Abstract
Objective
Inflammatory bowel diseases (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC), are chronic conditions primarily affecting the intestines. This study aims to identify the nature and frequency of oral symptoms and signs in patients with ulcerative colitis and to explore the connection between these complications and the activity of the disease.
Methods
This descriptive-analytical study involved ulcerative colitis patients who visited the gastroenterology clinic at Shahid Sayad Shirazi Hospital in Gorgan, along with an equal number of healthy companions as a control group in 2019. A gastroenterology internist assessed the disease severity through clinical examination and a simple clinical disease activity index for colitis patients. The collected data were then analyzed statistically using SPSS version 19.
Results
The average age of patients in the case group was 41.84 ± 11.66 years, while in the control group it was 40.43 ± 12.67 years. There was a significant correlation between difficulty swallowing, burning sensation in the mouth, vomiting, acidic taste, the presence of oral ulcers, and a coated tongue with the severity of disease activity (p < 0.05). However, no significant relationship was found between other variables and the severity of disease activity (p > 0.05).
Conclusion
The results of this study confirm that as the severity of ulcerative colitis increases, the occurrence of oral lesions also rises, particularly during severe disease activity. Among oral symptoms, dry mouth had the highest incidence, followed by bad breath and changes in taste. The most common oral lesions observed were a coated tongue, grooved tongue, and oral ulcers, respectively.
Keywords: Inflammatory Bowel diseases, Colitis, Ulcerative, Oral manifestations, Oral Ulcer, Oral health
Introduction
Ulcerative Colitis (UC) is a long-term inflammatory condition that predominantly impacts the large intestine, particularly the colon. The key feature of this disease is the inflammation and formation of ulcers on the innermost layer of the colon [1]. The precise cause of UC remains incompletely understood, but it is thought to result from a combination of genetic and environmental factors that provoke an abnormal immune response [2]. Risk factors for developing UC include a family history of the disease, smoking, dietary factors, and specific infections [3]. The peak age of onset for UC is typically between 30 and 40 years. Globally, the incidence and prevalence of UC have increased over time [1].
The typical symptoms of UC include abdominal pain, diarrhea, rectal bleeding, an urgent need to have bowel movements, and weight loss [4]. The severity of symptoms can fluctuate, occurring in cycles of flare-ups and remission. UC diagnosis involves a combination of medical history, physical exams, lab tests, endoscopy, and imaging studies [5]. Treatment for UC focuses on reducing inflammation, managing symptoms, and maintaining remission. Approaches may involve medications, dietary adjustments, and occasionally, surgical interventions [6, 7].
Patients with UC may experience additional symptoms beyond gastrointestinal issues, known as extra-intestinal manifestations (EIM). These EIM can occur alongside typical gastrointestinal symptoms. The prevalence of EIM in ulcerative colitis ranges from 25 to 40%. These complications extend beyond the gut and can impact various organs and systems in the body, leading to conditions such as arthritis, skin disorders, eye inflammation, liver problems, and oral cavity issues [8]. Oral involvement in UC can present in diverse ways, with these symptoms sometimes appearing years before the onset of intestinal symptoms [9]. Oral lesions in UC patients can include UC-specific mucosal ulcers, Pyostomatitis Vegetans (PSV) (a specific manifestation linked to UC), and oral symptoms induced by medications used for gastrointestinal conditions. These oral manifestations play a crucial role as indicators for monitoring disease progression and guiding treatment strategies [10].
In various studies, several oral manifestations have been linked to UC. These include PSV—characterized by pustules and ulcerations on the oral mucosa—Recurrent Aphthous Ulcers (RAU), Atrophic Glossitis (AG) affecting taste perception, Burning Mouth Syndrome (BMS), Angular Cheilitis (AC) at the corners of the mouth, Dry Mouth (Xerostomia), altered taste perception, Halitosis (Bad Breath), and an increased risk of periodontitis. These manifestations can sometimes serve as early indicators of UC and are valuable for monitoring patients’ health [9]. Dentists and gastroenterologists may not always recognize the potential connection between the severity of gastrointestinal diseases and specific oral lesions in these patients. Consequently, this relationship often remains undiscovered [11]. Poor oral and dental health is linked to a reduced risk of future UC [12]. Patients with Crohn’s disease who have proximal gastrointestinal or perineal involvement exhibit a higher incidence of oral lesions, estimated to be between 20% and 50% [13]. Additionally, individuals with moderate and severe UC show a greater prevalence of oral manifestations [14].
