Abstract
Photochemical reaction is expected to become a promising type of green chemistry with merits of operational flexibility, excellent regioselectivity, high yield, and mild reaction condition. Recently, aggregation-induced emission (AIE)-based fluorescent molecules with photoresponsivity have been quickly developed and employed in the biomedical field. In this Review, photoresponsive AIE materials based on the inherent photochemical reactions are highlighted according to the photochemical pathways: photoisomerization, photocyclization, photodimerization, and multiple photoreactions. Following this, their biomedical applications are summarized and discussed, including photoactivatable bioimaging, diagnosis, and therapy. Finally, the challenges and future perspectives are presented.
Keywords: aggregation-induced emission, photoreactions, photoisomerization, photocyclization, photodimerization, fluorescence imaging, antitumor/antibacterial therapy, diphenylethene
1. Introduction
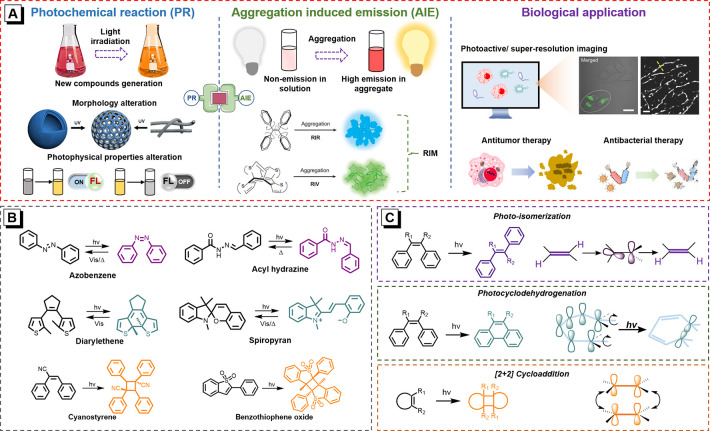
Photochemical reaction, as a distinctive category of chemical reactions, plays a significant role in advancing the development of organic synthetic chemistry and has garnered increasing attention based on the platform of biomedicine.1−3 Based on diverse photochemical reactions, a library of photoresponsive “smart” materials is explored and created. The photochemical activity endows these materials with various light-related merits, such as remote control, convenient and accurate adjustability, noninvasiveness, environmental friendliness, and so forth. Hence, photochemically active materials are suitable for a wide range of research applications, including bioimaging,4 information storage,5 anticounterfeiting,6 etc. In general, photochemical processes involve electronically excited species, formed by an effective excitation, who undergo a deactivation process to generate new chemicals.7 Since the electronic and chemical structures of photoreaction products are different from those before the reaction, the photophysical properties are greatly altered, generally accompanied by distinct photochromism and/or fluorescence “turn-on”/“turn-off” phenomena during the reaction, making the real-time molecular monitoring within a desired region via continuous irradiation possible. Interestingly, photochemical reactions can even change the self-assembled morphology of amphiphilic polymers, showing great potential in light-gated drug delivery (Scheme 1A, left).8 Nowadays, organic molecules with inherent photochemical reactions are proved to be powerful imaging tools for tracking molecular and cellular dynamics with high spatiotemporal resolution in biomedical systems, and photoactivatable fluorescence materials are also emerging as promising antibacterial/anticancer diagnosis and treatment candidates.
Scheme 1. (A) Overview of This Review;8,11,23,24 (B) Typical Photochemical Reactions; (C) Three Types of Photochemical Reactions Mentioned in This Review.
Reprinted from ref (8). Copyright 2021, American Chemical Society. Reprinted with permission from refs (11, 23, and 24). Copyright 2021 Oxford University Press. Copyright 2015 Wiley-VCH. Copyright 2016 Wiley-VCH.
Nevertheless, designing photochemically active molecules with high performance to promote practical biomedical application is still a big challenge. One of the reasons is that conventional organic fluorescent molecules usually adopt large flat disc-like structures suffering from the aggregation-caused quenching (ACQ) problem, which may lead to significantly weakened or quenched emission in the aggregated state. Besides, they usually have shortages of poor photostability and small Stokes shifts, greatly restricting their practical application. Delightfully, an intriguing aggregation-induced emission (AIE) phenomenon was reported for some propeller-like silole derivatives in 2001,9 which paved a new avenue toward efficient luminescent materials free of the ACQ problem. Different from classical fluorescent dyes that possess planar structures, AIE luminogens (AIEgens) have twisted structures, which can hamper the excited state energy dissipation by inhibiting a strong intermolecular interaction and restricting intramolecular motion (RIM), resulting in enhanced fluorescence intensity in aggregates (Scheme 1A, middle).10,11 Specifically, the RIM mechanism can include the following four modes: restriction of vibronic coupling, restriction of access to conical intersection, restriction of access to dark state, and suppression of photochemical reaction.11 Furthermore, most AIEgens can be simply synthesized and modified to achieve multifunctionalities and superior properties such as intense emission, large Stokes shift, good photostability, and excellent biocompatibility, which make them great candidates in biomedical areas. Up to now, various AIEgens have been developed for application in chemical sensing,12,13 bioimaging,14,15 photodynamic/photothermal therapy,16−19 etc.
Multifunctional materials that integrate the advantages of AIE and photochemical activity in a single formulation for synchronous biomedical applications are highly desired, which can be employed in photoactivation imaging, super-resolution imaging, photodynamic therapy, etc (Scheme 1A, right).20−26 In the past few years, there are several kinds of organic photochemical groups that have been reported, such as azobenzene, acyl hydrazine, diarylethenes, spiropyrans, cyanostyrene, benzothiophene oxide, and so on, as shown in Scheme 1B.27−29 Generally, utilizing these photochemical groups to construct photochemical-active AIE materials is one of the most effective design strategies. Up to now, many AIEgens have been designed and synthesized based on these photochemical cores.30−36 It is noteworthy that the relationship between AIE and photochemical reactions is indispensable. As a dominant nonradiative transition pathway, photochemical reaction plays a vital role in the excited state inactivation. As discussed above, the suppression of photochemical reaction is one of the important pathways to achieve AIE. Moreover, there are a few similarities between AIEgens and photochemically active molecules. Diphenylethene (DPE) is one of the most typical molecules. For instance, the rotation of Ph and torsion/twisting of C=C under light irradiation can promote the photochemical reaction, which is also the basis of AIE phenomenon. Therefore, DPE and its derivatives can serve as photochromic cores to create AIEgens with multiple photochemical activities (Scheme 1C).37 In this Review, we mainly focus on the photochemical reactions and the biomedical application of AIEgens with photochemical activity. According to the photochemical pathways, three major categories of photochemical reactions are introduced: (i) reversible conformational photoisomerization; (ii) photocyclization; (iii) photodimerization, including the reaction mechanisms and concrete examples. We extensively describe the photoresponsive AIEgens containing DPE groups as the photoisomerization/photocyclization units, and then, we highlight the most typical and promising applications of these AIEgens in the biomedical field. Finally, the prospects for future research directions and challenges in this field are discussed.
