Abstract
Comparison of the extent of leaf senescence depending on the genetic background of different recombinant inbred lines (RILs) of Arabidopsis (Arabidopsis thaliana) is described. Five RILs of the Bay-0 × Shahdara population showing differential leaf senescence phenotypes (from early senescing to late senescing) were selected to determine metabolic markers to discriminate Arabidopsis lines on the basis of senescence-dependent changes in metabolism. The proportion of γ-aminobutyric acid, leucine, isoleucine, aspartate, and glutamate correlated with (1) the age and (2) the senescence phenotype of the RILs. Differences were observed in the glycine/serine ratio even before any senescence symptoms could be detected in the rosettes. This could be used as predictive indicator for plant senescence behavior. Surprisingly, late-senescing lines appeared to mobilize glutamine, asparagine, and sulfate more efficiently than early-senescing lines. The physiological basis of the relationship between leaf senescence and flowering time was analyzed.
Leaf senescence is a key developmental step in the life of annual plants. During growth, green leaves accumulate nutrients. The main purpose of senescence is the mobilization and recycling of these nutrients to the developing seeds to prepare the next generation. Developmental signals, aging, or stress can induce leaf senescence. The final stage of this process is death, but cell death is actively delayed until nutrients have been removed (Buchanan-Wollaston et al., 2003).
During senescence, cell constituents are dismantled in an ordered progression. Chlorophyll degradation is the first visible symptom of senescence, but by the time yellowing can be seen, some senescence has already occurred. Chlorophyll, protein, and lipid degradation processes have been largely investigated (Hörtensteiner and Feller, 2002). Protein and mRNA degradation parallels the loss in photosynthetic activity and participates in nutrient mobilization. Nucleic acids, especially RNA, form a valuable source of phosphorus in mature leaves. Total RNA levels fall rapidly during senescence, whereas nuclear DNA is maintained to allow gene expression to continue, until late in the process. Nutrients such as sulfur, phosphorus, metal ions, and minerals are also transferred out of the leaves (Himelblau and Amasino, 2001). Accelerated metabolism of membrane lipids results in a decline in the structural and functional integrity of cellular membranes. Thylakoid membranes provide an abundant source of carbon that can be mobilized for use as an energy source during senescence. Rubisco is one of the major sources of nitrogen for mobilization. A major question in leaf senescence is how leaf proteins, up to 75% of which are located within the chloroplast, are degraded and mobilized (Mae, 2004).
The molecular events that induce and contribute to the senescence process have recently been extensively investigated. The genomic resources that are now available for Arabidopsis (Arabidopsis thaliana) have allowed the rapid identification of novel genes and mutants. From genomic and mutant approaches, a partial picture of the molecular basis for the regulation and progress of senescence has emerged. A recent transcriptome study showed that the major functional category of leaf senescence expressed sequence tags in Arabidopsis was for metabolism (Guo et al., 2004) and then for cell rescue and defense. Analysis of the transcriptome associated with leaf senescence helped to unravel the biochemical pathways and regulatory mechanisms that underlie the senescence process (Buchanan-Wollaston and Ainsworth, 1997; Guo et al., 2004; Lin and Wu, 2004). However, many unanswered questions remain, e.g. how changes in transcript levels affect leaf metabolism during senescence.
Until now, the different studies interested in leaf senescence focused almost exclusively on the time course of the successive events occurring during senescence. The markers usually used to monitor the senescence time course were a decrease in chlorophyll or changes in gene expression patterns (Buchanan-Wollaston and Ainsworth, 1997; Buchanan-Wollaston et al., 2003). Except for mutants, attempts to compare senescence between different plants, different ecotypes, or different lines are rare. Levey and Wingler (2005) showed that substantial differences exist in the timing and regulation of leaf senescence in different Arabidopsis ecotypes. Recently, we used yellowing as a marker to compare recombinant inbred lines and to map quantitative trait loci (QTL) related to senescence in Arabidopsis (C. Diaz, V. Saliba-Colombani, O. Loudet, P. Belluomo, L. Moreau, F. Daniel-Vedele, J.-F. Morot-Gaudry, and C. Masclaux-Daubresse, unpublished data). The difficulty of this work was to find markers suitable for monitoring the variability in the extent of senescence in lines that showed different phenology and life span. The use of the plant model Arabidopsis for the study of developmental senescence was also criticized since the developmental signals that initiate leaf senescence may be weaker in Arabidopsis, and aging or stress may have a more significant role than in other plants (Noodén et al., 1996). However, using a different Arabidopsis accession and looking specifically at one individual leaf, Ye et al. (2000) showed that leaf chlorosis was delayed in plants from which the developing bolts were constantly removed. A normal senescence process does occur in Arabidopsis, including the degradation of macromolecules and efficient nutrient mobilization (Himelblau and Amasino, 2001).
Despite the importance of nutrient mobilization during leaf senescence, metabolite profiles for senescing leaves are not available. We therefore decided to identify senescence markers using a metabolite profiling approach rather than proteomic or genomic tools. These metabolic markers allow us to estimate the extent of leaf senescence throughout the life of a plant and also to discriminate between lines or ecotypes. Results presented in this work propose tools suitable for the characterization of the extent of leaf senescence in different contexts, such as (1) time-course studies, (2) mutant characterization, and (3) QTL research.
RESULTS
Selection of Five Recombinant Lines with Distinct Senescence Phenotypes
We have observed that the 415 recombinant inbred lines raised from the cross between the two ecotypes Bay-0 and Shahdara (Loudet et al., 2002) displayed differential yellowing of their old leaves when cultivated under low nitrate conditions (C. Diaz, V. Saliba-Colombani, O. Loudet, P. Belluomo, L. Moreau, F. Daniel-Vedele, J.-F. Morot-Gaudry, and C. Masclaux-Daubresse, unpublished data). Yellowing of the rosette was studied at 35 d after sowing (DAS) on the whole recombinant inbred line (RIL) population using both a visual scale of notation and an imaging process (C. Diaz, V. Saliba-Colombani, O. Loudet, P. Belluomo, L. Moreau, F. Daniel-Vedele, J.-F. Morot-Gaudry, and C. Masclaux-Daubresse, unpublished data). This, together with data from Loudet et al. (2002, 2003), allowed us to choose five lines (RIL045, RIL083, RIL232, RIL272, and RIL310) with distinct leaf yellowing symptoms (Table I; Fig. 1). The lines RIL310, RIL232, and RIL083 turned yellow sooner than RIL272 and RIL045. Yellowing was also more intense for RIL310 than for RIL083 and RIL232 (Fig. 1; Table I). The lines also differed for flowering time and biomass (Table I). Lines RIL083 and RIL272 flowered at a similar time. Line RIL310 flowered very late, whereas line RIL045 flowered early. Line RIL232 showed similar senescence symptoms at 35 DAS as line RIL083, but RIL083 had a higher biomass than RIL232 at the same time. In the same way, line RIL272 and RIL045 were both green at 35 DAS, but line RIL045 had a lower biomass than RIL272.
