Abstract
Background:
Intrahepatic cholangiocarcinoma (iCCA) is the second most common hepatic malignancy and has a poor prognosis. Surgical resection is the standard of care for patients with resectable disease, representing 30–40% of cases. Increasingly, neoadjuvant systemic therapy is being utilized in patients due to high-risk anatomic or biologic considerations. However, data on the clinical effect of this approach are limited. We performed a cohort study to evaluate the effect of neoadjuvant therapy in patients with oncologically high-risk iCCA.
Methods:
iCCA patients (n = 181) between the years 2014–2020 were reviewed for clinical, histopathologic, treatment, and outcome-related data. Tumor regression grade was scored per CAP criteria for gastrointestinal carcinomas.
Results:
47 iCCA patients received neoadjuvant therapy and 72 did not. Neoadjuvant treatment led to objective response and tumor regression by CAP score. After adjustment for age, clinical stage, and tumor size, the outcomes of patients who had neoadjuvant therapy followed by surgery were not significantly different from those patients who had surgery first.
Discussion:
In conclusion, neoadjuvant therapy in iCCA facilitated surgical care. The progression-free and overall survival for surgical patients with and without neoadjuvant therapy were not significantly different suggesting this approach needs further exploration as an effective treatment paradigm.
Background
Cholangiocarcinoma (CCA) is a primary biliary tract malignancy and is the second most common primary hepatic neoplasm. CCAs are classified into three subtypes based on their anatomic location: intrahepatic, perihilar, and distal. Intrahepatic cholangiocarcinoma (iCCA) comprises about 10–20% of all cholangiocarcinoma subtypes.1 The incidence and mortality of iCCA has been increasing in the USA and worldwide despite advances in treatment options.2–4 Currently, surgical resection with curative intent is the standard of care for patients with resectable tumor burden. Approximately 30–40% of iCCA patients have resectable disease at presentation with the 5-year survival post-resection varying from 20 to 40% depending on the multiple prognostic factors such as margin status, presence of lymph node metastases, tumor multifocality, and/or vascular invasion.3 In patients with advanced disease, the median survival is only 12–15 months.3
Due to high recurrence rates combined with the morbidity of major hepatectomy, neoadjuvant therapy is considered at some institutions in the setting of locally advanced disease or in patients with high-risk oncologic features such as lymphadenopathy or tumor multifocality. In these centers neoadjuvant therapy is used as a tool to convert patients with unresectable/borderline resectable disease into potential surgical candidates..5–7 This follows an approach that has been utilized in other gastrointestinal tumor types such as pancreatic ductal adenocarcinoma and colorectal adenocarcinoma.8 Gemcitabine in combination with platinum-based chemotherapy has been the long-standing first line treatment for advanced stage, unresectable cholangiocarcinoma and this regimen has also been used for neoadjuvant therapy for iCCA.9–12 Triplet regimen including gemcitabine, cisplatin and nab-paclitaxel has been increasingly utilized recently.13 The current first-line therapy includes immune checkpoint inhibition with durvalumab based on a positive phase 3 study, however the use of this regimen in the neoadjuvant setting is only starting to undergo evaluation.14
Only a few previous small retrospective studies have described the outcomes of neoadjuvant therapy for locally advanced iCCA and subsequent surgery.11,15,16 Therefore, we performed a retrospective cohort analysis to examine the oncologic outcomes of neoadjuvant therapy for the treatment of patients with locally advanced or oncologically high-risk iCCA.
Methods
Patient selection
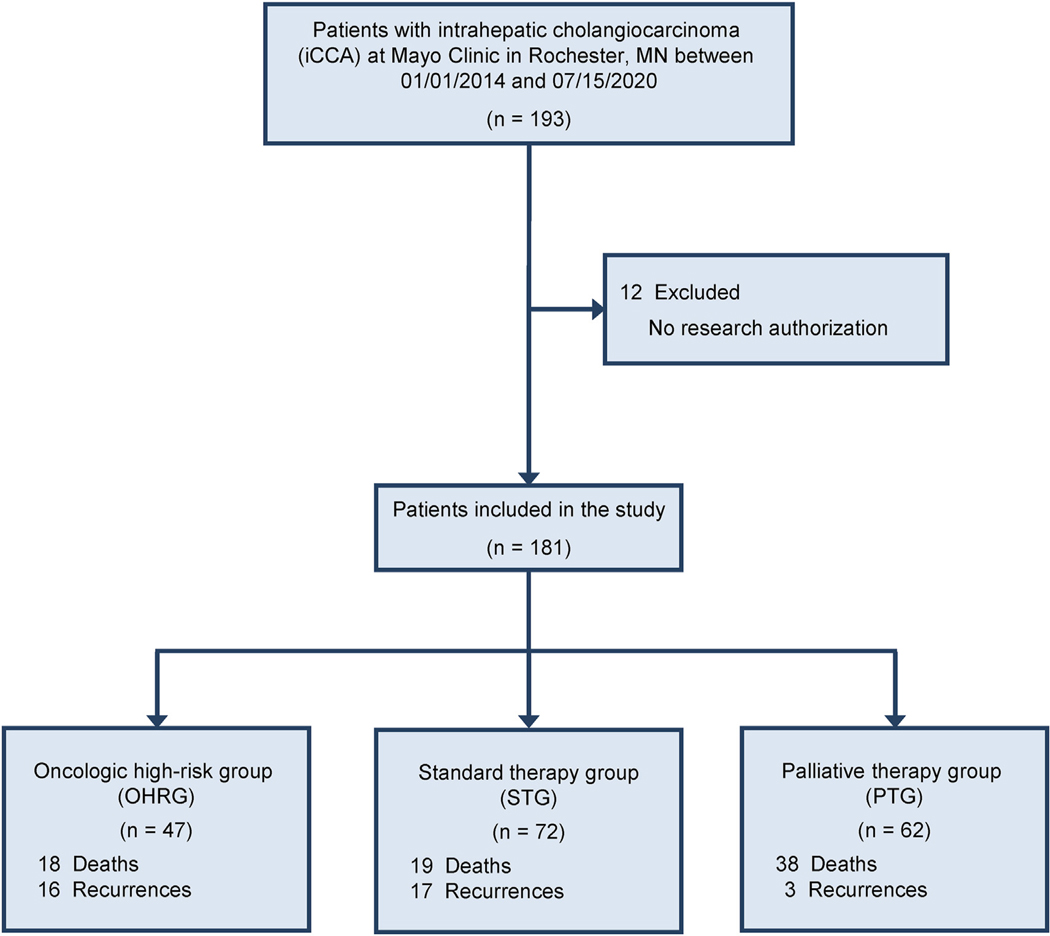
All iCCA patients who underwent partial hepatectomy at Mayo Clinic in Rochester, Minnesota between the years 2014–2020 were identified retrospectively (Fig. 1). Medical records, histologic slides, and pathology reports were retrieved and reviewed. Histopathological diagnosis was made based on H&E slides and available clinicopathologic information. The pathological tumor stage was determined using the American Joint Comossion on Cancer (AJCC) 8th edition criteria. A control group consisting of patients with iCCA who did not undergo partial hepatectomy and only received palliative chemotherapy were also selected (Fig. 1). Patients with combined hepatocellular carcinoma and iCCA or mixed neuroendocrine non-neuroendocrine neoplasms were excluded.
