Abstract
Microplastics are a globally emerging contaminant in the environment, but little is known about the potential risks of microplastics to human health. Possible exposure routes of microplastics to humans include ingestion, inhalation, and dermal penetration, with the last of these needing equal attention as the other two main routes. Evidence showed the presence of microplastics in human-derived biological samples (i.e., excrement, biofluids, and tissues). Most of the toxicological studies of microplastics on humans were based on laboratory rodents and human-derived cells. Energy homeostasis, intestinal microflora, and the reproductive, immune, and nervous systems were regarded as targets of microplastics. The toxicity of microplastics on microstructures including lysosomes, mitochondria, endoplasmic reticulum, and the nucleus further revealed the potential risks of microplastics on human health at the cellular levels. As a carrier, microplastics also had the potential to magnify the toxicity of other contaminants in the environment (e.g., plasticizer, metals, antibiotics, and microorganisms). Studies of microplastics at environmentally realistic conditions are still in their infancy with many unsolved questions to predict their risks on human health.
Keywords: Microplastics, cell lines, human health, risk, toxicity
1. Introduction
The term “microplastics” was first introduced in 2004, being defined as the microscopic plastic fragments which are ubiquitous and accumulated in the ocean.1 The National Oceanic and Atmospheric Administration (NOAA) further specified “microplastics” in terms of size distribution, i.e., the small plastic pieces of less than 5 mm. Based on the origin of microplastics, they are classified as either primary microplastics, which are typically applied for consumer and industrial purposes, or secondary microplastics resulting from the mechanical/chemical/biological transformation of larger plastics.2−4 Both primary microplastics and secondary microplastics could be transported for a long distance due to their inert chemical properties and light weight.5 These microplastics are thus readily moved across air, land, freshwater, and the ocean.6−8 The ubiquitous presence of microplastics in the environment further enhances the exposure risks of microplastics on humans.
Besides environmental exposure, humans are also directly exposed to microplastics via domestic products. Microfibers, plastic foams, and microbeads are widely used in clothing, food containers, and toiletries,9 enhancing the risks of personal exposure to microplastics. The content of microplastics in tap water was measured to be 4.2 items/L, while the value increased by over 20 folds to 94 items/L in bottled water.10 Due to their small sizes, microplastics are readily accumulated in both aquatic and terrestrial organisms,11,12 facilitating the transfer of microplastics via the food chain and finally ending up in human consumers. Domestic food with additives is another origin of microplastics (0.10–1.48 items/g), resulting in an annual individual consumption reaching 52,000 microplastics from food and drink.10 If the inhalable microplastics are considered, the figure could reach up to 121,000 items per year.2 The ubiquitous presence of microplastics around humans thus results in inevitable exposure to humans, leading to the high necessity to explore the side effects of microplastics on human health.
Here, we searched articles mainly via Google Scholar with no publication year restrictions, and 3 types of articles (review articles, original articles, and reports by WHO) are included. The searching was based on each section and used a combination of keywords including “microplastics”, “nanoplastics”, “human health”, “human exposure”, one specific human biological sample like “liver”, “cytotoxicity”, and “mice”. We examined the main routes of exposure to humans, revealing the accessibility of environmental microplastics to human tissues and cells. Then, evidence of the presence of microplastics in humans and in vivo translocation of microplastics were summarized. Given the comprehensive studies on toxicity of microplastics on mammalian model and human-derived cell lines, possible toxic mechanisms of microplastics on human health were presented, and directions of future studies were proposed.
2. Exposure Routes of Microplastics to Humans
Exposure routes of microplastics to humans include ingestion, inhalation, and dermal penetration (Figure 1). Among all the exposure routes, the ingestion of microplastics was regarded as the primary route.8 Due to the intensive presence of microplastics in the ocean (up to 102,000 particles/m3),9 seafood was considered as one main source of microplastics by the ingestion route. Yearly microplastics uptake of each family from mollusks, crustaceans, and fish reached up to 27,825 items, 17,716 items, and 8,323 items, respectively.11 Although a recent World Health Organization’s report found no proof of harmful effects caused by microplastics in drinking water,13 the long-lasting effect of continuous exposure of tap water (4.23 items/L) on humans required more attention. The eating habits of humans directly influenced the quantity of ingested microplastics. The annual individual ingestion of table salt was 37 items in Europe, in contrast to a nearly 3-fold increase of microplastics ingested by Chinese.14,15 The source of table salt also largely determined the ingested quantity of microplastics. The content of microplastics in sea salt, lake salt, and rock/well salt was determined to be over 550, 43, and 7 items/kg, suggesting that sea products were more likely to be contaminated by microplastics in the ocean.15 In addition to the direct ingestion of microplastics from food items, microplastics released from plastic containers aggravated the human exposure, as illustrated by the increased 90,000 items ingested by consumers who preferred bottled water to tap water.10 Despite of the low concentration of microplastics, their sizes in the tap water from groundwater sources was larger than the sizes of water packaged in bottles (50–150 μm vs 6.5–20 μm).16,17 The difference was possibly due to the different purification and transportation procedures. For drinks (e.g., soft drinks, cold teas, and energy drinks) with more complicated manufacturing procedures, the size of microplastics was further increased to 0.1–3 mm.18 Similarly, compared with the size of microplastics directly derived from seafood (38.2–820 μm), the size of microplastics in canned seafood was bigger and reached 3800 μm.19,20 In addition to the direct release of microplastics from food packaging, migration of chemical plasticizers from food packaging to food further facilitated the exposure of those chemical additives to humans.21
Figure 1.
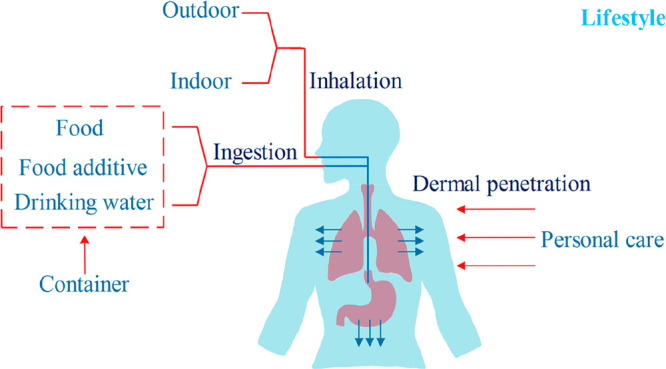
Exposure routes of microplastics to humans.
Synthetic textiles and city dust were considered as the most important sources of primary microplastics, while plastic fragments shedding from clothes, furniture, textiles, and building materials led to secondary exposure to humans via inhalation.22 Inhalable plastic fibers (19.6 fibers/m3) of polypropylene nature were found in indoor air, consistent with the chemical composition of carpets, sofas, and chairs in the indoor environment.23 The human lung is one of the main destinations of inhaled microplastics, with 87% of lungs containing plastic fibers made of petrochemicals.24 Simulating the human tidal volume at 6 L/min, up to 130 airborne microplastics could be individually inhaled per day, directly posing risks to the human lung.22 Compared with the suspended MPs in an indoor environment (1583 MPs/m3), the contents of microplastics in outdoor areas were much lower at 224 MPs/m3 and 101 MPs/m3 in urban and rural areas, respectively.25 In terms of particulate shape, bigger fractions (30–3000 μm, mostly fibers) were observed in the urban air compared with those found in rural air, consistent with the large size of suspended atmospheric microplastics up to 2191 μm in megacities.26 In both indoor air and outdoor air, the size of suspended particles was mostly less than 300 μm and abundant at approximately 30 μm,25,27 suggesting the high possibility of these MPs reaching humans by inhalation.
Particles that are internalized via a transdermal route should pass sublayers of epidermis before reaching microcirculation of the dermis and being transported through the human body via the circulatory system.28 Compared with the exposure routes of ingestion and inhalation, absorption of microplastics via dermal contact received much less attention since the dermal barrier inhibited the absorption of particles larger than 100 nm.29 However, nanoplastics less than 100 nm in size were an emerging environmental contaminant and had the potential to pass through the dermal barrier.30 Microbeads up to 40 nm could penetrate into epidermal Langerhans cells around hair follicles, in contrast to the limited uptake of larger counterparts including 750 nm/1500 nm particles.31 Dermal absorption occurred mostly when humans used personal care products, including hand cleanser, facial/body scrubs, face masks, and toothpaste, which may result in local toxicity and possible absorption.32 Due to the size limitation of microplastics available for dermal penetration, the dermal route was thus more associated with the absorption of released monomers or organic plasticizers like phthalates and bisphenols which were endocrine disruptors.33 As the biggest organ of the human body,34 human skin has a surface area of 1.5–2 m2 and provides an interface with ubiquitous microplastics in the environment. Thus, potential absorption of microplastics via dermal penetration requires equal attention as the other two routes in future research.
