (percentage) was used. Subsequently, the data were transformed into mean and SD format using an equation in an Excel spreadsheet.
Table 1.
Baseline axial length and refractive error changes in violet light and single vision spectacles single vision spectacles groups among myopic subjects studies from Japan (2021–2022)
| Study ID | Arm | Subjects | Age (years), mean±SD (range) | Baseline AL, mean±SD | Baseline SER, mean±SD | Baseline VA, mean±SD |
|---|---|---|---|---|---|---|
| Mori K et al. Japan 2021[26] | VL group | 45 | 9.8±1.7 (06–12) | 24.58±0.61 | −2.53±0.99 | −0.09±0.03 |
| SVS group | 46 | 9.6±1.3 (06–12) | 24.59±0.75 | −2.57±0.80 | −0.08±0.03 | |
| Torri H et al. Japan 2022[27] | VL group | 19 | 9.3±1.5 (06–12) | 24.45±0.97 | −2.66±0.87 | −0.09±0.03 |
| SVS group | 16 | 9.5±1.5 (06–12) | 24.54±0.67 | −2.90±0.92 | −0.09±0.03 |
VL: Violet light, SVS: Single-vision spectacles, SD: Standard deviation, SER: Spherical equivalent refraction, VA: Visual acuity, AL: Axial length
The posttest SD is referred to as “SD final,” the pretest SD is referred to as “SD baseline,” and the mean changes from baseline are referred to as “SD change.” For Randomized Controlled Trials (RCTs) which did not report a SD, it was calculated using the formula below. By inputting the values of Credible interval (CI) and sample size, the SD was obtained using Excel version 16 with the following code: (CI_Width/(2 * 1.96) * SQRT (Sample_ Size).
An online effect size calculation software was used to determine the effect size using Cohen’s D formula. For a t-test, an online statistical calculator was used to determine the effect size (Cohen’s D). The values of mean, SD, and sample size for both the intervention and control groups were entered from an Excel sheet to calculate the effect size.
The same process was used to determine the standard error of the effect size; an Excel formula was utilized for this purpose. are the respective sample sizes of the VL and SVSs groups, the standard error (SEg) was calculated using the following formula: SEg = \sqrt(\frac[n_1+ n_2][n_1\times n_2] + \frac[d^2][2(n_1+ n_2)])
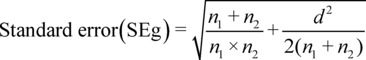
A separate column in the Excel spreadsheet was created for the effect size and standard error of effect size, and all these calculations were entered for the studies. These calculations, along with study labels, were imported from the Excel spreadsheet to Jaffrey Amazing Statistical Package (JASP version) for the calculation of effect size to draw a forest plot and funnel plot along with the pooled effect size. Data in the Excel sheet, including the study label, were imported to JASP. A fixed effect model was employed to calculate the effect size.
RESULTS
Literature search results
Each database was searched using a highly targeted approach, yielding a total of 136 studies. After eliminating all duplicates, only ten studies remained for full-text screening.
After further eliminating irrelevant studies, a selection of two research publications was made available for data extraction and analysis. The results of the search are visually represented in a PRISMA flowchart [refer to Figure 1 for the PRISMA flowchart].
Characteristics of the included studies
The meta-analysis included two randomized controlled trials (RCTs), each consisting of 126 participants. The treatment group comprised 64 individuals, while the control group included 62 individuals. Each study included in the analysis was an RCT. Figure 4a-c provide a comprehensive summary of the research conducted on the three primary parameters: AL, SER, and VA. In addition, Table 1 offers a baseline analysis of the features. The participants were individuals aged between 3 and 18 years. None of the baseline variables used for the children identified in the study showed any statistically significant differences.
Figure 4.