2. Photochemical Reactions of AIEgens
2.1. Photoisomerization
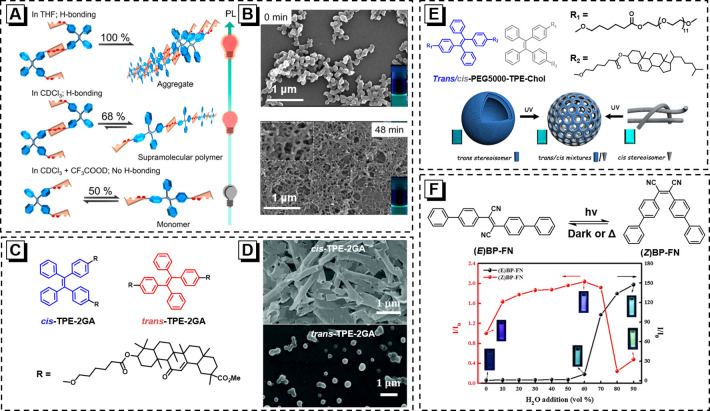
Regarding the photoisomerization process of alkenes, it is recognized that the cis–trans photoisomerization of the C=C double bond is typical for primary photochemical reactions in the S1 (π, π*) and T1 (π, π*) states. Similarly, photoisomerization can also occur for stilbene under light irradiation, which is a temperature-dependent process. Trans- and cis-isomers generally have different photophysical properties. Compared to cis-isomer, the trans product has a larger molar absorption coefficient and a longer absorption maximum. It is worth noting that the trans/cis-stereoisomers with distinct geometrical structures can be used to construct stimuli-responsive functional materials because of different intermolecular interactions and packing patterns. Better yet, the AIE feature allows the photoisomerization process to be “seen” and controlled. Two AIE-active isomers named (Z)-TPE-UPy and (E)-TPE-UPy with supramolecular assembly capacity are designed to realize this goal.38 Due to the presence of quadruple hydrogen-bonding interactions from the 2-ureido-4[1H]-pyrimidinone (UPy) group, (Z)-TPE-UPy and (E)-TPE-UPy own self-assembly abilities and show dramatically different aggregate morphologies. The experimental results prove that the conversion efficiency of (Z)-TPE-UPy to (E)-TPE-UPy can be well controlled by changing the solvent. Concretely, the conversion from (Z)-TPE-UPy to (E)-TPE-UPy would be inhibited when the hydrogen bonds are destructed by trifluoroacetic acid (Figure 1A) but can be promoted in tetrahydrofuran (THF) due to the poor solubility of (E)-isomer. Meanwhile, the obvious fluorescence “turn-on” effect in the isomerization from (Z)-TPE-UPy to (E)-TPE-UPy allows us to directly “see” the ongoing photoisomerization process. Interestingly, benefiting from the different aggregate morphologies of (Z)-TPE-UPy to (E)-TPE-UPy, the photoisomerization process can also be monitored by scanning electron microscopy (SEM) (Figure 1B).
Figure 1.
(A) Schematic representation of controlling the photoisomerization efficiency of (Z)-TPE-UPy by different states of the isomers. (B) SEM images of self-assembly nanostructures prepared by precipitating (Z)-TPE-UPy into chloroform/hexane (1/99, v/v) before and after UV irradiation. Insets show the associated fluorescent pictures of the nanostructures in chloroform/hexane mixtures. Reprinted from ref (38). Copyright 2019 American Chemical Society. (C) Chemical structures of trans- and cis-TPE-2GA isomers. (D) SEM images of cis-TPE-2GA (top) and trans-TPE-2GA (bottom) at fw = 60%. Reprinted with permission from ref (39). Copyright 2021 Royal Society of Chemistry. (E) Chemical structures of the two stereoisomers, trans-PEG550-TPE-Chol and cis-PEG550-TPE-Chol, and the schematic representation of the perforated membrane and nanoporous polymersomes. Reprinted from ref (8). Copyright 2021 American Chemical Society. (F) Schematic depiction of photoisomerization and the AIE effect of the (E) BP-FN and (Z) BP-FN acquired in THF solution with the addition of H2O. Reprinted with permission from ref (40). Copyright 2015 Elsevier.
The structures of substituents have a great influence on material properties. For instance, two isomers (cis/trans-TPE-2GA) with rigid triterpenoid units are prepared (Figure 1C), showing distinct behaviors in fluorescence, assembly morphology (Figure 1D), and photoisomerization rate.39 Besides, for cis-TPE-2GA, the mechanical stimulation can lead to the condensed state changing from crystalline to amorphous phases, accompanied by a red-shift emission. Notably, the emission can be reversibly recovered after cis-TPE-2GA powder is treated with methanol vapor due to the restoration of crystalline structure. Oppositely, the crystalline structure of pristine trans-TPE-2GA has a more regular molecular packing and stronger intermolecular interactions, rendering an insensitive mechanochromic response.
Benefiting from their distinct self-assembly behaviors of stereoisomers, trans–cis photoisomerization can be used to prepare vesicles with various morphologies. For example, the two stereoisomers, trans-PEG550-TPE-Chol and cis-PEG550-TPE-Chol, show different self-assembly behaviors in water.8trans-PEG550-TPE-Chol can form classical vesicles, while cis-PEG550-TPE-Chol self-assembles into cylindrical micelles. Interestingly, the ordered cylindrical micelles of cis-PEG550-TPE-Chol can converse to meshes and nanoporous membranes due to the generation of trans-isomers under UV irradiation (Figure 1E). This work provides a valid strategy to control pores to “open” for supramolecular capsules by UV irradiation, which shows great potential as light-controlled delivery vehicles in biotechnology. Additionally, some small organic molecules are also characterized by photoisomerism, and these two isomers show different photophysical properties (Figure 1F).40
With the development of research, the mechanism behind the photophysical phenomena is constantly being explored. In general, it is considered that E–Z isomerization causes fluorescence quenching of stilbene in the solution state. A series of photochemical experiments and quantum chemical calculations are conducted to study the role of C=C double bond twisting in the fluorescence quenching of AIE-active TPE derivatives in the solution state. The experimental results prove that the E–Z isomerization is the major factor for the quenching of the photoexcited state of TPE derivatives in the solution state, differently from the well-accepted propeller-like rotation of the side phenyl rings in earlier research.41 In contrast, experimental results from other researchers show that the fluorescence intensity during the normal PL spectral measurement has a negligible effect on the E–Z isomerization, so the isomerization process is not involved in its AIE process under this condition42 even though the photochemical reaction plays an important role as a nonradiation pathway in the photophysical process.
2.2. Photocyclization
In the photochemical reaction, molecules experience chemical bond formation and breakage under light illumination, which is considered a reversible cyclization process. Photocyclization is one of the representatives of electrocyclization. As a particular type of position isomerization, the electrocyclization reaction is highly stereoselective. During the process of electrocyclization, the positions of π and σ bonds change, accompanied by the generation of a new σ bond. According to the Woodward–Hoffmann rule, for the 6π electron system, the molecules in the excited state (under light irradiation) are favorable for a cis spin.43 Stilbene is a typical molecule that can undergo electrocyclization into the unstable dihydrophene (DHP) under light stimulation, which can return to stilbene by thermal or photochemical treatments. However, the existence of oxidant can irreversibly transfer DHP to a more stable dehydrogenation product (PHI). Interestingly, according to NEER’s rule,44 there will be more than one photochemical product formed when the phenyl of stilbene is replaced by another group, which is due to their diverse isomers in the ground state.
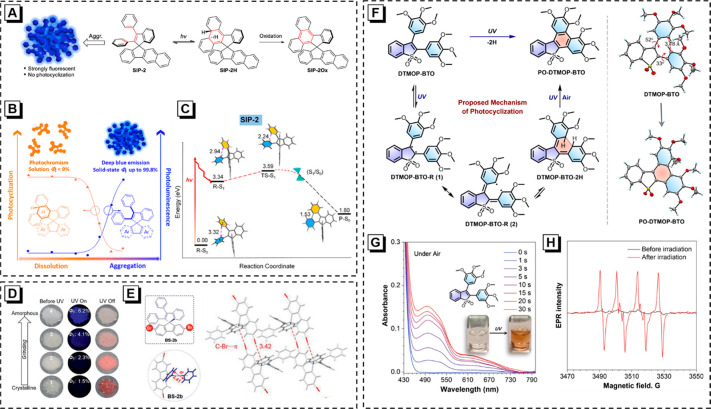
DPE-based molecules generally undergo the typical reversible cyclization reactions, which have received the most attention and have been extensively explored. By merging a flexible DPE moiety with a rigid spiro scaffold, Tang et al. designed a novel class of AIEgens (DPI and SIPs), as shown in Figure 2A.34 Compared with DPI, SIPs own ultrahigh solid-state fluorescence quantum yields (ΦFs), and the extension of the π-system is avoided due to the sp3-hybridized spiro. As expected, such a rigid spiro scaffold can efficiently restrict conformational relaxation of the molecules in the excited state and thus suppress nonradiative transition. Besides the AIE feature, DPE can undergo reversible photocyclization. However, this photochemical cyclization is enhanced in solutions but blocked by aggregation. During the irradiation on SIP-2, an obvious absorption at 475 nm is captured, which is ascribed to the cyclized intermediate (SIP-2H). Therefore, photocyclization activity is evaluated by monitoring the change of absorption at 475 nm. As shown in Figure 2B, the change of PL intensity is negligible at low fws (0–50 vol%), and the photocyclization activity still maintains its maximum value. While aggregates are formed with a further increase of fws, the PL intensity increases progressively but photocyclization-based photochromism is limited. Notably, SIP-2/4 shows a cyclization-favorable antiparallel conformation and small distances between the photocyclization reactive carbon atoms (d ∼ 3.40 Å). However, due to the constraint of both DPE rotors by the benzofluorene unit from adjacent molecules, the photochromism is highly suppressed in their crystalline states. In addition, the theoretical calculation is a prominent method to explore the photochemical reaction pathways and mechanisms. As shown in Figure 2C, there is a S1/S0 conical intersection (CI) point between the lowest excited state of transition state (TS-S1) and the ground state of product (P-S0), indicating SIP-2 undergoes a process of excited reactant (*R)–CI-P under UV irradiation.45
Figure 2.