Table I.
Flowering time, biomass, and yellowing traits determined on the whole rosettes of RIL045, RIL083, RIL232, RIL272, and RIL310, 35 DAS
References: Loudet et al. (2003); C. Diaz, V. Saliba-Colombani, O. Loudet, P. Belluomo, L. Moreau, F. Daniel-Vedele, J.-F. Morot-Gaudry, and C. Masclaux-Daubresse (unpublished data).
| RILs | Flowering Time | DW | Yellowing |
|---|---|---|---|
| DAS | g plant−1 | % | |
| RIL310 | 86 | 5.4 | 25.73 |
| RIL083 | 61 | 6.1 | 12.65 |
| RIL232 | 58.5 | 4.4 | 11.47 |
| RIL45 | 46 | 4.8 | 4.23 |
| RIL272 | 61 | 7.9 | 1.58 |
Figure 1.
Frequency distribution of the yellowing of the whole rosette in the Bay-0 × Shahdara population at 35 DAS. The yellowing classes of the lines RIL045, RIL083, RIL232, RIL272, and RIL310 are indicated by arrows, and their yellowing values (as %) are presented.
Characterization of Whole-Rosette Senescence Dependent on Carbon and Nitrogen Supply
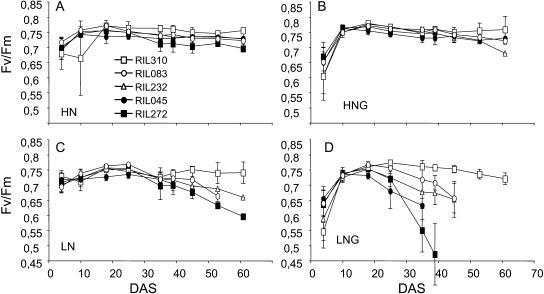
Nitrogen and carbon supply plays an important role in the regulation of leaf senescence. To investigate whole-rosette senescence of the five lines depending on carbon/nitrogen balance, we measured maximum photosynthetic efficiency (Fv/Fm) under low or high nitrogen and Glc availability on plants grown in vitro. We have shown previously that visible senescence observed on individual leaves is correlated with a decline in Fv/Fm (Wingler et al., 2004). As shown previously for other Arabidopsis ecotypes (Pourtau et al., 2004; Wingler et al., 2004), addition of 2% Glc in combination with low but not high nitrogen supply accelerated senescence (Fig. 2). Line RIL310 was an exception, showing earlier senescence in the absence than in the presence of Glc (Fig. 2, A and B). The five lines showed differential patterns in their Fv/Fm decrease when grown in the low nitrogen conditions (Fig. 2, C and D). Differences between lines were not as significant in the high nitrogen conditions (Fig. 4, A and B). The whole-rosette Fv/Fm decrease appeared however accelerated in the late-senescing lines RIL045 and RIL272 compared to the early-senescing lines RIL310, RIL083, and RIL232, whatever the growth media.
Figure 2.
Comparison of Fv/Fm between lines RIL045 (black circle), RIL083 (white circle), RIL232 (white triangle), RIL272 (black square), and RIL310 (white square) depending on Glc and nitrogen supply. Fv/Fm ratio was estimated on the whole rosettes of plants grown on agar medium containing 30 mm nitrogen without sugar (HN; A) or 30 mm nitrogen with 111 mm Glc (HNG; B), or 4.7 mm nitrogen without sugar (LN; C) or 4.7 mm nitrogen with 111 mm Glc (LNG; D). Data are the means of at least 10 plants ± sd. The same biological experiment was repeated four times and gave similar results.
Figure 4.
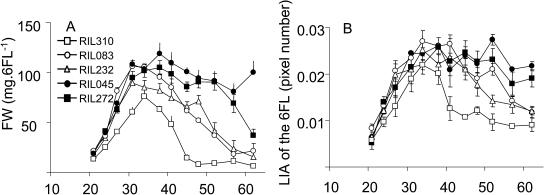
Comparison of the FW (A) and LAI (B) of the 6FL of the lines RIL045 (black circle), RIL083 (white circle), RIL232 (white triangle), RIL272 (black square), and RIL310 (white square). Data are the means of at least three repeats ± sd.
Characterization of Leaf Senescence under Low Nitrogen Nutrition
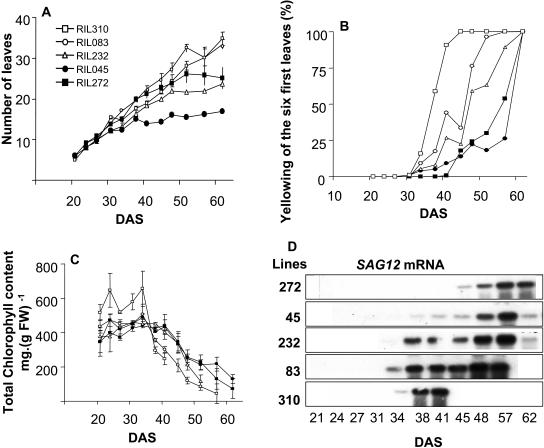
It has been suggested that leaf senescence in Arabidopsis might be controlled by development and also by aging and stress. To compare the RILs for developmental leaf senescence, we harvested leaves of the same age in a time-course experiment. We observed that the time when the six first leaves (6FL) emerged was similar for all the RILs, whereas the date of emergence of further leaves was different (Fig. 3A). As physiological studies require a lot of plant material, we decided to use the 6FL of the rosettes to study leaf senescence.
Figure 3.
Comparison of the leaf senescence progress of lines RIL045 (black circle), RIL083 (white circle), RIL232 (white triangle), RIL272 (black square), and RIL310 (white square). A, Total leaf numbered from rosettes. B, Leaf yellowing (%) measured on the 6FL of the rosettes. C, Determination of chlorophyll contents in the 6FL. D, SAG12 mRNA contents in the 6FL. Data are the means of three repeats ± sd.
