Abstract
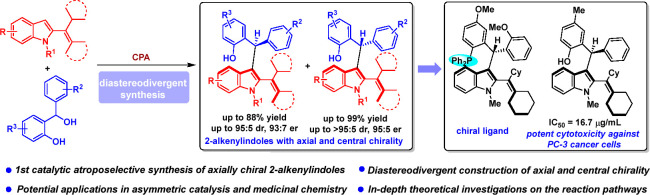
The catalytic asymmetric diastereodivergent synthesis of axially chiral 2-alkenylindoles was established via chiral phosphoric acid-catalyzed addition reactions of C3-unsubstituted 2-alkenylindoles with o-hydroxybenzyl alcohols under different reaction conditions. Using this strategy, two series of 2-alkenylindoles bearing both axial and central chirality were synthesized in a diastereodivergent fashion with moderate to high yields and good stereoselectivities (up to 99% yield, 95:5 er, >95:5 dr). Moreover, theoretical calculations were performed on the key transition states leading to different stereoisomers, which provided an in-depth understanding of the origin of the observed stereoselectivity and diastereodivergence of the products under different reaction conditions. More importantly, these 2-alkenylindoles were utilized in asymmetric catalysis as chiral organocatalysts and in medicinal chemistry for evaluation of their cytotoxicity, which demonstrated their potential applications. This study has not only established the catalytic atroposelective synthesis of axially chiral 2-alkenylindoles, but also provided an efficient strategy for catalytic asymmetric diastereodivergent construction of indole-based scaffolds bearing both axial and central chirality.
Keywords: 2-Alkenylindole, Axial chirality, Central chirality, Diastereodivergent synthesis, Chiral phosphoric acid
Introduction
Chiral compounds bearing multiple stereogenic centers are widely found in pharmaceuticals and materials.1,2 The absolute and relative configurations of these compounds have an impact on their physiological or pharmacological properties.3,4 Therefore, developing efficient strategies to access all stereoisomers of chiral compounds bearing multiple stereocenters is crucial.
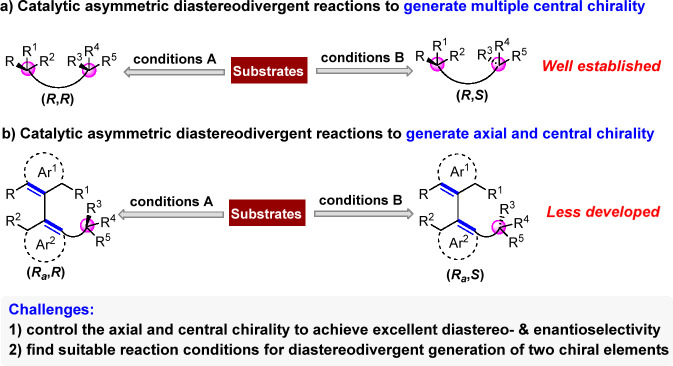
Catalytic asymmetric diastereodivergent reactions have recently been recognized as a class of powerful methods for synthesizing each stereoisomer of chiral compounds using the same set of starting materials by slightly modulating the reaction conditions.5−10 Thus, developing catalytic asymmetric diastereodivergent reactions has attracted intensive attention from the scientific community.11−28 Among them, catalytic asymmetric diastereodivergent reactions for the synthesis of chiral compounds bearing multiple central chirality have been well-developed (Scheme 1a).17−28 However, in stark contrast, catalytic asymmetric diastereodivergent reactions for the synthesis of chiral compounds bearing both axial and central chirality remain largely unexplored (Scheme 1b)29,30 in spite of the importance of atropisomers bearing multiple chiral elements.31 The challenges in realizing such diastereodivergent reactions mainly include: 1) simultaneously controlling the axial chirality and central chirality to achieve excellent diastereoselectivity and enantioselectivity; 2) finding suitable reaction conditions for diastereodivergent generation of two chiral elements. Therefore, it is highly desired to develop efficient strategies toward settling these challenges and realize catalytic asymmetric diastereodivergent reactions for the synthesis of chiral compounds bearing both axial and central chirality.
Scheme 1. Profile of Catalytic Asymmetric Diastereodivergent Reactions.
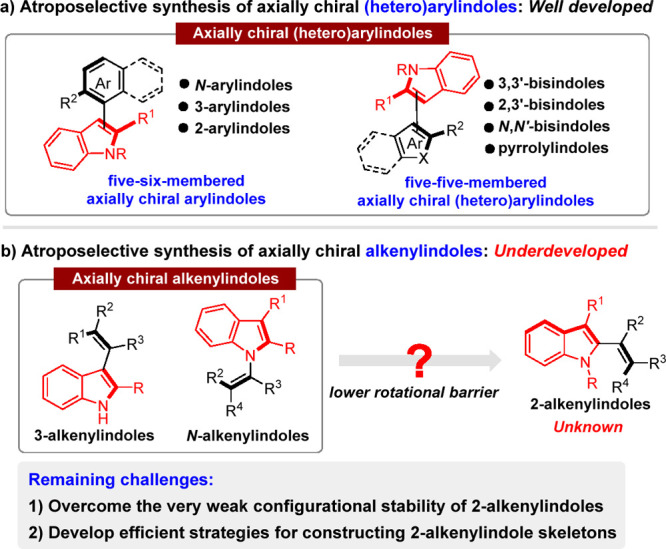
Axially chiral indole-based scaffolds, a class of unique axially chiral skeletons,31−45 are widely found in natural products,46−48 pharmaceutically relevant molecules,49−51 and chiral catalysts.52−55 Therefore, catalytic asymmetric construction of axially chiral indole-based scaffolds has become an emerging field.56,57 As shown in Scheme 2a, various elegant strategies have been developed for the enantioselective construction of axially chiral (hetero)arylindoles, such as five-six-membered axially chiral arylindoles49,58−103 and five-five-membered axially chiral (hetero)arylindoles.51,52,104−116 However, in sharp contrast, axially chiral alkenylindoles, more challenging axially chiral indole-based scaffolds,50,117−123 remain rarely studied except for a few examples on the synthesis of axially chiral 3-alkenylindoles117 and N-alkenylindoles118−123 (Scheme 2b). Despite this progress, catalytic atroposelective synthesis of axially chiral 2-alkenylindoles is still unknown due to the much lower rotational barrier of this class of molecules. Therefore, how to overcome the very weak configurational stability of axially chiral 2-alkenylindoles and to develop efficient strategies for constructing such skeletons in a catalytic asymmetric manner remain challenging.
Scheme 2. Profile of Catalytic Atroposelective Construction of Axially Chiral Indole-Based Scaffolds.
To overcome the challenges in developing catalytic asymmetric diastereodivergent reactions for the synthesis of chiral compounds bearing both axial and central chirality, and to construct axially chiral 2-alkenylindole skeletons, we proposed the concept of our strategy as designing new platform molecules for diastereodivergent synthesis of 2-alkenylindoles bearing both axial and central chirality. As shown in Scheme 3a, C3-unsubstituted 2-alkenylindoles were designed as indole-based platform molecules. The structural features of C3-unsubstituted 2-alkenylindoles mainly include: (1) the unsubstituted C3-position of the indole ring could not only act as a nucleophilic site, but also make the indole ring and the alkenyl group be able to rotate freely around the axis and result in rapid racemization; (2) the bulky cyclohexyl group and the N-substituent could serve as steric groups to generate rotational restriction for the products and increase the configurational stability of the products; (3) this kind of C3-unsubstituted 2-alkenylindoles could be easily synthesized from the corresponding 2-indolylmethanols124−126 via dehydration. These structural features enable C3-unsubstituted 2-alkenylindoles to undergo catalytic asymmetric diastereodivergent addition reactions with bulky electrophiles bearing a prochiral center under different reaction conditions, thus leading to the generation of two series of 2-alkenylindoles bearing both axial and central chirality in a diastereodivergent manner. Although this strategy seems feasible, there are still some challenging issues to be solved, such as finding suitable bulky electrophiles bearing a prochiral center and selecting suitable chiral catalysts to control the reactivity and the stereoselectivity.
Scheme 3. Our Strategy for Catalytic Asymmetric Diastereodivergent Synthesis of 2-Alkenylindoles Bearing Both Axial and Central Chirality.
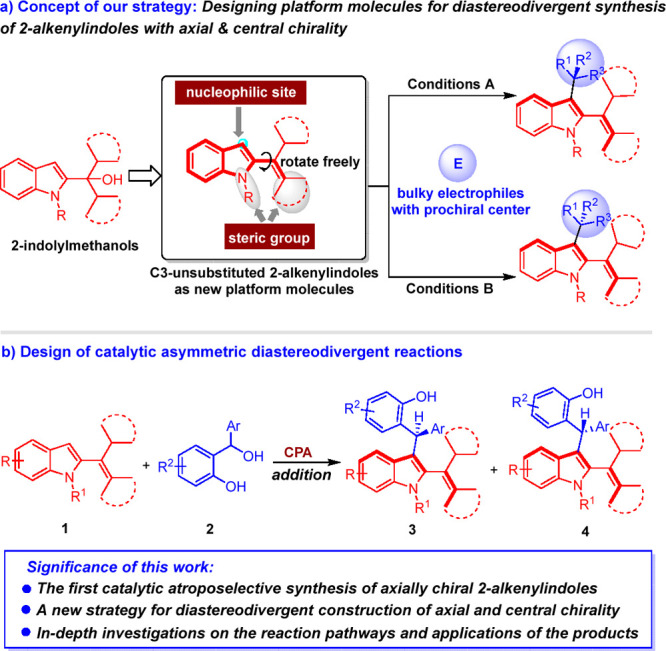
To address these issues, based on our long-lasting efforts in chiral indole chemistry,127−130 we designed a chiral phosphoric acid (CPA)-catalyzed asymmetric diastereodivergent addition reaction of C3-unsubstituted 2-alkenylindoles 1 with o-hydroxybenzyl alcohols 2 (Scheme 3b). In this design, the selection of o-hydroxybenzyl alcohols as suitable bulky electrophiles bearing prochiral center is based on that o-hydroxybenzyl alcohols can be converted into highly reactive o-quinone methides (o-QMs) in the presence of Brønsted acids and generate a new chiral center by the addition reaction.131−133 CPA was selected as a suitable chiral catalyst due to its ability to activate o-hydroxybenzyl alcohols, thus simultaneously controlling both the axial chirality and central chirality of products 3 and 4.134−141 Therefore, the significance of this study lies in that it will not only establish the first catalytic atroposelective synthesis of axially chiral 2-alkenylindoles, but also provide a new strategy for diastereodivergent construction of indole-based scaffolds bearing both axial and central chirality. Moreover, the in-depth investigations on the reaction pathways and potential applications of the products will be carried out.
