Abstract
Cytokines play important roles in the clearance of herpes simplex virus (HSV) infections and in virus-induced immunopathology. One cytokine known to contribute to resistance against HSV is interleukin-6 (IL-6). Here we have investigated virus-cell interactions responsible for IL-6 induction by HSV in leukocytes. Both HSV type 1 and type 2 are potent inducers of IL-6, and this phenomenon is augmented in the presence of gamma interferon. The ability to induce IL-6 is dependent on de novo protein synthesis and is sensitive to UV irradiation of the virus. Virus mutants lacking the virion-transactivating protein VP16 or any of the immediate-early proteins ICP0, ICP4, or ICP27 displayed unaltered capacities to induce IL-6. However, wild-type virus was unable to induce IL-6 in a macrophage cell line overexpressing a mutant of double-stranded RNA-activated protein kinase (PKR). This suggests a role for PKR in HSV-induced IL-6 expression. HSV infection led to enhanced binding to the κB, CRE, and AP-1 sites of the IL-6 promoter, and inhibitors against NF-κB and the p38 kinase strongly reduced accumulation of IL-6 mRNA in infected cells. Moreover, macrophage cell lines expressing dominant negative mutants of IκBα and p38 responded to HSV-1 infection with reduced IL-6 expression compared to the control-vector-transfected cell line. The results show that induction of IL-6 by HSV in leukocytes is dependent on PKR and cellular signaling through NF-κB and a p38-dependent pathway.
Efficient elimination of virus infections occurs through a highly controlled host response relying on both the innate and acquired immune defense systems. For instance, mice infected in the eye with herpes simplex virus type 1 (HSV-1) require both macrophages and T lymphocytes to resolve the infection (19). It is believed that the cross talk between different cell types of the immune system is highly dependent on cytokines.
Interleukin-6 (IL-6) is a pleiotropic cytokine supporting a range of functions in the host response to infection and various forms of stress. These include differentiation and proliferation of B cells and T cells, multipotent colony formation by hematopoietic stem cells and the acute-phase response (3). Recently it was shown that IL-6 switches the differentiation of monocytes from dendritic cells to macrophages (8). The role of IL-6 in clearance of infections with intracellular bacteria and viruses has been demonstrated through studies with IL-6-deficient mice (20). Specifically, it was shown that such mice are unable to control infections with Listeria monocytogenes and vaccinia virus. Moreover, the mice mount an impaired T-cell-dependent antibody response against vesicular stomatitis virus. Recently, it has been demonstrated that IL-6 is also required for an optimal immune response after ocular HSV-1 infection (23). Despite similar viral titers in the eye, the knockout mice were less able than their wild-type littermates to survive the infection.
As to the cell types responsible for IL-6 production, many cell populations have been reported to produce this cytokine, with monocytes and macrophages representing an important source (3). The molecular mechanism of IL-6 induction has been studied in great detail for a number of nonviral proinflammatory agents (11, 15, 27, 30, 37), whereas the regulation by viral infections is less well understood. The IL-6 promoter contains a region with adjacent binding sites for nuclear factor κB (NF-κB) and NF-IL6, and the participation of these two factors in IL-6 expression in response to many stimuli is well documented (27). Moreover, binding sites for activator protein 1 (AP-1), cAMP responsive element binding protein, and activating transcription factor 2 (ATF2/Jun) are present, and potential roles for these in IL-6 gene transcription have been suggested (11, 21).
A number of studies have addressed which viral entities elicit cytokine expression (reviewed in reference 28). For instance, it has been shown that cytomegalovirus induces IL-6 production through interaction between the viral glycoprotein gB and a cellular receptor (6), while hepatitis B virus triggers the response by a mechanism dependent on the viral X protein (24). Human immunodeficiency virus is particularly interesting in this respect since it induces IL-6 by no fewer than four distinct mechanisms involving the viral proteins gp120, Tat, Nef, and Vpr (4, 10, 35, 38).
In this study we have investigated the ability of leukocytes to produce IL-6 in response to HSV infection and have studied viral components responsible for the induction. In addition, our work addresses the cellular signaling pathways leading to IL-6 expression in HSV-infected leukocytes.
MATERIALS AND METHODS
Reagents.
The recombinant cytokines used were murine IL-6 (Genzyme), murine gamma interferon (IFN-γ) (Pharmingen), and human IFN-γ (Genzyme). Antibodies used were neutralizing polyclonal rabbit anti-tumor necrosis factor alpha (TNF-α) (Genzyme), mouse monoclonal anti-gD (Virusys), rat monoclonal anti-mouse IL-6 (Genzyme), biotinylated monoclonal rat anti-mouse IL-6 (Pharmingen), and horseradish peroxidase-conjugated rabbit polyclonal anti-mouse immunoglobulin (Transduction Laboratories). RNA was purified with Trizol (Life Technologies) and reverse transcribed using Expand Reverse Transcriptase (Roche). For PCR amplification, Taq2000 DNA polymerase (Stratagene) was used. The chemical inhibitors of diverse cellular functions were cyclosporine (CsA; Sigma), 1 μM; SB203580 (Calbiochem), 10 μM; pyrollidine dithiocarbamate (PDTC; Sigma), 10 μM; N-tosyl-l-phenylalanine chloromethyl ketone (TPCK; Sigma), 3 μM; H89 (Biomol), 5 μM; GF109203X (Biomol) and cycloheximide (CHX; Sigma), 10 μg of each/ml. DNA primers and probes were obtained from DNA Technology. LumiGLO was purchased from New England BioLabs, and the polyvinylidene difluoride membranes were from Novex. Heparin was from Leo Pharmacies and G418 was obtained from Roche. Cells were transfected with LipofectAMINE (Life Technologies).
Cell culture.