(A) AIE and photocyclization processes of SIP-2 in the dissolved and aggregated states. (B) The plot of photocyclization activity and fluorescent intensity of SIP-2 along with the formation of aggregate. Reprinted from ref (34). Copyright 2019 American Chemical Society. (C) Energy profiles for the photocyclization in the singlet states of SIP-2. Reprinted with permission from ref (45). Copyright 2022 Royal Society of Chemistry. (D) Images of BS-2b in crystals and amorphous powders before and after UV irradiation at 365 nm. (E) Single crystal structures of BS-2b and the C–Br···π intermolecular interactions. Reprinted with permission from ref (46). Copyright 2020 Wiley-VCH. (F) Proposed photocyclization mechanism of DTMOP-BTO and crystal structures of DTMOP-BTO and PO-DTMOP-BTO. (G) UV/vis absorption spectral changes of DTMOP-BTO in THF (1.0 × 10–3 M) upon 365 nm UV light irradiation and corresponding photographs of color changes. (H) EPR spectra of DMPO in the presence of DTMOP-BTO before and after 365 nm UV light irradiation. Reprinted from ref (47). Copyright 2023 American Chemical Society.
The photoresponsive solid-state fluorescent materials have become a research focus in the field of intelligent materials in recent years. However, it is still a challenge to build solid-state photochromic structures. For instance, DPE can easily undergo photochemical transformations in the solution state, but the photochemical activities are sensitively suppressed due to the conformational constraints of the surrounding matrix in aggregate. The intermolecular C–Br···π interaction can be employed to modulate photochemical and photophysical reactivities. Specifically, by introducing through-space Br-mediated halogen−π interactions on the DPE moiety, a solid-state photoresponsive molecule (BS-2b) with AIE feature is well designed.46 The crystal of BS-2b shows a reversible colorless-to-red photochromism with high fatigue resistance, benefiting from the perpendicular configuration of the paired C–Br···π bonding interactions (Figure 2D,E). In contrast, BS-2b amorphous powders are completely inactive in terms of photochromism but show enhanced fluorescence. This design strategy provides unique insights into through-space halogen-π interactions and expands the structure scope of advanced solid-state photoresponsive materials. In addition, introducing other halogen atoms (e.g., Cl) into TPE derivatives to construct proper intermolecular interaction can also realize photocyclization in a solid.35
Monitoring the intermediates/transition states is helpful to explore the intrinsic reaction mechanism and pathway hidden in the photochemical process, while relevant investigations are limited in previous work. Recently, a serious of AIE-featured benzothiophene derivatives with sensitive photoresponsive behavior are prepared.47 It is confirmed that DTMOP-BTO can undergo photocyclization with the participation of more than one intermediate (Figure 2F). As illustrated in Figure 2G, two new absorption bands appear upon UV irradiation, one is attributed to the cyclized intermediate (DTMOP-BTO-2H). The other with a longer wavelength is associated with the formation of triplet diradical species. Moreover, the obvious electron paramagnetic resonance (EPR) signal in the mixture of 5,5-dimethyl-1-pyrroline-1-oxide (DMPO) and DTMOP-BTO further confirms the formation of free radicals (Figure 2H). By combining with the theoretical calculation, a reasonable photocyclization mechanism is proposed. DTMOP-BTO undergoes photochemical C=C bond cleavage to generate a transition state with a diradical character after excitation. The diradical is highly unstable and tends to migrate and recombine to form cyclized intermediate DTMOP-BTO-2H, which can be easily oxidated to finally yield the ring-closed PO-DTMOP-BTO neatly. Interestingly, these newly formed diradical intermediates can effectively inhibit the growth of S. aureus (Gram-positive bacteria), showing great potential in antibacterial therapy.
Due to the highly specific regioselectivity, photocyclization is a good choice to construct polycyclic compounds under mild conditions. Although there is more than one reactive site in o-TPBQ, an unexpected five-membered azaheterocycle (c5-TPBQ) is produced.48 It is generally recognized that the reactive site of photocyclization tends to locate at the electron-deficient region of a molecule. Interestingly, different from most cyclization products exhibiting the ACQ effect, c5-TPBQ shows an obvious AIE feature.
2.3. Photodimerization
For the photodimerization, it is necessary to arrange the double bonds of neighboring molecules in a parallel manner with a distance smaller than 4.2 Å.49 Upon photoexcitation, the molecule undergoes intramolecular [2 + 2] cycloaddition, rehybridizing the orbitals from sp2 to sp3, and finally generates the photodimerization product. Among various reported photochemical reactions, photodimerization owns distinct merits, including solvent-free, high stereoselectivity, and so forth. Recently, tremendous progress has been made to design fluorescent molecules with photodimerization characteristics.
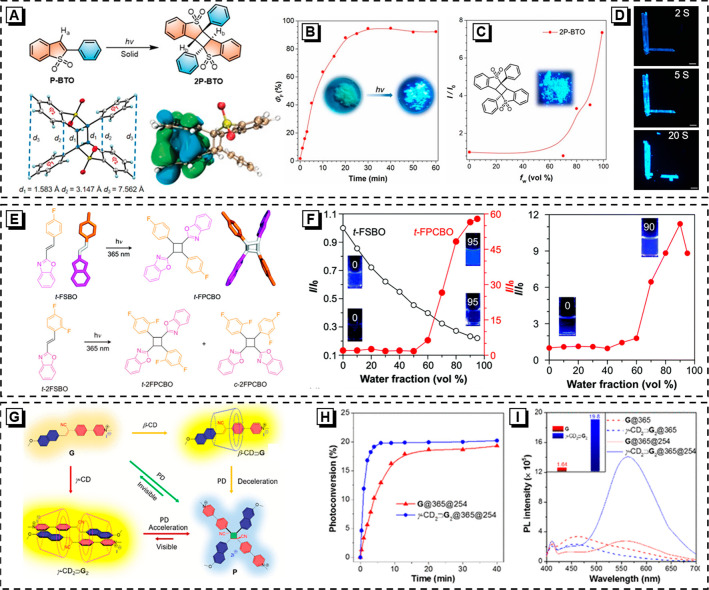
During the photodimerization process, the conversion of molecular structure can drive the mechanical motions of molecular crystals, making it possible to track molecular motions on a macroscopic scale. Regrettably, during the photodimerization process, the π-conjugation of the precursor will be broken with the formation of the cyclobutane ring, which invalidates the photomechanical luminescence in most molecular crystals. Interestingly, the microcrystalline powder of 2-phenylbenzo[b]thiophene 1,1-dioxide (P-BTO) could undergo [2 + 2] cycloaddition to generate 2P-BTO under 365 nm UV irradiation (Figure 3A),50 and the photodimerization product (2P-BTO) shows stronger fluorescence intensity than P-BTO. As illustrated in Figure 3B, the value of ΦF increases along with the elongation of the irradiation time (2P-BTO formation). Although it is weakly through-bond conjugated for 2P-BTO, it exhibits a remarkable AIE feature (Figure 3C), which is beneficial from the through-space interaction between two stacked phenyl rings.51−53 Meanwhile, the crystal of P-BTO shows multifarious photomechanical behaviors (splitting, jumping, and bending) under UV irradiation, which are driven by the slow release of strain accumulated by atomic motion associated with the transformation of crystal structures during [2 + 2] cycloaddition (Figure 3D). [2 + 2] cycloaddition is a novel strategy to construct solid-state fluorescent materials. Additionally, it could also realize in situ ACQ-to-AIE transformation. Two styrylbenzoxazole derivatives, trans-2-(4-fluorostyryl)benzo[d]oxazole (t-FSBO) and trans-2-(2,4-difluorostyryl)benzo[d]oxazole (t-2FSBO), suffer from a severe ACQ problem due to the strong π–π interaction in their planar rigid structure.54 Interestingly, upon UV irradiation, t-FSBO and t-2FSBO can undergo photodimerization (Figure 3E). Delightfully, as shown in Figure 3F, all the cycloaddition products show typical AIE properties, and their emission intensity is dramatically increased with increasing fws. Their intramolecular or intermolecular through-space conjugation has contributed significantly to their strong emission in the aggregated state. This fascinating in situ ACQ-to-AIE transformation strategy provides new opportunities to construct intelligent solid-state emitters in the future.