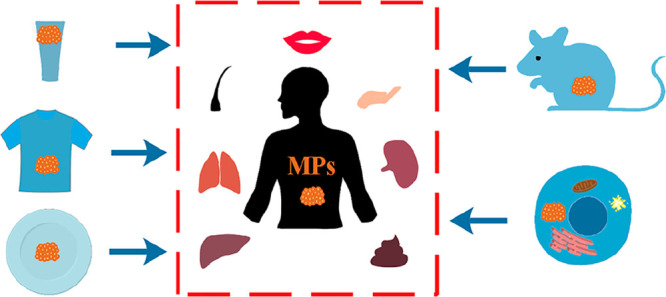
3. Abundance and Translocation of Microplastics in Human Body
The first publication of microplastics in a human biological sample (feces) was in 2019, in which up to 50 items/g and 9 plastic types were found in the fecal sample.35 After that, studies to quantify microplastics in human biological samples accelerated, especially from 2021 to 2022 when 80% of related studies were published.3 Due to the complicated preparations for human biomonitoring studies, such as volunteer recruitment and ethics approval, noninvasive biological sampling was preferred, leading most studies to evaluate human feces. Besides human feces, it was also feasible to sample saliva, sputum, hair, skin, and hands noninvasively compared with sampling microplastics from blood, bronchoalveolar lavage fluid, and tissues.36,37
In human feces, over 95.8% of samples tested positive, with the contents of microplastics up to 138.9 items/g in the form of fragments, films, and fibers.35,38−40 The size of fecal microplastics varied from 20 to 4813 μm, and over 15 types of microplastics were detected in fecal samples. In contrast to the high abundance of microplastics in human feces, limited microplastics were measured in human head hair, hands, face skin, and saliva at 0.33–3.5 items per individual per day.41 The category of plastic shape was also limited to fragments and fibers with sizes mostly less than 100 μm, shorter than those found in the feces. The detected microplastics in sputum sampled in deeper places of the body were narrower in shape at up to 9 items/mL.42 The average contents of microplastics extracted from bronchoalveolar lavage fluid were approximately 9 items/100 mL.43 However, the size of those detectable microplastics was mainly over 1000 μm as long plastic fibers had higher penetration ability,44 in contrast to the shorter fibers (<500 μm) found in sputum. The size of microplastics determined in human lung tissue was smaller than those in bronchoalveolar lavage fluid, mostly within 500 μm at up to 2.84 items/g.36,45
Unlike the respiratory tracts, which contained plastic fibers with high aspect ratios and large sizes, fragmented microplastics with a diameter less than 30 μm were mainly distributed in the human liver (4.6 items/g) and spleen (1.1 items/g).46 Large plastic fibers emerged again in colon tissue, with approximately 28.1 items/g and a size range of 800 to 1600 μm,47 which were consistent with the microfibers determined in feces. Although previous reports showed the ubiquitous presence of microplastics in human tissues including lung, liver, spleen, and colon, no microplastics were observed in the kidney tissue,46 suggesting the limited penetration of microplastics to kidneys. Fecal matter would be the main pathway to remove microplastics from human body. Besides various microplastics in different tissues, the individual’s health status also made a difference on the quantity of microplastics in the same tissue. Healthy liver contained <1.9 items/g microplastics, while the concentration increased to 11.9 items/g in patients with liver cirrhosis.46 A higher content of microplastics (41.8 items/g) was found in the feces of patients with inflammatory bowel disease compared with those in healthy people (28 items/g).39
The fate of microplastics in the human body was dependent on the in vivo translocation pattern, and crossing the epithelial layers of the skin, lungs, and gastrointestinal tract was the first step for translocation.48 Four layers (stratum corneum, viable dermis, dermis, and subcutaneous connective tissues) were included in human skin, rendering the difficulty for penetration of microplastics.49 Simulating human skin with pigskin, the negatively charged surface of microplastics facilitated the translocation through the skin by enhancing the density of particles, even when the particle size was as large as 500 nm.50 Only ultrasmall particles (<4 nm) could pass across the skin, and larger ones (<45 nm) could penetrate only if the skin was predamaged.51 Before being transported to the epithelial layer, a mucus layer which served to capture the exogenous particles entrapped the microplastics in both the gastrointestinal tract and lungs.52 Once ingested by humans, microplastics reached the gastrointestinal system and could be either engulfed by M-cells or directly adsorbed to gastrointestinal mucus.53 Numerous microplastics would be trapped in the mucus and rapidly removed due to the barrier effects of penetration by mucus layers.54 Except for the direct ingestion from foodstuff to the gastrointestinal system, the ciliary movement of the respiratory tract facilitated the transport of microplastics to the gastrointestinal system after inhalation.55 After being inhaled by humans, the deposition of microplastics was the first step affected by the physical–chemical properties of the particles, physiological state of humans, and lung anatomy.56 Small-size and low-density microplastics such as polyethylene were more easily able to reach the low airway.22 The size distribution of microplastics could also influence the mechanisms of deposition in airways such as Brownian motion-dependent diffusion (<1 μm), gravity-dependent sedimentation (1–5 μm), and momentum-dependent impaction (5–30 μm).56,57 Clearance of inhaled microplastics from the respiratory system relied on mucus progression to the pharynx, alveolar macrophage phagocytosis, and lymphatic transport.58,59 Despite the barrier effect of mucus layers, an abnormal thickness of the mucus layer could be induced by microplastics-caused dysbiosis via altering the microbiome’s composition,60,61 facilitating the interaction between microplastics and epithelial layers. Penetration may be facilitated with increasing epithelial permeability due to inflammation caused by microplastics.22 Transportation through the epithelium was largely dependent on size, with small plastics more likely to be internalized by endocytosis and large plastics more likely to be transferred via paracellular ways.48 The above results also supported that larger-sized microplastics were found in dermal contact portions and excreted feces, while smaller-sized ones within 50 μm were mostly translocated within the body.3
Following the penetration of epithelial layers of the skin, lungs, and gastrointestinal tract, various immune cells were responsible for capturing the invasive microplastics followed by translocation of microplastics in the circulatory system (as well as lymphatic system).62 Transportation from the nasal cavity to the lymphatic system and finally to the bloodstream was found in BALB/c mice after exposure to microspheres via an intranasal approach.63 After oral administration, latex microspheres at 0.87 μm could be transferred to the circulatory system of a Wistar rat via the gastrointestinal tract within 15 min.64 A recent study documented the presence of microplastics over 700 nm in human blood at 1.6 μg/mL,37 confirming the possible translocation from the environment to systemic circulation. After that, circulation carried microplastics to distant tissues, where microplastics and pathological change were observed. Microparticles (5 and 20 μm) were fed to ICR mice, and high concentrations of microplastics (up to 107 items/g) were accumulated in liver, kidney, and gut tissues through the circulatory system.65 Although no evidence showed the presence of microplastics in the lung of stillborns,36 newborns might be exposed to microplastics before contacting a contaminated environment. Approximately 12 plastic fragments (within 10 μm) were found in 4 placentas, and 10 types of microplastics were present in meconium.66,67 Additionally, over 76% of human breastmilk samples contained small plastic fragments (2–12 μm).68 The above evidence thus indicated the high possibility of translocation of microplastics from mother to newborns via maternal transfer. Abundances of microplastics in different parts of the human body are summarized in Figure 2.
Figure 2.