The 6FL were dissected every 3 or 4 d and leaves were photographed. The yellowing of the leaves was observed visually and measured through imaging. Yellowing of the 6FL appeared sooner and was more intense for RIL310 (41 DAS) than for RIL232 and RIL083. During early stages, yellowing was undetectable in RIL045 and RIL272. At 52 DAS, the leaves of RIL310 were completely dead; at the same time, yellowing symptoms were detectable in RIL272 and RIL045. The quantitative measurement of the rate of yellow area in the 6FL (%Y6FL) is presented in Figure 3B. The first positive %Y6FL values appeared 34 DAS for all the lines except RIL272. The %Y6FL was higher for RIL310 than for RIL232, RIL083, and RIL045. Yellowing of RIL272 was late and detected 45 DAS. The patterns for %Y6FL were sigmoid, and the slope of the linear part of the curve was greatest for RIL310, followed by RIL083 and RIL232 (Fig. 3B), indicating that yellowing spread more rapidly in RIL310 than in RIL083 and RIL232.
Yellowing of leaves is commonly associated with a loss in total chlorophyll. In the 6FL of RIL310, total chlorophyll content was high and stable until 34 DAS, when it suddenly dropped (Fig. 3C). Chlorophyll in the 6FL of RIL310 had declined to 60% by 41 DAS; afterward chlorophyll was undetectable. A decrease in chlorophyll in the 6FL of RIL083 was detectable 34 DAS and progressed faster than for RIL310. Chlorophyll declined from 38 DAS for RIL232 and 41 DAS for RIL045 and RIL272. Chlorophyll content in the 6FL was equal for RIL232, RIL045, and RIL272 during early stages, but the loss was more rapid for RIL232 after 48 DAS.
Among the senescence-associated genes described in the literature, SAG12 is the marker whose expression is most specific for developmental senescence. SAG12 transcripts were detected 34 DAS for RIL310 and RIL083, 38 DAS for RIL232 and RIL045, and 45 DAS for RIL272 (Fig. 3D). However, the amount of transcripts was not related to the onset of SAG12 expression since only very small amounts of transcript were detectable in line RIL045 until 48 DAS, whereas high expression was observed in RIL232.
Taken together, these results show that RIL310, RIL232, and RIL083 represent three early-senescing lines. Especially, the 6FL of RIL310 were senescing sooner and far more rapidly than others. The 6FL of RIL083 were senescing more rapidly than those of RIL232. Lines RIL045 and RIL272 were late-senescing lines, and, when the senescence process finally began, RIL272 stayed green longer and expressed SAG12 later than RIL045.
Characterization and Comparison of the Biomass and Leaf Surface of the Six First Leaves
Senescence onset is often associated to the enhancement of nutrient mobilization, and biomarkers such as fresh weight (FW) or leaf area index (LAI) have been proposed as senescence-related markers (Masclaux et al., 2000; Buchanan-Wollaston et al., 2003). We observed that the FW loss appeared as soon as 31 DAS for RIL083, 34 DAS for RIL310 and RIL232, and 37 DAS for RIL045 and RIL272 (Fig. 4A). The loss of FW of the 6FL of RIL310 was very rapid, reaching minimum values 48 DAS, when leaves were considered to be dead. The FW decrease for the 6FL of RIL232 was more rapid and drastic than for RIL083. FW decreased later and more slowly for RIL272 and RIL045. Changes in dry weight (DW) were similar to FW (data not shown). The LAI of the 6FL was measured through imaging to estimate leaf expansion and to detect the time when leaf growth stopped (Fig. 4B). The LAI stopped to increase at 34 DAS for all the lines. The LAI of early-senescing lines decreased earlier and more rapidly than the LAI of late-senescing lines that remained stable for a longer time. The LAI decrease paralleled the decrease in FW and is explained by the withered state of the leaves.
Characterization of Metabolic Markers Associated with Leaf Senescence
Leaf senescence is characterized by a decrease in photosynthesis and changes in carbohydrate levels. We analyzed metabolic changes to investigate whether changes in metabolite profiles of leaves with aging could (1) inform us about the progress of leaf senescence and (2) allow us to classify different lines on the basis of the degree of senescence. This would also allow us to discover new senescence markers.
Sugar Contents in the Six First Leaves
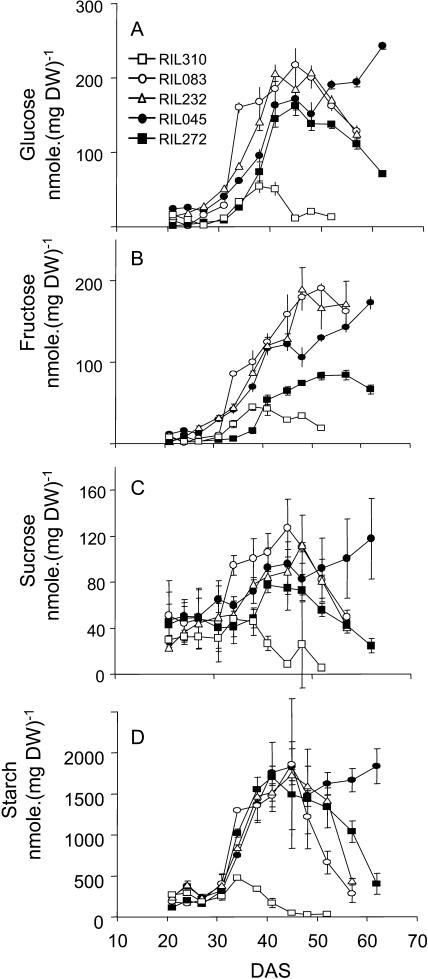
In lines RIL310, RIL083, RIL232, and RIL272, the pattern of Glc (Fig. 5A), Fru (Fig. 5B), Suc (Fig. 5C), and starch (Fig. 5D) contents formed bell-shaped curves. Sugars accumulated from 31 DAS. Sugar contents culminated at about the same time for all the lines except for RIL310, in which the optimum was reached sooner and sugar contents were untypically low. In contrast to the other lines, RIL045 continued to accumulate sugars throughout development.
Figure 5.
