Abstract
Ambient ozone (O3) exposure may be associated with a reduction of semen quality, yet the potential biological mechanisms remain unclear. We investigated the effects of certain seminal plasma metabolites on mediating the links between O3 exposure and the deterioration of semen quality. The untargeted metabolomics analysis was performed on semen samples of 200 Chinese adult men to determine candidate metabolites associated with characteristics of semen quality. Mediation analysis was adopted to examine whether these metabolites modulated the links between O3 exposure and semen quality. We found a significant reduction in sperm concentration by −28.1% (95% CI: −41.7%, −11.3%), and sperm count by −29.2% (95% CI: −43.7%, −11.0%) associated with each 10 μg/m3 increase in ambient O3 concentration during the period of sperm development. We delineated 7 metabolites in seminal fluid that substantially mediated the links between O3 exposure and declined semen quality, including myristoleic acid, aspartyl-isoleucine, phenylethyl primeveroside, ACar (18:2), ACar (18:1), FAHFA (22:6/22:3), and LPS (22:5). Among these, myristoleic acid exhibited the most pronounced mediation effects, with its indirect effect of which accounts for 46.4% of the overall association. Our findings suggested that exposure to ozone decreased sperm quality by disrupting fatty acid metabolism, particularly myristoleic acid.
Keywords: ozone exposure, sperm quality, adult men, metabolomics
The decline in sperm quality has emerged as a critical concern in male health in recent decades, posing significant threats to human reproduction and socioeconomic development. It has been estimated that approximately 15%–20% of couples globally, nearly 48.5 million, are affected by infertility attributed to inferior semen quality.1 Environmental determinants can lead to abnormalities in semen quality, such as nonoptimal ambient temperature,2,3 exposure to heavy metals/metalloids,4 and ambient air pollution.5−7
Urbanization and industrialization have led to widespread air pollution. While there have been significant reductions in concentrations of particulate matter in recent years, ozone (O3) has emerged as a growing public health concern. O3, a natural atmospheric component, is primarily derived from anthropogenic emissions and is predicted to increase with global warming.8 Numerous epidemiological studies have linked O3 exposure to morbidity and mortality, including respiratory diseases, cardiovascular ailments, and reproductive abnormality.9−11 Several epidemiological studies, including a meta-analysis by Xu et al.,12 a retrospective longitudinal study by Qiu et al.,13 and cross-sectional studies by Sokol et al.14 and Sun et al.,15 have associated O3 exposure with decreased sperm quality. Nevertheless, their estimates varied and even contradictory due to disparities in exposure sources, sample sizes, and methods of air pollutant assessment.12−15 The adverse effects of O3 could decrease semen quality by exacerbating inflammation responses, inducing oxidative stress, disrupting the blood-testis barrier, and resulting in diminished sperm quality.16,17 However, despite efforts to understand these associations, the biological mechanisms linking O3 exposure to impaired semen quality are yet to be fully elucidated.
Emerging omics technologies are being utilized to identify potential biomarkers and elucidate the pathogenesis of various diseases. Among approaches like transcriptomics, metabolomics, and proteomics, metabolomics offers distinct advantages for unraveling biochemical activities and cellular metabolism in living organisms by aligning with metabolite profiles and biological characteristics.18 Some studies have investigated how metabolites mediate the links between exogenous exposure and sperm abnormality.19,20 For instance, Wang et al. pinpointed that urinary phthalate metabolites could alter the expression of metabolites in seminal plasma, consequently decreasing the proportion of spermatozoa with abnormal head morphology.19 In our previous study, our findings indicated that PM2.5 exposure was positively linked to decreased sperm progressive and total motility, mediated by a reduction in d-Aspartate levels.20 To our understanding, there are few studies that have explored the intermediary roles of metabolites in seminal plasma regarding the links between O3 exposure and sperm abnormality.
Leveraging the seminal plasma metabolome, we sought to investigate the potential mediation by specific metabolites in the links between O3 exposure and decreased sperm levels within a cohort of 200 male adults for metabolomics analysis.
Methods
Study Participants
We recruited reproductive-age couples from the Tongji Reproductive and Environment study (TREE),21,22 which was initially constructed and followed-up for the investigation of determinants related to reproduction among Chinese population. Briefly, 200 male partners of couples were recruited and underwent assisted reproductive treatment at the Reproductive Center of Tongji Hospital in Wuhan, China from July 1, 2019 to November 30, 2019. Inclusion criteria were age ≥20 years. Exclusion criteria included a history of occupational exposure to hazardous chemicals, reproductive organ malformations or injuries, a family history of hereditary diseases, autoimmune diseases, and chromosomal karyotype abnormalities. All participants free from male reproductive-related diseases. Additionally, they were required to have complete questionnaire and clinical examination. The study comprised 200 eligible participants primarily residing in urban areas of Wuhan, China (Figure 1). Demographic data were gathered through a uniform and methodically designed questionnaire administered by certified nurses. Approval from the Tongji Medical College Ethics Committee was granted. Informed consent was obtained from all participants.
Figure 1.
Spatial distribution of participants in Wuhan, Hubei province, China.
Sample Collection
Semen samples were collected before the medical or surgical treatments and adhered to the professional guideline, as described as previously.23,24 Specifically, participants were given privacy in a separate room next to the laboratory, where semen sample were gathered by masturbation in a sterile plastic container. The clinical laboratory centrifuged the sample and the seminal sample was gathered into a sterilized cryotube, and then instantly stored using liquid nitrogen at −80 °C for metabolomic examination. We measured four sperm parameters as outcomes, including sperm concentration (106/mL), sperm count (106), progressive motility (%), and total motility (%). The computer-assisted analysis system was used for semen quality test. Sperm density, progressive motility, and nonprogressive motility were measured using a microcell slide and computer-assisted semen analysis. Sperm total motility was defined as the sum of progressive motility and nonprogressive motility. Quantitative analysis was conducted independently in separate laboratory rooms by two skilled technicians following a blinded approach.
The Identification of Seminal Plasma Metabolites
The samples were collected in 2019 and used for metabolomics analysis in 2022. During storage and analysis, the extracts were kept at −80 °C to prevent metabolite degradation.
Since the samples were processed in batches and stored together, the condition of each sample can be ensured to be consistent. To analyze the seminal plasma metabolites, a 100 μL portion of the sample was transferred into an EP tube. Subsequently, 300 μL of an extraction solution containing methanol, along with a mixture of isotopically labeled internal standards, was added. The specimens were vortexed for 30 s and then sonicated for 10 min in an ice–water bath. Afterward, the samples were incubated at −40 °C for 1 h to induce protein precipitation, and then centrifuged the sample at 12 000 rpm for 15 min at 4 °C to extract supernatant fluid and we transferred fluid to a fresh glass vial. A quality control sample was generated by mixing equal amounts of supernatant fluid from all the samples. Internal standards included [RING-2H5]-l-Phenylalanine, 2-Chloro-l-phenylalanine, DiisobutylPhthalate-3,4,5,6-D4, 2-Chloro-l-phenylalanine, Decanoic Acid-d19, and [RING-2H5]-l-Phenylalanine.
