Abstract
Long day (LD) exposure of rosette plants causes rapid stem/petiole elongation, a more vertical growth habit, and flowering; all changes are suggestive of a role for the gibberellin (GA) plant growth regulators. For Arabidopsis (Arabidopsis thaliana) L. (Heynh), we show that enhancement of petiole elongation by a far-red (FR)-rich LD is mimicked by a brief (10 min) end-of-day (EOD) FR exposure in short day (SD). The EOD response shows red (R)/FR photoreversibility and is not affected in a phytochrome (PHY) A mutant so it is mediated by PHYB and related PHYs. FR photoconversion of PHYB to an inactive form activates a signaling pathway, leading to increased GA biosynthesis. Of 10 GA biosynthetic genes, expression of the 20-oxidase, AtGA20ox2, responded most to FR (up to a 40-fold increase within 3 h). AtGA20ox1 also responded but to a lesser extent. Stimulation of petiole elongation by EOD FR is reduced in a transgenic AtGA20ox2 hairpin gene silencing line. By contrast, it was only in SD that a T-DNA insertional mutant of AtGA20ox1 (ga5-3) showed reduced response. Circadian entrainment to a daytime pattern provides an explanation for the SD expression of AtGA20ox1. Conversely, the strong EOD/LD FR responses of AtGA20ox2 may reflect its independence of circadian regulation. While FR acting via PHYB increases expression of AtGA20ox2, other GA biosynthetic genes are known to respond to R rather than FR light and/or to other PHYs. Thus, there must be different signal transduction pathways, one at least showing a positive response to active PHYB and another showing a negative response.
The light environment of plants, particularly its quality and daily duration, regulates many aspects of plant development including seed germination, shoot elongation, shoot architecture, and flowering. Of the plant photoreceptors involved in such light responses, the phytochromes (PHYs), cryptochromes, and phototropins are most important (see Quail, 2002).
Typically, PHY acts by regulating the synthesis of the GA class of plant growth regulators. For example, a brief (5–10 min) exposure to PHY-active far-red (FR) light at the end-of-day (EOD) increases bioactive GA content in isolated, elongating epicotyls of cowpea (Vigna unguiculata; Martínez-García et al., 2000), a system in which applied GA stimulates elongation, as is also known for a number of rosette dicot species (for review, see Smith, 1995; García-Martínez and Gil, 2002). Similarly, a prolonged (>6 h) FR-rich long day (LD) exposure causes petiole and stem elongation and an increase in GA content in spinach (Spinacia oleracea; Wu et al., 1996), bean (Phaseolus vulgaris; Beall et al., 1996), and Arabidopsis (Arabidopsis thaliana; Xu et al., 1997; Gocal et al., 2001). In addition, FR-rich LD exposures increase the activity of a key group of GA biosynthetic enzymes, the 20-oxidases (Gilmour et al., 1986), and there are matching increases in expression of 20-oxidase mRNAs (Wu et al., 1996; Xu et al., 1997; Lee and Zeevaart, 2002).
In contrast to the FR stimulation of 20-oxidase expression in shoots, in germinating seed (Toyomasu et al., 1993; Yamaguchi et al., 1998), and during seedling deetiolation (Reid et al., 2002), red (R) light acting via PHY may regulate expression of 3-oxidases, another class of GA biosynthetic gene. In germinating lettuce (Lactuca sativa) seed, for example, GA 3-oxidase expression and its biosynthetic product, GA1, increase within 4 h of a brief R exposure (Toyomasu et al., 1993, 1998). Similarly, during Arabidopsis seed germination, expression of two 3-oxidase genes increases rapidly on exposure to R light (Yamaguchi et al., 1998). In a parallel manner, during deetiolation in pea, inhibition of stem elongation by R light involves a reduction in GA content and down-regulation of the expression of various GA biosynthetic genes (Reid et al., 2002). Interestingly, in germinating lettuce seed, R promotes the expression of a 3-oxidase during the early hours of germination but also inhibits the expression of two GA 20-oxidase genes, one of these showing an increase in FR (Toyomasu et al., 1998). Thus, depending on the physiological response and the wavelengths of light used, expression of any GA biosynthetic gene might increase or decrease.
Genetic studies have provided clear evidence that the PHY family of photoreceptors are responsible for responses to brief R or FR exposures. In Arabidopsis, PHYB, D, E, and possibly PHYC, act redundantly in such regulation of shoot elongation, leaf growth, and flowering (Franklin et al., 2003). R/FR photoreversibility is also diagnostic of this action of the PHYB-type genes whereby, in a sequence of brief exposures (i.e. 5–10 min), the effect of R can be fully reversed by a following FR exposure (see Borthwick et al., 1952; Smith, 1995). Unlike the other four PHY, Arabidopsis PHYA is unstable in light, it is important in responses involving prolonged exposure to FR light, it is considered to act during LD exposures (see Quail, 2002), and it might be active in the FR responsive, GA-regulated responses discussed above.
In our earlier studies with Arabidopsis, we showed that FR-rich light during an LD caused rapid increases (within 2 d) in both petiole elongation and their content of bioactive GAs (Gocal et al., 2001), while application of an inhibitor of GA biosynthesis abolished the LD stimulation of elongation. Thus, it was important to establish which photoreceptor(s) were active and on which GA biosynthetic gene(s). As a model plant, Arabidopsis provides the necessary molecular information and genetic tools for such a study and, here, we have analyzed GA biosynthetic gene expression and used mutants and gene silencing lines to examine the way light regulates plant growth. The four questions that provide a focus for this study are: (1) Which genes of GA metabolism show substantial increases in expression in response to FR exposure? (2) Does expression of GA biosynthetic genes oscillate in a circadian manner? (3) Does decreasing the expression of GA biosynthetic genes reduce petiole elongation? (4) Which photoreceptors are important in the stimulation of both petiole elongation and the expression of GA biosynthetic genes?
