Abstract
Several different cellular processes determine the size of the metabolically available nitrate pool in the cytoplasm. These processes include not only ion fluxes across the plasma membrane and tonoplast but also assimilation by the activity of nitrate reductase (NR). In roots, the maintenance of cytosolic nitrate activity during periods of nitrate starvation and resupply (M. van der Leij, S.J. Smith, A.J. Miller [1998] Planta 205: 64–72; R.-G. Zhen, H.-W. Koyro, R.A. Leigh, A.D. Tomos, A.J. Miller [1991] Planta 185: 356–361) suggests that this pool is regulated. Under nitrate-replete conditions vacuolar nitrate is a membrane-bound store that can release nitrate to the cytoplasm; after depletion of cytosolic nitrate, tonoplast transporters would serve to restore this pool. To study the role of assimilation, specifically the activity of NR in regulating the size of the cytosolic nitrate pool, we have compared wild-type and mutant plants. In leaf mesophyll cells, light-to-dark transitions increase cytosolic nitrate activity (1.5–2.8 mm), and these changes were reversed by dark-to-light transitions. Such changes were not observed in nia1nia2 NR-deficient plants indicating that this change in cytosolic nitrate activity was dependent on the presence of functional NR. Furthermore, in the dark, the steady-state cytosolic nitrate activities were not statistically different between the two types of plant, indicating that NR has little role in determining resting levels of nitrate. Epidermal cells of both wild type and NR mutants had cytosolic nitrate activities that were not significantly different from mesophyll cells in the dark and were unaltered by dark-to-light transitions. We propose that the NR-dependent changes in cytosolic nitrate provide a cellular mechanism for the diurnal changes in vacuolar nitrate storage, and the results are discussed in terms of the possible signaling role of cytosolic nitrate.
Nitrogen is the mineral nutrient required in the highest amounts by plants and is most frequently limiting growth and yield. Nitrate is often the most abundant form of nitrogen available to roots especially in temperate agricultural soils. Nitrate incorporation into biological molecules involves the reduction of nitrate to nitrite via the cytosolic enzyme nitrate reductase (NR). The key position of this enzyme early in the pathway and the cellular toxicity of the product, nitrite, has stimulated research to identify if NR is a critical regulatory step in nitrate assimilation (Stitt et al., 2002). Measurements of both gene expression and protein levels of NR have shown that the amounts of the enzyme are not what limit the NR activity in cells (for review, see Kaiser and Huber, 2001; MacKintosh and Meek, 2001). NR is known to be under complex regulation that occurs at both transcription and posttranscriptional levels. The expression of NR is induced by the application of nitrate, and the activity of the protein is altered in response to changes in environmental conditions, such as light, dark, anoxia, pH, and carbon dioxide concentration (for review, see Kaiser et al., 1999; Kaiser and Huber, 2001).
A link between leaf tissue NR activity and nitrate concentration has been described in several papers (e.g. Riens and Heldt, 1992; Ferrario et al., 1995, and refs. therein). The sophisticated regulation of NR activity suggests this step may be important in the regulation of nitrogen metabolism. Nitrogen status, or at least nitrate availability and internal concentrations of Gln, have been shown to regulate NR degradation (Scheible et al., 1997). This fact together with the ability of NR activity to change rapidly and reversibly by phosphorylation in response to changing environmental conditions implies a potential capacity to coordinate associated metabolism. The tight control of NR activity may result in changes in cytoplasmic nitrate pools that may have a signaling role.
The dramatic effects of nitrate on growth, development, and germination were recognized more than 30 years ago and led to the proposal that the ion was like a plant hormone (Trewavas, 1983). More recently, nitrate has been identified as a signal to initiate changes in carbon and nitrogen metabolism (Redinbaugh and Campbell, 1991; Crawford, 1995; Scheible et al., 1997; Stitt, 1999; Coruzzi and Bush, 2001). The cytosolic activities of well-known signaling ions, such as free Ca2+ (Sanders et al., 2002) and H+, change in response to environmental stimuli in a distinctive repeatable fashion (Felle, 2001). The ability of these ions to signal changes are dependent upon certain physical characteristics. First, there must be homeostatic control of cytosolic ion activity when signal stimuli are absent; hence, there must be mechanisms to restore cytosolic ion activities after a signaling event. Secondly, there must be sufficient gradients across membranes surrounding the cytosol to facilitate sufficient ion change during a signal. Thirdly, there must be a change in the cytosolic-signaling ion activity in response to environmental stimuli. The cytosolic nitrate pool does have some of the physical characteristics required to provide a signal, but until now there has been no direct measure of a link between any environmental stimuli and a change in cytosolic nitrate. Finally, implicit in the concept of signaling is that a small change in the signal is amplified through a receptor cascade, and cytosolic nitrate does not fulfill this requirement at the moment.
The aim of this work was to investigate whether changes in cytosolic nitrate activities could be detected in conditions known to produce changes in the NR activity. NR is activated in the light and deactivated in the dark; therefore, light-to-dark transitions were used as the stimulus (Kaiser and Spill, 1991). To determine whether functional NR played a role, the measurements were made in wild type and a mutant that has 0.5% to 2% of the NR activity found in the wild type. We demonstrated a light-dependent change in the cytosolic nitrate pool of leaf mesophyll cells, and these results are discussed in terms of the diurnal changes in vacuolar nitrate pools and the signaling role of nitrate.