Ultraperformance liquid chromatography (UPLC) (Vanquish, Thermo Fisher Scientific) was performed using UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm). Mobile phase for UPLC consisted of a mixture of 5 mmol/L ammonium acetate and 5 mmol/L acetic acid in water (phase A), combined with acetonitrile (phase B). The temperature of the autosampler was set to 4 °C, and sample injection volume was 2 μL. In addition, the Orbitrap Exploris 120 mass spectrometer was employed to perform and collect MS/MS spectra at information-dependent acquisition (IDA) mode for full-scan MS spectrum. Electrospray ionization (ESI) source conditions were set as follows: sheath gas flow rate 50 Arb; auxiliary gas flow rate 15 Arb; capillary temperature 320 °C; full MS resolution 60 000; MS/MS resolution 15 000; collision energy 10/30/60 in NCE mode; spray voltage 3.8 kV (positive) or −3.4 kV (negative).
All liquid chromatography-tandem mass spectrometry (LC-MS) data were processed using ProteoWizard for imputing raw to obtain peak intensities for retention time and m/z metrics.
ProteoWizard software version was 3.0, and parameters were configured as “proteowizard/pwiz-skyline-i-agree-to-the-vendor-licenses wine msconvert --mzXML --filter ‘peakPicking vendor msLevel=1-’ ”.
Annotation of candidate metabolites was performed using an internal MS2 database, BiotreeDB, which incorporated reference spectra and information for known metabolites. Annotation relied on analog scores derived from comparing acquired MS2 spectra with database entries. A threshold of 0.3 was set for annotation, requiring similarity score above this threshold for a successful metabolite annotation. To account for instrument drift, the original peak areas were normalized using specific internal standards for each metabolite class. Additionally, identified metabolites were cross-referenced and annotated using the Human Metabolome Database (HMDB) (https://www.hmdb.ca) or PubChem (https://pubchem.ncbi.nlm.nih.gov/) for further validation. Among the 984 metabolites qualitatively identified through MS/MS matching, 684 can be identified by the HMDB database (69%). We calculate the minimum number of samples required to detect a statistically significant difference between normal and abnormal populations, based on FDR < 0.1. We found that the predictive power can reach 0.8 when sample sizes were 100 per group (Figure S1 of the Supporting Information).
The Assessment of O3 Exposure
We collected daily ambient O3 data at a 10 km × 10 km resolution in Wuhan, Hubei province, China from the Tracking Air Pollution in China (TAP) data sets in 2019. The daily ambient PM2.5 data at a 1 km × 1 km resolution was also retrieved from this data set. These data sets, known as a comprehensive source of China’s air pollutants, offer extensive coverage, near real-time updates, and demonstrate exceptional model performance.25,26 TAP data sets used a machine learning model that predicts full-coverage daily maximum 8-h average O3 concentrations by merging data from multiple sources, including two random forest models and a spatiotemporal Kriging interpolation model.27,28 The accuracy of the O3 estimation was high, exhibiting a strong correlation with direct measurement of daily maximum 8-h averaged O3 (R2 = 0.7) validated via a 5-fold cross-validation.28
The residential address of each subject was geocoded into geographical coordinates of latitude and longitude, which enable alignment with the estimates from the predicted grided ambient O3 data. Given that spermatogenesis spans about 90 days and involves three pivotal stages before ejaculation: epididymal storage (0–9 days), sperm motility development (10–14 days), and the spermatogenesis phase (70–90 days), we assessed the mean O3 levels during the sperm maturation period. Additionally, we determined the mean O3 levels for each critical phase of sperm development for each subject. Beyond O3 concentration, we also gathered data on daily ambient temperature from the nearest monitoring stations provided by China’s meteorological bureau (https://data.cma.cn).
Statistical Analysis
Given the skewed distributions of sperm concentration and count, we performed a log transformation on these metrics prior to regression analysis. We employed a multivariate linear regression model to assess the associations of O3 exposure with semen quality. The model was adjusted for a range of variables identified from previous research,7,15 such as age (under 33 vs 33 and older), body mass index (BMI) (below 24.0, between 24.0 and 28.0, above 28.0 kg/m2), ever having fathered a child (no vs yes), educational level (below college vs college and above), smoking status (no vs yes), monthly income (below 5000, 5001–10 000, over 10 001 CNY), and daily ambient air temperature with 3 degrees of freedom using a natural cubic spline fitting,6 along with the number of days of abstinence (less than 4 vs 4 or more days).
To explore the nonlinear relationship, we modeled restricted cubic spline function for O3 concentration in the regression analysis while controlling for the same set of covariates as in the main models.6,29 We tested the nonlinearity through visual examination and likelihood ratio tests that compares models incorporating nonlinear terms against those using linear terms.30
To select candidate metabolites, a univariate linear regression model was modeled between O3 levels and semen quality. Before conducting the analysis, all metabolite data underwent logarithmic transformation and standardization to z-scores. To control for false positives, Benjamini-Hochberg method was adopted to adjusted p values using the false discovery rate (FDR).31 An FDR < 0.05 was considered as statistically significant.
Subsequently, the Least Absolute Shrinkage and Selection Operator (LASSO) regression was performed to remaining metabolites with FDR < 0.05 to identify metabolites predictive of semen quality.32 LASSO is a regularization technique that identifies key metabolite signals from a highly dimensional and correlated set of predictors.32 To ensure the robustness of results, we employed 10-fold cross-validation to determine the threshold that reduces the error in model predictions to a minimum within 1 standard deviation, utilizing normalized metabolomics data.33 Metabolites chosen through LASSO regression were considered potential mediators.
We conducted mediation analyses using “RMediation” package in the R statistical software platform to assess the potential indirect effects of metabolites. The mediating effects were estimated by multiplying two direct effects: the links between O3 exposure and candidate metabolites, and the links between candidate metabolites and semen parameters. To calculate the direct effects of O3 exposure on these candidate metabolites, we conducted multivariate regression model adjusting for the same covariates as in the main analysis. To estimate the direct effects of candidate metabolites on semen quality, we configured another multivariate regression model that included the O3 term. The proportion of mediating effects was determined as the ratio of mediating effects (i.e., indirect effect in the mediation analysis) to the total effects, representing the extent to which the mediators contribute to the overall association observed.20,34
Two sensitivity analyses were conducted. First, we dichotomized subjects into normal and abnormal participants following standard guidelines.23 For the normal participants, reference metrics including total motility exceeding 40% motile sperms, progressive motility over 32% motile sperms, sperm concentration over 15 × 106/mL, and sperm count above 39 × 106. On the contrary, participants were classified as abnormality if their values were below any of these benchmarks. We then applied logistic regression, adjusting for the identical covariates as in the primary analysis, to assess the links between semen quality categories and specific sperm quality indicators. The normal group served as the reference category in these analyses. Second, we modeled dual-pollutant models with further adjustments for PM2.5. Third, we included the relative humidity in the models to test the association between O3 exposure and semen quality. Forth, due to the small sample size, it is difficult to conduct subgroup analysis. Therefore, we considered dividing the daily ambient temperature into two groups (high vs low temperature) based on the median and including them in the models.
