Abstract
Current sensors for Zn2+ detection are largely based on the photoinduced electron transfer (PET) mechanism, which can effectively change the fluorescence intensity, without inducing a significant spectral shift. By coupling the PET mechanism with an excited state intramolecular proton transfer (ESIPT), a near-infrared fluorescent sensor was developed for Zn2+ detection. Upon binding to the Zn2+ cation, the sensor was able to generate two well-separated emission bands (λem ≈ 540 and 770 nm), whose ratio was quite sensitive to the probe’s environments. The finding offers an advanced tool for in vitro and in vivo imaging of the Zn2+ cation, which is desirable for future discovery of the biological functions of zinc.
Keywords: fluorescent probe, zinc sensing, near-infrared emission, proton transfer, environmental sensitivity, fluorescence imaging
Molecular imaging is widely used to visualize the fundamental biological processes.1−3 Due to its high sensitivity, quick response, and excellent spatial and temporal resolution, fluorescent sensing has become one of the top choices to detect the analytes of interest.4,5 Near-infrared (NIR) fluorescent sensors are especially attractive for in vivo detection of biologically relevant species,6,7 as the NIR photon has deep tissue penetration and low autofluorescence.8−16 For applications, the emission should be well-separated from the excitation (i.e., large Stokes’ shift), in order to minimize the interference from the excitation light.17−19 Although cyanine-based dyes give strong NIR absorption and emission, they exhibit small Stokes’ shifts (usually 20–50 nm).19−24 Development of NIR fluorescent dyes with a large Stokes’ shift (e.g., >200 nm) remains a challenge.
As an active component in enzymes and proteins,25−27 the zinc cation (Zn2+) plays an important role in various biological28 and pathological processes, including Alzheimer’s disease, epilepsy, infantile diarrhea, and ischemic stroke.29−33 Free zinc pools exist in some tissues, such as the brain, intestines, pancreas, and retina.34 There is significant interest in sensing Zn2+ in living systems.35−42 However, the majority of Zn2+ sensors give emissions in the visible region, which often suffer interference from autofluorescence.43 Most zinc sensors are based on the photoinduced electron transfer (PET) mechanism,35,44 which can induce a large fluorescence change. It should be noticed that metal ion binding typically induces a small spectral shift.44,45 In addition, PET often exhibits incomplete fluorescence quenching that limits the performance of the sensors. Solving the problem requires integration of other mechanisms in the sensor design.
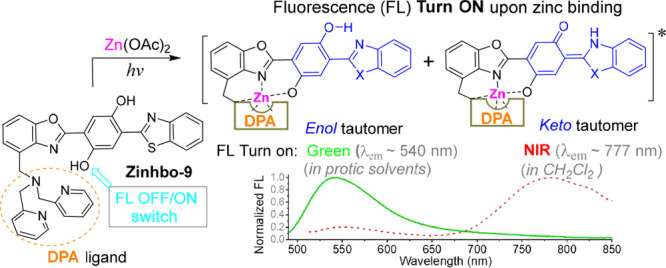
In order to overcome the deficiency of PET-based sensors, a potential solution is based on a bis(HBO) 3 (Scheme 1). In the central phenyl ring of 3, one −OH group acts as a fluorescence OFF/ON switch when it binds to the Zn2+ cation. The second −OH group in 3 is reserved for the excited state intramolecular proton transfer (ESIPT) that can trigger a large spectral shift. The probe design utilizes Zn2+ binding to the −OH switching group, thereby turning on the fluorescence of the ESIPT fluorophore, which can give two fluorescence signals.
Scheme 1. Convergent Synthesis of Zinhbo-9.
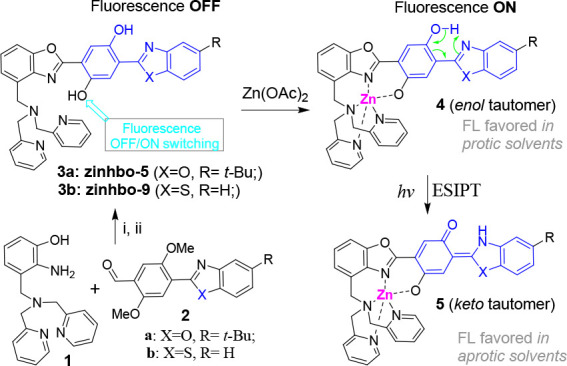
Conditions: (i) xylenes 120 °C, O2/activated carbon, 48 h, 52%; (ii) BBr3, DCM, −78 °C to rt; overnight, 87%.