Comparison of the Glc (A), Fru (B), Suc (C), and starch (D) contents between the lines RIL045 (black circle), RIL083 (white circle), RIL232 (white triangle), RIL272 (black square), and RIL310 (white square). Data are the means of three repeats ± sd.
Organic Nitrogen in the Six First Leaves
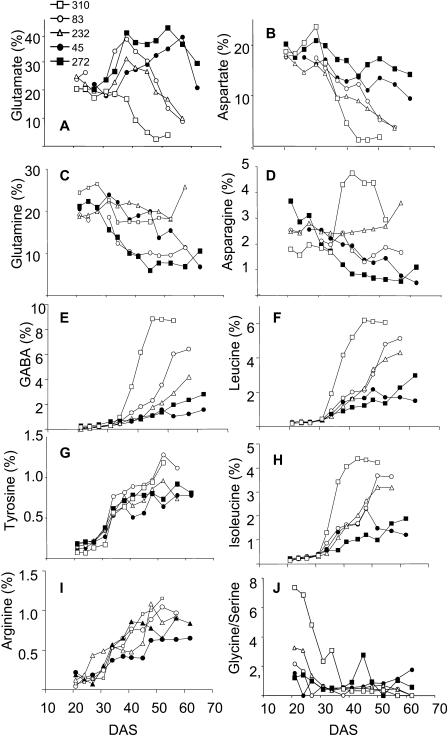
The amount and the decrease of protein and total amino acid contents with age were similar for the five lines (data not shown). The metabolic profiling of individual amino acids was far more informative. Figure 6, A to J, presents the time course of the amino acid proportions that allowed us to differentiate the lines according to the severity of the senescence symptoms observed. The major amino acids found in the 6FL leaves of Arabidopsis lines during early development were Glu, Gln, Asp, and Asn.
Figure 6.
Comparison of the amino acids proportions of Glu (A), Asp (B), Gln (C), Asn (D), GABA (E), Leu (F), Tyr (G), Ile (H), Arg (I), and of the Gly/Ser ratio, between the 6FL of the lines RIL045 (black circle), RIL083 (white circle), RIL232 (white triangle), RIL272 (black square), and RIL310 (white square). >From three repeats, the same amount of total amino acids was pooled, and the pools were analyzed for individual amino acids by chromatography.
For all the lines, the Glu and Asp proportions increased just after the onset of senescence (Fig. 6, A and B). Afterward, Glu and Asp decreased. The decrease in Glu and Asp was differential according to the lines and correlated with senescence. The Gln proportion decreased steadily in the 6FL for all the lines except RIL232, in which it remained stable (Fig. 6C). The Asn proportion of the 6FL also decreased except for RIL310, in which it suddenly rose, and for RIL232, in which it remained stable (Fig. 6D). Conversely, the γ-aminobutyric acid (GABA), Leu, Ile, Tyr, and Arg proportions rose with age (Fig. 6, E–I). Their increase appeared to be correlated with the senescence phenotype of the RILs. The Gly/Ser ratio is commonly used as an indicator of photorespiratory activity (Fig. 6J). Amazingly, results showed that the Gly/Ser ratio measured at early stage of development (20 DAS) is indicative of senescence at a later stage. For example, the Gly/Ser ratio is highest in RIL310, followed by RIL232 and RIL083.
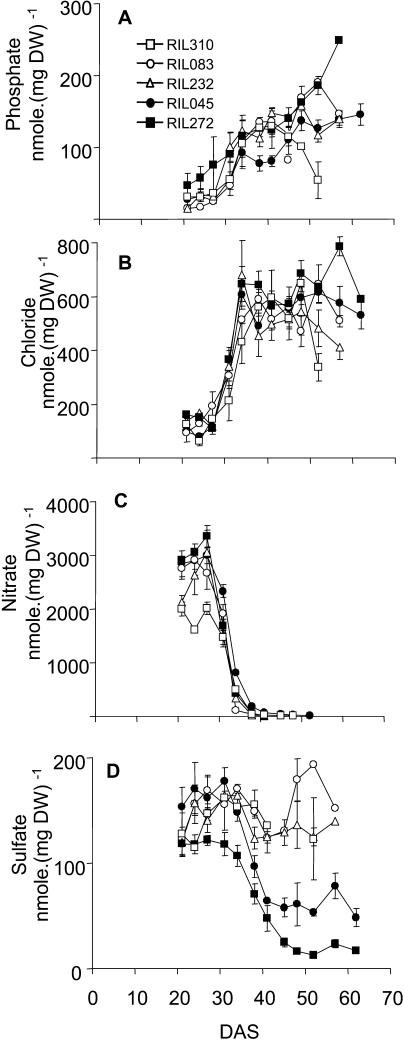
Anion Content in the Six First Leaves
The contents in nitrate, sulfate, phosphate, and chloride were investigated. Whereas phosphate (Fig. 7A) and chloride (Fig. 7B) accumulated continuously with aging, nitrate content decreased (Fig. 7C). A bell-shaped curve for phosphate and chloride was observed, showing that both compounds culminated before they slightly decreased in early-senescing lines. Interestingly, the sulfate patterns were different between the early-senescing lines and the late-senescing lines (Fig. 7D). The early-senescing lines RIL310, RIL232, and RIL083 showed high sulfate contents all along their development, whereas sulfate dropped as the 6FL of RIL045 and RIL272 were aging. This drop was initiated about 31 DAS and was more pronounced for RIL272 than RIL045, indicating that the loss of sulfate was higher in lines that stayed green longer.
Figure 7.
Comparison of the phosphate (A), chloride (B), nitrate (C), and sulfate (D) contents between the lines RIL045 (black circle), RIL083 (white circle), RIL232 (white triangle), RIL272 (black square), and RIL310 (white square). Data are the mean of three repeats ± sd.
Correlations of Markers for Monitoring the Extent of Senescence
To determine which markers can be used to evaluate the senescence extent of the leaves, correlations were determined (Table II). Correlations show that the total chlorophyll, total protein, and total amino acids contained in the 6FL are negatively correlated with the yellowing intensity of the 6FL. More interestingly, important and significant positive correlations between Leu (%), Arg (%), Tyr (%), or GABA (%) and yellowing (%Y6FL) were revealed. Glu (%), Asp (%), and Gln (%) also correlated with yellowing, but negatively. The total number of leaves correlated with yellowing, thus suggesting the involvement of rosette development and emergence of new leaves in the control of the senescence progress.