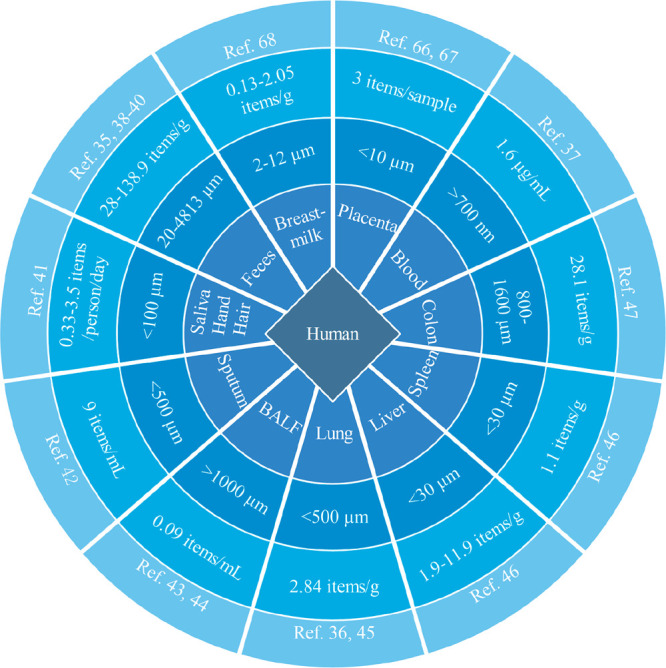
Abundance of microplastics in the human body. “BALF” indicates bronchoalveolar lavage fluid.
4. Potential Toxicity of Microplastics on Human Health
Due to the widely detected microplastics in various human tissues and the possible translocation of microplastics in the human body, there are considerable concerns of the toxicity of microplastics on human health. To evaluate the toxicity of microplastics on human health, laboratory rodents and human-derived cells were generally used as the model animals/cell lines.
4.1. Toxicity of Microplastics on Laboratory Rodents
The most conventional model organisms were ICR mice, C57bl/6 mice, C57 mice, CD-1 mice, Wistar rats, C57/B6 mice, Balb/c mice, Sprague–Dawley rats, and Swiss mice, and almost all applied microplastics were the commercially synthesized polystyrene and polyethylene.7 The administration routes in these studies included water drinking (ingestion), gastrostomy tube (ingestion), food source (ingestion), intratracheal instillation (inhalation), air exposure (inhalation), and intraperitoneal injection. Both male and female (including pregnant female) mice were employed in these toxicological studies of microplastics. Detailed information on the toxicity of microplastics on laboratory rodents is shown in Table 1.
Table 1. Toxicity of Microplastics on Laboratory Rodentsa.
| Organism | MPs | Size/Shape | Dose | Route | Toxicity |
|---|---|---|---|---|---|
| 5-week-old ICR mice | PS | 0.5 and 50 μm | 100 and 1000 μg/L | Drinking water 5 weeks | 1000 μg/L 0.5 and 50 μm: energy homeostasis; |
| Spherical shape | 100 μg/L 0.5 and 50 μm: intestinal disturbance60 | ||||
| 5-week-old male ICR mice | PS | 5 and 20 μm | 0.1 mg/d | Oral gavage 28 d | Energy homeostasis65 |
| Spherical shape | |||||
| 7-week-old pregnant ICR mice | PS | 5 μm | 100 and 1000 μg/L | Drinking water 6 weeks | Energy homeostasis and intestinal disturbance70 |
| Spherical shape | |||||
| 5-week-old ICR mice | PS | 5 μm | 100 and 1000 μg/L | Drinking water 6 weeks | Energy homeostasis and intestinal disturbance71 |
| Spherical shape | |||||
| 8-week-old male C57/B6 mice | PS | 0.07/5 μm | 0.2 and 2 mg/kg | Oral gavage 28 d | Intestinal disturbance72 |
| Spherical shape | |||||
| 6-week-old male BALB/C mice | PS | 0.5, 4, 10 μm | 1 mg/d | Oral gavage 28 d | Reproductive toxicity74 |
| Spherical shape | |||||
| 6-week-old female Wistar rats | PS | 0.5 μm | 0.015, 0.15, 1.5 mg/kg | Drinking water 90 d | 0.15 and 1.5 mg/kg: reproductive toxicity75 |
| Spherical shape | |||||
| 5 to 6-week-old male Balb/c mice | PS | 5.0–5.9 μm | 0.01, 0.1, 1 mg/d | Oral gavage 42 d | 0.01, 0.1, 1 mg/d: reproductive toxicity76 |
| Spherical shape | |||||
| 6-week-old female Wistar rats | PS | 0.5 μm | 0.015, 0.15, 1.5 mg/d | Drinking water 90 d | 0.15 and 1.5 mg/d: reproductive toxicity77 |
| Spherical shape | |||||
| 5-week-old male C57BL/6 mice | PS | 0.5 μm | 0.1 and 0.5 mg/d | Oral gavage 28 d | 0.5 mg/d/individual: immunotoxicity78 |
| Spherical shape | |||||
| 6-week-old- Sprague–Dawley rats | PS | 0.10 μm | 0.75 × 105, 1.5 × 105 and 3 × 105 particle/cm3 | Inhalation 60 h | Immunotoxicity (insignificant)79 |
| Spherical shape | |||||
| 6-week-old male ICR mice | PE | 40–48 μm | 0.125, 0.5, or 2 mg/d | Oral gavage 90 h | 0.125, 0.5, or 2 mg/d: immunotoxicity80 |
| Irregular shape | |||||
| 3-month-old male Swiss mice | PE | 35.46 μm | 60 mg/L via water | Oral gavage 7 d | Neurotoxicity81 |
| Irregular shape (mostly in spherical shape) | |||||
| 4 to 6-week-male Swiss mice | PS | 23.03 nm | 14.6 ng/kg | Intraperitoneal route 3 d | Neurotoxicity82 |
| Spherical shape |
PS: polystyrene; PE: polyethylene.
4.1.1. Energy Homeostasis and Intestinal Disturbance
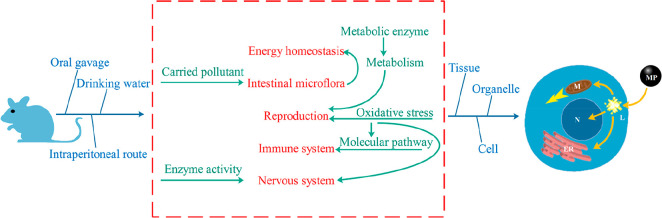
One important effect of microplastics on laboratory rodents was the disrupted energy homeostasis between available energy and expenditure. There was a decreased body, liver, and lipid weight in mice associated with disorders of hepatic lipid.60 Similarly, the liver lost weight and degenerated due to the disorders of energy and lipid metabolism.65 One mechanism resulting in the energy imbalance was attributed to the side effects of microplastics on metabolic enzymes. Anaerobic metabolism in both fish and mice was observed with activation of lactate dehydrogenase enzyme accompanied by a rapid reduction of ATP production.65,69 However, humans require higher energy than rodents, resulting in possibly different metabolic responses facing microplastics. When the pregnant female mice were exposed to microplastics, the first and second offspring may have a disordered fatty acid metabolism,70 indicating the possibly maternal transfer of these ingested microplastics. The administration routes were also important in the microplastics toxicity. Microplastics administered to mice by drinking water or food sources induced energy imbalance, which then caused intestinal inflammation, disturbance of the intestinal barrier, decreased mucin secretion, and most importantly, the pronounced changes of intestinal microflora.60,71 At the genus level, a total of 15 types of bacteria were significantly altered, with most altered genera belonging to beneficial microbes performing maintaining functions of the intestine.71,72 Specifically, short-chain fatty acids produced by genera such as Anaerotruncus and Ruminiclostridium were responsible for maintaining intestinal barrier functions, while these producers were decreased by microplastics. The decreased genera, including Anaerotruncus and Roseburia with tight junction-promoting functions, were also decreased by microplastics. The mechanism for plastic-induced change of intestinal microflora was mostly due to the plastic-associated pollutants including organics, metals, and even colonized microbes that could be desorbed in the gut environment and interacted with local microbes in the gut.73
4.1.2. Reproductive Toxicity
Another main target of microplastics was reproduction by ingestion routes such as drinking water and food sources. Degeneration of reproduction in male mice included a decreased testosterone level, decreased number and mobility of viable spermatozoa, enhanced deformation, atrophy, and apoptosis of spermatozoa.74−76 Microplastics were also detected in ovarian granulosa cells, resulting in the apoptosis, pyroptosis and development of ovarian fibrosis.75,77 Side effects of microplastics on reproduction were mostly attributed to the induced oxidative stress (evidenced by the decreased antioxidants and increased level of malondialdehyde) and loss of metabolism-related enzyme activity (including succinate dehydrogenase and lactate dehydrogenase).75,76
4.1.3. Immunotoxicity
The immune system was another target of microplastics via both the ingestion and inhalation routes. Hematopoiesis was influenced by microplastics, with a decreased number of leukocytes, increased Pit count, and inhibition of the growth of the colony-forming unit with over 40 differentially expressed genes.78 Similarly, a decrease of leukocyte and lymphocyte was found in the blood.79 Polystyrene microplastics were used in these two studies, but the administration routes were different (i.e., ingestion vs inhalation). Therefore, the exposure route might not influence the immune response, as microplastics successfully penetrating the epithelial layers of either the lungs or gastrointestinal tract could eventually reach the circulatory system. In contrast to the reduction of leukocyte number, the proportion of neutrophils in the bloodstream increased along with the altered lymphocytes subpopulation.80
4.1.4. Neurotoxicity
The study of the neurotoxicity of microplastics was still in its infancy, although some studies focused on the effects of microplastics on the brain. When the microplastics were consumed via a gastric tube, the locomotor activity decreased in mice, associated with a high anxiety and loss of behavior.81 The concentrations of microplastics used in most studies on mice (1.2 × 1011 items/m3) were 106-fold higher than the realistic concentration in the environment (2 × 104 items/m3).7 By lowering the concentration of exposed microplastics to an environmentally relevant dose (14.6 ng/kg), there was no change of locomotor activity or anxiogenic behaviors in mice.82 However, the use of nanoplastics (approximately 20 nm) still influenced the cognitive function with a decreased activity of acetylcholinesterase in the mouse brain, suggesting the importance of the small size of microplastics in inducing neurotoxicity.
