Abstract
Homeostasis of brassinosteroids (BRs) is essential for normal growth and development in higher plants. We examined responsiveness of 11 BR metabolic gene expressions to the decrease or increase of endogenous BR contents in Arabidopsis (Arabidopsis thaliana) to expand our knowledge of molecular mechanisms underlying BR homeostasis. Five BR-specific biosynthesis genes (DET2, DWF4, CPD, BR6ox1, and ROT3) and two sterol biosynthesis genes (FK and DWF5) were up-regulated in BR-depleted wild-type plants grown under brassinazole, a BR biosynthesis inhibitor. On the other hand, in BR-excessive wild-type plants that were fed with brassinolide, four BR-specific synthesis genes (DWF4, CPD, BR6ox1, and ROT3) and a sterol synthesis gene (DWF7) were down-regulated and a BR inactivation gene (BAS1) was up-regulated. However, their response to fluctuation of BR levels was highly reduced (DWF4) or nullified (the other eight genes) in a bri1 mutant. Taken together, our results imply that BR homeostasis is maintained through feedback expressions of multiple genes, each of which is involved not only in BR-specific biosynthesis and inactivation, but also in sterol biosynthesis. Our results also indicate that their feedback expressions are under the control of a BRI1-mediated signaling pathway. Moreover, a weak response in the mutant suggests that DWF4 alone is likely to be regulated in other way(s) in addition to BRI1 mediation.
Brassinosteroids (BRs) are steroid hormones that play important roles in plant growth and development activities that include stem elongation, leaf expansion, vascular differentiation, stress tolerance, and senescence (Clouse and Sasse, 1998). In the past decade, molecular geneticists have discovered and characterized many BR-related genes that function in BR biosynthesis, inactivation, and signaling (Bishop and Yokota, 2001; Clouse, 2002). Furthermore, recent transcriptome studies have identified numerous BR-responsive genes (Hu et al., 2001; Goda et al., 2002; Mussig et al., 2002). These studies have provided insight into molecular mechanisms of BR action.
It is crucial to adjust to and maintain appropriate levels of endogenous BRs for normal plant growth and development. For example, BR-deficient mutants of several plants exhibit strong dwarfism with curly and dark-green leaves in the light and deetiolation with short hypocotyls and open cotyledons in the dark (Bishop and Yokota, 2001). Abnormal growth that is apparent in the mutants is attributable to the constitutive decrease of endogenous BRs. Administration of excess amounts of bioactive BRs such as brassinolide (BL) and castasterone also causes abnormal organ growth, such as swelling and twisting of hypocotyls and roots in both light and dark (Hata et al., 1986; Wang et al., 2002; Tanaka et al., 2003). These results anticipate that endogenous BR contents are checked and balanced constantly via control of BR biosynthesis and inactivation rates in normally growing plants.
Recently, several studies using Arabidopsis (Arabidopsis thaliana) have shown that expressions of some BR metabolic genes are modulated at mRNA levels for BR homeostasis. For instance, the mRNAs of BR biosynthesis genes such as DWARF4 (DWF4) and CONSTITUTIVE PHOTOMORPHOGENESIS AND DWARFISM (CPD) increase in response to lowered amounts of endogenous BRs in BR-deficient mutants as well as in wild-type Arabidopsis treated with a BR biosynthesis inhibitor, brassinazole (Brz; Noguchi et al., 2000; Asami et al., 2001; Choe et al., 2001). In contrast, mRNAs of DWF4 and CPD are decreased rapidly; that of a BR inactivation gene, phyB activation-tagged suppressor1 (BAS1), is increased when BL is applied to wild-type plants (Mathur et al., 1998; Goda et al., 2002). Furthermore, it has been reported that BL-induced down-regulation of CPD expression is annulled in BR-insensitive bri1 mutants in which BR perception is defective (Li et al., 2001; Bancos et al., 2002), suggesting that feedback expressions of BR metabolic genes require BR-insensitive1 (BRI1) function. Indeed, accumulation of biologically active BRs and mRNA elevation of BR biosynthesis genes DEETIOLATED2 (DET2), DWF4, and CPD are observed in bri1 mutants (Noguchi et al., 1999, 2000; Choe et al., 2001; Bancos et al., 2002). Similar results have also been reported for other plant species including pea (Pisum sativum), rice (Oryza sativa), tomato (Lycopersicon esculentum), and barley (Hordeum vulgare; Nomura et al., 1997; Yamamuro et al., 2000; Montoya et al., 2002; Chono et al., 2003).
In this study, we attempted to examine the expression of 11 BR metabolic genes in Brz-treated and BL-treated Arabidopsis seedlings to better understand molecular mechanisms underlying BR homeostasis. We report here that mRNA levels of the nine genes were altered in response to temporarily fluctuating endogenous BR contents and that intact BRI1 function is necessary for their respective feedback expressions.
