Abstract
Toxic air pollutants (TAPs) are a class of airborne chemicals known or suspected to cause serious health issues. This study, applying positive matrix factorization and inhalation unit risk estimates of TAPs, quantifies the changes in significant sources contributing to inhalation cancer risks (ICRs) from 2000 to 2020 in Hong Kong, China. Total ICR decreased from 1701 to 451 cases per million between 2000–2004 and 2016–2020, largely attributed to the reduction in diesel particulate matter (DPM), gasoline and solvent use-related volatile organic compounds (VOCs), and coal/biomass combustion-related polycyclic aromatic hydrocarbons and metal(loid)s. The regional contribution of VOCs associated with industrial and halogenated solvent sources increased substantially, representing the largest non-DPM ICR contributor (37%) in 2016–2020, stressing the need for a more comprehensive risk evaluation across the fast-growing and densely populated Greater Bay Area (GBA). ICRs in Hong Kong and the GBA will likely remain over 100 cases per million by 2050. The contributions to ozone formation potential of VOC/carbonyl sources were quantified, which show a notable shift from being solvent/gasoline-dominant in 2000–2004 to being more evenly shared by various sources in 2016–2020. Establishing a similar TAP monitoring network in the GBA is anticipated to provide the monitoring data needed to facilitate the development of more informed air quality management strategies.
Keywords: toxic air pollutant, hazardous air pollutant, health risk assessment, source apportionment, ozone formation potential, air quality management
1. Introduction
Toxic air pollutants (TAPs), also referred to as hazardous air pollutants or air toxics, are a subset of pollutants that are known or suspected to cause cancer and various adverse health, environmental, and ecological effects.1 Exposure to these pollutants is linked to increased risk of developing cancer and various developmental, neurological, respiratory, reproductive, and other chronic health effects.1 Certain TAPs that have been extensively monitored worldwide include diesel particulate matter (DPM) and certain volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), and metal(loid)s. Quantification of inhalation cancer risks (ICRs) based on ambient TAP concentrations represents a viable approach for health risk assessment, providing guidance for risk management.2−4 The United States Environmental Protection Agency (USEPA) is required to regulate 188 TAPs, of which 30 are further categorized as urban air toxics, requiring regulatory measures to address their sources.5 In 2019, the Ministry of Ecology and Environment of China issued the first list of toxic and hazardous air pollutants with six VOC species and five classes of metal(loid)s and their compounds, requiring enterprises and institutions that emit these air pollutants to both implement risk management at the source and put into practice an environmental management system for pollution sources.6
We previously conducted the first long-term assessment (2000–2020) of the ambient characteristics of TAPs and their associated health risks in Hong Kong, a core coastal city in the Greater Bay Area (GBA), one of the leading megalopolises located in southern China.7 The study observed a considerable risk reduction resulting from the continuous decline in numerous TAPs, attributed to the multifaceted interventions to control both local and regional air pollution sources. Meanwhile, the evolution of ambient concentrations exhibited varying patterns for individual TAPs, implying that the contributions of the associated sources had also changed over the years. However, the long-term variations in TAP sources have not been formally investigated, prohibiting an evidence-based evaluation of the effectiveness of the relevant control policies.
Many of the VOC and carbonyl species classified as TAPs are photochemically reactive and, hence, can promote the formation of ozone (O3) under the presence of nitrogen oxides and sunlight, leading to tropospheric O3 air pollution.8 Surface O3, being a powerful oxidant, has long been recognized for its detrimental impacts on human health and vegetation.8 In particular, increasingly severe surface O3 pollution has been observed in Hong Kong and across the GBA over the past decade.9,10 The ozone formation potential (OFP) scale has been widely used to quantify the relative effects of individual VOCs on O3 production, providing guidance needed for establishing cost-effective O3 mitigation strategies.11−13 Although there has been a large body of literature examining the sources of OFP in the GBA, the long-term source variations of OFP have remained largely unexplored. The extended TAP monitoring effort in Hong Kong hence offered an opportunity to understand the long-term OFP source variations, providing the information required for further management and control of O3 air pollution.
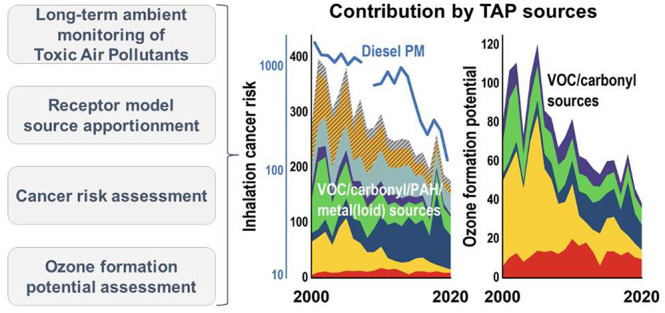
The objective of this study is to conduct a systematic analysis to identify and quantify the sources of TAPs in Hong Kong from 2000 to 2020. Analyzed TAPs include DPM, VOCs, carbonyls, PAHs, and metal(loid)s. The temporal variations in source contributions to ICR and OFP (VOCs and carbonyls only) are evaluated to make connections with the known emission change and associated control policies. Trends in VOC source contributions by mass, ICR, and OFP are also compared to identify how the rate of change differs across different metrics over the last two decades. A hypothetical case of ICR source contributions in 2050 is also deduced to speculate to what extent the ICR could reach by assuming zero vehicular emissions and a best-health-effect pollution control scenario. We propose and discuss reasons based on the study findings that establishing a pan-GBA TAP monitoring network is highly beneficial to the development of more informed, nuanced air quality management strategies.
2. Methods
2.1. Ambient Sampling of TAPs
VOC, carbonyl, and PAH samples were collected by the Hong Kong Environmental Protection Department (HKEPD) following USEPA Methods TO-14A, TO-11A, and TO-13A, respectively. VOC samples were collected twice a month, whereas carbonyl and PAH samples were collected once a month. Metals/metalloids and carbonaceous components (for deriving the DPM contribution) were obtained from Hong Kong’s PM10 chemical speciation network with a sampling frequency of once every six days.14 In each sampling event, 24 h midnight-to-midnight samples were collected at the Central/Western (CW) and Tsuen Wan (TW) air quality monitoring stations. Both sites are part of Hong Kong’s air quality monitoring network and are situated in typical urban environments with a high density of population, roads, and residential and commercial buildings in the vicinity. The ambient air samplers were held 16–17 m above ground level. Field blanks were collected for each sampling event from both locations. Collocated samples collected quarterly from both sites showed a measurement precision of within ±30%. Because of site renovation, the sampling of VOCs and carbonyls at TW was temporarily shifted to a nearby station in Kwai Chung with similar neighborhood characteristics during January 2015–June 2020. The data sets were merged, given that no noticeable spatiotemporal discontinuity is shown.
2.2. Chemical Analysis of TAPs and Data Quality Assurance and Control
The TAP data analyzed in this work span 21 years from 2000 to 2020. Table S1 lists all of the TAPs evaluated in this study. Over the years, the entirety of chemical analyses for the TAP monitoring has been conducted by the Government Laboratory of Hong Kong, adhering to consistent analytical methods complying with the USEPA standards (Methods TO-14A, TO-11A, and TO-13A for VOCs, carbonyls, and PAHs). The TAP monitoring started in mid-1997, but the data before 2000 were excluded from the current analysis because of a major upgrade in the VOC analytical system in 2000, which expanded the target analytes from 41 to 143 compounds with notable improvement in detection limits.15 The upgrade enabled the robust detection of a number of health-significant VOCs (e.g., 1,2-dichloroethane and 1,2-dichloropropane) and a much wider range of alkanes and alkenes that had not been detected reliably before. As a data quality control step, the time series of all TAPs was examined visually to identify any outliers not following the expected temporal patterns. Abnormal spikes (typically more than 10 times higher than the standard deviation) that were not documented to be associated with any renovation and construction activities at or near the sampling sites were deemed questionable data and, thus, were censored. Table S2 provides a summary of the average concentrations in CW and TW for 2000–2004 and 2016–2020 for a subset of TAPs with unit risk estimate values given.