4.2. Cytotoxicity of Microplastics
Some connections were found between tissue damage of mice and subcellular structures. Microplastics caused damage of cardiac structure and impaired the mitochondrial integrity in cardiomyocytes.83 Histopathological lesions in the kidneys of mice were associated with endoplasmic reticulum stress in kidney cells.84 To study the underlying mechanisms of tissue damage, the subcellular effects of microplastics should be examined. Several studies on the cytotoxicity of pristine microplastics on human-derived cells showed insignificant toxicity except at high concentrations of microplastics.85 The diameter of human cells ranged between 5 μm (e.g., lymphocytes) and 200 μm (e.g., mature egg cells),86 which may limit the internalization of microplastics. Thus, the size of microplastics available to cells should be within 5 μm, and the largest microplastics found in the cells was 4.5 μm.87 Compared with large polystyrene microplastics, smaller nanoplastics were internalized by A549 cells more rapidly.88 Surface modification of microplastics also influenced the cellular uptake efficiency, as evidenced by the 20–40 times faster uptake of positively charged microplastics than the uptake of negatively charged ones.89
Cellular uptake of microplastics included passive transportation and active endocytosis, which was the most common pathway. Contacting with the lipid bilayer of the plasma membrane, microplastics might physically disrupt the membrane and damage the important structures on the cell surface such as proteoglycans or other extracellular matrix components.85 Similar to other metal-based nanoparticles, microplastics were first entrapped in the early endosomes, followed by the maturation and differentiation of the early endosomes to late endosomes for intracellular transportation.90 After being transported to digestive organelles such as lysosomes, microplastics would interact with hydrolytic enzymes in lysosomes, as evidenced by the overlay of polystyrene nanoplastics and lysosomes in rat basophilic leukemia cells.91 Nanoplastics ingested in lysosomes caused the imbalance of the lysosomal environment, in association with the enhanced permeabilization of embryonic zebrafish fibroblasts and the subsequent lysosomal escape of microplastics.92 Microplastics escaped from lysosomes or endosomes were transported to cytosol and other organelles such as mitochondria, the endoplasmic reticulum, and the nucleus. The overproduction of ROS represented mitochondrial damage by microplastics as a byproduct of mitochondrial metabolism. With the decreased size of microplastics, oxidative stress in Caco-2 cells increased as well as the enhanced mitochondrial depolarization.93
In addition to the size-dependent cytotoxicity caused by microplastics, the surface chemistry of microplastics also made a difference. After irradiation-promoted aging, obvious surface oxidation and surface microcracks could be found in secondary MPs and the changed characters would result in the different bioeffects of MPs.94 After aging by UV irradiation, aged microplastics exhibited higher cytotoxicity compared with pristine microplastics.95 The produced ROS further caused mitochondrial damage; for example, the membrane damage was closely related to cellular storage of energy. Exposure of positively charged nanoplastics reduced the mitochondrial membrane potential which was a prerequisite for maintaining the mitochondrial oxidative phosphorylation procedure.96 Cell apoptosis was induced by polystyrene nanoplastics due to mitochondrial damage and mitochondria-mediated autophagy.96 Apart from the mitochondrial damage, stress of the endoplasmic reticulum was found in the RAW 264.7 cells after exposure to polyethylene nanoplastics, with an overexpression of IRE1-α, Bip, and CHOP proteins.97 In addition, the internalized microplastics could even attach to the nuclear envelope following interaction with the inside components. Nanoplastics as small as 20 nm were observed in the vicinity of chromosomes with no surrounding nuclear membrane, in contrast to the insignificant contact with chromosomes by 200 nm ones,98 suggesting the possible genotoxicity caused by nanoplastics. Polystyrene nanoplastics further upregulated the gene expression relating to DNA proliferation, synthesis and repair.99 Detailed information about the toxicity of microplastics on human/rodent-derived cells is shown in Table 2.
Table 2. Cytotoxicity of Microplasticsa.
| Cell | MPs | Size/Shape | Dose | Subcellular Damage |
|---|---|---|---|---|
| Myocardial cells of 6-week-old Wistar rats | PS | 510.4 nm | 0.5, 5, and 50 mg/L in water for rats 90 d | 5 and 50 mg/L: Mitochondrial damage83 |
| Spherical shape | ||||
| Human kidney proximal tubular epithelial cells (HK-2 cells) | PS | 2 μm | 0.025–0.8 mg/L | Mitochondrial damage (>0.2 mg/L); |
| Spherical shape | Endoplasmic reticulum stress84 | |||
| Human alveolar type II epithelial cell line (A549) | PS | 25 and 70 nm | 25 mg/L for 25 nm and 160 mg/L for 70 nm | Mitochondrial damage and genotoxicity88 |
| Spherical shape | ||||
| Rat basophilic leukemia (RBL-2H3) cells | PS | 50 nm, 500 nm and 5 μm | 10 mg/L | Mainly located in lysosome91 |
| Spherical shape | ||||
| Embryonic zebrafish fibroblast cell lines (ZF4) | PS | 100 and 1000 nm | 20 mg/L | Lysosomal and mitochondrial damage92 |
| Spherical shape | ||||
| Caco-2 cells | PS | 300 nm–6 μm | 20 mg/L | Mitochondrial damage93 |
| Spherical shape | ||||
| Human alveolar type II epithelial A549 cells | PS | 100 nm | 5, 50, and 100 mg/L | Genotoxicity; |
| Spherical shape | Mitochondrial damage caused by aged MPs > 50 mg/L95 | |||
| Murine splenic lymphocytes of female wild-type BALB/c mice | PS | 20 and 50 nm | 5–160 mg/L for 20 nm and 25–800 mg/L for 50 nm | Mitochondrial damage96 |
| Spherical shape | ||||
| Mouse macrophage-like cell line RAW 264.7 | PE | 65 nm | 300–1000 mg/L | Endoplasmic reticulum stress97 |
| Spherical shape | ||||
| Human alveolar type II epithelial cell line (A549) | PS | 20 and 200 nm | 250 mg/L | 20 nm: Membrane disruption and interaction with chromosome98 |
| Spherical shape |
PS: polystyrene; PE: polyethylene.