This study investigates the relationship between oral signs and symptoms and disease severity, while also determining the prevalence of such signs and symptoms in these patients.
Materials and methods
This case-control study was conducted among patients with UC who visited the gastrointestinal center at Gorgan Shahid Sayad Shirazi Hospital in 2019. The sample size included 99 patients in the case group and 99 healthy individuals in the control group. The sample size was determined using the formula for equal sample sizes for each group, based on Elahi et al.‘s article [14], with a confidence level of 0.95 and a test power of 0.8.
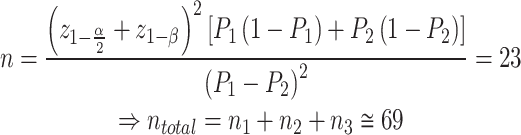 |
Taking into account the difference in the total volume of qualified patients in each group, based on the information obtained from Sayad Shirazi Medical Center, and applying the unequal ratio of sample sizes in each group, we will have:
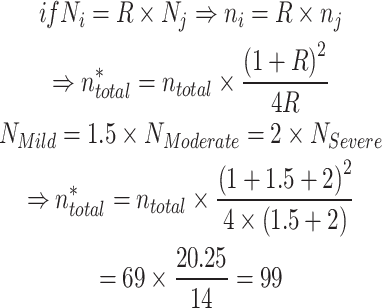 |
Within each group, an unequal sample size ratio was selected: 22, 33, and 44 patients for the Severe, Moderate, and Mild groups, respectively. Subjects were chosen through available sampling. Inclusion criteria comprised UC patients who consented to participate and had no systemic or local causes for oral manifestations. All cases underwent oral examination within 48 h of disease severity assessment.
The ethical dimensions of the research were examined and received approval from the Golestan University of Medical Sciences’ ethics committee. The study complies with the ethical guidelines outlined in the Declaration of Helsinki (Reference: IR.GOUMS.REC.1397.318).
Patients were informed about the study’s purpose, and written consent was obtained. Gastroenterologists assessed illness severity using the Simple Clinical Colitis Activity Index (SCCAI). Based on individual symptoms, the SCCAI scores ulcerative colitis severity, ranging from mild to severe. For instance:
Daily defecation frequency: 1–3 times (score 0), 4–6 times (score 1), 7–9 times (score 2), more than 9 times (score 3).
Night defecation frequency: 1–3 times (score 1), 4–6 times (score 2).
Urgency of defecation: Hurried (score 1), immediate (score 2), incontinent (score 3).
Blood in feces: Very low (score 1), sometimes (score 2), often (score 3).
General condition: Very good (score 0), a little unbalanced (score 1), poor (score 2), very poor (score 3), severe (score 4).
Extra-colonic manifestations: 1 point.
The sum of scores indicated the severity of UC: mild (total score < 6), moderate (6–9), or severe (> 9).
Following severity assessment, oral medicine specialists examined patients with UC. Clinical signs (e.g., Burning Sensation, Dysphagia, Taste changes, Dry mouth, Nausea, Vomiting, Regurgitation, Acidic taste, Halitosis) and symptoms (e.g., Oral ulceration, Coated tongue, Geographic tongue, Fissured tongue, Pyostomatitis vegetans) were recorded. Patients were categorized into four treatment groups: “no medication,” “oral medication,” “injectable medication,” and “combined oral and injectable medication.” Duration of illness and age groups (17–32, 33–47, 48–62, 63 years and older) were also documented.