RESULTS
Steady-State Nitrate-Selective Microelectrode Measurements Made in Wild Type and nia1nia2 Arabidopsis Leaves
Wild-type plants were grown in both hydroponic and sterile culture (see “Materials and Methods”), and measurements were not significantly different with either culture method, and so the data were pooled for all subsequent analysis. The mean nitrate activities of the apoplastic, cytosolic, and vacuolar populations from epidermal and mesophyll cells of wild-type and nia1nia2 plants in the dark are shown in Table I. There are no statistically significant differences between the mean cytosolic nitrate activities between both cell and plant types in the dark. The mean vacuolar nitrate activity in both the wild-type and nia1nia2 epidermal and mesophyll cells was considerably higher than that of the cytosol, between 25 and 50 mm. The measurements of nitrate activity in wild-type and nia1nia2 leaf apoplast were much lower than the cytosol and vacuole, with means of 0.3 and 0.7 mm, respectively, but these were not significantly different. The cell membrane potentials were also not significantly different between the cell types or type of plant. Subtracting the mean membrane potential measured in the vacuole from that in the cytoplasm for wild type, we obtain transtonoplast potentials of −48 ± 9 mV (mean ± sd) for the epidermal cells and −31 ± 13 mV (mean ± sd) for mesophyll cells. These two values are not significantly different.
Table I.
Mean values of steady-state nitrate-selective microelectrode measurements in wild-type and nia1nia2 epidermal and mesophyll cells in the dark
| Compartment | Plant | Cell Type | Mean Nitrate Activity | Mean Membrane Potential |
|---|---|---|---|---|
| mm | mv | |||
| Cytoplasm | Wild type | Epidermal | 2.2 (±0.5)a | −147.0 (±15.8) |
| Mesophyll | 2.8 (±0.7) | −128.6 (±20.3) | ||
| nia1nia2 | Epidermal | 3.2 (±1.6) | −123.3 (±11.6) | |
| Mesophyll | 3.9 (±0.6) | −118.6 (±24.4) | ||
| Vacuole | Wild type | Epidermal | 35.0 (±7.1) | −99.5 (±7.8) |
| Mesophyll | 31.8 (±11.8) | −98.0 (±27.6) | ||
| nia1nia2 | Epidermal | 50.0 (*)b | −60.0 (*) | |
| Mesophyll | 32.0 (*) | −100.0 (*) | ||
| Apoplast | Wild type | 0.3 (±0.2) | – | |
| nia1nia2 | 0.7 (±0.3) | – |
sds shown in brackets n > 5.
*, No replication.
Dynamic Nitrate-Selective Microelectrode Measurements in Arabidopsis Leaves during Light-Dark and Dark-Light Transitions
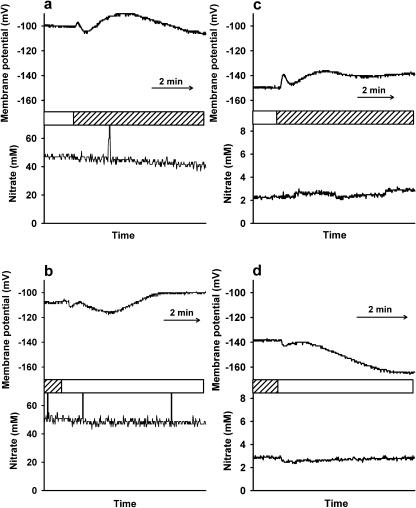
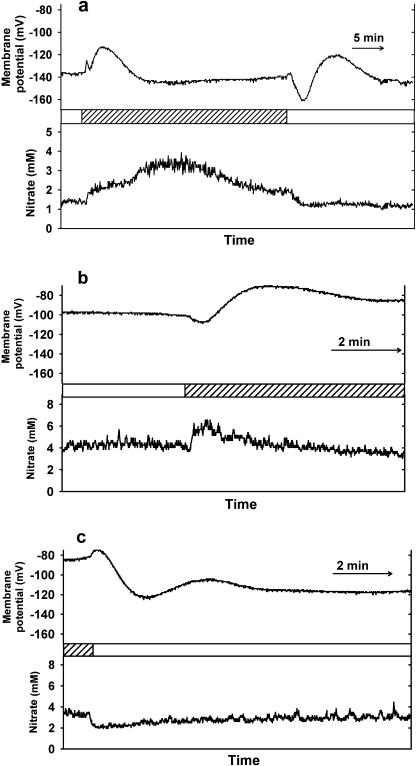
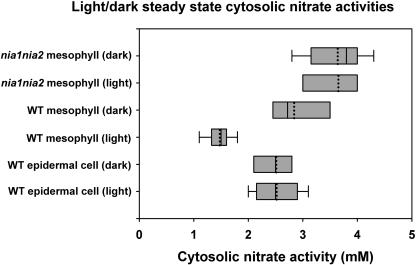
Figures 1 and 2 show some typical examples of microelectrode recordings in Arabidopsis (Arabidopsis thaliana) leaves during light/dark transitions in epidermal and mesophyll cells, respectively. The top trace of each figure shows the membrane potential and the bottom trace shows the nitrate activity of the cell compartment. Figure 3 summarizes the mean steady-state nitrate activities in the light and dark for the electrode measurements in wild-type and nia1nia2 leaf cells, of which examples are shown in Figures 1 and 2. Further examples of microelectrode recordings during dark and light transitions are given as supplemental data.
Figure 1.
Typical examples of nitrate-selective microelectrode recordings in Arabidopsis epidermal leaf cells during light/dark transitions; bars indicate the time at which the light or dark (shaded) treatment was applied. a, Wild-type epidermal vacuole during light-to-dark transition; b, Wild-type epidermal vacuole during dark-to-light transition; c, Wild-type epidermal cytosol during light-to-dark transition; d, Wild-type epidermal cytosol during dark-to-light transition.
