Abstract
Two-dimensional (2D) transition metal dichalcogenides (TMDs) have garnered widespread interest in the scientific community and industry for their exceptional physical and chemistry properties, and great potential for applications in diverse fields including (opto)electronics, electrocatalysis, and energy storage. Chemical vapor deposition (CVD) is one of the most compelling growth methods for the scalable growth of high-quality 2D TMDs. However, the conventional CVD process for synthesis of 2D TMDs still encounters significant challenges, primarily attributed to the high melting point of precursor powders, and achieving a uniform distribution of precursor atmosphere on the substrate to obtain controllable smaple domains is difficult. The spin-coating precursor mediated chemical vapor deposition (SCVD) strategy provides refinement over traditional methods by eliminating the use of solid precursors and ensuring a more clean and uniform distribution of the growth material on the substrate. Additionally, the SCVD process allows fine-tuning of material thickness and purity by manipulating solution composition, concentration, and the spin coating process. This Review presents a comprehensive summary of recent advances in controllable growth of 2D TMDs with a SCVD strategy. First, a series of various liquid precursors, additives, source supply methods, and substrate engineering strategies for preparing atomically thin TMDs by SCVD are introduced. Then, 2D TMDs heterostructures and novel doped TMDs fabricated through the SCVD method are discussed. Finally, the current challenges and perspectives to synthesize 2D TMDs using SCVD are discussed.
Keywords: Spin-coating, Chemical vapor deposition, Liquid precursor, Substrate, Two-dimensional materials, Heterostructure, Doping, Film
1. Introduction
The discovery of graphene1 marked a significant milestone in two-dimensional (2D) materials research. Since then, other 2D materials, including transition metal dichalcogenides (TMDs), with the formula MX2 (M: transition metal atom; X: chalcogen atom), have garnered considerable interest in various fields such as electronics, optoelectronics, magnetics, and catalysis research due to their ultrathin thickness, unique crystal structures, excellent physical and chemical properties.2−10 It is widely recognized that material preparation is a fundamental prerequisite for meeting the increasing demands of various applications. Therefore, the exploration of diverse synthesis methods for materials is crucial. Conventional mechanical exfoliation methods prove to be inadequate in fulfilling the demands for the precise construction of large-area thin films and heterostructures of 2D TMDs, rendering them unsuitable for practical applications.
Chemical vapor deposition (CVD) method stands out as a preferred method for the synthesis of 2D TMDs and their heterostructures due to relatively good controllability, high quality, and high yield of samples. However, controlling the gas phase distribution and reaction rate of the gas phase during the traditional CVD growth process presents a challenge. The nonuniform distribution of the precursor concentration on the substrate will directly affect the nucleation sites, resulting in undesirable nucleation and poor control of the nucleation density. Research indicates that incorporating additives into the CVD process can lower the melting point of solid precursors and enhance source delivery. For instance, the incorporation of salt additives with metal oxides has been utilized to form intermediate volatile compounds, which can reduce the reaction temperature and promote the reaction.11,12 However, this method introduces unwanted and unavoidable byproducts or impurities, leading to a notable reduction in the quality of ultrathin TMDs, which affects their stability and results in poor electrical and optical performance.13,14
To address this issue, an updated CVD synthesis technique termed spin-coating-mediated precursor chemical vapor deposition (SCVD) is gradually coming into view for the growth of TMDs and has made rapid progress in recent years. In SCVD, one or multiple suitable precursors are dissolved in solvents to form uniform mixed solutions serving as metal-containing sources. Subsequently, the precursor solutions are spin-coated onto the pretreated substrates, reacting with chalcogen to produce 2D TMDs. And the thickness of the synthesized 2D TMDs can be regulated by adjusting the concentration of solution and spin coating speed. As the synthesis process involves heating, the solutes gradually transform into a molten liquid state, facilitating more complete reactions with chalcogen feedings. Unlike the conventional CVD growth method where the positioning of substrates with precursors is crucial, SCVD method enables direct reaction and nucleation on the substrate, which eliminates the issues related to position-dependent substrates with precursors and reduces the formation of undesirable byproducts and additional contaminants during growth. It enables precise control of the precursor atmosphere, ensuring a clean and uniform distribution of the resulting sample on the substrate, thus achieving controllability and repeatability of material preparation. It is worth noting that SCVD technology is particularly advantageous for synthesizing large-area single-layer or multilayer TMD thin films. For example, the liquid precursor (NH4)6H2W12O40·x(H2O) (AMT) was reported to be deposited on Au to synthesize WS2 monolayers with domain sizes up to ∼420 μm.15 The wafer-scale MoS2 layers were obtained through thermolysis of a spin-coated (NH4)2MoS4 solution film.16 Furthermore, by regulating the tunable doping concentrations of various dopants in mixed solutions, the properties and functionalities of 2D TMDs can be engineered. Overall, TMDs grown by SCVD have the advantages of precise controllability of composition, thickness, and other key parameters.
Herein, we present the recent advancements in controllable SCVD growth of 2D TMDs. The first part focuses on the exploration of liquid precursors of SCVD, and introduces the different kinds of liquid precursors, additives, and new source supply methods, such as bubble method, dissolution–precipitation method, and monomer feeding method. The second part discusses the substrate engineering strategies including substrate choice, surface treatment, and patterning. It is worth noting that substrate engineering plays a crucial role in controlling the nucleation and growth of TMD films. The third part mainly introduces 2D TMDs heterostructures and the novel doped TMDs in the SCVD. Finally, we provide insights into the challenges and potential opportunities for fabricating and commercializing 2D TMDs using SCVD in the future.
2. SCVD Growth of 2D TMDs
Nowadays, the SCVD technique has demonstrated its superiority in synthesizing 2D TMDs with excellent reproducibility and homogeneity under mild conditions. In this section, our primary focus is on the liquid precursor categories, additives, and new source supply method in SCVD. And the implementation of effective substrate pretreatment strategies can significantly increase the size of the synthesized material, potentially extending it to the centimeter level even to the wafer-level films.
2.1. Liquid Precursors for SCVD
2.1.1. Importance of Liquid Precursors in Determining TMDs Quality
Utilizing liquid precursors in the SCVD method effectively overcomes the challenge of unstable nucleation that arises from the difficulty in controlling the evaporation of solid precursors in the CVD method. The selection of liquid-phase precursor is a critical factor in the success of SCVD. In addition, a lot of progress has been made in the growth of various 2D TMDs in monolayer and few-layer forms using SCVD.
It is worth summarizing that the precursors generally should (i) have a melting point lower or close to the growth temperature, (ii) be enabled to form stable melts in the reaction, and (iii) be soluble in the target solvent and become stable compounds in solutions. The first two conditions promote the growth of the target material, while the last condition facilitates the formation of a homogeneous solution mixture. In the literature, precursors such as (NH4)2MoS4,17 Na2MoO4,18 Na2WO4,19 NaReO4,20 and C4H4NNbO921 have been used for the SCVD growth of 2D TMDs. The possible chemical reactions in SCVD growth of 2D TMDs are illustrated in Table 1.
Table 1. Possible Chemical Reactions in SCVD Growth of 2D TMDs.
| Reactions | References | |
|---|---|---|
| MoS2 | (NH4)2MoO4 + 4S + 2KOH + H2O → MoO3 + 3H2S + K2SO4 + 2NH3 | (22) |
| 2MoO3 + 7S → 2MoS2 + 3SO2 | ||
| (NH4)6Mo7O24·4H2O → 6NH3 + 7H2O + 7MoO3 | (23) | |
| 2MoO3 + 7S → 2MoS2 + 3SO2 | ||
| MoSe2 | 2(NH4)2MoO4 → 2MoO3 + 4NH3 + 2H2O | (24) |
| 2MoO3 + 2KI → MoO2I2 + K2MoO4 | ||
| MoO2I2 + 4H2 + 3Se → MoSe2 + 2HI + 2H2O + H2Se | ||
| Na2MoO4 + 2Na2SeO3 + 3Se + 10H2 → MoSe2 + 3Na2Se + 10H2O | (20) | |
| WS2 | (NH4)10W12O41 → 10NH3 + 5H2O + 12WO3 | (25) |
| 2WO3 + 7S → 2WS2 + 3SO2 | ||
| MoTe2 | Na2MoO4 + 3Te + 3H2O → TeO2 + 2H2Te + MoO3 + 2NaOH | (26) |
| MoO3 + 2TeO2 + 7H2 → MoTe2 + 7H2O | ||
| WTe2 | Na2WO4 + 2Na2TeO3 + 3Te + 10H2 → WTe2 + 3Na2Te + 10H2O | (20) |
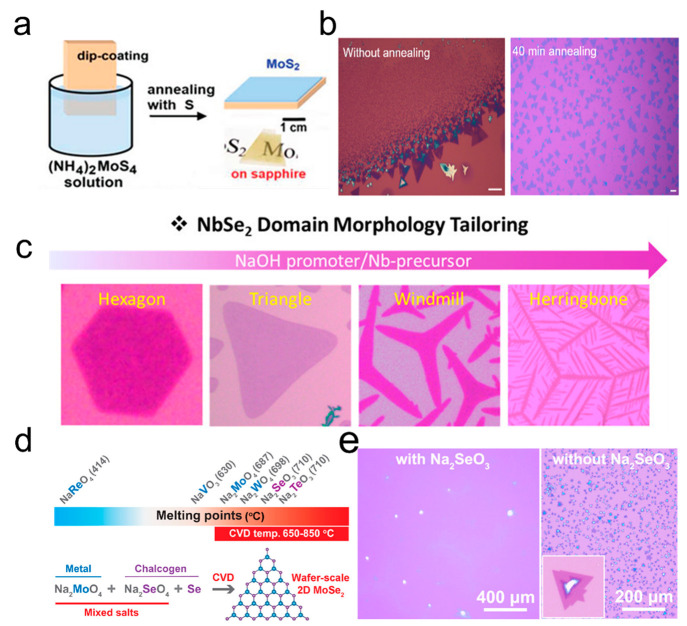
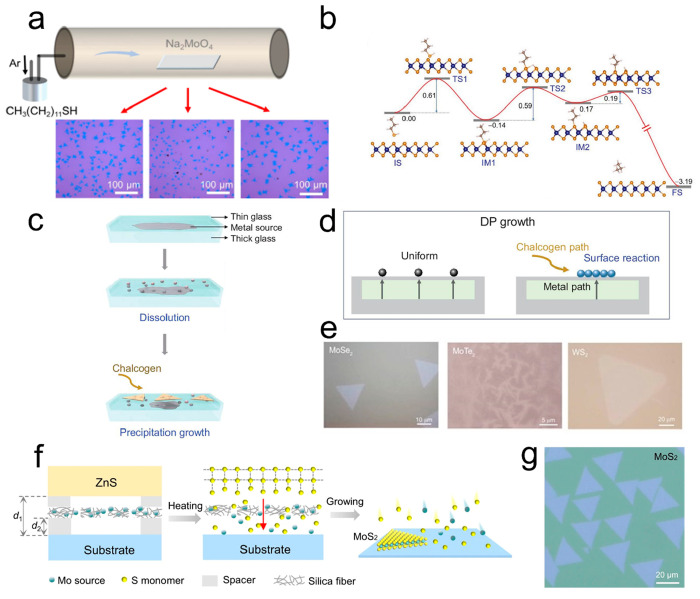
In 2012, (NH4)2MoS4 solution was dip-coated onto an insulating substrate and subsequently decomposed through high-temperature annealing, resulting in the production large-area MoS2 thin layers (Figure 1a).17 However, the dip-coating method frequently results in nonuniform distribution of precursor on the substrate, leading to the formation of multidomains with different layer numbers. To tackle this challenge, Liu et al. utilized a Na2WO4 solution as a precursor for spin coating and successfully synthesized monolayer WS2 crystals after annealing the precursor.18Figure 1b displays the OM images of a WS2 crystal with and without annealing. The precursor annealing process resulted in the redistribution of the molten liquid precursor, effectively preventing aggregation and promoting monolayer growth. Park et al. utilized mixed solutions of C4H4NNbO9·xH2O with different concentrations to construct the different morphologies of metallic NbSe2 from hexagonal, triangular, windmill-like, to herringbone-like (Figure 1c).21 The concentration of the Nb precursor gradually increases and surpasses that of the Se precursor. As a result, Se-terminated facets grow faster than Nb-terminated facets under Nb-rich conditions. Monolayer WTe2 and MoTe2 have also been successfully synthesized using (NH4)6H2W12O40·xH2O and (NH4)6Mo7O24·4H2O as precursors, respectively.27,28 Various precursors with the formula AxMOy (where A is an alkali metal or ammonium and M is a transition metal) have been employed in the growth of TMDs, such as Na2MoO4,19 Na2WO419 and (NH4)2MoO4.29
Figure 1.
Synthesis of TMDs with SCVD. (a) Schematic illustration of decomposing the dip-coating (NH4)2MoS4 to produce multilayer MoS2. Reproduced from ref (17). Copyright 2012 American Chemical Society. (b) OM image of WS2 crystal before and after annealing using Na2WO4 solution as a precursor; scale bar: 20 μm. Reproduced from ref (18). Copyright 2020 American Chemical Society. (c) Change in the morphology of NbSe2 at various NaOH/C4H4NNbO9·xH2O ratios. Reproduced from ref (21). Copyright 2020 American Chemical Society. (d) Melting points of several precursors and synthetic strategy of MoSe2 with mixed-salt precursors. (e) OM image of MoSe2 monolayer grown with or without adding Na2SeO3. The inset shows a typical MoSe2 nanoflake in an area of 10 × 10 μm2. Reproduced from ref (20). Copyright 2021 American Chemical Society.
On the other hand, the synthesis of high-quality 2D TMDs is also inseparable from the precise feeding of chalcogen. Similarly, liquid precursors can also be used as a source of chalcogen supply, such as Na2SeO3 and Na2TeO3. Taniguchi et al. proposed various groups of mixed precursors to synthesis transition metal selenides and tellurides. Figure 1d shows the melting points of several precursors. The 2D MoSe2, ReSe2, and WTe2 were successfully produced with Na2MoO4–Na2SeO3, NaReO4–Na2SeO3, and Na2WO4–Na2TeO3 as precursors, respectively.20 The OM images of the MoSe2 monolayer grown with or without the addition of Na2SeO3 are shown in Figure 1e. Despite the SCVD technique being a universal option for growing large groups of 2D TMDs, the limited suitable precursors degrade the versatility of this strategy. Notably, along with 2D TMDs, a group of transition metal carbides (TMNxs) including MoNx, WNx, CrNx, VNx, and transition metal carbides (TMCs) like MoC2 have been fabricated via SCVD technique,30,31 which suggests the potential diversification of the 2D material family. The more detailed information regarding synthesized 2D TMDs by SCVD is illustrated in Table 2.