2.3. Source Apportionment Analysis
Source apportionment analyses of VOCs, carbonyls, PAHs, and PM10 were conducted with the USEPA Positive Matrix Factorization (PMF) 5.0 software.16 The working principle of PMF is to decompose an ambient speciation data matrix into factor profiles and factor contribution matrices, with non-negative constraints and the objective of minimizing the uncertainty weighted differences between observed and apportioned species concentrations.17 Given that the sampling schedules of different TAP groups were different, the analyses were conducted for each chemical group separately. PMF was conducted on CW and TW sites separately because the two sites may have different source combinations and characteristics. Separating the two sites would reduce source ambiguity in the PMF solution, producing more robust source factors. The source factors resolved from both sites can also be compared to help identify common and different sources. Further information regarding the model inputs and source identification methods are detailed in Section S1 of the Supporting Information.
The DPM contribution was estimated using elemental carbon (EC) from PM10 speciation as the source tracer.18 The organic carbon (OC) and EC in PM10 were determined with a thermal-optical transmittance method with the NIOSH 5040 temperature program.19 DPM was estimated as the sum of ambient EC and DPM-related organic matter:
| 1 |
where [OC/EC]DPM is equal to 0.35, representing the OC-to-EC mass ratio in DPM determined from a previous local roadside study spanning 2011–2017, and the factor 1.2 is to account for the non-carbon fraction in vehicular organic matter.20,21 We acknowledge that this method is associated with uncertainties arising from neglecting EC contribution from non-diesel sources and varying OC-to-EC ratio in DPM over time, resulting from the evolution of DPM control technologies, which have been discussed in our previous work and warrant further investigation.7
2.4. Health Risk Assessment
To assess the health risks of TAPs, we converted the concentrations of TAPs into a cancer-risk-weighted concentration by multiplying the ambient concentration with a unit risk estimate (URE). The weighted concentration represents the inhalation cancer risks (ICRs), as expressed below:
| 2 |
where UREi, expressed in units of (μg/m3)−1, represents the upper-bound excess cancer incidence over a population of 1 million subjected to lifetime (70 years) continuous exposure to 1 μg/m3 of species i through inhalation. The URE values were compiled from multiple databases: the Office of Air Quality Planning and Standards of the USEPA, the Office of Environmental Health Hazard Assessment of the California Environmental Protection Agency, and the Guidelines for Air Quality of the World Health Organization.22−24 It should be emphasized that the URE values typically derived from epidemiologic or animal studies are considered to be conservative estimates for the benefit of protecting public health.25 Consequently, it is unlikely that the actual ICR exceeds the estimated ICR. To align with this approach, the largest URE was taken when more than one value is reported. The final URE values adopted in this study are summarized in Table S2. Source contribution to ICRs was subsequently calculated as the linear sum of individual ICRs associated with the TAPs apportioned to the source. We note that when individuals are exposed to a mixture of toxic substances, the combined effect can exceed the sum of the individual effects.
We acknowledge that not all of the TAPs included in the cancer risk assessment are classified as group 1 human carcinogens by the International Agency for Research on Cancer (IARC). The human carcinogenicity classifications and definitions by the IARC for the TAPs considered in the source apportionment analysis are provided in Table S3. Of the 26 TAPs considered, nine are classified as group 1 carcinogens (carcinogenic to humans), four as group 2A (probably carcinogenic to humans), 12 as group 2B (possibly carcinogenic to humans), and one as group 3 (not classifiable as to its carcinogenicity to humans). The URE values of these TAPs are derived from available epidemiologic or animal studies. Consequently, the UREs for some TAPs are extrapolations from animal response to humans, which have considerable uncertainties that can vary across multiple magnitudes and are an active area of research.1 This gives rise to the situation that some TAPs have UREs while there is still insufficient evidence to classify them as group 1 human carcinogens. Nevertheless, the merit of deriving cancer risks using these UREs is to provide a cautious estimate of an upper-bound excess cancer rate resulting from lifetime inhalation exposure to specific TAPs in a population, regardless of whether the TAPs are classified as a definite human carcinogen.25 This approach is generally considered to provide a measure to help identify risks that will guide further actions for safeguarding public health.
2.5. Ozone Formation Potential Assessment
We evaluated the relative impacts of individual VOC sources on tropospheric O3 formation by adopting the extensively used ozone formation potential (OFP) scale method.12 OFP can be defined as the gram(s) of O3 formed (or consumed) per gram of a given VOC added to a base atmospheric system. We applied the maximum incremental reactivity (MIR) scale to represent this quantitative relationship, given that O3 formation in our study location has been in the VOC-limited regime (i.e., O3 reduction is more effective toward suppressing VOCs than suppressing NOx) in the last two decades.9 The OFP of a VOC i was calculated as follows:
| 3 |
where [VOCi] is the ambient concentration of VOC i in units of μg/m3, MIRi is a unitless value, and OFPi is the maximum amount of O3 in μg/m3 that can be formed potentially. MIR values are typically determined with a chemical box model built upon a predetermined chemical mechanism and atmospheric conditions. Therefore, these values are dependent on the configuration of the model, such as the VOC composition and relative availability of VOCs and NOx. To obtain a best-estimate OFP, we first considered the MIR values that were developed based on VOC observation data in Guangzhou.13 If observation-based values were not reported, those derived from an emission-based model were considered.13 MIR values from the United States were taken if neither observation- nor emission-based values were available.26 Source contributions to OFP were eventually calculated as the linear sum of individual OFPi values associated with the source.
3. Results and Discussion
3.1. Inhalation Cancer Risks and Comparison with Other Locations
The total ambient concentration of all the TAPs analyzed by PMF is equivalent to an ICR of 415 and 315 cases per million in the first five and last five years (i.e., 2000–2004 and 2016–2020), respectively. The TAP concentrations modeled by PMF produced an ICR of 358 and 278 cases per million in the corresponding periods, representing 86% and 88% of the ICRs based on the observed concentrations. Within the PMF-derived ICRs, 54 (15%) and 87 (31%) of the cancer risks are apportioned to construction/renovation at or near the sampling location. While this highly localized contribution will not be considered in subsequent assessments to avoid biased interpretation of the risks associated with general air quality, it demonstrates the merits of identifying the health risks in microenvironments where TAP composition can be very different.