Peripheral blood mononuclear cells (PBMCs) were isolated from blood obtained from a healthy 28-year-old male donor by Isopaque-Ficoll separation. Briefly, the blood was laid on top of Ficoll and centrifuged at 600 × g for 30 min at 20°C. The PBMC-containing interphase was isolated, and the cells were washed in phosphate-buffered saline (PBS) containing 100 μg of heparin per ml. Subsequently, the cells were centrifuged at 200 × g for 15 min at 20°C and resuspended in RPMI 1640 medium containing 5% fetal calf serum (FCS) and antibiotics (200 IU of penicillin per ml and 200 μg of streptomycin per ml). The cells were seeded in 10-cm2 tissue culture wells at a density of 7 × 106 cells/well and left overnight before stimulation and infection.
Resting peritoneal cells (PC) were harvested from C57BL/6 mice by lavage of the peritoneal cavities with cold PBS supplemented with 2% FCS and 20 IU of heparin per ml. Inflammatory macrophages were induced by injection of 2 ml of 10% thioglycolate into the peritoneal cavities of C57BL/6 mice, and five days later the cells were harvested as described above. The cells were washed once in RPMI 1640 medium plus 5% FCS and seeded at a density of 7 × 106 cells/10 cm2 and left overnight before stimulation and infection.
The cell lines RAW 264.7 and J774A.1 were maintained in Dulbecco's minimal essential medium with 1% Glutamax I (Life Technologies), antibiotics, and 5% FCS. For experiments, the cells were seeded in 10-cm2 tissue culture wells at a density of 2 × 106 cells/well and left 16 to 20 h before further treatment. THP-1 cells were grown in RPMI 1640 containing 2 mM glutamine, 10% FCS, and antibiotics. For experiments the cells were seeded in 10-cm2 tissue culture wells at a density of 5 × 106 cells per well. Vero cells were maintained in minimal essential medium supplemented with 200 IU of penicillin per ml, 200 μg of streptomycin per ml, and 5% FCS. For virus amplification the cells were seeded at a density of 2 × 107 cells per 175-cm2 tissue culture flask 5 h prior to infection. For plaque assays, the cells were seeded at a density of 2 × 106 cells per 22.5-cm2 tissue culture plate.
The RAW 264.7-derived cell lines were grown in the same manner as the parental cell line, with the addition of 300 μg of G418 per ml. The protein kinase (PKR)-mutant cell line RAW-PKR-M7 and empty vector control cell line RAW-pBK-CMV, kindly donated by J. A. Corbett, Washington University (25), were grown in the presence of 200 μg of G418 per ml.
Viruses
The wild-type viruses used in this study were the MS strain of HSV-2 and the KOS and 17+ strains of HSV-1. The ICP0 mutant dl1403, the VP16 mutant in1814, and the rescued virus in1814R are on a 17+ genetic background (1, 41). The mutants lacking ICP4 or ICP27 or gL are on a KOS genetic background (29, 34, 39). The viruses were produced essentially as previously described (12). Just before usage, virus was thawed and used as infectious virus, subjected to heat inactivation at 56°C for 30 min, or inactivated by UV light for 15 min.
Isolation of RNA and RT-PCR.
RNA was isolated using Trizol according to the manufacturer's recommendations. Two micrograms of RNA was subjected to reverse transcription (RT) using oligo(dT)15 and Expand Reverse Transcriptase. The cDNA was amplified by PCR with the following primers: human IL-6, 5′-ACA AAT TCG GTA CAT CCT C-3′ (sense) and 5′-GCA GAA TGA GAT GAG TTG T-3′ (antisense); murine IL-6, 5′-TTC TGG AGT ACC ATA GCT AC-3′ (sense) and 5′-AGT TCT TCG TAG AGA ACA AC-3′ (antisense); β-actin, 5′-CCA ACC GTG AAA AGA TGA CC-3′ (sense) and 5′-GCA GTA ATC TCC TTC TGC ATC C-3′ (antisense). The products spanned 421 bp (human IL-6), 370 bp (murine IL-6), and 616 bp (β-actin). The RT-PCR protocol was tested with serial twofold dilutions of RNA prepared from RAW 264.7 cells treated with HSV-1 and IFN-γ for 4 h. Twenty cycles of PCR were found to be required to detect IL-6 mRNA, and a twofold difference in input material could be detected on the final agarose gel when performing 25 PCR cycles (data not shown).
ELISA.
Murine IL-6 was detected by enzyme-linked immunosorbent assay (ELISA). Maxisorp plates were coated overnight at 4°C with 2 μg of anti-IL-6 per ml in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 0.2% sodium azide [pH 9.6]). After blocking for 2 h at 37°C with PBS (pH 7.4) containing 1% (wt/vol) bovine serum albumin, samples and standard dilutions of IL-6 (3.9 to 2,000 pg/ml) were added to the wells and the plates were incubated at 37°C for 1 h. Subsequently the wells were incubated for 1 h at 37°C with a biotin-labeled IL-6 detection antibody at a concentration of 1 μg/ml in blocking buffer. Finally, horseradish peroxidase-conjugated streptavidin, diluted in blocking buffer, was added and incubated for 20 min at 20°C, and the result was visualized by the TMB system. After 10 min the color reaction was stopped with 5% H2SO4. Between each step the plates were washed three times with PBS containing 0.05% (vol/vol) Tween 20. The results were quantified by reading the absorbance at 450 nm.
Isolation of nuclear extracts.
To isolate nuclear proteins, the cell monolayer was washed twice with ice-cold PBS, scraped off the plate, and spun down (2,000 × g for 1 min). The cells were resuspended in a hypotonic buffer (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT], 0.2 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 0.2 mM leupeptin, 0.2 mM pepstatin A, 0.1 mM Na3VO4) and left on ice for 15 min. NP-40 was added to 0.6%, and the mixture was vortexed 15 s and centrifuged at 10,000 × g for 1 min. Extraction buffer (20 mM HEPES [pH 7.9], 20% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM DTT, 0.2 mM EDTA, 0.2 mM PMSF, 0.2 mM leupeptin, 0.2 mM pepstatin A, 0.1 mM Na3VO4, 0.2% NP-40) was added to the nuclei, and incubated 30 min at 4°C with rocking. The samples were centrifuged at 10,000 × g for 15 min at 4°C and the supernatants were harvested as nuclear extracts.
Electrophoretic mobility shift assay.