RESULTS
Effect of Brz on Expression of BR Metabolic Genes in Wild-Type Arabidopsis
Phytohormones' homeostasis, including BR's, should be attained by balance of their biosynthesis and inactivation rates. Therefore, to elucidate the feedback control of BR homeostasis at mRNA levels, we examined the expression of BR metabolic genes in response to alternation of endogenous BR contents, which are involved either in sterol biosynthesis, BR-specific biosynthesis, or BR inactivation pathways.
We used Brz to reduce endogenous BR contents (Asami and Yoshida, 1999). Figure 1 shows that seedling growth was apparently retarded when Brz was given to 14-d-old Arabidopsis plants. Growth arrest of the leaf petioles was most evident; they did not elongate at all in the presence of Brz, irrespective of Brz concentrations of 1 or 5 μm (Fig. 1A). Morphological observation further disclosed dwarfism of Brz-treated seedlings accompanied by typical characteristics of BR mutants, such as curly and dark-green leaves and shortened leaf blades and petioles (Fig. 1B). These morphological aberrations became detectable 2 d after Brz administration (Fig. 1B, subsections a and c). The aberrations were apparent after 4 d (Fig. 1B, subsections b and d). Abnormality was recovered by feeding of BL (data not shown). These traits of Brz-treated seedlings resembled those of the previously reported BR-deficient mutants (Li et al., 1996; Szekeres et al., 1996). Therefore, endogenous BR contents of the Brz-treated seedlings are likely to be decreased in our condition.
Figure 1.
Effects of Brz on the growth of Arabidopsis seedlings. Wild-type seedlings (14 d old) were cultured in the presence of 1 or 5 μm Brz for the indicated days as described in “Materials and Methods.” A, Lengths of the third and fourth leaf petioles. Each value represents mean of 20 seedlings with se. B, Photographs show wild-type seedlings treated with (subsections a and b) or without (subsections c and d) Brz. Nontreated wild-type (subsection e; 14 d old) and bri1-401 mutant seedlings (subsection f; 14 d old) are shown as controls. Scale bars indicate 5 mm.
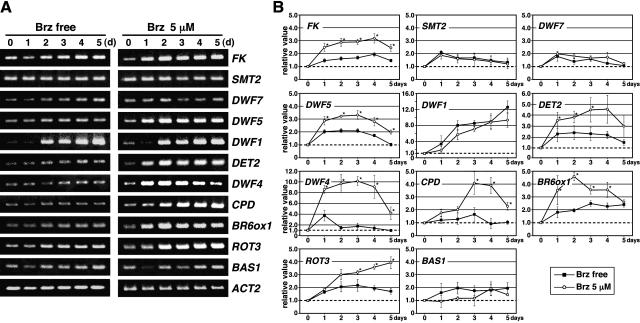
We performed reverse transcription (RT)-PCR analysis to examine mRNA levels of 11 BR metabolic genes in 5 μm Brz-treated seedlings. Figure 2 shows that three BR-specific biosynthesis genes, DET2, DWF4, and CPD, were up-regulated in response to Brz, which is consistent with accumulation of their mRNAs in BR-deficient mutants (Noguchi et al., 2000; Choe et al., 2001). In addition, we discovered Brz-induced expressions of the other two BR-specific biosynthesis genes, BR-6-oxidase (BR6ox1) and ROTUNDFOLIA3 (ROT3). Interestingly, expressions of FACKEL (FK) and DWARF5 (DWF5) were induced remarkably by Brz application, both of which function in sterol biosynthesis to provide precursor compounds of BR production (Schaller, 2003).
Figure 2.
Expressions of BR metabolic genes in response to Brz in Arabidopsis seedlings. RT-PCR analysis was performed to examine steady-state levels of mRNAs for 11 BR metabolic genes in Brz-administered wild-type seedlings. Amplified PCR products were fractionated through an agarose gel and stained with ethidium bromide. Fluorescence on the gel was scanned with a fluoro-image analyzer. Fluorescence intensity of each band was measured and converted into a relative value [initial (0 d) = 1.0] after normalization with that of ACT2 band used as an internal control. A, Photographs show representative gel images in three replicated experiments. B, Each value in the graphs shows mean with se of three experiments. Asterisks indicate statistically significant differences compared with nontreated control (*P < 0.05).
Brassinazole-induced expression differed kinetically among the seven genes (Fig. 2). These genes can be classified into at least two groups. The first group of genes includes FK, DWF5, DET2, DWF4, and BR6ox1, for which inductions were recognized 1 d after Brz treatment. The second group of genes, including CPD and ROT3, were induced 2 to 3 d after Brz administration. We chose DWF4 and CPD as representatives of the two groups and further analyzed their expressions in the early period of Brz treatment to confirm differences in their induction modes. Figure 3 shows that DWF4 expression was increased following 1 h lag time. It attained a plateau 8 h after Brz addition. In contrast, CPD expression was not up-regulated, even after 24 h.
Figure 3.