RESULTS
Enhanced Expression of GA Biosynthetic Genes with FR Exposure
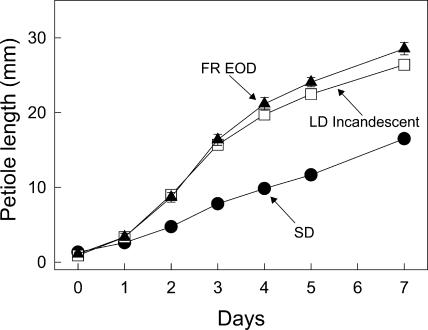
Following exposure to a single low-irradiance FR-rich LD from incandescent lamps, Arabidopsis plants double their petiole elongation compared with plants kept in short day (SD; Fig. 1). Furthermore, since a 10-min EOD FR exposure in SD gave a comparable stimulation to that from a LD (Fig. 1), we can conclude that the enrichment for FR wavelengths in incandescent lamps accounts for their effectiveness. We return to this matter later but first examine the effect of LD/FR exposure on the expression of GA biosynthesis genes.
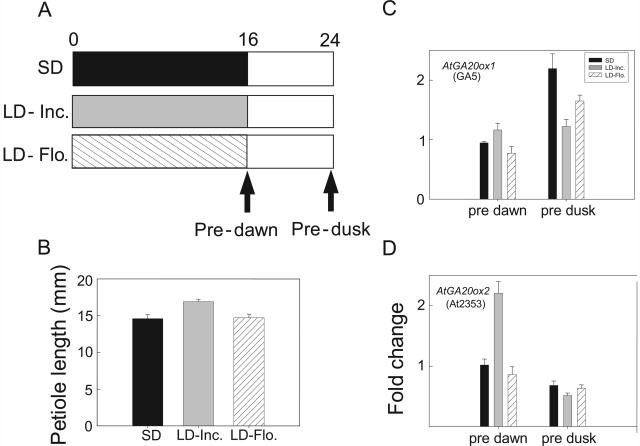
Figure 1.
Effect of daylength (SD versus LD) or a brief EOD FR light exposure on elongation of young petioles of Arabidopsis. Plants of ecotype Columbia were 7 weeks old. The EOD FR exposure was for a 10-min duration at the end of the 8-h SD. The LD incandescent exposure was for 16 h at the end of the main 8-h day. Values in this and subsequent figures are means ± se, the latter shown as bars which, when not evident, were smaller than the symbol.
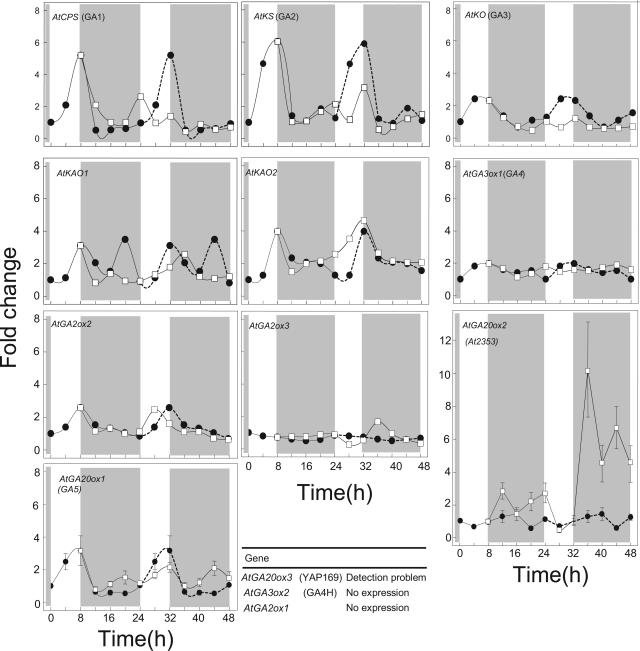
Using quantitative real time PCR (Q-PCR) and specific primers for 13 of the known GA biosynthetic genes, the effect of daylength on their mRNA expression level was examined in young elongating petioles of Arabidopsis (Fig. 2). We could detect little or no transcript of AtGA3ox2 and AtGA2ox1, while AtGA20ox3 was barely detectable and we could not detect expression of AtGA20ox4 or AtGA20ox5. The ACTIN standard was effectively detected in all samples, which excludes the possibility of failed assays. The lack of detection of AtGA3ox2 may reflect differences in tissue specificity as it has only been detected previously in germinating seeds (Yamaguchi et al., 2001).
Figure 2.
Effect of daylength on the pattern of expression in petioles of some GA biosynthetic genes. Arabidopsis plants were grown in SD and then either maintained in SD (•) or exposed to two LD cycles (□).The shaded areas on each graph indicate the 16-h period of either darkness in SD or low intensity (10 μmol m−2 s−1) light from incandescent lamps for LD treatments. Changes in gene expression are shown as fold increases relative to the time zero value, which, generally, was the lowest value. The dashed line on the second day is the SD samples from the day before, although a repeat of this SD sampling to cover 2 SD (Fig. 3) shows that there will be small variations from day to day. Other conditions as in Figure 1.
The major effect of LD is to increase expression of AtGA20ox2 in elongating petioles, especially after the second LD exposure (Fig. 2). These findings fit with earlier evidence that GA 20-oxidases are important for the LD-regulated increases in GA biosynthesis that are required for increases in petiole and stem elongation (Gilmour et al., 1986; Xu et al., 1997; Lee and Zeevaart, 2002). However, it appears that not all 20-oxidases show such a large LD response. For AtGA20ox1, there was only a limited effect of LD on its expression (Fig. 2).
Despite the fact that the AtGA3ox genes are important for increased GA biosynthesis during R-light-regulated seed germination in Arabidopsis (Yamaguchi et al., 1998), our study shows that LD had no effect on expression of AtGA3ox1 in petioles (Fig. 2). Also, there is no change in expression in response to EOD FR treatments (Fig. 7) or in stems of Arabidopsis plants exposed to up to eight cycles of LD (Xu et al., 1997).
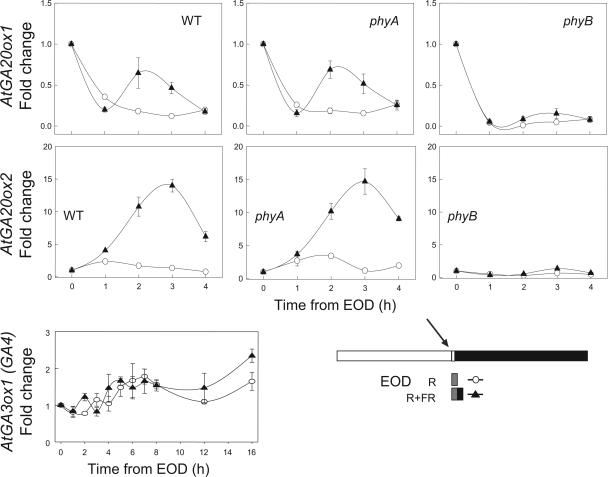
Figure 7.