The Receiver Operator Characteristic (ROC) curve analysis was performed to determine the diagnostic efficacy of mediated metabolites for sperm quality.
All statistical analyses were completed on the R software platform (version 4.2.1).
Results
Among 200 male participants, the average age, BMI and abstinence days were 33.4 years (SD: 5.0), 24.6 kg/m2 (SD: 2.9), and 4.6 days (SD: 4.3), respectively. Notably, about 35.0% of them had an income above 10 000 CNY, about 68.5% of them obtained the degree above high school and 48.0% of them were inclined to no smoking. Most participants reported having no children (90.0%) (Table 1). The observed averages for sperm concentration, sperm count, progressive motility, and total motility were 50.3 × 106/mL, 147 × 106 per sample, 39%, and 41.7%, respectively. The O3 concentration noted was 136.0 ± 8.7 μg/m3, and the ambient temperature averaged at 25.8 ± 2.9 °C throughout the duration of the study (Table 2).
Table 1. Demographic Characteristics and Semen Quality of the Study Population (n = 200)a.
| Variables | Overall (n = 200) |
|---|---|
| Characteristics | |
| Age, n (%) | |
| <33 y | 104 (52.0%) |
| ≥33 y | 96 (48.0%) |
| BMI, kg/m2, n (%) | |
| <24 | 82 (41.0%) |
| 24–28 | 93 (46.5%) |
| >28 | 25 (12.5%) |
| Education level, n (%) | |
| University/college or above | 137 (68.5%) |
| Below university/college | 63 (31.5%) |
| Income, RMB, n (%) | |
| <5000 | 61 (30.5%) |
| 5000–10 000 | 69 (34.5%) |
| >10 000 | 70 (35.0%) |
| Smoking status, n (%) | |
| Yes | 96 (48.0%) |
| No | 104 (52.0%) |
| Ever having fathered a child, n (%) | |
| Yes | 20 (10.0%) |
| No | 180 (90.0%) |
| Abstinence time, days, n (%) | |
| <4 | 96 (48.0%) |
| ≥4 | 104 (52.0%) |
| Semen quality | |
| Semen qualityb, n (%) | |
| Normal | 102 (51.0%) |
| Abnormal | 98 (49.0%) |
| Sperm concentration (106/mL), mean ± SD | 50.3 ± 33.7 |
| Sperm count (106), mean ± SD | 147.0 ± 113.0 |
| Total motility (%), mean ± SD | 41.7 ± 16.4 |
| Progressive motility (%), mean ± SD | 39.3 ± 16.1 |
Abbreviations: RMB = Renminbi; BMI = Body Mass Index; SD = Standard deviation.
The binary semen quality is based on World Health Organization reference. Abnormal sperm quality was classified as being below the World Health Organization reference values: sperm concentration (15 × 106/mL), total sperm count (39 × 106/sample), progressive motility (32% motile sperm), and total motility (40% motile sperm).
Table 2. Descriptive Statistics on the Concentrations of O3 and Ambient Temperature Across Three Critical Sperm Development Periodsa.
| Exposure | Critical period | Mean ± SD | Min | Median | Max | IQR |
|---|---|---|---|---|---|---|
| O3 (μg/m3) | 0–9 day | 126.2 ± 37.5 | 39.5 | 132.4 | 193.8 | 50.5 |
| O3 (μg/m3) | 10–14 day | 129.4 ± 34.8 | 51.2 | 127.1 | 215 | 41.7 |
| O3 (μg/m3) | 70–90 day | 137.5 ± 15.3 | 109.8 | 136.1 | 176.5 | 25.6 |
| O3 (μg/m3) | 0–90 day | 136.0 ± 8.7 | 110 | 136.6 | 155.8 | 10.5 |
| Ambient temperature (°C) | 0–9 day | 23.5 ± 6.7 | 9.2 | 25.8 | 32.4 | 12.8 |
| Ambient temperature (°C) | 10–14 day | 24.3 ± 6.0 | 12.2 | 25.4 | 33.1 | 11.5 |
| Ambient temperature (°C) | 70–90 day | 26.1 ± 3.8 | 17.7 | 26.7 | 31.6 | 4.8 |
| Ambient temperature (°C) | 0–90 day | 25.8 ± 2.9 | 19.1 | 26.8 | 28.8 | 4.7 |
Abbreviations: IQR = interquartile ranges; O3= ozone; and SD = standard deviation.
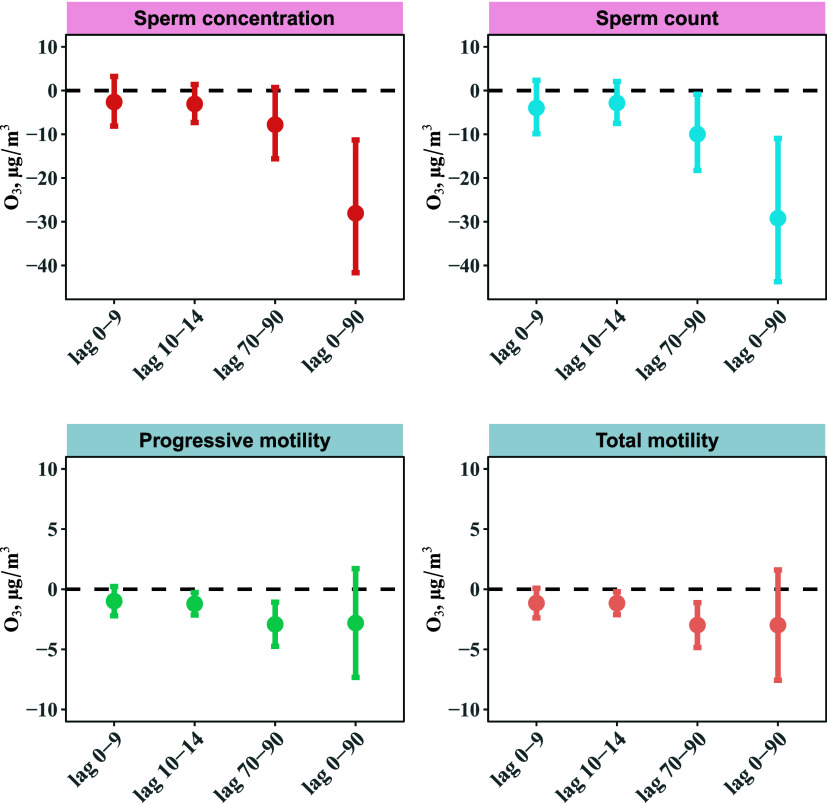
Exposure to O3 was associated with decreased sperm quality parameters (Figure 2). A 10 μg/m3 increase in O3 concentration was linked with declines in various sperm quality parameters. Specifically, during the entire sperm development (0–90 days before semen collection), there was a decrease of −28.06% (95% CI: −41.65%, −11.32%) in sperm concentration, −29.20% (95% CI: −43.71%, −10.95%) in sperm count, −2.98% (95% CI: −7.57%, 1.61%) in sperm total motility, and −2.81% (95% CI: −7.33%, 1.71%) in sperm progressive motility.