Kinetic difference between DWF4 and CPD expressions in Brz-applied Arabidopsis seedlings. RT-PCR analysis was performed to compare the expression of DWF4 and CPD in an early period of Brz treatment. Experimental procedures are identical to those in Figure 2. A, Gel photographs show amplified PCR bands with those of ACT2. B, Fluorescence intensity of each band is shown as a relative value [initial (0 h) = 1.0] after normalization with that of ACT2 band. Results are representative of three independent experiments.
Effect of BL on Expression of BR Metabolic Genes in Wild-Type Arabidopsis
Following examination of Brz-affected expression of BR metabolic genes, we tested the effect of exogenously applied BL on their expressions. It has long been argued that endogenous phytohormones often perturb effects of exogenous identical hormones (Mussig and Altmann, 2003). Therefore, we used Brz prior to BL application for reduction of endogenous BR contents. As mentioned before, Brz up-regulated five BR metabolic genes and caused growth aberrations of the seedlings within 2 d of Brz administration (Figs. 1 and 2), suggesting that endogenous BR contents in the seedlings were reduced sufficiently to bring about these phenomena by that time. Based on these results, we applied BL to Arabidopsis seedlings that had been cultured for 2 d in the presence of Brz.
We performed RT-PCR analysis to quantify mRNA levels of BR metabolic genes in the BL-treated seedlings described above. Figure 4 shows that expressions of four BR-specific biosynthesis genes, DWF4, CPD, BR6ox1, and ROT3, were reduced by 0.1 μm BL treatment. These repressions were apparent 1 to 2 h after BL administration and reached their lowest levels within 4 h. The DWF4 and CPD expressions were decreased most remarkably: they shrank to less than 7% of their initial levels. Reductions of BR6ox1 and ROT3 expressions were more moderate than those of DWF4 and CPD. The DET2 expression was not altered by BL supplementation (Fig. 4), but it responded to Brz treatment (Fig. 2). On the other hand, expression of BAS1 involved in BR inactivation was elevated rapidly 1 h after BL administration; it reached a plateau level at 8 h, at which time the transcript abundance was 4.15 times the initial value (Fig. 4). The DWARF7 (DWF7) expression alone was reduced by BL supplementation among five sterol biosynthesis genes (Fig. 4). The transcript level reached its minimum level within 2 h after BL addition and was about 50% of the initial value. Expressions of FK and DWF5 were little affected by exogenously applied BL (Fig. 4), whereas they were increased when the seedlings were grown in the presence of Brz (Fig. 2). Expressions of the other sterol biosynthesis genes, STEROL METHYLTRANSFERASE2 (SMT2) and DWARF1 (DWF1), did not respond to BL (Fig. 4) or to Brz (Fig. 2).
Figure 4.
Expressions of BR metabolic genes in response to BL in Arabidopsis seedlings. Following 5 μm Brz treatment for 2 d, 0.1 μm BL was given to wild-type seedlings for the indicated periods. The steady-state mRNA levels of 11 BR metabolic genes were examined using RT-PCR. Presentation styles of graphs are identical to those in Figure 2.
Based on the results described thus far, 11 BR metabolic genes were divided into four groups according to their respective responses to endogenous BR contents: the first group includes genes such as DWF4, CPD, BR6ox1, and ROT3, for which expressions were altered by both Brz and BL treatments; elements of the second (FK, DWF5, and DET2) and third (DWF7 and BAS1) groups responded to Brz and BL, respectively; the fourth one includes genes such as SMT2 and DWF1, for which expressions were unaffected by the two chemical treatments.
Expression of BR Metabolic Genes in a BR Signaling Mutant, bri1-401
BR-induced down-regulation of CPD is reportedly nullified in BR-insensitive bri1 mutants (Li et al., 2001; Bancos et al., 2002). Therefore, we examined responsiveness of other BR metabolic genes to fluctuation of endogenous BR contents in a bri1 mutant to clarify whether intact BRI1 function is required for their feedback expressions (Figs. 2 and 4). A newly identified bri1 mutant was used for this experiment, which was designated as bri1-401 with Wassilewskija (WS) background (Fig. 1B, subsection f) because we have used the WS ecotype throughout our research (Tanaka et al., 2003). DNA sequence analysis of bri1-401 revealed two point mutations (data not shown), which caused amino acid substitutions of G895R in kinase domain I and G1194E in the C-terminal region. First, we tested expressions of seven BR-metabolic genes in Brz-treated bri1-401 seedlings because they all increased dependently on Brz administration in wild-type plants (Fig. 2). Figure 5 shows that expressions of the other six genes except for DWF4 (FK, DWF5, DET2, CPD, BR6ox1, and ROT3) were unaffected by Brz application in bri1-401 mutants. DWF4 expression alone was up-regulated by Brz application in bri1-401 and became 3.2 times the initial level (Fig. 5). However, this level of increase was approximately one-third of that (10 times the initial level) in wild-type seedlings (Fig. 2).
Figure 5.
Expression profiles of BR metabolic genes in a BR signaling mutant fed with Brz. Five micromolar Brz were administered to bri1-401 seedlings for the indicated days. RT-PCR was performed to examine the expressions of BR metabolic genes. The experimental procedure and graph styles are identical to those in Figure 2.