Table 1 provides an overview of the ICRs derived from TAP measurements in this study and those from other locations. The measurement periods can be roughly divided into pre-2008, 2008–2013, and post-2013, representing early, middle, and recent years. In the early years, Hong Kong had a DPM-associated ICR that was much lower than that of South Coast Air Basin (the Basin) and modestly higher than that of California. Both Hong Kong and the Basin had seen a substantial reduction in DPM emissions as they transitioned into recent years, resulting in an ICR reduction of 82–86%. As shown in the table, the range of examined TAPs varies by study, prohibiting a direct comparison of non-DPM ICRs between the sites. Still, it is of note that Hong Kong had a lower non-DPM ICR than the Basin in recent years (210 vs 370 cases per million), even though more VOCs are considered in the evaluation for Hong Kong. The non-DPM ICRs in Hong Kong were approximately 2 times lower than those in the Yangtze River Delta and the North China Plain of China and comparable to those in other regions in China, despite having many more TAP species included in the assessment, highlighting that a comprehensive risk assessment is worth considering in mainland China. The variety of TAPs measured in the studies of Hong Kong, Korea, and the United States in recent years are comparable, except there are fewer PAHs being measured in Hong Kong. Generally, the non-DPM ICRs in Hong Kong were modestly lower than those in Incheon, comparable to those in Seoul, and substantially higher than those in urban and rural areas across the United States.
Table 1. Summary of Inhalation Cancer Risks Reported and Number of TAPs Measured in Various Studies across Different Locations.
| cancer
risks per million |
number
of TAPs included in the assessment |
|||||
|---|---|---|---|---|---|---|
| location | period | DPMa | non-DPM sources | VOCs and carbonyls | PAHs | metals/metalloids |
| Hong Kong, China (this study) | 2000–2004 | 1362 | 339 | 24 | 8 | 6 |
| 2009–2013 | 784 | 264 | 24 | 8 | 6 | |
| 2016–2020 | 241 | 210 | 24 | 8 | 6 | |
| South Coast Air Basin, United States27 | 2004–2006 | 2610 | 970 | 14 | 8 | 7 |
| 2012–2013 | 1260 | 790 | 14 | 8 | 7 | |
| 2018–2019 | 370 | 370 | 14 | 8 | 7 | |
| California, United States3 | 2000–2004 | 1054 | 446 | 5 | 0 | 1 |
| 2008–2012 | 626 | 238 | 5 | 0 | 1 | |
| Yangtze River Delta, China2 | 2008b | - | 545 | 13 | 0 | 0 |
| North China Plain, China2 | 2013b | - | 396 | 13 | 0 | 0 |
| Greater Bay Area, China2 | 2007b | - | 151 | 11 | 0 | 0 |
| other regions, China2 | 2012b | - | 263 | 13 | 0 | 0 |
| Incheon, Korea28 | 2014–2015 | - | 270 | 29 | 14 | 6 |
| Seoul, Korea28 | 2013–2014 | - | 200 | 29 | 14 | 6 |
| 21 urban sites in the United States4 | 2013–2017 | - | 67 | 22 | 15 | 5 |
| six rural sites in the United States4 | 2013–2017 | - | 42 | 22 | 15 | 5 |
| Vancouver, Canada29 | 2013–2018 | - | 81c | 9 | 0 | 0 |
| Calgary, Canada29 | 2013–2018 | - | 53c | 9 | 0 | 0 |
Diesel particulate matter.
Median sampling year of studies reviewed by the investigator.
Cancer risks were retrieved by multiplying the TAP concentrations reported with unit risk estimates.
3.2. Source Contributions to Inhalation Cancer Risks
3.2.1. Sources of TAPs Identified by PMF Analysis
The details of the source identification process are provided in Section S2 of the Supporting Information. The sources are mainly identified based on the source markers present in the factor profiles resolved and the interannual and seasonal variation patterns in source contributions, which often show source-indicative characteristics. These characteristics may include alignment with emission changes and policy interventions over the years and seasonal differences, reflecting whether the source is associated with local emissions or regional transport. Four VOC sources named LPG (liquified petroleum gas), solvent/gasoline, industry/halogenated solvent, and freon/solvent/gasoline/biogenic and four carbonyl sources named primary formation and secondary formations I–III were identified. Identified sources of PAHs include road traffic, coal/biomass combustion, and chrysene-dominated sources, whereas those for metal(loid)s include road traffic, coal/biomass combustion, secondary PM formation, ship emissions, the metal industry, sea salt, and soil dust.
3.2.2. ICR Source Contributions by TAPs
Figure 1a,b shows the changes in source contributions to ICRs averaged from both the CW and TW sites for individual TAPs between 2000–2004 and 2016–2020. Eleven out of 14 VOCs and carbonyls exhibited a notable reduction in ICRs of 19–63%. The improvement is mainly attributed to the reduction in solvent/gasoline and freon/solvent/gasoline/biogenic factors that dominated the ICRs from the VOCs in 2000–2004. Formaldehyde remained a top cancer risk contributor in both periods, with its ICRs decreasing from 60 to 40 cases per million. The decrease was mainly attributed to the continuous reduction in primary contribution (p < 0.001, as shown in Figure S3e). Meanwhile, secondary formations I–III evolved to dominate the formaldehyde contribution in recent years, accounting for 79%. Given the importance of secondary contribution, a more detailed investigation into the origins of formaldehyde precursors and the formation and removal pathways of formaldehyde in the relevant environment would support further management and control for this compound.
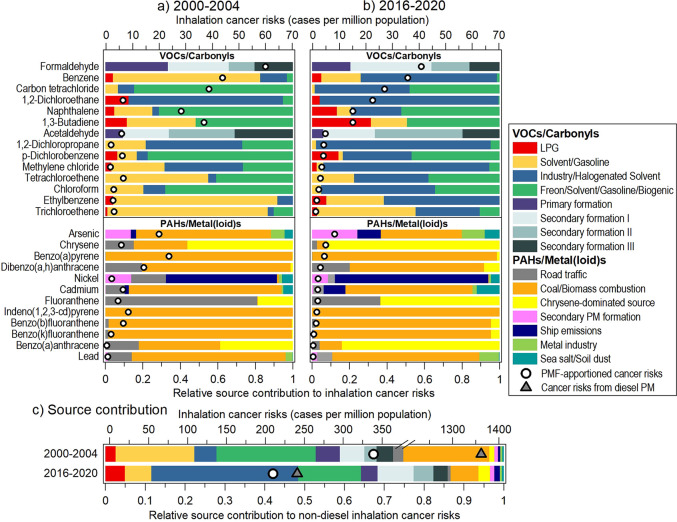
Figure 1.
Source contributions to inhalation cancer risks averaged from the CW and TW sites. (a, b) Apportioned risks (circle markers) and relative source contributions for individual TAPs during (a) 2000–2004 and (b) 2016–2020. The species are arranged in descending order of ICRs based on 2016–2020 values. (c) Cancer risk contributions by source. Cancer risks associated with diesel PM are also shown in (c) as triangle markers for comparison with the risks aggregated from all other sources, represented by circle markers.
The source contributions changed remarkably in 2016–2020, where the industry/halogenated solvent factor had become the major risk contributor in many of the measured VOCs (Figure 1b). Such a change is driven concurrently by the considerable reduction in solvent/gasoline and freon/solvent/gasoline/biogenic contributions (p < 0.001, see Figure S3b,d) and the steep increase in industry/halogenated solvent contribution (p < 0.01, Figure S3c). The rise of industry/halogenated solvent contribution had resulted in a 95–257% increase in the ICRs associated with methylene chloride, 1,2-dichloroethane, and 1,2-dichloropropane. It is of note that this single source category contributed to 73% of the ICR related to benzene, 51% for carbon tetrachloride, and 95% for 1,2-dichloroethane, which are all among the leading VOCs identified to present concerning levels of cancer risks. The disproportionately large cancer risks derived from industry/halogenated solvents call for further evaluation of the potential health burdens imposed by the relevant emission sources to be protective of public health.