Figure 2.
Percent Changes (95% CI) in semen quality parameters associated with a 10 μg/m3 increase in O3 in Chinese adult men. Abbreviation: O3 = ozone. Models were adjusted for age (<33 versus ≥33 years), BMI (<24.0, 24.0–28.0, > 28.0 kg/m2), ever having fathered a child (yes versus no), education (below college or university, and college or university above), smoking status (no versus yes), household income (≤5000, 5001–10 000, ≥ 10 001 RMB), daily ambient temperature using a natural cubic spline with 3 degrees of freedom, and abstinence time (<4 versus ≥4 days).
The exposure window most susceptible to O3 was during the spermatogenesis period. For example, for each 10 μg/m3 increase in O3 concentration, there was an associated decline of −1.21% (95% CI: −2.15%, −0.27%) in sperm total motility during sperm motility development period compared to a decline of −2.92% (95% CI: −4.75%, −1.08%) during the spermatogenesis period.
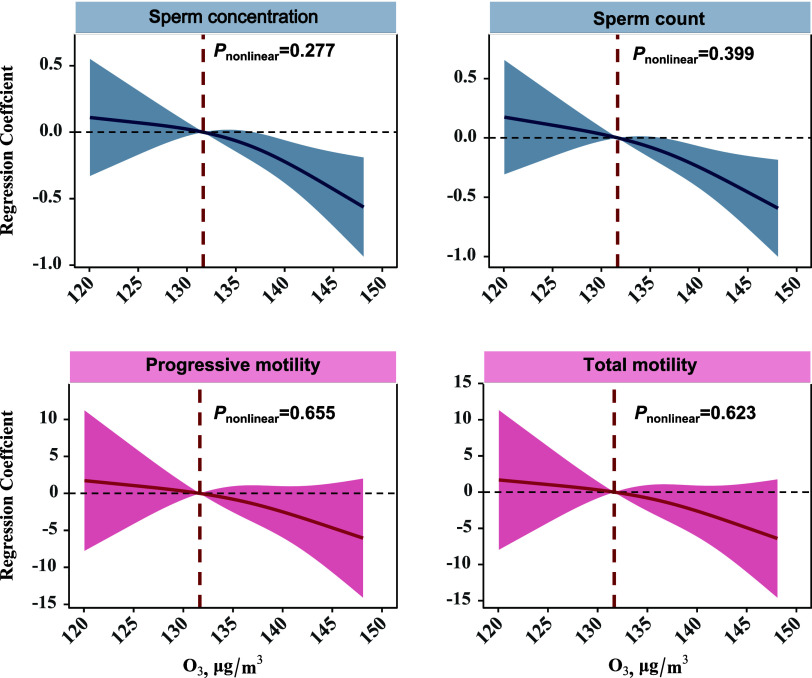
Using a restricted cubic spline function for O3 exposure, we observed an approximate downward linear relationship of sperm concentration, sperm count, and sperm progressive and total motility associated with O3 exposure (Figure 3).
Figure 3.
Adjusted restricted cubic splines for the association of 90-day average O3 concentration with semen quality parameters. Abbreviation: O3=ozone. Models were adjusted for age (<33 versus ≥33 years), BMI (<24.0, 24.0–28.0, > 28.0 kg/m2), ever having fathered a child (yes versus no), education (below college or university, and college or university above), smoking status (no versus yes), household income (≤5000, 5001–10 000, ≥ 10 001 RMB), daily ambient temperature using a natural cubic spline with 3 degrees of freedom, and abstinence time (<4 versus ≥4 days).
To select candidate metabolites, we used univariate regression and LASSO regression with 10-fold cross-validation (Figure S1). We found that 94 metabolites were significantly associated with sperm concentration (FDH < 0.05), and 9 metabolites were retained after LASSO screening. We identified 45 metabolites significantly associated with sperm count (FDH < 0.05), and 11 metabolites were retained after LASSO screening. We detected 17 metabolites associated with sperm total motility (FDH < 0.05), and 9 metabolites were retained after LASSO screening. We screened 16 metabolites associated with sperm progressive motility (FDH < 0.05), and 11 metabolites were retained after LASSO screening. Thus, the candidate metabolites for mediation analysis were 9 for sperm concentration, 11 for sperm count, 9 for sperm total motility, and 9 for sperm progressive motility.
The mediation analyses revealed that 7 metabolites played a negative regulatory role in the association between O3 exposure and semen quality (Table 3). Myristoleic acid acted as a significant mediator in the association between O3 exposure and sperm concentration across the entire period of sperm development. For sperm count, asparyl-isoleucine and phenylethyl primeveroside were identified as mediators during the periods of spermatogenesis and the entire sperm development. For sperm progressive motility, the mediated metabolites included ACar (18:2) and ACar (18:1) during the periods of sperm motility development and spermatogenesis, and FAHFA (22:6/22:3), LPS (22:5), aspartyl-isoleucine, and myristoleic acid were observed as mediators during the period of spermatogenesis. Similarly, for total motility, ACar (18:2) was identified as a mediator during periods of sperm motility development and spermatogenesis, and FAHFA (22:6/22:3), LPS (22:5), myristoleic acid, and aspartyl-isoleucine during spermatogenesis period (Table 3). The most pronounced mediation proportion was observed with myristoleic acid, which mediated proportion of 46.4% for the association between O3 exposure and sperm concentration. We found the correlation between metabolites was minimal (Figure S2).