Statistical analysis
Based on the normality of the data distribution, we employed either the T-test or the Mann-Whitney test for quantitative variables. Given the binary nature of the response variables (i.e., “have” or “don’t have”), we utilized the chi-square test and, if needed, Fisher’s exact test to compare the proportions between the two groups. A p-value of less than 0.05 was considered statistically significant.
Results
Demographic analysis
Two groups, one with UC patients of varying severity and a control group, were assessed for demographic information and oral signs and symptoms. The Shapiro-Wilk test revealed a non-normal age distribution for both groups. The Mann-Whitney test indicated no significant age difference between the groups, with the patient group averaging 41.84 ± 11.66 years and the control group 40.43 ± 12.67 years (p = 0.24). Each group consisted of 50 males (50.5%) and 49 females (49.5%), showing no significant gender disparity (p = 1.00, Chi-square test) (Table 1).
Table 1.
Demographic characteristics of
| Groups\Variables | Type of Medication | Duration of Illness | Gender | Age | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Drug Intake | Edible | Injectable | Edible & Injectable | Mean | SD | Female | Male | Mean | SD | |||
| Control | Count | — | — | 49 | 50 | 40.43 | 12.67 | |||||
| % | 49.5% | 50.5% | ||||||||||
| Case | Mild | Count | 2 | 36 | 3 | 2 | 7.68 | 4.94 | 26 | 18 | 39.07 | 13.45 |
| % | 4.7% | 83.7% | 7.0% | 4.7% | 59.1% | 40.9% | ||||||
| Moderate | Count | 2 | 19 | 2 | 9 | 11.98 | 8.67 | 14 | 19 | 45.33 | 10.21 | |
| % | 6.3% | 59.4% | 6.3% | 28.1% | 42.4% | 57.6% | ||||||
| Severe | Count | 4 | 8 | 2 | 7 | 8.64 | 6.83 | 9 | 13 | 42.14 | 10.51 | |
| % | 19.0% | 38.1% | 9.5% | 33.3% | 40.9% | 59.1% | ||||||
| P-Value | 0.008* | 0.057*** | 0.240** | 0.271*** | ||||||||
* Fisher’s Exact Test **Chi square test *** Kruskal – Wallis H test
Oral symptoms correlation
An increase in UC severity correlated with a rise in symptoms such as taste alteration, dysphagia, burning sensation, nausea, vomiting, acidic taste, oral ulcers, and coated tongue, which were statistically significant according to the Chi-square test. Symptoms such as dry mouth, halitosis, and food and acid reflux, along with geographic and fissured tongue, also increased with disease severity but were not statistically significant.
Disease duration and oral findings
When considering disease duration categorized into 0–5, 6–10, and over 10 years, a direct but not statistically significant relationship was observed between disease severity and various oral symptoms across all time intervals.
Gender- and age-specific findings
The study found direct but not statistically significant associations between disease severity and symptoms such as dysphagia, nausea, halitosis, and food and acid reflux in both genders. Vomiting, acidic taste, and oral ulcers showed similar patterns, with no statistical significance in males. Dry mouth and taste changes were directly related to disease severity in both genders but lacked statistical significance in females. An inverse relationship was noted for geographic tongue in males and fissured tongue in both genders, neither of which was statistically significant.
Statistically significant relationships were observed in specific age groups:
In the 17–32 age group, severity of illness correlated significantly with oral ulcers, acid reflux, and food reflux (p = 0.033 and 0.021, respectively).
In the 33–47 age group, taste change and acidic taste were significantly related to disease severity (p = 0.014 and p < 0.001, respectively).
Both the 17–32 and 48–62 age groups showed a significant relationship between severity of illness and burning sensation (p = 0.020 and 0.023, respectively).
In the 63 and older age group, dysphagia was significantly associated with disease severity (p = 0.036).
Medication impact
The severity of UC was directly related to taste changes across all medication usage groups, with a significant correlation in the oral medication group (p = 0.031), but not in the combined oral and injectable medication group.