Figure 3.
(A) Chemical and crystal structures of 2P-BTO and the photodimerization route; the electron cloud distribution of 2P-BTO in the excited state. (B) The ΦF values of P-BTO microcrystalline powders under irradiation with 365 nm UV for different times. Inset: fluorescent photos of P-BTO before and after UV light irradiation. (C) Plots of relative PL intensity (I/I0) versus fws (I0 = intensity at fw = 0%). Inset: Fluorescent image of pure 2P-BTO under UV light irradiation. (D) Fluorescence microscopy images of P-BTO crystals under 365 nm irradiation. Reprinted with permission from ref (50). Copyright 2019 Wiley-VCH. (E) Photochemical reaction of t-FSBO and t-2FSBO. (F) Plots of relative I/I0 value versus fws of t-FSBO, t-FPCBO (left) and t-2FPCBO (right). Inset: fluorescent photos of t-FSBO, t-FPCBO, and t-2FPCBO in DMSO/water mixtures at fw = 0% and fw = 95% under 365 nm UV light irradiation. Reprinted with permission from ref (54). Copyright 2020 Royal Society of Chemistry. (G) Proposed mechanism of visible and rate-controllable photodimerization. (H) Photoconversion of dimer to monomer with irradiation time evaluated from the absorbance intensity change at λ = 380 nm. (I) PL intensity at λ = 570 nm of G in water solution with and without γ-CD before and after 365 nm UV irradiation followed by exposure to 254 nm UV light. Inset in panel I shows the contrast of PL intensity at 570 nm of G with and without γ-CD before and after 254 nm UV irradiation. Reprinted from ref (55). Copyright 2019 American Chemical Society.
Regulating photochemical reaction rates is highly desirable but challenging. Delightfully, this can be achieved by host–guest chemistry.55 Cyclodextrin (CD), a typical macrocyclic compound, tends to form host–guest complexes with hydrophobic molecules. β-CD (7 glucopyranose units) and γ-CD (8 glucopyranose units) are used as hosts to form 1:1 and 2:2 complexes with a guest (molecule G) in the aqueous mixture, respectively. According to the experimental result, for β-CD⊃G (1:1 complex), the host efficiently restricts the dimerization (Figure 3G). Otherwise, for γ-CD2⊃G2 (2:2 complex), the average distance between two double bonds is shortened to 3.9 Å, which is beneficial for photodimerization. As expected, the reaction rate constants are as follows: Kγ-CD > KG > Kβ-CD. Interestingly, the reversible photochemical reaction can also occur upon irradiation by 254 nm UV light. Moreover, compared with molecule G, the host–guest complex (γ-CD2⊃G2) shows more obvious emission enhancement due to the RIM effect. Accordingly, the photoconversion process of γ-CD2⊃G2 can be captured by PL spectra or even by the naked eye (Figure 3H,I). Moreover, CD-based hydrogels are prepared to further explore their photochemical activity. Surprisingly, the β-CD-based hydrogel can completely inhibit the photodimerization reaction, whereas the photodimerization and its inverse process of the γ-CD-based hydrogel can perform efficiently. In sum, the synergy of AIE and host–guest chemistry amplifies the signal of the photochemical reaction and makes it possible to visualize a “tiny” ring-opening product.
2.4. Multiple Photoreactions
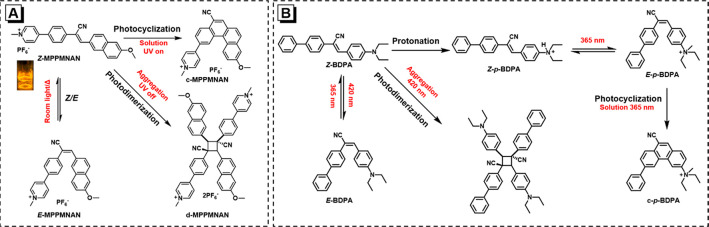
Incorporation of two or three independent photoreactions into one system to impart multiple specific functionalities while maintaining controllability is not only of academic interest but also of practical implication. Cyanostilbene-containing molecules are one of the promising candidates to realize such a wonderful conception. As depicted in Figure 4A, the AIE-active molecule (MPPMNAN) exhibits efficient, multiple, and controllable photoresponsive behaviors under different conditions.56Z-MPPMNAN can undergo reversible photoisomerization under room light in CH3CN to form its E-isomer, which can convert to the initial Z-state by heating. To our surprise, Z-MPPMNAN can efficiently transform to its E-form, followed by a photocyclization reaction to generate the cyclized product (c-MPPMNAN) by continuous UV irradiation. During this process, the fluorescence intensity is gradually enhanced with the elongation of exposure time. Moreover, this photocyclization occurs at low fws in the mixture of CH3CN/H2O but is highly inhibited at high fws. Oppositely, at fw = 99%, photodimerization activity is enhanced due to the increase of microcrystals. Z-MPPMNAN can form tiny nanocrystals and then gradually changes to its crystalline dimer under ambient condition, accompanied by the emission color change from yellow to green.
Figure 4.
(A) Schematic illustration of multiple photoreactions of Z-MPPMNAN. Reprinted from ref (56). Copyright 2018 American Chemical Society. (B) Schematic illustration of multiple photoreactions of Z-BDPA Reprinted with permission from ref (57). Copyright 2022 Science China Press and Springer-Verlag GmbH Germany, part of Springer Nature.
Likewise, similar results are also observed in another cyanostyrene-based AIEgen (Z-BDPA) (Figure 4B).57 Upon 420 nm light irradiation, Z-BDPA can undergo Z/E-tautomerization to form E-isomer in THF, accompanied by an obvious blue shift in the PL spectrum, and this photoisomerization is reversible under 365 nm UV in THF. Additionally, by prolonging 420 nm light irradiation on Z-BDPA, the [2 + 2] cycloaddition can be observed in the THF/H2O mixtures with fw = 90%. Benefiting from the diethylamine moiety, Z-BDPA is pH-responsive, tending to be protonated and transform into Z-p-BDPA under acidic conditions. Surprisingly but expectedly, Z-p-BDPA can also undergo Z/E-isomerization under 365 nm UV irradiation, followed by photocyclization to generate c-p-BDPA, but noteworthily, the improved water solubility and extensive intermolecular electrostatic repulsion are averse to the formation of dimers of Z-p-BDPA in an aqueous solution. Additionally, there are many cyanostilbene-based molecules showing similar characteristics.58
3. Biomedical Applications of Photoactivatable AIEgens
The AIEgens with photochemical activity combining the advantages of both photoactivation and AIE properties are highly favorable in biomedical applications, including photoactivation bioimaging, super-resolution fluorescent imaging, photocontrolled diagnosis, and cancer/bacteria therapy.59 In this section, we mainly focus on the recent biomedical applications of photochemically active AIEgens.