Table 2. Summary of SCVD Synthesis of 2D TMDs.
| TMDs | Source materials | Phase | Morphology | Thickness | Temperature (°C) | Substrate | Carrier gas (sccm) | Growth time [min] |
|---|---|---|---|---|---|---|---|---|
| MoS2 | (NH4)2MoS4 + S32 | 2H | Nanosheet | 1.5–2 nm | 700 | SiO2/Si | 300Ar | 14 |
| Na2MoO4 + NaOH + S33 | 2H | Nanosheet | 2.07 nm | 800 | Sapphire | 2 | ||
| (NH4)2MoO4 + KOH + S22 | 2H | Film | 0.7 nm | 750 | Sapphire/SiO2/Si | 30Ar | 10 | |
| MoO3 + NH4OH + S34 | 2H | Nanosheet | Monolayer | 760–880 | Glass | 150Ar | 5 | |
| (NH4)6Mo7O24·4H2O + S35 | 2H | Film | 0.6–2.5 nm | 800 | SiO2/Si | 100Ar | 10 | |
| Na2MoO4·2H2O + C12H25SH36 | 2H | Nanosheet | Monolayer | 850 | SiO2/Si | 10Ar | 60 | |
| (NH4)2MoO4 + KI + S37 | 2H | Film | 1 nm | 720 | SiO2/Si | 30Ar | 3–5 | |
| (NH4)2MoS417 | 2H | Film | 2 nm | 1000 | Sapphire, SiO2/Si | 80%Ar/20%H2 | 30 | |
| (NH4)2MoO4 + S29 | 2H | Nanosheet | 0.78 nm | 750 | Sapphire | Ar | 20 | |
| (NH4)2MoS438 | 2H | Film | 2–30 nm | 700 | SiO2/Si | 96%Ar/4% H2 | 60 | |
| Na2MoO4·2H2O + (C2H5)2S39 | 2H | Film | 0.7 nm | 850 | Sapphire, SiO2/Si | 350Ar/10 H2 | 20 | |
| Na2MoO4 + Mo(CO)6 + CH3SSCH340 | 2H | Film | 0.9 nm | 850 | SiO2/Si | 350Ar/15H2 | 20 | |
| MoO3 + NH4OH + S41 | 2H | Film | 0.8 nm | 800 | SiO2/Si | 150Ar | 5 | |
| Na2MoO4 + S19 | 2H | Nanosheet | Monolayer | 730–750 | soda lime glass slide | 80Ar | 10–20 | |
| Na2MoO4 + ZnS42 | 2H | Nanosheet | Monolayer | 780 | Sapphire/SiO2/Si | 100Ar | 10–60 | |
| (NH4)2MoO4 + KI + S24 | 2H | Film | Monolayer | 800 | Sapphire | Ar | 10 | |
| MoSe2 | (NH4)2MoO4 + KI + Se24 | 2H | Film | Monolayer | 800 | sapphire | Ar/H2 | 10 |
| Na2MoO4 + Se19 | 2H | Nanosheet | Monolayer | 750–800 | soda lime glass slide | 80Ar/8 H2 | 10–20 | |
| Na2MoO4 + ZnSe42 | 2H | Nanosheet | Monolayer | 800 | Sapphire/SiO2/Si | 100Ar | 10–60 | |
| Na2MoO4 + Na2SeO320 | 2H | Nanosheet | Monolayer | 775–850 | SiO2/Si | 190Ar/10H2 | 5–10 | |
| WS2 | (NH4)10H2(W2O7)6 + S32 | 2H | Nanosheet | 1.5–2 nm | 700 | SiO2/Si | 300Ar | 14 |
| Na2WO4·H2O + N2H4 + (CH3)2S243 | 2H | Film | 0.65 nm | 850 | SiO2/Si | 350Ar | 30 | |
| Na2WO4 + S19 | 2H | Nanosheet | Monolayer | 730–780 | soda lime glass slide | 80Ar/4H2 | 10–20 | |
| Na2WO4 + ZnS42 | 2H | Nanosheet | Monolayer | 930 | Sapphire/SiO2/Si | 100Ar | 10–60 | |
| (NH4)2WO4 + KI + S24 | 2H | Film | Monolayer | 850 | sapphire | Ar/H2 | 10 | |
| WSe2 | Na2WO4 + ZnSe42 | 2H | Nanosheet | Monolayer | 820 | Sapphire/SiO2/Si | 100Ar | 10–60 |
| (NH4)2WO4 + KI + Se24 | 2H | Film | Monolayer | 850 | sapphire | Ar/H2 | 10 | |
| MoTe2 | Na2MoO4 + Te19 | 1T’ | Nanosheet | 730–750 | soda lime glass slide | 120Ar/20H2; | 10–20 | |
| (NH4)6Mo7O24·4H2O + C24H39NaO5 + Te27 | 1T’ | Nanosheet | 0.8 nm | 700/500 | SiO2/Si | 400N2/25H2; | 5 | |
| Na2MoO4 + ZnTe42 | 2H | Nanosheet | Monolayer | 750–800 | Sapphire/SiO2/Si | 100Ar | 10–60 | |
| Na2MoO4 + ZnTe42 | 1T’ | Nanosheet | Monolayer | 800 | Sapphire/SiO2/Si | 100Ar | 10–60 | |
| (NH4)6Mo7O24 + NaOH + Te44 | 1T’ | Nanosheet | 0.82 ± 0.04 nm | 730 | SiO2/Si | 100Ar/3H2 | 15 | |
| WTe2 | Na2WO4 + ZnTe42 | 1T’ | Nanosheet | Monolayer | 780 | Sapphire/SiO2/Si | 100Ar | 10–60 |
| (NH4)6H2W12O40 + Te28 | 1T’ | Nanosheet | 0.8 nm | 650 | SiO2/Si | N2/H2 | 6 | |
| Na2WO4 + Na2TeO320 | 1T’ | Nanosheet | 2.1 nm | 775–825 | sapphire | 95Ar/5H2 | 5 | |
| NbSe2 | C4H4NNbO9·xH2O + NaOH + Se21 | 2H | Nanosheet | 0.7 nm | 800 | SiO2/Si | 600N2/15H2 | 20 |
| ReSe2 | NaReO4 + Na2SeO320 | 1T’ | Nanosheet | 750 | sapphire | 190Ar/10H2 | 5–10 |
2.1.2. Overview of Additives Used in SCVD Growth
Many research results indicate that the addition of additives can significantly improve the interaction between the precursor vapor and substrate during the growth of materials.11,45−47 Here, the additives used during SCVD growth are divided into inorganic and organic compounds for further discussion and analysis.
In recent years, researchers have discovered that inorganic additives play multifaceted roles in SCVD growth, including growth promotion, acting as reducing agents, and acting as solvents. Notably, additives such as KI and NaOH have been widely studied.
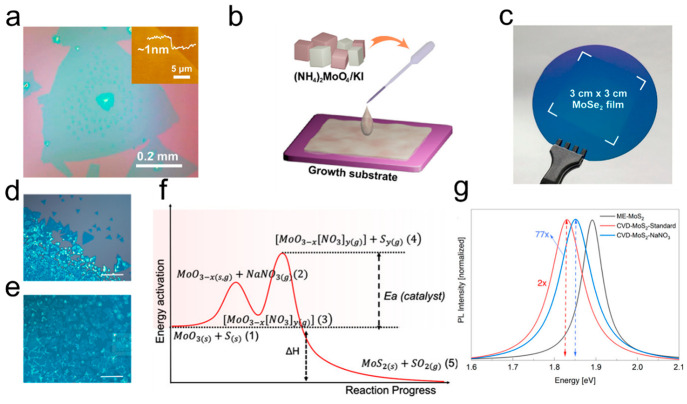
For example, Kong et al. reported that through introducing KI into the (NH4)2MoO4 solution, the domain size of synthesized MoS2 monolayers could reach up to approximately 0.62 mm from vertex to vertex (Figure 2a),37 which demonstrates the effectiveness of KI as an additive in promoting the growth of large-area monolayer MoS2. Kim et al. reported the synthesis of large-area monolayer MoSe2 with good uniformity on 3 cm × 3 cm sapphire substrate via KI promoter assisted as shown in Figure 2b,c.24 Further research indicates that KI not only facilitates the formation of reactive transition metal oxyhalide, reducing the energy barrier of chalcogenization, but also promotes the growth along the in-plane direction. The addition of KI is also suitable for other TMDs families, including MoS2, WS2, and WSe2. Similarly, centimeter scale Nb1–xWxSe2 monolayers have been reported with the assistance of promoter KI, allowing for tunable composition to achieve continuously adjustable bandgaps.48 In another study, Zhao et al. added NaCl salt into (NH4)6Mo7O24 (AHM)) solution to achieve rapid growth of large-area monolayer MoSe2 films.49 Nguyen et al. found that the presence of NaNO3 suppressed nucleation and subsequently increased crystal size significantly. Figure 2d,e shows OM images of MoS2 nanoflakes with and without NaNO3 catalyst.50 As illustrated in Figure 2f, NaNO3 reacts with MoO3–x to form a metal oxynitrate [MoO3–x[NO3]y], which has a melting point lower than that of the metal oxide, reducing the overall activation energy. The formation of the metal oxynitrate affected the reaction kinetics and expedited the reaction equilibrium, resulting in an increase in the density and size of the MoS2 monolayers. However, the corresponding PL intensity is significantly reduced due to the presence of excess residues (Figure 2g).
Figure 2.
Synthesis of MoS2 using inorganic additives. (a) OM image of MoS2 crystal with introducing KI into the (NH4)2MoO4 solution precursor, inset is the atomic force microscopy with height of 1 nm. Reproduced from ref (37). Copyright 2021 American Association for the Advancement of Science (AAAS). (b,c) Schematic illustration of mixing (NH4)2MoO4/KI liquid precursors (b) to synthesize centimeter-scale monolayer MoSe2 film (c). Reproduced from ref (24). Copyright 2021 American Chemical Society. (d,e) OM image of synthesized MoS2 monolayers without and with the addition of NaNO3 catalyst. Scale bars: 20 μm. (f) The reaction mechanism of synthesis of MoS2 monolayers with the addition of NaNO3. (g) Photoluminescence spectra of MoS2 obtained by mechanical exfoliation and grown by SCVD. Reproduced from ref (50). Copyright 2022 American Chemical Society.
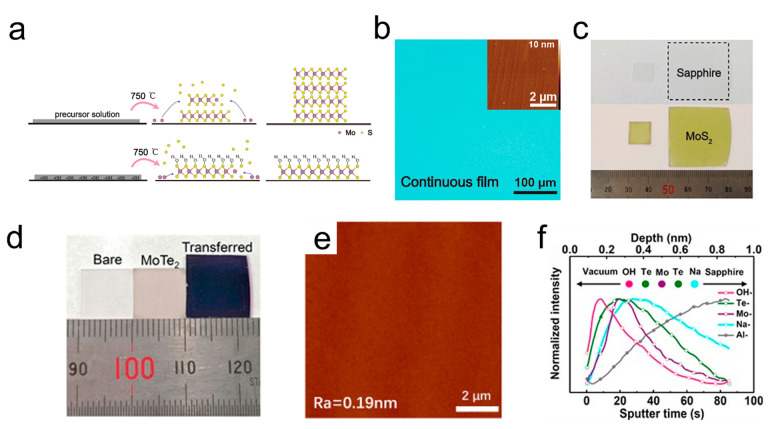
Besides, NaOH(KOH) is also commonly used as an additive because it could improve the uniform distribution of precursors on the substrate, which is important especially in growing centimeter-scale uniform monolayer TMDs films.33,51 Zhu et al. brought KOH (or NaOH) to the liquid-phase precursor to introduce −OH groups.22 As illustrated in Figure 3a, −OH groups in solution aimed at attaching to the (001) surface of MoS2 to form an S–Mo–S-OH bilayer structure during the growth process, which leads to preferential growth of MoS2 layers in the lateral direction instead of in the vertical direction, further promoting the generation of large area monolayer MoS2. The OM and AFM images in Figure 3b verified the uniform atomically flat MoS2 film with a surface roughness of about 0.12 nm. Figure 3c shows representative uniform MoS2 monolayers grown with 100% coverage on 1 × 1 and 3 × 3 cm2 sapphire. Moreover, the test of devices fabricated from the obtained MoS2 films suggested that the −OH layer on the monolayer MoS2 surface could effectively suppress oxidation of MoS2 in air, resulting in the highest carrier mobility up to 30 cm2 V–1 s–1 in air. Hu et a reported a method for preparing nonfilter light storage transistors using a hydrophilic monolayer molybdenum disulfide film with strong charge trapping capability.52 The hydroxyl groups covalently bonded with MoS2 possess strong charge trapping capabilities, which can control the capture and release of electrons by laser and electric operation, thus achieving stable image memory functions, and promoting the development of artificial visual systems in the future. Furthermore, 1 × 1 cm2 MoTe2 monolayer can be grown using NaOH as additive (Figure 3d).26 The spin-coated precursors, which can be dehydrated into immobilized particles during the reaction, were consumed continually to provide a source for the growth of the monolayer. The small roughness of 0.19 nm calculated from the AFM image in Figure 3e indicates the smoothness of the fabricated monolayer. The time-of-flight secondary ion mass spectrometry (ToF-SIMS) data in Figure 3f showed that −OH groups could be adsorbed on the surface of the MoTe2 layer, which can inhibit the growth of MoTe2 in the vertical direction. Ammonium salts have also been proved to be a clean and nondestructive additives for the synthesis of MoS2.53 Overall, the novel additives in the SCVD technique provide a new opportunity for the growth of inch-sized monolayer TMDs films, moving us closer to the realization of inch-scale functional nanomaterials.