Our recent studies showed that Zinhbo-5 (i.e., 3a in Scheme 1) could be a useful NIR probe, as its binding to the Zn2+ cation can induce two fluorescence signals at ∼540 and ∼730 nm (in ∼1:1 ratio), attributed to its enol and keto tautomers (Figure 1).46 However, all the known bis(HBO) probes still exhibited relatively low NIR response upon Zn2+ binding, despite the efforts for over a decade.46,47 Herein, we demonstrate that the probe’s response to Zn2+ could be dramatically improved by using Zinhbo-9 (3b), giving strong green (λem ≈ 540 nm) or NIR fluorescence (λem ≈ 750–780 nm), depending on the environments. The large NIR fluorescence turn on, in addition to its potential to sense its environment change, makes the probe a very attractive candidate for Zn2+ sensing.
Figure 1.
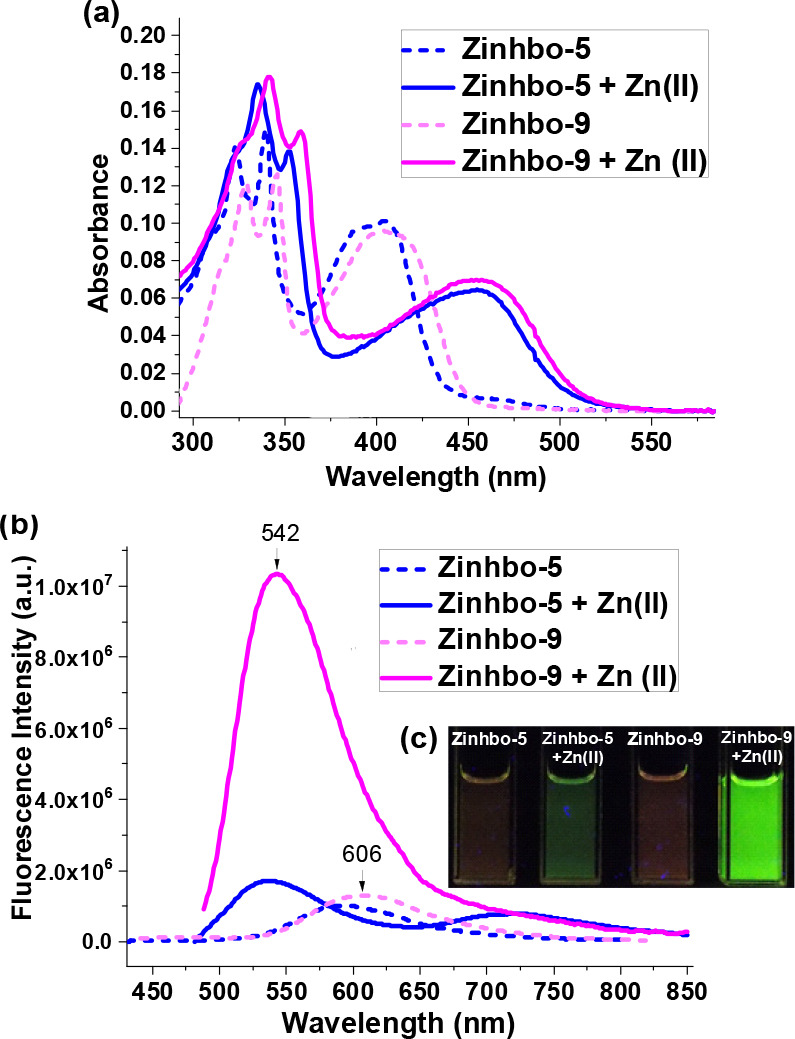
UV–vis (a) and fluorescence spectra (b) of 10 μM Zinhbo-5 and Zinhbo-9 (broken line) in aqueous solution containing 50% EtOH and their zinc complex (solid line). (c) Fluorescent images taken when the same samples (for spectra) were irradiated with a 365 nm UV lamp at room temperature.