Results and Discussion
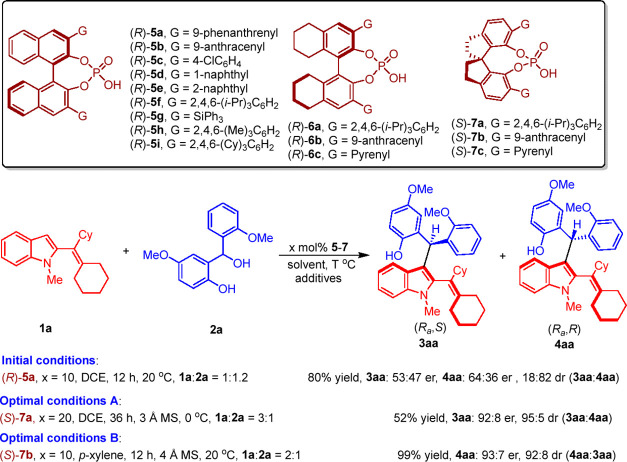
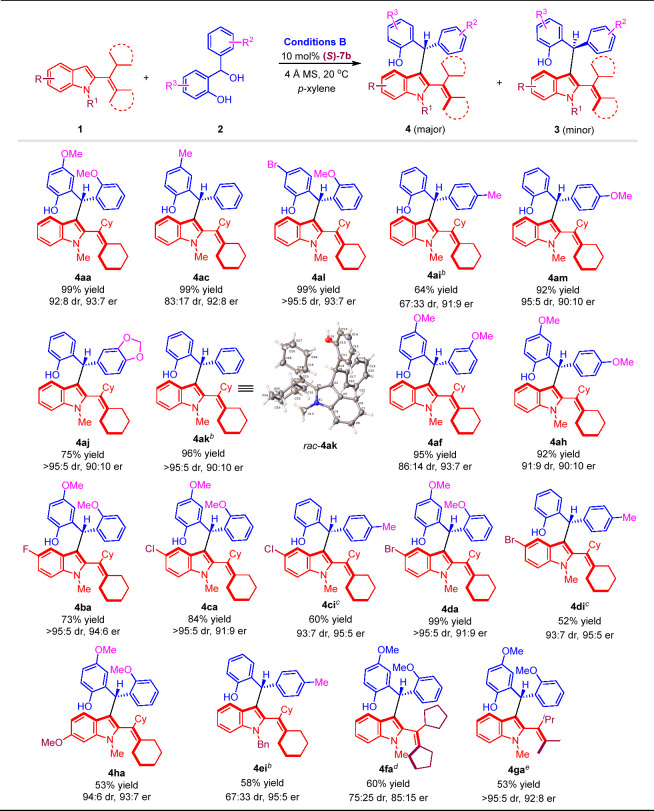
To verify the feasibility of our design, the reaction of C3-unsubstituted 2-alkenylindole 1a with o-hydroxybenzyl alcohol 2a was conducted (Scheme 4). As anticipated, this addition reaction occurred under the catalysis of CPA (R)-5a in 1,2-dichloroethane (DCE) at 20 °C and delivered axially chiral 2-alkenylindoles 3aa and 4aa in high total yields albeit with low enantioselectivity and moderate diastereoselectivity (80% yield, 3aa: 53:47 er, 4aa: 64:36 er, 18:82 dr). To realize asymmetric diastereodivergent addition reaction, various reaction parameters such as catalysts, solvents, additives, reagents ratio, reaction temperature, and catalyst loading were screened (see the Supporting Information for details). Finally, product 3aa was obtained as the major diastereomer in a moderate yield of 52% with a high enantioselectivity of 92:8 er and an excellent diastereoselectivity of 95:5 dr under the optimal conditions A (1a:2a = 3:1, 20 mol % (S)-7a, DCE, 36 h, 3 Å MS, 0 °C). On the other hand, product 4aa could be obtained as the major diastereomer in an excellent yield of 99% with a high enantioselectivity of 93:7 er and a good diastereoselectivity of 92:8 dr under the optimal conditions B (1a:2a = 2:1, 10 mol % (S)-7b, p-xylene, 12 h, 4 Å MS, 20 °C).
Scheme 4. Catalysts and Model Reaction Employed for Condition Optimization.
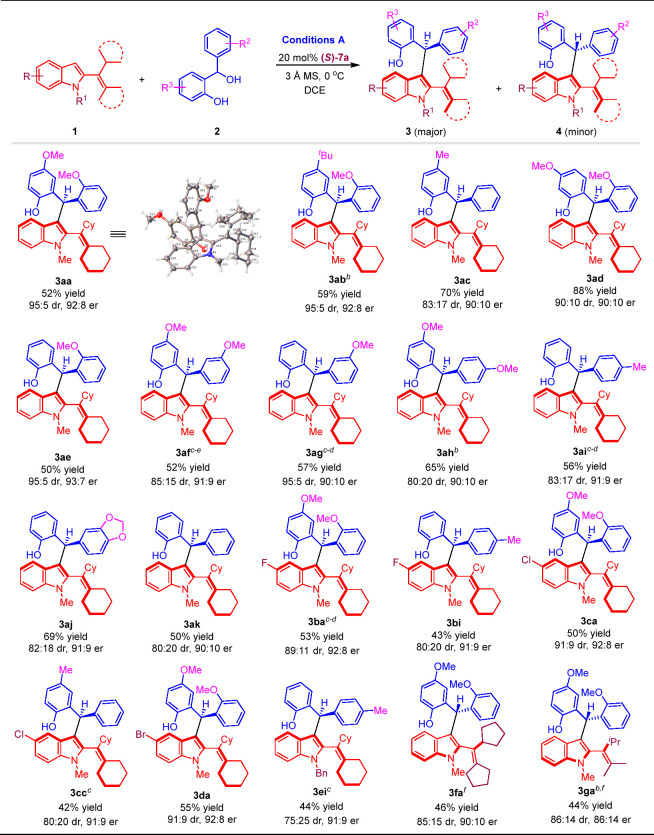
After establishing the optimal reaction conditions, we investigated the substrate scope of this catalytic asymmetric diastereodivergent addition reaction. First, the substrate scope for atroposelective synthesis of 2-alkenylindoles 3 as the major diastereomers bearing both axial and central chirality was examined under optimal conditions A. As shown in Table 1, a series of o-hydroxybenzyl alcohols 2a–2k bearing various R2/R3 groups were successfully employed to generate chiral 2-alkenylindoles 3ab–3ak in moderate to good yields (up to 88%) with high enantioselectivities (up to 93:7 er) and diastereoselectivities (up to 95:5 dr). For C3-unsubstituted 2-alkenylindoles 1, substrates 1b–1d with different R groups on the C5-position of the indole ring were suitable reactants for this addition reaction to produce chiral 2-alkenylindoles 3ba–3da in moderate yields (up to 55%) with good enantioselectivities (up to 92:8 er) and diastereoselectivities (up to 91:9 dr). Besides, N-benzyl substituted 2-alkenylindole 1e could participate in the reaction to afford chiral 2-alkenylindole 3ei in a moderate yield (44%) with a high enantioselectivity (91:9 er) and a moderate diastereoselectivity (75:25 dr).
Table 1. Substrate Scope for Atroposelective Synthesis of Chiral 2-Alkenylindoles 3a.
Reaction conditions: 1 (0.3 mmol), 2 (0.1 mmol), (S)-7a (20 mol %), 3 Å MS (100 mg), DCE (2 mL), 0 °C for 36 h. Isolated yields were provided, the er value was determined by HPLC and the dr value (3:4) was determined by 1H NMR. The absolute configuration of 3aa was determined as (Ra,S) via single-crystal X-ray diffraction analysis (see the Supporting Information for details).142
4 mL of DCE was used.
1 mL of DCE was used.
Catalyzed by 30 mol % (S)-7a for 48 h.
Using 5 Å MS (100 mg) as additives.
Catalyzed by 20 mol % (S)-5i.
To investigate the generality of this strategy for atroposelective synthesis of chiral 2-alkenylindoles 4 as the major diastereomers bearing both axial and central chirality, a wide scope of C3-unsubstituted 2-alkenylindoles 1 with different R/R1 groups and o-hydroxybenzyl alcohols 2 bearing various R2/R3 groups were employed as substrates under optimal conditions B. As shown in Table 2, a variety of 2-alkenylindoles 4 bearing axial and central chirality were synthesized in good yields (up to 99%) with high enantioselectivities (up to 95:5 er) and moderate to excellent diastereoselectivities (up to >95:5 dr).
Table 2. Substrate Scope for Atroposelective Synthesis of Chiral 2-Alkenylindoles 4a.
Reaction conditions: 1 (0.2 mmol), 2 (0.1 mmol), (S)-7b (10 mol %), 4 Å MS (100 mg), p-xylene (4 mL), 20 °C for 12 h. Isolated yields were provided, the er value was determined by HPLC and the dr value (4:3) was determined by 1H NMR. The structure of rac-4ak was confirmed by single-crystal X-ray diffraction analysis,142 and the absolute configuration of 4ak was determined to be (Ra, R) by comparison with a known chiral compound (see the Supporting Information for details).
Catalyzed by 10 mol % (S)-7c.
Catalyzed by 10 mol % (S)-7c at −30 °C in mesitylene for 24 h.
Catalyzed by 10 mol % (R)-6b.
Catalyzed by 20 mol % (R)-6b at 0 °C in mesitylene for 24 h, 1g:2a = 3:1.
Notably, cyclopentyl- and isopropyl-substituted 2-alkenylindoles 1f–1g successfully participated in this catalytic asymmetric diastereodivergent addition reaction to deliver axially chiral 2-alkenylindoles 3fa–3ga and 4fa–4ga, respectively, in acceptable yields with good to excellent enantioselectivities and diastereoselectivities.
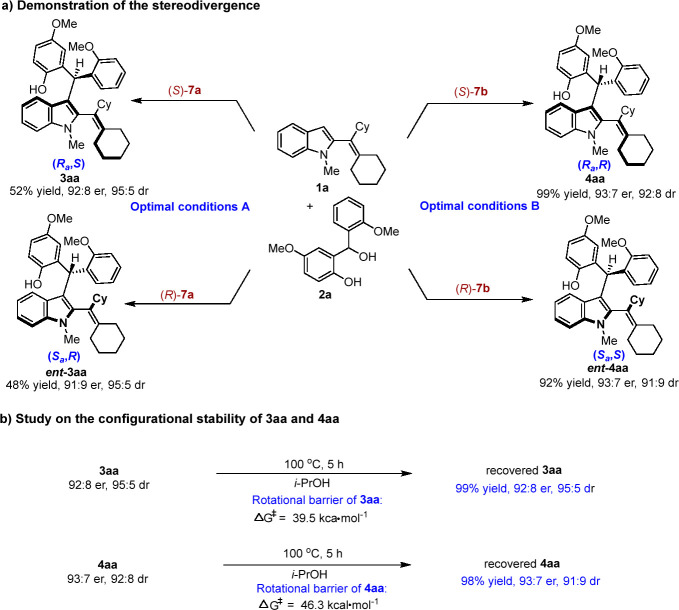
Subsequently, we evaluated the stereodivergence of this catalytic asymmetric addition reaction. As shown in Scheme 5a, the addition reactions of 1a and 2a under conditions A or B in the presence of CPA 7a or 7b with different absolute configurations were carried out, which delivered four stereoisomers of 2-alkenylindoles 3aa and 4aa bearing both axial and central chirality in moderate to good yields with high enantioselectivities and diastereoselectivities, respectively. In addition, we investigated the configurational stability and rotational barriers of axially chiral 2-alkenylindoles 3aa and 4aa (Scheme 5b). Products 3aa and 4aa were stirred in i-PrOH at 100 °C for 5 h and recovered in high yields with retained enantioselectivities and diastereoselectivities. These results indicated that products 3aa and 4aa have high configurational stability. The rotational barriers of products 3aa and 4aa were theoretically calculated as 39.5 and 46.3 kcal mol–1, respectively, which were in accordance with the experimentally observed high configurational stability of these products.