4.3. Pollutants Carried by Microplastics
Microplastics could also act as a carrier to harbor endogenous chemical additives and other contaminants in the environment. Additives, dyes, and pigments incorporated into microplastics during the manufacturing process could cause toxicity, carcinogenicity, and mutagenicity.100 For example, plasticizers including phthalates, bisphenol A, and styrene have been applied in the manufacturing of plastics. Microplastics enhanced the toxicity of phthalates by facilitating their transport and release to intestines of mice.101 Over 56% of polymers were composed of monomers with severe bioeffects, such as the carcinogenic polyvinyl chloride.102 With hydrophobic properties, microplastics were found to adsorb the persistent organic pollutants, facilitating the migration and ingestion of mixtures to organisms.103 Similarly, various metals and antibiotics could be adsorbed on the surface of microplastics, resulting in unpredictable bioeffects during transportation in the environment. There was no chemical bond between microplastics and those chemicals, suggesting that the chemicals would leach from the microplastics to environment during transportation.100 In addition to the potentially enhanced toxicity of chemicals, microplastics could also act as a colony of pathogens to protect them from identification by the immune system. For instance, vibrio spp. could colonize the surface of microplastics,104 which then altered the gut microbiome in the presence of microplastics. The toxicity of microplastics on laboratory rodents and human-derived cells is summarized in Figure 3.
Figure 3.
Toxicity of microplastics on laboratory rodents and human-derived cells. “MP”, “M”, “L”, “N”, and “ER” represent microplastics, mitochondria, lysosome, nucleus, and endoplasmic reticulum, respectively.
5. Conclusions and Future Perspectives
Due to the widespread distribution of microplastics in the environment, humans are inevitably exposed to microplastics. Different tissues of the human body accumulate microplastics, highlighting the necessity to study the potential side effects of microplastics on human health. Intensive studies on mammals, especially using laboratory rodents and human-derived cells, have been conducted to reveal the toxicity targets and the underlying mechanisms. To further improve our understanding of potential toxicity to humans, the following recommendations for experimental designs are proposed.
In terms of properties (i.e., chemical composition and shape) of microplastics applied in previous studies, microplastics made of polystyrene or polyethylene were mostly applied, with primary spherical shapes to facilitate the procurement. However, reported types of microplastics are updated based on an increasingly standard definition of microplastics. Types of microplastics should not be limited to the conventional petroleum-based plastics. Carried by personal care products, artificially synthesized polymers with solid state, insoluble, and more complicated chemical structures require further identification and assessment. Also, microplastics with different types do not exist independently; studies on which type of microplastics could be preferentially taken up and accumulated by organisms are required. Additionally, UV irradiation and weathering during the transportation of microplastics in the environment induce cleavage of the chemical bonds of microplastics, suggesting that in addition to spherical microplastics, other irregularly shaped microplastics should be introduced in toxicological studies. Furthermore, the environmentally relevant properties of microplastics should be considered. Due to the complexity of the environment and human biofluid, the surface of microplastics is inevitably modified with eco-corona or biocorona during transportation, leading to totally different bioeffects caused by modified microplastics compared with the pristine ones. Studies reporting biodegradation of microplastics were mostly related to microorganisms; hence, understanding the fate of microplastics in the intestines and feces of biota could help to predict the environmental fate of microplastics in terms of biology. As a result, properties of these secondary microplastics such as translocation capacity, bioavailability, and biocompatibility should be re-evaluated.
To make toxicological studies of microplastics on humans more relevant, a comparison of the toxic effects with different administration routes should be conducted. The administration route of microplastics to the laboratory rodent was mostly via oral paths including drinking water, food source, and gastric tube, followed by administering by inhalation, intratracheally, or intraperitoneally. Although it was difficult for microplastics to penetrate the dermal barrier, in vivo studies on the effects of microplastics on laboratory rodents via dermal penetration are rather limited. Although different types of cells were used to evaluate the cytotoxicity of microplastics, the most used ones were the adherent cells that attached on the culture dish. However, microplastics with low density were more likely to float in the culture medium, leading to inadequate contact of microplastics with the cells. Therefore, more types of cells, such as suspension cells, should be used to simulate the interaction between cells and microplastics. Also, the cell density would influence the final toxicity of microplastics by affecting the number of plastics assigned to each cell; thus, cell density in the cytotoxicity test should be standardized.
In addition to the optimization of the experimental design, some key questions remain to be further explained. First, microplastics have been detected in various tissues of the human body, but it remains unclear whether the health conditions of these volunteers could be connected to the detected contents of microplastics. Although high accumulation of microplastics could be associated with human disease, it was uncertain whether the damaged tissue in humans would lose the ability to resist the penetration of microplastics. Also, the potential side effects of these accumulated microplastics on the recovery of human diseases remain unknown. Thus, follow-up studies on participants, especially those with chronic diseases and high exposure risks, are required. Also, the most direct approach to connect human health with exposure of microplastics is based on real-time wearable devices that could accurately differentiate microplastics from other particulates and measure physiological indicator simultaneously. Since microplastics potentially carry other pollutants and facilitate their transportation in the environment, a long-term epidemiological study is required on a certain human population which is considered at high risk of coexposure of microplastics and conventional pollutants. Additionally, few studies have illustrated the bioeffects of plastic debris directly sampled from the environment. This was largely due to the limited standardized extraction approach across diverse environmental sample types. High heterogeneity of environmentally relevant microplastics would make the result of following toxicological studies difficult to explain; thus, the proper identification and classification methods would facilitate screening the key factors of microplastics determining the resulting toxicity.
Acknowledgments
This study was supported by the National Science Foundation of China (22076159), Shenzhen Municipal Science and Technology Innovation Commission (JCYJ20210324134000001), and the Hong Kong Research Grants Council (CityU 1102321).
Biography

Prof. Wen-Xiong Wang: TUYF Chair Professor in Oceanography and Chair Professor of Environmental Toxicology in City University of Hong Kong.
Prof. Wen-Xiong Wang received his BSc and MSc in Marine Biology from Xiamen University and PhD in Coastal Oceanography from the State University of New York at Stony Brook (USA). Prof. Wang has authored >570 peer-reviewed publications on subjects related to metal ecotoxicology and biogeochemistry. He is the corresponding or first author for the majority of his publications. His work is widely cited (∼28,000 times) with a current H-index of 86 (Google Scholar).
The authors declare no competing financial interest.
References
- Thompson R. C.; Olsen Y.; Mitchell R. P.; Davis A.; Rowland S. J.; John A. W. G.; McGonigle D.; Russell A. E. Lost at sea: Where is all the plastic?. Science 2004, 304, 838–838. 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- Yee M. S. L.; Hii L. W.; Looi C. K.; Lim W. M.; Wong S. F.; Kok Y. Y.; Tan B. K.; Wong C. Y.; Leong C. O. Impact of microplastics and nanoplastics on human health. Nanomaterials-Basel 2021, 11, 496. 10.3390/nano11020496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G.; Shruti V. C.; Perez-Guevara F.; Roy P. D. Microplastic diagnostics in humans: ″The 3Ps″ Progress, problems, and prospects. Sci. Total Environ. 2023, 856, 159164 10.1016/j.scitotenv.2022.159164. [DOI] [PubMed] [Google Scholar]
- Jiang B. R.; Kauffman A. E.; Li L.; McFee W.; Cai B.; Weinstein J.; Lead J. R.; Chatterjee S.; Scott G. I.; Xiao S. Health impacts of environmental contamination of micro- and nanoplastics: a review. Environ. Health Prev. 2020, 25, 25. 10.1186/s12199-020-00870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbalaei S.; Hanachi P.; Walker T. R.; Cole M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. Int. 2018, 25, 36046–36063. 10.1007/s11356-018-3508-7. [DOI] [PubMed] [Google Scholar]
- Sarijan S.; Azman S.; Said M. I. M.; Jamal M. H. Microplastics in freshwater ecosystems: a recent review of occurrence, analysis, potential impacts, and research needs. Environ. Sci. Pollut. R. 2021, 28, 1341–1356. 10.1007/s11356-020-11171-7. [DOI] [PubMed] [Google Scholar]
- Zolotova N.; Kosyreva A.; Dzhalilova D.; Fokichev N.; Makarova O. Harmful effects of the microplastic pollution on animal health: a literature review. Peerj 2022, 10, 13503. 10.7717/peerj.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata J. C.; da Costa J. P.; Lopes I.; Duarte A. C.; Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- Rahman A.; Sarkar A.; Yadav O. P.; Achari G.; Slobodnik J. Potential human health risks due to environmental exposure to nano-and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872 10.1016/j.scitotenv.2020.143872. [DOI] [PubMed] [Google Scholar]
- Cox K. D.; Covernton G. A.; Davies H. L.; Dower J. F.; Juanes F.; Dudas S. E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- Danopoulos E.; Jenner L. C.; Twiddy M.; Rotchell J. M. Microplastic contamination of seafood intended for human consumption: A systematic review and meta-analysis. Environ. Health Persp. 2020, 128, 126002 10.1289/EHP7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwanga E. H.; Vega J. M.; Quej V. K.; Chi J. D.; del Cid L. S.; Chi C.; Segura G. E.; Gertsen H.; Salanki T.; van der Ploeg M.; Koelmans A. A.; Geissen V. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep-Uk 2017, 7, 14071. 10.1038/s41598-017-14588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Microplastics in drinking-water. 2019.