Burning sensation and medication usage
The severity of illness showed direct relationships with burning sensation in both the non-medication and combined medication usage groups. However, this relationship was inverse in the injectable medication group and significantly direct in the oral medication group (p = 0.017).
Vomiting, acidic taste, and other symptoms in relation to medication usage
Across all four medication usage groups, there were direct relationships between the severity of illness and vomiting. However, this relationship was inverse in the injectable medication group. Only the oral medication group exhibited a significant relationship (p = 0.010). Similar to vomiting, the severity of illness correlated directly with acidic taste in all four medication usage groups. However, this relationship was inverse in the combined oral and injectable medication group (Table 2). Only the oral medication group had a significant relationship (p < 0.001). No significant relationships were found between the severity of illness and dysphagia, nausea, halitosis, food and acid reflux, oral ulcers, geographic tongue, fissured tongue, or coated tongue across all medication usage groups.
Table 2.
Correlation of oral signs and symptoms with severity of ulcerative colitis
| Variables | Disease Severity | P-value (p < 0.05) | Test | |||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||||
| Yes | No | Yes | No | Yes | No | |||
| Taste change | 8 (18.2) | 36 (81.8) | 15 (45.5) | 18 (54.5) | 13 (59.1) | 9 (0.9) | 0.002 | Chi-square |
| Dysphagia | 10 (22.7) | 34 (77.3) | 7 (21.2) | 26 (78.8) | 11 (50) | 11 (50) | 0.037 | Chi-square |
| Burning sensation | 3 (6.8) | 41 (93.2) | 0 | 33 (100) | 9 (40.9) | 13 (59.1) | < 0.001 | Fisher’s exact |
| Dry mouth | 20 (45.5) | 24 (54.5) | 15 (45.5) | 18 (54.5) | 15 (68.2) | 7 (31.8) | 0.171 | Chi-square |
| Nausea | 13 (29.5) | 31 (70.5) | 13 (39.4) | 20 (60.6) | 13 (59.1) | 9 (40.9) | 0.068 | Chi-square |
| Vomiting | 2 (4.5) | 42 (95.5) | 4 (12.1) | 29 (87.9) | 8 (36.4) | 14 (63.6) | 0.003 | Fisher’s exact |
| Halitosis | 17 (38.6) | 27 (61.4) | 16 (48.5) | 17 (51.5) | 13 (59.1) | 9 (40.9) | 0.28 | Chi-square |
| Acidic taste | 2 (4.5) | 42 (95.5) | 4 (12.1) | 29 (87.9) | 11 (50) | 11 (50) | < 0.001 | Chi-square |
| Regurgitation | 15 (34.1) | 29 (65.9) | 9 (27.3) | 24 (72.7) | 11 (50) | 11 (50) | 0.219 | Chi-square |
| Oral ulceration | 9 (20.5) | 35 (79.5) | 14 (42.4) | 19 (57.6) | 11 (50) | 11 (50) | 0.029 | Chi-square |
| Geographic tongue | 3 (6.8) | 41 (93.2) | 1 (3) | 32 (97) | 0 | 22 (100) | 0.116 | Fisher’s exact |
| Fissured tongue | 27 (61.4) | 17 (38.6) | 22 (66.7) | 11 (33.3) | 10 (45.5) | 12 (54.5) | 0.277 | Chi-square |
| Coated tongue | 19 (43.2) | 25 (56.8) | 25 (75.8) | 8 (24.2) | 17 (77.3) | 5 (22.7) | 0.003 | Chi-square |
Discussion
In this investigation, we aimed to explore the relationship between oral signs and symptoms and disease severity in patients with UC. Our study revealed significant associations between dysphagia, burning sensation, vomiting, acidic taste, oral ulcers, and coated tongue with disease severity. However, no significant relationships were found between other variables and illness severity. Interestingly, our results align with Elahi et al.‘s study [14], which also reported similar associations. Similarly, Kumar et al. [15] observed significant links between variables such as acidic taste, oral ulcers, and coated tongue, consistent with our findings.