3.1. Bioimaging
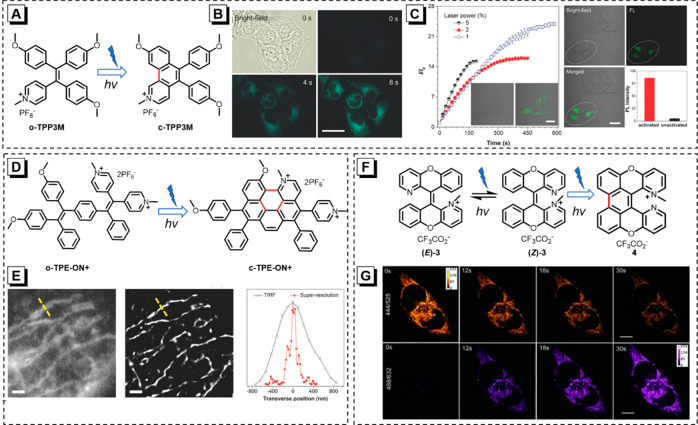
A pyridinium-bearing photoactivatable fluorescence probe (o-TPP3M) with mitochondrion targeting ability is well-designed and prepared (Figure 5A).23 The introduction of strong electron-donor and electron-acceptor pairs endows o-TPP3M with a typical twisted intramolecular charge-transfer effect, resulting in weak emission in a high-polar solution. Meanwhile, strong green fluorescence from the cyclized product (c-TPP3M) is observed upon irradiation by UV light, presenting a typical fluorescence “turn on” feature. Interestingly, with the aid of the pyridinium salt, o-TPP3M can penetrate the cell membrane and accumulate in the mitochondria region selectively, whose photocyclization product (c-TPP3M) can also generate in situ inside the living cells upon UV irradiation. During this process, the fluorescence signal is enhanced with the elongation of irradiation time, implying that this photoactivation is efficient in living HeLa cells (Figure 5B). Following this, o-TPP3M is chosen to image selected cells in space and time. As expected, molecules can be activated by the UV light in the optical window, and the fluorescence intensity is 17-fold higher than that in areas without irradiation (Figure 5C).
Figure 5.
(A) Photocyclization process of o-TPP3M. (B) Bright-field and fluorescent images of living HeLa cells stained with o-TPP3M after UV irradiation for 4 and 8 s. Scale bar: 30 μm. (C) The plot of I/I0 against irradiation time at different 405 nm laser power in HeLa cells. Insets: images of HeLa cells before and after irradiation with 405 nm 5% power laser beam. CLSM images of HeLa cells. The cells in the white ellipse region are selected to expose to 405 nm 35% power laser light for 1 s. Scale bar: 20 μm. Reprinted with permission from ref (23). Copyright 2015 Wiley-VCH. (D) Photocyclization process of o-TPE-ON+. (E) Diffraction-limited TIRF image and super-resolution image of mitochondria in HeLa cells and the corresponding transverse profiles of signal mitochondrion along the yellow dotted line. Reprinted with permission from ref (24).Copyright 2016 Wiley-VCH. (F) E–Z interconversion and photocyclization/oxidation reaction of (E)-3/(Z)-3. (G) Confocal microscopy images of HeLa cells stained with (E)-3/(Z)-3, continuous observation at 200 alternating 150 ms pulses. Reprinted with permission from ref (36). Copyright 2015 Wiley-VCH.
Compared with traditional imaging technology, super-resolution imaging possesses higher resolution even at the nanometer scale, attracting tremendous attention in recent years. Organelle-specific small-molecule probes with the photoactivatable feature are a great candidate for super-resolution imaging. As shown in Figure 5D, o-TPE-ON+, a novel mitochondria-targeted AIEgen exhibits a similar light-up property upon UV irradiation.24 With a lipophilic positive charge, o-TPE-ON+ is highly cell permeable and biocompatible. Better yet, it can spontaneously blink without any additives, which makes it a promising material for single-molecule localization microscopy imaging of mitochondria on nanoscale. As shown in Figure 5E, the mitochondria exhibit a clear structure for super-resolution images that is obtained by stochastic optical reconstruction microscopy with the full width of 104.5 nm. However, the diffraction-limited total internal reflection fluorescence (TIRF) image shows a blurred structure with low resolution (full-width is 697.1 nm). More importantly, there is no unfriendly additive that participates in this super-resolution imaging process, so the fission and fusion behaviors of mitochondria could also be monitored in living cells. This work proves the feasibility of AIEgens with photochemical activity to realize super-resolution imaging of living cells.
In general, short-wavelength emission of the photoproduct is undesirable in biomedical applications. Different from the most photoactivatable fluorescent probes, (E)-3/(Z)-3 has emerged as a good alternative for photoresponsive mitochondrial-specific imaging of living cells because its photocyclization product has red-shift absorption and emission.36 Since only (Z)-3 can fulfill the requirement for the photocyclization, (E)-3 first undergoes isomerization to form a Z-isomer for facilitating the photocyclization, as illustrated in Figure 5F. There is a large red shift in the PL spectrum during the photochemical process, and thus, two excitation channels can be applied to the imaging without signal overlap. To be specific, the signal intensity of the photoproduct can light up, while the fluorescence from (E)-3/(Z)-3 is reduced under continuous irradiation (Figure 5G). Such a wonderful fluorescent molecule has great potential in photoresponsive cellular imaging.
3.2. Antitumor/Antibacterial Therapy
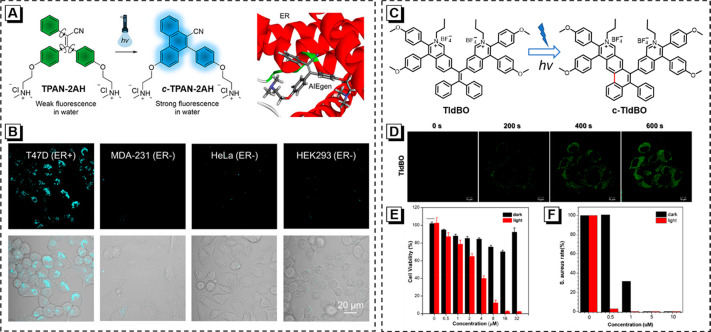
Apart from fluorescence imaging in living cells, photoresponsive AIEgens are highly desirable to provide more opportunities for realizing precise diagnosis and therapy. Estrogen receptors (ERs) can be activated by estrogen to perturb the transcription process upon binding to DNA. It is reported that more than 60% of breast cancer patients are ER-positive, indicating that ERs have great potential to be the targeted proteins to develop a therapeutic agent for hormone receptor-dependent breast cancer. In response, TPAN-2AH, an ER antagonist analogue, is prepared with good binding affinity for ER (Figure 6A). More fascinatingly, TPAN-2AH undergoes a photocyclization reaction to emit blue fluorescence under UV irradiation (Figure 6B) and shows obvious cytotoxicity toward ER-positive cells.60
Figure 6.
(A) The photocyclization process of TPAN-2AH and the computational docking model of TPAN-2AH and ER. (B) Fluorescent images and merged images of ER-positive cells (T47D) or ER-negative cells (MDA-231, HeLa, HKE293) stained with TPAN-2AH for 24 h. Reprinted from ref (60). Copyright 2022 American Chemical Society. (C) Chemical structure of TIdBO and the photoactivation process. (D) CLSM images of HeLa cells stained with TIdBO under continuous excitation and sequential scanning by 405 nm laser. (E) Cell viability after treatment with a range of concentrations with or without white light irradiation. (F) The killing efficiency of TIdBO on S. aureus at different concentrations; the photographs of S. aureus cultured on an agar plate supplemented with different concentrations of TIdBO. Reprinted from ref (63). Copyright 2021 American Chemical Society.
Photodynamic therapy (PDT) with plenty of advantages is expected to become a promising modality for cancer treatment. During the PDT process, the generation efficiency of reactive oxygen species (ROS) of photosensitizer (PS) directly affects the therapeutic effect.61,62 A novel AIE-active PS (TIdBO) with fantastic photoactivation properties is developed (Figure 6C).63 It is verified that the ROS generation process is competitive with photocyclization. In general, TIdBO undergoes photocyclization in solution exposed under UV light, while it generates Type I ROS (free radical ROS) in aggregate by white light irradiation. Such an excellent Type I ROS generation ability is owed to the occurrence of electron transfer during the photocyclization process, which makes it a good PS to realize PDT. Better yet, together with its AIE effect, TIdBO can efficiently achieve the integration of diagnosis and therapy and show light-up fluorescence after 405 nm laser irradiation in cells (Figure 6D). More importantly, TIdBO shows good biocompatibility in the dark condition, while exhibiting excellent PDT performance in cancer cells (Figure 6E). Surprisingly, as illustrated in Figure 6F, TIdBO owns obvious dark and light toxicity toward S. aureus, demonstrating the potential to be an antimicrobial drug.