Figure 3.
Synthesis of TMDs films using inorganic additives. (a) Schematic mechanism of MoS2 growth process with −OH or not. (b) OM image of a continuous MoS2 film grown on sapphire. The inset is an AFM image showing the atomically flat surface. (c) Photographs of 1 × 1 and 3 × 3 cm2 MoS2 monolayer films on sapphire. Reproduced from ref (22). Copyright 2019 American Chemical Society. (d) Photograph of 1 cm × 1 cm bare sapphire, as-grown MoTe2 monolayer on sapphire, and transferred MoTe2 monolayer on SiO2/Si substrate. (e) Typical AFM image of monolayer MoTe2 with small roughness (Ra = 0.19 nm). (f) ToF-SIMS depth profile of the MoTe2–OH sample. Reproduced from ref (26). Copyright 2021 American Chemical Society.
Inorganic additives in the SCVD process for TMDs are multifaceted, acting not just as growth promoters but also as reducing and solubilizing agents. For example, Kim et al. incorporated hydrazine (N2H4) as a strong reducing agent into an aqueous Na2WO4 solution to prereduce Na2WO4 and facilitate the synthesis of WS2.43 The introduction of hydrazine into the reaction provides regulation over the synthesis, yielding a notable enhancement in the quality of the WS2 product. The reduction of sodium tungstate by hydrazine generates enough volatile tungsten suboxides to directly participate in the growth of WS2, avoiding the etching caused by H2 reduction, thus ensuring the attainment of full coverage of the WS2 film. Another method of forming a precursor solution is dissolving the oxide in an alkaline solvent.34,41 Lee et al. reported the synthesis of MoS2 monolayer with a size of up to 500 μm using the preprocessed solution composed of MoO3 and NH4OH.41 However, the oxides dissolved in alkaline solvent as precursors are somewhat more complex in nature. Clearly, the role of inorganic additives during the SCVD growth of TMDs is versatile and extends beyond a singular function, underscoring their critical importance in tailoring and refining the quality and dimensions of the resulting materials.
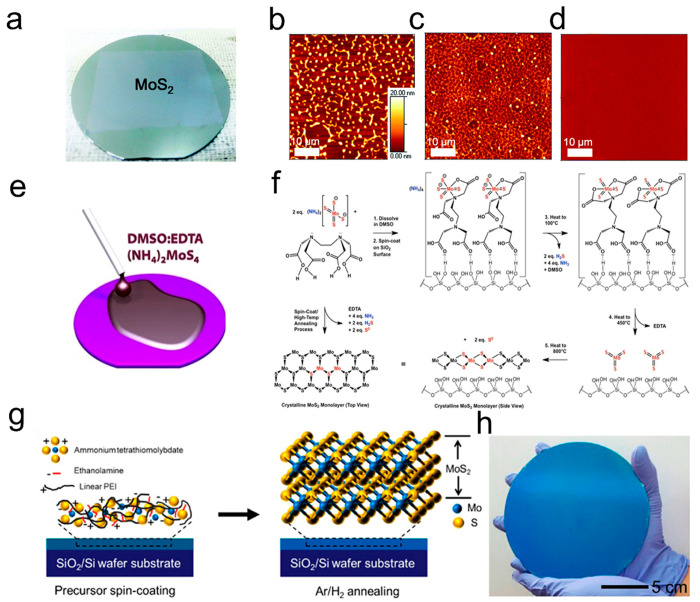
Furthermore, organic additives such as dimethylformamide (DMF),54,55 ethylene glycol,56 and n-methylpyrollidone (NMP)16 could be also used as effective agents for dissolving poorly water-soluble precursors. Theses precursors and the organic solvents can chemically bond to the precursor to form a stable solute in the mixture solution. Kim et al. utilized (NH4)2MoS4-DMF solution with additional amine- and amino alcohol-based solvents (n-butylamine and 2-aminoethanol) to synthesize 2 in. wafer-scale MoS2 thin films (Figure 4a).54 As shown in Figure 4b–d, the spin-coated precursor film exhibits dewetted regions and pinholes when only DMF was used as solvent. After adding 2-aminoethanol to DMF, thiomolybdate clusters formed with a hydrogen bond with a hydroxide group, which enhanced the solubility of (NH4)2MoS4. However, microsize defects still existed in this case. With the further addition of n-butylamine again, a defect-free spin-coating precursor film was finally obtained, and the 2 in. wafer-scale MoS2 thin films were obtained in the following thermal process. Ozkan et al. used ethylenediaminetetraacetic acid (EDTA) and dimethyl sulfoxide (DMSO) as a solvent that dissolved (NH4)2MoS4 to fabricate MoS2 films (Figure 4e).57Figure 4f illustrates the mechanism for the synthesis of MoS2 crystals through this strategy. Tetrathiomolybdate coordinates with EDTA, where the presence of both coordinated and uncoordinated carboxylic groups could enhance the interaction with the silanol-functionalized silica surface. Therefore, with EDTA and DMSO, the precursor solution can have superior wettability on the hydroxylated surface. Besides, Jeong et al. designed a precursor-polymer complex film consisting of anhydrous ammonium tetrathiopolybdate (ATM) precursor and linear polyimide (L-PEI) (Figure 4g,h),38 which facilitates uniform coating of precursor on substrate over large areas and LPEI fully decomposes at 400 °C without residual carbon. From the above examples, it is evident that the utilization of organic additives can enhance the interaction of the precursors in the solution and enhance the wettability of the precursor on the substrate, thereby contributing to the synthesis of wafer-level TMDs film. However, while some organic additives can be decomposed without leaving residue, most of them still remain as contaminants and potentially compromise the quality of the crystal.
Figure 4.
Synthesis of MoS2 films using organic additives. (a) Optical image of a 2 in.-sized MoS2 wafer thin film from SCVD using (NH4)2MoS4-DMF solution with additional amine- and amino alcohol-based solvents (n-butylamine and 2-aminoethanol). (b–d) AFM images of the different spin-coated precursor films with additive of (b) only DMF, (c) DMF:2-aminoethanol, and (d) DMF:n-butylamine:2-aminoethanol. Reproduced from ref (54). Copyright 2015 Royal Society of Chemistry. (e) Schematic of wet behavior of solution of (NH4)2MoS4-EDTA-DMSO. (f) Mechanism for the synthesis of MoS2 crystal. Reproduced from ref (57). Copyright 2017 Springer Nature. (g) Schematic illustration of the formation of MoS2 thin film from the anhydrous ammonium tetrathiopolybdate (ATM) precursor and linear polyimide (L-PEI) complex film. (h) Image of a 6 in.-sized MoS2 thin film synthesized through the method illustrate in (g). Reproduced from ref (38). Copyright 2017 American Chemical Society.
In summary, the strategic incorporation of the appropriate additives into precursor solutions in the SCVD process is remarkably beneficial. These additives can lower the melting point of the precursor, increase the vapor pressure, enable the formation of intermediates, reduce the activation energy, and increase the wettability of the precursor solution on substrate. Collectively, these effects synergistically contribute to the efficient synthesis of 2D TMDs.
2.1.3. Recent Advances in Developing the Source Supply Method
Not only will the metal or chalcogen source itself affect the material preparation but also the source supply method can affect the growth process. In recent years, new developed source supply methods such as bubble method, dissolution–precipitation method and monomer feeding method have introduced advanced capabilities in SCVD growth.19,36,42,58 The supply of CH3SSCH3 and C12H25SH precursor have been precisely controlled by bubble method during SCVD growth.36,40 As Figure 5a shows, Liu et al. utilized bubbling suppling C12H25SH as a continuous sulfur sources to react with Na2MoO4 during the synthesis of 2D MoS2.36 The synthesized MoS2 crystals possess the lower density of sulfur vacancies (0.32 nm–2) and the quality of that can even be close to that of exfoliated MoS2. As simulated in Figure 5b, this is because thiol molecules could be chemically absorbed into the S vacancy to repair these defects in MoS2. It offers a new idea to repair defects and decrease vacancies density in TMDs. Liu et al. reported the growth of uniform monolayer TMDs and their monolayer alloys, such as MoSe2, WS2, MoTe2, and MoxW1–xS2, using the dissolution–precipitation (DP) method (Figure 5c–e).19 The embedded metal source melted and diffused through the molten glass to its surface and reacted with chalcogen sources to attain TMDs. The soda lime glass acts as both a spin-coated substrate and metal source transferred layer, which achieves a uniform feed of the metal source while eliminating unwanted gas-phase reaction via independent diffusion path of the metal and chalcogen sources. On account of the inhibition of secondary nucleation by the DP method, the synthesized MoS2 monolayers appear cleaner. Another strategy is to introduce an active chalcogen monomer supply.42 As shown in Figure 5f (left panel), Zuo et al. put forward a stacked sandwiched model of ZnS, Na2MoO4 coated silica fiber fabric, mica spacer, and the substrate. When heated to growth temperature, the released S monomers from the ZnS and vaporized Na2MoO4 passed through the fiber fabric to form monolayer MoS2 on the substrate (Figure 5f, right panel). Figure 5g shows the OM image of as-grown MoS2. The density of sulfur vacancy defects in MoS2 were measured as ∼2 × 1012 cm–2 from STEM characterizations, which is among the lowest value of as-grown monolayer TMDs in previous literatures. Subsequently, a series of TMDs and alloys were synthesized in the same way by changing the transition metal sources (e.g., Na2MoO4 and Na2WO4) and chalcogenide plates (e.g., ZnS, ZnSe, and ZnTe). Moreover, Liu et al. produced a new bright spot in nonlinear optical fibers.59
Figure 5.
TMDs synthesized through new resource supply. (a) Schematic image of the bubbling setup and OM images of grown MoS2 monolayers through the bubble method. (b) Simulation of in situ repairing process by thiol molecules. Reproduced from ref (36). Copyright 2020 John Wiley and Sons. (c) Illustration of the DP growth process. (d) Detail of DP growth during nucleation. (e) OM images of synthesized MoSe2, MoTe2, and WS2 nanosheets through the DP method, respectively. Reproduced from ref (19). Copyright 2021 Oxford University Press. (f) Schematic model of sulfur (S) monomer supply for the growth of MoS2. (g) OM image of the as-grown MoS2 monolayers through monomer feeding method. Reproduced from ref (42). Copyright 2022 Springer Nature.
The development of new source supply methods overcomes the uncontrollable nature of vaporization of solid metal and chalcogen precursors and therefore ensures the constant delivery of precursor vapors to the substrate surface. This steady delivery is essential for achieving uniformity and high quality in the synthesis of materials.
2.2. Substrate Engineering Strategies in SCVD Growth
The substrate plays a crucial role in the adsorption of source materials, promoting nucleation, and stimulating the epitaxial growth of nucleus during synthesis of 2D TMDs.60 In the SCVD technique, various substrate engineering strategies have been developed to control nucleation, enhance growth, and improve the film quality. These strategies include careful selection of the substrate material, surface treatment, and substrate patterning.
2.2.1. Substrate Selection
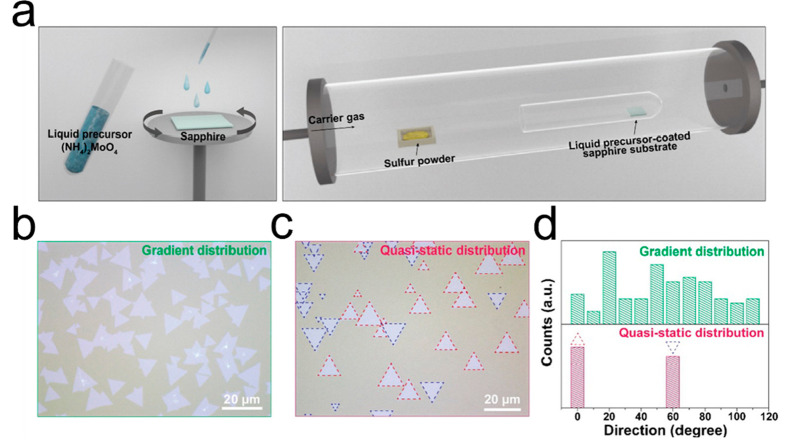
Commonly used substrates in the SCVD technique include SiO2/Si, sapphire, glass, and quartz. Given the requirements of SCVD growth, SiO2/Si and sapphire stand out as the preferred substrate materials, meeting the specific requirements of growth of SCVD. The use of SiO2/Si as a substrate for large-scale SCVD growth facilitates subsequent nanopatterning and integration and is highly compatible with metal-oxide-semiconductor (CMOS) technology. However, due to the amorphous surface of the SiO2/Si substrate, the TMDs films grown on the SiO2/Si are mostly polycrystalline with randomly oriented grains. The C-plane sapphire substrate, characterized by lattice symmetry that matches well with TMDs, proves to be more conducive for the growth of wafer-scale single crystal TMDs. Park group regulated chalcogen vapor pressure to guide MoS2 seeds to grow along the preferred orientation of the lattice structure of the sapphire substrate, resulting in the well-aligned MoS2 crystals (Figure 6a).29 As shown in Figure 6b–d, the chalcogen atoms are inclined to low potential energy sites guided by the sapphire lattice structure, enabling the alignment of TMDs under an orientation of 0°/60°. Numerous studies61−63 have demonstrated the crucial influence of substrates on the crystal orientation. It is expected to further investigate the impact of substrates on the orientation of TMDs in the SCVD technique.
Figure 6.
Well-aligned MoS2 crystals on sapphire substrate. (a) Schematic illustration of controlling the chalcogen vapor pressure to grow MoS2 on a sapphire substrate. (b–d) OM images (b,c) and the orientation histogram (d) of as-grown MoS2 under gradient and quasi-static distributions of chalcogen vapor pressure. Reproduced from ref (29). Copyright 2021 American Chemical Society.
2.2.2. Substrate Pretreatment Strategy
To ensure uniform deposition of precursors, substrates are typically pretreated using simple methods such as O2 plasma,18,42,64 piranha solution,17,65 alkaline solution39,40 and UV–O366−68 to enhance hydrophilicity. These treatments improve the surface properties of the substrate and promote the adsorption of source materials, resulting in improved nucleation and growth of 2D TMDs.