Table II.
Correlations between senescence and metabolic traits
NS, Not significant; *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
| Yellow | Leaves No. | DW | Chlorophyll | Protein | Total Amino Acids | Glu | Gln | Asp | GABA | Leu | Tyr | Arg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | |||||||||||||
| Yellow | 1 | ||||||||||||
| Leaves no. | 0.79*** | 1 | |||||||||||
| DW | NS | 0.38* | 1 | ||||||||||
| Chlorophyll | −0.83*** | −0.71*** | NS | 1 | |||||||||
| Protein | −0.71*** | −0.77*** | −0.53*** | 0.84*** | 1 | ||||||||
| Amino acids | −0.56*** | −0.83*** | −0.66*** | 0.68*** | 0.85*** | 1 | |||||||
| Glu | −0.46*** | NS | 0.50*** | NS | NS | −0.31* | 1 | ||||||
| Gln | −0.34** | −0.64*** | −0.46** | 0.52*** | 0.60*** | 0.67*** | −0.52*** | 1 | |||||
| Asp | −0.84*** | −0.65*** | NS | 0.71*** | 0.70*** | 0.54*** | 0.45** | NS | 1 | ||||
| GABA | 0.87*** | 0.69*** | NS | −0.75*** | −0.62*** | −0.41* | −0.58*** | NS | −0.84*** | 1 | |||
| Leu | 0.90*** | 0.74*** | NS | −0.74*** | 0.68*** | −0.55*** | −0.50*** | NS | −0.91*** | 0.95*** | 1 | ||
| Tyr | 0.74*** | 0.93*** | 0.51*** | −0.71*** | −0.83*** | −0.87*** | NS | −0.64*** | −0.75*** | 0.68*** | 0.77*** | 1 | |
| Arg | 0.78*** | 0.91*** | 0.39* | −0.74*** | −0.80*** | −0.79*** | NS | −0.57*** | −0.77*** | 0.73*** | 0.81*** | 0.9*** | 1 |
DISCUSSION
To select plants that show differential leaf senescence symptoms, the Bay-0 × Shahdara RIL population was screened. When plants were grown in low nitrogen (3 mm) and short (8 h) photoperiods, they exhibited stronger visual yellowing symptoms and longer leaf life span than under high nitrogen (10 mm) conditions (data not shown). Five RIL were selected and low nitrogen condition was chosen for this study.
The senescence of the whole rosettes of chosen RIL was monitored on a time-course experiment by measuring the decline in Fv/Fm. We observed that the Fv/Fm values obtained by fluorescence imaging of the whole rosette were mainly representative of the younger leaves. Indeed, younger leaves partially covered the old leaves, and early-senescing lines produced a larger number of leaves (Table II). Moreover, the Fv value of the highly-senescing and no-more photosynthetic leaves of the early-senescing lines might be very low and might not contribute in a large extent to the whole-rosette Fv/Fm. This explains why early-senescing rosettes showed higher total Fv/Fm than late-senescing lines. The classification of the RIL on the basis of Fv/Fm was similar on low and high nitrogen nutrition and with or without Glc feeding, even if more significant differences between lines were observed under low nitrogen nutrition. This suggested that the differences observed between the five RIL for senescence progress were genetically determined and amplified by low nitrogen nutrition.
Taking into consideration the markers usually used to monitor leaf senescence, we then determined the progress of senescence in individual leaves for the five RILs. The 6FL were harvested regularly, every 3 or 4 d to measure the kinetics of marker changes. Markers such as yellowing, chlorophyll and protein contents, biomass, foliar expansion, and SAG12 expression allowed us to estimate both the onset and the evolution of leaf senescence for the five RILs. The major difference between the five lines was the difference in the life span of their 6FL and the duration of their senescence process. Under low nitrogen nutrition, the phenotyping of RIL310, RIL083, and RIL232 as early-senescing lines and RIL045 and RIL272 as late-senescing lines was then confirmed.
The time course of the yellowing of the 6FL was significantly correlated with the total number of leaves that compose the rosettes, indicating that resources released during senescence can be used for the formation of additional leaves. This suggested that the source/sink relationship might have an impact on leaf senescence and life span, through the management of nutrient and resource allocation and recycling. The drastic change in leaf metabolism that occurs during senescence led us to investigate leaf senescence from a metabolic point of view. Many genomic and some proteomic studies have been performed to investigate senescence-related events (Hinderhofer and Zentgraf, 2001; Guo et al., 2004; Lin and Wu, 2004). However, the impact on metabolism of senescing leaves cannot be assessed solely on the basis of transcript levels, but requires analysis of metabolite contents. While the metabolomic finger-printing approaches to determine overall changes in metabolites are still being developed, we chose to concentrate on metabolite profiling of specific groups of compounds.
It has been debated extensively if sugar accumulation triggers senescence. The involvement of sugars in a feedback control of photosynthesis has already been reported (Wingler et al., 1998). Sugars are also a central element in source/sink relationships (Balibrea Lara et al., 2004; Roitsch and Gonzalez, 2004), and their implication in the positive and negative regulation of the early and late SAGs was shown (Masclaux-Daubresse et al., 2005; for review, see Paul and Pellny, 2003). In tobacco (Nicotiana tabacum), we have observed that senescence-related enzymes involved in nutrient mobilization were induced when sugar contents reached maximum values (Masclaux et al., 2000) and that sugars were involved in the regulation of the nitrogen-mobilization enzymes (Masclaux-Daubresse et al., 2005).
Similar to tobacco leaves (Masclaux et al., 2000), Arabidopsis leaves accumulated sugars during the first day following leaf yellowing. After a peak in sugar was reached, sugars dropped rapidly. The drop in hexose appeared slightly sooner in the early-senescing lines. Contrary to the other lines, RIL045 kept high contents of sugars, thus confirming its status as a late-senescing line. By contrast, the sugar pool accumulated by RIL310 was much lower. The interpretation proposed to explain the peaks of carbohydrate is that leaves use the sugars they synthesize as long as they grow and build structures, such as cell walls. When full expansion is attained, sugars accumulate and repress photosynthesis, thereby inducing senescence (Wingler et al., 1998, 2004). As a consequence of reduced photosynthesis, sugar formation eventually declines and stored carbohydrates are used to sustain respiration.