4. Conclusion and Perspective
In this Review, we have summarized recent developments and progress of AIEgens with photochemical activity, especially those containing DPE groups as the photoisomerization/photocyclization units. The integration of photochemical activity and AIE feature through elegant design into one molecule not only holds advantages such as remote control, adjustability, and noninvasiveness but also avoids the troublesome ACQ phenomenon. Based on their excellent performances, we then introduce typical applications of photoresponsive AIEgens in biomedical applications, including bioimaging, diagnosis, and therapy. Nevertheless, AIEgens with photochemical activity are still at the infant stage including structural design and practical application, and there are still challenges and perhaps future opportunities to further advance AIEgens for practical applications.
-
(1)
One of the challenges for AIE-featured photoactivatable materials is that their structures are still far from diverse, and it is urgent to develop novel photoresponsive molecules. Besides, to broaden their practical applications, the performances of existing photoactivatable AIEgens, including fluorescence quantum yield, thermal stability, switching speed, and fatigue resistance, need to be further improved.
-
(2)
Moreover, most photoactivatable AIEgenes only respond to UV/vis light, suffering limited penetration depth and even exhibiting damage to normal cells and tissues, which is undesirable in biomedical applications. Worse still, their photoproducts usually show obvious blue-shifted emissions after photoexcitation. This can be overcome by precise molecular engineering to construct molecules containing strong electronic donor–acceptor pairs to develop photoactivatable AIEgens with long-wavelength activation, especially near-infrared light responsiveness.
-
(3)
It is also suggested to substantially expand the field of practical application. In addition to designing novel photoactivatable AIEgens, it is also important to develop advanced devices or diagnosis kits appropriate for the application of photoactivatable materials in the future.
Up to now, a great deal of progress has been made in the systematic investigation of the mechanisms and applications of photochemical reactions, and we believe that AIEgens with photochemical activity can provide an ideal platform from which to fabricate “smart” stimuli-responsive fluorescent materials.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21788102), the GuangDong Basic and Applied Basic Research Foundation (2023B1515040003, 2022A1515010315 and 2019B030301003), and the Fundamental Research Funds for the Central Universities (2022ZYGXZR107).
Glossary
Vocabulary
- conical intersection
the point where two potential energy surfaces intersect and their energies become degenerate
- electron paramagnetic resonance
a magnetic resonance technique that utilizes the magnetic moment of unpaired electrons to detect the presence of free radicals in substances
- through-space conjugation
a crucial category of π-electron delocalization systems (the inter-ring interaction among aromatic rings relies on their ring overlap rather than electron transport via covalent bonds)
- photodynamic therapy
a novel therapeutic modality that employs photosensitizers to interact with surrounding oxygen molecules at specific wavelengths, generating reactive oxygen species and inducing non-specific damage to tumor tissues
- stochastic optical reconstruction microscopy
an ultra-resolution imaging technology capable of acquiring two- or three-dimensional images in multiple colors, even within living cells
The authors declare no competing financial interest.
References
- Bach T.; Hehn J. P. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew. Chem., Int. Ed. 2011, 50, 1000–1045. 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]
- Karkas M. D.; Porco J. A. Jr; Stephenson C. R. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. 2016, 116, 9683–747. 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latrache M.; Hoffmann N. Photochemical Radical Cyclization Reactions with Imines, Hydrazones, Oximes, and Related Compounds. Chem. Soc. Rev. 2021, 50, 7418–7435. 10.1039/D1CS00196E. [DOI] [PubMed] [Google Scholar]
- Yang H.; Li M.; Li C.; Luo Q.; Zhu M. Q.; Tian H.; Zhu W. H. Unraveling Dual Aggregation-Induced Emission Behavior in Steric-Hindrance Photochromic System for Super Resolution Imaging. Angew. Chem., Int. Ed. 2020, 59, 8560–8570. 10.1002/anie.201909830. [DOI] [PubMed] [Google Scholar]
- Ma L.; Li C.; Yan Q.; Wang S.; Miao W.; Cao D. Unsymmetrical Photochromic Bithienylethene-Bridge Tetraphenylethene Molecular Switches: Synthesis, Aggregation-Induced Emission and Information Storage. Chin. Chem. Lett. 2020, 31, 361–364. 10.1016/j.cclet.2019.07.040. [DOI] [Google Scholar]
- Huang G.; Xia Q.; Huang W.; Tian J.; He Z.; Li B. S.; Tang B. Z. Multiple Anti-Counterfeiting Guarantees from a Simple Tetraphenylethylene Derivative-High-Contrasted and Multi-State Mechanochromism and Photochromism. Angew. Chem., Int. Ed. 2019, 58, 17814–17819. 10.1002/anie.201910530. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Xu Y.; Zhao C.; Jia C.; Guo X. Recent Advances in Photochemical Reactions on Single-Molecule Electrical Platforms. Macromol. Rapid Commun. 2022, 43, 2200017. 10.1002/marc.202200017. [DOI] [PubMed] [Google Scholar]
- Chen H.; Fan Y.; Yu X.; Semetey V.; Trepout S.; Li M. H. Light-Gated Nano-Porous Capsules from Stereoisomer-Directed Self-Assemblies. ACS Nano 2021, 15, 884–893. 10.1021/acsnano.0c07400. [DOI] [PubMed] [Google Scholar]
- Luo J.; Xie Z.; Lam J. W.; Cheng L.; Chen H.; Qiu C.; Kwok H. S.; Zhan X.; Liu Y.; Zhu D.; Tang B. Z. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 1740–1741. 10.1039/b105159h. [DOI] [PubMed] [Google Scholar]
- Hong Y.; Lam J. W. Y.; Tang B. Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- Tu Y.; Zhao Z.; Lam J. W. Y.; Tang B. Z. Mechanistic Connotations of Restriction of Intramolecular Motions (RIM). Natl. Sci. Rev. 2021, 8, nwaa260. 10.1093/nsr/nwaa260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giel M.-C.; Zhang S.; Hu Q.; Ding D.; Tang Y.; Hong Y. Synthesis of a β-Arylethenesulfonyl Fluoride-Functionalized AIEgen for Activity-Based Urinary Trypsin Detection. ACS Appl. Bio Mater. 2022, 5, 4321–4326. 10.1021/acsabm.2c00513. [DOI] [PubMed] [Google Scholar]
- Hu J.; Jiang W.; Yuan L.; Duan C.; Yuan Q.; Long Z.; Lou X.; Xia F. Recent Advances in Stimuli-Responsive Theranostic Systems with Aggregation-Induced Emission Characteristics. Aggregate 2021, 2, 48–65. 10.1002/agt2.10. [DOI] [Google Scholar]