All of the individual ICRs associated with PAHs and metal(loid)s exhibited a discernible reduction between 2000–2004 and 2016–2020 (Figures 1a,b). In general, the relative source contributions have remained similar over the years. In 2016–2020, seven out of the 12 air toxics were dominated by coal/biomass combustion, which accounted for over 70%. This source is also responsible for ∼40% of the ICRs associated with arsenic, which is the top ICR-contributing species among the PAHs/metal(loid)s group. Ship emissions dominated the ICRs for nickel in both periods (over 60%), as expected. The relative contribution to several PAHs including chrysene, fluoranthene, and benzo(a)anthracene from road traffic decreased noticeably as a result of the increased contribution from the chrysene-dominated source, which is speculated to be associated with gasoline evaporation and/or traffic exhaust, as discussed in Section S2 of the Supporting Information.
3.2.3. ICR Contribution by Source Category
Figure 1c compares the site-averaged contributions to ICRs by source category between the first five-year and last five-year periods. The relative contribution bars represent ICRs for all sources identified in the PMF analysis with the total absolute contribution denoted by a circle marker. The ICR of DPM is indicated separately by a triangle marker. Note that the ICRs from the PMF-derived road traffic should have already been accounted for by DPM to some extent. Still, we retain the road traffic contribution here, given that gasoline vehicles are also known to contribute to PAHs and metal(loid)s.30 It is noticed that in 2000–2004, the ICR associated with DPM was substantially higher than the total of all other sources, showing a contrast of 1362 vs 339 cancer cases per million, with DPM constituting 80% of the risks. The disparity narrowed to being almost equivalent in 2016–2020, with ICRs at 241 vs 210 cases per million. The remarkable decrease in DPM-related cancer risks is attributed to the sustained control efforts targeting high-emission diesel vehicles by the local government over the last two decades. The benefits of such control efforts to air quality have been evidenced by various field measurement studies.20,31
Benefiting from the sustained and ambitious local and regional air pollution control measures, a remarkable decrease in cancer risks equivalent to 191 cases per million was recorded between 2000–2004 and 2016–2020. The decrease was primarily driven by coal/biomass combustion showing an 80% reduction from 73 to 15 cases per million (including PAHs and metals/metalloids), solvent/gasoline with a 79% reduction from 67 to 14 cases per million, and freon/solvent/gasoline/biogenic with a 61% reduction from 84 to 33 cases per million. These three sources combined represented 85% of the total reduction. It is noted from Figure 1c that the relative contributions to non-DPM cancer risks of all PAHs/metal(loid)s sources decreased from a quarter in 2000–2004 to ∼15% in 2016–2020, equivalent to 30 cancer cases per million, with coal/biomass combustion accounting for half of the risks.
The substantial cancer risk reduction over the years is partially counteracted by the increase in industry/halogenated solvent contribution, in which the ICRs has risen 4-fold from 19 cases per million in 2000–2004 to 77 cases per million in 2016–2020. The offset discernibly undermined the ICR reduction contributed by other sources, resulting in a net reduction in total non-DPM cancer risks of merely 38% from 339 cases per million in 2000–2004 to 210 cases per million in 2016–2020. As shown in Figure 1c, the relative source contributions also altered substantially as a result of the dramatic change in the industry/halogenated solvent contribution. This source became the largest contributor to non-DPM cancer risks, accounting for 37%. Such a percentage share is double the contributions of the following sources, which are the secondary carbonyl formation (i.e., total of secondary formations I–III) and freon/solvent/gasoline/biogenic sources, each accounting for 16–18%. As discussed in Section S2 of the Supporting Information, this factor is attributed to downwind transport of industrial and solvent use-related emissions from mainland China. This source is particularly enriched in halogenated VOCs, particularly the major cancer-causing species 1,2-dichloroethane and methylene chloride, leading to the large ICR contribution of this source (Figures S2a and 1b). Indeed, two-thirds of the ICRs of this source come from halogenated VOCs, highlighting that it is important to identify and control the specific sources releasing these VOCs. In China, generally, the major sources of these halocarbons are industrial activities (e.g., waste incineration and the petrochemical industry) and coal combustion.2 Associated industrial activities include, for example, the production of 1,2-dichloroethane, which serves as the primary chemical for the production of polyvinyl chloride. The industrial application of methylene chloride covers a wide range of processes, such as producing paint remover, pharmaceuticals, and process solvent, foam blowing of polyurethane, and stripping and degreasing in the electronics industry.
In various emission inventory studies in China, industry and solvent use indeed represent two very broad source categories, each entailing a large number of unique processes.32,33 The nationwide VOCs emission inventory for China spanning 1990–2017 considered 52 and 17 individual sources in industry and solvent use sectors, respectively.34 The industry sector includes the following: the chemical industry with 24 sources related to the production of raw materials such as glass, rubber, plastic, wool, etc.; industrial coal use with nine different coal types or use processes; industrial fuel combustion of five different fuel types other than coal, such as fuel oil and natural gas; the petroleum industry including seven oil production, distribution, and refinery processes; and other industrial processes with seven production sources such as iron, steel, sugar, etc. The solvent use sector includes industrial paint use with seven processes (e.g., architecture paint, vehicle refinish paint, wood furniture, etc.) and solvent use other than paint, which includes 10 sources (e.g., printing ink, pharmaceutical production, pesticide use, domestic solvent, etc.). China’s VOCs emission inventories at present have the capability of depicting emissions on a very fine scale. They can generate source contribution information for individual VOC species at the provincial level.32−35 While these inventories have often been coupled with the MIR scale to quantify the source contribution to OFP, their application for studying the sources responsible for cancer risks has remained largely unexplored. Extending the scope of current emission inventories for cancer risk assessment is recommended to aid the development of more health-oriented control measures.
3.3. Source Contributions to Ozone Formation Potential
3.3.1. OFP Source Contributions by TAPs
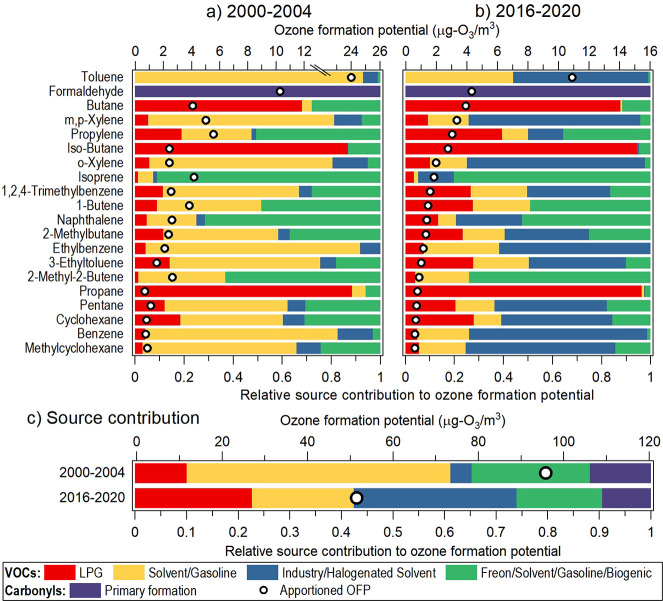
Panels a and b of Figure 2 show the site-averaged source contributions to OFP of the 20 most important VOCs/carbonyls in 2000–2004 and 2016–2020, respectively. These compounds represent ∼85% of the OFP calculated, with the remaining 44 compounds representing the rest. The secondary formation I–III carbonyls factors are not included in the OFP quantification to avoid double counting the secondary sources.13 Most VOCs exhibited a noticeable decrease in OFP contribution as a result of the reduction in solvent/gasoline and freon/solvent/gasoline/biogenic contributions, which dominated the OFPs in the early years, as shown in Figures S3b,d and 2a. The decrease in contribution from primary carbonyl formation also contributes to a discernible reduction in OFP of formaldehyde, which ranked second in both periods. Toluene was the top OFP contributor, showing an OFP 2 times higher than that of formaldehyde in both periods. This result, deriving from residential locations, differs from that observed in a roadside and coastal background location in Hong Kong primarily because the VOC compositions are different.36,37 Specifically, formaldehyde and isoprene were, respectively, the most important OFP contributor at the roadside and background locations, whereas toluene was beyond the top five list in both cases. The formaldehyde and isoprene concentrations observed in this study (2016–2020 average) were one-third (3.14 vs 9.48 μg/m3) and one-fifth (0.25 vs 1.31 μg/m3) those observed in the roadside and background studies, whereas the toluene concentration was comparable to that in the roadside study and was 5 times higher than that in the background study (4.67 vs 0.94 μg/m3).36,37 The variability in VOC composition and the consequential OFP contribution underline the importance of characterizing VOCs across a broader range of locations.