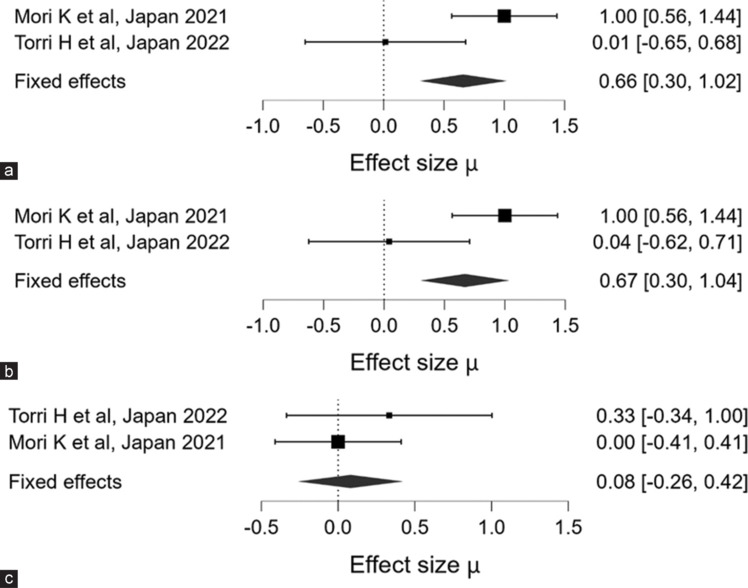
(a-c) shows the Forest Plot for the overall effect size for axial length, spherical equivalent refraction, and Visual Acuity in Log-MAR
The experimental group consisted of youngsters who were exposed to both VL and SVSs, while the control group only used SVS.
Quality assessments
In the statistical analysis, the VL and SVSs groups were compared using the mean change and SD as the key statistical measures.
Statistical analysis
Statistical measures, such as mean change and SD, are predominant in the published literature. Differential analysis of the three key components: AL, SER, and VA enables a comprehensive examination of the outcomes. These components are measured at intervals of 6 months.
Axial length
The meta-analysis included data from two studies examining the effect sizes for AL. Mori et al. (Japan, 2021)[26] reported significant findings with 45 participants in the VL group (mean ± SD: 0.73 ± 0.03) and 46 participants in the SVS group (mean ± SD: 0.76 ± 0.03). The estimated mean difference (MD) for AL was 0.659 ± 0.184, with a 95% credible interval (CI) ranging from 0.299 to 1.023. This result indicates a statistically significant difference between the two groups. Furthermore, the Bayes Factor (BF₁₀) of 140.066 suggests strong evidence in favor of the hypothesis that the VL intervention has a meaningful impact on AL.
In contrast, the study by Torri et al. (Japan, 2022)[27] included 19 participants in the VL group (mean ± SD: 24.58 ± 0.61) and 16 participants in the SVS group (mean ± SD: 24.59 ± 0.75). However, this study did not provide an effect size or CI, rendering its individual significance less interpretable. Despite this, Torri et al.’s contribution to the overall meta-analysis weightage was 27.78%, indicating that it still provides valuable context to the collective findings [refer to Table 2 and Figure 4a].
Table 2.
Effect sizes of axial length - a review of studies and meta-analysis
| Study ID | RLRL | SVS | Estimated | BF10 | Weightage (%) | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| n | Mean±SD | n | Mean±SD | Mean | 95% CI lower-upper | |||
|
Effect sizes per study for AL | ||||||||
| Mori K et al. Japan 2021[26] | 45 | 0.73±0.03 | 46 | 0.76±0.03 | 0.659±0.184 | 0.299–1.023 | 140.066 | 72.22 |
| Torri H et al. Japan 2022[27] | 19 | 24.58±0.61 | 16 | 24.59±0.75 | 27.78 | |||
|
Effect sizes per study for SER | ||||||||
| Mori K et al. Japan 2021[26] | 45 | 1.42±0.11 | 46 | 1.53±0.11 | 0.669±0.188 | 0.303–1.036 | 164.308 | 72.22 |
| Torri H et al. Japan 2022[27] | 19 | 2.53±0.99 | 16 | 2.57±0.88 | 27.78 | |||
|
Effect sizes per study for VA | ||||||||
| Mori K et al. Japan 2021[26] | 45 | 1.42±0.11 | 46 | 1.53±0.11 | 0.082±0.171 | −0.262–0.423 | 0.214 | 72.22 |
| Torri H et al. Japan 2022[27] | 19 | 2.53±0.99 | 16 | 2.57±0.88 | 27.78 | |||
SER: Spherical equivalent refraction, VA: Visual acuity, AL: Axial length, RLRL: Repeated low-level red light, SD: Standard deviation, BF: Bayes factor, CI: Confidence interval, SVS: Single-vision spectacles
Spherical equivalent refraction
The meta-analysis also evaluated the effect sizes for SER across the same two studies. Mori et al. (2021)[26] found significant differences in SER with 45 participants in the VL group (mean ± SD: 1.42 ± 0.11) and 46 participants in the SVS group (mean ± SD: 1.53 ± 0.11). The estimated MD was 0.669 ± 0.188, with a 95% CI of 0.303–1.036, indicating a significant effect. The Bayes Factor (BF₁₀) of 164.308 further corroborates this, suggesting very strong evidence in favor of the effect of the VL intervention on SER.