Effect of EOD FR light on GA 20-oxidase gene expression in elongating petioles of the Columbia wild type and of the phyA-211 and phyB-9 mutants in the Columbia background. Harvests were made over the first 4 h of the dark period following a single EOD R or an R/FR treatment. A further experiment is included that shows that EOD FR did not enhance expression of the 3-oxidase gene, AtGA3ox1, in elongating petioles of Columbia. Other conditions as in Figure 2.
Does Expression of GA Biosynthetic Genes Oscillate in a Circadian Manner?
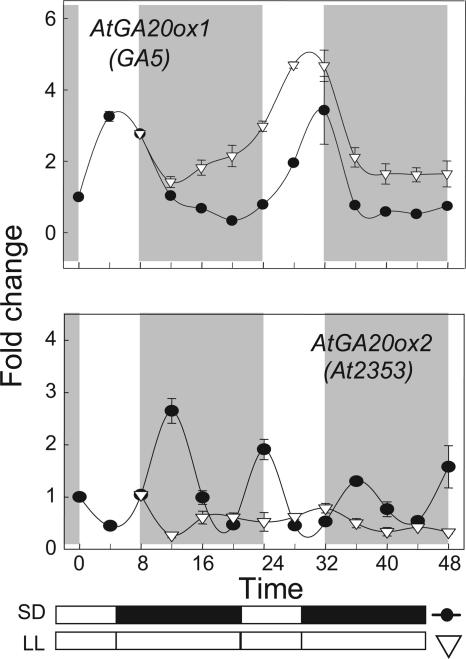
Given the clear diurnal oscillations we observed in expression of at least one 20-oxidase gene and possibly some of the genes for early biosynthetic steps (Fig. 2), control by a circadian oscillator is likely. Therefore, some 7-week-old plants held in 8-h SD from high intensity light were transferred to continuous high intensity light (LL) and at the same constant temperature. Young petioles were harvested every 4 h and, as shown in Figure 3, in constant conditions, expression of AtGA20ox1 clearly oscillates with a circadian periodicity (approximately 24 h). The phase over the first 2 d was the same as for plants harvested at the same time but kept in SD (Figs. 2 and 3). There was little or no diurnal oscillation in expression of the second 20-oxidase, AtGA20ox2 (Figs. 2 and 3), and it did not show any circadian rhythmicity in constant conditions (Fig. 3). Rather, in SD, there was a noncircadian cycling in expression of AtGA20ox2 and this was reproduced in both the experiments shown (Figs. 2 and 3). Expression of some 20-oxidases is down-regulated by feedback by bioactive GA products (see Phillips et al., 1995), which may explain this noncircadian cycling of AtGA20ox2.
Figure 3.
Oscillation in 20-oxidase gene expression in petioles of plants of Columbia transferred from SD cycles (8:16 h light:dark, •) to constant LL (continuous fluorescent light at 100 μmol m−2 s−1 at 22°C; ▿). Other conditions as in Figure 2.
Based on our spectrophotometric measurements, input RNA amounts were equal and the constancy of the ACTIN assays supports this claim. For the three replicates of all 23 samples in Figure 3, the average ACTIN expression was 0.90 ± 0.06, after normalization across assays to the value of the first SD sample taken as 1.0. Thus, there is little variation in the loading control and the circadian oscillation must be in the expression of AtGA20ox1. Furthermore, the absence of any cycling in AtGA20ox2 expression in the same RNA samples supports this argument. Previously, Blazquez et al. (2002) failed to find evidence of oscillations in expression of AtGA20ox1 with seedlings of Columbia grown in almost identical conditions, but their use of total shoot tissue could account for this difference.
To restate the above findings, AtGA20ox1 is entrained by the circadian clock and expresses more during the 8-h main SD-light period (Figs. 2 and 3). By contrast, expression of AtGA20ox2 is not entrained by the circadian clock, it expresses at low levels in darkness or during any R-rich fluorescent exposure (Figs. 2 and 3), and it expresses most during the FR-rich LD, increasing up to 7-fold, whereas expression of AtGA20ox1 barely doubled in LD (Fig. 2). To emphasize these differences between AtGA20ox1 and AtGA20ox2 in their response to daylength and light quality, a petiole sample was taken just before the start of the main 8-h-SD light period (predawn) when AtGA20ox2 expression should be elevated. A second sample was taken at the end of the main 8-h-light period (predusk) when AtGA20ox1 expression should be elevated. The results in Figure 4 confirm these expectations and, most importantly, show that only an FR-rich incandescent LD exposure activated AtGA20ox2 expression; a comparable low irradiance LD exposure (10 μmol m−2 s−1) from R-rich fluorescent lamps gave no LD increase. Furthermore, in the same experiment, the R-rich fluorescent light gave no enhancement of petiole elongation (Fig. 4). Thus, not only are these LD photoresponses linked closely to FR wavelengths and not to the R of fluorescent lamps, but these differences indicate that the blue wavebands of the fluorescent lamps may also be unimportant. Later, we return to the identity of photoreceptors. A further implication of these findings is that blocking AtGA20ox2 expression should impact on the LD enhancement of petiole elongation, while mutation of AtGA20ox1 should only impact on petiole growth in SD and we consider these predictions below.
Figure 4.
Effect of LD light quality on petiole elongation and on expression in the petiole of two GA 20-oxidase genes in ecotype Columbia. The LD was at a low irradiance of 10 μmol m−2 s−1 from red-rich fluorescent (Flo) or FR-rich incandescent (Inc) lamps as shown in A. The arrows in A indicate harvest times of the harvests shown in C and D for analysis of gene expression. The predawn, morning harvests were taken after a single overnight LD or at the same time in SD conditions. The predusk samples were taken 8 h later at the end of the daily 8-h fluorescent light period. Petiole length (B) was determined 4 d after the start of the LD exposure. Other conditions as in Figure 2.
Does Decreasing the Expression of GA Biosynthetic Genes Affect Petiole Elongation?