Figure 2.
Source contributions to the ozone formation potential (OFP) averaged from CW and TW sites. (a, b) Apportioned OFP (circle markers) and relative source contributions of the 20 most important compounds during (a) 2000–2004 and (b) 2016–2020. The compounds are arranged in descending order of OFP contribution in 2016–2020. (c) OFP contributions by source, including contributions from compounds beyond the top 20 list.
On the other hand, the increasing contribution of industry/halogenated solvent over the years is accompanied by a noticeable enhancement in OFP contribution in many VOCs (Figure 2b). Note that different from ICRs, where halogenated VOCs play the key role in the contribution, the OFP contributions of this source category are mostly attributed to BTEX, alkenes, or alkanes, which are much more reactive than halocarbons. The growing importance of industry/halogenated solvent as an OFP source is supported by a recent emission inventory study in Guangdong province, which is the broader geographical region to the north of Hong Kong where a multiplicity of regional sources is situated.35 The study showed that in 2017, the manufacturing and solvent use-related industrial processes accounted for 70–90% of the OFPs associated with m,p-xylene, toluene, o-xylene, and ethylbenzene, all of which were in the top 10 OFP-contributing species. A noteworthy point is that while butane and iso-butane are among the most important OFP contributors identified in this study, they did not appear in the top 10 list of the study.35 And the difference points to the significant contribution of local sources in Hong Kong to these OFP-significant VOCs.
3.3.2. OFP Contribution by Source Category
Figure 2c presents the site-averaged OFP contributions by source category in 2000–2004 and 2016–2020. The OFPs from species beyond the top 20 list are included. The OFP nearly halved across both periods, decreasing from 96 μg O3/m3 to 52 μg O3/m3. Such a change is driven by the reduction in solvent/gasoline, freon/solvent/gasoline/biogenic, and primary carbonyl formation sources. The reduction can be attributed to a series of VOC control policies that have been implemented by the local government since the mid-2000s.38 Example policies include controlling the VOC content of architectural paints, printing inks, consumer products, and other VOC-containing products. Control measures were also implemented to recover gasoline vapor released during vehicle refueling at gas stations and to tighten the emission standards of motor vehicles. Combined with the growing emissions from industry/halogenated solvents, as noted in Section 3.2, the OFP source contributions exhibited a noticeable shift from being solvent/gasoline-dominant in the early years to being more evenly distributed among different sources in recent years, making it more challenging to prioritize sources for control for O3 mitigation based on OFP. In 2016–2020, industry/halogenated solvents represent the most important OFP contributor, accounting for one-third of the OFP, followed by LPG (23%), solvent/gasoline (20%), freon/solvent/gasoline/biogenic (17%), and primary carbonyl formation (9%).
It should be noted that the OFP contributions reported here are obtained from the measured VOCs only. The OFP from other unmeasured species, for example, ethanol and ethyl acetate, which are among the top VOC components in solvent use emissions, are not accounted for here, possibly leading to underestimation.32 Nevertheless, as shown in the nationwide emission inventory in 2017, neither of these two compounds ranked among the top 30 OFP-contributing VOCs.34 Moreover, all of the top 30 OFP-contributing VOCs estimated from the nationwide inventory have been covered by the VOCs analyzed in this study, except for ethylene and butyl cellosolve (or 2-butoxyethanol), which ranked third and 26th in 2017, respectively. Therefore, the OFP analysis should represent a reasonable assessment of the potential impact of different source sectors on tropospheric O3 air pollution. Still, as in many observation-based OFP assessment studies, our OFP estimates for the more reactive VOCs such as alkenes could be underestimated because a certain fraction of the species might have been reacted prior to sampling.13
3.4. Policy Implications and Recommendations
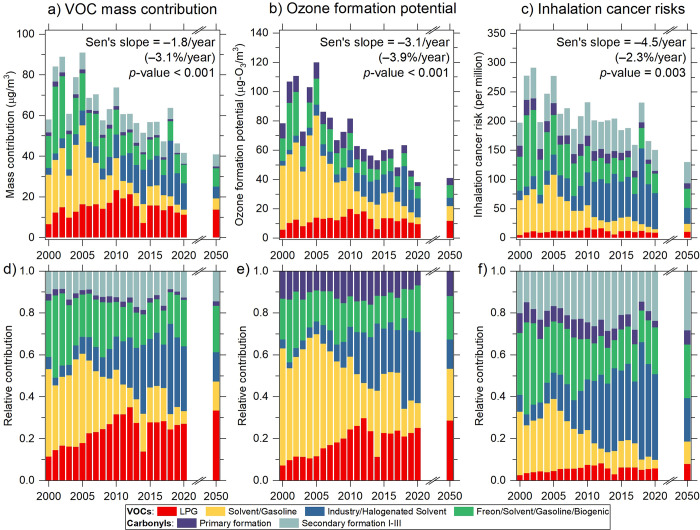
Figure 3 presents the two-decadal trends in the source contributions of VOCs/carbonyls by mass, OFP, and ICRs. For each metric, the upper and lower panels show the absolute and relative contributions, respectively. We quantify the trends by calculating the Theil–Sen’s slope and determine whether the trend is monotonic based on the significance level obtained from the Mann–Kendall trend test.39,40 These statistics are presented in Figure 3a–c for total source contributions and in Table S6 for individual sources. Overall, the total mass contributions showed a monotonic decrease (p < 0.001) at a rate of −1.8 μg/m3/year over the last two decades (Figure 3a), primarily driven by the reduction of the solvent/gasoline and freon/solvent/gasoline/biogenic factors (Table S6). Such a decrease has resulted in a simultaneous reduction in OFP of −3.1 μg O3/m3/year (p < 0.001) and ICRs of −4.5 cancer cases per million/year (p < 0.01) (Figure 3b,c). Noticeably, the industry/halogenated solvent source became increasingly important in terms of contribution in all three metrics, as illustrated in Figure 3d–f. To compare the interannual change across the three metrics, we normalize the Sen’s slope by the level in 2000, as shown in the parentheses in Figure 3a–c. The results show that OFP had a higher rate of reduction than mass reduction (−3.9 vs −3.1%/year). However, the reduction of ICRs was less than the reduction by mass, showing only −2.3%/year. The disparity in reduction rates indicates the growing industry/halogenated solvent source has a stronger effect on impeding the cancer risk reduction than the O3 reduction, benefited from the progress in air pollution control, emphasizing the importance of monitoring and controlling this source category more closely.
Figure 3.