The study by Torri et al. (2022),[27] which included 19 participants in the VL group (mean ± SD: 2.53 ± 0.99) and 16 participants in the SVS group (mean ± SD: 2.57 ± 0.88), did not provide sufficient data for effect size and CI. Nonetheless, it accounted for 27.78% of the total weightage in the meta-analysis, adding to the robustness of the overall findings despite the lack of detailed data [refer to Table 2 and Figure 4b].
Visual acuity
In the assessment of VA, the results were less conclusive. Mori et al. (2021)[26] included 45 participants in the VL group (mean ± SD: 1.42 ± 0.11) and 46 participants in the SVS group (mean ± SD: 1.53 ± 0.11). The estimated MD for VA was 0.082 ± 0.171, with a 95% CI of −0.262–0.423, indicating no significant difference between the groups. The Bayes Factor (BF₁₀) of 0.214 suggests very weak evidence against the null hypothesis, implying that the RLRL intervention did not significantly affect VA.
Similarly, Torri and et al. (2022)[27] reported VA data for 19 participants in the VL group (mean ± SD: 2.53 ± 0.99) and 16 participants in the SVS group (mean ± SD: 2.57 ± 0.88). However, without sufficient effect size and CI data, the significance of these results remains unclear. Despite the lack of detailed reporting, the study contributed 27.78% to the overall meta-analysis, indicating its relevance in the broader analysis.
Overall, the findings from Mori et al.[26] provide robust evidence for significant differences in AL and VL, while VA does not appear to be significantly affected. The contributions from Torri and et al., despite limited data, reinforce the need for further detailed investigations to conclusively determine the effects of VL interventions [refer to Table 2 and Figure 4c].
Discussion
This systematic review and meta-analysis was conducted with the aim to find out the effect of VL on controlling myopia progression among children. The exposure to VL shows a statistically significant effect on myopia progression by controlling axial elongation, reducing the SER, and improving VA. However, no difference in VA between the VL and SVSs was observed, as visual improvement was seen with both modalities.
Publication bias was assessed with a funnel plot, which revealed no bias. A fixed-effect model using Bayesian statistics was employed because the same age group of Japanese children was enrolled in both included studies for the meta-analysis. Moreover, the risk of bias was assessed using the Cochrane tool (ROB2), which revealed no bias.