To assay for DNA-binding activity, 5 μg of protein in 3 μl of nuclear extraction buffer was mixed with 4 μg of poly(dI-dC) and 20,000 cpm of 32P-labeled probe in 18 μl. The final concentrations were 4 mM Tris-HCl, 23 mM HEPES (pH 7.9), 66 mM NaCl, 5 mM MgCl2, 0.7 mM EDTA, 1 mM DTT, and 14% glycerol. After 25 min of incubation at room temperature, the reaction mixture was subjected to electrophoresis on a nondenaturing 5% polyacrylamide gel in 0.5× TBE buffer (45 mM Tris base, 45 mM boric acid, 1 mM EDTA). The gel was dried and analyzed by autoradiography. For competition assays 100-fold excess of cold probe was added together with the labeled probe. The probes corresponding to sites in the murine IL-6 promoter are as follows: κB, 5′-ATG TGG GAT TTC CCA TG-3′; AP-1, 5′-AGT GCT GAG TCA CTT TT-3′; CRE, 5′-CTA AAC GAC GTC ACA TTG-3′; C/EBP, 5′-CGT CAC ATT GTG CAA TCT TAA T-3′.
Stable transfections.
The DNA plasmids used for transfections encoded dominant negative versions of p38 and IκBα. The p38 construct was a kind gift from Helmut Holtmann (43). To generate the dominant negative IκBα expression construct, mutant IκBα, generously donated by John Hiscott (22), was amplified by PCR with the high-fidelity polymerase Pfu using the following primers: 5′-GAA TTC ATG TTC CAG GCG GCC GAG-3′ (sense), 5′-GTC GAC TTA GAA CTC TGA CTC TGT GTC-3′ (antisense) (restriction sites used for subcloning are underlined). The PCR fragment was cloned into the EcoRV site of pBluescript KS and subcloned into the appropriate sites in pcDNA3. For the empty vector, control pcDNA3 was used. For transfections the cells were seeded at a density of 5 × 106 cells/25-cm2 plate and left for 24 h. The cells were transfected using LipofectAMINE (7.5 μg of DNA and 20 μl of LipofectAMINE) and serum-free medium. Four hours after transfection, FCS was added to 10%, and the cells were incubated 24 h before application of selection with 300 μg of G418 per ml. Surviving colonies were pooled in order to avoid single-clone abnormalities, and cells were grown through five to eight passages in Dulbecco's modified Eagle's medium supplemented with 5% FCS in the presence of selection. The levels of ectopic expression were assessed by Western blotting prior to use of the cell lines.
RESULTS
Expression of IL-6 in human and murine leukocyte populations after HSV infection.
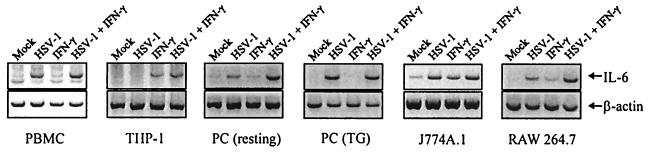
It has previously been shown that HSV infections induce expression of IL-6 in vivo (14, 40), and we wanted to examine if selected leukocyte populations responded to HSV infection in vitro with expression of IL-6. As seen from Fig. 1 human PBMCs, murine PCs, J774A.1, and RAW 264.7 cells all produced IL-6 mRNA following HSV-1 infection. In contrast, no IL-6 mRNA was produced by THP-1 cells after HSV-1 infection. In most of the HSV-responsive cells, cotreatment with IFN-γ enhanced the levels of accumulated IL-6 mRNA. Similar results were obtained with HSV-2 (data not shown). These results show that many human and murine leukocyte populations express IL-6 after HSV infection.
FIG. 1.
Expression of IL-6 mRNA in various human and murine leukocyte populations. Primary cells were isolated as described in Materials and Methods. The primary cells and cell lines were seeded and left overnight to settle. The cells were infected with 3 × 106 PFU of HSV-1 (KOS) per ml and stimulated with 10 IU of human or murine IFN-γ per ml. After 4 h of treatment, total RNA was isolated and subjected to RT-PCR using oligo(dT)15 RT priming and PCR primers specific for murine β-actin, human IL-6, and murine IL-6. PBMC, peripheral blood mononuclear cells; PC, peritoneal cells; TG, thioglycolate.
Induction of IL-6 in macrophages is dependent on a functional viral genome and intermediary de novo protein synthesis.
We also examined if the enhanced IL-6 mRNA accumulation was associated with secretion of IL-6 protein. Whereas RAW 264.7 cells left untreated or treated with heat-inactivated virus or mock preparation produced little or no IL-6, infection with either HSV-1 or HSV-2 led to a strong induction of IL-6 protein (Fig. 2A). IFN-γ alone did induce some IL-6, and an appreciable synergy was observed between IFN-γ and HSV infection. The ability of HSV-1 to induce IL-6 expression did not occur through phagocytosis-mediated uptake of virus particles, since gL-deficient HSV-1, which can adsorb but not penetrate cells, was unable to affect IL-6 protein levels in supernatants from resting or IFN-γ-treated RAW 264.7 cells (data not shown).
FIG. 2.
Characterization of IL-6 induction by HSV-kinetics and effects of cycloheximide, anti-TNF-α, and UV-irradiation of the virus. RAW 264.7 cells were seeded and left overnight to settle. (A) The cells were treated with 3 × 106 PFU of HSV-1 (KOS) per ml, 3 × 106 PFU of HSV-2 (MS) per ml, and 10 IU of IFN-γ per ml. After 24 h, supernatants were harvested and analyzed for IL-6 by ELISA. The results are shown as means ± standard errors of the means. (B) The cells were treated as indicated with the following concentrations: 3 × 106 PFU of HSV-1 (KOS) per ml or equivalent amount of UV-irradiated virus, 10 IU of IFN-γ per ml, 1,000 U of neutralizing anti-TNF-α per ml, 10 μg of CHX per ml. After 4 h of treatment total RNA was isolated. (C) The cells were treated with 3 × 106 PFU of HSV-1 (KOS) per ml and 10 IU of IFN-γ per ml. After the indicated time points total RNA was isolated. (B and C) The RNA was subjected to RT-PCR using oligo(dT)15 RT-priming and PCR primers specific for murine β-actin and IL-6.