Interannual trends in source contributions to VOCs and carbonyls by mass concentration, ozone formation potential, and inhalation cancer risks per million, averaged from CW and TW sites from 2000 to 2020. Prediction for 2050, assuming zero vehicular emissions and a best-health-effect pollution control scenario, is presented. The upper panels (a–c) and lower panels (d–f), respectively, show the absolute and relative contributions. The Sen’s slope values of the total source contributions and corresponding significance level of the Mann–Kendall trend test displayed in panels (a–c) are for 2000–2020. The percentage change in parentheses represents the Sen’s slope values normalized by the 2000 level.
For the years 2016–2020, the total ICRs derived from DPM and non-DPM sources were 451 cases per million. This value substantially exceeds the guideline value of 100 cases per million, which is considered to pose definite cancer risks with lifetime exposure.41 The local government recently set out policy objectives aiming to achieve zero vehicular emissions by 2050.42 This implies a possible ICR reduction of 241 cases per million, resulting from the full elimination of the DPM contribution (Table 1). LPG and gasoline vehicular emissions are not considered because their contributions are mixed with some other sources in the PMF factors and cannot be separated. The contribution of industry/halogenated solvent in 2050 can be estimated using the projected emission data retrieved from the Dynamic Projection model for Emissions in China (DPEC database: http://meicmodel.org.cn). From the DPEC database v1.1, the total emissions of non-methane VOCs associated with industry and solvent use combined in Guangdong province for 2015–2020 are 1.84 Tg. Assuming a socioeconomic transitions scenario under China’s carbon neutrality goals and best-health-effect local pollution control, the emissions would reduce to 0.64 Tg in 2050, equivalent to a 65% reduction relative to 2015–2020.43 This emission reduction is translated to an ICR reduction of 77 cases per million × 65% = 50 cases per million in 2050. Assuming that ICRs from other sources remain the same, the ICRs in 2050 would become 451 – 241 – 50 = 160 cases per million, with VOC and carbonyl sources accounting for 81% (130 cases per million) of the risks. As shown in Figure 3f, secondary carbonyl formation and freon/solvent/gasoline/biogenic factors would become the major risk contributors (26–28%), followed by industry/halogenated solvent (21%) and other sources (7–11%).
The reduction in public health risks resulting from the targeted control of key cancer-causing sources (mainly DPM, gasoline, solvent use, and coal/biomass combustion) over the last two decades is substantial. Remarkably, the ICR estimate had dropped by 73% from 1701 cases per million in 2000–2004 to 451 cases per million in 2016–2020. However, it is important to acknowledge that these are the excess risks that are in addition to the baseline cancer risks and are not the overall absolute cancer risks of a population. To provide some context, the crude incidence rate of cancer in Hong Kong in 2020 was 4569 cases per million.44 Moreover, these risks are expected to be higher in the GBA, where TAP sources are more widespread. To better evaluate the health risks associated with exposure to ambient air toxics and understand their sources in the GBA, establishing a pan-GBA TAP monitoring program would be highly beneficial. While emission inventory could potentially be used to help identify sources of TAPs, studies showed large discrepancies between the emission-based and observation-based source apportionment of VOCs, emphasizing that ambient monitoring is needed to complement source analysis.45,46 In addition to health protection, many of the TAP species also play a crucial role in other environmental issues, such as global warming and stratospheric ozone depletion. Having a pan-GBA TAP monitoring network would yield important guidance to address many of these environmental issues at a regional level.
4. Conclusions
This study investigated the sources of a comprehensive set of TAPs in Hong Kong, China, from 2000 to 2020 with PMF modeling. A considerable reduction in ICR contributions was identified in DPM, solvent/gasoline VOCs, freon/solvent/gasoline/biogenic VOCs, and coal/biomass combustion PAHs and metal(loids), supporting the effectiveness of air pollution control measures implemented by local and regional governments over the last two decades. The contrast in DPM vs non-DPM ICRs had narrowed substantially, which highlights the emerging importance of VOC sources as ICR contributors. A regional industry/halogenated solvent VOC source exhibiting a steep increasing trend was identified. This source has become the largest non-DPM ICR contributor in recent years. The rise of this source had a more significant impact in counteracting the reduction in ICRs from VOCs than in offsetting the reductions in mass and OFP. These results underline that a more extensive evaluation of the health risks deriving from this source is necessary. This undertaking can be aided by extending the emission inventory for health risk assessment application. Source contributions to OFP were found to change from being solvent/gasoline-dominant in the past to being more evenly shared by various VOC and carbonyl sources in recent years, challenging the use of the OFP scale for developing O3 mitigation strategies. This 21 year long TAP monitoring in Hong Kong unveiled the changes in significant sources contributing to inhalation risks and O3 formation potential. We have demonstrated, by way of using Hong Kong as an example, the importance and necessity of TAP monitoring. Given that the ICRs in Hong Kong and across the GBA are expected to remain above 100 cases per million by 2050, we anticipate that establishing a similar TAP monitoring network in the GBA will provide the monitoring data needed to develop more effective air quality management strategies.
Acknowledgments
We gratefully acknowledge the Environmental Protection Department and Government Laboratory of HKSAR, China, for unwaveringly maintaining the TAP program. Appreciation is given to the excellent research assistance of Chi Kit Chan, Ka Kit Cheng, Shun Ching Cheuk, Ming Hin Leung, and Hau Yin Wong.
Data Availability Statement
The TAP monitoring data cannot be made publicly accessible by the authors at the moment, as the data are exclusively owned by the Hong Kong Environmental Protection Department. However, the data can be requested by emailing enquiry@epd.gov.hk or by contacting the corresponding authors (alau@ust.hk, jian.yu@ust.hk).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/envhealth.3c00209.
Additional details on PMF model inputs, source interpretation, and model evaluation; ambient concentrations and unit risk estimates of TAPs measured in this study; and statistics for VOC and carbonyl source contribution trends (PDF)
Author Contributions
Y.K.W.: data curation, formal analysis, and writing—original draft preparation. W.W.C. and D.G.: data curation and writing—review and editing. J.Z.Y.: conceptualization, supervision, and writing—review and editing. A.K.H.L.: funding acquisition, conceptualization, supervision, and writing—review and editing.
This research has been supported by the Hong Kong Environmental Protection Department (Project 20-00424). The work described in this paper was partially supported by a fellowship award from the Research Grants Council of the HKSAR, China (HKUST PDFS2223-6S10).
The content of this paper does not necessarily reflect the views and policies of the HKSAR government, nor does the mention of trade names or commercial products constitute an endorsement or recommendation of their use.
The authors declare no competing financial interest.
Supplementary Material
References
- U.S. EPA . Health Effects Notebook for Hazardous Air Pollutants. https://www.epa.gov/haps/health-effects-notebook-hazardous-air-pollutants (accessed 2023-05-13).
- Lyu X.; Guo H.; Wang Y.; Zhang F.; Nie K.; Dang J.; Liang Z.; Dong S.; Zeren Y.; Zhou B.; Gao W.; Zhao S.; Zhang G. Hazardous Volatile Organic Compounds in Ambient Air of China. Chemosphere 2020, 246, 125731. 10.1016/j.chemosphere.2019.125731. [DOI] [PubMed] [Google Scholar]
- Propper R.; Wong P.; Bui S.; Austin J.; Vance W.; Alvarado Á.; Croes B.; Luo D. Ambient and Emission Trends of Toxic Air Contaminants in California. Environ. Sci. Technol. 2015, 49 (19), 11329–11339. 10.1021/acs.est.5b02766. [DOI] [PubMed] [Google Scholar]
- Weitekamp C. A.; Lein M.; Strum M.; Morris M.; Palma T.; Smith D.; Kerr L.; Stewart M. J. An Examination of National Cancer Risk Based on Monitored Hazardous Air Pollutants. Environ. Health Perspect. 2021, 129 (3), EHP8044 10.1289/EHP8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA . Initial List of Hazardous Air Pollutants with Modifications. https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications (accessed 2024-02-14).