The mechanism of axial elongation in myopia using the VL transmission eyeglass frame was apparently due to OPN5-expressing retinal ganglion cells emitting VL from the frame, as shown in the animal study. The thickening of the choroid actually prevented this. Moreover, the change in the choroidal thickness in the VL group was noticeably more than in the control group. The fundamental mechanism of myopia causing ocular ischemia is choroidal thinning brought on by accommodation.[29] Wu et al. found that in the myopia model, HIF-1α in the sclera was elevated, which in turn caused collagen remodeling resulting in scleral weakness, and ocular elongation soon followed.[29] Ischemia of the choroidal blood perfusion causes this ischemia or HIF-1 upregulation in the sclera.[30] According to reports, myopia is typified by a thin choroid; extremely myopic patients have a particularly thin choroid.[31] Recent studies have revealed that choroidal thinning proceeds before ocular elongation.[31,32] For myopia prevention, maintaining the choroidal blood flow and choroidal thickness is essentially therapeutic. Jiang et al. found in the mouse model of myopia that nonvisual photoreceptor OPN5 stimulation by VL resulted in maintenance of the choroidal thickness.[25] As shown in previous investigations in animals as well as people, this study inhibited axial elongation with choroidal thickening in the VL group wearers, therefore, preserving the choroidal blood flow and at least partially resolving ischemia. One basic therapy technique to reduce myopia is the VL produced by the eyeglass frames. There has been some earlier research on VL. Using chicks, Torii et al. showed that VL reduced axial elongation and myopic shift of the refractive error in a lens-induced myopia (LIM) model.[19] Other studies utilizing mouse models have produced the same findings.[25,33] It was shown that VL’s mode of action in slowing down myopia development preserves choroidal thickness across OPN5 in the retina.[25,34] Sensitive to VL, OPN5 is an opsin among the photoreceptors found in the retina.[25] OPN5 is reported to be linked with the circadian rhythm, vasculogenesis, and thermogenesis. VL is linked, according to another study, to the myopia-suppressive gene EGR1.[21,25,35] In chicks, EGR1 expression was prominent in the myopia-suppressed enucleated eyes lit by VL.[19]
Human research often requires reports on animal experimental models as a prerequisite. For instance, red light was found to cause a myopia response in chicks,[20] while exposure to long-wavelength red light caused hyperopic responses in Rhesus monkeys and tree shrews.[36,37] Short-wavelength light exposure, on the other hand, caused hyperopia in guinea pigs, poultry, and fish.[20,38,39,40] Moreover, LIM models in chicks, mice, and guinea pigs revealed an inhibition of axial elongation and myopic shift of refractive error upon exposure to VL.[19,25,33,41] Among visible lights, VL was the most successful wavelength in LIM to slow down myopia development.[25]
Retarding axial elongation helps physicians limit myopia advancement by means of AL, which is the main determinant of this process of myopia therapy.[42] According to a well-accepted idea, bright light is thought to stimulate the retina to increase the creation and release of dopamine,[43] which in turn prevents axial elongation. Current data suggests that oxygen-derived free radicals and the inflammatory process affect myopia progression.[44] On the other hand, blue light, which has a shorter wavelength, has been shown to raise retina dopamine levels. Blue light influences the activation of melanopsin-containing intrinsically photosensitive retinal ganglion cells axons in the optic nerve head.[45] Enhanced contrast sensitivity and changes in retinal electrical activity are associated with the activation of these cells, thereby suggesting a possible increase in the dopamine release in the retina.[45]
On one hand, the present systematic review and meta-analysis have strengths in terms of the included randomized control trials (RCTs). The risk of bias was assessed with the standardized tool ROB-2, developed by Cochrane. In addition, Bayesian analysis was used to estimate the effect size, and publication bias was assessed with a funnel plot. However, several limitations were also present. Long-term follow-up and monitoring of AL and SER were needed. The rebound effect also needs to be evaluated. Both of the included studies were based in Japan, so there exists a gap in the representation of other populations. Furthermore, one of the included RCTs had a small sample size.