Scheme 5. Demonstration of the Stereodivergence and Study on the Configurational Stability.
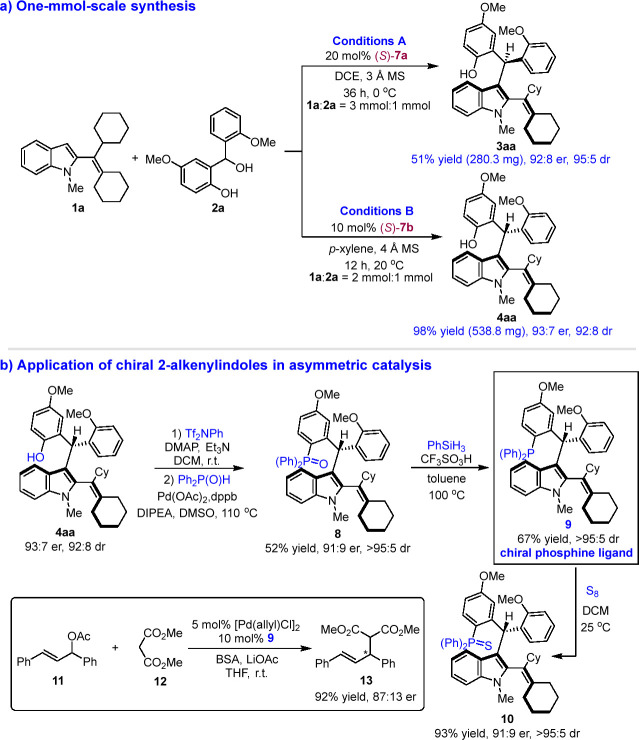
To demonstrate the utility of the reaction, we performed the synthesis of products 3aa and 4aa on 1 mmol scale (Scheme 6a). Compared with the small-scale reactions, these 1 mmol scale reactions smoothly afforded products 3aa and 4aa in similar yields with maintained high enantioselectivities and diastereoselectivities. Moreover, we further investigated the utility of this new class of 2-alkenylindoles bearing both axial and central chirality (Scheme 6b). For instance, product 4aa was transformed into the corresponding chiral phosphine ligand 9 bearing multiple chiral elements via a two-step reaction. The preliminary application of chiral ligand 9 was verified in a palladium-catalyzed enantioselective allylic alkylation reaction, which afforded chiral product 13 in a high yield of 92% with a good enantioselectivity of 87:13 er. Therefore, this result demonstrated the promising applications of this kind of 2-alkenylindole skeletons bearing both axial and central chirality in asymmetric catalysis.
Scheme 6. Synthesis (1 mmol Scale) and Application of Axially Chiral 2-Alkenylindoles in Asymmetric Catalysis.
To find the possible bioactivities of this class of chiral 2-alkenylindoles, a preliminary evaluation on the cytotoxicity of some selected products 3 and 4 was carried out (see the Supporting Information for details). As shown in Figure 1a, several products 3 and 4 exhibited some extent of cytotoxicity against HepG2 cancer cells, and the IC50 values were ranging from 20.0 to 50.4 μg/mL. Besides, the cytotoxicity of some selected products 3 and 4 against PC-3 cancer cells was also investigated (Figure 1b). Among them, product 3ac exhibited potent cytotoxicity against PC-3 cancer cells with a very low IC50 value of 16.7 μg/mL. These results indicated that this class of chiral 2-alkenylindoles might find their potential applications in medicinal chemistry.
Figure 1.
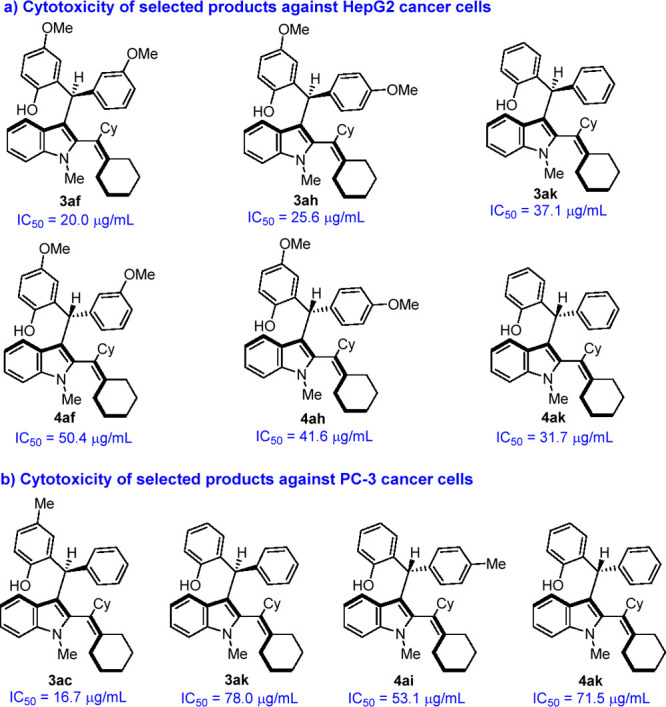
Chiral 2-alkenylindoles 3 and 4 with promising anticancer activity
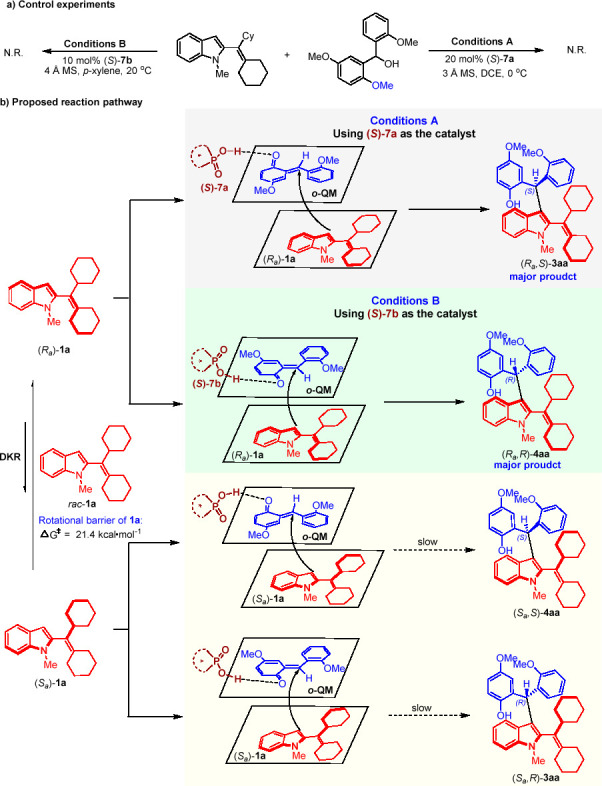
To elucidate the possible activation mode, we conducted some control experiments (Scheme 7a). When methyl-protected o-hydroxybenzyl alcohol 2n was allowed to react with C3-unsubstituted 2-alkenylindole 1a under standard conditions A and B, no reaction occurred. This indicated that the OH group in o-hydroxybenzyl alcohols played a crucial role in controlling the reactivity, possibly by forming hydrogen-bonding interactions with CPA. Based on the control experiments, we proposed a possible reaction pathway for this catalytic asymmetric diastereodivergent reaction. As shown in Scheme 7b, (Ra)-1a and (Sa)-1a were two enantiomers of racemic C3-unsubstituted 2-alkenylindole 1a, and the rotational barrier of 1a was calculated as 21.4 kcal mol–1, which verified our proposal that (Ra)-1a and (Sa)-1a could rapidly transform to each other, resulting in rapid racemization for the dynamic kinetic resolution (DKR) process. Under the activation of (S)-7a via hydrogen-bonding interaction (conditions A), a fast nucleophilic addition between (Ra)-1a and o-quinone methide (o-QM) (generated via dehydration of o-hydroxybenzyl alcohol 2a) occurred to give product (Ra,S)-3aa. When using (S)-7b as the catalyst (conditions B), diastereomer (Ra,R)-4aa was obtained, thus realizing the diastereodivergence process and the generation of two types of 2-alkenylindoles bearing both axial and central chirality. On the other hand, in the presence of CPA (S)-7a or (S)-7b, the nucleophilic addition of (Sa)-1a with o-QM was very slow and (Sa)-1a continuously transformed into (Ra)-1a via rapid racemization, resulting in the DKR process.
Scheme 7. Control Experiments and Proposed Reaction Pathway.
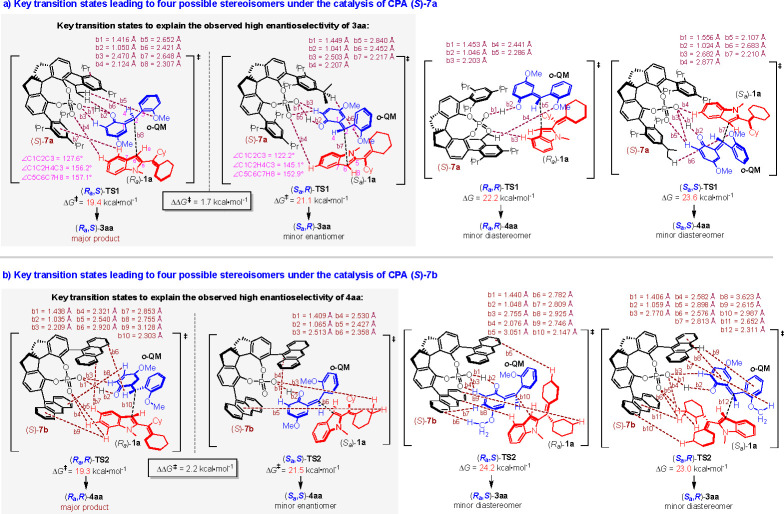
To explain the observed high enantioselectivity of products 3, 4 and the diastereodivergence of this addition reaction under different reaction conditions, we performed density functional theory (DFT) calculations on the key transition states leading to four possible stereoisomers in the catalytic asymmetric diastereodivergent reactions between 1a and 2a under the catalysis of different CPAs (S)-7a and (S)-7b, respectively (Figure 2). As shown in Figure 2a, the Gibbs free energy barrier of transition state (Ra,S)-TS1, leading to the formation of major product (Ra,S)-3aa, was calculated as 19.4 kcal·mol–1, which was the lowest energy barrier among the transition states leading to four possible stereoisomers under the catalysis of CPA (S)-7a (see Figure S4 in the Supporting Information for details). Notably, the C=O group in o-QM could form hydrogen-bonding interactions with CPA in (Ra,S)-TS1, demonstrating that the OH group in o-hydroxybenzyl alcohol 2a played a crucial role in controlling the reactivity, which was consistent with the control experiments.