To further characterize the HSV-induced IL-6 expression, we tested if a functional virus genome was required for the induction. This is normally done by irradiating the virus with UV light prior to infection. Such experiments have previously revealed that the ability of HSV to induce IL-12 p40 expression is sensitive to UV light (26) whereas the virus induces secretion of IFN-α/β and TNF-α through a mechanism displaying only little sensitivity to this treatment (12). When RAW 264.7 macrophage-like cells were infected with UV-irradiated HSV-1, we observed that no IL-6 was induced (Fig. 2B). UV treatment totally prevented the accumulation of mRNA for ICP27 (α), ICP8 (β), and gD (γ1) normally observed after infection of RAW 264.7 cells with live HSV-1 and HSV-2 (data not shown). We also examined if virus-induced TNF-α was involved in the induction, since it is known that TNF-α supports IL-6 expression (37). However, the presence of neutralizing TNF-α antibodies had no appreciable effect on the IL-6 mRNA levels. Given the UV-sensitive nature of the response, we also wanted to examine if de novo protein synthesis was required for the induction of IL-6 mRNA expression. We found that the protein synthesis inhibitor CHX per se did enhance the IL-6 mRNA levels marginally. More importantly, however, the ability of infection to affect IL-6 mRNA levels was abolished by CHX treatment. Finally, we examined the kinetics of IL-6 mRNA accumulation after HSV-1 infection. In the experiment shown, a detectable level of IL-6 mRNA was present constitutively (Fig. 2C). After 2 h of infection a significant increase in the IL-6 mRNA levels was observed, and they increased further between 2 and 5 h postinfection and remained high through the 8 h of the experiment.
Induction of IL-6 by mutant viruses in wild-type cells and by wild-type HSV-1 in macrophages unable to respond to double-stranded RNA.
In order to identify the virus-cell interactions responsible for IL-6 induction, we analyzed the ability of a number of HSV-1 mutants to induce expression of the cytokine in macrophages. First, we tested mutants with defects in the tegument protein VP16 (in1814), the immediate-early proteins ICP0 (dl1403), ICP4 (vi13) or ICP27 (d27-1). As seen in Fig. 3A, all 4 mutants retained the capacity to induce IL-6, demonstrating that any of the four proteins alone was redundant for IL-6 induction.
FIG. 3.
Induction of IL-6 by mutant viruses in wild-type cells and by wild-type HSV-1 in macrophages unable to activate PKR in response to double-stranded RNA. (A) RAW 264.7 cells were seeded and left overnight to settle. The cells were treated with 10 IU of IFN-γ per ml and 3 × 106 PFU of wild-type or mutant HSV-1 per ml. After 4 h of treatment, total RNA was isolated and subjected to RT-PCR using oligo(dT)15 RT-priming and PCR primers specific for murine β-actin and IL-6. (B) The RAW 264.7-derived cell lines pBK-CMV and PKR-M7 were seeded as above. Sixteen hours later the cells were treated with 10 IU of IFN-γ per ml and 3 × 106 PFU of HSV-1 (KOS) per ml. After 4 h of treatment, total RNA was extracted and analyzed for IL-6 and β-actin by RT-PCR.
We also tested if macrophages unable to activate the double-stranded RNA-activated kinase PKR in response to infection were capable of producing IL-6. To this end we used a RAW 264.7-derived cell line stably expressing a PKR mutant (PKR-M7) lacking the first double-stranded RNA-binding domain which is required for maximal kinase activity (25). We found that whereas the cell line transfected with the parental vector was fully capable of producing IL-6 in infected cells, the PKR-M7-harboring cells did not respond to infection or IFN-γ stimulation with expression of IL-6 (Fig. 3B). Cotreatment with IFN-γ and HSV-1 did allow production of low amounts of IL-6.
Activation of DNA-binding activity to the IL-6 promoter after HSV infection.
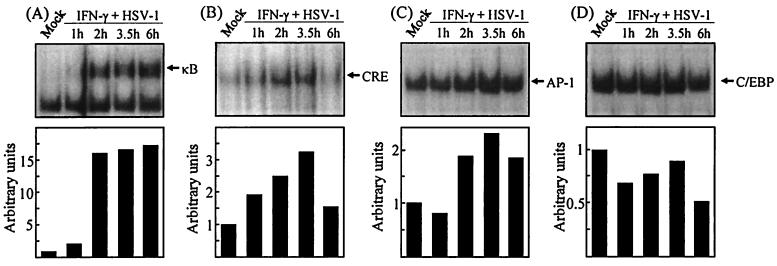
To identify virus-induced cellular signal transduction responsible for IL-6 expression, we performed an electrophoretic mobility shift assay using probes derived from the murine IL-6 promoter. Nuclear extracts were prepared at different time points after HSV and IFN-γ treatment, and the extracts were examined for DNA-binding activity. When using the probe corresponding to the κB site localized between 73 and 65 nucleotides (nt) upstream of the transcriptional start site, we found that binding was not induced or was only weakly induced after 1 h but was strongly enhanced after 2 h and remained elevated through the 6 h of the experiment (Fig. 4A). The CRE site (167 to 160 nt) and AP-1-binding site (277 and 271 nt) were also analyzed, and we observed that a modest yet specific and reproducible induction occurred (Fig. 4B and C). This was most apparent after between 2 and 3.5 h, which interestingly correlates with the onset of IL-6 mRNA accumulation (Fig. 2C). Finally we examined the binding pattern of a C/EBP-binding site (158 and 145 nt) in the promoter. As seen in Fig. 4D, no induction of C/EBP binding to the probe was detected within the 6-h time frame chosen for the experiment. All the above-described protein-DNA complexes were sequence specific as assessed by competition with identical and unrelated cold probes (data not shown). Together these results open up the possibility of a role for NF-κB-, AP-1-, and CRE-binding proteins in virus-induced IL-6 expression.
FIG. 4.