Subsequently, we tested expressions of six BR metabolic genes in BL-administered bri1-401 seedlings as they all responded to exogenous BL in wild-type plants (Fig. 4). Again, DWF4 expression alone was altered in response to BL and down-regulated to 0.31-fold of the initial level in bri1-401 seedlings (Fig. 6), but this reduction level was much less than that (0.07 times that of the initial level) in wild type (Fig. 4). Expressions of the other five genes (DWF7, CPD, BR6ox1, ROT3, and BAS1) were not affected in bri1-401 by BL application (Fig. 6). In all, eight BR metabolic genes among the nine we tested did not respond to BL, Brz, or both in bri1-401, whereas they clearly did so in the wild type. Expression of DWF4 alone is still affected by both chemicals in the mutant, but the responsiveness was much weaker than that in wild type.
Figure 6.
Expression profiles of BR metabolic genes in a BR signaling mutant fed with BL. The bri1-401 seedlings were treated with 0.1 μm BL for the indicated periods after culture for 2 d in 5 μm Brz. Then, expressions of BR metabolic genes were examined by RT-PCR. Graph styles are identical to those in Figure 2.
DISCUSSION
Phytohormone homeostasis is believed to be crucial for normal growth and development in higher plants. For that reason, it is readily inferred that BR biosynthesis and inactivation can be modulated in a feedback manner to reduce elevated BR concentrations when bioactive BRs are given excessively to the plants. We observed BL-induced down-regulation of four BR-specific biosynthesis genes, DWF4, CPD, BR6ox1, and ROT3, as well as BL-induced up-regulation of a BR inactivation gene, BAS1, in our experimental system (Fig. 4; Table I), which are consistent with data in previous reports (Mathur et al., 1998; Bancos et al., 2002; Goda et al., 2002). Taken together, these results indicate that the increase of endogenous BR contents results in a feedback regulation at mRNA levels of these BR metabolic genes to maintain BR homeostasis. Furthermore, we found that a sterol biosynthesis gene, DWF7, was markedly down-regulated by BL addition (Fig. 4). Mussig et al. (2002) also observed BL-induced down-regulation of DWF7 in DNA microarray analysis. Nevertheless, that study did not refer to this phenomenon. Our time-course experiment showed that the kinetic behavior of DWF7 differed greatly from those of the four BR-specific biosynthesis genes described above (Fig. 4); DWF7 mRNA level was decreased rapidly upon BL addition, but it never reached as low as the levels of BR-specific biosynthesis genes. Kinetic differences between DWF7 and other BR-specific biosynthesis genes probably reflect that the former affects not only BR synthesis, but also sterol synthesis, whereas the latter influences only BR production.
Table I.
Expression profiles of BR-metabolic genes in Brz- or BL-treated Arabidopsis seedlings
Symbols are indicated as up-regulation (↑), down-regulation (↓), or no significant change (–) of gene expression.
| Category
|
Gene Name
|
Wild Type
|
bri1-401
|
||
|---|---|---|---|---|---|
| +Brz | +BL | +Brz | +BL | ||
| Sterol biosynthesis | FK | ↑ | – | – | – |
| SMT2 | – | – | – | – | |
| DWF7 | – | ↓ | – | – | |
| DWF5 | ↑ | – | – | – | |
| DWF1 | – | – | – | – | |
| BR-specific biosynthesis | DET2 | ↑ | – | – | – |
| DWF4 | ↑ | ↓ | ↑a | ↓a | |
| CPD | ↑ | ↓ | – | – | |
| BR6ox1 | ↑ | ↓ | – | – | |
| ROT3 | ↑ | ↓ | – | – | |
| BR inactivation | BAS1 | – | ↑ | – | – |
Responsiveness of DWF4 expression in bri1-401 to both chemicals was much weaker than that in wild type.
Little information is available regarding responses of BR metabolic genes when endogenous BR concentrations are reduced. A few studies have shown that some BR-specific biosynthesis genes, DET2, DWF4, and CPD, are up-regulated in BR-deficient mutants such as dwf1, dwf4, and cpd (Noguchi et al., 2000; Choe et al., 2001). Our study found that BR6ox1 and ROT3, in addition to the three genes described above, were up-regulated when endogenous BR concentrations were temporarily reduced by Brz (Fig. 2; Table I). All five BR-specific biosynthesis genes we tested responded to a decrease of endogenous BR contents; four of them, aside from DET2, also respond to their increase. These results suggest that the BR-specific biosynthesis genes play important roles in maintaining BR homeostasis. Although Choe et al. (2001) reported lower expression of BAS1 in a dwf4 mutant than that in wild type, we did not detect Brz-induced down-regulation of BAS1 in our system (Fig. 2). Currently, causes of the discrepancy in BAS1 response to the depletion of endogenous BR contents between the two laboratories remain unknown. Those different results might reflect different experimental conditions with respect to the use of either a BR-deficient mutant or a Brz-fed wild-type Arabidopsis. We observed that Brz caused up-regulation of sterol biosynthesis genes FK and DWF5 (Fig. 2). FK expression in Brz-fed plants is consistent with results of He et al. (2003). Their study demonstrated that FK::GUS expression is higher in BR-deficient det2 mutants than in wild type. Taken together, our results indicate that BR homeostasis is finely modulated by the feedback expressions of multiple (at least nine) BR metabolic genes, each of which is involved not only in BR-specific biosynthesis and inactivation, but also in sterol biosynthesis.