For example, Figure 7 presents the OM of water contact angles on the SiO2 surface and corresponding schematic illustration of the mechanism before and after O2 plasma treatment.64 After the O2 plasma treatment, the SiO2 surface becomes more hydrophilic. Oxygen plasma removes organic contaminants and breaks siloxane group (Si–O–Si) through chemical reactions with oxygen radicals, thus promoting the formation of Si–OH. That is, SiO2 surface treated by oxygen plasma could form hydrogen bond with water molecules.69 In particular, the water contact angles of the treated SiO2 surface are 11.4°, which is lower than the water contact angle of the untreated SiO2 surface of 45.2°, indicating an enhanced surface energy of the substrate. It is noteworthy that O2 plasma treatment can not only eliminate natural contaminants on the surface of SiO2, but also increase the surface energy, thereby improving the hydrophilicity. Moreover, Sodium cholate and iodixanol have been reported to function as a surfactant for increasing the wettability and viscosity of the aqueous precursor on the SiO2/Si substrate.21,70
Figure 7.
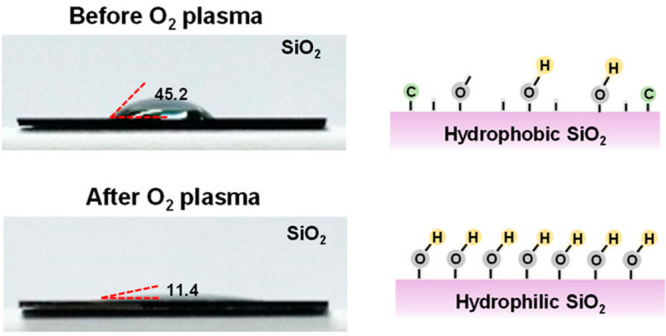
Optical images of the water contact angle on the SiO2 surface and corresponding schematic illustrations before and after O2 plasma treatment, respectively. Reproduced from ref (64). Copyright 2021 American Chemical Society.
2.2.3. Substrate Patterning
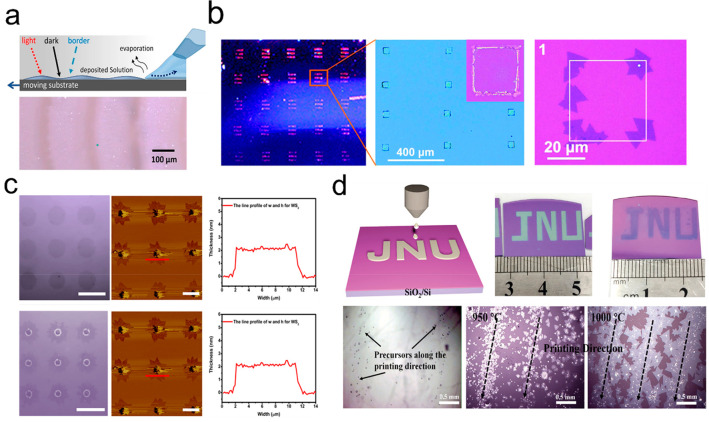
Precise patterned growth is crucial for the integration of functional materials into circuit devices but is difficult for the TMDs monolayers grown using typical CVD method due to the uncontrollable random nucleation seeds. While there have been reports of successful growth of patterned TMDs using predesigned Au, Cr, and Pt–Ti seeds, the complete removal of residual seeds remains exceedingly difficult, thereby limiting further research in this area.71−73 People utilize micropipette tip, photolithography, and inkjet printing to pattern the substrate in the SCVD technique, enabling seed-free synthetic patterned TMDs. As is shown in Figure 8a, the strip patterned MoS2 layers were obtained by dragging a precursor solution droplet via micropipette tip.74 The droplet, dragged with variable velocities, induces an asymmetric shape as it moves across the substrate, resulting in spatial material deposition. It is well-known that lithography is an available patterning method.51,64,75Figure 8b shows arrays of MoS2 nanoflakes grown from patterned Na2MoO4 squares. After arrays of square windows was fabricated on SiO2/Si substrate by photolithography, the molybdenum-containing seed material was spin-coated with Na2MoO4 aqueous solution.75 The seeds accumulated at the edges of the patterned squares to form MoS2 nanoflakes. However, it is obviously observed that the arrays of MoS2 are not regular. Another mask-free approach seems simpler compared with photolithography. It is utilized to deposit arrays of patterns on preselected locations via the direct write patterning (DWP) technique.32Figure 8c shows the representative images of grown patterned MoS2 and WS2 nanoflakes with a domain size of 8–10 μm and a thickness of average 2 nm. As for inkjet printing, it is supposed to be a desirable patterning way. The corresponding printed patterns in centimeter size like the word “JNU” were displayed in Figure 8d. When printed aqueous precursors along parallel direction began to melt, the inkjet-printed small nucleation centers appeared moving, coalescing, and merging with each other under maintaining the initial printing patterns.23 However, further research is still needed to explore how to achieve high-precision control of precursors, enabling the preparation of single crystal patterns with high quality and industrial prospects.
Figure 8.
Synthesis of patterned TMDs via patterned substrate. (a) Schematic illustration and OM image of patterned MoS2 layers by dragging a precursor solution droplet via micropipette tip. Reproduced from ref (74). Copyright 2021 Springer Nature. (b) Optical image of arrays of MoS2 nanoflakes grown from patterned Na2MoO4 squares. Reproduced from ref (75). Copyright 2019 Royal Society of Chemistry. (c) OM and the corresponding atomic force microscope (AFM) images of patterned MoS2 (upper) and WS2 (bottom) nanoflakes via the DWP. Reproduced from ref (32). Copyright 2019 IOP Publishing Ltd. (d) Schematic model and OM images of monolayer “JNU” MoS2 based on patterned aqueous precursors (upper) and the OM images of as-grown TMDs with different growth temperature along the printed aqueous precursors direction on the transparent glass substrate (bottom) via inkjet printing. Reproduced from ref (23). Copyright 2021 John Wiley and Sons.
Currently, the growth dynamics of 2D TMDs on substrates, particularly nucleation and growth processes aiming at wafer scale production of 2D TMDs, still need being explored. With the appropriate selection of substrates, modification of surface properties, or the utilization of prepatterned substrates, it is expected to gain more control over the nucleation and growth of 2D TMDs, ultimately enhancing film quality and achieving desired material properties.
3. Novel Structure of TMDs Obtained by SCVD
3.1. TMDs-Based Heterostructures
Combining different atomically thin transition metal dichalcogenides (TMDs) into vertically stacked or seamlessly connected planar structures can form van der Waals heterostructures (vdWHs) or lateral heterostructures. These heterostructures have significantly expanded the range of available material systems and provided a reliable platform for exploring novel physical and chemical properties, greatly broadening the application prospects of 2D TMDs. In recent developments, the SCVD growth method has been successfully applied in producing TMD heterostructures with large crystal sizes and clean interfaces.
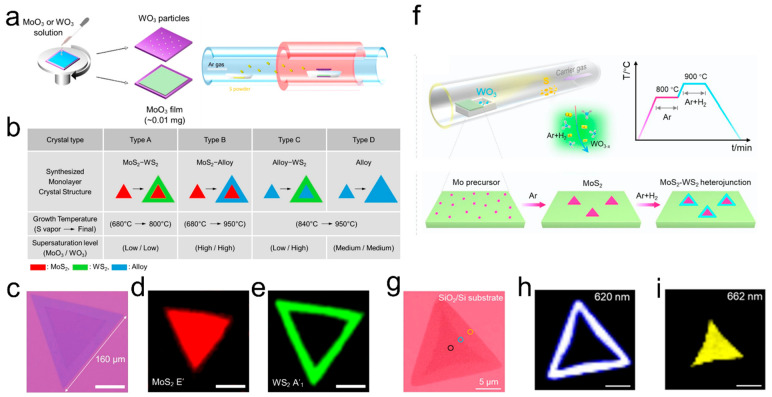
Several studies have been reported on the successful synthesis of high-quality MoS2–WS2 lateral heterostructures using the SCVD method.76−78 Kim et al. reported the preparation of various lateral heterostructures and alloys (WS2–MoS2, MoS2-alloy, alloy-WS2) (Figure 9a,b) by precisely adjusting growth parameters including the subsequent growth temperature, and the relative supersaturation level of MoO3 and WO3 precursors.76 The MoO3 and WO3 precursor solutions were prepared by dissolving MoO3 and WO3 powders in NH4OH. Following regular spin- coating treatment, the substrates containing MoO3 film and WO3 particles were positioned at the bottom and top of the crucible in the furnace, respectively (Figure 9a). Four types of as-grown monolayer lateral structures can be obtained: MoS2/WS2 heterostructures (type A), MoS2/alloy heterostructures (type B), alloy/WS2 heterostructures (type C), and alloy monolayer structures (type D) (Figure 9b). At low growth temperatures (∼680 °C), due to the relatively high vaporization temperature of WO3, there is almost no W precursor arrive the substrate coated with MoO3, resulting in the formation of a single MoS2 monolayer inside (referred to as type A and B). Upon heating to ∼840 °C, both MoO3 and WO3 simultaneously vaporize and react with gaseous sulfur, leading to the formation of alloy-type monolayers in the core (referred to as types C and D). Then at the second growth step, at a low growth temperature of about 800 °C, WO3 vaporize while MoO3 do not, MoS2/WS2 lateral heterostructure with a clean interface (referred to as type A) can be synthesized. At higher growth temperatures of about 950 °C for the second growth step, alloy often appears at the interface of lateral heterostructure (referred to as type B and D) because MoO3 violently vaporizes at high temperatures and coexists with the WO3 precursor in the gas phase. Until MoO3 is completely consumed, WS2 will grow from the lateral side of the alloy monolayer, and a type C heterostructure can be formed. Figure 9c−e shows the OM images and the corresponding Raman mappings of the well-crystallized in-plane heterostructure consisting of MoS2 monolayer inside and WS2 outside. In another example, it is reported by Xu et al. that through H2-triggered reaction, MoS2–WS2 lateral heterostructures with an ultraclean and defect-free narrow interface were synthesized.77Figure 9f schematically shows the two stages of the growth process. On the first stage, the MoS2 monolayers were grown at 800 °C under pure Ar flow. Once exhausting Mo precursor completely, the H2 was then introduced to trigger second growth of WS2 at 900 °C. In fact, the sequential growth of MoS2 and WS2 is due to the different reaction conditions for the Mo-based precursor and the W-based precursor. Without H2, only MoS2 can grow on substrate. Under the presence of H2, conversion of WO3 to low-volatile W-based precursors happened and WS2 can grow from the side of MoS2. The typical OM image of the lateral MoS2–WS2 is shown in Figure 9f. The associated photoluminescence (PL) intensity mappings at 620 and 662 nm verified well the spatial distribution of MoS2 and WS2 domains (Figure 9g,h).
Figure 9.
Synthesis of MoS2–WS2 lateral heterostructure with the SCVD strategy. (a) Growth process diagram of MoS2–WS2 lateral heterostructures and alloys. (b) Summary table of conditions for each monolayer structure. (c-e) OM image of a large sized typical MoS2–WS2 lateral heterostructure and the corresponding Raman mapping images of the E′ mode of MoS2 and the A′1 mode of WS2. Scale bar: 50 μm. Reproduced from ref (76). Copyright 2019 American Chemical Society. (f) Schematic diagram of MoS2–WS2 lateral heterostructure synthesized with hydrogen-triggered reaction. (g–i) OM image of MoS2–WS2 lateral heterostructure on SiO2/Si substrate and the corresponding PL intensity mapping at 620 and 662 nm. Scale bar: 5 μm. Reproduced from ref (77). Copyright 2021 American Chemical Society.
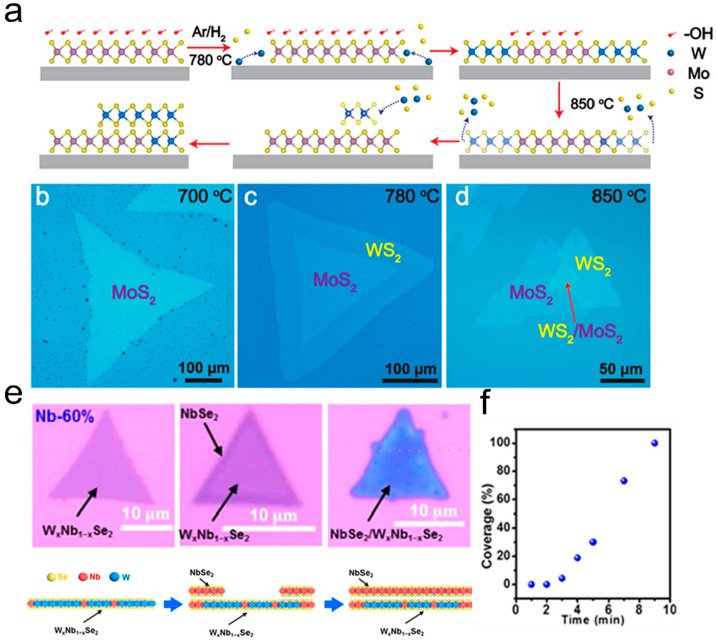
Changing the synthesis conditions to realize the sequential growth of varied materials is an important idea for synthesizing high quality vertical heterostructure with the SCVD method. Zhu et al. realized the optional growth of large WS2/MoS2 vertical heterostructures via hydroxide-assisted nucleation.79 The agents KOH, (NH4)2MoO4, and Na2WO4 were dissolved together in deionized water to obtain an aqueous solution containing −OH groups. Subsequently, the solution was spin-coated onto a clean sapphire substrate and then went through a three-stage growth process with sulfur atmospheres, resulting in vertical growth of WS2/MoS2. The specific reaction process and mechanism are described in Figure 10a. During the first stage, a large MoS2–OH bilayer was obtained in an Ar + S atmosphere of about 700 °C. Figure 10b shows the OM image of a MoS2–OH triangular domain with a length of about 600 μm. In the second stage, as the growth temperature was raised to 780 °C in the S + Ar/H2 mixed atmosphere, the growth of WS2 was activated, promoting the lateral epitaxy of WS2 on the MoS2 edges under the protection of the −OH groups protection. Figure 10c presents the as-obtained WS2–MoS2 lateral heterostructures with a large lateral size of 700 μm. Finally, in the third stage, as the temperature continued to rise to 850 °C, the immediate decomposition of WS2 and the OH– layer occurred, leading to the vertical nucleation of WS2 on the MoS2 surface and the formation of WS2/MoS2 vertical heterostructures (Figure 10d). Here, the numerous OH– ions derived from KOH in the precursor solution are crucial for the selective growth of the desired heterostructure. Besides, the NbSe2/WxNb1–xSe2 vertical heterostructures has been successfully synthesized with (NH4)6(H2W12O42)·4H2O and C4H4NNbO9·xH2O as W and Nb precursors.80 As depicted in Figure 10e, at first the 2D WxNb1–xSe2 domains formed; and when the reaction time increased, the Nb precursor continued to evaporate to preferentially nucleate at the edge of the as grown WxNb1–xSe2 to form the NbSe2/WxNb1–xSe2 vertical heterostructures. Figure 10f shows the corresponding coverage of NbSe2 on the WxNb1–xSe2 monolayer with growth time.