Sensor Zinhbo-9 was synthesized in good yield by coupling the key intermediate aminophenol 1 with aldehyde 2b in two steps (Scheme S1) using a convergent synthetic strategy.48 UV–vis of Zinhbo-9 exhibited an absorption of λmax ≈ 405 nm, which was red-shifted to λmax ≈ 455 nm upon addition of the Zn2+ cation (Figure 1a, solid lines), indicating the complex formation. On the basis of the Job plot and ESI-MS data (Figure S1), the complex structure was assumed to be 4b with a 1:1 ligand-to-metal ratio (i.e., Zinhbo-9/Zn, Figure S2), which is consistent with the reported crystal structure for 4a.46 In the aqueous solution (EtOH:H2O = 1:1), Zinhbo-9 alone gave a relatively weak fluorescence (λem ≈ 600 nm, φfl ≈ 0.11 by using quinine sulfate as a reference). However, formation of the zinc complex drastically increased the fluorescence when being excited at 455 nm to give bright green emission (λem ∼ 542 nm; φfl ≈ 0.72; Figure 1b,c). Although both ligands exhibited similar emission profile with λem ≈ 600 nm, the zinc complex of Zinhbo-9 gave one bright emission peak (λem ≈ 542 nm), while that of Zinhbo-5 gave two weak emission peaks (λem ≈ 540 and 725 nm) in about a 1:1 ratio (Figure 1b). The large difference in responding to the Zn2+ cation indicated the large impact of a heteroatom substitution on the ESIPT process, as Zinhbo-9/Zn in the aqueous buffer gave nearly exclusive emission from its enol tautomer.
The fluorescence of the new probe was further examined in different solvents (Figure S3). Interestingly, Zinhbo-9/Zn gave mainly an enol emission (λem ∼ 540 nm) in a protic solvent such as EtOH. The enhanced enol emission from Zinhbo-9/Zn in a protic solvent was in sharp contrast to that from Zinhbo-5/Zn, whose ESIPT emission (from the keto tautomer) was enhanced in EtOH (Figures S4–S6). It was assumed that the hydrogen bonding with protic solvents inhibited the ESIPT process effectively in Zinhbo-9/Zn, making it possible to achieve a predominant enol emission.
More interestingly, Zinhbo-9/Zn exhibited a major NIR emission peak at ∼770 nm in CH2Cl2 and dual emission (λem ≈ 534 and 770 nm in about 1:1 ratio) in CH3CN, showing that the emission of the zinc complex was quite sensitive to its environments (Figure 2 and Figure S3). The NIR emission peak at ∼770 nm could be attributed to its keto tautomer 5. The result thus illustrated a rare probe that gave strong NIR emission with a very large Stokes’ shift (Δλ = (777 – 492) nm = 285 nm) upon binding to Zn2+ by selectively turning on its keto emission in an aprotic solvent. In addition, the emission ratio of the resulting Zn2+ complex at 534 and 770 nm was quite sensitive to its environment. Thus, the NIR-emitting new probe not only is capable of detecting zinc cation but also raising the possibility of detecting zinc in different bioenvironments. The Zn2+ binding induced NIR emission can be clearly observed by using an “in vivo imaging spectrum (IVIS)” system (Figure 2B).
Figure 2.
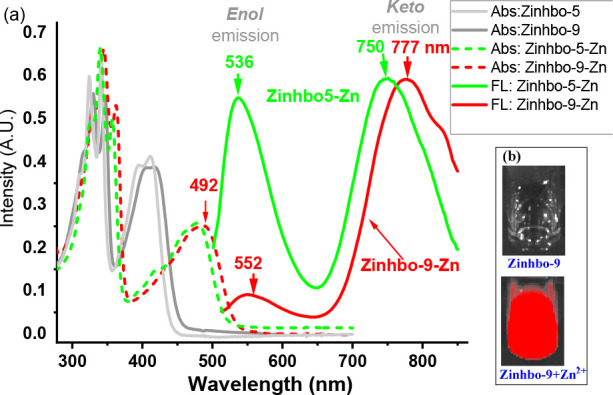
(a) Normalized absorption (ABS) and fluorescence (FL) spectra of Zinhbo-5 and Zinhbo-9 upon addition of 1.0 equiv of Zn2+ in CH2Cl2 excited at λmax (480 and 492 nm). (b) Zinhbo-9 and Zinhbo-9/Zn2+ CH2Cl2 solution (1.0 μM) in glass scintillation vials were imaged with Ex/Em = 500/780 nm (NIR channel) using an IVIS imaging system.