CONCLUSION
Violet transmission glasses play a crucial role in managing myopia in children. They contribute to the shortening of the eye, which reduces refractive error and enhances visual clarity. Nevertheless, further research is required to analyze the potential long-term rebound effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Flaxman SR, Bourne RR, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, et al. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221–34. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 2.Dolgin E. The myopia boom. Nature. 2015;519:276–8. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Wu LJ, You QS, Duan JL, Luo YX, Liu LJ, Li X, et al. Prevalence and associated factors of myopia in high-school students in Beijing. PLoS One. 2015;10:e0120764. doi: 10.1371/journal.pone.0120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62:6. doi: 10.1167/iovs.62.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird PN, Saw SM, Lanca C, Guggenheim JA, Smith EL, Iii, Zhou X, et al. Myopia. Nat Rev Dis Primers. 2020;6:99. doi: 10.1038/s41572-020-00231-4. [DOI] [PubMed] [Google Scholar]
- 7.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–48. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 8.Ohno-Matsui K, Wu PC, Yamashiro K, Vutipongsatorn K, Fang Y, Cheung CM, et al. IMI pathologic myopia. Invest Ophthalmol Vis Sci. 2021;62:5. doi: 10.1167/iovs.62.5.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pärssinen O, Kauppinen M. Risk factors for high myopia: A 22-year follow-up study from childhood to adulthood. Acta Ophthalmol. 2019;97:510–8. doi: 10.1111/aos.13964. [DOI] [PubMed] [Google Scholar]
- 10.Hu Y, Ding X, Guo X, Chen Y, Zhang J, He M. Association of age at myopia onset with risk of high myopia in adulthood in a 12-year follow-up of a Chinese cohort. JAMA Ophthalmol. 2020;138:1129–34. doi: 10.1001/jamaophthalmol.2020.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikuno Y. Overview of the complications of high myopia. Retina. 2017;37:2347–51. doi: 10.1097/IAE.0000000000001489. [DOI] [PubMed] [Google Scholar]
- 12.Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y, et al. Prevalence and causes of low vision and blindness in a Japanese adult population: The Tajimi study. Ophthalmology. 2006;113:1354–62. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Bullimore MA, Brennan NA. Myopia control: Why each diopter matters. Optom Vis Sci. 2019;96:463–5. doi: 10.1097/OPX.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 14.Bullimore MA, Richdale K. Myopia control 2020: Where are we and where are we heading? Ophthalmic Physiol Opt. 2020;40:254–70. doi: 10.1111/opo.12686. [DOI] [PubMed] [Google Scholar]
- 15.Xiong S, Sankaridurg P, Naduvilath T, Zang J, Zou H, Zhu J, et al. Time spent in outdoor activities in relation to myopia prevention and control: A meta-analysis and systematic review. Acta Ophthalmol. 2017;95:551–66. doi: 10.1111/aos.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankaridurg P, Conrad F, Tran H, Zhu J. Controlling progression of myopia: Optical and pharmaceutical strategies. Asia Pac J Ophthalmol (Phila) 2018;7:405–14. doi: 10.22608/APO.2018333. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Lan W, Liao Y, Zhao F, Chen C, Yang Z. Treatment outcomes of myopic anisometropia with 1% atropine: A pilot study. Optom Vis Sci. 2013;90:1486–92. doi: 10.1097/OPX.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 18.Chan TC, Li EY, Wong VW, Jhanji V. Orthokeratology-associated infectious keratitis in a tertiary care eye hospital in Hong Kong. Am J Ophthalmol. 2014;158:1130–5.e2. doi: 10.1016/j.ajo.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–9. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013;54:8004–12. doi: 10.1167/iovs.13-12476. [DOI] [PubMed] [Google Scholar]