Figure 10.
Synthesis of vertical heterostructures with SCVD strategy. (a) Schematic of the structural evolution of WS2/MoS2 heterostructures. (b–d) OM images of typical triangular MoS2–OH and WS2/MoS2 lateral and vertical heterostructures. Reproduced from ref (79). Copyright 2020 American Chemical Society. (e) The Optical images and growth mechanism of NbSe2/WxNb1–xSe2 vdWHs (Nb-60%). (f) Coverage of NbSe2 on the WxNb1–xSe2 monolayer with growth time. Reproduced from ref (80). Copyright 2021 American Chemical Society.
Overall, 2D TMDs heterostructures provide a versatile platform for the integration of different materials with specific properties. To further broaden the applications of these materials, continued efforts are necessary to utilize SCVD technology to develop more extensive and various heterostructures.
3.2. Transition Metal Substitution of TMDs in SCVD
Compared with traditional CVD, the SCVD technique offers great flexibility in obtaining controllable transition metal (TM) doped TMDs. The utilization of mixed precursor solutions enables the tunability of doping concentrations for various dopants in different host materials, which resulting in tunable electrical,65,68,80−82 optoelectrical,83,84 catalytic85,86 and magnetic87−89 properties of TMDs beyond their intrinsic characteristics.
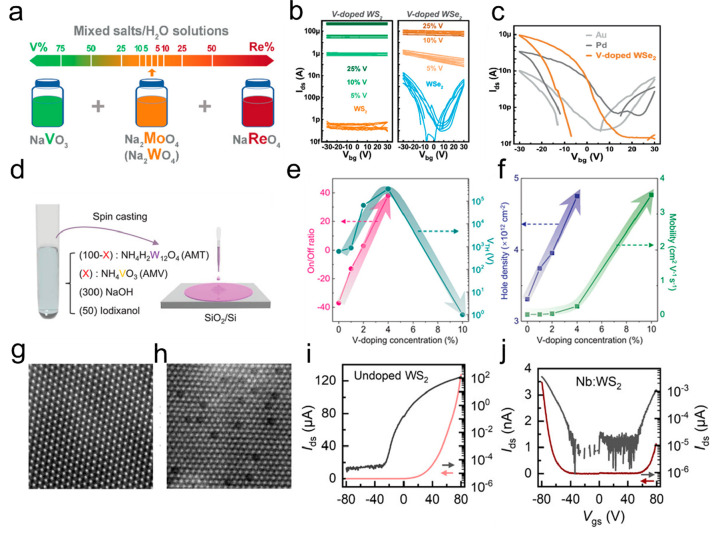
Recently, high-quality and tunable Re- and V-doped 2D TMDs68 are synthesized using liquid precursor solutions (NaReO4, NaVO3, Na2MoO4, and Na2WO4) with controlled dopants molar ratio, as shown in Figure 11a. Figure 11b shows the typical FET transfer curves of V-doped WS2 and WSe2 monolayers. The V-doped WSe2-contacted WSe2 FETs has the high on-state current and on/off ratio than that of Au/Pd-contacted WSe2 FETs in the Figure 11c, which indicated the SCVD grown V-doped WSe2 monolayers could be as promising low-resistance electrode material for the p-type WSe2 FETs. Such doping strategy is obvious convenient than doping through gas phase dopant, such as synthesis of Re-doped WSe2 with precise control of the partial pressure of Re2(CO)10.81 Similarly, Fan et al. used SCVD method to regulate the electrical properties of V-doped WSe2 with different V doping concentration and thus realizing various band arrangements.82Figure 11d exhibits schematic for preparation of V-doped WSe2 monolayers. The threshold voltage of the V-doped WSe2 monolayer shifted to a positive value and showed obvious p-type transport characteristics (Figure 11e), and the hole mobility and carrier density increased with the increase of V doping concentration (Figure 11f). Additionally, Vu et al. precisely manipulated the ratio of Nb/(W + Nb) in the solution to control the hole carrier concentration and the corresponding contact resistance RC value.80
Figure 11.
Synthesis of transition meatal doped TMDs. (a) Schematic illustration of preparing different ratios of mixed salt solutions for the fabrication of Re- and V-doped 2D TMDs. (b) Typical transfer curves of V-doped WS2 and WSe2 monolayers. (c) Transfer characteristics of WSe2–FETs with three kinds of contacts including Au, Pd, and V-doped WSe2. Reproduced from ref (68). Copyright 2021 John Wiley and Sons. (d) Schematic for preparation of V-doped WSe2. (e,f) On/off ratio and threshold voltage (e), as well as the field-effect hole mobility and intrinsic hole carrier concentration (f), vary as a function of V-doping concentration. Reproduced from ref (82). Copyright 2020 John Wiley and Sons. (g,h) HAADF-STEM images of undoped (g) and doped (h) monolayer WS2 crystals. Scale bars are 2 nm. (i,j) Transfer characteristics of undoped (i) and doped (j) monolayer WS2 crystals. Reproduced from ref (65). Copyright 2019 American Chemical Society.
In another example, different proportions of Na2WO4·2H2O and Nb(HC2O4)5·yH2O mixed solutions were spin-coated on the SiO2/Si to obtain Nb-doped WS2 nanosheets with different Nb concentrations.65 The STEM images reveal that 10.3% Nb-doped WS2 exhibited darker spots at the W atom position compared to the undoped WS2 (Figure 11g,h), confirming the random in situ atomic substitution of Nb doping. Furthermore, the FETs based on 10.3% Nb-doped WS2 displayed clear indications of hole doping, while the undoped WS2 FETs exhibited n-type behavior (Figure 11 i,j), providing evidence that the Nb dopant acts as a p-type dopant. Additionally, V-doped MoS2 and Nb-doped WS2 nanosheets displayed p-type conductivity,65,90 whereas Re-doped MoS2 presented n-type behavior.68 In addition to the tunable electrical properties, there have also been reports of changes in optical properties. Zhang et al. continuously increased the proportion of Nb components in the Nb1-XWXSe2 alloy to realize the continuous shift of PL peak from 760 to 845 nm.48 The Nb atoms act as electron acceptors in the WSe2, resulting in a change of the energy band gap. Eda et al. observed a well-defined impurity-induced emission in Re-doped WS2 monolayers as neutral donors.83 Gao et al. realized a fascinating up-converted luminescence on the Yb–Er codoped bilayer WSe2 excited by 980 nm laser.84 A series of experimental reports on as-grown doped-TMDs using the SCVD technique is summarized in Table 3.
Table 3. Summary of Common Substitutionally Doped TMDs by the SCVD Technique.
| Dopant | Host | Precursor | Dopant type | Density (%) | References |
|---|---|---|---|---|---|
| P | WSe2 | (NH4)6H2W12O40·4H2O + P2O5 + Se | p | (91) | |
| V | MoS2 | Na2MoO4 + NaVO4 + S | p | 2.0∼6.0 | (90) |
| MoS2 | (NH4)6Mo7O24·4H2O + NaVO3 + S | p | 0.8–7.6 | (92) | |
| MoS2 | Na2MoO4 + NaVO3 + S | p | 2.9 | (90) | |
| WS2 | Na2WO4 + NaVO3 + S | 4.7 | |||
| WSe2 | Na2WO4 + NaVO3 + Se | p | 2.7 | ||
| WS2 | (NH4)6H2W12O40·xH2O + VO[SO4] + S | p | 0.4–12 | (87) | |
| WSe2 | (NH4)6H2W12O40 + VO[SO4] + Se | 0.5∼8 | (88) | ||
| WSe2 | (NH4)6H2W12O40·xH2O + NH4VO3 + Se | p | 0.1∼10 | (89) | |
| WSe2 | NH4H2W12O4 + NH4VO3 + Se | p | 1.0∼10 | (82) | |
| Cr | MoS2 | Na2MoO4 + Na2CrO3 + S | (90) | ||
| Fe | MoS2 | Na2MoO4 + FeCl3 + S | (90) | ||
| MoSe2 | Na2MoO4 + FeCl3·6H2O + Se | n | 0.93–6.10 | (93) | |
| WS2 | (NH4)6H2W12O40 + FeCl3·6H2O + S | 0.7–2.8 | (70) | ||
| Nb | WSe2 | (NH4)6H2W12O40·xH2O + C4H4NNbO9·xH2O + Se | p | 0.9∼9.1 | (21) |
| WS2 | Na2WO4·2H2O + Nb(HC2O4)5·yH2O + S | p | (65) | ||
| Re | WS2 | Na2WO4·2H2O + NaReO4 + S | n | 1 | (83) |
| WS2 | Na2WO4 + NaReO4 + S | (68) | |||
| MoS2 | Na2MoO4 + NaReO4 + S | n | 2.1 | ||
| WSe2 | Na2WO4 + NaReO4 + Se | ||||
| MoS2 | (NH4)6Mo7O24 + NaReO4 + S | n | 0.3 | (70) | |
| Co | MoSe2 | Na2MoO4 + CoCl2·6H2O + Se | (93) | ||
| Ni | MoSe2 | Na2MoO4 + NiCl2 + Se | (93) | ||
| Yb/Er | WSe2 | WO3 + NaCl + Yb2O3/Er2O3 | (84) |
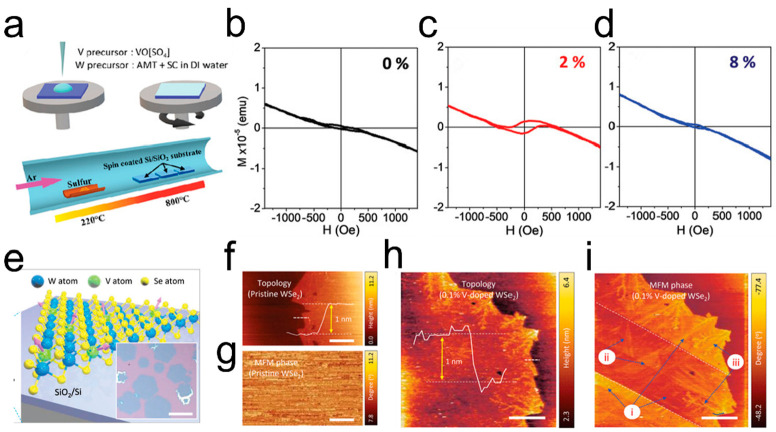
Apart from altering the electrical properties of 2D TMDs, the incorporation of suitable doping atoms into the host can also change the magnetic properties of these materials. Both theoretical and experimental reseach have indicated that doping with transition metal elements such as Cr, Mn, Fe, and Co can enable the realization of ferromagnetism in inherently nonferromagnetic TMDs.94−99 The SCVD technique has been proven to be an effective and straightforward method for the controlled synthesis of dilute magnetic semiconductors (DMSs). V doping is one of the common magnetic doping.87,89 Zhang et al. prepared V-doped WS2 monolayers with V concentration ranges from 0 to 12% to obtain room-temperature ferromagnetic order.87Figure 12a shows a schematic of the synthetic process of V-doped WS2 monolayers. Figure 12b–d depicts the relationship between ferromagnetism and the V doping concentration at room temperature. It can be seen that, with the increase of V doping concentration, the ferromagnetic intensity of V-doped WS2 increases. Specifically, when the concentration of V doping in WS2 reaches 2%, the coercivity and saturation magnetization of the material reach their maximum values, which is also applied in the WSe2 host. Yun et al. reported V-doped WSe2 monolayer induces long-range ferromagnetism at room temperature. The OM image of V-doped WSe2 monolayers is shown in Figure 12e. Figure 12f,g shows topography and MFM phase images of pristine WSe2 at room temperature.89 With the increasing V concentration from 0.5 to 8 atom % WSe2, the magnetization gradually realized the enhancement. Figure 12h,i exhibits the existence of the ferromagnetic order from the microscopic scale of magnetic force microscopy (MFM). And the ferromagnetic enhancement of the V-doped WSe2 monolayer has been achieved by adjusting the complex Se vacancies structure through post heat-treatment.100 In addition to V atoms, Fe, Co and Ni has been successfully doped into MoSe2 using the SCVD technique to modulate the magnetism.93
Figure 12.
Synthesis of transition meatal doped TMDs. (a) Schematic of synthetic process of V-doped WS2 monolayers. (b–d) Magnetization versus field loops of V-doped WS2 monolayers at 0, 2, and 8 atom % at 300 K. Reproduced from ref (87). Copyright 2020 John Wiley and Sons. (e) Schematic of fabricated V-doped WSe2 monolayers. The inset shows an optical image of SCVD-grown V-doped WSe2 monolayer. Scale bar, 50 μm. (f,g) Topography and MFM phase images of pristine WSe2 at room temperature, respectively. (h,i) Topography and MFM phase images of 0.1% V-doped WSe2 at room temperature, respectively. Reproduced from ref (89). Copyright 2020 John Wiley and Sons.
4. Conclusions and Perspectives
In summary, we discussed recent advancements in the growth of 2D TMDs using the SCVD technique. A variety of liquid precursors, additives, and new source supply methods for the synthesis of larger area and higher quality monolayer TMDs, even wafer-level homogeneous films, are reviewed. Substrate engineering strategies, including selection of the substrate, surface treatment, and substrate patterning, are studied to precisely control the nucleation and growth of TMD films. In addition, we provide an overview of the latest progress in the SCVD growth of 2D TMDs heterostructures and 2D doped TMDs. Despite these advancements, challenges still exist in the SCVD technique, and it is important to address these challenges to explore new synthesis method of 2D TMD with wafer scale area, high crystal quality, high reproductivity, and low cost:
-
(I)
The SCVD technique offers numerous opportunities for synthesizing atomically thin and high-quality 2D TMDs. Further research should focus on finding more novel liquid precursors or utilizing additives which could increase the solubility of precursors to expand the materials library. Additionally, exploring the growth of ternary or multielement 2D materials by mixing multiple precursors could be a promising direction.