The apparent association constant, Ka = 6.41 nM, for Zn2+ was determined, which reveals strong binding of Zinhbo-9 toward the Zn2+ cation, and the detection limit was found to be as low as 0.5 nM in the buffered aqueous (Figure S25).
The sensor’s response to other metal ions was also examined in both CH2Cl2 and aqueous solutions (Figures S9–S11). The probe was silent to other physiologically important metal ions, such as Mg2+, Na+, and K+ cations. The heavy and transition metal ions, such as Mn2+, Ni2+, Co2+, Cu2+, Fe2+, Fe3+, Pb2+, Ba2+, and Cr3+ cations, which are well-known fluorescence quenchers, did not show a fluorescence quenching effect on Zinhbo-9 in both CH2Cl2 and aqueous solutions. Furthermore, significant fluorescence turn-on was observed in the presence of 10 equiv of the other metal ion and 1 equiv of Zn2+ in aqueous solution, which indicates that all the tested metal ions did not interfere with Zn2+ sensing. Therefore, Zinhbo-9 is a highly selective NIR fluorescence turn-on chemosensor for the Zn2+ cation.
For Zn2+ sensing, a significant challenge is to differentiate Zn2+ from Cd2+, as both cations have similar electron configuration. Interestingly, Zinhbo-9 did not exhibit notable change in absorption, λmax, upon binding Cd2+ in CH2Cl2, and the resulting Cd2+ complex did not give NIR emission (Figures S12–S15). The dissociation constant using the Benesi–Hildebrand plot revealed that Zinhbo-9 exhibited stronger binding toward Zn2+ [e.g., Kd ≈ 17.9 μM for Zn2+ and Kd ≈ 86.0 μM for Cd2+ in CH2Cl2 (Figure S17)]. The competitive binding of the probe toward Zn2+ and Cd2+ was also examined in HEPEs buffer (Figures S22–S26), revealing the same trend. Replacement of Cd2+ by Zn2+ in the preformed Zinhbo-9/Cd2+ complex solution was observed, which further indicated the stronger binding of Zn2+ over Cd2+. Therefore, Zinhbo-9 provided a rare NIR sensor that can selectively detect the Zn2+ cation.
In order to examine its biological applications, the probe was applied to cells by staining human mesenchymal stem cells (hMSCs). When the samples were excited with a 488 nm laser, strong NIR fluorescence (700–800 nm) was observed from the intracellular area (Figure S26), and only a very weak signal was observed in the green channel. In a control experiment, negligible intracellular fluorescence was detected from the cells after being treated with 10 μM Zinhbo-9 for 60 min (Figure S26c). The weak signal observed in the control experiment was attributed to the natural presence of an endogenous zinc cation. These results demonstrated that the probe could be a suitable tool for determining intracellular Zn2+ activities. An interesting feature is that Zinhbo-9 appeared to pass through the karyotheca (or nuclear membrane) into the nuclei, which was quite different from that of Zinhbo-5 (mainly spreads in the cytoplasm).49 To further confirm this feature, Zinhbo-9 was applied to a more divisive cell line (HeLa cells) with a shortened staining time (Figure 3). A strong NIR signal was observed in nuclei, which further proved the nuclear staining by Zinhbo-9. Interestingly, the chromosomes were found to be brighter in the metaphase during mitosis, which indicates that zinc plays an important role in cell proliferation where an advanced zinc probe would be a useful tool.50
Figure 3.
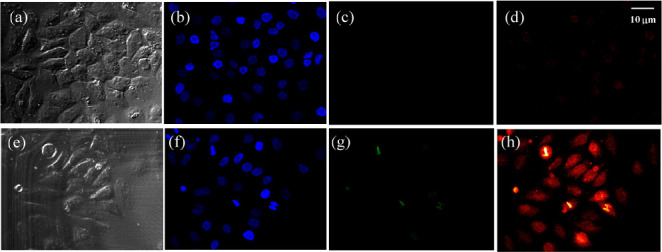
Confocal fluorescence images of HeLa cells excited with a 488 nm laser. The images were collected at bright field (a,e), DAPI (b,f), green channel (c,g), and NIR channel (d,h: 700–800 nm). Top (control): Cells were incubated with Zinhbo-9 for 60 min at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM). Bottom: Cells were first treated with Zn2+ (10 μM) for 30 min and further exposed to Zinhbo-9 (10 μM) for another 60 min at 37 °C in DMEM.