The differences in sugar metabolism between lines RIL045 and RIL310 are also interesting with respect to the relationship between sugars and flowering. It has been shown that sugar availability is important for floral transition (Roldán et al., 1999; Ohto et al., 2001). Indeed, line RIL045 accumulated sugars for a longer time than the other lines and flowered earlier, whereas line RIL310 only transiently accumulated small amounts of sugars and flowered very late.
It is well known that leaf senescence is of major importance for nitrogen management and recycling. The nitrate, amino acid, and protein pools contained in the 6FL did indeed decrease with aging. Himelblau and Amasino (2001) reported that more than 80% of the total nitrogen contained in the leaves of Arabidopsis is exported during senescence. Protein breakdown is necessary for organic nitrogen recycling. Mechanisms involved in nitrogen mobilization are under investigation, and many works reported the involvement of both proteolysis and reactive oxygen species in protein breakdown. During protein degradation, free amino acids are released and probably interconverted, hydrolyzed, catabolized, or exported without any modifications. Modifications that occur during amino acid recycling and nitrogen export are poorly known. By now, only few senescence-related enzymes that hydrolyze amino acids have been detected. Glutamate dehydrogenase and Thr deaminase are the best candidates for such a process (Samach et al., 1991; Masclaux-Daubresse et al., 2002).
Whereas the amino acid proportions were relatively stable for all the RILs studied during the 31st DAS, major changes were detected as soon as the senescence process had started. Glutamate proportions increased transiently but decreased at the end of senescence. A steady decrease in Asp was also observed. Asn and Gln are usually designated as the major amino acids translocated in the phloem sap. Gln and Asn proportions also changed in leaves with aging and appeared to decrease much more in late-senescing than in early-senescing lines (except for Gln in line RIL083). This suggests that these amino acids, which are rich in nitrogen, were stored in the leaves of senescing lines and much more efficiently interconverted or exported from leaves of the late-senescing lines. This observation poses questions about the role of nitrogen-mobilization efficiency with regards to senescence. The nitrogen-remobilization mechanisms in the five RILs are under investigation. The GABA, Leu, Ile, Tyr, and Arg proportions increased. Glu is an important amino acid that, together with Gln, has a central role in mineral nitrogen assimilation. Glu is the primary amino donor for the synthesis of all the other amino acids, and Asp is a direct product of Glu transamination. Therefore, any change in the Glu content might modify the Asp pool. The Asp pathway is involved in the biosynthesis of Lys, Thr, Met, Ile, and Leu (Miflin and Lea, 1982). Whereas Asp proportion decreased with aging, the pools of Leu and Ile were increasing, suggesting that Ile/Leu biosynthesis had exhausted the Asp pool. Tyr is a product of the shikimate pathway and is a precursor for vitamin E (α-tocopherol) biosynthesis. The role of vitamin E as a powerful antioxidant is well known (Collakova and DellaPenna, 2003a, 2003b), and, interestingly, the accumulation of vitamin E in leaf tissue with aging has been shown (Rise et al., 1989; Molina-Torres and Martinez, 1991; Tramontano et al., 1992). The increase in Tyr proportions with aging might play a role in supporting vitamin E biosynthesis.
GABA is a direct product of the Glu decarboxylase reaction. GABA accumulation is triggered by various stresses, such as mechanical damage, cold, anoxia, heat, drought, salt stress, cadmium stress, or viral attack (Kinnersley and Turano, 2000; Chaffei et al., 2004). Accumulation of GABA with leaf aging has been reported for legumes and tobacco plants (Lahdesmaki, 1968; Masclaux et al., 2000), and this work clearly demonstrates a major accumulation of GABA in senescing leaves of Arabidopsis. Several roles have been proposed for GABA: (1) as amplifier of stress signals through the enhancement of ethylene synthesis; (2) as transient nitrogen-storage compound; and (3) as anaplerotic compound in stress-related metabolism (Kathiresan et al., 1997; for review, see Kinnersley and Turano, 2000). Since sugar pools are decreasing during late senescence, accumulation of GABA in senescing leaves might be important for anaplerotic reactions (Bown and Shelp, 1997). Recently, Ansari et al. (2005) showed that the Osl2 gene, encoding a GABA/pyruvate transaminase, was specifically up-regulated during rice leaf senescence, thus suggesting an anaplerotic role for GABA. The GABA proportion was especially high in the 6FL of RIL310 that were rich in amino acids, also suggesting a role for nitrogen storage.
In addition to important physiological information obtained by determination of amino acid contents, comparison of the five lines revealed that Glu, Asp, GABA, Leu, and Ile can serve as chemical markers to discriminate relative senescence severity. The relative contents in GABA, Asp, Leu, Ile, and Glu were highly correlated with the degree of leaf yellowing. This finding opens new perspectives for such indicators as quantitative traits for QTL analysis.
The discrimination of early-senescing and late-senescing lines was also possible through the measurement of the Gly/Ser ratio during early stages following the emergence of the 6FL. Gly and Ser are two amino acids formed during photorespiration. The high Gly/Ser ratio we observed for early-senescing lines compared to late-senescing lines indicates high rates of photorespiration. Increased photorespiration could e.g. be caused by stress (Wingler et al., 1999, 2000). The possibility that the Gly/Ser ratio might be a predictive indicator for further senescence behavior of the leaves is very attractive, and studies to test this hypothesis are in progress.
When aging, leaves do not only degrade organic compounds. They also export the anionic compounds stored in the vacuoles. In the 6FL, nitrate, chloride, and phosphate remained stable until the onset of senescence. When leaf senescence was initiated, nitrate dropped dramatically in a similar manner for all the lines, whereas chloride and phosphate remained stable. Surprisingly, sulfate contents did not develop in a similar way for all the lines. Sulfate was efficiently mobilized from the 6FL of the late-senescing lines RIL272 and RIL045. Conversely, no change in the sulfate pools of the 6FL of RIL310, RIL232, and RIL083 could be detected with aging. The mechanisms by which sulfate is mobilized and exported during leaf senescence are not known, and our results suggest that sulfate is differentially managed in early-senescing and late-senescing lines and is more efficiently mobilized or reduced and assimilated when the leaves stay alive for a longer time.
Several metabolic makers to discriminate Arabidopsis lines on the basis of senescence progress and severity were identified. Based on the changes in metabolite contents we have identified, it is possible to combine metabolite profiling with transcriptome analysis to obtain a complete picture of the regulation of plant metabolism during senescence.