-
(II)
In view of the fact that monolayer MoS2 and WS2 single crystal films have been successfully achieved on single-crystal Au substrates and well-designed sapphire substrates, it seems to be a feasible approach to explore new or pretreated growth substrates that have good contact with precursor solutions, and then seamlessly grow and link unidirectional domains into wafer-level single crystal films by inhibiting nucleation rate and promoting edge-induced growth.
-
(III)
The development of patterned growth of TMDs using substrate patterning in the SCVD technique has shown many superiorities. However, there is still a need to realize large-area with well-controllable size and morphology arrays for the application in integrated circuits. Further advancements in patterning techniques will be crucial for enabling the practical implementation of TMD-based devices.
-
(IV)
More in-depth studies on doped/alloyed 2D TMDs are necessary. Controlling the doping concentrations and defect density of doped TMDs is crucial as it can effectively regulate the electrical and optical properties of TMDs. Further research efforts should be dedicated to understanding and optimizing the doping processes to unlock the full potential of doped TMDs in various applications.
Acknowledgments
We acknowledge the support from the National Key R&D Program of the Ministry of Science and Technology of China (No. 2022YFA1203801), the National Natural Science Foundation of China (grant numbers 51991340, 51991343, 52221001, 62205055), the Hunan Key R&D Program Project (No. 2022GK2005), and Natural Science Foundation of Jiangsu Province (BK20220860).
Author Contributions
‡ D.S. and Y.J. contributed equally.
The authors declare no competing financial interest.
References
- Novoselov K. S.; Geim A. K.; Morozov S. V.; Jiang D.; Zhang Y.; Dubonos S. V.; Grigorieva I. V.; Firsov A. A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306 (696), 666–669. 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- Duan X.; Wang C.; Pan A.; Yu R.; Duan X. Two-Dimensional Transition Metal Dichalcogenides as Atomically Thin Semiconductors: Opportunities and Challenges. Chem. Soc. Rev. 2015, 44 (24), 8859–8876. 10.1039/C5CS00507H. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov D.; Allain A.; Huang Y. S.; Dumcenco D.; Kis A. Electrical Transport Properties of Single-Layer WS2. ACS Nano 2014, 8 (8), 8174–8181. 10.1021/nn502362b. [DOI] [PubMed] [Google Scholar]
- Lee C. H.; Lee G. H.; van der Zande A. M.; Chen W.; Li Y.; Han M.; Cui X.; Arefe G.; Nuckolls C.; Heinz T. F.; Guo J.; Hone J.; Kim P. Atomically Thin p-n Junctions with van der Waals Heterointerfaces. Nat. Nanotechnol. 2014, 9 (9), 676–681. 10.1038/nnano.2014.150. [DOI] [PubMed] [Google Scholar]
- Migliato Marega G.; Zhao Y.; Avsar A.; Wang Z.; Tripathi M.; Radenovic A.; Kis A. Logic-In-Memory Based on An Atomically Thin Semiconductor. Nature 2020, 587 (7832), 72–77. 10.1038/s41586-020-2861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan V. K.; Lee H. S.; Bergeron H.; Balla I.; Beck M. E.; Chen K. S.; Hersam M. C. Multi-Terminal Memtransistors from Polycrystalline Monolayer Molybdenum Disulfide. Nature 2018, 554 (7693), 500–504. 10.1038/nature25747. [DOI] [PubMed] [Google Scholar]
- Bonaccorso F.; Colombo L.; Yu G.; Stoller M.; Tozzini V.; Ferrari A. C.; Ruoff R. S.; Pellegrini V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347 (6217), 1246501. 10.1126/science.1246501. [DOI] [PubMed] [Google Scholar]
- Waldrop M. M. More than moore. Nature 2016, 530 (7589), 144–148. 10.1038/530144a. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Duan X.; Shin H. J.; Park S.; Huang Y.; Duan X. Promises and prospects of two-dimensional transistors. Nature 2021, 591 (7848), 43–53. 10.1038/s41586-021-03339-z. [DOI] [PubMed] [Google Scholar]
- Cao W.; Bu H.; Vinet M.; Cao M.; Takagi S.; Hwang S.; Ghani T.; Banerjee K. The future transistors. Nature 2023, 620 (7974), 501–515. 10.1038/s41586-023-06145-x. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Lin J.; Huang X.; Zhou Y.; Chen Y.; Xia J.; Wang H.; Xie Y.; Yu H.; Lei J.; Wu D.; Liu F.; Fu Q.; Zeng Q.; Hsu C. H.; Yang C.; Lu L.; Yu T.; Shen Z.; Lin H.; Yakobson B. I.; Liu Q.; Suenaga K.; Liu G.; Liu Z. A library of atomically thin metal chalcogenides. Nature 2018, 556 (7701), 355–359. 10.1038/s41586-018-0008-3. [DOI] [PubMed] [Google Scholar]
- Li S.; Wang S.; Tang D.-M.; Zhao W.; Xu H.; Chu L.; Bando Y.; Golberg D.; Eda G. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Applied Materials Today 2015, 1 (1), 60–66. 10.1016/j.apmt.2015.09.001. [DOI] [Google Scholar]
- Zhang K.; Bersch B. M.; Zhang F.; Briggs N. C.; Subramanian S.; Xu K.; Chubarov M.; Wang K.; Lerach J. O.; Redwing J. M.; Fullerton-Shirey S. K.; Terrones M.; Robinson J. A. Considerations for Utilizing Sodium Chloride in Epitaxial Molybdenum Disulfide. ACS Applied Mater. Interfaces 2018, 10 (47), 40831–40837. 10.1021/acsami.8b16374. [DOI] [PubMed] [Google Scholar]
- Fang H.; Tosun M.; Seol G.; Chang T. C.; Takei K.; Guo J.; Javey A. Degenerate n-doping of few-layer transition metal dichalcogenides by potassium. Nano Lett. 2013, 13 (5), 1991–1995. 10.1021/nl400044m. [DOI] [PubMed] [Google Scholar]
- Yun S. J.; Chae S. H.; Kim H.; Park J. C.; Park J. H.; Han G. H.; Lee J. S.; Kim S. M.; Oh H. M.; Seok J.; Jeong M. S.; Kim K. K.; Lee Y. H. Synthesis of Centimeter-Scale Monolayer Tungsten Disulfide Film on Gold Foils. ACS Nano 2015, 9 (5), 5510–5519. 10.1021/acsnano.5b01529. [DOI] [PubMed] [Google Scholar]
- George A. S.; Mutlu Z.; Ionescu R.; Wu R. J.; Jeong J. S.; Bay H. H.; Chai Y.; Mkhoyan K. A.; Ozkan M.; Ozkan C. S. Wafer Scale Synthesis and High Resolution Structural Characterization of Atomically Thin MoS2 Layers. Adv. Funct. Mater. 2014, 24 (47), 7461–7466. 10.1002/adfm.201402519. [DOI] [Google Scholar]
- Liu K. K.; Zhang W.; Lee Y. H.; Lin Y. C.; Chang M. T.; Su C. Y.; Chang C. S.; Li H.; Shi Y.; Zhang H.; Lai C. S.; Li L. J. Growth of large-area and highly crystalline MoS2 thin layers on insulating substrates. Nano Lett. 2012, 12 (3), 1538–1544. 10.1021/nl2043612. [DOI] [PubMed] [Google Scholar]
- Liu H.; Qi G.; Tang C.; Chen M.; Chen Y.; Shu Z.; Xiang H.; Jin Y.; Wang S.; Li H.; Ouzounian M.; Hu T. S.; Duan H.; Li S.; Han Z.; Liu S. Growth of Large-Area Homogeneous Monolayer Transition-Metal Disulfides via a Molten Liquid Intermediate Process. ACS Applied Mater. Interfaces 2020, 12 (11), 13174–13181. 10.1021/acsami.9b22397. [DOI] [PubMed] [Google Scholar]
- Cai Z.; Lai Y.; Zhao S.; Zhang R.; Tan J.; Feng S.; Zou J.; Tang L.; Lin J.; Liu B.; Cheng H. M. Dissolution-precipitation growth of uniform and clean two dimensional transition metal dichalcogenides. National Science Review 2021, 8 (3), nwaa115. 10.1093/nsr/nwaa115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Lin Y.-C.; Hong J.; Gao B.; Lim H. E.; Yang X.; Liu S.; Tateyama Y.; Tsukagoshi K.; Sakuma Y.; Suenaga K.; Taniguchi T. Mixed-Salt Enhanced Chemical Vapor Deposition of Two-Dimensional Transition Metal Dichalcogenides. Chem. Mater. 2021, 33 (18), 7301–7308. 10.1021/acs.chemmater.1c01652. [DOI] [Google Scholar]
- Park S.; Yun S. J.; Kim Y. I.; Kim J. H.; Kim Y. M.; Kim K. K.; Lee Y. H. Tailoring Domain Morphology in Monolayer NbSe2 and WxNb1-xSe2 Heterostructure. ACS Nano 2020, 14 (7), 8784–8792. 10.1021/acsnano.0c03382. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Xu H.; Zou G.; Zhang W.; Chai R.; Choi J.; Wu J.; Liu H.; Shen G.; Fan H. MoS2-OH Bilayer-Mediated Growth of Inch-Sized Monolayer MoS2 on Arbitrary Substrates. J. Am. Chem. Soc. 2019, 141 (13), 5392–5401. 10.1021/jacs.9b00047. [DOI] [PubMed] [Google Scholar]
- Wan X.; Miao X.; Yao J.; Wang S.; Shao F.; Xiao S.; Zhan R.; Chen K.; Zeng X.; Gu X.; Xu J. In Situ Ultrafast and Patterned Growth of Transition Metal Dichalcogenides from Inkjet-Printed Aqueous Precursors. Adv. Mater. 2021, 33 (16), e2100260. 10.1002/adma.202100260. [DOI] [PubMed] [Google Scholar]
- Kim M.; Seo J.; Kim J.; Moon J. S.; Lee J.; Kim J. H.; Kang J.; Park H. High-Crystalline Monolayer Transition Metal Dichalcogenides Films for Wafer-Scale Electronics. ACS Nano 2021, 15 (2), 3038–3046. 10.1021/acsnano.0c09430. [DOI] [PubMed] [Google Scholar]
- Chen K.; Wan X.; Xie W.; Wen J.; Kang Z.; Zeng X.; Chen H.; Xu J. Lateral Built-In Potential of Monolayer MoS2-WS2 In-Plane Heterostructures by a Shortcut Growth Strategy. Adv. Mater. 2015, 27 (41), 6431–6437. 10.1002/adma.201502375. [DOI] [PubMed] [Google Scholar]
- Ma L.; Zhu J.; Li W.; Huang R.; Wang X.; Guo J.; Choi J. H.; Lou Y.; Wang D.; Zou G. Immobilized Precursor Particle Driven Growth of Centimeter-Sized MoTe2 Monolayer. J. Am. Chem. Soc. 2021, 143 (33), 13314–13324. 10.1021/jacs.1c06250. [DOI] [PubMed] [Google Scholar]
- Naylor C. H.; Parkin W. M.; Ping J.; Gao Z.; Zhou Y. R.; Kim Y.; Streller F.; Carpick R. W.; Rappe A. M.; Drndic M.; Kikkawa J. M.; Johnson A. T. Monolayer Single-Crystal 1T’-MoTe2 Grown by Chemical Vapor Deposition Exhibits Weak Antilocalization Effect. Nano Lett. 2016, 16 (7), 4297–4304. 10.1021/acs.nanolett.6b01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor C. H.; Parkin W. M.; Gao Z.; Kang H.; Noyan M.; Wexler R. B.; Tan L. Z.; Kim Y.; Kehayias C. E.; Streller F.; Zhou Y. R.; Carpick R.; Luo Z.; Park Y. W.; Rappe A. M.; Drndic M.; Kikkawa J. M.; Johnson A. T. C. Large-area synthesis of high-quality monolayer 1T’-WTe2 flakes. 2D Materials 2017, 4 (2), 021008. 10.1088/2053-1583/aa5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.; Lee J.; Baek S.; Jung W.; Oh N. K.; Son E.; Park H. Liquid Precursor-Mediated Epitaxial Growth of Highly Oriented 2D van der Waals Semiconductors toward High-Performance Electronics. ACS Applied Electronic Materials 2021, 3 (12), 5528–5536. 10.1021/acsaelm.1c00946. [DOI] [Google Scholar]
- Zhao C.; Meng C.; Wang B.; Wang C.; Li R.; Fu Q. Vapor–Liquid–Solid Growth of Thin and Epitaxial Transition Metal Nitride Nanosheets for Catalysis and Energy Conversion. ACS Applied Nano Materials 2021, 4 (10), 10735–10742. 10.1021/acsanm.1c02185. [DOI] [Google Scholar]
- Wang B.; Zhao C.; Wang C.; Li R.; Zhang G.; Mu R.; Fu Q. Low-temperature growth of ultrathin and epitaxial Mo2C nanosheets via a vapor-liquid-solid process. Nanoscale 2022, 14 (25), 9142–9149. 10.1039/D2NR02389J. [DOI] [PubMed] [Google Scholar]