Binding activity to κB, CRE, AP-1, and C/EBP sites of the murine IL-6 promoter. RAW 264.7 cells were treated with 10 IU of IFN-γ per ml and infected with 3 × 106 PFU of HSV-1 (KOS) per ml for the indicated time periods, and nuclear extracts were prepared. The extracts were tested for binding to the following probes: κB (A), CRE (B), AP-1 (C), and C/EBP (D). Five micrograms of nuclear proteins and 20,000 cpm of 32P-labeled probe were used per reaction. The binding was quantified by densitometric measurements of the autoradiographs. The results are shown as histograms using arbitrary units.
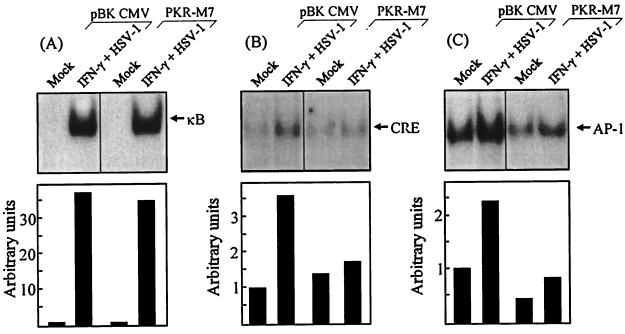
To evaluate the potential involvement of PKR in activation of these transcription factors, we used the PKR mutant cell line. This cell line (PKR-M7) and the empty-vector control cell line (pBK-CMV) were treated with mock preparation or HSV-1 plus IFN-γ for 3.5 h, and nuclear extracts were prepared. When examining for κB-binding activity, we found that the constitutive band observed in the parental RAW 264.7 cells was absent (compare Fig. 4A and 5A). However, both pBK-CMV and PKR-M7 activated κB-binding activity to at least the same extent as RAW 264.7 did. In contrast to this, we found that the abilities of HSV-1 infection and IFN-γ treatment to induce binding to CRE and AP-1 probes were impaired in PKR-M7 cells compared to the pBK-CMV cells (Fig. 5B and C). Therefore, RAW 264.7 cells harboring the PKR mutant M7, which lacks the first RNA-binding domain, are fully capable of activating NF-κB in response to HSV-1 infection but display reduced activation of AP-1- and CRE-binding factors.
FIG. 5.
PKR dependency of binding activity on the κB, CRE, and AP-1 sites of the murine IL-6 promoter. RAW-pBK-CMV or RAW-PKR-M7 cells were mock infected or treated with 10 IU of IFN-γ per ml and 3 × 106 PFU of HSV-1 (KOS) per ml. Nuclear extracts were prepared 3.5 h later, and the extracts were tested for binding to κB (A), CRE (B), and AP-1 (C) probes. Five micrograms of nuclear proteins and 20,000 cpm of 32P-labeled probe were used per reaction. The binding was quantified by densitometric measurements of the autoradiographs. The results are shown as histograms using arbitrary units.
HSV-induced IL-6 expression is repressed by inhibitors against NF-κB and the p38 MAP kinase.
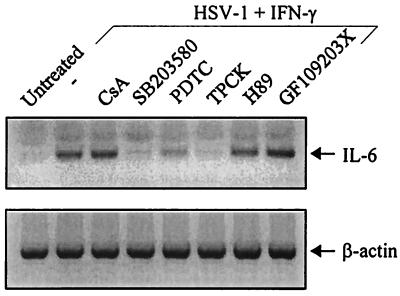
To further analyze the signaling pathways involved in HSV-induced IL-6 expression, we tested the influence of a range of chemical inhibitors on IL-6 mRNA accumulation. For this experiment, the cells were pretreated with the inhibitors 15 min prior to cytokine stimulation and HSV infection. Four hours after infection and stimulation, RNA was extracted and analyzed for IL-6 and β-actin mRNA (Fig. 6). We found that virus-induced accumulation of IL-6 mRNA was not significantly affected by inhibitors against NF-AT activation (CsA), protein kinase A (H89), or protein kinase C (GF109203X). In contrast, the p38 kinase inhibitor SB203580 and TPCK, which prevents NF-κB activation, totally abolished IL-6 production. Another inhibitor of NF-κB activation, PDTC, had a more modest effect. Together these results support a role of NF-κB and a p38-dependent pathway in HSV-induced IL-6 induction.
FIG. 6.
Effects of chemical inhibitors on HSV-induced IL-6 expression. RAW 264.7 cells were seeded and left overnight to settle. The cells were treated with inhibitors and left for 15 min prior to stimulation with 10 IU of IFN-γ per ml and infection with 3 × 106 PFU of HSV-1 (KOS) per ml. Four hours later, total RNA was extracted and IL-6 and β-actin were detected by RT-PCR using primers specific for the two mRNA species.
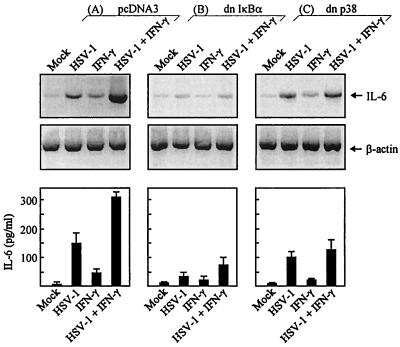
Induction of IL-6 in RAW 264.7 cells expressing dominant negative IκBα and p38.
To corroborate the above results with other data, we generated RAW 264.7-derived cell lines stably expressing dominant negative mutants of IκBα and p38. The cells were infected with HSV-1 and stimulated with IFN-γ. After 4 h, one-half of the cell cultures were harvested and RNA was extracted. The remaining cell cultures were left for 24 h prior to collection of the culture supernatants. The RNA and supernatants were analyzed for the presence of IL-6 mRNA and protein, respectively. The cell line transfected with empty vector displayed an expression pattern similar to that of the parental RAW 264.7 cell line (compare Fig. 7A with Fig. 2A and 3). In contrast, if the cells expressed the IκBα mutant, the ability of HSV-1 to induce IL-6 alone or in concert with IFN-γ was severely compromised (Fig. 7B). Finally we found that overexpression of dominant negative p38 only had a moderate effect on IL-6 induction by virus alone but significantly compromised the synergy between IFN-γ and HSV-1 (Fig. 7C). Similar results were obtained with HSV-2 (data not shown).