The study findings suggest a positive correlation between illness severity and pronounced taste changes, supported by the chi-square test indicating statistical significance. However, discrepancies exist in related studies; for instance, Katz et al. [16] reported no significant link between taste alterations and disease severity in both case and control groups. In contrast, Elahi et al. [14] noted a significant association between taste changes and disease severity, consistent with our results. Notably, inflammatory bowel disease is recognized as an etiological factor for taste variations, though not all patients experience such changes. Gender-specific analyses revealed no significant relationship between illness severity and vomiting or acidic taste in males, and no significant associations with nausea or dry mouth in females. These outcomes align with some studies, such as those by Katz [16] and Lankarani [17], while discrepancies in findings, like those in the study by Mortada, cast uncertainty on gender’s role in illness severity among patients [18].
Our study found that as illness severity increases, dysphagia becomes more pronounced. The chi-square test confirms this trend’s statistical significance. Interestingly, other studies by Katz et al. [16] and Elahi et al. [14] also reported significant associations between illness severity and dysphagia, aligning with our results. Swallowing relies on digestive tract muscles, which environmental factors, including ion levels, can affect. Inflammation-induced changes in ion concentrations may disrupt these muscles and impact swallowing. Additionally, we observed an increasing relationship between illness severity and taste changes across all age groups. While significant in some age groups, this relationship was insignificant in others. Notably, the higher proportion of individuals in the 31–45 age group contributes to its greater significance, a finding not consistently reported in similar studies [15, 19, 20].
Our study found that illness severity was directly related to the duration of illness, as well as taste changes, dysphagia, dry mouth, halitosis, and food reflux. However, these associations lacked statistical significance. Conversely, an inverse relationship existed between illness severity and geographic tongue, but it also lacked statistical significance. Notably, within the 5-year to 10-year interval, the relationship between illness severity, duration, and fissured tongue was significant, while the burning sensation did not show significance. Furthermore, acidic taste and nausea were not significantly associated with illness severity in patients with less than 10 years of disease duration, nor were oral ulcers significant in those with more than 10 years of illness. Interestingly, oral ulcers were significant in patients using both oral and injectable medications, consistent with Lankarani et al.‘s review study [17].
Our study also revealed that increasing illness severity correlated with a higher incidence of vomiting. Fisher’s exact test confirmed the statistical significance of this relationship. However, Hearty et al.‘s research on Crohn’s disease patients [19] yielded insignificant results in this regard. In a comparison between colitis and Crohn’s disease patients by Katz et al. [16], vomiting rates were significantly higher in Crohn’s patients but not in colitis patients. Given the multifactorial nature of vomiting, further extensive retrospective or prospective cohort studies should explore additional contributing factors beyond the disease itself.
Regarding mucosal changes in UC, the oral cavity may exhibit stomatitis, glossitis, cheilitis, aphthous ulcers, and pyostomatitis vegetans—a specific sign of UC. However, the exact nature of this association remains unclear [14]. Additionally, deep linear ulcers and hyperplastic folds, which cause pain upon touch or when consuming acidic, spicy, or hot foods, should not be confused with the shallow, round-to-elliptical aphthous ulcers that typically heal within 7 to 14 days [17].
UC may precede oral or cutaneous lesions by months or years, sometimes with minimal symptoms hindering early diagnosis. Identifying pyostomatitis vegetans can prompt further investigation for subclinical intestinal diseases [14]. Treatment aims to manage associated gastrointestinal conditions, and controlling colitis leads to gradual oral lesion improvement. Aphthous ulcers, affecting up to 20% of the population, are wider and more persistent in UC. Adult patients with a history of recurrent aphthous ulcers may experience concurrent aphthous stomatitis alongside colitis [15]. Mucosal changes include stomatitis, glossitis, cheilitis, aphthous ulcers, and pyostomatitis vegetans. Dental care should prioritize prevention, regular visits, and avoiding non-steroidal anti-inflammatory drugs (NSAIDs) [18].