Figure 2.
Key transition states to explain the enantioselectivity and diastereodivergence of the products.
To explain the observed absolute configuration and good enantioselectivity of product (Ra,S)-3aa, we compared the key transition states (Ra,S)-TS1 and (Sa,R)-TS1, which ultimately led to the formation of two enantiomers of 3aa. The energy difference between (Ra,S)-TS1 and (Sa,R)-TS1 was calculated as 1.7 kcal mol–1, which was in accordance with the observed er value (92:8). Moreover, the origin of the energy difference leading to the observed enantioselectivity of 3aa was studied by energy decomposition analysis and noncovalent interaction (NCI) plots (see Figures S5 and S6 in the Supporting Information for details). Energy decomposition analysis indicated that the energy difference between (Ra,S)-TS1 and (Sa,R)-TS1 could be mainly attributed to the difference of distortion energies of the substrates and the catalyst. Structure analysis in Figure 2a indicated that the planar carbon center of C3-unsubstituted 2-alkenylindole 1a (∠C5C6C7H8 = 179.2°) and o-QM (∠C1C2H4C3 = 180.0°) as well as the angle in o-QM (∠C1C2C3 = 127.7°) were ready to distort to pyramidal structures during the nucleophilic addition. The dihedral angles of these two carbon centers in (Ra,S)-TS1 (∠C5C6C7H8 = 157.1° and ∠C1C2H4C3 = 156.2°) and the angle of o-QM (∠C1C2C3 = 127.6°) were bigger than those in (Sa,R)-TS1 (∠C5C6C7H8 = 152.9°, ∠C1C2H4C3 = 145.1°, ∠C1C2C3 = 122.2°), indicating more structural distortions in (Sa,R)-TS1 than those in (Ra,S)-TS1. Besides, the hydrogen bond between the oxygen atom and the hydrogen atom of CPA (S)-7a in (Ra,S)-TS1 (b1 = 1.416 Å) was considerably shorter than that in (Sa,R)-TS1 (b1 = 1.449 Å), further indicating the bigger structural distortion in (Sa,R)-TS1. These results well explain the observed absolute configuration and good enantioselectivity of the major enantiomer of product 3aa. Moreover, the energy barrier of (Ra,S)-TS1 was much lower than those of (Ra,R)-TS1 and (Sa,S)-TS1, which corresponded to the formation of minor diastereomers (Ra,R)-4aa and (Sa,S)-4aa. So, these calculation results also explained the observed high diastereoselectivity of 3aa in the presence of CPA (S)-7a.
In addition, Figure 2b shows the calculated transition states leading to four possible stereoisomers under the catalysis of CPA (S)-7b. Obviously, the energy barrier of (Ra,R)-TS2 leading to the formation of major product (Ra,R)-4aa was lower (19.3 kcal·mol–1) than those of other three transition states (see Figure S7 in the Supporting Information for details). The energy difference between the transition states (Ra,R)-TS2 and (Sa,S)-TS2, leading to the generation of two enantiomers of 4aa, was 2.2 kcal·mol–1, which well explained the observed absolute configuration of major enantiomer (Ra,R)-4aa and its good enantioselectivity of 93:7 er. Similarly, the origin of the energy difference leading to the observed enantioselectivity of 4aa was also investigated by energy decomposition analysis (see Figure S8 in the Supporting Information for details). Energy decomposition analysis indicated that the energy difference between (Ra,R)-TS2 and (Sa,S)-TS2 mainly comes from the difference of interaction energies between the substrates and CPA (S)-7b. As shown in Figure 2b, three C–H···O (b3 = 2.209 Å, b4 = 2.321 Å, b5 = 2.540 Å) and four C–H···π interactions (b6 = 2.920 Å, b7 = 2.853 Å, b8 = 2.755 Å, b9 = 3.128 Å) between the substrates [(Ra)-1a and o-QM] and CPA (S)-7b were observed in (Ra,R)-TS2. In contrast, only one C–H···O (b3 = 2.513 Å) and two C–H···π interactions (b4 = 2.530 Å, b5 = 2.427 Å) were observed in (Sa,S)-TS2, indicating that the interactions between the substrates and CPA (S)-7b in (Sa,S)-TS2 were weaker than those in (Ra,R)-TS2. These noncovalent interactions could also be visualized from the NCI plots (see Figure S9 in the Supporting Information for details). These results well explained the observed absolute configuration and good enantioselectivity of the major product (Ra,R)-4aa. Evidently, under the catalysis of CPA (S)-7b, the energy barrier of (Ra,R)-TS2 was much lower than those of (Ra,S)-TS2 and (Sa,R)-TS2 corresponding to the generation of minor diastereomers (Ra,S)-3aa and (Sa,R)-3aa, which explained the observed high diastereoselectivity of 4aa in the presence of CPA (S)-7b. Therefore, the theoretical calculations provided an in-depth understanding of the origin of the observed stereoselectivity and diastereodivergence of the products under different reaction conditions.
Conclusions
In summary, we have established the first catalytic asymmetric diastereodivergent synthesis of 2-alkenylindoles bearing both axial chirality and central chirality via CPA-catalyzed addition reactions of C3-unsubstituted 2-alkenylindoles with o-hydroxybenzyl alcohols. Using this approach, two series of 2-alkenylindoles bearing multiple chiral elements were synthesized in a diastereodivergent fashion with moderate to high yields and excellent stereoselectivities. Moreover, such 2-alkenylindoles bearing both axial and central chirality could be converted into new chiral ligand, and several 2-alkenylindole products displayed potent anticancer activities, which demonstrated their promising applications in asymmetric catalysis and medicinal chemistry. Besides, theoretical calculations have been performed on the key transition states leading to different stereoisomers, which provided an in-depth understanding of this catalytic asymmetric diastereodivergent reaction. Therefore, this work has not only established the first catalytic atroposelective synthesis of axially chiral 2-alkenylindoles with potential applications, but also provided a new strategy for diastereodivergent construction of indole-based scaffolds bearing both axial and central chirality, thus offering an efficient tactic toward settling the challenges in developing catalytic asymmetric diastereodivergent reactions for the synthesis of chiral compounds bearing both axial and central chirality.
Acknowledgments
We are grateful for financial support from NSFC (22125104 and 22101103), Project for Excellent Scientific and Technological Innovation Team of Jiangsu Province, the open research fund of Songshan Lake Materials Laboratory (2023SLABFN16) and the STU Scientific Research Foundation for Talents (NTF20022). We are grateful for Prof. Shu Zhang for her help in biological evaluation.
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/prechem.4c00008.
Accession Codes
CCDC 2326447 (3aa) and CCDC 2326448 (rac-4ak) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
Author Contributions
¶ S.Y., J.-B.H., and D.-H.W. contributed equally to the work.
The authors declare no competing financial interest.
Supplementary Material
References
- Abram M.; Jakubiec M.; Kamiński K. Chirality as an Important Factor for the Development of New Antiepileptic Drugs. ChemMedChem. 2019, 14, 1744–1761. 10.1002/cmdc.201900367. [DOI] [PubMed] [Google Scholar]
- Bogaerts J.; Aerts R.; Vermeyen T.; Johannessen C.; Herrebout W.; Batista J. M. Tackling Stereochemistry in Drug Molecules with Vibrational Optical Activity. Pharmaceuticals 2021, 14, 877–901. 10.3390/ph14090877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foye’s Principles of Medicinal Chemistry, 7th ed.; Lemke T. L., Williams D. A., Roche V. F., Zito S. W., Eds.; Lippincott Williams & Wilkins, Wolters Kluwer: Baltimore, 2013. [Google Scholar]
- Waldeck B. Biological Significance of the Enantiomeric Purity of Drugs. Chirality 1993, 5, 350–355. 10.1002/chir.530050514. [DOI] [PubMed] [Google Scholar]
- Wei L.; Zhu Q.; Xu S.-M.; Chang X.; Wang C.-J. Stereodivergent Synthesis of α,α-Disubstituted α-Amino Acids via Synergistic Cu/Ir Catalysis. J. Am. Chem. Soc. 2018, 140, 1508–1513. 10.1021/jacs.7b12174. [DOI] [PubMed] [Google Scholar]