Table 3. Mediated Proportion of Semen Metabolites on the Associations between O3 Exposure and Semen Qualitya,b.
| Total effect | Direct effect Ac | Direct effect Bd | Mediation effect | ||||
|---|---|---|---|---|---|---|---|
| Sperm parameters | Critical period | Metabolites | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | Mmediated proportion |
| Sperm concentration | 0–90 days | myristoleic acid | –0.28 (−0.42, −0.11) | –0.49 (−0.76, −0.21) | 0.27 (0.17, 0.37) | –0.13 (−0.23, −0.05) | 0.464 |
| Sperm count | 70–90 days | aspartyl-isoleucine | –0.10 (−0.18, −0.01) | 0.12 (0.001, 0.23) | –0.38 (−0.48, −0.27) | –0.04 (−0.09, −0.001) | 0.400 |
| 0–90 days | phenylethyl primeveroside | –0.29 (−0.44, −0.11) | 0.29 (0.01, 0.57) | –0.22 (−0.33, −0.11) | –0.06 (−0.14, −0.001) | 0.207 | |
| Progressive motility | 10–14 days | ACar (18:2) | –1.21 (−2.15, −0.28) | –0.07 (−0.13, −0.02) | 3.29 (0.93, 5.64) | –0.24 (−0.55, −0.03) | 0.198 |
| 10–14 days | ACar (18:1) | –1.21 (−2.15, −0.28) | –0.08 (−0.13, −0.02) | 5.15 (2.84, 7.46) | –0.41 (−0.79, −0.11) | 0.339 | |
| 70–90 days | ACar (18:2) | –2.92 (−4.75, −1.08) | –0.12 (−0.23, −0.01) | 3.21 (0.82, 5.59) | –0.39 (−0.94, −0.01) | 0.134 | |
| 70–90 days | FAHFA (22:6/22:3) | –2.92 (−4.75, −1.08) | –0.15 (−0.27, −0.04) | 4.01 (1.78, 6.24) | –0.61 (−1.28, −0.12) | 0.209 | |
| 70–90 days | ACar (18:1) | –2.92 (−4.75, −1.08) | –0.12 (−0.23, −0.01) | 5.07 (2.77, 7.38) | –0.60 (−1.30, −0.04) | 0.205 | |
| 70–90 days | LPS (22:5) | –2.92 (−4.75, −1.08) | –0.16 (−0.27, −0.05) | 3.24 (0.88, 5.59) | –0.52 (−1.14, −0.08) | 0.178 | |
| 70–90 days | aspartyl-isoleucine | –2.92 (−4.75, −1.08) | 0.12 (0.001, 0.23) | –5.00 (−7.15, −2.86) | –0.59 (−1.30, −0.01) | 0.202 | |
| 70–90 days | myristoleic acid | –2.92 (−4.75, −1.08) | –0.12 (−0.23, −0.01) | 3.04 (0.67, 5.40) | –0.35 (−0.89, −0.001) | 0.120 | |
| Total motility | 10–14 days | ACar (18:2) | –1.16 (−2.11, −0.21) | –0.07 (−0.13, −0.02) | 3.34 (0.94, 5.74) | –0.25 (−0.56, −0.03) | 0.216 |
| 70–90 days | FAHFA (22:6/22:3) | –2.98 (−4.84, −1.12) | –0.15 (−0.27, −0.04) | 4.10 (1.84, 6.37) | –0.62 (−1.30, −0.12) | 0.208 | |
| 70–90 days | ACar (18:2) | –2.98 (−4.84, −1.12) | –0.12 (−0.23, −0.01) | 3.22 (0.80, 5.64) | –0.39 (−0.95, −0.01) | 0.131 | |
| 70–90 days | LPS (22:5) | –2.98 (−4.84, −1.12) | –0.16 (−0.27, −0.05) | 3.42 (1.03, 5.81) | –0.55 (−1.19, −0.09) | 0.185 | |
| 70–90 days | myristoleic acid | –2.98 (−4.84, −1.12) | –0.12 (−0.23, −0.01) | 3.22 (0.82, 5.62) | –0.38 (−0.93, −0.01) | 0.128 | |
| 70–90 days | aspartyl-isoleucine | –2.98 (−4.84, −1.12) | 0.12 (0.001, 0.23) | –5.26 (−7.43, −3.08) | –0.62 (−1.36, −0.01) | 0.208 |
Abbreviation: O3=ozone; CI = confidence interval
Models were adjusted for age (<33 versus ≥33 years), BMI (<24.0, 24.0–28.0, > 28.0 kg/m2), ever having fathered a child (yes versus no), education (below college or university, and college or university above), smoking status (no and yes), household income (≤5000, 5001–10,000, ≥ 10,001 RMB), daily ambient temperature using a natural cubic spline with 3 degrees of freedom, and abstinence days (<4 days versus ≥4 days).
Indicates the coefficient of O3 exposure on metabolites.
Indicates the coefficient of metabolites exposure on semen quality.
In our sensitivity analyses, we found that exposure to O3 increased the risk of sperm abnormality specifically during spermatogenesis period (Table S1). Additionally, after adjusting for ambient PM2.5 exposure and relative humidity, respectively, our results were not materially different (Tables S2 and S3). Likewise, the associations were not significantly various when daily ambient temperature was included as a binary variable (Table S4). The Area Under the Curve (AUC) of metabolites for semen quality ranged from 0.67 to 0.71 (Table S5).
Discussion
The seminal plasma metabolome provided a comprehensive profile of the sperm microenvironment in response to exposure to air pollutants.35 In our cross-sectional analysis of 200 Chinese adults, we found an association between O3 exposure and a reduction in sperm concentration, sperm count, sperm total motility, and sperm progressive motility across critical periods of sperm development. In addition, we identified 7 seminal plasma metabolites that mediated the association between O3 exposure and the decline in sperm parameters. These metabolites include myristoleic acid, aspartyl-isoleucine, phenylethyl primeveroside, ACar (18:2), ACar (18:1), FAHFA (22:6/22:3), and LPS (22:5), with myristoleic acid showing the highest mediation proportion throughout the entire sperm development.
We found that O3 exposure was associated with a reduction in sperm concentration and sperm count during the entire sperm development. This finding aligns with a growing body of studies indicating O3 is an environmental risk factor for semen quality.7,9,12 In a multicenter study with a large cohort of 33 234 male participants in China, Cai et al. reported that exposure to O3 was associated with reduced sperm concentration.7 Similarly, a retrospective study involving 1068 sperm donors conducted by Lu et al. found adverse effects of O3 exposure on sperm concentration and sperm count during the period of sperm development.9 However, our study did not find a significant association between O3 exposure and sperm progressive and total motility. Our findings were corroborated by a recent comprehensive meta-analysis consisting of 11 studies, conducted by Xu et al., which reported that the pooled effects of O3 exposure were not linked to a reduction in total and progressive motility during sperm development. This conclusion contrasts with some previous studies that reported a positive association between O3 exposure and sperm motility.12 The observed disparities in findings could be attributed to differences in O3 sources and the characteristics of the study populations.