Why are sterol biosynthesis genes FK, DWF5, and DWF7 up-regulated or down-regulated by respective administration of Brz or BL (Figs. 2 and 4; Table I)? Two plant sterols, campesterol and its epimer, are precursor compounds for BR biosynthesis (Schaller, 2003). For that reason, endogenous BR concentrations, whether excessive or depleted, may affect expression of these sterol biosynthesis genes to decrease or increase precursor contents and thereby control the BR biosynthesis rate. However, their altered expressions should accessorily influence contents of sterols other than two precursor sterols for BR production because the three genes are also proven to function in synthesis of two major sterols: sitosterol and stigmasterol (Catterou et al., 2001; Schaller, 2003). Otherwise, both the rise and fall of endogenous BR concentrations may affect sterol contents and compositions positively. In animal systems, specialized areas called lipid rafts of cellular membranes, which are rich in both cholesterol and sphingolipids, are known to function as membrane scaffolds in signal transduction and protein sorting (Simons and Toomre, 2000). Furthermore, Mongrand et al. (2004) reported recently that tobacco plasma membranes possess such lipid rafts that are rich in sitosterol, stigmasterol, and 24-methylcholesterol as well as in cholesterol and sphingolipids. Therefore, changes of sterol contents and compositions that depend on endogenous BR concentrations may alter the properties and number of lipid rafts and then indirectly modulate BR perception on membrane-bound BR receptors. It also cannot be ruled out that endogenous BR-dependent expressions of the sterol biosynthesis genes may merely reflect crosstalk between BRs and signaling plant sterols, as in the case of interactions between BRs and other phytohormones (Tanaka et al., 2003). Increasingly numerous reports indicate that plant sterols function as signal molecules, but not as membrane constituents for plant growth and development, especially in embryogenesis (Diener et al., 2000; Jang et al., 2000; Schrick et al., 2000, 2002; Souter et al., 2002). Therefore, BRs may crosstalk with signaling plant sterols by means of the control of sterol biosynthesis. In any case, further studies are necessary to elucidate these conjectures relating to BR content-dependent expression of sterol biosynthesis genes.
Feedback regulation of endogenous BR concentrations is probably achieved through successful perception and transduction of BR signals because bioactive BRs, BL, and castasterone accumulate in BR-insensitive bri1/cbb2 and bin2/dwf12 mutants (Noguchi et al., 1999; Choe et al., 2002). Furthermore, a BR-specific biosynthesis gene, CPD, fails to be down-regulated in the same mutants fed with BL (Li et al., 2001; Bancos et al., 2002). This inference is supported strongly by our observation showing that expression of nine BR metabolic genes had a reduced or no response to fluctuation of endogenous BR contents in a bri1-401 mutant, whereas all of them clearly responded in wild type (Table I). It is interesting to speculate why DWF4 expression was regulated by fluctuation of endogenous BR contents in this mutant even though its responsiveness was much weaker than that in wild-type plants. One possible explanation for this phenomenon is that feedback expression of DWF4 is mediated by other receptor(s) in addition to a BRI1 receptor. Hu et al. (2000, 2004) reported that BR-responsive genes CycD3 and RAV1 are regulated by epi-BL in bri1 mutants. Furthermore, BRI1-like receptor genes have been shown to form a small gene family in the respective genomes of Arabidopsis, rice, and tomato (Yin et al., 2002; Nomura et al., 2003; Cano-Delgado et al., 2004; Zhou et al., 2004). These reports together suggest that feedback expression of DWF4 is also under the control of either BRI1 homologs or unknown receptors without homology to BRI1. Alternatively, DWF4 expression may be regulated directly in a feedback manner by end-products (BL and castasterone) or immediate products of DWF4-catalyzing reactions.