Figure 2.
Typical examples of nitrate-selective microelectrode recordings in Arabidopsis mesophyll cells during light/dark transitions; bars indicate the time at which the light or dark (shaded) treatment was applied. a, Wild-type mesophyll cytosol during light-to-dark and dark-to-light transition in one cell; b, nia1nia2 mesophyll cytosol during light-to-dark transition; c, nia1nia2 mesophyll cytosol during dark-to-light transition.
Figure 3.
Box plot of data showing the range of steady-state nitrate electrode measurements for leaf cells in the light and dark. The horizontal boxes show the fifth and 95th percentiles, the median is shown as a solid line, the mean as a dotted line, and the bars show the maximum and minimum values.
Figure 1, a and b, shows that there was no change in vacuolar nitrate activity (in these examples approximately 50 mm) in response to light-dark and dark-light transitions in wild-type epidermal cells. In a few epidermal cells, a slight decrease in vacuolar nitrate activity was reported during the recording, but these changes were independent of changes in the light supply (e.g. Fig. 1a). When these changes happened, the vacuolar nitrate concentration always decreased and the rate of change was about 0.5 mm per min. However, for the recordings from mesophyll cells, we found no changes in vacuolar nitrate activity for the duration of most of the recordings. One exception showed a slight increase in vacuolar nitrate, and this example is given in the supplemental data (Fig. Bb), but we have no explanation for these differences between the two types of leaf cell.
The membrane potential reported from the vacuole did change in response to light-dark and dark-light transitions in a multiphase, transient response. The examples shown are typical responses in terms of magnitude, duration, and shape of response. Epidermal cells responded to light-dark transitions (see Fig. 1a) with a fast, small initial depolarization (the membrane potential changes to a less negative value) followed by a subsequent small hyperpolarization (the membrane potential changes to a more negative value), then a more prolonged depolarization, and then another slow hyperpolarization before returning to a steady resting membrane potential. Epidermal cell membrane potentials respond to dark-light transitions (see Fig. 1b) in the reverse way to light-dark transitions. Generally, the resting membrane potential of each cell was slightly more negative in the dark compared with that measured in the light. These light/dark changes in membrane potential were obtained using double-barreled electrodes but are very similar to recordings made using single-barrel microelectrodes in Arabidopsis leaf cells (Spalding, 1995, 2000).
There was also little or no change in cytosolic nitrate activity (maintained at approximately 3 mm in these examples) in response to light-dark and dark-light changes in wild-type epidermal cells (Fig. 1, c and d). The membrane potential of the cell changed in response to the light-dark and dark-light transitions with the same response as described and shown previously for responses recorded from vacuoles of epidermal cells (Fig. 1, a and b).
In contrast, there was a change in cytosolic nitrate activity in response to light-dark and dark-light changes in wild-type mesophyll cells (Fig. 2a). Figure 2a shows the light-to-dark transition; during the light treatment, the cytosolic nitrate activity was 1.3 mm. Under the dark treatment, cytosolic nitrate activity was maintained at around 2 mm. In the reverse process, after the application of the light treatment, the cytosolic nitrate activity rapidly decreased to 1.3 mm (see Fig. 2a). On switching to the dark, cytosolic nitrate activity quickly increased to 2 mm and then more gradually changed to 3.5 mm over the following 7 min. A steady-state nitrate activity of 2 mm was restored after a further 7 min. The membrane potential of the cell changed in response to the illumination transitions in a pattern similar to that of the epidermal cells (as shown in Fig. 1, a–d). The mean magnitude and duration of the perturbation in the membrane potential to light off and light on in mesophyll cells was significantly greater than the response in epidermal cells (data not shown). Mean cytosolic nitrate activity in wild-type mesophyll cells was significantly different during the light and dark treatments. However, in the epidermal cells, there was no significant difference in cytosolic nitrate under the two treatments.
The magnitude, pattern, and duration of membrane potential perturbation in nia1nia2 mesophyll cells was not significantly different (data not shown) from those of the wild-type mesophyll cells. The mean cytosolic nitrate activity in the nia1nia2 mesophyll cells was not significantly different under the light and dark treatments. Cytosolic nitrate activity in the nia1nia2 mesophyll cells showed small, rapidly recovering changes (2 min) in response to light-dark and dark-light transitions (Fig. 2, b and c). Similar small, short, transient responses could be seen in epidermal cells (Fig. 1, c and d). These transients show a return to the same steady-state nitrate activities and are probably due to the differing response times of the nitrate-selective and the membrane potential recording barrels of the microelectrode (see supplemental data and “Discussion” for more details). The membrane potential of a mesophyll cell changes rapidly and considerably in response to illumination changes, and so the differing response times of the microelectrode barrels could have a considerable effect during the first few minutes of the light transition. The silver-chloride junctions of electrodes can respond to light (Janz, 1961), but this was unlikely to explain these changes (see “Materials and Methods”). After the apparent transient change, the nitrate activity returned to the pretreatment level, a response different from that of the wild-type mesophyll cell. As only wild-type plants show cytosolic nitrate activity changes in response to illumination transitions, this supports a role for NR activity in the response, and this is discussed below.
DISCUSSION
Nitrate in the Apoplast
Nitrate activity in the apoplast of wild-type Arabidopsis leaf tissue was approximately 0.3 mm, which is similar to the values measured in other species and using different methods (Kronzucker et al., 1995; Mühling and Sattelmacher, 1995). Low-apoplastic nitrate activity may result from the fact that the cell wall contains many fixed-negative charges and is therefore a compartment of high cation exchange capacity from which anions are excluded. In addition, the plasma membrane of leaf cells may contain high-affinity nitrate transporters that are able to access this pool and remove nitrate from the apoplast very effectively.