MATERIALS AND METHODS
Plant Material
The Bay-0 × Shahdara RIL population has been fully described in a previous publication (Loudet et al., 2002) and on http://www.inra.fr/qtlat. F8 seeds obtained from the last generation of single-seed descent for 415 lines were used. These seeds were harvested from plants grown at the same time for all lines, thus minimizing the maternal environment effect. The RIL310, RIL272, RIL232, RIL083, and RIL045 used in this study have been selected from the whole RIL population based on their senescence phenotype.
The homogeneous vegetative plant material was grown in a growth chamber under controlled conditions (Loudet et al., 2002). The experimental unit was a small pot (long = 60 mm, large = 65 mm, high = 60 mm) containing six plants of each RIL positioned in a circle. The seeds were stratified for 48 h in 0.1% agar solution (in water) at 4°C in the dark. Homogeneous germination occurred 2 DAS. Three times per week, the pots were watered (by immersion of the base of the pots) in a solution containing 3 mm nitrate. Phosphate and sulfate were present at the same concentration (0.25 mm), as well as magnesium (0.25 mm) and sodium ions (0.20 mm). The watering solution also contained 2.75 mm potassium, 0.5 mm calcium, and 0.7 mm chloride ions. The pH of the watering solutions remained between 5.1 and 5.5. The plants were maintained in short days with a photoperiod of 8 h throughout the culture. Day and night temperatures were regulated at 21°C and 17°C, respectively. Light was provided by 20 mercury-vapor bulbs, ensuring a photosynthetic photon flux density of approximately 160 μmol m−2 s−1.
About 20 DAS, the five RILs had formed six leaves plus cotyledons. For metabolic and molecular analysis, the 6FL of each rosette were dissected every 3 or 4 d and pooled. At each harvesting time, four different bulks of 6FL were harvested per RIL and stored at −80°C before further experiments. To analyze the effect of carbon and nitrogen supply on senescence in the RILs, plants were grown on agar (1% [w/v]) plates with high nitrogen (30 mm) or low (4.7 mm) nitrogen supply with or without addition of 2% Glc (Wingler et al., 2004). The plates were placed vertically in a growth chamber and illuminated for 16 h per day at a photon flux density of 100 μmol m−2 s−1. The temperature was 21°C during the day and 18°C at night.
Phenotyping through Yellowing and Fv/Fm Measurement
Yellowing rate of rosettes and individual leaves was measured by image processing. We measured the yellowing of the rosettes at 35 DAS by imaging each pot with a Nikon digital camera (model Coolpix800; Nikon Imaging Products Division, Tokyo). For individual leaf yellowing and LAI measurement, leaves were dissected and laid out individually on black squares of fixed size before imaging. Digital pictures of rosettes and leaves were then analyzed using a macro (Belluomo et al., 2003) running on the computer program Optimas (version 6.5; Media Cybernetics, Silver Spring, MD; http://www.mediacy.com).
Maximum photosynthetic efficiency, Fv/Fm, was analyzed using a FluorCam 700MF kinetic imaging fluorometer (Photon Systems Instruments, Brno, Czech Republic) as described before (Wingler et al., 2004).
Chlorophyll and Total Protein Determinations
Chlorophyll content was determined in crude leaf extracts (Arnon, 1949). Soluble protein was extracted in 100 mm Tris, pH 7.5, buffer and was determined using a commercially available kit (Coomassie Protein assay reagent; Bio-Rad, Hercules, CA).
Metabolite Extraction and Analysis
Amino acids were determined after extraction in 2% solution of 5-sulfosalicylic acid (50 mg FW mL−1). Total amino acid content was assayed by the Rosen colorimetric method using Gln as a reference (Rosen, 1957). Individual amino acid composition was determined in pooled samples extracted from equal dry weights of three individual repeats by ion-exchange chromatography using the AminoTac JLC-500/V amino acid analyzer according to the instructions of the manufacturer (JEOL [Europe], Croissy-sur-Seine, France).
Anions and carbohydrates were determined after ethanolic extraction (Loudet et al., 2003). Suc and hexoses were determined as described by Masclaux-Daubresse et al. (2002) in supernatants. Starch content was determined from pellets (Masclaux-Daubresse et al., 2002). Anions (nitrate, chloride, phosphate, and sulfate) were determined as described by Loudet et al. (2003).
Extraction of Total RNA and Northern-Blot Analysis
Total RNA was extracted from plant material stored at −80°C, and northern-blot analysis was performed as described previously (Masclaux et al., 2000). 32P-labeled SAG12 probes were used for mRNA detection (Hinderhofer and Zentgraf, 2001). 18S rRNA was used as a constitutive control (Masclaux et al., 2000). Hybridization was performed under high stringency conditions at 65°C. Filters were washed with 2× SET (0.06 m Tris-HCl, pH 8, 0.3 m NaCl, 4 mm EDTA) at room temperature for 5 min and at 65°C for 10 min. Additional washing was performed successively using 1× SET and 0.5× SET at 65°C for 15 min before drying and exposure to x-ray film.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number U37336.
Acknowledgments
We thank Marie-Thérèse Ledeycker and Stéphanie Boutet for technical help for anion and amino acid determination. Thanks to Joël Talbotec and François Gosse for help to grow plants. Thanks to Ulrike Zentgraf (Tuebingen University, Germany) for the gift of the SAG12 probe. We thank the Centre Technique Interprofessionnel des Oléagineux Metropolitains (http://www.cetiom.fr) and the Institut National de la Recherche Agronomique for providing financial support for C.D.'s thesis.
This work was supported by the Centre Technique Interprofessionel des Oléagineux Métropolitains (Ph.D. studentship supporting the work of C.D.), the Biotechnology and Biological Siences Research Council (grant no. 31/P16341 to the A.W. laboratory), and the Natural Environment Research Council (Ph.D. studentship supporting the work of S.P.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.060764.