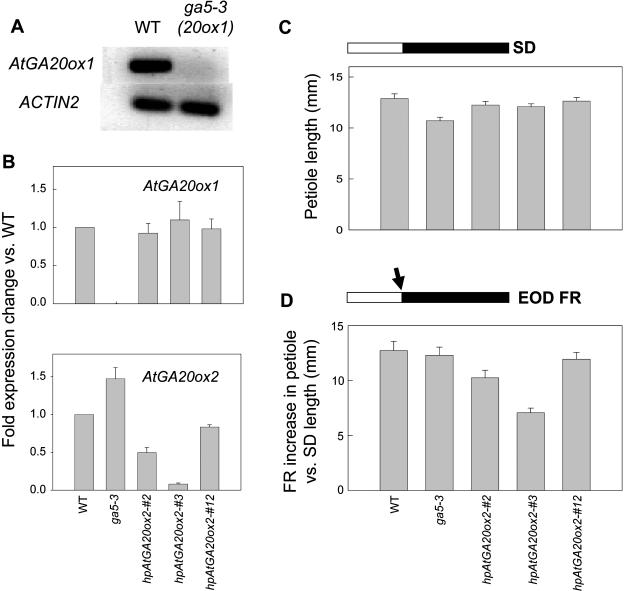
A Salk line (Alonso et al., 2003) with a T-DNA insert in the intron of the AtGA20ox1 gene was selected in the Columbia background and this mutant is subsequently designated ga5-3. Based on reverse transcription-PCR assays with petioles of ga5-3, we could detect no expression of AtGA20ox1 (Fig. 5A), as might be predicted. Conversely, expression of AtGA20ox2 did not decrease but increased by about 50% in the ga5-3 mutant (Fig. 5B), which may indicate a gene-specific reduction in feedback control of 20-oxidase gene expression by a lowering of bioactive GA product.
Figure 5.
Expression in petioles of GA 20-oxidases (A and B) and petiole elongation in SD (C) or with EOD FR (D). For AtGA20ox1 gene expression, petioles were harvested predusk in SD and, for AtGA20ox2, after 3 h in the dark following an EOD FR exposure. Petiole elongation is shown in D as the FR increment over that in SD (C). The lines examined were: WT, ecotype Columbia; ga5-3, a TDNA-insertional mutant of AtGA20ox1 in Columbia; and three Columbia lines, hpAtGA20ox2-2, -3, and -12, transformed with a hairpin construct designed to specifically block AtGA20ox2 expression. The extent of inhibition of AtGA20ox2 expression (virtually no effect in no. 12 to a 90% reduction in no. 3) is matched by a reduced FR increment in petiole growth.
We were unable to obtain a T-DNA insertional mutant of AtGA20ox2, although we examined two candidate lines available in the Salk Collection. Therefore, we used an RNA silencing approach and obtained 13 stably transformed second generation transgenic hairpin lines. As shown in Figure 5B, relative to wild type, AtGA20ox2 expression was reduced by 90% in line 3, to about 50% in line 2 and by about 10% in line 12 that was included as a transformed control. There was no effect of reduction in expression of AtGA20ox2 on expression of AtGA20ox1 (Fig. 5B).
For these lines with their reduced 20-oxidase expression, petiole growth in SD or following an EOD FR exposure is shown in Figure 5, C and D. For ga5-3, its elongation in SD was reduced by approximately 25% compared to the wild type (Fig. 5C). However, ga5-3 showed a normal increase in response to an EOD FR exposure (Fig. 5D) and to an FR-rich LD (data not shown), this claim being based on first removing the SD effect that we have done here by calculating the LD increment over that of the same line in SD. With the AtGA20ox2 transgenic hairpin lines, the converse response was evident. They responded as well as the control in SD (Fig. 5C) but showed reduced FR (Fig. 5D) or LD response (data not shown). Importantly, the extent of gene silencing for the three AtGA20ox2 lines (Fig. 5B) matches the reduction in their petiole elongation on exposure to EOD FR (Fig. 5D). This finding further supports our claim that AtGA20ox2 expression induced by FR-light is important for the synthesis of GAs required for the LD stimulation of petiole elongation. No other “growth” phenotypes were obvious in these gene silencing lines.
In a further, preliminary study with GA20ox antisense lines provided to us by A. Phillips and P. Hedden (Biotechnology and Biological Science Research Council, Rothamsted, UK), we have found a similar daylength differential in the regulation of petiole elongation. The AtGA20ox1 antisense line showed a 22% reduction in its petiole elongation in SD and no reduction for the AtGA20ox2 antisense line (data not shown). Conversely, LD response was reduced somewhat in the AtGA20ox2 antisense line but there was no reduction in LD response for the AtGA20ox1 antisense line (data not shown). That petiole elongation is reduced in these antisense lines was noted by Coles et al. (1999) based on photographic records with young seedlings, but no effects of daylength were established in that study.
Overall, circadian changes regulate AtGA20ox1 so that it expresses most in the “daytime” of SD. By contrast, AtGA20ox2 lacks this circadian pattern of response and shows a FR-rich light, LD response over the “nighttime” hours of each daily cycle. Thus, there could be significant differences between these genes in their circadian regulation and in their response to different light treatments. We examine the photoregulation of these genes in more detail in the following section.
Photoreceptors, Expression of GA Biosynthetic Genes, and Regulation of Petiole Elongation
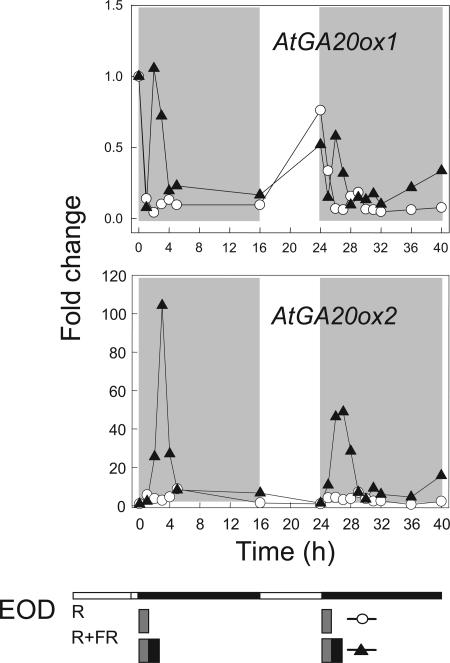
Of the various photoreceptors, we have examined only PHY action since petiole elongation and 20-oxidase expression increased in FR-rich LD or EOD FR conditions and not in R-rich conditions (Figs. 1 and 4). In an initial experiment, after exposure to a single 10-min EOD FR, AtGA20ox2 expression increased rapidly (within 3 h) and dramatically (up to 40-fold), and this increase was duplicated when the FR exposure was repeated the next day (Fig. 6). Importantly, the results in Table I show that the EOD FR promotion of AtGA20ox2 expression and of petiole elongation was fully reversed by a subsequent exposure to 10 min of R light. Both AtGA20ox genes showed increased expression after FR in the experiment shown in (Fig. 6), but AtGA20ox1 showed a weaker response and sometimes no change after FR whereas AtGA20ox2 always responded strongly (Fig. 6; Table I; a third experiment not shown here). In a further study, but with AtGA3ox1 that regulates a different GA biosynthetic step, there was no effect of R/FR on its expression in the petiole (Fig. 7), which confirms the evidence in Figure 2 for plants exposed to a FR-rich LD.