- PRCMoEE . List of Toxic and Hazardous Air Pollutants (2018. Edition). https://www.mee.gov.cn/xxgk2018/xxgk/xxgk01/201901/t20190131_691779.html (accessed 2024-02-14).
- Wong Y. K.; Chan W. W.; Gu D.; Wong T. W.; Chan K. J. D.; Yu J. Z.; Lau A. K. H. Characterization of Toxic Air Pollutants in Hong Kong, China: Two-Decadal Trends and Health Risk Assessments. Atmos. Environ. 2023, 314, 120129. 10.1016/j.atmosenv.2023.120129. [DOI] [Google Scholar]
- Monks P. S.; Archibald A. T.; Colette A.; Cooper O.; Coyle M.; Derwent R.; Fowler D.; Granier C.; Law K. S.; Mills G. E.; Stevenson D. S.; Tarasova O.; Thouret V.; von Schneidemesser E.; Sommariva R.; Wild O.; Williams M. L. Tropospheric Ozone and Its Precursors from the Urban to the Global Scale from Air Quality to Short-Lived Climate Forcer. Atmospheric Chem. Phys. 2015, 15 (15), 8889–8973. 10.5194/acp-15-8889-2015. [DOI] [Google Scholar]
- Feng X.; Guo J.; Wang Z.; Gu D.; Ho K.-F.; Chen Y.; Liao K.; Cheung V.; Louie P. K. K.; Leung K.; Yu J. Z.; Fung J.; Lau A. Investigation of the Multi-Year Trend of Surface Ozone and Ozone-Precursor Relationship in Hong Kong. Atmos. Environ. 2023, 315, 120139. 10.1016/j.atmosenv.2023.120139. [DOI] [Google Scholar]
- Li X.-B.; Yuan B.; Parrish D. D.; Chen D.; Song Y.; Yang S.; Liu Z.; Shao M. Long-Term Trend of Ozone in Southern China Reveals Future Mitigation Strategy for Air Pollution. Atmos. Environ. 2022, 269, 118869. 10.1016/j.atmosenv.2021.118869. [DOI] [Google Scholar]
- Louie P. K. K.; Ho J. W. K.; Tsang R. C. W.; Blake D. R.; Lau A. K. H.; Yu J. Z.; Yuan Z.; Wang X.; Shao M.; Zhong L. VOCs and OVOCs Distribution and Control Policy Implications in Pearl River Delta Region, China. Atmos. Environ. 2013, 76, 125–135. 10.1016/j.atmosenv.2012.08.058. [DOI] [Google Scholar]
- Carter W. P. L. Development of Ozone Reactivity Scales for Volatile Organic Compounds. Air Waste 1994, 44 (7), 881–899. 10.1080/1073161X.1994.10467290. [DOI] [Google Scholar]
- Zhang Y.; Xue L.; Carter W. P. L.; Pei C.; Chen T.; Mu J.; Wang Y.; Zhang Q.; Wang W. Development of Ozone Reactivity Scales for Volatile Organic Compounds in a Chinese Megacity. Atmospheric Chem. Phys. 2021, 21 (14), 11053–11068. 10.5194/acp-21-11053-2021. [DOI] [Google Scholar]
- Zhang X.; Yuan Z.; Li W.; Lau A. K. H.; Yu J. Z.; Fung J. C. H.; Zheng J.; Yu A. L. C. Eighteen-Year Trends of Local and Non-Local Impacts to Ambient PM10 in Hong Kong Based on Chemical Speciation and Source Apportionment. Atmospheric Res. 2018, 214, 1–9. 10.1016/j.atmosres.2018.07.004. [DOI] [Google Scholar]
- Sin D. W.-M.; Wong Y.-C.; Sham W.-C.; Wang D. Development of an Analytical Technique and Stability Evaluation of 143 C3-C12 Volatile Organic Compounds in Summa® Canisters by Gas Chromatography-Mass Spectrometry. Analyst 2001, 126 (3), 310–321. 10.1039/b008746g. [DOI] [PubMed] [Google Scholar]
- Norris G.; Duvall R.; Brown S.; Bai S. In EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide; U.S. EPA, 2014. [Google Scholar]
- Paatero P.; Tapper U. Positive Matrix Factorization: A Non-Negative Factor Model with Optimal Utilization of Error Estimates of Data Values. Environmetrics 1994, 5 (2), 111–126. 10.1002/env.3170050203. [DOI] [Google Scholar]
- Huang X. H. H.; Bian Q. J.; Louie P. K. K.; Yu J. Z. Contributions of Vehicular Carbonaceous Aerosols to PM2.5 in a Roadside Environment in Hong Kong. Atmospheric Chem. Phys. 2014, 14 (17), 9279–9293. 10.5194/acp-14-9279-2014. [DOI] [Google Scholar]
- Birch M. E.; Cary R. A. Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust. Aerosol Sci. Technol. 1996, 25 (3), 221–241. 10.1080/02786829608965393. [DOI] [PubMed] [Google Scholar]
- Wong Y. K.; Huang X. H. H.; Louie P. K. K.; Yu A. L. C.; Chan D. H. L.; Yu J. Z. Tracking Separate Contributions of Diesel and Gasoline Vehicles to Roadside PM2.5 through Online Monitoring of Volatile Organic Compounds and PM2.5 Organic and Elemental Carbon: A 6-Year Study in Hong Kong. Atmospheric Chem. Phys. 2020, 20 (16), 9871–9882. 10.5194/acp-20-9871-2020. [DOI] [Google Scholar]
- Lee B. P.; Li Y. J.; Yu J. Z.; Louie P. K. K.; Chan C. K. Characteristics of Submicron Particulate Matter at the Urban Roadside in Downtown Hong Kong—Overview of 4 Months of Continuous High-Resolution Aerosol Mass Spectrometer Measurements. J. Geophys. Res. Atmospheres 2015, 120 (14), 7040–7058. 10.1002/2015JD023311. [DOI] [Google Scholar]
- U.S. EPA . Dose-Response Assessment for Assessing Health Risks Associated With Exposure to Hazardous Air Pollutants. https://www.epa.gov/fera/dose-response-assessment-assessing-health-risks-associated-exposure-hazardous-air-pollutants (accessed 2022-12-29).
- CARB . Consolidated Table of OEHHA/CARB Approved Risk Assessment Health Values. https://ww2.arb.ca.gov/resources/documents/consolidated-table-oehha-carb-approved-risk-assessment-health-values (accessed 2022-12-29).