Figure 1.
Flowchart of patients with intrahepatic cholangiocarcinoma
Clinicopathologic data acquisition
After selecting patients who met the inclusion criteria, the patient’s medical charts were examined retrospectively from the date of diagnosis to either the date of last follow-up or death. Initial diagnosis was based on a tissue biopsy. Disease recurrence was based on imaging studies and/or tissue biopsy. The type of chemotherapy regimen used for neoadjuvant, adjuvant, and palliative purposes was collected.
Statistical analysis
The patients were separated into three categories as follows: 1) Palliative therapy group; Patients with locally advanced/unresectable iCCAwithout partial hepatectomy ± palliative chemotherapy (PTG), 2) Oncologic high-risk group (Neoadjuvant); Patients with locally advanced/initially surgically unresectable iCCA status post neoadjuvant therapy (OHRG), and 3) Standard therapy group; Patients with surgically resectable iCCA without neoadjuvant therapy ± adjuvant chemotherapy (STG). Patient characteristics were compared in a pairwise fashion using chi-square, Fisher’s exact, or Wilcoxon rank sum tests, as appropriate. Overall survival was defined as time from diagnosis or surgery to death from any cause. Progression-free survival was defined as time from date of diagnosis or surgery to disease recurrence or death from any cause. Patients without an event (death or progression) were censored at date of last follow-up. For analyses including surgical together with non-surgical patients, the diagnosis date was regarded as baseline. For analyses limited to surgical patients only, the date of partial hepatectomy was regarded as baseline. The Kaplan–Meier method was used to estimate the median time-to-event, as well as overall survival (OS) and progression-free survival (PFS) at 1- and 3-years post-baseline. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression. P-values less than 0.05 were considered significant. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Histopathologic tumor regression analysis
Histologic tumor regression grade was scored per the College of American Pathologists (CAP) criteria for gastrointestinal carcinomas.17 The CAP tumor regression grade categories are listed below:
Score 0: complete response - no viable cancer cells.
Score 1: near complete response - single cells or rare small groups of cancer cells.
Score 2: partial response - residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells.
Score 3: poor or no response - extensive residual cancer with no evident tumor regression.
In addition to the CAP tumor regression grade, the percent tumor regression was assessed in 5% increments. The tumor regression grade and percent tumor regression were calculated by two gastrointestinal pathologists (BJV and RPG) after performing a consensus review of all H&E-stained slides from each case.
Results
Clinical characteristics
A total of 181 cases of iCCA were included in the study with median age at diagnosis of 64 years (interquartile range [IQR] 57–72), 91 (50.3%) were male. Additional clinicopathologic details are listed in Table 1. Of the 181 iCCA cases, 62 patients were deemed to have locally advanced/oncologically high-risk iCCA (PTG: Palliative therapy group). The remaining 119 cases of iCCA underwent partial hepatectomy with 47 patients receiving neoadjuvant therapy (OHRG: Oncologic high-risk group) and 72 patients not receiving neoadjuvant therapy and undergoing upfront surgery (STG: Standard therapy group) (see Fig. 1). In the oncologic high-risk group who received neoadjuvant therapy, the median number of cycles was 4 (IQR 3–6, range 2–12). The neoadjuvant regimen used and information on adjuvant chemotherapy after partial hepatectomy are shown in Table 2. Among 72 patients within the standard group, 39 received adjuvant chemotherapy, 28 did not, and 5 had unknown adjuvant chemotherapy status (Supplemental Table 1). Of the 62 patients within the palliative therapy group, 40 received palliative chemotherapy, 21 did not, and 1 had unknown chemotherapy status (Supplemental Table 2).
Table 1.
Baseline and clinicopathologic characteristics of patients with intrahepatic cholangiocarcinoma
| Characteristic | OHRG (n = 47) | STG (n = 72) | PTG (n = 62) | P valuesa | ||
|---|---|---|---|---|---|---|