- Jiang X.; Boehm P.; Hartwig J. F. Stereodivergent Allylation of Azaaryl Acetamides and Acetates by Synergistic Iridium and Copper Catalysis. J. Am. Chem. Soc. 2018, 140, 1239–1242. 10.1021/jacs.7b12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.-M.; Wei L.; Shen C.; Xiao L.; Tao H.-Y.; Wang C.-J. Stereodivergent Assembly of Tetrahydro-γ-Carbolines via Synergistic Catalytic Asymmetric Cascade Reaction. Nat. Commun. 2019, 10, 5553–5563. 10.1038/s41467-019-13529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.-M.; Wang Y.-N.; Wang B.-C.; Chen X.-W.; Lu L.-Q.; Xiao W.-J. Synergetic Iridium and Amine Catalysis Enables Asymmetric [4 + 2] Cycloadditions of Vinyl Aminoalcohols with Carbonyls. Nat. Commun. 2019, 10, 2716–2724. 10.1038/s41467-019-10674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha S.; Serrano E.; Mondal S.; Daniliuc C. G.; Glorius F. Diastereodivergent Synthesis of Enantioenriched α,β-Disubstituted γ-Butyrolactones via Cooperative N-Heterocyclic Carbene and Ir Catalysis. Nat. Catal. 2020, 3, 48–54. 10.1038/s41929-019-0387-3. [DOI] [Google Scholar]
- Peng Y.; Huo X.; Luo Y.; Wu L.; Zhang W. Enantio- and Diastereodivergent Synthesis of Spirocycles through Dual-Metal-Catalyzed [3 + 2] Annulation of 2-Vinyloxiranes with Nucleophilic Dipoles. Angew. Chem., Int. Ed. 2021, 60, 24941–24949. 10.1002/anie.202111842. [DOI] [PubMed] [Google Scholar]
- Lin L.; Feng X. Catalytic Strategies for Diastereodivergent Synthesis. Chem.—Eur. J. 2017, 23, 6464–6482. 10.1002/chem.201604617. [DOI] [PubMed] [Google Scholar]
- Krautwald S.; Carreira E. M. Stereodivergence in Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 5627–5639. 10.1021/jacs.6b13340. [DOI] [PubMed] [Google Scholar]
- Zhan G.; Du W.; Chen Y.-C. Switchable Divergent Asymmetric Synthesis via Organocatalysis. Chem. Soc. Rev. 2017, 46, 1675–1692. 10.1039/C6CS00247A. [DOI] [PubMed] [Google Scholar]
- Beletskaya I. P.; Nájera C.; Yus M. Stereodivergent Catalysis. Chem. Rev. 2018, 118, 5080–5200. 10.1021/acs.chemrev.7b00561. [DOI] [PubMed] [Google Scholar]
- Nájera C.; Foubelo F.; Sansano J. M.; Yus M. Stereodivergent Routes in Organic Synthesis: Carbohydrates, Amino Acids, Alkaloids and Terpenes. Org. Biomol. Chem. 2020, 18, 1232–1278. 10.1039/C9OB02419K. [DOI] [PubMed] [Google Scholar]
- Huo X.; Li G.; Wang X.; Zhang W. Bimetallic Catalysis in Stereodivergent Synthesis. Angew. Chem., Int. Ed. 2022, 61, e202210086 10.1002/anie.202210086. [DOI] [PubMed] [Google Scholar]
- krautwald S.; Sarlah D.; Schafroth M. A.; Carreira E. M. Enantio- and Diastereodivergent Dual Catalysis: α-Allylation of Branched Aldehydes. Science 2013, 340, 1065–1068. 10.1126/science.1237068. [DOI] [PubMed] [Google Scholar]
- Huo X.; He R.; Zhang X.; Zhang W. An Ir/Zn Dual Catalysis for Enantio- and Diastereodivergent α-Allylation of α-Hydroxyketones. J. Am. Chem. Soc. 2016, 138, 11093–10096. 10.1021/jacs.6b06156. [DOI] [PubMed] [Google Scholar]
- Rong Z.-Q.; Wang M.; Chow C. H. E.; Zhao Y. A Catalyst-Enabled Diastereodivergent Aza-Diels-Alder Reaction: Complementarity of N-Heterocyclic Carbenes and Chiral Amines. Chem.—Eur. J. 2016, 22, 9483–9487. 10.1002/chem.201601626. [DOI] [PubMed] [Google Scholar]
- Kim B.; Kim Y.; Lee S. Y. Stereodivergent Carbon-Carbon Bond Formation between Iminium and Enolate Intermediates by Synergistic Organocatalysis. J. Am. Chem. Soc. 2021, 143, 73–79. 10.1021/jacs.0c11077. [DOI] [PubMed] [Google Scholar]
- He Z.; Peng L.; Guo C. Catalytic Stereodivergent Total Synthesis of Amathaspiramide D. Nat. Synth. 2022, 1, 393–400. 10.1038/s44160-022-00063-y. [DOI] [Google Scholar]
- Hu Y.; Huang J.-Y.; Yan R.-J.; Chen Z.-C.; OuYang Q.; Du W.; Chen Y.-C. Diastereodivergent cis- and trans-Fused [4 + 2] Annulations of Cyclic 1,3-Dienes and 1-Azadienes via Ligand-Controlled Palladium Catalysis. Chem. Sci. 2023, 14, 1896–1901. 10.1039/D2SC06813C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L.; Wei L.; Wang C. Stereodivergent Synthesis of Enantioenriched γ-Butyrolactones Bearing Two Vicinal Stereocenters Enabled by Synergistic Copper and Iridium Catalysis. Angew. Chem., Int. Ed. 2021, 60, 24930–24940. 10.1002/anie.202107418. [DOI] [PubMed] [Google Scholar]
- Wen Y.; Zhang Z.; Li S.; Song J.; Gong L. Stereodivergent Propargylic Alkylation of Enals via Cooperative NHC and Copper Catalysis. Nat. Commun. 2022, 13, 1344–1353. 10.1038/s41467-022-29059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Wang P.; Zhang Q.; Tang W.; Zi W. Diastereodivergent Aldol-Type Coupling of Alkoxyallenes with Pentafluorophenyl Esters Enabled by Synergistic Palladium/Chiral Lewis Base Catalysis. Angew. Chem., Int. Ed. 2022, 61, e202207621 10.1002/anie.202207621. [DOI] [PubMed] [Google Scholar]
- Hu Q.; He Z.; Peng L.; Guo C. Combining Nickel and Squaramide Catalysis for the Stereodivergent α-Propargylation of Oxindoles. Nat. Synth. 2022, 1, 322–331. 10.1038/s44160-022-00050-3. [DOI] [Google Scholar]
- Shen G.; He F.; Xie W.; Gu H.; Yang X. Diastereodivergent Asymmetric [4 + 2] Cycloaddition of In Situ Generated Ortho-Quinone Methides and Allenyl Ketones Enabled by Chiral Phosphoric Acid Catalysis. ACS Catal. 2023, 13, 12472–12480. 10.1021/acscatal.3c02851. [DOI] [Google Scholar]
- Wen Y.; Yang F.; Li S.; Yao X.; Song J.; Gong L. Diastereodivergent Desymmetric Annulation to Access Spirooxindoles: Chemical Probes for Mitosis. J. Am. Chem. Soc. 2023, 145, 4199–4207. 10.1021/jacs.2c12648. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zheng S.; Rajkumar S.; Xie J.; Yu N.; Peng Q.; Yang X. Chiral Phosphoric Acid-Catalyzed Stereodivergent Synthesis of Trisubstituted Allenes and Computational Mechanistic Studies. Nat. Commun. 2020, 11, 5527–5538. 10.1038/s41467-020-19294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Huo X.; Xiao J.; Zhao L.; Ma S.; Zhang W. Enantio- and Diastereodivergent Construction of 1,3-Nonadjacent Stereocenters Bearing Axial and Central Chirality through Synergistic Pd/Cu Catalysis. J. Am. Chem. Soc. 2021, 143, 12622–12632. 10.1021/jacs.1c05087. [DOI] [PubMed] [Google Scholar]
- Zhang H.-H.; Li T.-Z.; Liu S.-J.; Shi F. Catalytic Asymmetric Synthesis of Atropisomers Bearing Multiple Chiral Elements: An Emerging Field. Angew. Chem., Int. Ed. 2024, 63, e202311053 10.1002/anie.202311053. [DOI] [PubMed] [Google Scholar]
- Wang Y.-B.; Tan B. Construction of Axially Chiral Compounds via Asymmetric Organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. 10.1021/acs.accounts.7b00602. [DOI] [PubMed] [Google Scholar]
- Liao G.; Zhou T.; Yao Q.; Shi B.-F. Recent Advances in the Synthesis of Axially Chiral Biaryls via Transition Metal-Catalysed Asymmetric C-H Functionalization. Chem. Commun. 2019, 55, 8514–8523. 10.1039/C9CC03967H. [DOI] [PubMed] [Google Scholar]
- Bonne D.; Rodriguez J. Enantioselective Syntheses of Atropisomers Featuring a Five-membered Ring. Chem. Commun. 2017, 53, 12385–12393. 10.1039/C7CC06863H. [DOI] [PubMed] [Google Scholar]
- Bonne D.; Rodriguez J. A Bird’s Eye View of Atropisomers Featuring a Five-Membered Ring. Eur. J. Org. Chem. 2018, 2018, 2417–2431. 10.1002/ejoc.201800078. [DOI] [Google Scholar]
- Zhang S.; Liao G.; Shi B.-F. Enantioselective Synthesis of Atropoisomers Featuring Pentatomic Heteroaromatics. Chin. J. Org. Chem. 2019, 39, 1522–1528. 10.6023/cjoc201904030. [DOI] [Google Scholar]
- Da B.; Xiang S.; Li S.; Tan B. Chiral Phosphoric Acid Catalyzed Asymmetric Synthesis of Axially Chiral Compounds. Chin. J. Chem. 2021, 39, 1787–1796. 10.1002/cjoc.202000751. [DOI] [Google Scholar]
- Carmona J.; Rodríguez-Franco C.; Fernández R.; Hornillos V.; Lassaletta J. M. Atroposelective Transformation of Axially Chiral (Hetero)Biaryls. From Desymmetrization to Modern Resolution Strategies. Chem. Soc. Rev. 2021, 50, 2968–2983. 10.1039/D0CS00870B. [DOI] [PubMed] [Google Scholar]
- Cheng J. K.; Xiang S.-H.; Li S.; Ye L.; Tan B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805–4902. 10.1021/acs.chemrev.0c01306. [DOI] [PubMed] [Google Scholar]
- Liu C.-X.; Zhang W.-W.; Yin S.-Y.; Gu Q.; You S.-L. Synthesis of Atropisomers by Transition-Metal-Catalyzed Asymmetric C-H Functionalization Reactions. J. Am. Chem. Soc. 2021, 143, 14025–14040. 10.1021/jacs.1c07635. [DOI] [PubMed] [Google Scholar]
- Wu Y.-J.; Liao G.; Shi B.-F. Stereoselective Construction of Atropisomers Featuring a C-N Chiral Axis. Green. Synth. Catal. 2022, 3, 117–136. 10.1016/j.gresc.2021.12.005. [DOI] [Google Scholar]
- Rodríguez-Salamanca P.; Fernández R.; Hornillos V.; Lassaletta J. M. Asymmetric Synthesis of Axially Chiral C-N Atropisomers. Chem.—Eur. J. 2022, 28, e202104442 10.1002/chem.202282861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W.; Liu Y.; Yan H. Enantioselective Synthesis of Atropisomers via Vinylidene Ortho-Quinone Methides (VQMs). Acc. Chem. Res. 2022, 55, 2780–2795. 10.1021/acs.accounts.2c00486. [DOI] [PubMed] [Google Scholar]