FIG. 7.
Induction of IL-6 by HSV-1 in macrophages stably transfected with dominant negative IκBα and p38. RAW 264.7-derived cell lines with the following characteristics were generated: pcDNA3 (A), dominant negative IκBα (B), dominant negative p38V (C). The cells were seeded, infected with 3 × 106 PFU of HSV-1 (KOS) per ml, and stimulated with 10 IU of IFN-γ per ml. After 4 h, cells to be analyzed for mRNA expression were lysed and total RNA was extracted. IL-6 and β-actin were detected by RT-PCR using primers specific for the two mRNA species (upper panel). Other cells were left for 24 h, at which point supernatants were harvested and analyzed for IL-6 protein by ELISA. The results (lower panel) are shown as means ± standard errors of the means.
DISCUSSION
The first line of defense against virus infections involves NK cells and macrophages. These cells are able to directly inhibit virus replication through a number of antiviral effector mechanisms and also to cross-activate other cells of the immune system. This latter function is primarily mediated by cytokines. One cytokine with a role in the immune response to virus infections is IL-6, which is a cytokine with pleiotropic activities. For instance, this cytokine supports differentiation of B lymphocytes, plays a role in T-cell differentiation, inhibits myeloid-cell growth while inducing differentiation of these cells into macrophages, and induces an acute-phase response in hepatocytes (3, 8). It has been shown that IL-6-deficient mice exhibit an impaired T-cell-dependent antibody response to vesicular stomatitis virus infections, which are accompanied by increased virus titers in these mice (20). During an HSV infection IL-6 is produced in two phases: one early after infection, dependent on the presence of virus, and one at later stages of infection, in a manner independent of the presence of virus (40). It has been demonstrated that IL-6 does contribute to resistance against HSV-1 infections since IL-6-deficiency and transgenic IL-6 expression enhances susceptibility and resistance, respectively, to HSV-1 infections (7, 23).
Here we have investigated the molecular mechanisms underlying the early IL-6 expression in various leukocyte populations. Among the cell types tested, all except one expressed IL-6 in response to HSV infections. Costimulation with IFN-γ further augmented IL-6 mRNA accumulation. Thus, IL-6 is produced by leukocytes of both mouse and human origins as a primary response to HSV infections.
The virus-host interactions responsible for IL-6 production were investigated by two approaches. First, we examined mutant viruses for their capacities to induce IL-6. Second, IL-6 expression by a macrophage cell line expressing a dominant negative mutant of the double-stranded RNA-activated protein kinase PKR was tested. The PKR mutant used lacks the first double-stranded RNA-binding domain of PKR (25). The results obtained suggest that neither the virion-associated transactivator VP16 nor any one of the immediate-early proteins ICP0, ICP4, or ICP27 alone was essential for IL-6 induction. This is in agreement with previous studies with a permissive murine epithelial cell line (17). Despite the nonessential role of any of the three immediate-early proteins alone for induction of IL-6, a functional viral genome was required. This was evidenced by the lack of IL-6 expression after treatment with UV-irradiated virus.
When examining the role of PKR, we found that overexpression of a dominant negative PKR mutant abolished the ability of HSV-1 to induce IL-6. However, the effect was not virus specific since IFN-γ was also unable to induce IL-6 in the mutant cell line. Cotreatment with HSV-1 and IFN-γ did allow some production of IL-6. This result could suggest that PKR senses the accumulation of viral double-stranded RNA in the infected cell and induces signal transduction leading to cytokine induction. In fact, through a mechanism dependent on PKR, double-stranded RNA is known to activate the signal transduction cascades found here to be involved in IL-6 induction (13, 45). The kinetics of IL-6 mRNA production also allows a role for double-stranded RNA in the process, since accumulation of immediate-early viral mRNA precedes IL-6 mRNA (J. Melchjorsen and S. R. Paludan, unpublished data). However, we also observed that IFN-γ was unable to induce IL-6 expression in the mutant cell line, which argues for a double-stranded RNA-independent role of PKR in the process. HSV-1 has been reported to inhibit PKR in vitro through at least two different mechanisms (9, 33). Yet, PKR-deficient mice display significantly enhanced susceptibility to HSV-1 infections (18). Our study did not address whether PKR activity was affected by HSV infection in macrophages, but the reduced IL-6 expression in the PKR mutant cell line may help to explain why PKR deficiency renders mice more susceptible to HSV-1 infections.
As to the cellular signaling and transcription factors responsible for IL-6 induction by HSV, our results suggest that NF-κB and a p38-dependent pathway, likely ATF2/Jun, are important. NF-κB is a cardinal mediator of proinflammatory gene expression. The dimeric transcription factor is present in the cytoplasm in resting cells in a latent form bound to the inhibitory subunit IκB (5). Upon stimulation, IκB is phosphorylated by the IκB kinase and subsequently degraded, allowing NF-κB to migrate to the nucleus and promote transcription. HSV-1 and HSV-2 have previously been shown to activate NF-κB in various cell types (16, 31, 32), and we have shown that this does contribute to expression of proinflammatory mediators (26, 31). Here we show that NF-κB also contributes to induction of IL-6 expression. As to the mechanism of NF-κB activation, we found that it was independent of PKR. Others have reported that ICP4 and ICP27 are essential for sustained NF-κB activity in HSV-1-infected C33 cells (32). In macrophages, however, other mechanisms seem to be involved, since viruses deficient in ICP4 and ICP27 displayed no apparent defect in their ability to induce IL-6, which is dependent on NF-κB.