Despite we found no evidence of an association between O3 exposure and sperm motility during the entire period of sperm development, we found that exposure to O3 was linked to decreased sperm progressive and total motility in the periods of spermatogenesis and sperm motility development. Limited and inconclusive evidence exists regarding the critical exposure windows for the association between O3 exposure and sperm motility.7,9,36,37 For example, Cai et al. reported an association between O3 exposure and an increase in total motility during spermatogenesis period,7 and Lu et al. found that O3 exposure improved sperm total and progressive motility during spermatogenesis period.9 Conversely, two retrospective cohort studies by Huang et al. (2020) and Zhang et al. (2019) found no evidence of an association between O3 exposure and a decrease in sperm motility in the early stage of sperm development.36,37 The discrepancies in results could arise from variations in study design, study populations and sample sizes, and distinct approaches to exposure assessment.7,9,36,37
In our mediation analysis, we reported that exposure to O3 reduced semen quality by changing levels of seminal plasma metabolites associated with fatty acid metabolism. For example, an increase in myristoleic acid was found to promote sperm concentration, and O3 exposure appeared to disrupt this process. This observation aligns with a case-control study conducted in Italy, where significant differences in levels of myristoleic acid were found between oligoasthenoteratozoospermic and asthenozoospermic groups.38 Myristoleic acid, a fatty acid present in certain plant oils, is commonly used as a dietary supplement to maintain normal cholesterol levels and reduce inflammation.39,40 Therefore, the potential mechanism underlying our findings suggests that O3 exposure may induce an inflammatory reaction, disrupting the pathway associated with myristoleic acid metabolism and subsequently impacting sperm concentration.
In addition to myristoleic acid, we also found that ACar (18:2) and ACar (18:1), two unsaturated fatty acid, were associated with a reduction in progressive motility and total motility. Acylcarnitine (ACar), one of the acetyl derivatives of l-carnitine, plays a pivotal role in the β-oxidation of long-chain fatty acids across the inner mitochondrial membrane.41,42 The accumulation of ACar is regarded as an indicator of dysregulation in fatty acid oxidation, reflecting changes in mitochondrial metabolism.43 Such metabolic disruption in the mitochondria can result in inadequate energy production, ultimately leading to reduced sperm motility and impaired male fertility.44 Additionally, exposure to O3 could intervene mitochondrial bioenergetics.45
Branched fatty acid esters of hydroxy fatty acids (FAHFAs) are a new class of lipid molecules, and their specific function and mechanism on semen quality remains largely unknown. However, considering the established role of FAHFAs in regulating metabolism and inflammatory responses, it can be speculated that they may play a role in influencing the metabolic state and functionality of sperm, particularly in aspects related to sperm maturation and motility.46
In addition to metabolites associated with fatty acid metabolism, our study also found the involvement of other metabolites in regulating the association between O3 exposure and a decrease in semen quality. For example, aspartyl-isoleucine is a dipeptide composed of aspartate and isoleucine, which is linked to the declined sperm count, progressive motility and total motility associated with O3 exposure in our analyses. Although considered as intermediates in specific amino acid degradation pathways, the function of aspartyl-isoleucine has not been thoroughly studied.47 Additionally, we found that phenylethyl primeveroside could alleviate the adverse effects of O3 exposure on sperm count. Phenylethyl primeveroside is commonly identified in tea and is recognized as beneficial supplement for improved semen quality.48,49 Moreover, Lysophosphatidylserines (LPS) (22:5) were associated with the decline in sperm progressive and total motility associated with O3 exposure. LPS is involved in glycerophospholipid metabolism and has been identified in seminal plasma metabolome studies.50,51 In summary, our study identified a series of novel metabolites enriched in crucial signaling pathways, mediating the impact of O3 exposure on semen quality. However, the specific mechanisms underlying these associations warrant further confirmation through additional studies.
This study has several limitations. First, O3 exposure data was obtained from 10 km × 10 km gridded data in TAP. This relatively crude resolution may reduce the precision of exposure assessment and lead to exposure misclassification. Second, our sample size was confined to a total of 200 adult men, which might impact the generalizability and robustness of our findings. Third, the seminal plasma specimens for metabolomic assessment were obtained only once, which might not adequately reflect the variation in metabolite concentrations over time. Fourth, this is a cross-sectional study, and we cannot confirm the causal association between O3 exposure, seminal plasma metabolites, and sperm quality. Fifth, this study has a small sample size, which makes it impossible to conduct subgroup analyses of important risk factors, including different age groups and smoking status.
Conclusions
In an analysis of seminal plasma metabolomes with 200 adult males, we found that O3 exposure was associated with a reduction in sperm concentration and sperm count during the entire course of sperm development, and these associations were mediated by myristoleic acid, aspartyl-isoleucine, and phenylethyl primeveroside. We also found that exposure to O3 reduced sperm motility during the periods of sperm motility development and spermatogenesis by altering levels of certain fatty acid and other metabolites, such as myristoleic acid. While our findings provide valuable insights into the adverse effects of O3 exposure on semen quality, future studies are recommended to delve deeper into the pathogenesis underlying these associations.
Acknowledgments
The TREE Study Team refers to the research group behind the Tongji Reproductive and Environmental (TREE) cohort, a prospective cohort study based at the Reproductive Medicine Center of Tongji Hospital in Wuhan, Hubei Province. This cohort focuses on couples who visit the center for the first time to undergo assisted reproductive technology, aiming to study the impacts of lifestyle and environmental pollutant exposure on reproductive health and their mechanisms. The TREE Study Team played a crucial role in the research described in the manuscript. The team was responsible for the design and implementation of the cohort study, data collection, analysis, and interpretation, as well as drafting and revising the manuscript. Their collective expertise in reproductive medicine, environmental health, and epidemiology was essential to the success of this research. We thank those members who participated in the TREE study team, including Wen-Qing Lu, Yu-Feng Li, Qiong Luo, Xiao-Qiong Yuan, Pan-Pan Chen, Fei-Peng Cui, Tian Shi, Ting–Ting Lu, Yang-Cheng Yao, Lin-Jing Wu, Hua-Hua Jiang, Qing-Yun Yao, Dan-Yu Qin, Wen Yao, Yong Huang, Ni-Jie Li, Wen-Tao Rao, Yu-Ying Li, Hong-Mei Liao, and Qin-Ling Tian, as well as the doctors and nurses from the hospital. We also thank all the study participants for providing the biological samples for analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/envhealth.4c00066.
Sensitivity analysis between O3 exposure and semen quality; power analysis of metabolites; metabolites selection methods based on LASSO; and correlation analysis of candidate metabolites (DOCX)
The authors declare no competing financial interest.