Membrane Potentials in Leaf Cells
The membrane potential changes presented here were similar to previous reports of the responses for light-to-dark and dark-to-light transitions in Arabidopsis and other species in terms of shape, magnitude, and duration of response (e.g. Spalding 1995, 2000; Blom-Zandstra et al., 1997; Stahlberg and Van Volkenburgh, 1999). Although the pattern was similar, the magnitude and duration of the membrane potential response to light-dark transitions in mesophyll cells was different from that measured in epidermal cells. This tissue difference has been reported previously for other plant species (for example, Pisum sativum; Elzenga et al., 1995). The transtonoplast membrane potentials reported here are very similar to those described previously for Arabidopsis leaf mesophyll cells measured using pH-selective microelectrodes (Miller et al., 2001).
The membrane potential changes in response to illumination transitions for the wild-type and nia1nia2 mesophyll cells were similar in terms of magnitude, direction, and duration of response. This is important as it suggests that although the NR enzyme has been inactivated the plant cell responds normally to light transitions in terms of membrane potential changes.
Leaf Cell Nitrate Pools
Cytosolic nitrate activities have now been recorded in leaf cells, previous measurements have been conducted in nitrate-replete root cells (e.g. Zhen et al., 1991; Miller and Smith, 1992; van der Leij et al., 1998). Values for the cytosol in leaf cells are similar to those in root cells and are in the low millimolar range (Table I). In those earlier papers on roots, and now in leaf cells, the cytosolic measurements are associated with more negative membrane potentials, and the vacuolar measurements with higher nitrate activity and a less negative membrane potential (Table I). A comparison of the compartmental nitrate pools (vacuole, cytosol, and apoplast) between wild-type and nia1nia2 plants in the dark (Table I) shows no significant differences and suggests that the overall distribution is independent of NR activity.
The Response of Leaf Cell Nitrate Pools to Light-Dark Transitions
The most interesting result reported here is the change seen in mesophyll cytosolic nitrate activity. Wild-type mesophyll cells showed large changes maintained for at least 20 min in response to light transitions, whereas mutant plants with decreased NR activity did not show such changes. There are three possible means by which cytosolic nitrate activity could be changed: by increased uptake at the plasma membrane, by release of nitrate from the vacuole, or by reduction to nitrite by NR. Because we show here a requirement for NR in these changes, the most likely explanation for the change in cytosolic nitrate in mesophyll cells is that the shift to the dark inactivates NR, leading to a transient buildup of nitrate due to a slower reduction rate.
For ion-selective microelectrode measurements, the differing response times of the ion-selective and reference barrels can produce artefacts in the recordings of transient changes in ion concentrations (Sanders and Slayman, 1982; Miller and Sanders, 1987), but the duration of the changes in cytosolic nitrate activity in wild-type mesophyll cells negate this possibility. These artefacts produce transient changes that return to the prestimulus ion activities and can explain the light- and dark-elicited transient spikes obtained in wild-type epidermal cells and NR mutant cell responses. In wild-type mesophyll cells, the cytosolic nitrate activity was maintained at approximately 1.5 and 2.0 mm during the light and dark treatment, respectively (Fig. 2A). The onset of darkness also triggered a transient increase in nitrate activity from 2 to 3.5 mm, which peaked after 7 min of darkness and then returned to the steady-state level. The different steady-state nitrate activities during the light and dark treatments and the transient increase in nitrate activity caused by the onset of darkness may be examples of cytosolic signaling events in association with illumination transitions. Vacuolar nitrate activity in wild-type epidermal cells and mesophyll cells (data not shown) did not change in response to light transitions. This result is not surprising as the vacuole stores a large amount of nitrate, so it would be unlikely to change quickly under different environmental conditions.
The only previous dynamic microelectrode measurements investigating changes in cytosolic nitrate activity under changing environmental conditions did not demonstrate changes during the removal of the external nitrate supply in barley (Hordeum vulgare) root epidermal cells (van der Leij et al., 1998). In this study, cytosolic nitrate activity changes were also not detected in epidermal cells of Arabidopsis leaves. Epidermal cells are less likely to have detectable nitrate signaling events associated with changing NR activity under light transitions when compared with mesophyll cells. This is because mesophyll cells are very photosynthetically active with a large number of chlorophyll-containing plastids, whereas epidermal cells are not. Furthermore, almost all the NR activity of Arabidopsis leaves is located in the mesophyll not epidermal cells (Roy et al., 2003).
The Role of NR
Cytosolic nitrate activity is lower in the light when NR is actively reducing nitrate. The rate of NR activity change in response to illumination transitions is rapid, with a half-life of 2 to 15 min in spinach (Spinacia oleracea; Huber et al., 1992; Kaiser et al., 1992; Riens and Heldt, 1992). The duration of cytosolic nitrate activity responses to illumination transitions shown here also ranges from 2 to 15 min, which is consistent with the timescale for NR activity changes. Huber et al. (1992) showed that the rate of NR activation change in response to dark-light transitions was faster than that of light-dark transitions. In this study, the establishment of steady-state cytosolic nitrate activity in mesophyll cells of wild type was faster in response to dark-light than light-dark transitions, which is in agreement with the results for NR activity in spinach (Huber et al., 1992). Furthermore, supporting evidence for the role of NR in regulating cytosolic nitrate activity is provided by the higher mean cytosolic nitrate activity under the light treatment in nia1nia2 leaf cells compared with those of the wild type containing active NR (Fig. 3). Mean cytosolic nitrate activity in nia1nia2 leaf cells under the light treatment was not significantly different to that of the wild type under the dark treatment when NR was inactive. This suggests that active NR is essential for lowering cytosolic nitrate activity in wild-type leaf cells in the light treatment.