- Cheng J. K.; Xiang S.-H.; Tan B. Organocatalytic Enantioselective Synthesis of Axially Chiral Molecules: Development of Strategies and Skeletons. Acc. Chem. Res. 2022, 55, 2920–2937. 10.1021/acs.accounts.2c00509. [DOI] [PubMed] [Google Scholar]
- Feng J.; Lu C.-J.; Liu R.-R. Catalytic Asymmetric Synthesis of Atropisomers Featuring an Aza Axis. Acc. Chem. Res. 2023, 56, 2537–2554. 10.1021/acs.accounts.3c00419. [DOI] [PubMed] [Google Scholar]
- Norton R.; Wells R. A Series of Chiral Polybrominated Biindoles from the Marine Blue-green Alga Rivularia Firma. Application of Carbon-13 NMR Spin-lattice Relaxation Data and Carbon-13-proton Coupling Constants to Structure Elucidation. J. Am. Chem. Soc. 1982, 104, 3628–3635. 10.1021/ja00377a014. [DOI] [Google Scholar]
- Ito C.; Thoyama Y.; Omura M.; Kajiura I.; Furukawa H. Alkaloidal Constituents of Murraya koenigii. Isolation and Structural Elucidation of Novel Binary Carbazolequinones and Carbazole Alkaloids. Chem. Pharm. Bull. 1993, 41, 2096–2100. 10.1248/cpb.41.2096. [DOI] [Google Scholar]
- Zhang Q.; Mándi A.; Li S.; Chen Y.; Zhang W.; Tian X.; Zhang H.; Li H.; Zhang W.; Zhang S.; Ju J.; Kurtán T.; Zhang C. N-N-Coupled Indolo-sesquiterpene Atropo-Diastereomers from a Marine-Derived Actinomycete. Eur. J. Org. Chem. 2012, 2012, 5256–5262. 10.1002/ejoc.201200599. [DOI] [Google Scholar]
- Jiang F.; Chen K.-W.; Wu P.; Zhang Y.-C.; Jiao Y.; Shi F. A Strategy for Synthesizing Axially Chiral Naphthyl-Indoles: Catalytic Asymmetric Addition Reactions of Racemic Substrates. Angew. Chem., Int. Ed. 2019, 58, 15104–15110. 10.1002/anie.201908279. [DOI] [PubMed] [Google Scholar]
- Wang C.-S.; Li T.-Z.; Liu S.-J.; Zhang Y.-C.; Deng S.; Jiao Y.; Shi F. Axially Chiral Aryl-Alkene-Indole Framework: A Nascent Member of the Atropisomeric Family and Its Catalytic Asymmetric Construction. Chin. J. Chem. 2020, 38, 543–552. 10.1002/cjoc.202000131. [DOI] [Google Scholar]
- Chen K.-W.; Chen Z.-H.; Yang S.; Wu S.-F.; Zhang Y.-C.; Shi F. Organocatalytic Atroposelective Synthesis of N-N Axially Chiral Indoles and Pyrroles by De Novo Ring Formation. Angew. Chem., Int. Ed. 2022, 61, e202116829 10.1002/anie.202116829. [DOI] [PubMed] [Google Scholar]
- Baumann T.; Brückner R. Atropselective Dibrominations of a 1,1′-Disubstituted 2,2′-Biindolyl with Diverging Point-to-Axial Asymmetric Inductions. Deriving 2,2′-Biindolyl-3,3′-Diphosphane Ligands for Asymmetric Catalysis. Angew. Chem., Int. Ed. 2019, 58, 4714–4719. 10.1002/anie.201806294. [DOI] [PubMed] [Google Scholar]
- Xia W.; An Q.-J.; Xiang S.-H.; Li S.; Wang Y.-B.; Tan B. Chiral Phosphoric Acid Catalyzed Atroposelective C-H Amination of Arenes. Angew. Chem., Int. Ed. 2020, 59, 6775–6779. 10.1002/anie.202000585. [DOI] [PubMed] [Google Scholar]
- He T.; Peng L.; Li S.; Hu F.; Xie C.; Huang S.; Jia S.; Qin W.; Yan H. Chiral Naphthyl-C2-Indole as Scaffold for Phosphine Organocatalysis: Application in Asymmetric Formal [4 + 2] Cycloaddition Reactions. Org. Lett. 2020, 22, 6966–6971. 10.1021/acs.orglett.0c02519. [DOI] [PubMed] [Google Scholar]
- Liu S.-J.; Chen Z.-H.; Chen J.-Y.; Ni S.-F.; Zhang Y.-C.; Shi F. Rational Design of Axially Chiral Styrene-Based Organocatalysts and Their Application in Catalytic Asymmetric [2 + 4] Cyclizations. Angew. Chem., Int. Ed. 2022, 61, e202112226 10.1002/anie.202112226. [DOI] [PubMed] [Google Scholar]
- Li T.-Z.; Liu S.-J.; Tan W.; Shi F. Catalytic Asymmetric Construction of Axially Chiral Indole-Based Frameworks: An Emerging Area. Chem.—Eur. J. 2020, 26, 15779–15792. 10.1002/chem.202001397. [DOI] [PubMed] [Google Scholar]
- Zhang H.-H.; Shi F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022, 55, 2562–2580. 10.1021/acs.accounts.2c00465. [DOI] [PubMed] [Google Scholar]
- Ototake N.; Morimoto Y.; Mokuya A.; Fukaya H.; Shida Y.; Kitagawa O. Catalytic Enantioselective Synthesis of Atropisomeric Indoles with an N-C Chiral Axis. Chem.—Eur. J. 2010, 16, 6752–6755. 10.1002/chem.201000243. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhong J.; Lin X. Atroposelective Phosphoric Acid Catalyzed Three-Component Cascade Reaction: Enantioselective Synthesis of Axially Chiral N-Arylindoles. Angew. Chem., Int. Ed. 2019, 58, 15824–15828. 10.1002/anie.201909855. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Xu Q.; Wu J.; Fan J.; Xie M. Construction of N-C Axial Chirality through Atroposelective C-H Olefination of N-Arylindoles by Palladium/Amino Acid Cooperative Catalysis. Org. Lett. 2019, 21, 6361–6365. 10.1021/acs.orglett.9b02243. [DOI] [PubMed] [Google Scholar]
- Sun L.; Chen H.; Liu B.; Chang J.; Kong L.; Wang F.; Lan Y.; Li X. Rhodium-Catalyzed Atroposelective Construction of Indoles via C-H Bond Activation. Angew. Chem., Int. Ed. 2021, 60, 8391–8395. 10.1002/anie.202012932. [DOI] [PubMed] [Google Scholar]
- Kim A.; Kim A.; Park S.; Kim S.; Jo H.; Ok K. M.; Lee S. K.; Song J.; Kwon Y. Catalytic and Enantioselective Control of the C-N Stereogenic Axis via the Pictet-Spengler Reaction. Angew. Chem., Int. Ed. 2021, 60, 12279–12283. 10.1002/anie.202100363. [DOI] [PubMed] [Google Scholar]
- Ren Q.; Cao T.; He C.; Yang M.; Liu H.; Wang L. Highly Atroposelective Rhodium(II)-Catalyzed N-H Bond Insertion: Access to Axially Chiral N-Arylindolocarbazoles. ACS Catal. 2021, 11, 6135–6140. 10.1021/acscatal.1c01232. [DOI] [Google Scholar]
- Wang Z.-S.; Zhu L.-J.; Li C.-T.; Liu B.-Y.; Hong X.; Ye L.-W. Synthesis of Axially Chiral N-Arylindoles via Atroposelective Cyclization of Ynamides Catalyzed by Chiral Brønsted Acids. Angew. Chem., Int. Ed. 2022, 61, e202201436 10.1002/anie.202201436. [DOI] [PubMed] [Google Scholar]
- Corti V.; Thøgersen M. K.; Enemærke V. J.; Rezayee N. M.; Barløse C. L.; Jørgensen K. A. Construction of C-N Atropisomers by Aminocatalytic Enantioselective Addition of Indole-2-Carboxaldehydes to o-Quinone Derivatives. Chem.—Eur. J. 2022, 28, e202202395 10.1002/chem.202202395. [DOI] [PubMed] [Google Scholar]
- Li Y.; Liou Y.-C.; Chen X.; Ackermann L. Thioether-Enabled Palladium-Catalyzed Atroposelective C-H Olefination for N-C and C-C Axial Chirality. Chem. Sci. 2022, 13, 4088–4094. 10.1039/D2SC00748G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Zhou X.; Shan W.; Liao R.; Deng Y.; Peng F.; Shao Z. Construction of Axially Chiral Indoles by Cycloaddition-Isomerization via Atroposelective Phosphoric Acid and Silver Sequential Catalysis. ACS. Catal. 2022, 12, 8094–8130. 10.1021/acscatal.2c02574. [DOI] [Google Scholar]
- Wang P.; Huang Y.; Jing J.; Wang F.; Li X. Rhodium(III)-Catalyzed Atroposelective Synthesis of C-N Axially Chiral Naphthylamines and Variants via C-H Activation. Org. Lett. 2022, 24, 2531–2535. 10.1021/acs.orglett.2c00686. [DOI] [PubMed] [Google Scholar]
- Niu C.; Zhou Y.; Chen Q.; Zhu Y.; Tang S.; Yu Z.; Sun J. Atroposelective Synthesis of N-Arylindoles via Enantioselective N-H Bond Insertion. Org. Lett. 2022, 24, 7428–7433. 10.1021/acs.orglett.2c03003. [DOI] [PubMed] [Google Scholar]
- Kim A.; Lee C.; Song J.; Lee S. K.; Kwon Y. All-Round Catalytic and Atroposelective Strategy via Dynamic Kinetic Resolution for N-/2-/3-Arylindoles. Nat. Commun. 2023, 14, 5502–5512. 10.1038/s41467-023-41299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.-J.; Li S.-W.; Oliveira J. A.; Li Y.; Chen X.; Zhang S.-Q.; Xu L.-C.; Rogge T.; Hong X.; Ackermann L. Data-Driven Design of New Chiral Carboxylic Acid for Construction of Indoles with C-Central and C-N Axial Chirality via Cobalt Catalysis. Nat. Commun. 2023, 14, 3149–3157. 10.1038/s41467-023-38872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Guo C.-Q.; Yao W.; Lu C.-J.; Li Y.; Paton R. S.; Liu R.-R. Pd-Catalyzed Asymmetric Amination of Enamines: Expedient Synthesis of Structurally Diverse N-C Atropisomers. ACS Catal. 2023, 13, 7680–7690. 10.1021/acscatal.3c00732. [DOI] [Google Scholar]
- Zhang M.; Zhao P.; Wu D.; Qiu Z.; Zhao C.; Zhang W.; Li F.; Zhou J.; Liu L. Brønsted Acid-Catalyzed Reaction of N-Arylnaphthalen-2-Amines with Quinone Esters for the Construction of Carbazole and C-N Axially Chiral Carbazole Derivatives. J. Org. Chem. 2023, 88, 2841–2850. 10.1021/acs.joc.2c02518. [DOI] [PubMed] [Google Scholar]
- Wang L.; Yuan W.-K.; Wang Z.-K.; Luo J.; Zhou T.; Shi B.-F. Synthesis of C-N Axial Chirality N-Arylindoles via Pd(II)-Catalyzed Free Amine-Directed Atroposelective C-H Olefination. Chin. J. Chem. 2023, 41, 2788–2792. 10.1002/cjoc.202300355. [DOI] [Google Scholar]