Supplementary Material
References
- Agarwal A.; Mulgund A.; Hamada A.; Chyatte M. R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Meng T.; Wu L.; Duan Y.; Li G.; Shi C.; et al. Association between ambient temperature and semen quality: A longitudinal study of 10 802 men in China. Environ. Int. 2020, 135, 105364. 10.1016/j.envint.2019.105364. [DOI] [PubMed] [Google Scholar]
- Zhou N.; Jiang C.; Chen Q.; Yang H.; Wang X.; Zou P.; et al. Exposures to Atmospheric PM10 and PM10–2.5 Affect Male Semen Quality: Results of MARHCS Study. Environ. Sci. Technol. 2018, 52 (3), 1571–1581. 10.1021/acs.est.7b05206. [DOI] [PubMed] [Google Scholar]
- Carvalho T. C.; Peters J. I.; Williams R. O. 3rd Influence of particle size on regional lung deposition-what evidence is there?. Int. J. Pharm. 2011, 406 (1–2), 1–10. 10.1016/j.ijpharm.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Zhu Q.; Lin J.; Cai J. Association of Exposure to Particulate Matter Air Pollution With Semen Quality Among Men in China. JAMA Netw Open 2022, 5 (2), e2148684 10.1001/jamanetworkopen.2021.48684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Xu T.; Wang Q.; Ni H.; Yu X.; Song C.; et al. Exposure to Fine Particulate Matter Constituents and Human Semen Quality Decline: A Multicenter Study. Environ. Sci. Technol. 2023, 57 (35), 13025–13035. 10.1021/acs.est.3c03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X.; Ni H.; Wang Q.; Dai T.; Wang L.; Song C.; et al. Sperm quality decline associated with gaseous pollutant exposure: Evidence from a large cohort multicenter study. J. Hazard Mater. 2023, 460, 132330. 10.1016/j.jhazmat.2023.132330. [DOI] [PubMed] [Google Scholar]
- Orru H.; Ebi K.; Forsberg B. The interplay of climate change and air pollution on health. Current environmental health reports 2017, 4, 504–513. 10.1007/s40572-017-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z. H.; Sun B.; Wang Y. X.; Wu Y. R.; Chen Y. J.; Sun S. Z.; et al. Ozone exposure associates with sperm quality indicators: Sperm telomere length as a potential mediating factor. J. Hazard Mater. 2023, 459, 132292. 10.1016/j.jhazmat.2023.132292. [DOI] [PubMed] [Google Scholar]
- Xing Z.; Yang T.; Shi S.; Meng X.; Chai D.; Liu W.; et al. Combined effect of ozone and household air pollution on COPD in people aged less than 50 years old. Thorax 2024, 79 (1), 35–42. 10.1136/thorax-2022-219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Zhu Y.; Zhao B.; Wan W.; Shi S.; Xuan C.; et al. Long-term cardiometabolic effects of ambient ozone pollution in a large Chinese population. Ecotoxicol Environ. Saf 2023, 261, 115115. 10.1016/j.ecoenv.2023.115115. [DOI] [PubMed] [Google Scholar]
- Xu R.; Zhong Y.; Li R.; Li Y.; Zhong Z.; Liu T.; et al. Association between exposure to ambient air pollution and semen quality: A systematic review and meta-analysis. Sci. Total Environ. 2023, 870, 161892. 10.1016/j.scitotenv.2023.161892. [DOI] [PubMed] [Google Scholar]
- Qiu Y.; Yang T.; Seyler B. C.; Wang X.; Wang Y.; Jiang M.; et al. Ambient air pollution and male fecundity: A retrospective analysis of longitudinal data from a Chinese human sperm bank (2013–2018). Environ. Res. 2020, 186, 109528. 10.1016/j.envres.2020.109528. [DOI] [PubMed] [Google Scholar]
- Sokol R. Z.; Kraft P.; Fowler I. M.; Mamet R.; Kim E.; Berhane K. T. Exposure to environmental ozone alters semen quality. Environ. Health Perspect 2006, 114 (3), 360–5. 10.1289/ehp.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.; Zhao J.; Cao W.; Lu W.; Zheng T.; Zeng Q. Identifying critical exposure windows for ambient air pollution and semen quality in Chinese men. Environ. Res. 2020, 189, 109894. 10.1016/j.envres.2020.109894. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Jiang F.; Chen Q.; Yang H.; Zhou N.; Sun L.; et al. Associations of ambient air pollutant exposure with seminal plasma MDA, sperm mtDNA copy number, and mtDNA integrity. Environ. Int. 2020, 136, 105483. 10.1016/j.envint.2020.105483. [DOI] [PubMed] [Google Scholar]
- Liu X.; Ai Y.; Xiao M.; Wang C.; Shu Z.; Yin J.; et al. PM2.5 juvenile exposure-induced spermatogenesis dysfunction by triggering testes ferroptosis and antioxidative vitamins intervention in adult male rats. Environ. Sci. Pollut Res. Int. 2023, 30 (51), 111051–111061. 10.1007/s11356-023-30150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J.; Kim S. H.; Kim J. H.; Hwang S.; Yoo H. J. Understanding Metabolomics in Biomedical Research. Endocrinol Metab (Seoul) 2016, 31 (1), 7–16. 10.3803/EnM.2016.31.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. X.; Wu Y.; Chen H. G.; Duan P.; Wang L.; Shen H. Q.; et al. Seminal plasma metabolome in relation to semen quality and urinary phthalate metabolites among Chinese adult men. Environ. Int. 2019, 129, 354–363. 10.1016/j.envint.2019.05.043. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Shi W.; Zhang M.; Xu L.; Wu L.; Li C.; et al. Exposure to PM2.5, seminal plasma metabolome, and semen quality among Chinese adult men: Association and potential mediation analyses. J. Hazard Mater. 2024, 461, 132602. 10.1016/j.jhazmat.2023.132602. [DOI] [PubMed] [Google Scholar]
- Liu C.; Deng Y. L.; Yuan X. Q.; Chen P. P.; Miao Y.; Luo Q.; et al. Exposure to disinfection by-products and reproductive hormones among women: Results from the Tongji Reproductive and Environmental (TREE) study. Environ. Res. 2022, 209, 112863. 10.1016/j.envres.2022.112863. [DOI] [PubMed] [Google Scholar]
- Deng Y. L.; Luo Q.; Liu C.; Zeng J. Y.; Lu T. T.; Shi T.; et al. Urinary biomarkers of exposure to drinking water disinfection byproducts and ovarian reserve: A cross-sectional study in China. J. Hazard Mater. 2022, 421, 126683. 10.1016/j.jhazmat.2021.126683. [DOI] [PubMed] [Google Scholar]
- WHO . Laboratory manual for the examination and processing of human semen. World Health Organization, 2010.