- 21.Torii H, Ohnuma K, Kurihara T, Tsubota K, Negishi K. Violet light transmission is related to myopia progression in adult high myopia. Sci Rep. 2017;7:14523. doi: 10.1038/s41598-017-09388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ofuji Y, Torii H, Yotsukura E, Mori K, Kurihara T, Negishi K, et al. Axial length shortening in a myopic child with anisometropic amblyopia after wearing violet light-transmitting eyeglasses for 2 years. Am J Ophthalmol Case Rep. 2020;20:101002. doi: 10.1016/j.ajoc.2020.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–8. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Pardue MT, Mori K, Ikeda SI, Torii H, D’Souza S, et al. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc Natl Acad Sci U S A. 2021;118:e2018840118. doi: 10.1073/pnas.2018840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori K, Torii H, Hara Y, Hara M, Yotsukura E, Hanyuda A, et al. Effect of violet light-transmitting eyeglasses on axial elongation in myopic children: a randomized controlled trial. J Clin Med [Internet] 2021;10:5462. doi: 10.3390/jcm10225462. Available from: https://www.mdpi.com/2077-0383/10/22/5462. [Last accessed on 2024 May 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torii H, Mori K, Okano T, Kondo S, Yang HY, Yotsukura E, et al. Short-term exposure to violet light emitted from eyeglass frames in myopic children: a randomized pilot clinical trial. J Clin Med [Internet] 2022;11:6000. doi: 10.3390/jcm11206000. Available from: https://www.mdpi.com/2077-0383/11/20/6000. [Last accessed on 2024 May 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Chen W, Zhao F, Zhou Q, Reinach PS, Deng L, et al. Scleral hypoxia is a target for myopia control. Proc Natl Acad Sci U S A. 2018;115:E7091–100. doi: 10.1073/pnas.1721443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao F, Zhang D, Zhou Q, Zhao F, He M, Yang Z, et al. Scleral HIF-1α is a prominent regulatory candidate for genetic and environmental interactions in human myopia pathogenesis. EBioMedicine. 2020;57:102878. doi: 10.1016/j.ebiom.2020.102878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Wang L, Xu Y, Pang Z, Mu G. The influence of the choroid on the onset and development of myopia: From perspectives of choroidal thickness and blood flow. Acta Ophthalmol. 2021;99:730–8. doi: 10.1111/aos.14773. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, Zhang S, Yang F, Yang Y, Huang Q, Huang C, et al. Decreased choroidal blood perfusion induces myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2021;62:30. doi: 10.1167/iovs.62.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strickland R, Landis EG, Pardue MT. Short-wavelength (violet) light protects mice from myopia through cone signaling. Invest Ophthalmol Vis Sci. 2020;61:13. doi: 10.1167/iovs.61.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen MT, Vemaraju S, Nayak G, Odaka Y, Buhr ED, Alonzo N, et al. An opsin 5-dopamine pathway mediates light-dependent vascular development in the eye. Nat Cell Biol. 2019;21:420–9. doi: 10.1038/s41556-019-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): A novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–6. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- 36.Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith EL, 3rd, Hung LF, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015;56:6490–500. doi: 10.1167/iovs.15-17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kröger RH, Fernald RD. Regulation of eye growth in the African cichlid fish Haplochromis burtoni. Vision Res. 1994;34:1807–14. doi: 10.1016/0042-6989(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 39.Zou L, Zhu X, Liu R, Ma F, Yu M, Liu H, et al. Effect of altered retinal cones/opsins on refractive development under monochromatic lights in guinea pigs. J Ophthalmol. 2018;2018:9197631. doi: 10.1155/2018/9197631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu R, Qian YF, He JC, Hu M, Zhou XT, Dai JH, et al. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011;92:447–53. doi: 10.1016/j.exer.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Zhang S, Schaeffel F, Xiong S, Zheng Y, Zhou X, et al. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus) Vision Res. 2014;94:24–32. doi: 10.1016/j.visres.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Pugazhendhi S, Ambati B, Hunter AA. Pathogenesis and prevention of worsening axial elongation in pathological myopia. Clin Ophthalmol. 2020;14:853–73. doi: 10.2147/OPTH.S241435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramamurthy D, Lin Chua SY, Saw SM. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin Exp Optom. 2015;98:497–506. doi: 10.1111/cxo.12346. [DOI] [PubMed] [Google Scholar]
- 44.Francisco BM, Salvador M, Amparo N. Oxidative stress in myopia. Oxid Med Cell Longev. 2015;2015:750637. doi: 10.1155/2015/750637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schilling T, Torres CC, Wong N, Bahmani H, Huete F, Carracedo G. Selective stimulation of the optic nerve head with blue light increases vitreal dopamine levels in rabbits. Invest Ophthalmol Vis Sci. 2021;62:1385. [Google Scholar]