What molecular mechanisms are hidden under BR signaling mediated feedback expressions of its metabolic genes? Our results shed some light on those mechanisms. First, we found that seven BR metabolic genes that were up-regulated by the decrease of endogenous BR contents were classifiable into two groups with regard to their responsiveness: early response genes (FK, DWF5, DET2, DWF4, and BR6ox1) and late response genes (CPD and ROT3; Figs. 2 and 3). Results suggest that two independent feedback pathways modulate these two classes of genes separately when BR contents return to appropriate levels from its depletion state. Alternatively, a single pathway regulates both classes sequentially. Secondly, we clarified that nine BR metabolic genes responded to either excessive (DWF7 and BAS1), depleted (FK, DWF5, and DET2), or both levels (CPD, DWF4, BR6ox1, and ROT3) of endogenous BRs. This result gives rise to several questions concerning the feedback expressions of BR metabolic genes as described below. How are the rise and fall of BR concentrations sensed in plant cells? Are they monitored separately by different steps in BR signaling cascade or together detected by a single step, for example, perception of BR signals? How are the differences of BR levels distinguished and transmitted as distinct messages to each BR metabolic gene if the latter is the case? What signaling components are responsible for this process? Wang et al. (2002) reported that a single component downstream of BRI1, BZR1, is involved in feedback expression of CPD. More recently, BZR1 has been shown to act as a novel transcriptional repressor that binds directly to the promoters of BR-specific biosynthesis genes including CPD and DWF4 in BR homeostasis (He et al., 2005). Therefore, BZR1-interacting proteins and target genes of BZR1 may be good candidates for components that function in BR-signaling-mediated feedback expressions of BR metabolic genes. Based on our results, we are currently searching for missing links, including the above proteins (genes), that connect BR signaling and BR metabolism.
MATERIALS AND METHODS
Chemicals
All chemicals except those mentioned elsewhere were purchased from Wako Pure Chemical Industries, Osaka. Stock solutions of BL (Fuji Chemical, Tokyo) and Brz were prepared as described in our previous paper (Tanaka et al., 2003).
Plants
In this study, we used both a wild-type Arabidopsis (Arabidopsis thaliana) WS ecotype and a newly identified bri1 mutant with the same genetic background. A bri1 mutant was isolated as described below. Approximately 9,000 wild-type seeds were mutagenized using ethyl methanesulfonate (Kanto Chemical, Tokyo) as described by Redei and Koncz (1992). The mutagenized seeds (M1) were bulked in a pool of 1,000 each and propagated to obtain M2 seeds. The BR-insensitive dwarf plants were selected in M2 generation and applied to an allelism test with the known bri1 mutants [Arabidopsis Biological Resource Center (ABRC) stock no. CS3723 (bri1-1) and CS292 (cbb2)]. From a mutant candidate, the coding region of a bri1 gene was PCR-amplified with a primer set (5′-CTTCCCACACTTTTCTCTCTCACAA-3′ and 5′-GCTTTGGCTCTGTTTCTAACTCTCA-3′) using KOD-plus DNA polymerase (Toyobo, Osaka) with high fidelity and then sequenced to identify the mutation. A newly identified bri1 mutant was designated as bri1-401. The bri1-401 seeds are available upon request in a timely manner for noncommercial research purposes.
Chemical Treatments
Arabidopsis plants were cultured under growth conditions described in our previous paper (Tanaka et al., 2003). Chemical treatments were performed as follows. Twenty wild-type seedlings (10 in the case of a bri1 mutant) that had been grown for 14 d on a solidified Murashige and Skoog medium were further cultured with shaking at 80 rpm (Recipro Shaker NR-10; Taitec, Saitama, Japan) in Murashige and Skoog liquid medium (0.5 mL/plant) supplemented with either BL or Brz. Dimethylsulfoxide, a solvent used to dissolve BL and Brz, was added into each medium, not to exceed a final concentration of 0.1%.
RNA Isolation and RT-PCR
Total RNA was isolated from Arabidopsis seedlings using the guanidine thiocyanate method (McGookin, 1984). First-strand cDNA was synthesized from 2.5 μg total RNA using Moloney murine leukemia virus reverse transcriptase (ReverScript I; Nippon Gene, Tokyo) and subjected to PCR. The PCR program was composed of initial denaturation for 3 min at 94°C, amplification by each cycle of denaturation for 30 s at 94°C, annealing for 30 s at an appropriate temperature for each gene, elongation for 1.5 min at 72°C, and final elongation for 7 min at 72°C. The number of PCR cycles was determined through preliminary experiments to ensure linearity of DNA amplification. Primer sequences, annealing temperatures, and cycle numbers for each gene are given in Table II. The PCR products were fractionated in 0.7% (w/v) agarose gels and stained with ethidium bromide. Fluorescence signals of each band were measured using a fluoro-image analyzer (FLA 2000; Fuji Photo Film, Tokyo) and analyzed by the attached program ImageGauge version 3.4.
Table II.