Figure 6.
Enhancement by FR light of GA 20-oxidase gene expression in elongating petioles exposed twice over 2 d to 10 min EOD R or EOD R/FR light terminating the first and second of two 8-h SD (ecotype Columbia). Other conditions as in Figure 2.
Table I.
R/FR photoreversible regulation of elongation of young petioles and of the expression of two GA 20-oxidase genes
At the end of each 8-h SD cycle from fluorescent lamps, plants were exposed sequentially to 10 min of R and FR light. Petiole elongation was determined after 16 h in darkness following the second daily exposure to R/FR. Values are means ± se. Harvests for assays of gene expression were 3 h after the start of the dark period and these values have been normalized to the time zero value 3 h earlier. The differences shown for DAY I were also evident on DAY II (data not shown) and were replicated in other more detailed time courses shown in Figures 6 and 7.
| R | R + FR | R + FR + R | |
|---|---|---|---|
| Petiole length at 2 d (mm) | 10.0 ± 0.4 | 14.6 ± 0.7 | 10.6 ± 0.4 |
| AtGA20ox1 expression versus time zero | 0.4 | 1.4 | 0.5 |
| AtGA20ox2 expression versus time zero | 3.0 | 35.3 | 3,0 |
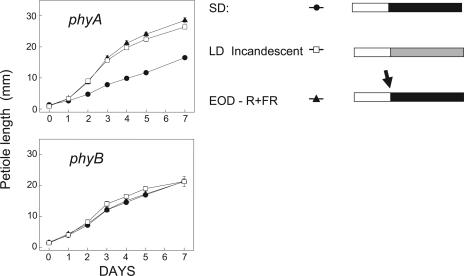
The R/FR photoreversibile control of gene expression as shown in Table I is diagnostic for PHY action (Borthwick et al., 1952) and, specifically, for the light stable PHY of the PHYB class (PHYB, C, D, and E; see Quail, 2002). To reinforce this claim, the wild type and a phyA mutant showed identical EOD FR effects on 20-oxidase expression and petiole elongation (Figs. 7 and 8). Thus, PHYA is not part of the response to either EOD FR or to FR-rich LD exposures. In a phyB null mutant, we expected the converse response, greater petiole elongation, and higher levels of gene expression in R light, particularly since hypocotyl elongation in young seedlings of this mutant is almost as great as for etiolated (dark) controls (Reed et al., 1993). The fact that light grown plants of the same mutant showed little or no increases in elongation or of gene expression when PHYB input was reduced in the mutant or when, in addition, the mutant was exposed to FR (Figs. 7 and 8), indicates that in adult plants, secondary, pleiotropic effects of the mutant make it unsuitable for testing for PHYB input. Nevertheless, our physiological demonstration of photoreversibility in the wild type stands as unequivocal evidence for the action of PHYB or of related PHYs.
Figure 8.
Effect of two PHY mutants, phyA-211 or phyB-9, on elongation of young petioles of Columbia exposed to an LD from incandescent lamps or to a 10-min EOD FR light treatment. For the same experiment, petiole elongation of wild type, Columbia, was shown in Figure 1, and it matches almost exactly that of the phyA mutant shown here.
The complex pattern of diurnal changes in expression of AtGA20ox1, particularly at the end of the SD (Figs. 2 and 6), could make it difficult to identify responses to FR exposure. Therefore, in further studies, the time of giving FR was either delayed by 3 h after the start of darkness or was given early when the main 8-h-SD light period was terminated after 4 h. The times to peak expression after such changes in the time of FR exposure were the same as before in Figures 6 and 7 (2 h in dark after FR for AtGA20ox1 and after 3 h for AtGA20ox2) and, again, the more substantial increases were for AtGA20ox2 (data not shown). Thus, the diurnal and circadian patterns of gene expression do not appear to influence the response to EOD FR.
DISCUSSION
Here, based both on gene expression analysis and on responses in a mutant or in gene silencing lines, we show that GA 20-oxidase biosynthetic genes are involved in the petiole elongation in Arabidopsis associated with exposure to EOD FR light or to LD from FR-rich incandescent lamps. The light input involves PHYB and related PHYs and not PHYA since responses were the same in the wild type and a phyA mutant. Furthermore, it is unlikely that there was an additional blue light action because petiole elongation and 20-oxidase expression could be accounted for as an R/FR response, an LD exposure from fluorescent tubes having the same effect as an R EOD treatment.
The rapid increase in elongation on exposure to FR fits with the scenario that increased expression of GA 20-oxidases caused increased GA synthesis and, in turn, greater elongation; gene expression increased within 3 h of a 10-min EOD FR exposure (Figs. 6 and 7) or within 4 h of the start of the first of two FR-rich LD exposures (Fig. 2). The first sign of increased petiole elongation is at 2 d (Fig. 1) by which time the GA1 and GA4 contents of the young petioles have increased up to 3-fold (Gocal et al., 2001). It is clear that GA is essential for elongation as the response is reduced in a mutant and in gene silencing lines (Fig. 5). Furthermore, in our earlier studies (Gocal et al., 2001), we showed that application of a GA biosynthesis inhibitor blocked the increase in petiole elongation on exposure to a FR-rich LD, the effect of the inhibitor being reversed by applied GA4, while GA4 alone applied to plants in SD increased petiole elongation (Gocal et al., 2001). Thus, we have provided substantial evidence that an increase in GA synthesis is central to the enhanced petiole elongation caused by a FR-rich LD or by exposure to EOD FR. It remains to be clarified whether the light acts directly on the petiole to increase GA synthesis there. In the most relevant study, García-Martínez and coworkers (1987) showed that light input and GA increase were directed to the growth-responsive epicotyl tissue of cowpea but they could not exclude an additional action of light via the leaf.