The potential limitations of this study include the relatively small sample size of 99 patients, which may not be representative of the broader population of UC patients. Additionally, the study’s cross-sectional design limits the ability to establish causality between oral manifestations and UC severity. The reliance on self-reported symptoms and clinical evaluations may introduce bias and variability in the data. Furthermore, the study was conducted in a single hospital in Gorgan, Iran, which may limit the generalizability of the findings to other regions or populations. Finally, the study did not account for potential confounding factors such as medication use, dietary habits, and other comorbid conditions that could influence the observed associations.
Conclusion
The results of this study confirm that an increase in the severity of UC is associated with higher odds of certain oral signs and symptoms. Further extensive studies using a retrospective or prospective cohort method are recommended to obtain better insights regarding other factors contributing to oral manifestations.
Acknowledgements
Not Applicable.
Abbreviations
- UC
Ulcerative Colitis
- SCCAI
Simple Clinical Colitis Activity Index
- EIM
Extraintestinal Manifestations
- NSAID
Non-steroidal anti-inflammatory drugs
Author contributions
NTT assisted in data collection and original draft preparation. NM assisted in conceptualization, methodology, supervision, original draft preparation, review, and editing. AN assisted in conceptualization, review, and editing. SB assisted in conceptualization, review, and editing. NB assisted in statistical analysis, review, and editing. NA assisted in data collection and original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Golestan University of Medical Sciences (Grant No. 110574). The funding source did not participate in the study design, data collection, analysis, interpretation, or manuscript preparation.
Data availability
Data transparency is provided.
Declarations
Ethics approval and consent to participate
The ethical dimensions of the research were examined and received approval from the Golestan University of Medical Sciences’ ethics committee. The study complies with the ethical guidelines outlined in the Declaration of Helsinki (Reference: IR.GOUMS.REC.1397.318). Patients were informed about the study’s purpose, and written consent was obtained.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2. Epub 2016 Dec 1. PMID: 27914657; PMCID: PMC6487890. [DOI] [PMC free article] [PubMed]
- 2.Caioni G, Viscido A, d’Angelo M, Panella G, Castelli V, Merola C, Frieri G, Latella G, Cimini A, Benedetti E. Inflammatory bowel disease: New insights into the interplay between environmental factors and PPARγ. Int J Mol Sci. 2021;22(3):985. 10.3390/ijms22030985. PMID: 33498177; PMCID: PMC7863964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9(6):367–74. PMID: 23935543; PMCID: PMC3736793. [PMC free article] [PubMed] [Google Scholar]
- 4.Dubinsky M, Bleakman AP, Panaccione R, Hibi T, Schreiber S, Rubin D, Dignass A, Redondo I, Gibble TH, Kayhan C, Travis S. Bowel urgency in Ulcerative Colitis: current perspectives and future directions. Am J Gastroenterol. 2023;118(11):1940–53. 10.14309/ajg.0000000000002404. Epub 2023 Jun 12. PMID: 37436151; PMCID: PMC10617668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchioni Beery R, Kane S. Current approaches to the management of new-onset ulcerative colitis. Clin Exp Gastroenterol. 2014;7:111 – 32. doi: 10.2147/CEG.S35942. PMID: 24872716; PMCID: PMC4026549. [DOI] [PMC free article] [PubMed]