- Alameri D.; Nasr J. R.; Karbach D.; Liu Y.; Divan R.; Das S.; Kuljanishvili I. Mask-free patterning and selective CVD-growth of 2D-TMDCs semiconductors. Semicond. Sci. Technol. 2019, 34 (8), 085010. 10.1088/1361-6641/ab28db. [DOI] [Google Scholar]
- Wang S.; Zhang Y.; Zhao D.; Li J.; Kang H.; Zhao S.; Jin T.; Zhang J.; Xue Z.; Wang Y.; Sui Y.; Chen Z.; Peng S.; Jin Z.; Liu X.; Wang J.; Chen Y.; Yu G. Fast and controllable synthesis of AB-stacked bilayer MoS2 for photoelectric detection. 2D Materials 2022, 9 (1), 015016. 10.1088/2053-1583/ac395f. [DOI] [Google Scholar]
- Lu Y.; Chen T.; Ryu G. H.; Huang H.; Sheng Y.; Chang R. J.; Warner J. H. Self-Limiting Growth of High-Quality 2D Monolayer MoS2 by Direct Sulfurization Using Precursor-Soluble Substrates for Advanced Field-Effect Transistors and Photodetectors. ACS Applied Nano Materials 2019, 2 (1), 369–378. 10.1021/acsanm.8b01955. [DOI] [Google Scholar]
- Khan H.; Medina H.; Tan L. K.; Tjiu W.; Boden S. A.; Teng J.; Nandhakumar I. A Single-Step Route to Single-Crystal Molybdenum Disulphide (MoS2) Monolayer domains. Sci. Rep. 2019, 9 (1), 4142. 10.1038/s41598-019-40893-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.; Tan J.; Zhao S.; Zhang S.; Khan U.; Tang L.; Zou X.; Lin J.; Cheng H. M.; Liu B. Synthesis of Ultrahigh-Quality Monolayer Molybdenum Disulfide through In Situ Defect Healing with Thiol Molecules. Small 2020, 16 (35), e2003357. 10.1002/smll.202003357. [DOI] [PubMed] [Google Scholar]
- Ji Q.; Su C.; Mao N.; Tian X.; Idrobo J. C.; Miao J.; Tisdale W. A.; Zettl A.; Li J.; Kong J. Revealing the Bro̷nsted-Evans-Polanyi relation in halide-activated fast MoS2 growth toward millimeter-sized 2D crystals. Science. Advances 2021, 7 (44), eabj3274. 10.1126/sciadv.abj3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Giri A.; Moon S.; Shin S.; Myoung J.-M.; Jeong U. Highly Scalable Synthesis of MoS2 Thin Films with Precise Thickness Control via Polymer-Assisted Deposition. Chem. Mater. 2017, 29 (14), 5772–5776. 10.1021/acs.chemmater.7b01605. [DOI] [Google Scholar]
- Cun H.; Macha M.; Kim H.; Liu K.; Zhao Y.; LaGrange T.; Kis A.; Radenovic A. Wafer-scale MOCVD growth of monolayer MoS2 on sapphire and SiO2. Nano Research 2019, 12 (10), 2646–2652. 10.1007/s12274-019-2502-9. [DOI] [Google Scholar]
- Boandoh S.; Choi S. H.; Park J. H.; Park S. Y.; Bang S.; Jeong M. S.; Lee J. S.; Kim H. J.; Yang W.; Choi J. Y.; Kim S. M.; Kim K. K. A Novel and Facile Route to Synthesize Atomic-Layered MoS2 Film for Large-Area Electronics. Small 2017, 13 (39), 1701306. 10.1002/smll.201701306. [DOI] [PubMed] [Google Scholar]
- Lee J.; Pak S.; Giraud P.; Lee Y. W.; Cho Y.; Hong J.; Jang A. R.; Chung H. S.; Hong W. K.; Jeong H. Y.; Shin H. S.; Occhipinti L. G.; Morris S. M.; Cha S.; Sohn J. I.; Kim J. M. Thermodynamically Stable Synthesis of Large-Scale and Highly Crystalline Transition Metal Dichalcogenide Monolayers and their Unipolar n-n Heterojunction Devices. Adv. Mater. 2017, 29 (33), 1702206. 10.1002/adma.201702206. [DOI] [PubMed] [Google Scholar]
- Zuo Y.; Liu C.; Ding L.; Qiao R.; Tian J.; Liu C.; Wang Q.; Xue G.; You Y.; Guo Q.; Wang J.; Fu Y.; Liu K.; Zhou X.; Hong H.; Wu M.; Lu X.; Yang R.; Zhang G.; Yu D.; Wang E.; Bai X.; Ding F.; Liu K. Robust growth of two-dimensional metal dichalcogenides and their alloys by active chalcogen monomer supply. Nat. Commun. 2022, 13 (1), 1007. 10.1038/s41467-022-28628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H.; Boandoh S.; Lee Y. H.; Lee J. S.; Park J. H.; Kim S. M.; Yang W.; Kim K. K. Synthesis of Large-Area Tungsten Disulfide Films on Pre-Reduced Tungsten Suboxide Substrates. ACS Applied Mater. Interfaces 2017, 9 (49), 43021–43029. 10.1021/acsami.7b12151. [DOI] [PubMed] [Google Scholar]
- Pace S.; Martini L.; Convertino D.; Keum D. H.; Forti S.; Pezzini S.; Fabbri F.; Miseikis V.; Coletti C. Synthesis of Large-Scale Monolayer 1T’-MoTe2 and Its Stabilization via Scalable hBN Encapsulation. ACS Nano 2021, 15 (3), 4213–4225. 10.1021/acsnano.0c05936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W.; Liu K.; Yang S.; Wang F.; Su J.; Jin B.; Li H.; Zhai T. Salt-assisted chemical vapor deposition of two-dimensional materials. Science China Chemistry 2019, 62 (10), 1300–1311. 10.1007/s11426-019-9525-y. [DOI] [Google Scholar]
- Aras F. G.; Yilmaz A.; Tasdelen H. G.; Ozden A.; Ay F.; Perkgoz N. K.; Yeltik A. A review on recent advances of chemical vapor deposition technique for monolayer transition metal dichalcogenides (MX2: Mo, W; S, Se, Te). Materials Science in Semiconductor Processing 2022, 148, 106829. 10.1016/j.mssp.2022.106829. [DOI] [Google Scholar]
- Wu Q.; Zhang J.; Tang L.; Khan U.; Nong H.; Zhao S.; Sun Y.; Zheng R.; Zhang R.; Wang J.; Tan J.; Yu Q.; He L.; Li S.; Zou X.; Cheng H.-M.; Liu B. Iodine-assisted ultrafast growth of high-quality monolayer MoS2 with sulfur-terminated edges. National Science Open 2023, 2 (4), 20230009. 10.1360/nso/20230009. [DOI] [Google Scholar]
- An B.; Ma Y.; Chu F.; Li X.; Wu Y.; You C.; Deng W.; Li S.; Zhang Y. Growth of centimeter scale Nb1-xWxSe2 monolayer film by promoter assisted liquid phase chemical vapor deposition. Nano Research 2022, 15 (3), 2608–2615. 10.1007/s12274-021-3825-x. [DOI] [Google Scholar]
- Zhang D.; Wen C.; McClimon J. B.; Masih Das P.; Zhang Q.; Leone G. A.; Mandyam S. V.; Drndic M.; Johnson A. T. C. Jr.; Zhao M. Q. Rapid Growth of Monolayer MoSe2 Films for Large-Area Electronics. Adv. Electron Mater. 2021, 7 (6), 2001219. 10.1002/aelm.202001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibowo A. A.; Tebyetekerwa M.; Bui A. D.; Kremer F.; Saji S.; Yin Z.; Lu Y.; Macdonald D.; Nguyen H. T. High-Luminescence and Submillimeter-Scale MoS2 Monolayer Growth Using Combinational Phase Precursors via Chemical Vapor Deposition. ACS Applied Electronic Materials 2022, 4 (10), 5072–5080. 10.1021/acsaelm.2c01162. [DOI] [Google Scholar]
- Han G. H.; Kybert N. J.; Naylor C. H.; Lee B. S.; Ping J.; Park J. H.; Kang J.; Lee S. Y.; Lee Y. H.; Agarwal R.; Johnson A. T. Seeded growth of highly crystalline molybdenum disulphide monolayers at controlled locations. Nat. Commun. 2015, 6, 6128. 10.1038/ncomms7128. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Dai M.; Feng W.; Zhang X.; Zhang S.; Tan B.; Shang H.; Fu Y. Q.; Hu P. Monolayer hydrophilic MoS2 with strong charge trapping for atomically thin neuromorphic vision systems. Materials Horizons 2020, 7 (12), 3316–3324. 10.1039/D0MH01472A. [DOI] [Google Scholar]
- Li G.; Zhang W.; Zhang Y.; Lee Y.; Zhao Z.; Song X. Z.; Tan Z.; Kim K.; Liu N. Ammonium Salts: New Synergistic Additive for Chemical Vapor Deposition Growth of MoS2. J. Phys. Chem. Lett. 2021, 12 (51), 12384–12390. 10.1021/acs.jpclett.1c03742. [DOI] [PubMed] [Google Scholar]
- Yang J.; Gu Y.; Lee E.; Lee H.; Park S. H.; Cho M. H.; Kim Y. H.; Kim Y. H.; Kim H. Wafer-scale synthesis of thickness-controllable MoS2 films via solution-processing using a dimethylformamide/n-butylamine/2-aminoethanol solvent system. Nanoscale 2015, 7 (20), 9311–9319. 10.1039/C5NR01486G. [DOI] [PubMed] [Google Scholar]
- Salazar R.; Varotto S.; Vergnaud C.; Garcia V.; Fusil S.; Chaste J.; Maroutian T.; Marty A.; Bonell F.; Pierucci D.; Ouerghi A.; Bertran F.; Le Fevre P.; Jamet M.; Bibes M.; Rault J. Visualizing Giant Ferroelectric Gating Effects in Large-Scale WSe2/BiFeO3 Heterostructures. Nano Lett. 2022, 22 (23), 9260–9267. 10.1021/acs.nanolett.2c02448. [DOI] [PubMed] [Google Scholar]
- Lim Y. R.; Song W.; Han J. K.; Lee Y. B.; Kim S. J.; Myung S.; Lee S. S.; An K. S.; Choi C. J.; Lim J. Wafer-Scale, Homogeneous MoS2 Layers on Plastic Substrates for Flexible Visible-Light Photodetectors. Adv. Mater. 2016, 28 (25), 5025–5030. 10.1002/adma.201600606. [DOI] [PubMed] [Google Scholar]
- Ionescu R.; Campbell B.; Wu R.; Aytan E.; Patalano A.; Ruiz I.; Howell S. W.; McDonald A. E.; Beechem T. E.; Mkhoyan K. A.; Ozkan M.; Ozkan C. S. Chelant Enhanced Solution Processing for Wafer Scale Synthesis of Transition Metal Dichalcogenide Thin Films. Sci. Rep 2017, 7 (1), 6419. 10.1038/s41598-017-06699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.; Nong H.; Zheng R.; Zhang R.; Wang J.; Yang L.; Liu B. Resolidified Chalcogen Precursors for High-Quality 2D Semiconductor Growth. Angew. Chem., Int. Ed. 2023, 62, e202301501. 10.1002/anie.202301501. [DOI] [PubMed] [Google Scholar]
- Zuo Y.; Yu W.; Liu C.; Cheng X.; Qiao R.; Liang J.; Zhou X.; Wang J.; Wu M.; Zhao Y.; Gao P.; Wu S.; Sun Z.; Liu K.; Bai X.; Liu Z. Optical fibres with embedded two-dimensional materials for ultrahigh nonlinearity. Nat. Nanotechnol. 2020, 15 (12), 987–991. 10.1038/s41565-020-0770-x. [DOI] [PubMed] [Google Scholar]
- Qin B.; Ma H.; Hossain M.; Zhong M.; Xia Q.; Li B.; Duan X. Substrates in the Synthesis of Two-Dimensional Materials via Chemical Vapor Deposition. Chem. Mater. 2020, 32 (24), 10321–10347. 10.1021/acs.chemmater.0c03549. [DOI] [Google Scholar]
- Li S.; Ouyang D.; Zhang N.; Zhang Y.; Murthy A.; Li Y.; Liu S.; Zhai T. Substrate Engineering for Chemical Vapor Deposition Growth of Large-Scale Two-Dimensional Transition Metal Dichalcogenides. Adv. Mater. 2023, 35, e2211855. 10.1002/adma.202211855. [DOI] [PubMed] [Google Scholar]
- Chen L.; Liu B.; Ge M.; Ma Y.; Abbas A. N.; Zhou C. Step-Edge-Guided Nucleation and Growth of Aligned WSe2 on Sapphire via a Layer-over-Layer Growth Mode. ACS Nano 2015, 9 (8), 8368–8375. 10.1021/acsnano.5b03043. [DOI] [PubMed] [Google Scholar]
- Shi J.; Huan Y.; Xiao M.; Hong M.; Zhao X.; Gao Y.; Cui F.; Yang P.; Pennycook S. J.; Zhao J.; Zhang Y. Two-Dimensional Metallic NiTe2 with Ultrahigh Environmental Stability, Conductivity, and Electrocatalytic Activity. ACS Nano 2020, 14 (7), 9011–9020. 10.1021/acsnano.0c03940. [DOI] [PubMed] [Google Scholar]
- Kang W. T.; Phan T. L.; Ahn K. J.; Lee I.; Kim Y. R.; Won U. Y.; Kim J. E.; Lee Y. H.; Yu W. J. Selective Pattern Growth of Atomically Thin MoSe2 Films via a Surface-Mediated Liquid-Phase Promoter. ACS Appl. Mater. Interfaces 2021, 13 (15), 18056–18064. 10.1021/acsami.1c04005. [DOI] [PubMed] [Google Scholar]
- Qin Z.; Loh L.; Wang J.; Xu X.; Zhang Q.; Haas B.; Alvarez C.; Okuno H.; Yong J. Z.; Schultz T.; Koch N.; Dan J.; Pennycook S. J.; Zeng D.; Bosman M.; Eda G. Growth of Nb-Doped Monolayer WS2 by Liquid-Phase Precursor Mixing. ACS Nano 2019, 13 (9), 10768–10775. 10.1021/acsnano.9b05574. [DOI] [PubMed] [Google Scholar]