- Thönnißen V.; Atodiresei I. L.; Patureau F. W. Atroposelective Nenitzescu Indole Synthesis. Chem.—Eur. J. 2023, 29, e202300279 10.1002/chem.202300779. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Salamanca P.; de Gonzalo G.; Carmona J. A.; Lopez-Serrano J.; Iglesias-Sigu̇enza J.; Fernandez R.; Lassaletta J. M.; Hornillos V. Biocatalytic Atroposelective Synthesis of Axially Chiral N-Arylindoles via Dynamic Kinetic Resolution. ACS Catal. 2023, 13, 659–664. 10.1021/acscatal.2c06175. [DOI] [Google Scholar]
- Zhang H.-H.; Wang C.-S.; Li C.; Mei G.-J.; Li Y.; Shi F. Design and Enantioselective Construction of Axially Chiral Naphthyl-Indole Skeletons. Angew. Chem., Int. Ed. 2017, 56, 116–121. 10.1002/anie.201608150. [DOI] [PubMed] [Google Scholar]
- Qi L.-W.; Mao J.-H.; Zhang J.; Tan B. Organocatalytic Asymmetric Arylation of Indoles Enabled by Azo Groups. Nat. Chem. 2018, 10, 58–64. 10.1038/nchem.2866. [DOI] [PubMed] [Google Scholar]
- Bisag G. D.; Pecorari D.; Mazzanti A.; Bernardi L.; Fochi M.; Bencivenni G.; Bertuzzi G.; Corti V. Central-to-Axial Chirality Conversion Approach Designed on Organocatalytic Enantioselective Povarov Cycloadditions: First Access to Configurationally Stable Indole-Quinoline Atropisomers. Chem.—Eur. J. 2019, 25, 15694–15701. 10.1002/chem.201904213. [DOI] [PubMed] [Google Scholar]
- Ding W.-Y.; Yu P.; An Q.-J.; Bay K. L.; Xiang S.-H.; Li S.; Chen Y.; Houk K. N.; Tan B. DFT-Guided Phosphoric-Acid-Catalyzed Atroposelective Arene Functionalization of Nitrosonaphthalene. Chem. 2020, 6, 2046–2059. 10.1016/j.chempr.2020.06.001. [DOI] [Google Scholar]
- Mao J.-H.; Wang Y.-B.; Yang L.; Xiang S.-H.; Wu Q.-H.; Cui Y.; Lu Q.; Lv J.; Li S.; Tan B. Organocatalyst-Controlled Site-Selective Arene C-H Functionalization. Nat. Chem. 2021, 13, 982–991. 10.1038/s41557-021-00750-x. [DOI] [PubMed] [Google Scholar]
- Xu W.-L.; Zhao W.-M.; Zhang R.-X.; Chen J.; Zhou L. Organocatalytic Cycloaddition-Elimination Cascade for Atroposelective Construction of Heterobiaryls. Chem. Sci. 2021, 12, 14920–14926. 10.1039/D1SC05161J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Sun H.-R.; He R.-Q.; Yu L.; Hu W.; Chen J.; Yang S.; Zhang G.-G.; Zhou L. Organocatalytic Cycloaddition of Alkynylindoles with Azonaphthalenes for Atroposelective Construction of Indole-Based Biaryls. Nat. Commun. 2022, 13, 632–640. 10.1038/s41467-022-28211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.; Yu L.; Gao C.-H.; Cheng Q.; Deng S.; Jiao Y.; Tan W.; Shi F. Design and Synthesis of Axially Chiral Aryl-Pyrroloindoles via the Strategy of Organocatalytic Asymmetric [2 + 3] Cyclization. Fundam. Res. 2023, 3, 237–l248. 10.1016/j.fmre.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; Liu M.; Qian C.; Li P.; Dong M.; Li W. Asymmetric Organocatalytic [3 + 2] Annulation of Propargylic Alcohols with Indolylnaphthalenols: Synergistic Construction of Axial and Central Chirality. Org. Chem. Front. 2022, 10, 30–34. 10.1039/D2QO01625G. [DOI] [Google Scholar]
- Woldegiorgis A. G.; Gu H.; Lin X. Atroposelective Synthesis of Axially Chiral Styrenes Connecting an Axially Chiral Naphthyl-Indole Moiety Using Chiral Phosphoric Acid Catalysis. Org. Lett. 2023, 25, 2068–2072. 10.1021/acs.orglett.3c00425. [DOI] [PubMed] [Google Scholar]
- Liu Y.-W.; Chen Y.-H.; Cheng J. K.; Xiang S.-H.; Tan B. Enantioselective Synthesis of 3-Arylindole Atropisomers via Organocatalytic Indolization of Iminoquinones. Chem. Synth. 2023, 3, 11–18. 10.20517/cs.2022.46. [DOI] [Google Scholar]
- He C.; Hou M.; Zhu Z.; Gu Z. Enantioselective Synthesis of Indole-Based Biaryl Atropisomers via Palladium-Catalyzed Dynamic Kinetic Intramolecular C-H Cyclization. ACS Catal. 2017, 7, 5316–5320. 10.1021/acscatal.7b01855. [DOI] [Google Scholar]
- Xi C.-C.; Zhao X.-J.; Tian J.-M.; Chen Z.-M.; Zhang K.; Zhang F.-M.; Tu Y.-Q.; Dong J.-W. Atroposelective Synthesis of Axially Chiral 3-Arylindoles by Copper-Catalyzed Asymmetric Cross-Coupling of Indoles with Quinones and Naphthoquinones. Org. Lett. 2020, 22, 4995–5000. 10.1021/acs.orglett.0c01558. [DOI] [PubMed] [Google Scholar]
- Shaaban S.; Li H.; Otte F.; Strohmann C.; Antonchick A. P.; Waldmann H. Enantioselective Synthesis of Five-Membered-Ring Atropisomers with a Chiral Rh(III) Complex. Org. Lett. 2020, 22, 9199–9202. 10.1021/acs.orglett.0c03355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Zhao L.; Qi Z.; Li X. Construction of Atropisomeric 3-Arylindoles via Enantioselective Cacchi Reaction. Org. Lett. 2021, 23, 5901–5905. 10.1021/acs.orglett.1c02012. [DOI] [PubMed] [Google Scholar]
- Wang C.-S.; Wei L.; Fu C.; Wang X.-H.; Wang C.-J. Asymmetric Synthesis of Axially Chiral Naphthyl-C3-Indoles via a Palladium-Catalyzed Cacchi Reaction. Org. Lett. 2021, 23, 7401–7406. 10.1021/acs.orglett.1c02574. [DOI] [PubMed] [Google Scholar]
- Surgenor R. R.; Liu X.; Keenlyside M. J. H.; Myers W.; Smith M. D. Enantioselective Synthesis of Atropisomeric Indoles via Iron-Catalysed Oxidative Cross-Coupling. Nat. Chem. 2023, 15, 357–365. 10.1038/s41557-022-01095-9. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Franco C.; Ros A.; Merino P.; Fernández R.; Lassaletta J. M.; Hornillos V. Dynamic Kinetic Resolution of Indole-Based Sulfenylated Heterobiaryls by Rhodium-Catalyzed Atroposelective Reductive Aldol Reaction. ACS Catal. 2023, 13, 12134–12141. 10.1021/acscatal.3c03422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.-L.; Wang Z.; Yang H.; Chen J.; Wu Z.-B.; Lei Y.; Zhou L. Conversion of Two Stereocenters to One or Two Chiral Axes: Atroposelective Synthesis of 2,3-Diarylbenzoindoles. Chem. Sci. 2019, 10, 6777–6784. 10.1039/C9SC00810A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L.; Li K.; Xie C.; Li S.; Xu D.; Qin W.; Yan H. Organocatalytic Asymmetric Annulation of Ortho-Alkynylanilines: Synthesis of Axially Chiral Naphthyl-C2-Indoles. Angew. Chem., Int. Ed. 2019, 58, 17199–17204. 10.1002/anie.201908961. [DOI] [PubMed] [Google Scholar]
- He Y.-P.; Wu H.; Wang Q.; Zhu J. Palladium-Catalyzed Enantioselective Cacchi Reaction: Asymmetric Synthesis of Axially Chiral 2,3-Disubstituted Indoles. Angew. Chem., Int. Ed. 2020, 59, 2105–2109. 10.1002/anie.201914049. [DOI] [PubMed] [Google Scholar]
- Xu D.; Huang S.; Hu F.; Peng L.; Jia S.; Mao H.; Gong X.; Li F.; Qin W.; Yan H. CCS Chem. 2022, 4, 2686–2697. 10.31635/ccschem.021.202101154. [DOI] [Google Scholar]
- Jia S.; Tian Y.; Li X.; Wang P.; Lan Y.; Yan H. Atroposelective Construction of Nine-Membered Carbonate-Bridged Biaryls. Angew. Chem., Int. Ed. 2022, 61, e202206501 10.1002/anie.202206501. [DOI] [PubMed] [Google Scholar]
- Yin S.-Y.; Pan C.; Zhang W.-W.; Liu C.-X.; Zhao F.; Gu Q.; You S.-L. Org. Lett. 2022, 24, 3620–3625. 10.1021/acs.orglett.2c01141. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li Q.; Shao Y.; Sun J. Atroposelective Synthesis of Axially Chiral C2-Arylindoles via Rhodium-Catalyzed Asymmetric C-H Bond Insertion. Org. Lett. 2022, 24, 4670–4674. 10.1021/acs.orglett.2c01818. [DOI] [PubMed] [Google Scholar]
- Yu L.; Liu J.; Xiang S.; Lu T.; Ma P.; Zhao Q. Silver-Catalyzed Direct Nucleophilic Cyclization: Enantioselective De Novo Synthesis of C-C Axially Chiral 2-Arylindoles. Org. Lett. 2023, 25, 522–527. 10.1021/acs.orglett.2c04234. [DOI] [PubMed] [Google Scholar]
- Bao W.; Chen Y.-H.; Liu Y.-W.; Xiang S.-H.; Tan B. Atroposelective Synthesis of 2-Arylindoles via Chiral Phosphoric Acid-Catalyzed Direct Amination of Indoles. Chin. J. Chem. 2024, 42, 731–735. 10.1002/cjoc.202300589. [DOI] [Google Scholar]
- Ma C.; Jiang F.; Sheng F.-T.; Jiao Y.; Mei G.-J.; Shi F. Design and Catalytic Asymmetric Construction of Axially Chiral 3,3′-Bisindole Skeletons. Angew. Chem., Int. Ed. 2019, 58, 3014–3020. 10.1002/anie.201811177. [DOI] [PubMed] [Google Scholar]
- Sheng F.-T.; Li Z.-M.; Zhang Y.-Z.; Sun L.-X.; Zhang Y.-C.; Tan W.; Shi F. Atroposelective Synthesis of 3,3′-Bisindoles Bearing Axial and Central Chirality: Using Isatin-Derived Imines as Electrophiles. Chin. J. Chem. 2020, 38, 583–589. 10.1002/cjoc.202000022. [DOI] [Google Scholar]
- Chen K.-W.; Wang Z.-S.; Wu P.; Yan X.-Y.; Zhang S.; Zhang Y.-C.; Shi F. Catalytic Asymmetric Synthesis of 3,3′-Bisindoles Bearing Single Axial Chirality. J. Org. Chem. 2020, 85, 10152–10166. 10.1021/acs.joc.0c01528. [DOI] [PubMed] [Google Scholar]
- Sheng F.-T.; Yang S.; Wu S.-F.; Zhang Y.-C.; Shi F. Catalytic Asymmetric Synthesis of Axially Chiral 3,3′-Bisindoles by Direct Coupling of Indole Rings. Chin. J. Chem. 2022, 40, 2151–2160. 10.1002/cjoc.202200327. [DOI] [Google Scholar]