- Zhuang Z.; Meng Z.; Li J.; Shen P.; Dai J.; Lou X.; Xia F.; Tang B. Z.; Zhao Z. Antibacterial Theranostic Agents with Negligible Living Cell Invasiveness: AIE-Active Cationic Amphiphiles Regulated by Alkyl Chain Engineering. ACS Nano 2022, 16, 11912–11930. 10.1021/acsnano.2c01721. [DOI] [PubMed] [Google Scholar]
- OwYong T. C.; Ding S.; Wu N.; Fellowes T.; Chen S.; White J. M.; Wong W. W. H.; Hong Y. Optimising Molecular Rotors to AIE Fluorophores for Mitochondria Uptake and Retention. Chem. Commun. 2020, 56, 14853–14856. 10.1039/D0CC06411D. [DOI] [PubMed] [Google Scholar]
- Zhuang Z.; Dai J.; Yu M.; Li J.; Shen P.; Hu R.; Lou X.; Zhao Z.; Tang B. Z. Type I Photosensitizers Based on Phosphindole Oxide for Photodynamic Therapy: Apoptosis and Autophagy Induced by Endoplasmic Reticulum Stress. Chem. Sci. 2020, 11, 3405–3417. 10.1039/D0SC00785D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Meng Z.; Zhuang Z.; Wang B.; Dai J.; Feng G.; Lou X.; Xia F.; Zhao Z.; Tang B. Z. Effective Therapy of Drug-Resistant Bacterial Infection by Killing Planktonic Bacteria and Destructing Biofilms with Cationic Photosensitizer Based on Phosphindole Oxide. Small 2022, 18, 2200743. 10.1002/smll.202200743. [DOI] [PubMed] [Google Scholar]
- Li J.; Dai J.; Zhuang Z.; Meng Z.; Hu J. J.; Lou X.; Xia F.; Zhao Z.; Tang B. Z. Combining PD-L1 Blockade with Immunogenic Cell Death Induced by AIE Photosensitizer to Improve Antitumor Immunity. Biomaterials 2022, 291, 121899. 10.1016/j.biomaterials.2022.121899. [DOI] [PubMed] [Google Scholar]
- Yao H.; Dai J.; Zhuang Z.; Yao J.; Wu X.; Wang S.; Xia F.; Zhou J.; Lou X.; Zhao Z. Red AIE Conjugated Polyelectrolytes for Long-Term Tracing and Image-Guided Photodynamic Therapy of Tumors. Sci. China Chem. 2020, 63, 1815–1824. 10.1007/s11426-020-9824-2. [DOI] [Google Scholar]
- Yan Q.; Wang S. Fusion of Aggregation-Induced Emission and Photochromics for Promising Photoresponsive Smart Materials. Mater. Chem. Front. 2020, 4, 3153–3175. 10.1039/D0QM00522C. [DOI] [Google Scholar]
- Wang J.; Zhang L.; Li Z. Aggregation-Induced Emission Luminogens with Photoresponsive Behaviors for Biomedical Applications. Adv. Healthcare Mater. 2021, 10, 2101169. 10.1002/adhm.202101169. [DOI] [PubMed] [Google Scholar]
- Tian H.; Yang S. Recent Progresses on Diarylethene Based Photochromic Switches. Chem. Soc. Rev. 2004, 33, 85–97. 10.1039/b302356g. [DOI] [PubMed] [Google Scholar]
- Gu X.; Zhao E.; Lam J. W.; Peng Q.; Xie Y.; Zhang Y.; Wong K. S.; Sung H. H.; Williams I. D.; Tang B. Z. Mitochondrion-Specific Live-Cell Bioprobe Operated in a Fluorescence Turn-On Manner and a Well-Designed Photoactivatable Mechanism. Adv. Mater. 2015, 27, 7093–7100. 10.1002/adma.201503751. [DOI] [PubMed] [Google Scholar]
- Gu X.; Zhao E.; Zhao T.; Kang M.; Gui C.; Lam J. W.; Du S.; Loy M. M.; Tang B. Z. A Mitochondrion-Specific Photoactivatable Fluorescence Turn-On AIE-Based Bioprobe for Localization Super-Resolution Microscope. Adv. Mater. 2016, 28, 5064–5071. 10.1002/adma.201505906. [DOI] [PubMed] [Google Scholar]
- Sun J.; Li H.; Gu X.; Tang B. Z. Photoactivatable Biomedical Materials Based on Luminogens with Aggregation-Induced Emission (AIE) Characteristics. Adv. Healthcare Mater. 2021, 10, 2101177. 10.1002/adhm.202101177. [DOI] [PubMed] [Google Scholar]
- Luo W.; Wang G. Photo-Responsive Fluorescent Materials with Aggregation-Induced Emission Characteristics. Adv. Optical Mater. 2020, 8, 2001362. 10.1002/adom.202001362. [DOI] [Google Scholar]
- Mandal A. K.; Gangopadhyay M.; Das A. Photo-Responsive Pseudorotaxanes and Assemblies. Chem. Soc. Rev. 2015, 44, 663–676. 10.1039/C4CS00295D. [DOI] [PubMed] [Google Scholar]
- Liubimov A. V.; Venidiktova O. V.; Valova T. M.; Shienok A. I.; Koltsova L. S.; Liubimova G. V.; Popov L. D.; Zaichenko N. L.; Barachevsky V. A. Photochromic and Luminescence Properties of a Hybrid Compound Based on Indoline Spiropyran of the Coumarin Type and Azomethinocoumarin. Photochem. Photobiol. Sci. 2018, 17, 1365–1375. 10.1039/c8pp00172c. [DOI] [PubMed] [Google Scholar]
- Gohy J. F.; Zhao Y. Photo-Responsive Block Copolymer Micelles: Design and Behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. 10.1039/c3cs35469e. [DOI] [PubMed] [Google Scholar]
- Li C.; Gong W.-L.; Hu Z.; Aldred M. P.; Zhang G.-F.; Chen T.; Huang Z.-L.; Zhu M.-Q. Photoswitchable Aggregation-Induced Emission of a Dithienylethene-Tetraphenylethene Conjugate for Optical Memory and Super-Resolution Imaging. RSC Adv. 2013, 3, 8967–8972. 10.1039/c3ra40674a. [DOI] [Google Scholar]
- Yu Q.; Su X.; Zhang T.; Zhang Y.-M.; Li M.; Liu Y.; Zhang S. X.-A. Non-Invasive Fluorescence Switch in Polymer films Based on Spiropyran-Photoacid Modified TPE. J. Mater. Chem. C 2018, 6, 2113–2122. 10.1039/C7TC05142E. [DOI] [Google Scholar]
- Huang L.; Qiu Y.; Wu C.; Ma Z.; Shen Z.; Jia X. A Multistate Fluorescent Switch with Multifunction of AIE, Methanol Responsiveness, Photochromism and Mechanochromism. J. Mater. Chem. C 2018, 6, 10250–10255. 10.1039/C8TC03728K. [DOI] [Google Scholar]
- Peng S.; Wen J.; Hai M.; Yang Z.; Yuan X.; Wang D.; Cao H.; He W. Synthesis and Application of Reversible Fluorescent Photochromic Molecules Based on Tetraphenylethylene and Photochromic Groups. New J. Chem. 2019, 43, 617–621. 10.1039/C8NJ05388J. [DOI] [Google Scholar]
- Zhou Z.; Xie S.; Chen X.; Tu Y.; Xiang J.; Wang J.; He Z.; Zeng Z.; Tang B. Z. Spiro-Functionalized Diphenylethenes: Suppression of a Reversible Photocyclization Contributes to the Aggregation-Induced Emission Effect. J. Am. Chem. Soc. 2019, 141, 9803–9807. 10.1021/jacs.9b04426. [DOI] [PubMed] [Google Scholar]
- Ou D.; Yu T.; Yang Z.; Luan T.; Mao Z.; Zhang Y.; Liu S.; Xu J.; Chi Z.; Bryce M. R. Combined Aggregation Induced Emission (AIE), Photochromism and Photoresponsive Wettability in Simple Dichloro-Substituted Triphenylethylene Derivatives. Chem. Sci. 2016, 7, 5302–5306. 10.1039/C6SC01205A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran M. N.; Chenoweth D. M. Photoelectrocyclization as An Activation Mechanism for Organelle-Specific Live-Cell Imaging Probes. Angew. Chem., Int. Ed. 2015, 54, 6442–6446. 10.1002/anie.201502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.; Niu Q.; Chen Y.; Lu M.; Zhang M.; Shi J.; Liu J.; Yan Y.; Li S.; Lan Y. Interpenetrating 3D Covalent Organic Framework for Selective Stilbene Photoisomerization and Photocyclization. J. Am. Chem. Soc. 2023, 145, 8860–8870. 10.1021/jacs.2c12313. [DOI] [PubMed] [Google Scholar]