Measurements of NR activity in leaf tissues have been reported for many different plant species, although the units used to express the values differ. For example, enzyme activity is often expressed per milligram of protein or in terms of the tissue fresh weight. Values using the latter units enable the calculation of NR activity in the cytosol of leaf cells. For leaves of Arabidopsis grown on 10 mm nitrate, and for those of a closely related species Brassica napus grown on 15 mm nitrate, NR activity in the light ranges from 20 to 60 nmol NO2 gfw−1 min−1 (Nejidat et al., 1997; Leleu and Vuylsteker, 2004). Using leaf cell volumes like those described for spinach (Winter et al., 1994), this gives single-cell cytosolic NR activities ranging from 2.6 to 7.8 fmol NO2 min−1. These NR activities are in considerable excess of that required to bring about the changes in cytosolic nitrate in Arabidopsis leaf mesophyll cells reported here (0.44 fmol min−1 using a cytosolic mesophyll cell volume of 4.1 pL; Winter et al., 1994). Additionally, single-cell measurements of NR activity in Arabidopsis mesophyll cells, although using another substrate for the enzyme, found values of 11 fmol NADH min−1 pL−1 (Roy et al., 2003). These NR activity estimates are in considerable excess of that required to bring about the changes in cytosolic nitrate in Arabidopsis leaf mesophyll cells reported here (0.44 fmol min−1) and suggest that changes in leaf NR activity can account for the light-elicited decrease in cytosolic nitrate.
A role of NR in the light-elicited changes in cytosolic nitrate reported here is clear; the precise mechanism by which these transients are achieved is not obvious. Light-to-dark transitions in photosynthetically active tissue are known to change cytosolic pH (Pallaghy and Lüttge, 1970), free Ca2+ (Miller and Sanders, 1987; Johnson et al., 1995), and Mg2+ (Igamberdiev and Kleczkowski, 2001). Changes in cellular pools of ATP are likely to occur during illumination, and these may also be signals (Demidchik et al., 2003). Changes in Ca2+, Mg2+, and pH may be involved with an illumination transition signal cascade to regulate NR activity in leaf cells; NR activity is affected by changes in pH (Kaiser and Brendle-Behnisch, 1995), and Mg2+/Ca2+-dependent NR kinase has been identified (Bachmann et al., 1995). Posttranslational changes, specifically 14-3-3 protein binding, can have a role in decreasing NR activity during transient shading events (Tucker et al., 2004), and the time scales for this type of response is very similar to that observed for the cytosolic nitrate changes reported here. There are direct effects on the activity of NR protein but there may also be more indirect effects via substrates for the enzyme. For example, recent work in tobacco (Nicotiana tabacum), Lemna (Lemna aequinoctialis), and Arabidopsis has shown that NR is activated by the oxidation state of an electron transport component located in the plastoquinone pool (Sherameti et al., 2002).
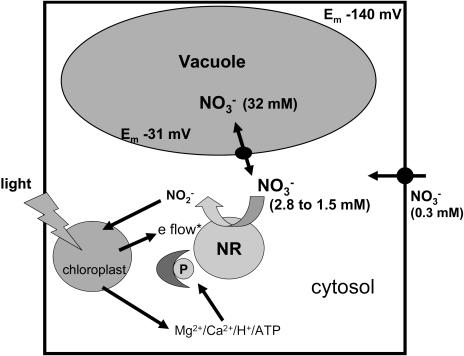
A Cellular Mechanism for Diurnal Changes in Vacuolar Nitrate Concentrations
The NR-dependent cytosolic nitrate activity changes reported here might be a signal for the release of nitrate from the vacuole. Diurnal changes in vacuolar leaf nitrate concentrations are well known, with vacuolar storage occurring during the night and release during the day (Steingröver et al., 1986; Niedziela et al., 1993). The fact that, in the light, cytosolic nitrate did not decrease to zero but rather appeared to establish a new steady-state suggests that uptake at the plasma membrane and/or remobilization of stored nitrate may be increased relative to that in the dark. We have summarized the factors that can influence cytosolic nitrate activity during dark-to-light changes via NR activity in a diagram (see Fig. 4). The first step after the dark-to-light transition is an increase in NR activity that results in a lower cytosolic nitrate activity. Nitrate moves thermodynamically downhill from the vacuole into the cytosol (Miller and Smith, 1992), and it is likely to be channel mediated (Geelen et al., 2000). In the light, the chemical gradient across the tonoplast is larger and nitrate remobilization is favored. Nitrate uptake at the plasma membrane continues throughout the light transition, but this process cannot match the increased demand for nitrate and assimilation. The low concentrations in the apoplast may be an important factor for this uptake limitation. Uptake at the plasma membrane requires an input of energy in both the light and dark (Miller and Smith, 1996); the lowering of cytosolic nitrate activity in the light decreases the chemical gradient across the plasma membrane and hence the amount of energy required for this process. The new steady state in the light aids both processes, efflux from the vacuole and uptake at the plasma membrane, to cope with the greater demand for assimilation. This model (Fig. 4) does not agree with a recent paper reporting that nitrate uptake at the plasma membrane in tobacco leaves can supply an increased rate of nitrate reduction (Lea et al., 2004). The tobacco work used cut leaf pieces from NR mutants immersed in 1 to 50 mm KNO3 solutions for 5 h in darkness with nitrite excretion giving a measure of NR activity. However, in common with the microelectrode measurements and our model, Lea et al. (2004) found that vacuolar stored nitrate was not readily accessible to NR.