- Karami A.; Golieskardi A.; Choo C. K.; Larat V.; Galloway T. S.; Salamatinia B. The presence of microplastics in commercial salts from different countries. Sci. Rep-Uk 2017, 7, 46173. 10.1038/srep46173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. Q.; Shi H. H.; Li L.; Li J. N.; Jabeen K.; Kolandhasamy P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. 10.1021/acs.est.5b03163. [DOI] [PubMed] [Google Scholar]
- Mintenig S. M.; Loder M. G. J.; Primpke S.; Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. 10.1016/j.scitotenv.2018.08.178. [DOI] [PubMed] [Google Scholar]
- Kankanige D.; Babel S. Smaller-sized micro-plastics (MPs) contamination in single-use PET-bottled water in Thailand. Sci. Total Environ. 2020, 717, 137232 10.1016/j.scitotenv.2020.137232. [DOI] [PubMed] [Google Scholar]
- Shruti V. C.; Perez-Guevara F.; Elizalde-Martinez I.; Kutralam-Muniasamy G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks-Future research and environmental considerations. Sci. Total Environ. 2020, 726, 138580 10.1016/j.scitotenv.2020.138580. [DOI] [PubMed] [Google Scholar]
- Karami A.; Golieskardi A.; Choo C. K.; Larat V.; Karbalaei S.; Salamatinia B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci. Total Environ. 2018, 612, 1380–1386. 10.1016/j.scitotenv.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Leung M. M. L.; Ho Y. W.; Lee C. H.; Wang Y. J.; Hu M. H.; Kwok K. W. H.; Chua S. L.; Fang J. K. H. Improved Raman spectroscopy-based approach to assess microplastics in seafood. Environ. Pollut. 2021, 289, 117648 10.1016/j.envpol.2021.117648. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Dietary and inhalation exposure to nano-and microplastic particles and potential implications for human health. 2022.
- Prata J. C. Airborne microplastics: Consequences to human health?. Environ. Pollut. 2018, 234, 115–126. 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Dris R.; Gasperi J.; Mirande C.; Mandin C.; Guerrouache M.; Langlois V.; Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Pauly J. L.; Stegmeier S. J.; Allaart H. A.; Cheney R. T.; Zhang P. J.; Mayer A. G.; Streck R. J. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidem. Biomar. 1998, 7, 419–428. [PubMed] [Google Scholar]
- Liao Z. L.; Ji X. L.; Ma Y.; Lv B. Q.; Huang W.; Zhu X.; Fang M. Z.; Wang Q.; Wang X. D.; Dahlgren R.; Shang X. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J. Hazard. Mater. 2021, 417, 126007 10.1016/j.jhazmat.2021.126007. [DOI] [PubMed] [Google Scholar]
- Liu K.; Wang X. H.; Wei N.; Song Z. Y.; Li D. J. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environ. Int. 2019, 132, 105127 10.1016/j.envint.2019.105127. [DOI] [PubMed] [Google Scholar]
- Vianello A.; Jensen R. L.; Liu L.; Vollertsen J. Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Sci. Rep-Uk 2019, 9, 8670. 10.1038/s41598-019-45054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoabi A.; Touitou E.; Margulis K. Recent advances in nanomaterials for dermal and transdermal applications. Colloid Interfac. 2021, 5, 18. 10.3390/colloids5010018. [DOI] [Google Scholar]
- Revel M.; Châtel A.; Mouneyrac C. Micro(nano)plastics: A threat to human health?. Curr. Opin. Environ. Sci. Health 2018, 1, 17–23. 10.1016/j.coesh.2017.10.003. [DOI] [Google Scholar]
- Cai H. W.; Xu E. G.; Du F. N.; Li R. L.; Liu J. F.; Shi H. H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208 10.1016/j.cej.2020.128208. [DOI] [Google Scholar]
- Vogt A.; Combadiere B.; Hadam S.; Stieler K. M.; Lademann J.; Schaefer H.; Autran B.; Sterry W.; Blume-Peytavi U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Invest. Dermatol. 2006, 126, 1316–1322. 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- Hernandez L. M.; Yousefi N.; Tufenkji N. Are there nanoplastics in your personal care products?. Environ. Sci. Technol. Lett. 2017, 4, 280–285. 10.1021/acs.estlett.7b00187. [DOI] [Google Scholar]
- Burgos-Aceves M. A.; Abo-Al-Ela H. G.; Faggio C. Physiological and metabolic approach of plastic additive effects: Immune cells responses. J. Hazard Mater. 2021, 404, 124114 10.1016/j.jhazmat.2020.124114. [DOI] [PubMed] [Google Scholar]
- Bouslimani A.; Porto C.; Rath C. M.; Wang M. X.; Guo Y. R.; Gonzalez A.; Berg-Lyon D.; Ackermann G.; Christensen G. J. M.; Nakatsuji T.; Zhang L. J.; Borkowski A. W.; Meehan M. J.; Dorrestein K.; Gallo R. L.; Bandeira N.; Knight R.; Alexandrov T.; Dorrestein P. C. Molecular cartography of the human skin surface in 3D. P. Natl. Acad. Sci. USA 2015, 112, E2120–E2129. 10.1073/pnas.1424409112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl P.; Koppel S.; Konigshofer P.; Bucsics T.; Trauner M.; Reiberger T.; Liebmann B. Detection of various microplastics in human stool a prospective case series. Ann. Int. Med. 2019, 171, 453. 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Amato-Lourenco L. F.; Carvalho-Oliveira R.; Ribeiro G.; Galvao L. D.; Ando R. A.; Mauad T. Presence of airborne microplastics in human lung tissue. J. Hazard Mater. 2021, 416, 126124 10.1016/j.jhazmat.2021.126124. [DOI] [PubMed] [Google Scholar]
- Leslie H. A.; van Velzen M. J. M.; Brandsma S. H.; Vethaak A. D.; Garcia-Vallejo J. J.; Lamoree M. H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- Zhang N.; Li Y. B.; He H. R.; Zhang J. F.; Ma G. S. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021, 767, 144345 10.1016/j.scitotenv.2020.144345. [DOI] [PubMed] [Google Scholar]
- Yan Z. H.; Liu Y. F.; Zhang T.; Zhang F. M.; Ren H. Q.; Zhang Y. Analysis of microplastics in human feces reveals a correlation between fecal microplastics and inflammatory bowel disease status. Environ. Sci. Technol. 2022, 56, 414–421. 10.1021/acs.est.1c03924. [DOI] [PubMed] [Google Scholar]
- Ho Y. W.; Lim J. Y.; Yeoh Y. K.; Chiou J. C.; Zhu Y. Y.; Lai K. P.; Li L.; Chan P. K. S.; Fang J. K. H. Preliminary findings of the high quantity of microplastics in faeces of Hong Kong residents. Toxics 2022, 10, 414. 10.3390/toxics10080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi S.; Turner A. Human exposure to microplastics: A study in Iran. J. Hazard Mater. 2021, 403, 123799 10.1016/j.jhazmat.2020.123799. [DOI] [PubMed] [Google Scholar]
- Huang S. M.; Huang X. X.; Bi R.; Guo Q. X.; Yu X. L.; Zeng Q. H.; Huang Z. Y.; Liu T. M.; Wu H. S.; Chen Y. L.; Xu J. L.; Wu Y. G.; Guo P. Detection and analysis of microplastics in human sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. 10.1021/acs.est.1c03859. [DOI] [PubMed] [Google Scholar]
- Baeza-Martinez C.; Olmos S.; Gonzalez-Pleiter M.; Lopez-Castellanos J.; Garcia-Pachon E.; Masia-Canuto M.; Hernandez-Blasco L.; Bayo J. First evidence of microplastics isolated in European citizens’ lower airway. J. Hazard Mater. 2022, 438, 129439 10.1016/j.jhazmat.2022.129439. [DOI] [PubMed] [Google Scholar]
- Chen Q. Q.; Gao J. N.; Yu H. R.; Su H.; Yang Y.; Cao Y. J.; Zhang Q.; Ren Y. J.; Hollert H.; Shi H. H.; Chen C.; Liu H. P. An emerging role of microplastics in the etiology of lung ground glass nodules. Environ. Sci. Eur. 2022, 34, 34. 10.1186/s12302-022-00605-3. [DOI] [Google Scholar]