It should be noted that the emission of the Zinhbo-9/Zn complex was quite sensitive to the probe’s environment (Figures 1 and 2). The strong NIR emission, coupled with very weak green emission, indicated that the Zinhbo-9/Zn complex was located in a hydrophobic cellular or tissue environment. In other words, the comparison of zinc binding patterns from the green and NIR signals could provide useful information about the local environment of Zn2+ (i.e., aqueous or hydrophobic environments), which the existing zinc probes cannot match.
Encouraged by the cell studies, we further investigated the potential of using Zinhbo-9 for in vivo imaging of zinc in a mouse model. The injection of Zinhbo-9 followed by further injection of a zinc ion generated strong NIR fluorescence. Mice treated only with the probe showed weaker NIR fluorescence under the same imaging conditions (Figure 4). In the control experiment, the NIR fluorescent signal around the injection site might derive from the tissue injury, which has been known to cause dramatic elevation of zinc.51 These results demonstrate that the Zinhbo-9 probe is a useful Zn2+-selective NIR probe for in vivo fluorescence imaging.
Figure 4.
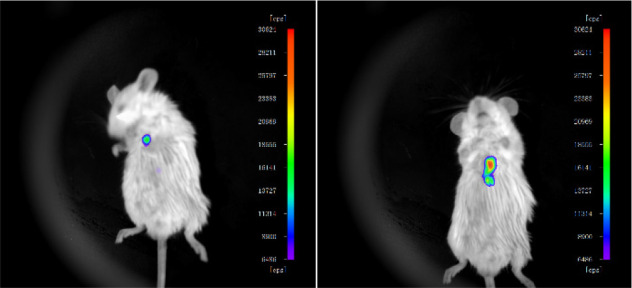
Fluorescent images of living mice. Left: Subcutaneous injection of 25 μL solution Zinhbo-9 (10 mM in DMSO). Right: Subcutaneous injection of 25 μL solution Zinhbo-9 (10 mM in DMSO), and then subcutaneous injection of 30 μL of Zn2+ solution (10 μM in DMSO). Images were taken 20 min after the subcutaneous injection, with Ex/Em = 500/780 nm (NIR channel) using an IVIS imaging system.
In addition, the Zinhbo-9/Zn complex also exhibited significant fluorescence at 777 nm when being excited at 980 nm in CH2Cl2 (Figures S6 and S7). The probe thus could have both excitation and emission within the NIR-I region (700–1000 nm), an ideal feature for an NIR sensor.
In conclusion, Zinhbo-9 has been shown to be a highly selective fluorescent turn-on sensor for Zn2+ cations, giving NIR emission at ∼777 nm. The finding leads to a promising molecular design which allows the probe to generate NIR emission (λem ≈ 777 nm) that is well-separated from its excitation (λmax ≈ 492 nm). In addition, the formed zinc complex Zinhbo-9/Zn exhibited attractive environmental sensing properties, giving emission at two drastically different wavelengths (i.e., 540 nm for protic and 770 nm for nonpolar environments). Use of the probe in cancer cells and stem cells further demonstrates its potential for biological applications. Since Zinhbo-9 possesses many desirable features that the current zinc sensors cannot match, the developed sensor could be of practical importance for future biological research.
Acknowledgments
Y.P. acknowledges the support of the Coleman endowment from the University of Akron.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/cbmi.3c00037.