| OHRG vs. STG | OHRG vs. PTG | STG vs. PTG | ||||
| Male (n, %) | 21 (44.7) | 38 (52.8) | 32 (51.6) | 0.388 | 0.473 | 0.893 |
| Race (n, %) | 0.677 | 0.334 | 1.000 | |||
| Asian | 0 (0.0) | 2 (2.9) | 2 (3.3) | |||
| Black | 2 (4.3) | 1 (1.4) | 0 (0.0) | |||
| White | 42 (91.3) | 64 (91.4) | 55 (91.7) | |||
| Other | 2 (4.3) | 3 (4.3) | 3 (5.0) | |||
| Unknown | 1 | 2 | 2 | |||
| Non-White race (n, %) | 4 (8.7) | 6 (8.6) | 5 (8.3) | 1.000 | 1.000 | 0.961 |
| Hispanic ethnicity (n, %) | 0 (0.0) | 0 (0.0) | 4 (6.6) | NA | 0.133 | 0.047 |
| Unknown | 1 | 4 | 1 | |||
| Age at diagnosis (years) (median, IQR) | 62.0 (55.0–68.0) | 67.5 (60.5–73.0) | 61.0 (52.0–73.0) | 0.012 | 0.915 | 0.033 |
| Age ≥64 years (n, %)b | 20 (42.6) | 46 (63.9) | 27 (43.5) | 0.022 | 0.917 | 0.018 |
| Age at surgery (years) (median, IQR) | 62.0 (55.0–69.0) | 68.0 (60.5–74.0) | NA | 0.024 | NA | NA |
| Age ≥65 years (n, %)c | 20 (42.6) | 45 (62.5) | NA | 0.033 | NA | NA |
| Months to surgery (median, IQR) | 5.0 (3.6–7.2) | 1.5 (0.9–2.2) | NA | <0.001 | NA | NA |
| Tumor characteristics d | ||||||
| Largest tumor size (cm) (median, IQR) | 6.9 (5.0–8.5) | 4.8 (3.5–6.6) | NA | <0.001 | NA | NA |
| Pre-treatment stages (n, %) | ||||||
| T stage | <0.001 | NA | NA | |||
| T1A | 2 (4.3) | 32 (44.4) | NA | |||
| T1B | 10 (21.3) | 12 (16.7) | NA | |||
| T2 | 32 (68.1) | 23 (31.9) | NA | |||
| T3 | 0 (0.0) | 0 (0.0) | NA | |||
| T4 | 3 (6.4) | 5 (6.9) | NA | |||
| T stage ≥ T2 | 35 (74.5) | 28 (38.9) | NA | <0.001 | NA | NA |
| N stage | <0.001 | NA | NA | |||
| N0 | 28 (59.6) | 67 (93.1) | NA | |||
| N1 | 19 (40.4) | 5 (6.9) | NA | |||
| M stage | NA | NA | NA | |||
| M0 | 47 (100.0) | 72 (100.0) | NA | |||
| M1 | 0 (0.0) | 0 (0.0) | NA | |||
| Clinical stagee | <0.001 | NA | NA | |||
| IA | 2 (4.3) | 29 (40.3) | NA | |||
| IB | 5 (10.6) | 13 (18.1) | NA | |||
| II | 19 (40.4) | 20 (27.8) | NA | |||
| IIIA | 0 (0.0) | 0 (0.0) | NA | |||
| IIIB | 21 (44.7) | 10 (13.9) | NA | |||
| IV | 0 (0.0) | 0 (0.0) | NA | |||
| Clinical stage ≥ II | 40 (85.1) | 30 (41.7) | NA | <0.001 | NA | NA |
| Post-surgical stages (n, %)e | ||||||
| Pathologic T stage | 0.126 | NA | NA | |||
| pT1A | 7 (14.9) | 25 (34.7) | NA | |||
| pT1B | 15 (31.9) | 17 (23.6) | NA | |||
| pT2 | 19 (40.4) | 19 (26.4) | NA | |||
| pT3 | 2 (4.3) | 3 (4.2) | NA | |||
| pT4 | 4 (8.5) | 8 (11.1) | NA | |||
| Pathologic T stage ≥ pT2 | 25 (53.2) | 30 (41.7) | NA | 0.218 | NA | NA |
| Pathologic stage | 0.377 | NA | NA | |||
| IA | 7 (14.9) | 22 (30.6) | NA | |||
| IB | 12 (25.5) | 14 (19.4) | NA | |||
| II | 12 (25.5) | 13 (18.1) | NA | |||
| IIIA | 1 (2.1) | 2 (2.8) | NA | |||
| IIIB | 15 (31.9) | 21 (29.2) | NA | |||
| IV | 0 (0.0) | 0 (0.0) | NA | |||
| Pathologic stage ≥ II | 28 (59.6) | 36 (50.0) | NA | 0.306 | NA | NA |
| Comparison of pre-treatment and pathologic stage | ||||||
| Clinical stage to pathologic stage | <0.001 | NA | NA | |||
| Downstaged | 22 (46.8) | 11 (15.3) | NA | |||
| No change | 14 (29.8) | 38 (52.8) | NA | |||
| Upstaged | 11 (23.4) | 23 (31.9) | NA | |||
| Clinical stage ≥ II to pathologic stage ≥ II | <0.001 | NA | NA | |||
| Downstaged (≥ II to < II) | 15 (31.9) | 10 (13.9) | NA | |||
| No change (< II to < II) | 4 (8.5) | 26 (36.1) | NA | |||
| No change (≥ II to ≥ II) | 25 (53.2) | 20 (27.8) | NA | |||
| Upstaged (< II to ≥ II) | 3 (6.4) | 16 (22.2) | NA | |||
OHRG, Oncologic high-risk group (Neoadjuvant), Patients with locally advanced/oncologically high-risk iCCA status post neoadjuvant therapy; iCCA, intrahepatic cholangiocarcinoma; STG, Standard therapy group, Patients with surgically resectable iCCA without neoadjuvant therapy ± adjuvant chemotherapy; PTG; Palliative therapy group, Patients with locally advanced/unresectable iCCA without partial hepatectomy ± palliative chemotherapy; NA, not applicable.
Pairwise P values were calculated using chi-square or Fisher’s exact tests for categorical characteristics or Wilcoxon rank sum tests for continuous characteristics.
Dichotomization based on the median age at diagnosis across all three groups pooled together.
Dichotomization based on the median age at surgery across both groups pooled together.
Tumor characteristics were not available for the PTG group.
Based on American Joint Commission on Cancer, 8th edition.
Table 2.