Our results also suggest that a p38-dependent pathway, which could involve ATF2/Jun, contributes to HSV-induced IL-6 expression. First, we found that binding to the CRE-like site of the murine IL-6 promoter was induced after infection in IFN-γ-activated macrophages. This was not observed in RAW-PKR-M7 cells, suggesting a role for PKR in HSV-1-induced CRE-binding activity. Second, inhibition of the ATF2 kinase p38 by SB203580 strongly reduces IL-6 expression. Third, macrophages stably transfected with dominant negative p38 are unable to induce maximal IL-6 expression in response to HSV-1 and IFN-γ. It is interesting, though, that IL-6 induction by HSV-1 per se is only marginally affected. This result indicates that a p38-dependent pathway, possibly mediated through ATF2/Jun activation and recruitment to the CRE site of the IL-6 promoter, is essential for the synergistic induction of IL-6 by HSV and IFN-γ. Finally, the transient nature of the CRE-binding activity, which is in contrast to the sustained expression of IL-6, suggests that ATF2/Jun contributes to induction of IL-6 gene transcription rather than sustained expression.
We have not directly addressed the involvement of AP-1 in IL-6 expression, but we did find that PKR plays an important role in HSV-1-induced AP-1 activation and IL-6 expression. However, indirect evidence suggests that AP-1 is not essential for IL-6 production. HSV-1 activates AP-1 in baby hamster kidney cells through a mechanism involving ICP0 and VP16 (16, 44). In our hands, viruses harboring mutants in the genes encoding these proteins exhibited unaltered ability to induce IL-6 expression. This suggests that AP-1 is not involved in HSV-induced IL-6 production.
As to the role of C/EBPβ in IL-6 expression, several studies have suggested that this transcription factor plays a central role in induction of many proinflammatory mediators (reviewed in reference 2). Different groups have reported that IFN-γ induces C/EBPβ expression in many cell types including macrophages (36, 42). However, we were unable to demonstrate any induction of C/EBP binding to the corresponding site in the IL-6 promoter. Although this suggests that initiation of IL-6 transcription in HSV-infected macrophages occurs independently of C/EBPs, it remains possible that C/EBPβ accumulates later after infection and/or stimulation and that the transcription factor is involved in sustained IL-6 expression.
In conclusion, the data presented here show that induction of IL-6 by HSV in leukocytes is triggered by a mechanism dependent on PKR, possibly accumulation of viral double-stranded RNA. The cellular signaling pathways involved include notably NF-κB and a p38-dependent pathway. Given the role of IL-6 and other proinflammatory cytokines in the host defense, this work contributes to the molecular understanding of the pathogenesis of HSV infections.
ACKNOWLEDGMENTS
The donation of mutant viruses, expression constructs, and cell lines by Bernard Roizman, Patricia G. Spear, David M. Knipe, Roger D. Everett, Chris M. Preston, Neal A. DeLuca, Paula Pitha, Helmut Holtmann, and John A. Corbett is greatly appreciated.
This work was supported by grants from the Danish Health Science Research Council (grant number 12-1622), The Carlsberg Foundation (grant number 990803/10-911), and The Leo Research Foundation.
REFERENCES
- 1.Ace C I, McKee T A, Ryan J M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Kishimoto T. NF-IL6 and NF-kB in cytokine gene regulation. Adv Immunol. 1997;65:1–46. [PubMed] [Google Scholar]
- 3.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 4.Ameglio F, Capobianchi M R, Castilletti C, Cordiali Fei P, Fais S, Trento E, Dianzani F. Recombinant gp120 induces IL-10 in resting peripheral blood mononuclear cells; correlation with the induction of other cytokines. Clin Exp Immunol. 1994;95:455–458. doi: 10.1111/j.1365-2249.1994.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 6.Carlquist J F, Edelman L, Bennion D W, Anderson J L. Cytomegalovirus induction of interleukin-6 in lung fibroblasts occurs independently of active infection and involves a G protein and the transcription factor, NF-κB. J Infect Dis. 1999;197:1094–1100. doi: 10.1086/314734. [DOI] [PubMed] [Google Scholar]
- 7.Carr D J, Campbell I L. Transgenic expression of interleukin-6 in the central nervous system confers protection against acute herpes simplex virus type-1 infection. J Neurovirol. 1999;5:449–457. doi: 10.3109/13550289909045373. [DOI] [PubMed] [Google Scholar]
- 8.Chomarat P, Banchereau J, Davoust J, Palucka A K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 9.Chou J, Chen J J, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De S K, Venkateshan C N, Seth P, Gajdusek D C, Gibbs C J. Adenovirus-mediated human immunodeficiency virus-1 Nef expression in human monocytes/macrophages and effect of Nef on downmodulation of Fcg receptors and expression of monokines. Blood. 1998;91:2108–2117. [PubMed] [Google Scholar]
- 11.Dokter W H A, Koopmans S B, Vellenga E. Effects of IL-10 and IL-4 on LPS-induced transcription factors (AP-1, NF-IL6 and NF-κB) which are involved in IL-6 regulation. Leukemia. 1996;10:1308–1316. [PubMed] [Google Scholar]
- 12.Ellermann-Eriksen S. Autocrine secretion of interferon-α/β and tumour necrosis factor-α synergistically activates mouse macrophages after infection with herpes simplex virus type 2. J Gen Virol. 1993;74:2191–2199. doi: 10.1099/0022-1317-74-10-2191. [DOI] [PubMed] [Google Scholar]
- 13.Goh K C, deVeer M J, Williams B R. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000;19:4292–4297. doi: 10.1093/emboj/19.16.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halford W P, Gebhardt B M, Carr D J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 15.Hu H-M, Baer M, Williams S C, Johnson P F, Schwartz R C. Redundancy of C/EBPα, -β and -δ in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J Immunol. 1998;160:2334–2342. [PubMed] [Google Scholar]
- 16.Jang K L, Pulverer B, Woodgett J R, Latchman D S. Activation of the cellular transcription factor AP-1 in herpes simplex virus infected cells is dependent on the viral immediate-early protein ICPO. Nucleic Acids Res. 1991;19:4879–4883. doi: 10.1093/nar/19.18.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanangat S, Babu J S, Knipe D M, Rouse B T. HSV-1-mediated modulation of cytokine gene expression in a permissive cell line: selective upregulation of IL-6 gene expression. Virology. 1996;219:295–300. doi: 10.1006/viro.1996.0250. [DOI] [PubMed] [Google Scholar]