Full experimental details, characterization data of all new compounds, spectroscopic assay conditions, and in vitro and in vivo experimental conditions (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lichtman J. W.; Conchello J. A. Nat. Methods 2005, 2, 910–19. 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- Loudet A.; Burgess K. Chem. Rev. 2007, 107, 4891–932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- Goncalves M. S. T Chem. Rev. 2009, 109, 190–212. 10.1021/cr0783840. [DOI] [PubMed] [Google Scholar]
- Boens N.; Leen V.; Dehaen W. Chem. Soc. Rev. 2012, 41, 1130–72. 10.1039/C1CS15132K. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Cheng T.; Zhu W.; Xu Y.; Qian X. Orgc Lett. 2011, 13, 264–7. 10.1021/ol102692p. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Lin W.; Zheng K.; He L.; Huang W. Chem. Soc. Rev. 2013, 42, 622–61. 10.1039/C2CS35313J. [DOI] [PubMed] [Google Scholar]
- Klohs J.; Wunder A.; Licha K. Basic Research in Cardiology 2008, 103, 144–51. 10.1007/s00395-008-0702-7. [DOI] [PubMed] [Google Scholar]
- Weissleder R. Nat. Biotechnol. 2001, 19, 316–7. 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- Frangioni J. V. Curr. Opin. Chem. Biol. 2003, 7, 626–34. 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hilderbrand S. A.; Weissleder R. Curr. Opin. Chem. Biol. 2010, 14, 71–9. 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Escobedo J. O.; Rusin O.; Lim S.; Strongin R. M. Curr. Opin. Chem. Biol. 2010, 14, 64–70. 10.1016/j.cbpa.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oushiki D.; Kojima H.; Terai T.; Arita M.; Hanaoka K.; Urano Y.; Nagano T. J. Am. Chem. Soc. 2010, 132, 2795–801. 10.1021/ja910090v. [DOI] [PubMed] [Google Scholar]
- Karton-Lifshin N.; Segal E.; Omer L.; Portnoy M.; Satchi-Fainaro R.; Shabat D. J. Am. Chem. Soc. 2011, 133, 10960–5. 10.1021/ja203145v. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Lin W. Y.; Zhao S.; Gao W. S.; Chen B.; He L. W.; Zhu S. S. J. Am. Chem. Soc. 2012, 134, 13510–23. 10.1021/ja305802v. [DOI] [PubMed] [Google Scholar]
- Hawrysz D. J.; Sevick-Muraca E. M. Neoplasia 2000, 2, 388–417. 10.1038/sj.neo.7900118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H.; Ogawa M.; Alford R.; Choyke P. L.; Urano Y. Chem. Rev. 2010, 110, 2620–40. 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X.; Song F.; Lu E.; Wang Y.; Zhou W.; Fan J.; Gao Y. J. Am. Chem. Soc. 2005, 127, 4170–1. 10.1021/ja043413z. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Achilefu S. Org. Lett. 2004, 6, 2067–70. 10.1021/ol049258a. [DOI] [PubMed] [Google Scholar]
- Tolosa L.; Nowaczyk K.; Lakowicz J.. An Introduction to Laser Spectroscopy, 2nd ed.; Kluwer: New York, 2002. [Google Scholar]
- Gonçalves M. S. T. Chem. Rev. 2009, 109, 190–212. 10.1021/cr0783840. [DOI] [PubMed] [Google Scholar]
- Sasaki E.; Kojima H.; Nishimatsu H.; Urano Y.; Kikuchi K.; Hirata Y.; Nagano T. J. Am. Chem. Soc. 2005, 127, 3684–5. 10.1021/ja042967z. [DOI] [PubMed] [Google Scholar]
- Kiyose K.; Kojima H.; Urano Y.; Nagano T. J. Am. Chem. Soc. 2006, 128, 6548–9. 10.1021/ja060399c. [DOI] [PubMed] [Google Scholar]
- Tang B.; Yu F.; Li P.; Tong L.; Duan X.; Xie T.; Wang X. J. Am. Chem. Soc. 2009, 131, 3016–23. 10.1021/ja809149g. [DOI] [PubMed] [Google Scholar]
- Weissleder R.; Ntziachristos V. Nat. Med. 2003, 9, 123–8. 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- Jiang P.; Guo Z. Coord. Chem. Rev. 2004, 248, 205–29. 10.1016/j.cct.2003.10.013. [DOI] [Google Scholar]
- Outten C. E.; O’Halloran T. V. Science 2001, 292, 2488–92. 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Finney L. A.; O’Halloran T. V. Science 2003, 300, 931–6. 10.1126/science.1085049. [DOI] [PubMed] [Google Scholar]