Characteristics of chemotherapy for patients with intrahepatic cholangiocarcinoma who underwent partial hepatectomy with neoadjuvant chemotherapy
| ’Characteristic | OHRGa (n = 47) |
|---|---|
| Neoadjuvant chemotherapy (n, %) | 47 (100.0) |
|
| |
| Number of cycles (n, %) | |
|
| |
| 1 | 0 (0.0) |
|
| |
| 2 | 4 (9.1) |
|
| |
| 3 | 13 (29.5) |
|
| |
| 4 | 11 (25.0) |
|
| |
| 5 | 0 (0.0) |
|
| |
| 6 | 8 (18.2) |
|
| |
| >6 | 8 (18.2) |
|
| |
| Unknown | 3 |
|
| |
| Number of cycles (median, IQR) | 4.0 (3.0–6.0) |
|
| |
| Range | 2–12 |
|
| |
| Chemotherapy regimen (n, %) | |
|
| |
| Gemcitabine + cisplatin | 30 (65.2) |
|
| |
| Gemcitabine + cisplatin + paclitaxel | 4 (8.7) |
|
| |
| R–CHOP | 1 (2.2) |
|
| |
| Capecitabine + oxaliplatin | 1 (2.2) |
|
| |
| Capecitabine + radiation | 1 (2.2) |
|
| |
| Gemcitabine + carboplatin | 1 (2.2) |
|
| |
| Gemcitabine + oxaliplatin | 1 (2.2) |
|
| |
| Rituximab + cyclophosphamide | 1 (2.2) |
|
| |
| Gemcitabine + cisplatin, then FOLFOX | 1 (2.2) |
|
| |
| Gemcitabine + cisplatin, then capecitabine + oxaliplatin | 1 (2.2) |
|
| |
| Gemcitabine + cisplatin, then gemcitabine + cisplatin + paclitaxel | 1 (2.2) |
|
| |
| Gemcitabine + oxaliplatin, then capecitabine + oxaliplatin | 1 (2.2) |
|
| |
| Gemcitabine + oxaliplatin, then gemcitabine | 1 (2.2) |
|
| |
| Gemcitabine, then dabrafenib + trametinib | 1 (2.2) |
|
| |
| Unknown | 1 |
|
| |
| Adjuvant chemotherapy (n, %) | |
|
| |
| No | 35 (77.8) |
|
| |
| Yes | 10 (22.2) |
|
| |
| Unknown | 2 |
|
| |
| Number of cycles (n, %) | |
|
| |
| 1 | 1 (16.7) |
|
| |
| 2 | 1 (16.7) |
|
| |
| 3 | 2 (33.3) |
|
| |
| 4 | 0 (0.0) |
|
| |
| 5 | 0 (0.0) |
|
| |
| 6 | 1 (16.7) |
|
| |
| >6 | 1 (16.7) |
|
| |
| Unknown | 4 |
|
| |
| Number of cycles (median, IQR) | 3.0 (2.0–6.0) |
|
| |
| Range | 1–12 |
|
| |
| Chemotherapy regimen (n, %) | |
|
| |
| Gemcitabine + cisplatin | 3 (30.0) |
|
| |
| Gemcitabine | 1 (10.0) |
|
| |
| Capecitabine + radiation | 1 (10.0) |
|
| |
| Gemcitabine + paclitaxel | 1 (10.0) |
|
| |
| FOLFIRI, then FOLFOX | 1 (10.0) |
|
| |
| FOLFOX, then gemcitabine + paclitaxel | 1 (10.0) |
|
| |
| Gemcitabine + carboplatin, then FOLFOX | 1 (10.0) |
|
| |
| Gemcitabine + cisplatin, then pembrolizumab, then FOLFOX | 1 (10.0) |
OHRG, Oncologic high-risk group (Neoadjuvant), Patients with locally advanced/oncologically high-risk iCCA status post neoadjuvant therapy; iCCA, intrahepatic cholangiocarcinoma; R–CHOP, rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisone; FOLFOX, leucovorin calcium, fluorouracil, and oxaliplatin; FOLFIRI, leucovorin calcium, fluorouracil, and irinotecan hydrochloride.
The distributions and percentages were calculated among applicable patients for whom this information was known.
The oncologic high-risk group patients were significantly younger at time of surgery (median 62 vs. 68 years, p = 0.024), had larger tumors (median 6.9 cm vs. 4.8 cm; p < 0.001), and were at higher pre-treatment T stage and clinical stage (both p < 0.001) than standard group patients. There was no significant difference in distributions of patient sex (p = 0.388), race (p = 0.677) or final post-surgical pathologic T stage (p = 0.126) between the oncologic high-risk patient group and standard patient group (Table 1).
Tumor regression analysis
Of the patients in the oncologic high-risk group, twenty-two patients showed response to therapy (46.8%) as defined by a final pathologic stage less than the initial clinical stage, 14 patients showed a pathologic stage that was the same as the initial clinical staging (29.8%) and a total of 11 patients developed tumor progression (23.4%). Within the standard therapy group, eleven patients showed a final pathology stage less than the initial clinical stage (n = 11, 15.3%), 38 patients showed no change in clinical staging and (52.8%), and a total of 23 patients developed tumor progression (31.9%).
Histologic tumor regression analysis was performed on 31 oncologic high-risk cases (where all of the histologic slides were available), including 7 cases of large duct type iCCA (22.6%) and 24 cases of small duct type iCCA (77.4%). Most cases (n = 20, 64.5%) showed CAP score 2. Near complete response (CAP score 1) was observed in 7 patients (22.6%) and CAP score 3 response was seen in 4 cases (12.9%). None of the patients had a complete response (CAP score 0). The median percent viable tumor was 60% (interquartile range: 30–80%, range 0–95%). Eighteen cases (58.1%) had 60% or more viable tumor cells, while 13 cases (41.9%) had less than 60% viable tumor cells.
Survival analysis
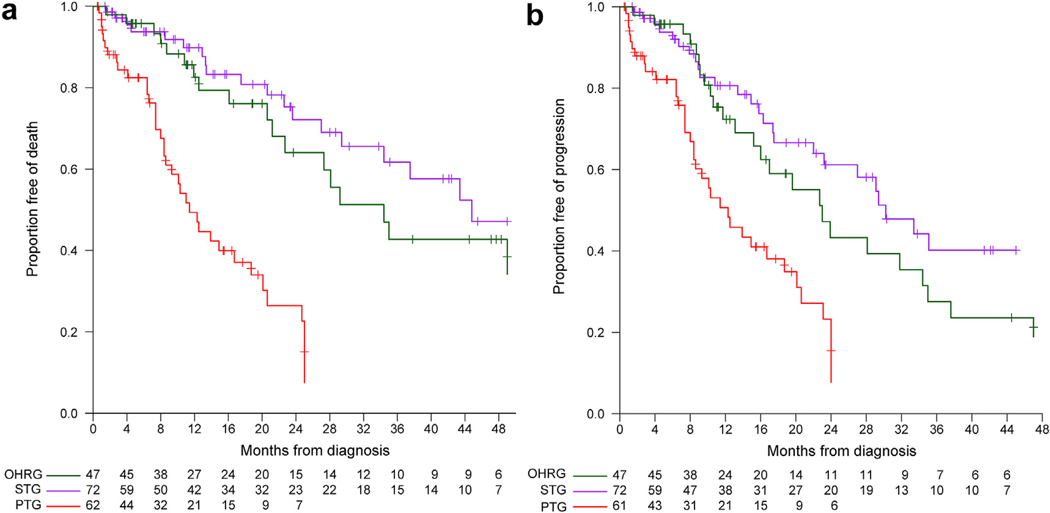
The follow up for all patients ranged from 0.5 months to 78.8 months. Overall survival (OS) was significantly lower for the palliative therapy group as compared to standard therapy and oncologic high-risk groups (p < 0.001), with median OS of 11.4, 44.8, and 34.4 months, respectively (as compared to the palliative therapy group: HR 0.20 [95% CI: 0.11–0.34] and HR 0.27 [95% CI: 0.15–0.48] for standard therapy and oncologic high-risk groups, respectively (Supplemental Table 3, Fig. 2a).
Figure 2.