- Kwon K. C.; Kim C.; Le Q. V.; Gim S.; Jeon J. M.; Ham J. Y.; Lee J. L.; Jang H. W.; Kim S. Y. Synthesis of Atomically Thin Transition Metal Disulfides for Charge Transport Layers in Optoelectronic Devices. ACS Nano 2015, 9 (4), 4146–4155. 10.1021/acsnano.5b01504. [DOI] [PubMed] [Google Scholar]
- Jo H. K.; Kim J.; Lim Y. R.; Shin S.; Song D. S.; Bae G.; Kwon Y. M.; Jang M.; Yim S.; Myung S.; Lee S. S.; Kim C. G.; Kim K. K.; Lim J.; Song W. Wafer-Scale Production of Two-Dimensional Tin Monoselenide: Expandable Synthetic Platform for van der Waals Semiconductor-Based Broadband Photodetectors. ACS Nano 2023, 17 (2), 1372–1380. 10.1021/acsnano.2c09854. [DOI] [PubMed] [Google Scholar]
- Li S.; Hong J.; Gao B.; Lin Y. C.; Lim H. E.; Lu X.; Wu J.; Liu S.; Tateyama Y.; Sakuma Y.; Tsukagoshi K.; Suenaga K.; Taniguchi T. Tunable Doping of Rhenium and Vanadium into Transition Metal Dichalcogenides for Two-Dimensional Electronics. Advanced Science 2021, 8 (11), 2004438. 10.1002/advs.202004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam A. U.; Howlader M. M. R.; Deen M. J. Oxygen Plasma and Humidity Dependent Surface Analysis of Silicon, Silicon Dioxide and Glass for Direct Wafer Bonding. ECS Journal of Solid State Science and Technology 2013, 2 (12), P515–P523. 10.1149/2.007312jss. [DOI] [Google Scholar]
- Zhang T.; Fujisawa K.; Zhang F.; Liu M.; Lucking M. C.; Gontijo R. N.; Lei Y.; Liu H.; Crust K.; Granzier-Nakajima T.; Terrones H.; Elias A. L.; Terrones M. Universal In Situ Substitutional Doping of Transition Metal Dichalcogenides by Liquid-Phase Precursor-Assisted Synthesis. ACS Nano 2020, 14 (4), 4326–4335. 10.1021/acsnano.9b09857. [DOI] [PubMed] [Google Scholar]
- Li Y.; Hao S.; DiStefano J. G.; Murthy A. A.; Hanson E. D.; Xu Y.; Wolverton C.; Chen X.; Dravid V. P. Site-Specific Positioning and Patterning of MoS2 Monolayers: The Role of Au Seeding. ACS Nano 2018, 12 (9), 8970–8976. 10.1021/acsnano.8b02409. [DOI] [PubMed] [Google Scholar]
- Sun D.; Nguyen A. E.; Barroso D.; Zhang X.; Preciado E.; Bobek S.; Klee V.; Mann J.; Bartels L. Chemical vapor deposition growth of a periodic array of single-layer MoS2 islands via lithographic patterning of an SiO2/Si substrate. 2D Materials 2015, 2 (4), 045014. 10.1088/2053-1583/2/4/045014. [DOI] [Google Scholar]
- Wang Z.; Huang Q.; Chen P.; Wang J.; Lu Y.; Zhang S.; Liang X.; Wang L. Patterned growth of tungsten diselenide flakes by chemical vapor deposition. Jpn. J. Appl. Phys. 2017, 56 (8), 080303. 10.7567/JJAP.56.080303. [DOI] [Google Scholar]
- Pareek D.; Roach K. G.; Gonzalez M. A.; Busing L.; Parisi J.; Gutay L.; Schafer S. Micro-patterned deposition of MoS2 ultrathin-films by a controlled droplet dragging approach. Sci. Rep. 2021, 11 (1), 13993. 10.1038/s41598-021-93278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Lin Y. C.; Liu X. Y.; Hu Z.; Wu J.; Nakajima H.; Liu S.; Okazaki T.; Chen W.; Minari T.; Sakuma Y.; Tsukagoshi K.; Suenaga K.; Taniguchi T.; Osada M. Wafer-scale and deterministic patterned growth of monolayer MoS2 via vapor-liquid-solid method. Nanoscale 2019, 11 (34), 16122–16129. 10.1039/C9NR04612G. [DOI] [PubMed] [Google Scholar]
- Lee J.; Pak S.; Lee Y. W.; Park Y.; Jang A. R.; Hong J.; Cho Y.; Hou B.; Lee S.; Jeong H. Y.; Shin H. S.; Morris S. M.; Cha S.; Sohn J. I.; Kim J. M. Direct Epitaxial Synthesis of Selective Two-Dimensional Lateral Heterostructures. ACS Nano 2019, 13 (11), 13047–13055. 10.1021/acsnano.9b05722. [DOI] [PubMed] [Google Scholar]
- Chen C.; Yang Y.; Zhou X.; Xu W.; Cui Q.; Lu J.; Jing H.; Tian D.; Xu C.; Zhai T.; Xu H. Synthesis of Large-Area Uniform MoS2-WS2 Lateral Heterojunction Nanosheets for Photodetectors. ACS Applied Nano Materials 2021, 4 (5), 5522–5530. 10.1021/acsanm.1c00890. [DOI] [Google Scholar]
- Wu W.; Zhang Q.; Zhou X.; Li L.; Su J.; Wang F.; Zhai T. Self-powered photovoltaic photodetector established on lateral monolayer MoS2-WS2 heterostructures. Nano Energy 2018, 51, 45–53. 10.1016/j.nanoen.2018.06.049. [DOI] [Google Scholar]
- Zhu J.; Li W.; Huang R.; Ma L.; Sun H.; Choi J. H.; Zhang L.; Cui Y.; Zou G. One-Pot Selective Epitaxial Growth of Large WS2/MoS2 Lateral and Vertical Heterostructures. J. Am. Chem. Soc. 2020, 142 (38), 16276–16284. 10.1021/jacs.0c05691. [DOI] [PubMed] [Google Scholar]
- Vu V. T.; Vu T. T. H.; Phan T. L.; Kang W. T.; Kim Y. R.; Tran M. D.; Nguyen H. T. T.; Lee Y. H.; Yu W. J. One-Step Synthesis of NbSe2/Nb-Doped-WSe2 Metal/Doped-Semiconductor van der Waals Heterostructures for Doping Controlled Ohmic Contact. ACS Nano 2021, 15 (8), 13031–13040. 10.1021/acsnano.1c02038. [DOI] [PubMed] [Google Scholar]
- Kozhakhmetov A.; Schuler B.; Tan A. M. Z.; Cochrane K. A.; Nasr J. R.; El-Sherif H.; Bansal A.; Vera A.; Bojan V.; Redwing J. M.; Bassim N.; Das S.; Hennig R. G.; Weber-Bargioni A.; Robinson J. A. Scalable Substitutional Re-Doping and its Impact on the Optical and Electronic Properties of Tungsten Diselenide. Adv. Mater. 2020, 32 (50), e2005159. 10.1002/adma.202005159. [DOI] [PubMed] [Google Scholar]
- Fan S.; Yun S. J.; Yu W. J.; Lee Y. H. Tailoring Quantum Tunneling in a Vanadium-Doped WSe2/SnSe2 Heterostructure. Advanced Science 2020, 7 (3), 1902751. 10.1002/advs.201902751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh L.; Chen Y.; Wang J.; Yin X.; Tang C. S.; Zhang Q.; Watanabe K.; Taniguchi T.; Wee A. T.; Bosman M.; Quek S. Y.; Eda G. Impurity-Induced Emission in Re-Doped WS2 Monolayers. Nano Lett. 2021, 21 (12), 5293–5300. 10.1021/acs.nanolett.1c01439. [DOI] [PubMed] [Google Scholar]
- Wang C.; Xu L.; Jin H.; Li C.; Zhang Z.; Li L.; Chen Y.; Su J.; Liu N.; Lai J.; Long F.; Jiang X.; Gao Y. Yb/Er coordinatively doping in bilayer WSe2 for fascinating up-conversion luminescence. Nano Energy 2020, 78, 105317. 10.1016/j.nanoen.2020.105317. [DOI] [Google Scholar]
- Taniguchi T.; Nurdiwijayanto L.; Li S.; Lim H. E.; Miyata Y.; Lu X.; Ma R.; Tang D. M.; Ueda S.; Tsukagoshi K.; Sasaki T.; Osada M. On/Off Boundary of Photocatalytic Activity between Single- and Bilayer MoS2. ACS Nano 2020, 14 (6), 6663–6672. 10.1021/acsnano.9b09253. [DOI] [PubMed] [Google Scholar]
- Son E.; Lee S.; Seo J.; Kim U.; Kim S. H.; Baik J. M.; Han Y. K.; Park H. Engineering the Local Atomic Configuration in 2H TMDs for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2023, 17 (11), 10817–10826. 10.1021/acsnano.3c02344. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Zheng B.; Sebastian A.; Olson D. H.; Liu M.; Fujisawa K.; Pham Y. T. H.; Jimenez V. O.; Kalappattil V.; Miao L.; Zhang T.; Pendurthi R.; Lei Y.; Elias A. L.; Wang Y.; Alem N.; Hopkins P. E.; Das S.; Crespi V. H.; Phan M. H.; Terrones M. Monolayer Vanadium-Doped Tungsten Disulfide: A Room-Temperature Dilute Magnetic Semiconductor. Advanced Science 2020, 7 (24), 2001174. 10.1002/advs.202001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham Y. T. H.; Liu M.; Jimenez V. O.; Yu Z.; Kalappattil V.; Zhang F.; Wang K.; Williams T.; Terrones M.; Phan M. H. Tunable Ferromagnetism and Thermally Induced Spin Flip in Vanadium-Doped Tungsten Diselenide Monolayers at Room Temperature. Adv. Mater. 2020, 32 (45), e2003607. 10.1002/adma.202003607. [DOI] [PubMed] [Google Scholar]
- Yun S. J.; Duong D. L.; Ha D. M.; Singh K.; Phan T. L.; Choi W.; Kim Y. M.; Lee Y. H. Ferromagnetic Order at Room Temperature in Monolayer WSe2 Semiconductor via Vanadium Dopant. Adv. Sci. (Weinh) 2020, 7 (9), 1903076. 10.1002/advs.201903076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y.; Tan J.; Cai Z.; Zhang R.; Teng C.; Zhao S.; Lin J.; Liu B. Dissolution–precipitation growth of doped monolayer molybdenum disulfide through double-faced precursor supply. APL Materials 2021, 9 (5), 051123. 10.1063/5.0048946. [DOI] [Google Scholar]
- Kang W. T.; Lee I. M.; Yun S. J.; Song Y. I.; Kim K.; Kim D. H.; Shin Y. S.; Lee K.; Heo J.; Kim Y. M.; Lee Y. H.; Yu W. J. Direct growth of doping controlled monolayer WSe2 by selenium-phosphorus substitution. Nanoscale 2018, 10 (24), 11397–11402. 10.1039/C8NR03427C. [DOI] [PubMed] [Google Scholar]
- Liu X.; Jiang X.; Shao G.; Xiang H.; Li Z.; Jin Y.; Chen Y.; Jiang H.; Li H.; Shui J.; Feng Y.; Liu S. Activating the Electrocatalysis of MoS2 Basal Plane for Hydrogen Evolution via Atomic Defect Configurations. Small 2022, 18 (22), e2200601. 10.1002/smll.202200601. [DOI] [PubMed] [Google Scholar]
- Shen D.; Zhao B.; Zhang Z.; Zhang H.; Yang X.; Huang Z.; Li B.; Song R.; Jin Y.; Wu R.; Li B.; Li J.; Duan X. Synthesis of Group VIII Magnetic Transition-Metal-Doped Monolayer MoSe2. ACS Nano 2022, 16 (7), 10623–10631. 10.1021/acsnano.2c02214. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Lin J.; Sims H.; Jiang C.; Cong C.; Brehm J. A.; Zhang Z.; Niu L.; Chen Y.; Zhou Y.; Wang Y.; Liu F.; Zhu C.; Yu T.; Suenaga K.; Mishra R.; Pantelides S. T.; Zhu Z. G.; Gao W.; Liu Z.; Zhou W. Synthesis of Co-Doped MoS2 Monolayers with Enhanced Valley Splitting. Adv. Mater. 2020, 32 (29), e1906536. 10.1002/adma.202003123. [DOI] [PubMed] [Google Scholar]
- Yun W. S.; Lee J. D. Unexpected strong magnetism of Cu doped single-layer MoS2 and its origin. Phys. Chem. Chem. Phys. 2014, 16 (19), 8990–8996. 10.1039/C4CP00247D. [DOI] [PubMed] [Google Scholar]
- Fan X.-L.; An Y.-R.; Guo W.-J. Ferromagnetism in Transitional Metal-Doped MoS2 Monolayer. Nanoscale Res. Lett. 2016, 11 (1), 1–10. 10.1186/s11671-016-1376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Xing T.; Zhong M.; Huang L.; Lei N.; Zhang J.; Li J.; Wei Z. A two-dimensional Fe-doped SnS2 magnetic semiconductor. Nat. Commun. 2017, 8 (1), 1958. 10.1038/s41467-017-02077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Wu H.; Zhang L.; Zhang W.; Li L.; Kawakami T.; Sugawara K.; Sato T.; Zhang G.; Gao P.; Muhammad Y.; Wen X.; Tao B.; Guo F.; Chang H. Highly Tunable Near-Room Temperature Ferromagnetism in Cr-Doped Layered Td-WTe2. Adv. Funct. Mater. 2021, 31 (13), 2008116. 10.1002/adfm.202008116. [DOI] [Google Scholar]
- Yang L.; Wu H.; Zhang L.; Zhang G.; Li H.; Jin W.; Zhang W.; Chang H. Tunable and Robust Near-Room-Temperature Intrinsic Ferromagnetism of a van der Waals Layered Cr-Doped 2H-MoTe2 Semiconductor with an Out-of-Plane Anisotropy. ACS Appl. Mater. Interfaces 2021, 13 (27), 31880–31890. 10.1021/acsami.1c07680. [DOI] [PubMed] [Google Scholar]
- Yun S. J.; Cho B. W.; Dinesh T.; Yang D. H.; Kim Y. I.; Jin J. W.; Yang S. H.; Nguyen T. D.; Kim Y. M.; Kim K. K.; Duong D. L.; Kim S. G.; Lee Y. H. Escalating Ferromagnetic Order via Se-Vacancies Near Vanadium in WSe2 Monolayers. Adv. Mater. 2022, 34 (10), e2106551. 10.1002/adma.202106551. [DOI] [PubMed] [Google Scholar]