- 6.Gordon M, Sinopoulou V, Ibrahim U, Abdulshafea M, Bracewell K, Akobeng AK. Patient education interventions for the management of inflammatory bowel disease. Cochrane Database Syst Rev. 2023;5(5):CD013854. 10.1002/14651858.CD013854.pub2. PMID: 37172140; PMCID: PMC10162698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas N, Shakil M, Akhtar Rana Z, Basharat Ali S, Ayub Awan A, Gul S. A systematic review of the role of Diet in Ulcerative Colitis. Cureus. 2023;15(5):e39350. 10.7759/cureus.39350. PMID: 37351247; PMCID: PMC10284595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7(4):235–41. PMID: 21857821; PMCID: PMC3127025. [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Wu Y, Xie Y, Zhang Y, Jiang S, Wang J, Luo X, Chen Q. Oral manifestations serve as potential signs of ulcerative colitis: a review. Front Immunol. 2022;13:1013900. 10.3389/fimmu.2022.1013900. PMID: 36248861; PMCID: PMC9559187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentini G, Ferrara SD’AgostinoE, Dolci M. 2023. Patients-reported oral manifestations in Coeliac Disease and Inflammatory Bowel diseases: an Italian survey oral 3, no. 3: 316–24. 10.3390/oral3030026
- 11.Tan CXW, Brand HS, Qaddour O, van der Bijl PML, De Boer NKH, Forouzanfar T, de Visscher JGAM. Knowledge and Interdisciplinary Communication of Gastroenterologists and dentists in the Netherlands about gastrointestinal diseases with oral manifestations. Crohns Colitis 360. 2022;4(1):otac006. 10.1093/crocol/otac006. PMID: 36777554; PMCID: PMC9802256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin W, Ludvigsson JF, Liu Z, Roosaar A, Axéll T, Ye W. Inverse Association Between Poor Oral Health and Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2017;15(4):525–531. 10.1016/j.cgh.2016.06.024. Epub 2016 Jul 5. PMID: 27392757. [DOI] [PubMed]
- 13.Ribaldone DG, Brigo S, Mangia M, Saracco GM, Astegiano M, Pellicano R. Oral manifestations of inflammatory bowel Disease and the role of non-invasive surrogate markers of Disease Activity. Med (Basel). 2020;7(6):33. 10.3390/medicines7060033. PMID: 32560118; PMCID: PMC7345678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elahi M, Telkabadi M, Samadi V, Vakili H. Association of oral manifestations with ulcerative colitis. Gastroenterol Hepatol Bed Bench. 2012 Summer;5(3):155–60. PMID: 24834217; PMCID: PMC4017478. [PMC free article] [PubMed]
- 15.Kumar KM, Nachiammai N, Madhushankari GS. Association of oral manifestations in ulcerative colitis: a pilot study. J Oral Maxillofac Pathol. 2018 May-Aug;22(2):199–203. 10.4103/jomfp.JOMFP_223_16. PMID: 30158772; PMCID: PMC6097373. [DOI] [PMC free article] [PubMed]
- 16.Katz J, Shenkman A, Stavropoulos F, Melzer E. Oral signs and symptoms in relation to disease activity and site of involvement in patients with inflammatory bowel disease. Oral Dis. 2003;9(1):34–40. 10.1034/j.1601-0825.2003.00879.x. PMID: 12617256. [DOI] [PubMed]
- 17.Lankarani KB, Sivandzadeh GR, Hassanpour S. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol. 2013;19(46):8571–9. 10.3748/wjg.v19.i46.8571. PMID: 24379574; PMCID: PMC3870502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortada I, Leone A, Gerges Geagea A, Mortada R, Matar C, Rizzo M, Hajj Hussein I, Massaad-Massade L, Jurjus A. Oral manifestations of inflammatory bowel disease. J Biol Regul Homeost Agents. 2017 Jul-Sep;31(3):817–21. PMID: 28958141. [PubMed]
- 19.Harty S, Fleming P, Rowland M, Crushell E, McDermott M, Drumm B, Bourke B. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3(9):886 – 91. 10.1016/s1542-3565(05)00424-6. PMID: 16234026. [DOI] [PubMed]
- 20.Muhvić-Urek M, Tomac-Stojmenović M, Mijandrušić-Sinčić B. Oral pathology in inflammatory bowel disease. World J Gastroenterol. 2016;22(25):5655–67. 10.3748/wjg.v22.i25.5655. PMID: 27433081; PMCID: PMC4932203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data transparency is provided.