Kaplan–Meier curves for overall survival and progression-free survival by patient group
Progression-free survival (PFS) also differed significantly between the three groups (p < 0.001), with a median of 12.3 months in the palliative therapy group patients compared to 30.2 months (HR 0.31, 95% CI: 0.18–0.51) in the standard therapy group, and 23.0 months (HR 0.45, 95% CI: 0.27–0.76) in the oncologic high-risk group (Supplemental Table 3, Fig. 2b).
OS and PFS in univariate models specified by selected characteristics for the standard therapy group are shown in Supplemental Table 4. Without any adjustment, OS was not significantly different between the oncologic high-risk and standard therapy patients (HR 1.68, 95% CI: 0.87–3.22, p = 0.118), while PFS was significantly worse for the oncologic high-risk group when compared with the standard therapy group (HR 1.93, 95% CI: 1.10–3.39, p = 0.021). The risk of both death and disease progression increased for larger tumor sizes, pre-treatment T stage ≥2, and pre-treatment clinical stage ≥2.
The oncologic high-risk group with near complete response (CAP score 1) had a median OS of 21.9 months and 1-year and 3-year percent survival of 83.3% (95% CI: 53.5–100%) and 41.7% (95% CI: 0–100%), respectively. The cases with CAP score 2, had a median OS of 18.2 months and 1 and 3-year percent survival of 57.9% (95% CI: 30.3–85.4%) and 14.5% (95% CI: 0–40%), respectively. This difference between CAP score 1 vs. 2 was not statistically significant (HR 2.14, 95% CI: 0.55–14.1, Table 3). There were insufficient cases in the poor or no response (score 3) category to calculate a median OS.
Table 3.
Overall survival and progression-free survival in patients with intrahepatic cholangiocarcinoma who underwent partial hepatectomy with neoadjuvant chemotherapy
| Characteristic | N | Events | Median survival (months) | Survival % (95% CI)a |
Univariate modelsb |
||
|---|---|---|---|---|---|---|---|
| 1 year | 3 years | HR (95% CI) | P value | ||||
| Overall survivalc | |||||||
|
| |||||||
| Gender | 0.899 | ||||||
|
| |||||||
| Female | 26 | 8 | 30.3 | 79.9% (61.9–97.9) | 38.8% (8.2–69.4) | -reference- | |
|
| |||||||
| Male | 21 | 10 | 21.9 | 70.7% (48.8–92.6) | 39.3% (13.3–65.3) | 1.06 (0.40–2.85) | |
|
| |||||||
| Age at surgery (years)d | 47 | 18 | 0.67 (0.43–1.06) 0.084 | ||||
|
| |||||||
| Largest tumor size (cm)d | 47 | 18 | 0.98 (0.82–1.17) 0.844 | ||||
|
| |||||||
| Pre-treatment T stage | 0.696 | ||||||
|
| |||||||
| <2 | 12 | 6 | 31.4 | 81.5% (58.1–100.0) | 46.6% (13.8–79.3) | -reference- | |
|
| |||||||
| ≥2 | 35 | 12 | 21.9 | 72.3% (54.1–90.4) | 36.8% (12.0–61.6) | 1.22 (0.46–3.55) | |
|
| |||||||
| Pre-treatment clinical stage | 0.467 | ||||||
|
| |||||||
| <2 | 7 | 3 | 59.5 | 85.7% (59.8–100.0) | 64.3% (23.0–100.0) | -reference- | |
|
| |||||||
| ≥2 | 40 | 15 | 21.9 | 72.3% (55.5–89.2) | 33.2% (11.5–54.9) | 1.57 (0.50–6.89) | |
|
| |||||||
| Pathologic T stage | 0.042 | ||||||
|
| |||||||
| <2 | 22 | 4 | NAe | 89.4% (75.5–100.0) | 62.6% (29.2–96.0) | -reference- | |
|
| |||||||
| ≥2 | 25 | 14 | 21.3 | 65.6% (44.6–86.6) | 26.3% (4.6–47.9) | 2.89 (1.04–10.2) | |
|
| |||||||
| Pathologic stage | 0.007 | ||||||
|
| |||||||
| <2 | 19 | 2 | NAe | 94.1% (82.9–100.0) | 75.3% (41.1–100.0) | -reference- | |
|
| |||||||
| ≥2 | 28 | 16 | 18.2 | 66.0% (46.6–85.4) | 25.7% (5.2–46.1) | 5.30 (1.50–33.6) | |
|
| |||||||
| Clinical vs. pathologic stage | 0.035 | ||||||
|
| |||||||
| Downstaged (≥2 to < 2) | 15 | 1 | NAe | 100.0% (NAe) | 66.7% (13.3–100.0) | 0.38 (0.01–9.93) | |
|
| |||||||
| No change (<2 to < 2) | 4 | 1 | NAe | 75.0% (32.6–100.0) | 75.0% (32.6–100.0) | -reference- | |
|
| |||||||
| No change (≥2 to ≥ 2) | 25 | 14 | 18.2 | 62.6% (41.8–83.5) | 23.5% (2.3–44.7) | 3.48 (0.69–63.4) | |
|
| |||||||
| Upstaged (<2 to ≥ 2) | 3 | 2 | 42.6 | 100.0% (NAe) | 50.0% (0.0–100.0) | 1.56 (0.11–36.8) | |
|
| |||||||
| Duct size | 0.296 | ||||||
|
| |||||||
| Large | 7 | 4 | 18.2 | 57.1% (8.3–100.0) | 0.0% (NAe) | -reference- | |
|
| |||||||
| Small | 24 | 9 | 31.4 | 63.5% (38.7–88.4) | 38.1% (7.0–69.2) | 0.50 (0.15–1.94) | |
|
| |||||||
| Tumor regression scoref | 0.467 | ||||||
|
| |||||||
| Score 1 | 7 | 2 | 21.9 | 83.3% (53.5–100.0) | 41.7% (0.0–100.0) | -reference- | |
|
| |||||||
| Score 2 | 20 | 10 | 18.2 | 57.9% (30.3–85.4) | 14.5% (0.0–40.0) | 2.14 (0.55–14.1) | |
|
| |||||||
| Score 3 | 4 | 1 | NAe | 50.0% (0.0–100.0) | 50.0% (0.0–100.0) | 0.97 (0.04–10.5) | |
|
| |||||||
| Tumor regression | 0.872 | ||||||
|
| |||||||
| <60% | 13 | 5 | 21.9 | 60.0% (21.3–98.7) | 20.0% (0.0–54.5) | -reference- | |
|
| |||||||
| ≥60% | 18 | 8 | 18.2 | 62.3% (34.6–90.0) | 31.1% (0.0–64.6) | 1.10 (0.36–3.66) | |
|
| |||||||
| Progression-free survival g | |||||||
|