- Guidelines for Air Quality (WHO/SDE/OEH/00.02); WHO, 2000. https://apps.who.int/iris/handle/10665/66537 (accessed 2022-12-23). [Google Scholar]
- Air Toxics Risk Assessment Reference Library; U.S. EPA, 2004; Vol 1. https://www.epa.gov/sites/default/files/2013-08/documents/volume_1_reflibrary.pdf (accessed 2024-02-17). [Google Scholar]
- Carter W. P. L. In Estimation of Ozone Reactivities for Volatile Organic Compound Speciation Profiles in the Speciate 4.2 Database; Center for Environmental Research and Technology, University of California, Riverside, 2013. [Google Scholar]
- MATES V Multiple Air Toxics Exposure Study; South Coast AQMD, 2021. http://www.aqmd.gov/home/air-quality/air-quality-studies/health-studies/mates-v (accessed 2023-08-19). [Google Scholar]
- Kim M.-J.; Baek K.-M.; Heo J.-B.; Cheong J.-P.; Baek S.-O. Concentrations, Health Risks, and Sources of Hazardous Air Pollutants in Seoul-Incheon, a Megacity Area in Korea. Air Qual. Atmosphere Health 2021, 14 (6), 873–893. 10.1007/s11869-021-00986-z. [DOI] [Google Scholar]
- Xiong Y.; Huang Y.; Du K. Health Risk-Oriented Source Apportionment of Hazardous Volatile Organic Compounds in Eight Canadian Cities and Implications for Prioritizing Mitigation Strategies. Environ. Sci. Technol. 2022, 56 (17), 12077–12085. 10.1021/acs.est.2c02558. [DOI] [PubMed] [Google Scholar]
- Schauer J. J.; Kleeman M. J.; Cass G. R.; Simoneit B. R. T. Measurement of Emissions from Air Pollution Sources. 5. C1-C32 Organic Compounds from Gasoline-Powered Motor Vehicles. Environ. Sci. Technol. 2002, 36 (6), 1169–1180. 10.1021/es0108077. [DOI] [PubMed] [Google Scholar]
- Wang X.; Ho K.-F.; Chow J. C.; Kohl S. D.; Chan C. S.; Cui L.; Lee S. F.; Chen L.-W. A.; Ho S. S. H.; Cheng Y.; Watson J. G. Hong Kong Vehicle Emission Changes from 2003 to 2015 in the Shing Mun Tunnel. Aerosol Sci. Technol. 2018, 52 (10), 1085–1098. 10.1080/02786826.2018.1456650. [DOI] [Google Scholar]
- Mo Z.; Cui R.; Yuan B.; Cai H.; McDonald B. C.; Li M.; Zheng J.; Shao M. A Mass-Balance-Based Emission Inventory of Non-Methane Volatile Organic Compounds (NMVOCs) for Solvent Use in China. Atmospheric Chem. Phys. 2021, 21 (17), 13655–13666. 10.5194/acp-21-13655-2021. [DOI] [Google Scholar]
- Simayi M.; Shi Y.; Xi Z.; Ren J.; Hini G.; Xie S. Emission Trends of Industrial VOCs in China since the Clean Air Action and Future Reduction Perspectives. Sci. Total Environ. 2022, 826, 153994. 10.1016/j.scitotenv.2022.153994. [DOI] [PubMed] [Google Scholar]
- Li M.; Zhang Q.; Zheng B.; Tong D.; Lei Y.; Liu F.; Hong C.; Kang S.; Yan L.; Zhang Y.; Bo Y.; Su H.; Cheng Y.; He K. Persistent Growth of Anthropogenic Non-Methane Volatile Organic Compound (NMVOC) Emissions in China during 1990–2017: Drivers, Speciation and Ozone Formation Potential. Atmospheric Chem. Phys. 2019, 19 (13), 8897–8913. 10.5194/acp-19-8897-2019. [DOI] [Google Scholar]
- Huang Z.; Zhong Z.; Sha Q.; Xu Y.; Zhang Z.; Wu L.; Wang Y.; Zhang L.; Cui X.; Tang M.; Shi B.; Zheng C.; Li Z.; Hu M.; Bi L.; Zheng J.; Yan M. An Updated Model-Ready Emission Inventory for Guangdong Province by Incorporating Big Data and Mapping onto Multiple Chemical Mechanisms. Sci. Total Environ. 2021, 769, 144535. 10.1016/j.scitotenv.2020.144535. [DOI] [PubMed] [Google Scholar]
- Han S.; Tan Y.; Gao Y.; Li X.; Ho S. S. H.; Wang M.; Lee S. Volatile Organic Compounds at a Roadside Site in Hong Kong: Characteristics, Chemical Reactivity, and Health Risk Assessment. Sci. Total Environ. 2023, 866, 161370. 10.1016/j.scitotenv.2022.161370. [DOI] [PubMed] [Google Scholar]
- Tan Y.; Han S.; Chen Y.; Zhang Z.; Li H.; Li W.; Yuan Q.; Li X.; Wang T.; Lee S. Characteristics and Source Apportionment of Volatile Organic Compounds (VOCs) at a Coastal Site in Hong Kong. Sci. Total Environ. 2021, 777, 146241. 10.1016/j.scitotenv.2021.146241. [DOI] [Google Scholar]
- HKEPD . An Overview on Air Quality and Air Pollution Control in Hong Kong. https://www.epd.gov.hk/epd/english/environmentinhk/air/air_maincontent.html (accessed 2023-11-09).
- Gilbert R. O. In Statistical Methods for Environmental Pollution Monitoring; John Wiley & Sons, Inc., New York, New York, U.S., 1987. [Google Scholar]
- Sen P. K. Estimates of the Regression Coefficient Based on Kendall’s Tau. J. Am. Stat. Assoc. 1968, 63 (324), 1379–1389. 10.1080/01621459.1968.10480934. [DOI] [Google Scholar]
- Sexton K.; Linder S. H.; Marko D.; Bethel H.; Lupo P. J. Comparative Assessment of Air Pollution-Related Health Risks in Houston. Environ. Health Perspect. 2007, 115 (10), 1388–1393. 10.1289/ehp.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HKEPD . Promotion of Electric Vehicles. https://www.epd.gov.hk/epd/english/environmentinhk/air/promotion_ev/promotion_ev.html (accessed 2024-02-19).
- Tong D.; Cheng J.; Liu Y.; Yu S.; Yan L.; Hong C.; Qin Y.; Zhao H.; Zheng Y.; Geng G.; Li M.; Liu F.; Zhang Y.; Zheng B.; Clarke L.; Zhang Q. Dynamic Projection of Anthropogenic Emissions in China: Methodology and 2015–2050 Emission Pathways under a Range of Socio-Economic, Climate Policy, and Pollution Control Scenarios. Atmospheric Chem. Phys. 2020, 20 (9), 5729–5757. 10.5194/acp-20-5729-2020. [DOI] [Google Scholar]
- Hong Kong Cancer Registry . Overview of Hong Kong Cancer Statistics of 2020. https://www3.ha.org.hk/cancereg (accessed 2024-02-12).
- Mo Z.; Shao M.; Liu Y.; Xiang Y.; Wang M.; Lu S.; Ou J.; Zheng J.; Li M.; Zhang Q.; Wang X.; Zhong L. Species-Specified VOC Emissions Derived from a Gridded Study in the Pearl River Delta, China. Sci. Rep. 2018, 8 (1), 2963. 10.1038/s41598-018-21296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J.; Zheng J.; Yuan Z.; Guan D.; Huang Z.; Yu F.; Shao M.; Louie P. K. K. Reconciling Discrepancies in the Source Characterization of VOCs between Emission Inventories and Receptor Modeling. Sci. Total Environ. 2018, 628–629, 697–706. 10.1016/j.scitotenv.2018.02.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The TAP monitoring data cannot be made publicly accessible by the authors at the moment, as the data are exclusively owned by the Hong Kong Environmental Protection Department. However, the data can be requested by emailing enquiry@epd.gov.hk or by contacting the corresponding authors (alau@ust.hk, jian.yu@ust.hk).