Expression profiling of elongation-related genes associated with exposure of Arabidopsis to FR-rich light (Devlin et al., 2003) indicated that a broad spectrum of genes regulate or are regulated by FR but, without detailed mutant and phenotype analysis, few conclusions can be drawn from such findings. On the other hand, the FR phenotype is consistently linked with increases in GA 20-oxidases and in GA content (see ref. in García-Martínez and Gil, 2002). Our data confirm this linkage as, based on analysis of 10 Arabidopsis GA biosynthetic genes, the 20-oxidases responded most to FR-rich LD (Fig. 2). Similarly, from an analysis of the expression of a 2-oxidase, a 3-oxidase, and a 20-oxidase in spinach petioles, Lee and Zeevaart (2002) concluded that a 20-oxidase was most significant. More importantly, however, our findings with a mutant and with hairpin gene silencing lines (Fig. 5) confirm that one 20-oxidase, AtGA20ox2, is central to FR-regulation of petiole elongation. Thus, a narrowed focus on the 20-oxidase step of GA biosynthesis is readily justified.
A unique aspect of our study is the contrast apparent between two of the Arabidopsis 20-oxidase genes. AtGA20ox1 responds weakly to FR and is regulated by a circadian clock such that its maximum expression and physiological effectiveness is seen in SD. By contrast, AtGA20ox2, which shows no circadian control, is mostly expressed when exposed to FR-rich EOD or LD conditions (Figs. 2 and 3). These distinctions explain the daylength-specific effects of the mutant and silencing lines in that rhythmic gating of gene expression and a weak FR response confer the SD phenotype of ga5-3 (Fig. 5). Conversely, there is an LD phenotype in the AtGA20ox2 hairpin silencing lines (Fig. 5) because of the greater FR response of AtGA2ox2 coupled with its lack of rhythmic gating. The daylength-specific effects of these two 20-oxidases genes also have implications for studies of flowering of Arabidopsis. For example, in an FR-rich LD, AtGA20ox2 should regulate a GA-dependent pathway and we will present such an analysis of flowering responses in a future publication.
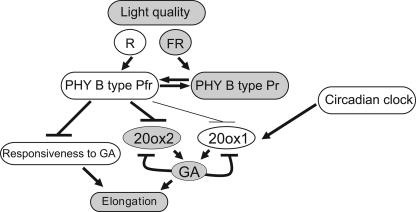
A model summarizing the interrelationships between LD or EOD FR light, GA metabolism, and elongation is presented in Figure 9. In relation to light regulation of GA biosynthesis, it is known that, in R light, PHY Pfr activates seed germination in association with increased expression of the 3-oxidase GA metabolism genes of Arabidopsis (Yamaguchi et al., 1998) and lettuce (Toyomasu et al., 1993, 1998). However, our findings show that expression of 3-oxidase in mature plants was unchanged by R/FR exposures, whereas photoconversion of PHYB to Pr in LD or with FR EOD increased 20-oxidase expression. Clearly, the lack of Pfr in FR activates a signal cascade that enhances the 20-oxidase step of GA biosynthesis and, so, elongation increases. Conversely, the presence of Pfr in R light activates GA biosynthesis but via increase in 3-oxidases, potentially only during germination and, apparently via a different signal transduction path.
Figure 9.
A model for the interactions between PHY-regulated light input, GA 20-oxidase expression, and EOD/LD regulation of petiole elongation in Arabidopsis. The shading emphasizes those changes shown to be essential for elongation in response to FR-rich conditions. Negative regulation is shown by the bar replacing an arrowhead.
The 20-oxidase, AtGA20ox2, is central to the LD/FR regulated PHY response associated with elongation, a claim also supported by our evidence of little or no change for the nine other GA metabolism genes studied here and including two 2-oxidases that regulate GA catabolism. As discussed above, the interaction between LD and circadian regulation of 20-oxidases can be significant but has received too little attention previously. The more significant LD-regulated changes occur rapidly, overnight (Fig. 2), and apparently independently of circadian regulation (Fig. 3). However, in earlier studies of Lee and Zeevaart (2002), 20-oxidase expression in LD was examined only at dawn and dusk, while the analysis of 20-oxidase rhythms by Carrera et al. (1999), although involving frequent sampling, did not focus on changes occurring during the switch between daylengths.
The complex ways PHY regulates different GA biosynthetic genes, different signal transduction pathways, and different physiological responses is further heightened by studies of deetiolation. In pea, R light acting via Pfr inhibits growth of etiolated plants and blocks GA synthesis (Reid et al., 2002), the expected converse of the effect of removal of Pfr to allow growth of Arabidopsis petioles. However, unlike the evidence that PHYB and not PHYA regulate petiole elongation (Figs. 7 and 8) and seed germination of Arabidopsis (Yamaguchi et al., 1998), for pea, PHYA is active and it regulates a decrease in a 3-oxidase. That R/FR photoreversible PHYB regulates both EOD FR and LD responses (Figs. 1, 6, 7, and 8; Table I) also raises questions about claims that flowering of Arabidopsis is mediated by PHYA; this claim must be treated with some skepticism.
An explanation for the clear evidence of R/FR photoreversible control of petiole elongation but the complete loss of response in the phyB mutant (Figs. 7 and 8) is not obvious. Were GA content to increase, as it appears to do in a sorghum phyB mutant (Lee et al., 1998), then 20-oxidase expression might be sufficiently down-regulated to become unresponsive to the removal of PHYB Pfr. Nevertheless, for an Arabidopsis phyB mutant, GA content of elongating hypocotyls is the same as the wild type (Reed et al., 1996). Perhaps PHYB also regulates some other component of GA signaling that alters responsiveness to GA but too little is known of this pathway for any conclusions to be drawn. Also, we cannot exclude the possibility of secondary changes given the well-documented pleiotropic effects of the phyB mutation (Reed et al., 1993).