Figure 4.
Diagrammatic representation showing how changes in NR activity can be responsible for nitrate-signaling events occurring during dark-to-light changes in a mesophyll cell. *e flow, Photosynthetic electron flow (Sherameti et al., 2002); P, phosphorylation.
Could Cytosolic Nitrate Changes Have a Signaling Role?
Nitrate has been proposed as a signal (Stitt, 1999), but could changes in cytosolic nitrate be the cellular part of this response? As reviewed earlier, the idea that nitrate could be a signal is not in itself new and a role as a signal metabolite associated with carbon partitioning between carbohydrates (principally Suc) and amino acid biosynthesis has been proposed (Champigny and Foyer, 1992). In this model, light activation by nitrate of protein kinase(s) alters the activity of enzymes that regulate carbon partitioning. The hypothesis was based upon the diversion of photosynthetic carbon away from carbohydrate synthesis toward organic acid and amino acid synthesis in leaves fed with nitrate. The regulation of carbon partitioning in leaves by nitrate required functional NR (van Quy et al., 1991). In this earlier work, nitrate-starved leaves were removed from plants and nitrate fed through the transpiration stream to illuminated leaves. Suc synthesis showed an inverse relationship to nitrate supply and this change in Suc synthesis was not seen in leaves that absorbed nitrate but were pretreated with tungstate to inactivate NR (van Quy et al., 1991).
The results reported here suggest that changes in cytosolic nitrate activity can act as a signal in plant cells. The changes in cytosolic nitrate do not necessarily imply a second messenger role but can be a signal regulating gene expression and triggering diurnal vacuolar nitrate release and storage. An NR-dependent nitrate signal gives the plant the capacity to change its metabolism in response to a wide range of environmental conditions.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) ecotype Columbia plants were obtained from the Nottingham Arabidopsis Stock Centre (University of Nottingham, UK). Nitrate-selective microelectrode measurements were made on 3- to 4-week-old Arabidopsis plants grown in either hydroponic or sterile culture, as described below. The leaf cell responses were not significantly different with either culture method. Plants were cultured in a controlled environment cabinet at 20°C, with 75% relative humidity, a 16-h day, and photosynthetically active radiation of 280 to 300 μmol m−2 s−1. Black 0.5 mL polypropylene tubes (Anachem, Luton, UK) with the lids removed were filled with 0.4 mL of 1% purified agar in deionized water solution. When the agar was solid, 3 mm from the bottom of each tube was cut off. The 0.8-L containers selected for hydroponic culture were blackened using electrical tape or paint and 10-mm-thick black polyethylene cross-linked closed-cell foam rubber (RS Components, Corby, UK) was cut to float in the top of these pots. A number of 4-mm-diameter holes were punched out of the foam float approximately 2 cm apart. The tubes were placed in the holes of the float and Arabidopsis seeds were placed on the surface of the agar. Nutrient solution was added to the container, the float was placed on the solution, and an airline, attached to an aquarium pump, was fed though an additional hole in the foam to gently aerate the nutrient solution. Wild-type plants were grown on a modified Hoagland nutrient solution (Gibeaut et al., 1997). This solution contains 1.25 mm KNO3, 0.5 mm KH2PO4, 0.75 mm MgSO4, 1.5 mm Ca(NO3)2, 50 μm KCl, 50 μm H3BO3, 10 μm MnSO4, 2 μm ZnSO4, 1.5 μm CuSO4, 0.075 μm (NH4)6Mo7O4, 72 μm ethylenediaminetetra-acetic acid ferric monosodium salt, and 0.1 mm Na2O3Si at pH 6.0.
The nia1nia2 plants could not be cultured in hydroponics and were grown only on sterile vertical agar plates. The agar solution contained the modified Hoagland nutrient solution used for wild-type plants; KNO3 and Ca(NO3)2 were removed from the nutrient solution and replaced by 1.25 mm KCl, 2.13 mm di-ammonium succinate, 0.8% (w/v) purified agar, and 2% (w/v) Suc. The nia1nia2 plants have only 0.5% to 2% of wild-type NR activity and so cannot assimilate nitrate normally and were supplied only with nitrate (KNO3 and Ca[NO3]2) for 3 d prior to measurement to fill the vacuoles with sufficient nitrate to allow the location of the microelectrode tip within the cell to be determined.
Manufacture and Use of Nitrate-Selective Double-Barreled Microelectrodes
Nitrate-selective membranes for the microelectrodes used in this project were constructed and calibrated according to the protocol described previously (Miller and Zhen, 1991). Leaves of intact plants were impaled with most of the leaf in the air (as described in Miller et al., 2001). The plants were placed in the chamber on the microscope stage; the light conditions required were maintained for at least 1 h before each measurement was taken to allow the leaf to adjust to the new environment of the chamber and recover from possible handling stress. After a successful calibration, the tip was positioned in the apoplast surrounding the cell, epidermal cells were easily identified and impaled. Mesophyll cells were impaled by maneuvering the microelectrode tip through the stomata; guard cells were easily identified on the leaf surface. Under these growth conditions two populations of microelectrode measurements can be easily identified and these have been shown to be randomly distributed and correspond to the cytosol and vacuole (Zhen et al., 1991; Walker et al., 1995; van der Leij et al., 1998).