| |||||||
| Gender | 0.839 | ||||||
|
| |||||||
| Female | 26 | 12 | 21.3 | 65.4% (41.2–89.6) | 12.5% (0.0–34.4) | -reference- | |
|
| |||||||
| Male | 21 | 13 | 10.0 | 48.8% (25.1–72.6) | 24.4% (3.7–45.1) | 0.92 (0.41–2.07) | |
|
| |||||||
| Age at surgery (years)d | 47 | 25 | 0.76 (0.52–1.12) | 0.161 | |||
|
| |||||||
| Largest tumor size (cm)d | 47 | 25 | 0.94 (0.81–1.10) | 0.448 | |||
|
| |||||||
| Pre-treatment T stage | 0.608 | ||||||
|
| |||||||
| <2 | 12 | 8 | 19.0 | 62.5% (32.9–92.1) | 31.3% (2.2–60.3) | -reference- | |
|
| |||||||
| ≥2 | 35 | 17 | 12.7 | 53.1% (31.5–74.7) | 14.8% (0.0–32.6) | 1.25 (0.54–3.14) | |
|
| |||||||
| Pre-treatment clinical stage | 0.732 | ||||||
|
| |||||||
| <2 | 7 | 4 | 19.0 | 53.6% (14.2–92.9) | 35.7% (0.0–74.5) | -reference- | |
|
| |||||||
| ≥2 | 40 | 21 | 12.8 | 56.7% (37.3–76.0) | 16.5% (0.1–32.9) | 1.20 (0.45–4.19) | |
|
| |||||||
| Pathologic T stage | 0.056 | ||||||
|
| |||||||
| <2 | 22 | 7 | 30.3 | 63.3% (35.2–91.4) | 39.6% (7.8–71.3) | -reference- | |
|
| |||||||
| ≥2 | 25 | 18 | 12.7 | 52.1% (30.4–73.9) | 11.6% (0.0–26.5) | 2.26 (0.98–5.85) | |
|
| |||||||
| Pathologic stage | 0.005 | ||||||
|
| |||||||
| <2 | 19 | 4 | NAe | 88.2% (72.9–100.0) | 55.1% (17.0–93.3) | -reference- | |
|
| |||||||
| ≥2 | 28 | 21 | 10.0 | 44.3% (23.7–64.9) | 9.8% (0.0–22.7) | 3.92 (1.47–13.6) | |
|
| |||||||
| Clinical vs. pathologic stage | 0.037 | ||||||
|
| |||||||
| Downstaged (≥2 to < 2) | 15 | 2 | 30.3 | 100.0% (NAe) | 37.5% (0.0–93.6) | 0.52 (0.06–4.39) | |
|
| |||||||
| No change (<2 to < 2) | 4 | 2 | NAe | 50.0% (1.0–99.0) | 50.0% (1.0–99.0) | -reference- | |
|
| |||||||
| No change (≥2 to ≥ 2) | 25 | 19 | 10.0 | 43.6% (21.9–65.2) | 10.9% (0.0–25.0) | 2.62 (0.73–16.8) | |
|
| |||||||
| Upstaged (<2 to ≥ 2) | 3 | 2 | 12.5 | 50.0% (0.0–100.0) | 0.0% (NAe) | 3.23 (0.37–28.5) | |
|
| |||||||
| Duct size | 0.262 | ||||||
|
| |||||||
| Large | 7 | 5 | 7.5 | 21.4% (0.0–58.4) | 0.0% (NAe) | -reference- | |
|
| |||||||
| Small | 24 | 10 | 19.0 | 51.9% (25.2–78.7) | 26.0% (0.0–54.7) | 0.51 (0.17–1.71) | |
|
| |||||||
| Tumor regression scoref | 0.265 | ||||||
|
| |||||||
| Score 1 | 7 | 2 | 7.7 | 41.7% (0.0–100.0) | 41.7% (0.0–100.0) | -reference- | |
|
| |||||||
| Score 2 | 20 | 12 | 7.5 | 37.3% (10.2–64.4) | 0.0% (NAe) | 2.44 (0.66–15.8) | |
|
| |||||||
| Score 3 | 4 | 1 | NAe | 66.7% (13.3–100.0) | 66.7% (13.3–100.0) | 0.78 (0.04–8.26) | |
|
| |||||||
| Tumor regression | 0.687 | ||||||
|
| |||||||
| <60% | 13 | 5 | 7.7 | 22.2% (0.0–59.3) | 22.2% (0.0–59.3) | -reference- | |
|
| |||||||
| ≥60% | 18 | 10 | 7.5 | 49.2% (20.9–77.5) | 12.3% (0.0–34.3) | 1.25 (0.44–4.05) | |
HR, hazard ratio; CI, confidence interval; CAP, College of American Pathologists; NA, not applicable.
Kaplan–Meier estimates at 1 and 3 years after surgery date.
Results from univariate Cox proportional hazards regression models.
For overall survival, patients are followed from the date of partial hepatectomy surgery to death, or are censored at last follow-up date.
Hazard ratio reported per 10 years of age and per 1 cm of tumor size.
Cannot be estimated due to the median not being reached (median survival) or due to insufficient variability (survival estimate 95% CI).
Based on College of American Pathologists criteria. Score 1: near complete response, single cells or rare small groups of cancer cells. Score 2: partial response, residual cancer with evident tumor regression, but more than single cells or rare small groups of cancer cells. Score 3: poor or no response, extensive residual cancer with no evident tumor regression.
For progression-free survival, patients are followed from the date of partial hepatectomy surgery to the earliest of recurrence or death, or are censored at last follow-up date.
Additionally, no significant difference in OS was seen between the large duct iCCA (median survival: 18.2 months; 1 year survival: 57.1%; 3-year survival: 0%) and small duct iCCA (median survival: 31.4 months; 1 year survival: 63.5%; 3-year survival: 38.1% (Table 3).
The OS and PFS of oncologic high risk and standard patients were not different
After adjustment for age at surgery, clinical stage, and tumor size, neoadjuvant therapy was not significantly associated with a difference in OS (HR 0.90, 95% CI: 0.41–1.99, p = 0.793); or with a difference in PFS (HR 1.20, 95% CI: 0.60–2.41, p = 0.609) when compared to the standard therapy group. Although the estimated effect of treatment on OS and PFS was slightly different between those with lower vs. higher stages, when stratified by clinical stage, the OS and PFS of the oncologic high-risk group did not differ significantly from the standard therapy group (p-values for treatment-by-stage interaction 0.668 and 0.271, respectively, Table 4).