- Yang P.; Chen D.; Wang Y. X.; Zhang L.; Huang L. L.; Lu W. Q.; et al. Mediation of association between polycyclic aromatic hydrocarbon exposure and semen quality by spermatogenesis-related microRNAs: A pilot study in an infertility clinic. J. Hazard Mater. 2020, 384, 121431. 10.1016/j.jhazmat.2019.121431. [DOI] [PubMed] [Google Scholar]
- Xiao Q.; Geng G.; Liu S.; Liu J.; Meng X.; Zhang Q. Spatiotemporal continuous estimates of daily 1 km PM2.5 from 2000 to present under the Tracking Air Pollution in China (TAP) framework. Atmos. Chem. Phys. 2022, 22 (19), 13229–13242. 10.5194/acp-22-13229-2022. [DOI] [Google Scholar]
- Geng G.; Xiao Q.; Liu S.; Liu X.; Cheng J.; Zheng Y.; et al. Tracking Air Pollution in China: Near Real-Time PM2.5 Retrievals from Multisource Data Fusion. Environ. Sci. Technol. 2021, 55 (17), 12106–12115. 10.1021/acs.est.1c01863. [DOI] [PubMed] [Google Scholar]
- Xiao Q.; Geng G.; Xue T.; Liu S.; Cai C.; He K.; et al. Tracking PM2.5 and O3 Pollution and the Related Health Burden in China 2013–2020. Environ. Sci. Technol. 2022, 56 (11), 6922–6932. 10.1021/acs.est.1c04548. [DOI] [PubMed] [Google Scholar]
- Xue T.; Zheng Y.; Geng G.; Xiao Q.; Meng X.; Wang M.; et al. Estimating Spatiotemporal Variation in Ambient Ozone Exposure during 2013–2017 Using a Data-Fusion Model. Environ. Sci. Technol. 2020, 54 (23), 14877–14888. 10.1021/acs.est.0c03098. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Wei J.; Liu C.; Cao W.; Zhang Z.; Li Y.; et al. Association between ambient PM1 and semen quality: A cross-sectional study of 27,854 men in China. Environ. Int. 2023, 175, 107919. 10.1016/j.envint.2023.107919. [DOI] [PubMed] [Google Scholar]
- Beelen R.; Raaschou-Nielsen O.; Stafoggia M.; Andersen Z. J.; Weinmayr G.; Hoffmann B.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014, 383 (9919), 785–95. 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- Glickman M. E.; Rao S. R.; Schultz M. R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin Epidemiol 2014, 67 (8), 850–7. 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression Shrinkage and Selection Via the Lasso. Journal of the Royal Statistical Society: Series B (Methodological) 1996, 58 (1), 267–288. 10.1111/j.2517-6161.1996.tb02080.x. [DOI] [Google Scholar]
- Stone M.Cross-validatory choice and assessment of statistical predictions. J. R. Stat. Soc. B 1974, 36. [Google Scholar]
- Albert J. M. Mediation analysis via potential outcomes models. Stat Med. 2008, 27 (8), 1282–304. 10.1002/sim.3016. [DOI] [PubMed] [Google Scholar]
- Yang W.; Hua R.; Cao Y.; He X. A metabolomic perspective on the mechanisms by which environmental pollutants and lifestyle lead to male infertility. Andrology 2023, 12 (4), 719–739. 10.1111/andr.13530. [DOI] [PubMed] [Google Scholar]
- Zhang H. T.; Zhang Z.; Cao J.; Tang W. H.; Zhang H. L.; Hong K.; et al. Ambient ozone pollution is associated with decreased semen quality: longitudinal analysis of 8945 semen samples from 2015 to 2018 and during pollution-control period in Beijing, China. Asian J. Androl 2019, 21 (5), 501–507. 10.4103/aja.aja_116_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G.; Zhang Q.; Wu H.; Wang Q.; Chen Y.; Guo P.; et al. Sperm quality and ambient air pollution exposure: A retrospective, cohort study in a Southern province of China. Environ. Res. 2020, 188, 109756. 10.1016/j.envres.2020.109756. [DOI] [PubMed] [Google Scholar]
- Zerbinati C.; Caponecchia L.; Rago R.; Leoncini E.; Bottaccioli A. G.; Ciacciarelli M.; et al. Fatty acids profiling reveals potential candidate markers of semen quality. Andrology 2016, 4 (6), 1094–1101. 10.1111/andr.12236. [DOI] [PubMed] [Google Scholar]
- HMDB Showing metabocard for Myristoleic acid (HMDB0002000). https://hmdb.ca/metabolites/HMDB0002000.
- Zhang H.; Wang C.; Sun H.; Zhou T.; Ma C.; Han X.; et al. Glutamine supplementation alleviated aortic atherosclerosis in mice model and in vitro. Proteomics 2023, 24 (5), e2300179 10.1002/pmic.202300179. [DOI] [PubMed] [Google Scholar]
- Bieber L. L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–83. 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- Steiber A.; Kerner J.; Hoppel C. L. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004, 25 (5–6), 455–73. 10.1016/j.mam.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Koves T. R.; Ussher J. R.; Noland R. C.; Slentz D.; Mosedale M.; Ilkayeva O.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008, 7 (1), 45–56. 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Amaral A.; Castillo J.; Estanyol J. M.; Ballesca J. L.; Ramalho-Santos J.; Oliva R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell Proteomics 2013, 12 (2), 330–42. 10.1074/mcp.M112.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez M. C.; Freeborn D.; Valdez J. M.; Johnstone A. F.M.; Snow S. J.; Tennant A. H.; Kodavanti U. P.; Kodavanti P. R. S. Mitochondrial Bioenergetics in Brain Following Ozone Exposure in Rats Maintained on Coconut, Fish and Olive Oil-Rich Diets. Int. J. Mol. Sci. 2019, 20 (24), 6303. 10.3390/ijms20246303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejchova K.; Balas L.; Paluchova V.; Brezinova M.; Durand T.; Kuda O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 101053. 10.1016/j.plipres.2020.101053. [DOI] [PubMed] [Google Scholar]
- HMDB Showing metabocard for Aspartyl-Isoleucine (HMDB0028756). https://hmdb.ca/metabolites/HMDB0028756.
- Liu X. R.; Wang X. L.; Zhao J.; Hu C. H.; Cao N. N.; Chen H. G.; Sun B.; Wang Y. X.; Xiong C. L.; Deng J.; Duan P.; et al. Association between tea consumption and semen quality among 1385 healthy Chinese men. Chemosphere 2022, 303 (2), 135140. 10.1016/j.chemosphere.2022.135140. [DOI] [PubMed] [Google Scholar]
- HMDB Showing metabocard for Phenylethyl primeveroside (HMDB0041274). https://hmdb.ca/metabolites/HMDB0041274.
- Guo Y.; Li J.; Hao F.; Yang Y.; Yang H.; Chang Q.; Kong P.; Liu W.; Jiao X.; Teng X.; et al. A new perspective on semen quality of aged male: The characteristics of metabolomics and proteomics. Front Endocrinol. 2023, 13, 1058250. 10.3389/fendo.2022.1058250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.; Liu L.; Wu Y.; Wang X.; Luo L.; Nan B.; et al. Seminal plasma metabolites mediate the associations of multiple environmental pollutants with semen quality in Chinese men. Environ. Int. 2019, 132, 105066. 10.1016/j.envint.2019.105066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.