- Wang H.-Q.; Wu S.-F.; Yang J.-R.; Zhang Y.-C.; Shi F. Design and Organocatalytic Asymmetric Synthesis of Indolyl-Pyrroloindoles Bearing Both Axial and Central Chirality. J. Org. Chem. 2023, 88, 7684–7702. 10.1021/acs.joc.2c02303. [DOI] [PubMed] [Google Scholar]
- Tian M.; Bai D.; Zheng G.; Chang J.; Li X. Rh(III)-Catalyzed Asymmetric Synthesis of Axially Chiral Biindolyls by Merging C-H Activation and Nucleophilic Cyclization. J. Am. Chem. Soc. 2019, 141, 9527–9532. 10.1021/jacs.9b04711. [DOI] [PubMed] [Google Scholar]
- He X.-L.; Zhao H.-R.; Song X.; Jiang B.; Du W.; Chen Y.-C. Asymmetric Barton-Zard Reaction To Access 3-Pyrrole-Containing Axially Chiral Skeletons. ACS Catal. 2019, 9, 4374–4381. 10.1021/acscatal.9b00767. [DOI] [Google Scholar]
- Zhang P.; Xu Q.; Wang X.-M.; Feng J.; Lu C.-J.; Li Y.; Liu R.-R. Enantioselective Synthesis of N-N Bisindole Atropisomers. Angew. Chem., Int. Ed. 2022, 61, e202212101 10.1002/anie.202212101. [DOI] [PubMed] [Google Scholar]
- Chen Z.-H.; Li T.-Z.; Wang N.-Y.; Ma X.-F.; Ni S.-F.; Zhang Y.-C.; Shi F. Organocatalytic Enantioselective Synthesis of Axially Chiral N,N′-Bisindoles. Angew. Chem., Int. Ed. 2023, 62, e202300419 10.1002/anie.202300419. [DOI] [PubMed] [Google Scholar]
- Wang L.-Y.; Miao J.; Zhao Y.; Yang B.-M. Chiral Acid-Catalyzed Atroposelective Indolization Enables Access to 1,1′-Indole-Pyrroles and Bisindoles Bearing a Chiral N-N Axis. Org. Lett. 2023, 25, 1553–1557. 10.1021/acs.orglett.3c00237. [DOI] [PubMed] [Google Scholar]
- Yao W.; Lu C.-J.; Zhan L.-W.; Wu Y.; Feng J.; Liu R.-R. Enantioselective Synthesis of N-N Atropisomers by Palladium-Catalyzed C-H Functionalization of Pyrroles. Angew. Chem., Int. Ed. 2023, 62, e202218871 10.1002/anie.202218871. [DOI] [PubMed] [Google Scholar]
- Yin S.-Y.; Zhou Q.; Liu C.-X.; Gu Q.; You S.-L. Enantioselective Synthesis of N-N Biaryl Atropisomers through Iridium(I)-Catalyzed C-H Alkylation with Acrylates. Angew. Chem., Int. Ed. 2023, 62, e202305067 10.1002/anie.202305067. [DOI] [PubMed] [Google Scholar]
- Wang C.-S.; Xiong Q.; Xu H.; Yang H.-R.; Dang Y.; Dong X.-Q.; Wang C.-J. Organocatalytic Atroposelective Synthesis of Axially Chiral N,N′-Pyrrolylindoles via De Novo Indole Formation. Chem. Sci. 2023, 14, 12091–12097. 10.1039/D3SC03686C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-Y.; Sun M.; Yu X.-Y.; Zhang Y.-C.; Tan W.; Shi F. Atroposelective Construction of Axially Chiral Alkene-Indole Scaffolds via Catalytic Enantioselective Addition Reaction of 3-Alkynyl-2-Indolylmethanols. Chin. J. Chem. 2021, 39, 2163–2171. 10.1002/cjoc.202100214. [DOI] [Google Scholar]
- Wang F.; Jing J.; Zhao Y.; Zhu X.; Zhang X.-P.; Zhao L.; Hu P.; Deng W.-Q.; Li X. Rhodium-Catalyzed C-H Activation-Based Construction of Axially and Centrally Chiral Indenes through Two Discrete Insertions. Angew. Chem., Int. Ed. 2021, 60, 16628–16633. 10.1002/anie.202105093. [DOI] [PubMed] [Google Scholar]
- Wu Y.-X.; Liu Q.; Zhang Q.; Ye Z.; He Y. Asymmetric Allylic Substitution-Isomerization for Accessing Axially Chiral Vinylindoles by Intramolecular π. . . π Stacking Interactions. Cell Reports Physical Science 2022, 3, 101005–101016. 10.1016/j.xcrp.2022.101005. [DOI] [Google Scholar]
- Mi R.; Chen H.; Zhou X.; Li N.; Ji D.; Wang F.; Lan Y.; Li X. Rhodium-Catalyzed Atroposelective Access to Axially Chiral Olefins via C-H Bond Activation and Directing Group Migration. Angew. Chem., Int. Ed. 2022, 61, e202111860 10.1002/anie.202111860. [DOI] [PubMed] [Google Scholar]
- Ji D.; Jing J.; Wang Y.; Qi Z.; Wang F.; Zhang X.; Wang Y.; Li X. Palladium-Catalyzed Asymmetric Hydrophosphination of Internal Alkynes: Atroposelective Access to Phosphine-Functionalized Olefins. Chem. 2022, 8, 3346–3362. 10.1016/j.chempr.2022.08.019. [DOI] [Google Scholar]
- Zhan L.-W.; Lu C.-J.; Feng J.; Liu R.-R. Atroposelective Synthesis of C-N Vinylindole Atropisomers by Palladium-Catalyzed Asymmetric Hydroarylation of 1-Alkynylindoles. Angew. Chem., Int. Ed. 2023, 62, e202312930 10.1002/anie.202312930. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Wu Qi.; Ren M.; Zhang H.; Zhang X.; Liu J.; Fu Z. N-Heterocyclic Carbene-Catalyzed Atroposelective Synthesis of 5-Indo-1-yl Pyran-2-ones with an N-C Axis from Enals. Adv. Synth.Catal. 2023, 365, 3467–3472. 10.1002/adsc.202300686. [DOI] [Google Scholar]
- Mei G.-J.; Shi F. Indolylmethanols as Reactants in Catalytic Asymmetric Reactions. J. Org. Chem. 2017, 82, 7695–7707. 10.1021/acs.joc.7b01458. [DOI] [PubMed] [Google Scholar]
- Zhang H.-H.; Shi F. Advances in Catalytic Asymmetric Reactions Using 2-Indolylmethanols as Platform Molecules. Chin. J. Org. Chem. 2022, 42, 3351–3372. 10.6023/cjoc202203018. [DOI] [Google Scholar]
- Shi Y.-C.; Yan X.-Y.; Wu P.; Jiang S.; Xu R.; Tan W.; Shi F. Design and Application of m-Hydroxybenzyl Alcohols in Regioselective [3 + 3] Cycloadditions of 2-Indolymethanols. Chin. J. Chem. 2023, 41, 27–36. 10.1002/cjoc.202200503. [DOI] [Google Scholar]
- Zhang Y.-C.; Jiang F.; Shi F. Organocatalytic Asymmetric Synthesis of Indole-Based Chiral Heterocycles: Strategies, Reactions, and Outreach. Acc. Chem. Res. 2020, 53, 425–446. 10.1021/acs.accounts.9b00549. [DOI] [PubMed] [Google Scholar]
- Hang Q.-Q.; Wu S.-F.; Yang S.; Wang X.; Zhong Z.; Zhang Y.-C.; Shi F. Design and Catalytic Atroposelective Synthesis of Axially Chiral Isochromenone-Indoles. Sci. China Chem. 2022, 65, 1929–1937. 10.1007/s11426-022-1363-y. [DOI] [Google Scholar]
- Zhang J.-Y.; Chen J.-Y.; Gao C.-H.; Yu L.; Ni S.-F.; Tan W.; Shi F. Asymmetric [4+n] Cycloadditions of Indolyldimethanols for the Synthesis of Enantioenriched Indole-Fused Rings. Angew. Chem., Int. Ed. 2023, 62, e202305450 10.1002/anie.202305450. [DOI] [PubMed] [Google Scholar]
- Wang J.-Y.; Gao C.-H.; Ma C.; Wu X.-Y.; Ni S.-F.; Tan W.; Shi F. Design and Catalytic Asymmetric Synthesis of Furan-Indole Compounds Bearing both Axial and Central Chirality. Angew. Chem., Int. Ed. 2024, 63, e202316454 10.1002/anie.202316454. [DOI] [PubMed] [Google Scholar]
- Wang H.-Q.; Yang S.; Zhang Y.-C.; Shi F. Advances in Catalytic Asymmetric Reactions Involving o-Hydroxybenzyl Alcohols. Chin. J. Org. Chem. 2023, 43, 974–999. 10.6023/cjoc202211022. [DOI] [Google Scholar]
- Yang S.; Wang N.-Y.; Hang Q.; Zhang Y.-C.; Shi F. Advances in Catalytic Asymmetric Reactions Involving o-Hydroxyphenyl Substituted p-Quinone Methides. Acta Chim. Sinica 2023, 81, 793–808. 10.6023/A23040192. [DOI] [Google Scholar]
- Dorsch C.; Schneider C. Asymmetric Brønsted Acid Catalyzed Cycloadditions of Ortho-Quinone Methides and Related Compounds. Synthesis 2022, 54, 3125–3141. 10.1055/a-1781-6538. [DOI] [Google Scholar]
- Akiyama T. Stronger Brønsted Acids. Chem. Rev. 2007, 107, 5744–5758. 10.1021/cr068374j. [DOI] [PubMed] [Google Scholar]
- Terada M. Binaphthol-Derived Phosphoric Acid as a Versatile Catalyst for Enantioselective Carbon-Carbon Bond Forming Reactions. Chem. Commun. 2008, 2008, 4097–4112. 10.1039/b807577h. [DOI] [PubMed] [Google Scholar]
- Terada M. Chiral Phosphoric Acids as Versatile Catalysts for Enantioselective Transformations. Synthesis 2010, 2010, 1929–1982. 10.1055/s-0029-1218801. [DOI] [Google Scholar]
- Yu J.; Shi F.; Gong L.-Z. Brønsted-Acid-Catalyzed Asymmetric Multicomponent Reactions for the Facile Synthesis of Highly Enantioenriched Structurally Diverse Nitrogenous Heterocycles. Acc. Chem. Res. 2011, 44, 1156–1171. 10.1021/ar2000343. [DOI] [PubMed] [Google Scholar]
- Parmar D.; Sugiono E.; Raja S.; Rueping M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. 10.1021/cr5001496. [DOI] [PubMed] [Google Scholar]
- Xia Z.-L.; Xu-Xu Q.-F.; Zheng C.; You S.-L. Chiral Phosphoric Acid-Catalyzed Asymmetric Dearomatization Reactions. Chem. Soc. Rev. 2020, 49, 286–300. 10.1039/C8CS00436F. [DOI] [PubMed] [Google Scholar]
- Lin X.; Wang L.; Han Z.; Chen Z. Chiral Spirocyclic Phosphoric Acids and Their Growing Applications. Chin. J. Chem. 2021, 39, 802–824. 10.1002/cjoc.202000446. [DOI] [Google Scholar]
- Cheng J. K.; Xiang S.-H.; Tan B. Imidodiphosphorimidates (IDPis): Catalyst Motifs with Unprecedented Reactivity and Selectivity. Chin. J. Chem. 2023, 41, 685–694. 10.1002/cjoc.202200618. [DOI] [Google Scholar]
- CCDC 2326447 for 3aa, CCDC 2326448 for rac-4ak; see the Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.