- Peng H. Q.; Liu B.; Liu J.; Wei P.; Zhang H.; Han T.; Qi J.; Lam J. W. Y.; Zhang W.; Tang B. Z. ″Seeing″ and Controlling Photoisomerization by (Z)-/(E)-Isomers with Aggregation-Induced Emission Characteristics. ACS Nano 2019, 13, 12120–12126. 10.1021/acsnano.9b06578. [DOI] [PubMed] [Google Scholar]
- Yu X.; Meng Y.; Zhang H.; Guo J.; Wang S.; Li H.; Hu J.; Li M. H. Trans/Cis-Stereoisomers of Triterpenoid-Substituted Tetraphenylethene: Aggregation-Induced Emission, Aggregate Morphology, and Mechano-Chromism. Nanoscale 2021, 13, 15257–15266. 10.1039/D1NR04353F. [DOI] [PubMed] [Google Scholar]
- Jeong H.; Kim K. M.; Cho S.; Lee J. K. In-Situ Photoisomerization for Aggregation-Induced Emission of Dibiphenyl Fumaronitrile. J. Photoch. Photobio. A 2015, 311, 199–202. 10.1016/j.jphotochem.2015.06.013. [DOI] [Google Scholar]
- Kokado K.; Machida T.; Iwasa T.; Taketsugu T.; Sada K. Twist of C=C Bond Plays A Crucial Role in the Quenching of AIE-Active Tetraphenylethene Derivatives in Solution. J. Phys. Chem. C 2018, 122, 245–251. 10.1021/acs.jpcc.7b11248. [DOI] [Google Scholar]
- Tseng N.-W.; Liu J.; Ng J. C. Y.; Lam J. W. Y.; Sung H. H. Y.; Williams I. D.; Tang B. Z. Deciphering Mechanism of Aggregation-Induced Emission (AIE): Is E-Zisomerisation Involved in an AIE process?. Chem. Sci. 2012, 3, 493–497. 10.1039/C1SC00690H. [DOI] [Google Scholar]
- Anslyn E. V.; Dougherty D. A.. Modern physical organic chemistry; University Science Books: Sausalito, 2006. [Google Scholar]
- Havinga E.; Schlatmann J. L. M. A. Remarks on the Specificities of the Photochemical and Thermal Transformations in the Vitamin D Field. Tetrahedron 1961, 16, 146–152. 10.1016/0040-4020(61)80065-3. [DOI] [Google Scholar]
- Wei H.; Zeng Y.; Li Q.; Zheng X. Suppression of Reversible Photocyclization Reaction Induced Fluorescence Enhancement: A Theoretical Study. Phys. Chem. Chem. Phys. 2022, 24, 25487–25494. 10.1039/D2CP03448D. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Liu Q.; Chen X.; Xu G.; Wang S.; Tu Y.; Zhang J.; Zheng X.; Xiang J.; Feng X.; Zhang Y.; Xie S.; Zeng Z.; Tang B. Z. Through-Space C-Br···π Halogen Interaction: Efficient Modulation of Reaction-Based Photochromism and Photoluminescence at Crystalline States for Irradiation Time-Dependent Anti-Counterfeiting. Adv. Funct. Mater. 2021, 31, 2009024. 10.1002/adfm.202009024. [DOI] [Google Scholar]
- Guo J.; Li J.; Wu T.; Peng X.; Wang S.; Zhao Z.; Hua Y.; Tang B. Z.; Zhao Y. Free Radical-Mediated Intramolecular Photocyclization of AIEgens Based on 2,3-Diphenylbenzo[b]thiophene S,S-Dioxide. J. Am. Chem. Soc. 2023, 145, 7837–7844. 10.1021/jacs.2c12738. [DOI] [PubMed] [Google Scholar]
- Li Q.; Gong J.; Li Y.; Zhang R.; Wang H.; Zhang J.; Yan H.; Lam J. W. Y.; Sung H. H. Y.; Williams I. D.; Kwok R. T. K.; Li M.-H.; Wang J.; Tang B. Z. Unusual Light-Driven Amplification through Unexpected Regioselective Photogeneration of Five-Membered Azaheterocyclic AIEgen. Chem. Sci. 2021, 12, 709–717. 10.1039/D0SC04725B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. M. J. Photodimerization in the Solid State. Pure Appl. Chem. 1971, 27, 647–678. 10.1351/pac197127040647. [DOI] [Google Scholar]
- Guo J.; Fan J.; Liu X.; Zhao Z.; Tang B. Z. Photomechanical Luminescence from Through-Space Conjugated AIEgens. Angew. Chem., Int. Ed. 2020, 59, 8828–8832. 10.1002/anie.201913383. [DOI] [PubMed] [Google Scholar]
- Li J.; Shen P.; Zhao Z.; Tang B. Z. Through-Space Conjugation: A Thriving Alternative for Optoelectronic Materials. CCS Chem. 2019, 1, 181–196. 10.31635/ccschem.019.20180020. [DOI] [Google Scholar]
- Zhuang Z.; Shen P.; Li J.; Li J.; Zhao Z.; Tang B. Z. Deciphering Benzene-Heterocycle Stacking Interaction Impact on the Electronic Structures and Photophysical Properties of Tetraphenylethene-Cored Foldamers. CCS Chem. 2022, 4, 286–303. 10.31635/ccschem.021.202000677. [DOI] [Google Scholar]
- Shen P.; Zhuang Z.; Jiang X.-F.; Li J.; Yao S.; Zhao Z.; Tang B. Z. Through-Space Conjugation: An Effective Strategy for Stabilizing Intramolecular Charge-Transfer States. J. Phys. Chem. Lett. 2019, 10, 2648–2656. 10.1021/acs.jpclett.9b01040. [DOI] [PubMed] [Google Scholar]
- Wang H.; Xing H.; Gong J.; Zhang H.; Zhang J.; Wei P.; Yang G.; Lam J. W. Y.; Lu R.; Tang B. Z. Living” Luminogens: Light Driven ACQ-to-AIE Transformation Accompanied with Solid-State Actuation. Mater. Horiz. 2020, 7, 1566–1572. 10.1039/D0MH00447B. [DOI] [Google Scholar]
- Wei P.; Li Z.; Zhang J.-X.; Zhao Z.; Xing H.; Tu Y.; Gong J.; Cheung T. S.; Hu S.; Sung H. H. Y.; Williams I. D.; Kwok R. T. K.; Lam J. W. Y.; Tang B. Z. Molecular Transmission: Visible and Rate-Controllable Photoreactivity and Synergy of Aggregation-Induced Emission and Host-Guest Assembly. Chem. Mater. 2019, 31, 1092–1100. 10.1021/acs.chemmater.8b04909. [DOI] [Google Scholar]
- Wei P.; Zhang J. X.; Zhao Z.; Chen Y.; He X.; Chen M.; Gong J.; Sung H. H.; Williams I. D.; Lam J. W. Y.; Tang B. Z. Multiple yet Controllable Photoswitching in a Single AIEgen System. J. Am. Chem. Soc. 2018, 140, 1966–1975. 10.1021/jacs.7b13364. [DOI] [PubMed] [Google Scholar]
- Luo W.; Xu X.; Tang Y.; Wu Z.; Wang G. Multiregulated Color and Fluorescence of a Cyanostilbene-Based AIEgen by Light and pH. Sci. China Mater. 2023, 66, 1180–1188. 10.1007/s40843-022-2215-1. [DOI] [Google Scholar]
- Bhaumik S. K.; Banerjee S. Multicolor-Luminescence Including White Light by Photomodulation of Supramolecular Assemblies in Aqueous Media. ACS Appl. Mater. Interfaces 2022, 14, 36936–36946. 10.1021/acsami.2c07836. [DOI] [PubMed] [Google Scholar]
- Cai X.; Liu B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem., Int. Ed. 2020, 59, 9868–9886. 10.1002/anie.202000845. [DOI] [PubMed] [Google Scholar]
- Xu C.; Zou H.; Hu L.; Shen H.; Sung H. H. Y.; Feng H.; Kwok R. T. K.; Lam J. W. Y.; Zheng L.; Tang B. Z. A Photoactivatable AIEgen for Selective Imaging and Killing of Estrogen Receptor-Positive Cells. ACS Mater. Lett. 2022, 4, 1831–1839. 10.1021/acsmaterialslett.2c00529. [DOI] [Google Scholar]
- Dai J.; Wu X.; Ding S.; Lou X.; Xia F.; Wang S.; Hong Y. Aggregation-Induced Emission Photosensitizers: From Molecular Design to Photodynamic Therapy. J. Med. Chem. 2020, 63, 1996–2012. 10.1021/acs.jmedchem.9b02014. [DOI] [PubMed] [Google Scholar]
- Wang S.; Wang Z.; Hou Y. Self-Assembled Magnetic Nanomaterials: Versatile Theranostics Nanoplatforms for Cancer. Aggregate 2021, 2, e18 10.1002/agt2.18. [DOI] [Google Scholar]
- Chen K.; He P.; Wang Z.; Tang B. Z. A Feasible Strategy of Fabricating Type I Photosensitizer for Photodynamic Therapy in Cancer Cells and Pathogens. ACS Nano 2021, 15, 7735–7743. 10.1021/acsnano.1c01577. [DOI] [PubMed] [Google Scholar]