- 18.Khabar K S, Dhalla M, Siddiqui Y, Zhou A, Al Ahdal M N, Der S D, Silverman R H, Williams B R. Effect of deficiency of the double-stranded RNA-dependent protein kinase, PKR, on antiviral resistance in the presence or absence of ribonuclease L: HSV-1 replication is particularly sensitive to deficiency of the major IFN-mediated enzymes. J Interferon Cytokine Res. 2000;20:653–659. doi: 10.1089/107999000414835. [DOI] [PubMed] [Google Scholar]
- 19.Kodukula P, Liu T, van Rooijen N, Jager M J, Hendricks R L. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 20.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 21.Krueger J, Ray A, Tamm I, Sehgal P B. Expression and function of interleukin-6 in epithelial cells. J Cell Biochem. 1991;45:327–334. doi: 10.1002/jcb.240450404. [DOI] [PubMed] [Google Scholar]
- 22.Kwon H, Pelletier N, DeLuca C, Genin P, Cisternas S, Lin R, Wainberg M A, Hiscott J. Inducible expression of IκBa repressor mutants interferes with NF-κB activity and HIV-1 replication in Jurkat T cells. J Biol Chem. 1998;273:7431–7440. doi: 10.1074/jbc.273.13.7431. [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc R A, Pesnicak L, Cabral E S, Godleski M, Straus S E. Lack of interleukin-6 (IL-6) enhances susceptibility to infection but does not alter latency or reactivation of herpes simplex virus type 1 in IL-6 knockout mice. J Virol. 1999;73:8145–8151. doi: 10.1128/jvi.73.10.8145-8151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, Park U S, Choi I, Yoon S K, Park Y M, Lee Y I. Human interleukin 6 gene is activated by hepatitis B virus-X protein in human hepatoma cells. Clin Cancer Res. 1998;4:1711–1717. [PubMed] [Google Scholar]
- 25.Maggi L B, Jr, Heitmeier M R, Scheuner D, Kaufman R J, Buller R M, Corbett J A. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J. 2000;19:3630–3638. doi: 10.1093/emboj/19.14.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malmgaard L, Paludan S R, Mogensen S C, Ellermann-Eriksen S. HSV-2 induces interleukin-12 in macrophages through a mechanism involving NF-κB. J Gen Virol. 2000;81:3011–3020. doi: 10.1099/0022-1317-81-12-3011. [DOI] [PubMed] [Google Scholar]
- 27.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines IL-6 and IL-8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mogensen T H, Paludan S R. Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev. 2001;65:131–150. doi: 10.1128/MMBR.65.1.131-150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 30.Munoz C, Pascual Salcedo D, Castellanos M C, Alfranca A, Aragones J, Vara A, Redondo M J, de Landazuri M O. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-κB and AP-1 transcription factors activity. Blood. 1996;88:3482–3490. [PubMed] [Google Scholar]
- 31.Paludan S R, Ellermann-Eriksen S, Mogensen S C. NF-κB activation is responsible for the synergistic effect of herpes simplex virus type 2 infection on interferon-γ-induced nitric oxide production. J Gen Virol. 1998;79:2785–2793. doi: 10.1099/0022-1317-79-11-2785. [DOI] [PubMed] [Google Scholar]
- 32.Patel A, Hanson J, McLean T I, Olgiate J, Hilton M, Miller W E, Bachenheimer S L. Herpes simplex type 1 induction of persistent NF-κB nuclear translocation increases the efficiency of virus replication. Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 33.Poppers J, Mulvey M, Khoo D, Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J Virol. 2000;74:11215–11221. doi: 10.1128/jvi.74.23.11215-11221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice S A, Knipe D M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus a protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux P, Alfieri C, Hrimech M, Cohen E A, Tanner J E. Activation of transcription factors NF-κB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol. 2000;74:4658–4665. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy S K, Wachira S J, Weihua X, Hu J, Kalvakolanu D V. CCAAT/enhancer-binding protein-β regulates interferon-induced transcription through a novel element. J Biol Chem. 2000;275:12626–12632. doi: 10.1074/jbc.275.17.12626. [DOI] [PubMed] [Google Scholar]
- 37.Sanceau J, Kaisho T, Hirano T, Wietzerbin J. Triggering of the human interleukin-6 gene by interferon-γ and tumor necrosis factor-α in monocytic cells involves cooperation between interferon regulatory factor-1, NF κB, and Sp1 transcription factors. J Biol Chem. 1995;270:27920–27931. doi: 10.1074/jbc.270.46.27920. [DOI] [PubMed] [Google Scholar]
- 38.Scala G, Ruocco M R, Ambrosino C, Mallardo M, Giordano V, Baldassarre F, Dragonetti E, Quinto I, Ventura S. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 tat protein. J Exp Med. 1994;179:961–971. doi: 10.1084/jem.179.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepard A A, DeLuca N A. Activities of heterodimers composed of DNA-binding- and transactivation-deficient subunits of the herpes simplex virus regulatory protein ICP4. J Virol. 1991;65:299–307. doi: 10.1128/jvi.65.1.299-307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimeld C, Whiteland J L, Williams N A, Easty D L, Hill T J. Cytokine production in the nervous system of mice during acute and latent infection with herpes simplex virus type 1. J Gen Virol. 1997;78:3317–3325. doi: 10.1099/0022-1317-78-12-3317. [DOI] [PubMed] [Google Scholar]
- 41.Stow N D, Stow E C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 42.Tengku-Muhammad T S, Hughes T R, Ranki H, Cryer A, Ramji D P. Differential regulation of macrophage ccaat-enhancer binding protein isoforms by lipopolysaccharide and cytokines. Cytokine. 2000;12:1430–1436. doi: 10.1006/cyto.2000.0711. [DOI] [PubMed] [Google Scholar]
- 43.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen C Y, Shyu A B, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zachos G, Clements B, Conner J. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J Biol Chem. 1999;274:5097–5103. doi: 10.1074/jbc.274.8.5097. [DOI] [PubMed] [Google Scholar]
- 45.Zamanian-Daryoush M, Mogensen T H, Didonato J A, Williams B R. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]