Light Treatments
Dynamic nitrate-selective microelectrode measurements (in which the responses to light were studied) were made after a minimum 5 min of steady recording of both the barrels of the microelectrode. The illumination treatment was then applied; initially measurements were made with the light on (photosynthetically active radiation 350 μmol m−2 s−1 photon flux density from a KL1500 fiber optic cold-light source [Schott, Mainz, Germany]), so the first treatment was to turn the light off. A new steady state was achieved, and then the treatment was reversed; the light was turned on, and recording continued until the previous steady state was restored. The electrode was then removed from the cell and recalibrated. A K-type 1-mm bead thermocouple connected to a thermometer (RS 206–3750, RS Components) was used to check that no temperature change occurred at the leaf surface during the light-to-dark transitions. The silver-chloride junctions of electrodes have been found to respond directly to light (Janz, 1961), but the fiber source delivered light to the leaf and not directly on to the metal electrode. We also checked that light-to-dark transitions did not elicit an electrical change from a nitrate-selective microelectrode placed in the leaf chamber filled with nutrient solution. During intracellular recordings the electrical resistance is much greater, but the lack of response to light in the nitrate activities of some cells indicates that there were no light-generated artifacts.
Statistical Analysis of Data
Data were analyzed using the statistical computer package Statistical Package for Social Sciences, version 6.0 (SPSS, Chicago) and significance testing used the Mann-Whitney U-test with P < 0.05 being considered statistically significant.
Supplementary Material
Acknowledgments
The authors wish to thank Susan Smith for technical help and the manuscript reviewers for their helpful comments.
This work was supported by the European Union (grant nos. BIO4CT972231 and HPRN–CT–2002–00247). Rothamsted Research is grant aided by the Biotechnology and Biological Sciences Research Council of the United Kingdom.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.062349.
References
- Bachmann M, McMichael RW, Huber JL, Kaiser WM, Huber SC (1995) Partial purification and characterization of a calcium-dependent protein kinase and an inhibitor protein required for the activation of spinach leaf nitrate reductase. Plant Physiol 108: 1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom-Zandstra M, Koot HTM, van Hattum J, Vogelzang SA (1997) Transient light-induced changes in ion channel and proton pump activities in the plasma membrane of tobacco mesophyll protoplasts. J Exp Bot 48: 1623–1630 [Google Scholar]
- Champigny ML, Foyer C (1992) Nitrate activation of cytosolic protein kinases diverts photosynthetic carbon from sucrose to amino acid biosynthesis. Plant Physiol 100: 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G, Bush DR (2001) Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol 125: 61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM (1995) Nitrate: nutrient and signal for plant growth. Plant Cell 7: 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JMD (2003) Is ATP a signaling agent in plants? Plant Physiol 133: 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzenga JTM, Prins HBA, van Volkenburgh E (1995) Light-induced membrane potential changes of epidermal and mesophyll cells in growing leaves of Pisum sativum. Planta 197: 127–134 [Google Scholar]
- Felle HH (2001) pH: signal and messenger in plant cells. Plant Biol 3: 577–591 [Google Scholar]
- Ferrario S, Valadier M-H, Morot-Gaudry J-F, Foyer CH (1995) Effects of constitutive expression of nitrate reductase in transgenic Nicotiana plumbaginifolia L. in response to varying nitrogen. Planta 196: 288–294 [Google Scholar]
- Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelievre F, Courtial B, Barbier-Brygoo H, Maurel C (2000) Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J 21: 259–267 [DOI] [PubMed] [Google Scholar]
- Gibeaut DM, Hulett J, Cramer GR, Seeman JR (1997) Maximal biomass of Arabidopsis thaliana using a simple, low maintenance hydroponic method and favorable environmental conditions. Plant Physiol 115: 317–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JL, Huber SC, Campbell WH, Redinbaugh MG (1992) Reversible light/dark modulation of spinach leaf nitrate reductase activity involves protein phosphorylation. Arch Biochem Biophys 296: 58–65 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Kleczkowski LA (2001) Implications of adenylate kinase-governed equilibrium of adenylates on contents of free magnesium in plant cells and compartments. Biochem J 360: 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz GJ (1961) Silver-silver halide electrodes. In DJG Ives, GJ Janz, eds, Reference Electrodes: Theory and Practice. Academic Press, New York, pp 218–220
- Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A (1995) Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269: 1863–1865 [DOI] [PubMed] [Google Scholar]
- Kaiser WM, Brendle-Behnisch E (1995) Acid-base modulation of nitrate reductase in leaf tissues. Planta 196: 1–6 [Google Scholar]
- Kaiser WM, Huber SC (2001) Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. J Exp Bot 52: 1981–1989 [DOI] [PubMed] [Google Scholar]
- Kaiser WM, Spill D (1991) Rapid modulation of spinach leaf nitrate reductase by photosynthesis in vitro modulation by ATP and AMP. Plant Physiol 96: 368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Spill D, Brendle-Behnisch E (1992) Adenine nucleotides are apparently involved in the light-dark modulation of spinach-leaf nitrate reductase. Planta 186: 236–240 [DOI] [PubMed] [Google Scholar]