Primer sequences, cycle numbers, and annealing temperatures used in the RT-PCR analysis
| Gene Name | Locus | Primer Sequences | No. of Cycles | Annealing Temperature |
|---|---|---|---|---|
| FK | At3g52940 | 5′-TCTCCTTTGAAAATCCTTCTTCTGC-3′ | 31 | 52 |
| 5′-AATCTGAAAGGATTCATGACTTGGC-3′ | ||||
| SMT2 | At1g20330 | 5′-TCTTCCTCACTCTTAACGAAAATGG-3′ | 31 | 52 |
| 5′-TTTTCCGGTGATGTTTCCTTTCTAC-3′ | ||||
| DWF7 | At3g02580 | 5′-ATCAATCCGGCGAATCTTCTTTCGT-3′ | 31 | 50 |
| 5′-CAATATAATTGGAAAGGTTGAGAGA-3′ | ||||
| DWF5 | At1g50430 | 5′-GACGAGAGAAGCAGAAGAAGAAAAT-3′ | 29 | 52 |
| 5′-GAGAACAACAGACTTCGTTACAATC-3′ | ||||
| DWF1 | At3g19820 | 5′-TTTCCTTTTTGGTTTGATGCAGTGA-3′ | 35 | 50 |
| 5′-ACATCAAACCAAGGCTTATTATTAC-3′ | ||||
| DET2 | At2g38050 | 5′-AATTCCATAACCCGAAAAATGGAAG-3′ | 29 | 50 |
| 5′-AGGTAGTTTGGAAACAAATTGACAC-3′ | ||||
| DWF4 | At3g50660 | 5′-TACCTCTTCTTCTTCTCCCATCGC-3′ | 29 | 55 |
| 5′-CGAGAAACCCTAATAGGCAAACCG-3′ | ||||
| CPD | At5g05690 | 5′-TCCTCCTCCTCTCTTCCATCGCCG-3′ | 27 | 55 |
| 5′-ACGGCGCTTCACGAAGATCGGGTA-3′ | ||||
| BR6ox1 | At5g38970 | 5′-AAAACAGAGCAGAAAACAGAGTGAG-3′ | 33 | 52 |
| 5′-TCTGTATCCTCTGCGTGCATAAAAT-3′ | ||||
| ROT3 | At4g36380 | 5′-AAGAAACCGGTTTAACTGTTTACGT-3′ | 31 | 50 |
| 5′-GCACAAACAGTTTTGTTCTTTGAAA-3′ | ||||
| BAS1 | At2g26710 | 5′-GCTCTCCTTTTTGTGTTTTCTCTCT-3′ | 31 | 52 |
| 5′-AGTCCGGAACAAATTTTTGACCGTT-3′ | ||||
| ACT2 | At5g09810 | 5′-TTCCGCTCTTTCTTTCCAAGCTCA-3′ | 31 | 50 |
| 5′-AAGAGGCATCAATTCGATCACTCA-3′ |
Statistical Analysis
All experiments were replicated at least three times. Data obtained were analyzed using ANOVA followed by paired or unpaired Student's t test. Difference with P < 0.05 was considered significant.
Acknowledgments
We are grateful to the ABRC at Ohio State University for providing the seeds of BR mutants. We also thank Dr. Satoru Taura at Research Center for Life Science Resources of Kagoshima University for technical assistance and valuable advice on fluoro-image analysis.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.058040.
References
- Asami T, Mizutani M, Fujioka S, Goda H, Min YK, Shimada Y, Nakano T, Takatsuto S, Matsuyama T, Nagata N, et al (2001) Selective interaction of triazole derivatives with DWF4, a cytochrome P450 monooxygenase of the brassinosteroid biosynthetic pathway, correlates with brassinosteroid deficiency in planta. J Biol Chem 276: 25687–25691 [DOI] [PubMed] [Google Scholar]
- Asami T, Yoshida S (1999) Brassinosteroid biosynthesis inhibitors. Trends Plant Sci 4: 348–353 [DOI] [PubMed] [Google Scholar]
- Bancos S, Nomura T, Sato T, Molnar G, Bishop GJ, Koncz C, Yokota T, Nagy F, Szekeres M (2002) Regulation of transcript levels of the Arabidopsis cytochrome P450 genes involved in brassinosteroid biosynthesis. Plant Physiol 130: 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Yokota T (2001) Plants steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant Cell Physiol 42: 114–120 [DOI] [PubMed] [Google Scholar]
- Cano-Delgado A, Yin Y, Yu C, Vafeados D, Mora-Garcia S, Cheng JC, Nam KH, Li J, Chory J (2004) BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131: 5341–5351 [DOI] [PubMed] [Google Scholar]
- Catterou M, Dubois F, Schaller H, Aubanelle L, Vilcot B, Sangwan-Norreel BS, Sangwan RS (2001) Brassinosteroids, microtubules and cell elongation in Arabidopsis thaliana. I. Molecular, cellular and physiological characterization of the Arabidopsis bull mutant, defective in the delta 7-sterol-C5-desaturation step leading to brassinosteroid biosynthesis. Planta 212: 659–672 [DOI] [PubMed] [Google Scholar]
- Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, Feldmann KA (2001) Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J 26: 573–582 [DOI] [PubMed] [Google Scholar]
- Choe S, Schmitz RJ, Fujioka S, Takatsuto S, Lee MO, Yoshida S, Feldmann KA, Tax FE (2002) Arabidopsis brassinosteroid-insensitive dwarf12 mutants are semidominant and defective in a glycogen synthase kinase 3β-like kinase. Plant Physiol 130: 1506–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chono M, Honda I, Zeniya H, Yoneyama K, Saisho D, Takeda K, Takatsuto S, Hoshino T, Watanabe Y (2003) A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol 133: 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD (2002) Brassinosteroids. In CR Somerville, EM Meyerowitz, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, http://www.aspb.org/publications/arabidopsis/toc.cfm, DOI/10.1199/tab.0009
- Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49: 427–451 [DOI] [PubMed] [Google Scholar]
- Diener AC, Li H, Zhou W, Whoriskey WJ, Nes WD, Fink GR (2000) STEROL METHYLTRANSFERASE 1 controls the level of cholesterol in plants. Plant Cell 12: 853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S (2002) Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 130: 1319–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Takagishi H, Egawa Y, Ota Y (1986) Effects of compactin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, on the growth of alfalfa (Medicago sativa) seedlings and the rhizogenesis of pepper (Capsicum annuum) explants. Plant Growth Regul 4: 335–346 [Google Scholar]