In conclusion, for Arabidopsis, we have shown that FR-rich LD or EOD FR conditions stimulate petiole elongation by regulating expression of GA20-oxidase biosynthetic genes, as was also implied in earlier studies of elongation of petioles or stems (for review, see García-Martínez and Gil, 2002). Here, by using PHY mutants and R/FR photoreversibility assays, we have established that both EOD FR treatment and FR-rich LD act through PHYB-type PHYs and not PHYA. In addition, our examination of expression of two 20-oxidase genes and of responses in their mutants has shown that their role in petiole elongation depends on light conditions: the one, AtGA20ox1, being more relevant in SD, the other, AtGA20ox2, responding to FR-rich LD daylengths/light qualities. There are important implications of these findings for studies of the role of GAs in LD-regulated flowering and this issue will be considered in a later publication.
MATERIALS AND METHODS
Plant Material, Growing Conditions, and Light Treatments
Plants of Arabidopsis (Arabidopsis thaliana) L. Heynh. ecotype Columbia and various mutant and transgenic lines of Columbia were grown for 5 to 7 weeks in 8-h SD at an irradiance of 100 μmol photons m−2 s−1 at 22°C. There was no flowering for plants held for up to 10 weeks in these SD conditions in light from red/blue-rich fluorescent tubes; however, flowering occurred within 3 weeks on transfer of 5-week-old plants to a 24-h LD from incandescent lamps (data not shown; Bagnall and King, 2001).
For studies of petiole growth, plants were either kept in the standard 8-h SD from fluorescent lamps or exposed to a LD given as an additional 16 h of light either from incandescent bulbs (FR-enriched light) or from fluorescent lamps (R-rich light) and both at an irradiance of 10 μmol m−2 s−1. For 10-min R or FR light EOD exposures terminating the 8-h SD, FR light was obtained using a plastic cutoff filter and R was from red fluorescent lamps, as we described previously (Bagnall and King, 2001). In one experiment, an LL exposure was given and this was from fluorescent lamps at 100 μmol m−2 s−1.
Although we found no evidence of a touch effect, to minimize any touching we used calipers for daily measurements of petiole elongation. Single young petioles from 16 or more plants were measured in studies of elongation and, for studies of gene expression, young elongating petioles were harvested from 16 or more plants. The studies of gene expression required the use of a green safe light when young petioles were harvested during a dark period. All our findings were confirmed in duplicate experiments some of which are presented here.
Q-PCR Analysis of RNA Expression
Total RNA was extracted from young elongating petioles using an RNeasy Plant Mini kit (Qiagen, Clifton Hills, Australia) and treated with RNase-free DNase (Qiagen) according to the manufacturer's instruction. One or two micrograms of total RNA were reverse-transcribed using Super Script II (Invitrogen, Mt. Waverley, Australia) according to the manufacturer's instruction. The cDNA was diluted 5- or 25-fold and 4 μL used in a 20-μL Q-PCR with QuantiTect SYBR Green PCR Master Mix (Qiagen) or in a 10-μL Q-PCR with SYBR Green JumpStart Taq ReadyMix (Sigma Aldrich, Castle Hill, Australia). Q-PCR was performed on a Rotor-Gene 2000 Real-Time Cycler (Corbett Research, Sydney). Cycling and reaction conditions were as described in Klok et al. (2002). The sequences of the forward and reverse primers are shown in Table II along with gene information. Product sizes matched those expected from the known gene sequence. Amplicon sequence was confirmed for each primer set. At least one of each primer pair bridged an intron so there was no amplification of genomic DNA and all assays included a no-template sample to ensure Q-PCR results were not influenced by primer dimer formation. The Q-PCR assays were repeated three times and for any claimed treatment effects the result was confirmed in at least one further independent experiment. All samples were normalized using the Comparative Quantification analysis method (Rotogene-5 software, Corbett Research, Sydney). This method uses information about the start of the exponential phase of amplification (take-off point) and the average reaction efficiency. Concentrations were compared directly after normalization against a loading standard and to the value at lights-on of the first SD. The loading standard was ACTIN-2 (At3g18780 forward primer, TCAGATGCCCAGAAGTCTTGTTCC and reverse, CCGTACAGATCCTTCCTGATATCC). Its expression was stable within and across experiments; as an example, in one experiment the normalized value was 0.9 ± 0.06 over 69 assays.
Table II.
Genes assayed and primers used for Q-PCR assays
| Gene | AGI Gene Code | Forward Primer | Reverse Primer | |
|---|---|---|---|---|
| AtCPS | (GA1) | At4g02780 | CTAAAGCTTACAAGGATACC | GACATTGGGAACTCCTCC |
| AtKS | (GA2) | At1g79460 | GGATCTTAAATGTGATAGTG | AAGGAACTGCAGCCTCG |
| AtKO | (GA3) | At5g25900 | TGCCAAAGAGGCCATGG | TTCGTTTCTGTGCATTAGC |
| AtKAO1 | (CYP88A3) | At1g05160 | AGAGCACTCAAGGCTAGG | CTTGTTCAGCCTTTGCTC |
| AtKAO2 | (CYP88A4) | At2g32440 | AGAGCTTTGAAGGCAAGG | TCTCTTGTTCTTCCTTAGC |
| AtGA20ox1 | (GA5) | At4g25420 | CTCATGAATACACGAGCC | TGATACACCTTCCCAAATG |
| AtGA20ox2 | (At2353) | At5g51810 | ATGCTCACCGTTTGATGG | CCTTCCCAAACTGCTCG |
| AtGA20ox3 | (YAP169) | At5g07200 | CCTATCTGCATATGGACTC | AAACCTTCCCGAAATCTTC |
| AtGA3ox1 | (GA4) | At1g15550 | CCACGGCGTGCCTTTGG | GATATCGCAGTAGTTGAGG |
| AtGA3ox2 | (GA4H) | At1g80340 | CCTCGCGACTTCTCGAC | AATAATTTCACAGTATTTGAGG |
| AtGA2ox1 | At1g78440 | CGGGAACTTTCAGAAACGC | ACATTCTTACCACCATTGG | |
| AtGA2ox2 | At1g30040 | TTTCCGTGAGTCGGTGG | CTCCGCCTCTTCCTCCG | |
| AtGA2ox3 | At2g34500 | GCAATTTTCAGAGAGGCAG | CTCTTCCTTGACCGGAG |
Mutant Selection and Production of Gene Silencing Lines
The phyA-211 and phyB-9 mutants in Columbia were described previously (Reed et al., 1993; Bagnall and King, 2001). A T-DNA-insertional mutant for AtGA20ox1 was isolated in the Columbia background using Salk lines (accession no. 016701) obtained from ABRC (see Alonso et al., 2003). Subsequently we refer to this line as ga5-3. Transgenic hairpin lines for silencing AtGA20ox2 were produced in the Columbia background using routine transvection techniques. Transformants were selected for kanamycin resistance and confirmation of the insert obtained by PCR for both the T-DNA and the hairpin sequence. Hairpin constructs were made using pHellsgate12 following the methods outlined by Helliwell and Waterhouse (2003). Primers for the hairpin construct (forward, GCACAACAACATCTCCGGCAG and reverse, AACTCTTGTCCTAATGTATCGG) were designed to span a unique coding region of AtGA20ox2 with no blocks of sequence identity of over 18 bases between the construct and nontarget gene sequences. Expression analysis was performed as above.