- Kaiser WM, Weiner H, Huber SC (1999) Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity. Physiol Plant 105: 385–390 [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY (1995) Compartmentation and flux characteristics of nitrate in spruce. Planta 196: 674–682 [Google Scholar]
- Lea US, ten Hoopen F, Provan F, Kaiser WM, Meyer C, Lillo C (2004) Mutation of the regulatory phosphorylation site in tobacco nitrate reductase results in high nitrite excretion and NO emission from leaf and root tissue. Planta 219: 59–65 [DOI] [PubMed] [Google Scholar]
- Leleu O, Vuylsteker C (2004) Unusual regulatory nitrate reductase activity in cotyledons of Brassica napus seedlings: enhancement of nitrate reductase activity by ammonium supply. J Exp Bot 55: 815–823 [DOI] [PubMed] [Google Scholar]
- MacKintosh C, Meek SEM (2001) Regulation of plant NR activity by reversible phosphorylation, 14-3-3 proteins and proteolysis. Cell Mol Life Sci 58: 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Cookson SJ, Smith SJ, Wells DM (2001) The use of microelectrodes to investigate compartmentation and the transport of metabolized inorganic ions in plants. J Exp Bot 52: 1–9 [PubMed] [Google Scholar]
- Miller AJ, Sanders D (1987) Depletion of cytosolic free calcium induced by photosynthesis. Nature 326: 397–400 [Google Scholar]
- Miller AJ, Smith SJ (1992) The mechanism of nitrate transport across the tonoplast of barley root cells. Planta 187: 554–557 [DOI] [PubMed] [Google Scholar]
- Miller AJ, Smith SJ (1996) Nitrate transport and compartmentation in cereal root cells. J Exp Bot 47: 843–854 [Google Scholar]
- Miller AJ, Zhen RG (1991) Measurements of intracellular nitrate concentrations in Chara using nitrate-selective microelectrodes. Planta 187: 47–52 [DOI] [PubMed] [Google Scholar]
- Mühling KH, Sattelmacher B (1995) Apoplastic ion concentrations of intact leaves of field bean (Vicia faba L.) as influenced by ammonium and nitrate nutrition. J Plant Physiol 147: 81–86 [Google Scholar]
- Nejidat A, Zhang GF, Grinberg M, Heimer YM (1997) Increased protein content in transgenic Arabidopsis thaliana over-expressing nitrate reductase activity. Plant Sci 130: 41–49 [Google Scholar]
- Niedziela CE, Nelson PV, Peet MM, Jackson WA (1993) Diurnal malate and citrate fluctuations as related to nitrate and potassium concentrations in tomato leaves. J Plant Nutr 16: 165–175 [Google Scholar]
- Pallaghy JE, Lüttge U (1970) Light-induced H+ fluxes and bioelectric phenomena in mesophyll cells of Atriplex spongiosa. Z Pflanzenphysiol 62: 417–425 [Google Scholar]
- Redinbaugh MG, Campbell WH (1991) Higher plant responses to environmental nitrate. Physiol Plant 82: 640–650 [Google Scholar]
- Riens B, Heldt HW (1992) Decrease of nitrate reductase activity in spinach leaves during a light-dark transition. Plant Physiol 98: 573–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SJ, Cuin TA, Leigh RA (2003) Nanolitre-scale assays to determine the activities of enzymes in individual plant cells. Plant J 34: 555–564 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Slayman CL (1982) Control of intracellular pH. J Gen Physiol 80: 377–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Gonzalez-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M (1997) Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 9: 783–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherameti I, Sopory SK, Trebicka A, Pfannschmidt T, Oelmuller R (2002) Photosynthetic electron transport determines nitrate reductase gene expression and activity in higher plants. J Biol Chem 277: 46594–46600 [DOI] [PubMed] [Google Scholar]
- Spalding EP (1995) An apparatus for studying rapid electrophysiological responses to light demonstrated on Arabidopsis leaves. Photochem Photobiol 62: 934–939 [DOI] [PubMed] [Google Scholar]
- Spalding EP (2000) Ion channels and the transduction of light signals. Plant Cell Environ 23: 665–674 [DOI] [PubMed] [Google Scholar]
- Stahlberg R, Van Volkenburgh E (1999) The effect of light on the membrane potential, apoplastic pH and cell expansion in leaves of Pisum sativum L. var. argenteum. Planta 208: 188–195 [Google Scholar]
- Steingröver E, Ratering P, Siesling J (1986) Daily changes in uptake, reduction and storage of nitrate in spinach grown at low light intensity. Physiol Plant 66: 550–556 [Google Scholar]
- Stitt M (1999) Nitrate regulation of metabolism and growth. Curr Opin Plant Biol 2: 178–186 [DOI] [PubMed] [Google Scholar]
- Stitt M, Muller C, Matt P, Gibon Y, Carillo P, Morcuende R, Scheible WR, Krapp A (2002) Steps towards an integrated view of nitrogen metabolism. J Exp Bot 53: 959–970 [DOI] [PubMed] [Google Scholar]
- Trewavas AJ (1983) Nitrate as a plant hormone. In MB Jackson, ed, British Plant Growth Regulator Group Monograph 9. Oxford University Press, Oxford, pp 97–110
- Tucker DE, Allen DJ, Ort DR (2004) Control of nitrate reductase by circadian and diurnal rhythms in tomato. Planta 219: 277–285 [DOI] [PubMed] [Google Scholar]
- van der Leij M, Smith SJ, Miller AJ (1998) Remobilisation of vacuolar stored nitrate in barley root cells. Planta 205: 64–72 [Google Scholar]
- van Quy L, Lamaze T, Champigny ML (1991) Short-term effects of nitrate on sucrose synthesis in wheat leaves. Planta 185: 53–57 [DOI] [PubMed] [Google Scholar]
- Walker DJ, Smith SJ, Miller AJ (1995) Simultaneous measurement of intracellular pH and K+ or NO3− in barley root cells using triple-barreled, ion-selective microelectrodes. Plant Physiol 108: 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193: 530–535 [Google Scholar]
- Zhen R-G, Koyro H-W, Leigh RA, Tomos AD, Miller AJ (1991) Compartmental nitrate concentrations in barley root cells measured with nitrate-selective microelectrodes and by single-cell sap sampling. Planta 185: 356–361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.