Table 4.
Multivariable modelsa for overall survival and progression-free survival in patients with intrahepatic cholangiocarcinoma who underwent partial hepatectomy
| Characteristics | Overall survivalb |
Progression-free survivalc |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Model 1d | ||||
|
| ||||
| Neoadjuvant chemotherapy | 0.90 (0.41–1.99) | 0.793 | 1.20 (0.60–2.41) | 0.609 |
|
| ||||
| Age at surgery (years)e | 0.94 (0.69–1.28) | 0.683 | 0.94 (0.73–1.23) | 0.657 |
|
| ||||
| Largest tumor size (cm)e | 1.12 (0.99–1.27) | 0.072 | 1.05 (0.94–1.17) | 0.391 |
|
| ||||
| Pre-treatment clinical stage ≥2 | 2.05 (0.97–4.52) | 0.066 | 2.00 (1.01–4.05) | 0.051 |
|
| ||||
| Model 2f | ||||
|
| ||||
| Neoadjuvant chemotherapy | 1.24 (0.62–2.52) | 0.542 | 1.37 (0.74–2.60) | 0.323 |
|
| ||||
| Pre-treatment clinical stage ≥2 | 2.13 (1.02–4.65) | 0.049 | 2.06 (1.05–4.13) | 0.037 |
|
| ||||
| Model 3g | ||||
|
| ||||
| Neoadjuvant chemotherapy | ||||
|
| ||||
| Clinical stage <2 | 1.60 (0.35–5.38) | 0.486 | 2.41 (0.67–6.95) | 0.129 |
|
| ||||
| Clinical stage ≥2 | 1.14 (0.52–2.62) | 0.746 | 1.14 (0.58–2.34) | 0.294 |
|
| ||||
| Interaction | 0.668 | 0.271 | ||
HR, hazard ratio; CI, confidence interval.
Results from multivariable Cox proportional hazards regression models.
For overall survival, patients are followed from the date of partial hepatectomy to death, or are censored at last follow-up date.
For progression-free survival, patients are followed from the date of partial hepatectomy to the earliest of recurrence or death, or are censored at last follow-up date.
Model including neoadjuvant chemotherapy status, age at partial hepatectomy, tumor size, and pre-treatment clinical stage (≥2 vs. <2).
Hazard ratio reported per 10 years of age and per 1 cm of tumor size.
Model including neoadjuvant chemotherapy status (yes vs. no) and pre-treatment clinical stage (≥2 vs. <2).
Model including neoadjuvant chemotherapy status (yes vs. no), pre-treatment clinical stage (≥2 vs. <2), and the interaction between neoadjuvant status and stage. Hazard ratios are presented as the risk of death or progression for neoadjuvant chemotherapy status (yes vs. no), stratified by pre-treatment clinical stage. The interaction p-value assesses the statistical significance of the difference for these stratified hazard ratios.
Discussion
Intrahepatic cholangiocarcinoma (iCCA) is the second most common primary liver malignancy, and its incidence is rising.18,19 The prognosis and long-term outcomes are highly dependent on the tumor resectability and unfortunately, more than a third of the patients present with locally advanced and unresectable disease.3 Neoadjuvant therapy and subsequent surgical resection have been reported as an effective downstaging approach in multidisciplinary therapy for pancreatic and colorectal cancer. Prior studies have shown that neoadjuvant therapy and/or presurgical liver-directed therapies may increase the proportion of iCCA patients who are eligible for surgery and improve the prognosis.20–22
Our retrospective cohort study compared outcomes of the use of neoadjuvant therapy for iCCA and found that neoadjuvant therapy led to objective tumor response and pathologic regression based on CAP regression criteria allowing to convert patients with advance disease into surgical candidates. Given that stage was the strongest predictor of OS and PFS, these data suggest that neoadjuvant therapy is potentially associated with apparent regression and reduction in final pathologic stage when compared with initial clinical parameters based on pre-treatment evaluation. This aligns with another observation from our cohort investigation; the clinical endpoints, OS and PFS in patients initially treated with neoadjuvant therapy prior to surgery (oncologic high-risk group) were not different from patients who had hepatectomy without neoadjuvant therapy (standard therapy group) after adjusting for pre-treatment stage, suggesting that neoadjuvant therapy could normalize the risk in patient’s who present with advanced stage disease allowing for surgical resection, obtaining similar outcomes than patients with lower stage disease.
The role of neoadjuvant therapy in iCCA has been evaluated in small single institution studies and in a database analysis.11,15,16 There has been only 1 clinical trial reported in resectable iCCA.13 However, the practice of neoadjuvant therapy in intrahepatic cholangiocarcinoma is not uniformly widespread within the US. Multiple hypotheses have been raised in the literature concerning the benefits of neoadjuvant therapy. Neoadjuvant therapy can downstage tumors and improve the probability of complete resection of tumors with negative margins. This theory has been demonstrated in different tumors such as such as pancreatic ductal adenocarcinoma, colorectal adenocarcinoma, and others, allowing to achieve resectable tumor burden.8 Kato et al. implemented this approach in a small series in Japan and showed that a proportion of patients achieved R0 resections, highlighting a potential benefit of chemotherapy allowing to achieve margin negative resections. Additionally, neoadjuvant therapy potentially aids in the selection of patients who may benefit from surgical resection, providing additional time to identify patients who have occult micrometastatic disease and are more likely to progress to higher stage disease.11,16
In a recent retrospective study of 74 advanced iCCA patients conducted in a single institution in France, 39 (53%) patients of the cohort considered initially to have unresectable disease were able to undergo surgery after neoadjuvant therapy.11 The authors reported similar results to ours with patients receiving neoadjuvant treatment having a similar outcome to those patients who did not need neoadjuvant therapy.23
A recently published US based single institution also evaluated this approach obtaining similar findings to ours, however only included 10 patients receiving neoadjuvant chemotherapy.22
Our study includes the largest number of patients receiving neoadjuvant therapy and specifically the widely used regimen in the US and incorporates evaluation of the histologic regression score and tumor subtype as part of the analysis. These data therefore add to our understanding of the impact of neoadjuvant therapy in intrahepatic cholangiocarcinoma and indicate the need for further study of a neoadjuvant approach in larger cohorts.