- Jenner L. C.; Rotchell J. M.; Bennett R. T.; Cowen M.; Tentzeris V.; Sadofsky L. R. Detection of microplastics in human lung tissue using mu FTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907 10.1016/j.scitotenv.2022.154907. [DOI] [PubMed] [Google Scholar]
- Horvatits T.; Tamminga M.; Liu B. B.; Sebode M.; Carambia A.; Fischer L.; Puschel K.; Huber S.; Fischer E. K. Microplastics detected in cirrhotic liver tissue. Ebiomedicine 2022, 82, 104147 10.1016/j.ebiom.2022.104147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim Y. S.; Anuar S. T.; Azmi A. A.; Khalik W. M. A. W. M.; Lehata S.; Hamzah S. R.; Ismail D.; Ma Z. F.; Dzulkarnaen A.; Zakaria Z.; Mustaffa N.; Sharif S. E. T.; Lee Y. Y. Detection of microplastics in human colectomy specimens. Jgh Open 2021, 5, 116–121. 10.1002/jgh3.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsperger A. F.; Bergamaschi E.; Panizzolo M.; Fenoglio I.; Barbero F.; Peters R.; Undas A.; Purker S.; Giese B.; Lalyer C. R.; et al. Nano-and microplastics: a comprehensive review on their exposure routes, translocation, and fate in humans. NanoImpact 2023, 29, 100441 10.1016/j.impact.2022.100441. [DOI] [PubMed] [Google Scholar]
- Desai P.; Patlolla R. R.; Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. 10.3109/09687688.2010.522203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli A. K.; Alpar H. O. Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int. J. Pharmaceut. 2004, 275, 13–17. 10.1016/j.ijpharm.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Filon F. L.; Mauro M.; Adami G.; Bovenzi M.; Crosera M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. 10.1016/j.yrtph.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Hussain N.; Jaitley V.; Florence A. T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliver Rev. 2001, 50, 107–142. 10.1016/S0169-409X(01)00152-1. [DOI] [PubMed] [Google Scholar]
- Ensign L. M.; Cone R.; Hanes J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliver. Rev. 2012, 64, 557–570. 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Lai S. K.; Wang Y. Y.; Zhong W. X.; Happe C.; Zhang M.; Fu J.; Hanes J. Biodegradable nanoparticles composed entirely of safe materials that rapidly penetrate human mucus. Angew. Chem. Int. Ed. 2011, 50, 2597–2600. 10.1002/anie.201006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S. Y.; Kaplan G. G.; Madsen K. L. Air pollution effects on the gut microbiota A link between exposure and inflammatory disease. Gut Microbes 2014, 5, 215–219. 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho T. C.; Peters J. I.; Williams R. O. Influence of particle size on regional lung deposition - What evidence is there?. Int. J. Pharmaceut. 2011, 406, 1–10. 10.1016/j.ijpharm.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Bakand S.; Hayes A.; Dechsakulthorn F. Nanoparticles: a review of particle toxicology following inhalation exposure. Inhal. Toxicol. 2012, 24, 125–135. 10.3109/08958378.2010.642021. [DOI] [PubMed] [Google Scholar]
- Tran C. L.; Buchanan D.; Cullen R. T.; Searl A.; Jones A. D.; Donaldson K. Inhalation of poorly soluble particles. II. Influence of particle surface area on inflammation and clearance. Inhal. Toxicol. 2000, 12, 1113–1126. 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- Donaldson K.; Stone V.; Gilmour P. S.; Brown D. M.; MacNee W. Ultrafine particles: mechanisms of lung injury. Philos. Trans. R. Soc., A 2000, 358, 2741–2748. 10.1098/rsta.2000.0681. [DOI] [Google Scholar]
- Lu L.; Wan Z. Q.; Luo T.; Fu Z. W.; Jin Y. X. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Herath M.; Hosie S.; Bornstein J. C.; Franks A. E.; Hill-Yardin E. L. The role of the gastrointestinal mucus system in intestinal homeostasis: Implications for neurological disorders. Front. Cell Infect. Mi. 2020, 10, 248. 10.3389/fcimb.2020.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethaak A. D.; Leslie H. A. Plastic debris is a human health issue. Environ. Sci. Technol. 2016, 50, 6825–6826. 10.1021/acs.est.6b02569. [DOI] [PubMed] [Google Scholar]
- Eyles J. E.; Bramwell V. W.; Williamson E. D.; Alpar H. O. Microsphere translocation and immunopotentiation in systemic tissues following intranasal administration. Vaccine 2001, 19, 4732–4742. 10.1016/S0264-410X(01)00220-1. [DOI] [PubMed] [Google Scholar]
- Eyles J.; Alpar H. O.; Field W. N.; Lewis D. A.; Keswick M. The transfer of polystyrene microspheres from the gastrointestinal-tract to the circulation after oral-administration in the rat. J. Pharm. Pharmacol. 2011, 47, 561–565. 10.1111/j.2042-7158.1995.tb06714.x. [DOI] [PubMed] [Google Scholar]
- Deng Y. F.; Zhang Y.; Lemos B.; Ren H. Q. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep-Uk 2017, 7, 46687. 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa A.; Svelato A.; Santacroce C.; Catalano P.; Notarstefano V.; Carnevali O.; Papa F.; Rongioletti M. C. A.; Baiocco F.; Draghi S.; D’Amore E.; Rinaldo D.; Matta M.; Giorgini E. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- Braun T.; Ehrlich L.; Henrich W.; Koeppel S.; Lomako I.; Schwabl P.; Liebmann B. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics 2021, 13, 921. 10.3390/pharmaceutics13070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa A.; Notarstefano V.; Svelato A.; Belloni A.; Gioacchini G.; Blondeel C.; Zucchelli E.; De Luca C.; D’Avino S.; Gulotta A.; Carnevali O.; Giorgini E. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers-Basel. 2022, 14, 2700. 10.3390/polym14132700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B.; Zhang N.; Jin S. R.; Chen Z. Z.; Gao J. Z.; Liu Y.; Liu H. P.; Xu Z. Microplastics have a more profound impact than elevated temperatures on the predatory performance, digestion and energy metabolism of an Amazonian cichlid. Aquat. Toxicol. 2018, 195, 67–76. 10.1016/j.aquatox.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Luo T.; Wang C.; Pan Z.; Jin C.; Fu Z.; Jin Y. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. 10.1021/acs.est.9b03191. [DOI] [PubMed] [Google Scholar]
- Jin Y. X.; Lu L.; Tu W. Q.; Luo T.; Fu Z. W. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- Qiao J. Y.; Chen R.; Wang M. J.; Bai R.; Cui X. J.; Liu Y.; Wu C. M.; Chen C. Y. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 2021, 13, 8806–8816. 10.1039/D1NR00038A. [DOI] [PubMed] [Google Scholar]
- Li W. X.; Chen X. F.; Li M. Q.; Cai Z. M.; Gong H.; Yan M. T. Microplastics as an aquatic pollutant affect gut microbiota within aquatic animals. J. Hazard Mater. 2022, 423, 127094 10.1016/j.jhazmat.2021.127094. [DOI] [PubMed] [Google Scholar]
- Jin H. B.; Ma T.; Sha X. X.; Liu Z. Y.; Zhou Y.; Meng X. N.; Chen Y. B.; Han X. D.; Ding J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard Mater. 2021, 401, 123430 10.1016/j.jhazmat.2020.123430. [DOI] [PubMed] [Google Scholar]
- Hou J. Y.; Lei Z. M.; Cui L. L.; Hou Y.; Yang L.; An R.; Wang Q. M.; Li S. D.; Zhang H. Q.; Zhang L. S. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotox. Environ. Safe 2021, 212, 112012 10.1016/j.ecoenv.2021.112012. [DOI] [PubMed] [Google Scholar]