Acknowledgments
We thank Cheryl Blundell for her help with maintaining plants and Drs. Tony Miller, Masumi Robertson, and Peter Waterhouse for guidance with molecular techniques. Drs. Peter Hedden and Andy Phillips (BBSRC, UK) are thanked for supplying the Columbia 20-oxidase antisense lines that were used previously in the study of Coles et al. (1999). For helpful comments on the manuscript, we thank Drs. Lloyd Evans, Neil Olszewski, and Masumi Robertson. During his stay in Canberra, T.H. was supported by a NARO Research Abroad Grant for Young Scientists.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.059055.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Bagnall DJ, King RW (2001) Phytochrome and flowering of Arabidopsis thaliana: photophysiological studies using mutants and transgenic lines. Aust J Plant Physiol 28: 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall FD, Yeung EC, Pharis RP (1996) Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can J Bot 74: 743–752 [Google Scholar]
- Borthwick HA, Hendricks SB, Parker MW, Toole EH, Toole VK (1952) A reversible photoreaction controlling seed germination. Proc Natl Acad Sci USA 38: 662–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez MA, Trenor M, Weigel D (2002) Independent control of gibberellin biosynthesis and flowering time by the circadian clock in Arabidopsis. Plant Physiol 130: 1770–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Jackson SD, Prat S (1999) Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato. Plant Physiol 119: 765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JP, Phillips AL, Croker SJ, Garcia-Lepe R, Lewis MJ, Hedden P (1999) Modification of gibberellin production and plant development in Arabidopsis by sense and antisense expression of gibberellin 20-oxidase genes. Plant J 17: 547–556 [DOI] [PubMed] [Google Scholar]
- Devlin PF, Yanofsky MJ, Kay SA (2003) A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133: 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC (2003) Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol 131: 1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JL, Gil J (2002) Light regulation of gibberellin biosynthesis and mode of action. J Plant Growth Regul 20: 354–368 [DOI] [PubMed] [Google Scholar]
- García-Martínez JL, Keith B, Bonner BA, Stafford AE, Rappaport L (1987) Phytochrome regulation of response to exogenous gibberellins by epicotyls of Vigna sinensis. Plant Physiol 85: 212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Zeevaart JAD, Schwenen L, Graebe JE (1986) Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol 82: 190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocal GFW, Sheldon C, Gubler F, Moritz T, Bagnall D, Song FL, Parish RW, Dennis ES, Weigel D, King RW (2001) GAMYB-like genes and gibberellin signaling in Arabidopsis. Plant Physiol 127: 1682–1693 [PMC free article] [PubMed] [Google Scholar]
- Helliwell C, Waterhouse P (2003) Constructs and methods for high-throughput gene silencing in plants. Methods 30: 289–295 [DOI] [PubMed] [Google Scholar]
- Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14: 2481–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Zeevaart JAD (2002) Differential regulation of RNA levels of gibberellin dioxygenases by photoperiod in spinach. Plant Physiol 130: 2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IJ, Foster KR, Morgan PW (1998) Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol 116: 1003–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García JF, Santes CM, García-Martínez JL (2000) The end-of-day far-red irradiation increases gibberellin A1 content in cowpea (Vigna sinensis) epicotyls by reducing inactivation. Physiol Plant 108: 426–434 [Google Scholar]
- Phillips AL, Ward DA, Uknes S, Appleford NEJ, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P (1995) Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol 108: 1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail PH (2002) Phytochrome photosensory signaling networks. Nat Rev Mol Cell Biol 3: 85–93 [DOI] [PubMed] [Google Scholar]
- Reed JW, Foster KR, Morgan PW, Chory J (1996) Phytochrome B affects responsiveness to gibberellins in Arabidopsis. Plant Physiol 112: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JB, Botwright NA, Smith JJ, O'Neill DP, Kerckhoffs LHJ (2002) Control of gibberellin levels and gene expression during de-etiolation in pea. Plant Physiol 128: 734–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H (1995) Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol 46: 289–315 [Google Scholar]
- Toyomasu T, Kawaide H, Mitsuhashi W, Inoue Y, Kamiya Y (1998) Phytochrome regulates gibberellin biosynthesis during germination of photoblastic lettuce seeds. Plant Physiol 118: 1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T, Tsuji H, Yamane H, Nakayama M, Yamaguchi I, Murofushi N, Takahashi N, Inoue Y (1993) Light effects on endogenous levels of gibberellins in photoblastic lettuce seeds. J Plant Growth Regul 12: 85–90 [Google Scholar]
- Wu K, Li L, Gage DA, Zeevaart JAD (1996) Molecular cloning and photoperiod-regulated expression of gibberellin 20-oxidase from the long-day plant spinach. Plant Physiol 110: 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y-L, Gage DA, Zeevaart JAD (1997) Gibberellins and stem growth in Arabidopsis thaliana. Effects of photoperiod on expression of the GA4 and GA5 loci. Plant Physiol 114: 1471–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Kamiya Y, Sun TP (2001) Distinct cell-specific expression patterns of early and late gibberellin biosynthetic genes during Arabidopsis seed germination. Plant J 28: 443–453 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Smith MW, Brown RGS, Kamiya Y, Sun TP (1998) Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell 10: 2115–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]