We acknowledge the limitations of our study, including a single institution and small cohort, making it challenging to properly adjust for important confounders in the comparison between survival outcomes with treatment. Specifically, Approximately 20% of our oncologic high risk cases cohort were large duct iCCA and there was no significant difference in outcome correlated with histologic type; however the limited number of cases may have obscured the prognostic impact of the large duct histologic type. Importantly, the retrospective nature of the study, and the possibility of selection bias due to a lack of well-defined standardized criteria for resectability is also identified. The heterogeneity in neoadjuvant therapy regimens might also represent a limitation, with few patients receiving triple chemotherapy regimen and majority receiving double chemotherapy regimen; different chemotherapy agents as well as duration of therapy regimens.
Neoadjuvant therapy can serve as a surgical selection instrument for patient selection who would benefit from surgical intervention. Our study demonstrates that neoadjuvant therapy can potentially improve survival outcomes. Further prospective studies are required to evaluate the impact on prognosis of neoadjuvant therapy not only in unresectable/borderline cases but also in resectable early-stage cases. Also, further studies are needed to investigate optimal neoadjuvant and adjuvant therapy regimens, and surgical intervention as well as the appropriate sequence to achieve a sustained increase in long-term survival in patients with iCCA diagnosis.
Conclusions
Our single institution-based study reveals that neoadjuvant therapy in iCCA lead to clinically objective tumor regression and pathologic regression based on CAP regression criteria and change in final pathologic stage when compared with initial clinical parameters based on pre-treatment evaluation allowing for surgical resectability. Importantly, surgical patients who underwent neoadjuvant therapy had similar clinical outcomes to surgical patients who did not need neoadjuvant therapy. These data provide evidence in support of neoadjuvant therapy and suggest further study is needed for this treatment approach in larger cohorts and prospective studies.
Supplementary Material
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.hpb.2024.04.003.
Conflicts of interest
None to declare.
References
- 1.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR et al. (2020) Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 17: 557–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SA, Toledano MB, Taylor-Robinson SD. (2008) Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 10: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T et al. (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60:1268–1289. [DOI] [PubMed] [Google Scholar]
- 4.Yang JD, Kim B, Sanderson SO, Sauver JS, Yawn BP, Larson JJ et al. (2012) Biliary tract cancers in olmsted county, Minnesota, 1976–2008. Am J Gastroenterol 107:1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nooijen LE, Franken LC, Belkouz A, Oulad Abdennabi I, Besselink MG, Busch OR et al. (2021) Efficacy and safety of gemcitabine plus cisplatin as potential preoperative chemotherapy in locally advanced intrahepatic, perihilar, and mid-cholangiocarcinoma: a retrospective cohort study. Am J Clin Oncol 44:526–532. [DOI] [PubMed] [Google Scholar]
- 6.Kato A, Shimizu H, Ohtsuka M, Yoshitomi H, Furukawa K, Takayashiki T et al. (2015) Downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer patients treated with gemcitabine plus cisplatin combination therapy followed by radical surgery. Ann Surg Oncol 22(Suppl 3):S1093–S1099. [DOI] [PubMed] [Google Scholar]
- 7.Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J et al. (2018) Chemoradiotherapy for initially unresectable locally advanced cholangiocarcinoma. World J Surg 42:2910–2918. [DOI] [PubMed] [Google Scholar]
- 8.Park W, Chawla A, O’Reilly EM. (2021) Pancreatic cancer: a review. JAMA 326:851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardiere C, Boucher E et al. (2014) Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 15:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki T, Takeda T, Okamoto T, Ozaka M, Sasahira N. (2021) Chemotherapy for biliary tract cancer in 2021. J Clin Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B et al. (2018) Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 105:839–847. [DOI] [PubMed] [Google Scholar]
- 12.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A et al. (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 13.Maithel SK, Keilson JM, Cao HST, Rupji M, Mahipal A, Lin BS et al. (2023) NEO-GAP: a single-arm, phase II feasibility trial of neoadjuvant gemcitabine, cisplatin, and nab-paclitaxel for resectable, high-risk intrahepatic cholangiocarcinoma. Ann Surg Oncol 30:6558–6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rimini M, Fornaro L, Lonardi S, Niger M, Lavacchi D, Pressiani T et al. (2023) Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: an early exploratory analysis of real-world data. Liver Int 43:1803–1812. [DOI] [PubMed] [Google Scholar]
- 15.Kato A, Shimizu H, Ohtsuka M, Yoshidome H, Yoshitomi H, Furukawa K et al. (2013) Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol 20:318–324. [DOI] [PubMed] [Google Scholar]
- 16.Yadav S, Xie H, Bin-Riaz I, Sharma P, Durani U, Goyal G et al. (2019) Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: a propensity score matched analysis. Eur J Surg Oncol 45:1432–1438. [DOI] [PubMed] [Google Scholar]
- 17.Lee SM, Katz MH, Liu L, Sundar M, Wang H, Varadhachary GR et al. (2016) Validation of a proposed tumor regression grading scheme for pancreatic ductal adenocarcinoma after neoadjuvant therapy as a prognostic indicator for survival. Am J Surg Pathol 40:1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vij M, Puri Y, Rammohan A, Gowripriya G, Rajalingam R, Kaliamoorthy I et al. (2022) Pathological, molecular, and clinical characteristics of cholangiocarcinoma: a comprehensive review. World J Gastrointest Oncol 14(3):607–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyson GL, Ilyas JA, Duan Z, Green LK, Younes M, El-Serag HB et al. (2014) Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci 59:3103–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ejaz A, Cloyd JM, Pawlik TM. (2020) Advances in the diagnosis and treatment of patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol 27:552–560. [DOI] [PubMed] [Google Scholar]
- 21.Cloyd JM, Ejaz A, Pawlik TM. (2020) The landmark series: intrahepatic cholangiocarcinoma. Ann Surg Oncol 27:2859–2865. [DOI] [PubMed] [Google Scholar]
- 22.Sutton TL, Billingsley KG, Walker BS, Enestvedt CK, Dewey EN, Orloff SL et al. (2021) Neoadjuvant chemotherapy is associated with improved survival in patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. Am J Surg 221:1182–1187. [DOI] [PubMed] [Google Scholar]
- 23.Jeong H, Kim KP, Jeong JH, Hwang DW, Lee JH, Kim KH et al. (2023) Adjuvant gemcitabine plus cisplatin versus capecitabine in node-positive extrahepatic cholangiocarcinoma: the STAMP randomized trial. Hepatology 77:1540–1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.