- Xie X. M.; Deng T.; Duan J. F.; Xie J.; Yuan J. L.; Chen M. Q. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotox. Environ. Safe 2020, 190, 110133 10.1016/j.ecoenv.2019.110133. [DOI] [PubMed] [Google Scholar]
- An R.; Wang X. F.; Yang L.; Zhang J. J.; Wang N. N.; Xu F. B.; Hou Y.; Zhang H. Q.; Zhang L. S. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665 10.1016/j.tox.2020.152665. [DOI] [PubMed] [Google Scholar]
- Sun R. L.; Xu K.; Yu L. L.; Pu Y. Q.; Xiong F.; He Y. H.; Huang Q. C.; Tang M. J.; Chen M. J.; Yin L. H.; Zhang J.; Pu Y. P. Preliminary study on impacts of polystyrene microplastics on the hematological system and gene expression in bone marrow cells of mice. Ecotox. Environ. Safe 2021, 218, 112296 10.1016/j.ecoenv.2021.112296. [DOI] [PubMed] [Google Scholar]
- Lim D.; Jeong J.; Song K. S.; Sung J. H.; Oh S. M.; Choi J. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere 2021, 262, 128330 10.1016/j.chemosphere.2020.128330. [DOI] [PubMed] [Google Scholar]
- Park E. J.; Han J. S.; Park E. J.; Seong E.; Lee G. H.; Kim D. W.; Son H. Y.; Han H. Y.; Lee B. S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. 10.1016/j.toxlet.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Araujo A. P. D.; Malafaia G. Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish. J. Hazard Mater. 2021, 401, 123263 10.1016/j.jhazmat.2020.123263. [DOI] [PubMed] [Google Scholar]
- Estrela F. N.; Guimaraes A. T. B.; Araujo A. P. D.; Silva F. G.; da Luz T. M.; Silva A. M.; Pereira P. S.; Malafaia G. Toxicity of polystyrene nanoplastics and zinc oxide to mice. Chemosphere 2021, 271, 129476 10.1016/j.chemosphere.2020.129476. [DOI] [PubMed] [Google Scholar]
- Wei J. L.; Wang X. F.; Liu Q.; Zhou N.; Zhu S. X.; Li Z. K.; Li X. L.; Yao J. P.; Zhang L. S. The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environ. Toxicol. 2021, 36, 935–944. 10.1002/tox.23095. [DOI] [PubMed] [Google Scholar]
- Wang Y. L.; Lee Y. H.; Hsu Y. H.; Chiu I. J.; Huang C. C. Y.; Huang C. C.; Chia Z. C.; Lee C. P.; Lin Y. F.; Chiu H. W. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 Mice. Environ. Health Persp. 2021, 129, 129. 10.1289/EHP7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong C. Q. Y.; Valiyaveettil S.; Tang B. L. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Env. Res. Pub. He. 2020, 17, 1509. 10.3390/ijerph17051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N.; Shi H. J.; Li X. X.; Gao C. Z.; Liu R. T. Combined toxicity of micro/nanoplastics loaded with environmental pollutants to organisms and cells: Role, effects, and mechanism. Environ. Int. 2023, 171, 107711 10.1016/j.envint.2022.107711. [DOI] [PubMed] [Google Scholar]
- Katsumiti A.; Losada-Carrillo M. P.; Barros M.; Cajaraville M. P. Polystyrene nanoplastics and microplastics can act as Trojan horse carriers of benzo(a)pyrene to mussel hemocytes in vitro. Sci. Rep-Uk 2021, 11, 22396. 10.1038/s41598-021-01938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. K.; Halimu G.; Zhang Q. R.; Song Y. B.; Fu X. H.; Li Y. Q.; Li Y. S.; Zhang H. W. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- Yacobi N. R.; Malmstadt N.; Fazlollahi F.; DeMaio L.; Marchelletta R.; Hamm-Alvarez S. F.; Borok Z.; Kim K. J.; Crandall E. D. Mechanisms of alveolar epithelial translocation of a defined population of nanoparticles. Am. J. Resp. Cell Mol. 2010, 42, 604–614. 10.1165/rcmb.2009-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X.; Wang D. Y. Cellular uptake, transport, and organelle response after exposure to microplastics and nanoplastics: Current knowledge and perspectives for environmental and health risks. Rev. Environ. Contam. T. 2022, 260, 260. 10.1007/s44169-022-00013-x. [DOI] [Google Scholar]
- Liu L.; Xu K. X.; Zhang B. W.; Ye Y. Y.; Zhang Q.; Jiang W. Cellular internalization and release of polystyrene microplastics and nanoplastics. Sci. Total Environ. 2021, 779, 146523 10.1016/j.scitotenv.2021.146523. [DOI] [PubMed] [Google Scholar]
- Yang M.; Wang W.-X. Differential cascading cellular and subcellular toxicity induced by two sizes of nanoplastics. Sci. Total Environ. 2022, 829, 154593 10.1016/j.scitotenv.2022.154593. [DOI] [PubMed] [Google Scholar]
- Wang Q. Q.; Bai J. L.; Ning B. A.; Fan L. X.; Sun T. Q.; Fang Y. J.; Wu J.; Li S.; Duan C. H.; Zhang Y. C.; Liang J.; Gao Z. X. Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere 2020, 254, 126788 10.1016/j.chemosphere.2020.126788. [DOI] [PubMed] [Google Scholar]
- Song Y. K.; Hong S. H.; Eo S.; Shim W. J. The fragmentation of nano- and microplastic particles from thermoplastics accelerated by simulated-sunlight-mediated photooxidation. Environ. Pollut. 2022, 311, 119847 10.1016/j.envpol.2022.119847. [DOI] [PubMed] [Google Scholar]
- Shi Q. Y.; Tang J. C.; Liu X. M.; Liu R. T. Ultraviolet-induced photodegradation elevated the toxicity of polystyrene nanoplastics on human lung epithelial A549. cells. Environ. Sci-Nano 2021, 8, 2660–2675. 10.1039/D1EN00465D. [DOI] [Google Scholar]
- Li Y.; Xu M.; Zhang Z.; Halimu G.; Li Y.; Li Y.; Gu W.; Zhang B.; Wang X. In vitro study on the toxicity of nanoplastics with different charges to murine splenic lymphocytes. J. Hazard Mater. 2022, 424, 127508 10.1016/j.jhazmat.2021.127508. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Bao D.; Li P.; Lu Z.; Pang L.; Chen Z.; Guo H.; Gao Z.; Jin Q. Particle-induced SIRT1 downregulation promotes osteoclastogenesis and osteolysis through ER stress regulation. Biomed. Pharmacother. 2018, 104, 300–306. 10.1016/j.biopha.2018.05.030. [DOI] [PubMed] [Google Scholar]
- Kihara S.; Ashenden A.; Kaur M.; Glasson J.; Ghosh S.; van der Heijden N.; Brooks A. E. S.; Mata J. P.; Holt S.; Domigan L. J.; Koper I.; McGillivray D. J. Cellular interactions with polystyrene nanoplastics-The role of particle size and protein corona. Biointerphases 2021, 16, 041001 10.1116/6.0001124. [DOI] [PubMed] [Google Scholar]
- Yang W. F.; Gao P.; Li H. X.; Huang J. Y.; Zhang Y.; Ding H. J.; Zhang W. H. Mechanism of the inhibition and detoxification effects of the interaction between nanoplastics and microalgae Chlorella pyrenoidosa. Sci. Total Environ. 2021, 783, 146919 10.1016/j.scitotenv.2021.146919. [DOI] [PubMed] [Google Scholar]
- Blackburn K.; Green D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio 2022, 51, 518–530. 10.1007/s13280-021-01589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y. F.; Yan Z. H.; Shen R. Q.; Wang M.; Huang Y. C.; Ren H. Q.; Zhang Y.; Lemos B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environ. Int. 2020, 143, 105916 10.1016/j.envint.2020.105916. [DOI] [PubMed] [Google Scholar]
- Lithner D.; Larsson A.; Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Velzeboer I.; Kwadijk C. J. A. F.; Koelmans A. A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- Kirstein I. V.; Kirmizi S.; Wichels A.; Garin-Fernandez A.; Erler R.; Loder M.; Gerdts G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 2016, 120, 1–8. 10.1016/j.marenvres.2016.07.004. [DOI] [PubMed] [Google Scholar]