- Berg J. M.; Shi Y. Science 1996, 271, 1081–5. 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- Bush A. I.; Pettingell W. H.; Multhaup G.; Paradis M.; Vonsattel J.-P.; Gusella J. F.; Beyreuther K.; Masters C. L.; Tanzi R. E. Science 1994, 265, 1464–7. 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- Koh J. Y.; Suh S. W.; Gwag B. J.; He Y. Y.; Hsu C. Y.; Choi D. W. Science 1996, 272, 1013–6. 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J.; Hernandez M. D.; McGinty J. F. Brain. Res. 1989, 480, 317–21. 10.1016/0006-8993(89)90199-6. [DOI] [PubMed] [Google Scholar]
- Walker C. F.; Black R. E. Annu. Rev. Nutr. 2004, 24, 255–75. 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- Lee J.-Y.; Cole T. B.; Palmiter R. D.; Suh S. W.; Koh J. -Y Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 7705–10. 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. C.; Yoon J. Y.; Spring D. R. Chem. Soc. Rev. 2010, 39, 1996–2006. 10.1039/b916287a. [DOI] [PubMed] [Google Scholar]
- Nolan E. M.; Lippard S. J. Acc. Chem. Res. 2009, 42, 193–203. 10.1021/ar8001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P.; Sreejith S.; Ajayaghosh A. Chem.-Asian J. 2007, 2, 338–48. 10.1002/asia.200600370. [DOI] [PubMed] [Google Scholar]
- Pluth M. D.; Tomat E.; Lippard S. J. Annu. Rev. Biochem. 2011, 80, 333–355. 10.1146/annurev-biochem-061009-091643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Peng X.; Wu Y.; Ren H.; Sun J.; Tong S.; Liu T.; Zhao Y.; Wang S.; Tang C.; Chen L.; Chen Z. Angew. Chem., Int. Ed. 2021, 60, 25846–25855. 10.1002/anie.202109510. [DOI] [PubMed] [Google Scholar]
- Fang H.; Geng S.; Hao M.; Chen Q.; Liu M.; Liu C.; Tian Z.; Wang C.; Takebe T.; Guan J. L.; Chen Y.; Guo Z.; He W.; Diao J. Nat. Commun. 2021, 12, 109. 10.1038/s41467-020-20309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J.; Li J.-Z.; Mao X.-M.; Wang Q.; Li S.-P.; Wang C.-Y. Analyst 2021, 146, 3971–3976. 10.1039/D1AN00508A. [DOI] [PubMed] [Google Scholar]
- Liu R.; Kowada T.; Du Y.; Amagai Y.; Matsui T.; Inaba K.; Mizukami S. ACS Sens. 2022, 7, 748–757. 10.1021/acssensors.1c02153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F.; Chen Y.; Jiang Z.; He W.; Guo Z. Acc. Chem. Res. 2023, 56, 258–269. 10.1021/acs.accounts.2c00643. [DOI] [PubMed] [Google Scholar]
- You Y.; Lee S.; Kim T.; Ohkubo K.; Chae W.-S.; Fukuzumi S.; Jhon G.-J.; Nam W.; Lippard S. J. J. Am. Chem. Soc. 2011, 133, 18328–42. 10.1021/ja207163r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. P.; Young A. M.; Palmer A. E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. 10.1021/cr400546e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyose K.; Kojima H.; Urano Y.; Nagano T. J. Am. Chem. Soc. 2006, 128, 6548–9. 10.1021/ja060399c. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Liu Q.; Dou B.; Wright B.; Wang J.; Pang Y. Adv. Healthcare Mater. 2012, 1, 485–92. 10.1002/adhm.201200025. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Pang Y. Chem. Commun. 2010, 46, 4070–4072. 10.1039/c003230a. [DOI] [PubMed] [Google Scholar]
- Wang J. F.; Pang Y. RSC Adv. 2013, 3, 10208–12. 10.1039/c3ra41080c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. F.; Baumann H.; Bi X.; Shriver L. P.; Zhang Z.; Pang Y. Bioorganic Chemistry 2020, 96, 103585. 10.1016/j.bioorg.2020.103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D.; Haase H. BioMetals 2001, 14, 331–341. 10.1023/A:1012905406548. [DOI] [PubMed] [Google Scholar]
- Sharir H.; Zinger A.; Nevo A.; Sekler I.; Hershfinkel M. J. Biol. Chem. 2010, 285, 26097–26106. 10.1074/jbc.M110.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