References
- Ansari MI, Lee R-H, Chen S-CG (2005) A novel senescence-associated gene encoding γ-aminobutyric acid (GABA):pyruvate transaminase is upregulated during rice leaf senescence. Physiol Plant 123: 1–8 [Google Scholar]
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris L. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balibrea Lara ME, Gonzalez Garcia MC, Fatima T, Ehness R, Lee TK, Proels R, Tanner W, Roitsch T (2004) Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16: 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluomo P, Robert C, Bancal MO (2003) Mise au point technique d'une méthode de repérage des couleurs sur des images numérisées pour quantifier la sévérité de maladies foliaires. Amélioration d'outils existants. Cahiers des Techniques de l'INRA 50: 15 [Google Scholar]
- Bown AW, Shelp BJ (1997) The metabolism and functions of γ-aminobutyric acid. Plant Physiol 115: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Ainsworth C (1997) Leaf senescence in Brassica napus: cloning of senescence related genes by subtractive hybridisation. Plant Mol Biol 33: 821–834 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Earl S, Harrison E, Mathas E, Navabpour S, Page T, Pink D (2003) The molecular analysis of leaf senescence—a genomics approach. Plant Biotechnol J 1: 3–22 [DOI] [PubMed] [Google Scholar]
- Chaffei C, Pageau K, Suzuki A, Gouia H, Ghorbel MH, Masclaux-Daubresse C (2004) Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol 45: 1681–1693 [DOI] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2003. a) Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol 131: 632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2003. b) The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol 133: 930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Cai Z, Gan S (2004) Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ 27: 521–549 [Google Scholar]
- Himelblau E, Amasino RM (2001) Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J Plant Physiol 158: 1317–1323 [Google Scholar]
- Hinderhofer K, Zentgraf U (2001) Identification of a transcription factor specifically expressed at the onset of leaf senescence. Planta 213: 469–473 [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53: 927–937 [DOI] [PubMed] [Google Scholar]
- Kathiresan A, Tung P, Chinnappa CC, Reid DM (1997) γ-Aminobutyric acid stimulates ethylene biosynthesis in sunflower. Plant Physiol 115: 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnersley AM, Turano FJ (2000) Gamma-aminobutyric acid (GABA) and plant response to stress. Crit Rev Plant Sci 19: 479–509 [Google Scholar]
- Lahdesmaki P (1968) The amount of γ-aminobutyric acid and the activity of glutamic carboxylase in ageing leaves. Physiol Plant 21: 1322–1327 [Google Scholar]
- Levey S, Wingler A (2005) Natural variation in the regulation of leaf senescence and relation to other traits in Arabidopsis. Plant Cell Environ 28: 223–231 [Google Scholar]
- Lin JF, Wu SH (2004) Molecular events in senescing Arabidopsis leaves. Plant J 39: 612–628 [DOI] [PubMed] [Google Scholar]
- Loudet O, Chaillou S, Camilleri C, Bouchez D, Daniel-Vedele F (2002) Bay-0 x Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis. Theor Appl Genet 104: 1173–1184 [DOI] [PubMed] [Google Scholar]
- Loudet O, Chaillou S, Krapp A, Daniel-Vedele F (2003) Quantitative trait loci analysis of water and anion contents in interaction with nitrogen availability in Arabidopsis thaliana. Genetics 163: 711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae T (2004) Leaf senescence and nitrogen metabolism. In LD Noodén, ed, Plant Cell Death Processes. Elsevier, Amsterdam, pp 157–168
- Masclaux C, Valadier M, Brugière N, Morot-Gaudry J, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211: 510–518 [DOI] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Carrayol E, Valadier M-H (2005) The two nitrogen mobilization- and senescence-associated GS1 and GDH genes are controlled by C and N metabolites. Planta (in press) [DOI] [PubMed]
- Masclaux-Daubresse C, Valadier M-H, Carrayol E, Reisdorf-Cren M, Hirel B (2002) Diurnal changes in the expression of glutamate dehydrogenase and nitrate reductase are involved in the C/N balance of tobacco source leaves. Plant Cell Environ 25: 1451–1462 [Google Scholar]
- Miflin BJ, Lea PJ (1982) Ammonia assimilation and amino acid metabolism. In D Boulter, B Parthier, eds, Nucleic Acids and Proteins in Plants I, Vol I. Springer-Verlag, Berlin, pp 5–64
- Molina-Torres J, Martinez ML (1991) Tocopherols and leaf age in Xanthium strumarium L. New Phytol 118: 95–99 [Google Scholar]
- Noodén LD, Hillsberg JW, Schneider MJ (1996) Induction of leaf senescence in Arabidopsis thaliana by long days through a light-dosage effect. Physiol Plant 96: 491–495 [Google Scholar]
- Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K (2001) Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol 127: 252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54: 539–547 [DOI] [PubMed] [Google Scholar]
- Pourtau N, Mares M, Purdy S, Quentin N, Ruel A, Wingler A (2004) Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta 219: 765–772 [DOI] [PubMed] [Google Scholar]
- Rise R, Cojocaru M, Gottlieb HE, Glodschmidt E (1989) Accumulation of alpha-tocopherol in senescing organs as related to chlorophyll degradation. Plant Physiol 89: 1028–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Gonzalez MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9: 606–613 [DOI] [PubMed] [Google Scholar]
- Roldán M, Gomez-Mena C, Ruiz-Garcia L, Salinas J, Martinez-Zapater JM (1999) Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J 20: 581–591 [DOI] [PubMed] [Google Scholar]
- Rosen H (1957) A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys 67: 10–15 [DOI] [PubMed] [Google Scholar]
- Samach A, Hareven D, Gutfinger T, Ken-Dror S, Lifschitz E (1991) Biosynthetic threonine deaminase gene of tomato: isolation, structure, and upregulation in floral organs. Proc Natl Acad Sci USA 88: 2678–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano WA, Ganci D, Pennino M, Dierenfeld ES (1992) Age dependent alpha-tocopherol concentrations in leaves of soybean and pinto beans. Phytochemistry 31: 3349–3351 [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355: 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Marès M, Pourtau N (2004) Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytol 161: 781–789 [DOI] [PubMed] [Google Scholar]
- Wingler A, Quick WP, Bungard RA, Bailey KJ, Lea PJ, Leegood RC (1999) The role of photorespiration during drought stress: an analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ 22: 361–373 [Google Scholar]
- Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP (1998) Regulation of leaf senescence by cytokinin, sugars, and light. Plant Physiol 116: 329–335 [Google Scholar]
- Ye Z, Rodriguez R, Tran A, Hoang H, De Los Santos D, Brown S, Vellanoweth RL (2000) The developmental transition to flowering repressed ascorbate peroxidase activity and induces enzymatic lipid peroxidation in leaf tissue in Arabidopsis thaliana. Plant Sci 158: 115–127 [DOI] [PubMed] [Google Scholar]