- He JX, Fujioka S, Li TC, Kang SG, Seto H, Takatsuto S, Yoshida S, Jang JC (2003) Sterols regulate development and gene expression in Arabidopsis. Plant Physiol 131: 1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YX, Bao F, Li JY (2000) Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J 24: 693–701 [DOI] [PubMed] [Google Scholar]
- Hu YX, Wang YH, Liu XF, Li JY (2004) Arabidopsis RAV1 is down-regulated by brassinosteroid and may act as a negative regulator during plant development. Cell Res 14: 8–15 [DOI] [PubMed] [Google Scholar]
- Hu YX, Wang ZK, Wang YH, Bao F, Li N, Peng ZH, Li JY (2001) Identification of brassinosteroid responsive genes in Arabidopsis by cDNA array. Sci China Ser C 44: 637–643 [DOI] [PubMed] [Google Scholar]
- Jang JC, Fujioka S, Tasaka M, Seto H, Takatsuto S, Ishii A, Aida M, Yoshida S, Sheen J (2000) A critical role of sterols in embryonic patterning and meristem programming revealed by the fackel mutants of Arabidopsis thaliana. Genes Dev 14: 1485–1497 [PMC free article] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398–401 [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J (2001) BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol 127: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C, et al (1998) Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J 14: 593–602 [DOI] [PubMed] [Google Scholar]
- McGookin R (1984) RNA extraction by the guanidine thiocyanate procedure. In JM Walker, ed, Methods in Molecular Biology. Humana Press, Totowa, NJ, pp 113–116 [DOI] [PubMed]
- Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ (2004) Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem 279: 36277–36286 [DOI] [PubMed] [Google Scholar]
- Montoya T, Nomura T, Farrar K, Kaneta T, Yokota T, Bishop GJ (2002) Cloning the tomato curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell 14: 3163–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussig C, Altmann T (2003) Genomic brassinosteroid effects. J Plant Growth Regul 22: 313–324 [DOI] [PubMed] [Google Scholar]
- Mussig C, Fischer S, Altmann T (2002) Brassinosteroid-regulated gene expression. Plant Physiol 129: 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Tax FE, Yoshida S, Feldmann KA (2000) Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol 124: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, Yuan H, Feldmann KA, Tax FE (1999) Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol 121: 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Bishop GJ, Kaneta T, Reid JB, Chory J, Yokota T (2003) The LKA gene is a BRASSINOSTEROID INSENSITIVE 1 homolog of pea. Plant J 36: 291–300 [DOI] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T (1997) Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol 113: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei GP, Koncz C (1992) Classical mutagenesis. In C Koncz, N-H Chua, J Schell, eds, Methods in Arabidopsis Research. World Scientific, Singapore, pp 16–82
- Schaller H (2003) The role of sterols in plant growth and development. Prog Lipid Res 42: 163–175 [DOI] [PubMed] [Google Scholar]
- Schrick K, Mayer U, Horrichs A, Kuhnt C, Bellini C, Dangl J, Schmidt J, Jurgens G (2000) FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev 14: 1471–1484 [PMC free article] [PubMed] [Google Scholar]
- Schrick K, Mayer U, Martin G, Bellini C, Kuhnt C, Schmidt J, Jurgens G (2002) Interactions between sterol biosynthesis genes in embryonic development of Arabidopsis. Plant J 31: 61–73 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39 [DOI] [PubMed] [Google Scholar]
- Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K (2002) hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell 14: 1017–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nakamura Y, Asami T, Yoshida S, Matsuo T, Okamoto S (2003) Physiological roles of brassinosteroids in early growth of Arabidopsis: Brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. J Plant Growth Regul 22: 259–271 [Google Scholar]
- Wang ZY, Nakano T, Gendron J, He J, Chen M, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T, et al (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2: 505–513 [DOI] [PubMed] [Google Scholar]
- Yamamuro C, Ihara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12: 1591–160511006334 [Google Scholar]
- Yin Y, Wu D, Chory J (2002) Plant receptor kinases: systemin receptor identified. Proc Natl Acad Sci USA 99: 9090–9092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Wang H, Walker JC, Li J (2004) BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J 40: 399–409 [DOI] [PubMed] [Google Scholar]