ABSTRACT
Background:
Understanding sexual maturity and bone fusion is crucial in forensic investigations and legal contexts. This study investigates the contemporary trend of sexual maturity and bone fusion in females, comparing the observed age-related characteristics with documented ages. Such analyses contribute to the accuracy of age estimation methods used in medico-legal scenarios.
Methods:
A comprehensive study was conducted involving a diverse sample of females, and their sexual maturity and bone fusion were assessed relative to documented ages. The research utilized a cross-sectional design, collecting data through interviews, medical examinations, and radiographic imaging. Ethical approval was obtained, and participants provided informed consent. Variables included documented age, age of menarche, stages of sexual maturity, dentition, and bone fusion. Validation of model performance was conducted.
Results:
Among 70 cases studied, the mean age of menarche was 10.48 years (range: 7–12 years). Maternal reports indicated menarche onset between 12–17 years. Tanner’s Staging 5 for pubic hair and fully developed breasts occurred at 14 years. Permanent teeth increased with age, with 28 or more teeth present at age 14 or above. Fusion of the distal end of the Radius and Ulna with the parent bone was positive from 15 years onwards. The regression model predicted age accurately (97.99% fit).
Conclusion:
The results highlight the early onset of sexual maturation, consistent teeth eruption, and bone fusion patterns in females. The observed correlations offer valuable insights for medicolegal practitioners and forensic experts.
Keywords: Bone fusion, dentition, menarche, sexual maturity
Introduction
Determining the precise age of individuals, particularly females, holds paramount importance across diverse fields including law, medicine, and social welfare. Age assessment methodologies traditionally rely on secondary sexual characteristics, teeth eruption patterns, and radiological examinations. The maturation of the dentition serves as a means to evaluate the maturity and approximate age across various fields, such as anthropology, archaeology, forensic science, pediatric dentistry, and orthodontics.[1] A child’s bone age provides a more accurate representation of their biological and structural maturity than the chronological age calculated from their date of birth.[2] The Tanner Staging, also known as Sexual Maturity Rating (SMR), is utilized by healthcare professionals as an objective method to document and track the development and order of secondary sexual traits in adolescents throughout puberty.[3] However, contemporary observations suggest a notable shift in these indicators, with even very young children displaying early manifestations of sexual maturation. The developmental stage of a maturing individual may not always align with their chronological age.[4] This phenomenon presents a formidable challenge in accurately gauging the age of females using conventional methods. The dynamic nature of biological development in females necessitates a comprehensive understanding of the current trends in sexual maturity, teeth eruption, and bone fusion. It is imperative to evaluate these age-related markers against documented ages to assess the reliability and validity of existing age-determination protocols. Thus, this study aims to scrutinize the contemporary trends in sexual maturity, teeth eruption patterns, and bone fusion in females, while juxtaposing these observations with documented ages. The main aim and objective of this study were (i) to investigate the current trend of sexual maturity, teeth eruption, and bone fusion in females, and (ii) to compare the observed age-related characteristics with documented ages.
Materials and Methods
Study design
This research adopts a prospective cross-sectional, observational study design to investigate the current trends of sexual maturity, teeth eruption, and bone fusion in females relative to documented age.
Data collection
Data collection involves a comprehensive approach utilizing interviews, medical examinations, and radiographic imaging techniques.
Ethical consideration
The study protocol has received approval from the Institutional Ethics Committee (IEC), ensuring adherence to ethical guidelines vide 065 / 2023/TMCH dated 09 / 09/2023. Informed consent is obtained from their legal guardians and assent has been obtained from the study participants before their inclusion in the study.
Variables
The variables under investigation include documented age, age of menarche, stages of sexual maturity (based on Tanner’s staging), dentition, and bone fusion.
Sampling method
A stratified random sampling technique is employed to ensure representation across different age groups. Participants consist of females aged between 5 and 18 years, selected from individuals undergoing medico-legal examination at the Department of Forensic Medicine of a tertiary care medical institute in Northeastern India. Five participants are selected from each age group.
Exclusion criteria
Individuals with growth-related disorders or a history of delayed milestones are excluded from the study to maintain the homogeneity of the sample population.
Study tools
Data collection tools include documented age proof certificates, a questionnaire regarding menarche, Tanner’s staging for sexual maturity assessment, the FDI (Fédération Dentaire Internationale) criteria for evaluating dentition, and radiographs for bone fusion assessment.[5]
Statistical analysis
Statistical analysis of the collected data is performed using Microsoft Excel version 10.0 for data organization and initial analysis.[6] Advanced statistical analyses are conducted using Python 3.9 programming language to explore relationships and patterns within the dataset, facilitating robust interpretation of the findings.[7] Multiple Linear regression Analysis has been performed to predict the age of an individual. F-Test (ANOVA)-Analysis of variance, in multiple linear regression model was used to determine if the complex model is performing better than simpler ones. Statsmodel has been used to calculate F statistics.
Model validation
After building the model, we need to validate its performance. We evaluated this model by looking at its coefficient of determination (R2), F-test (ANOVA), t-test, and residuals. We validated our model further by performing a Residual Analysis. Residuals are differences in the observed values and the corresponding predicted values in our model. We checked our data for Linearity, Normality, Multicollinearity, and Homoscedasticity.
Results
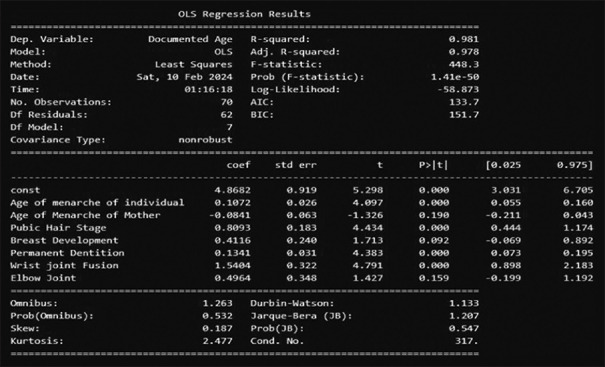
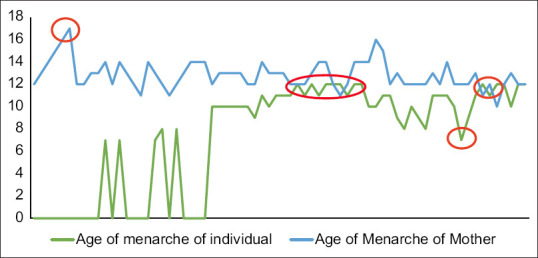
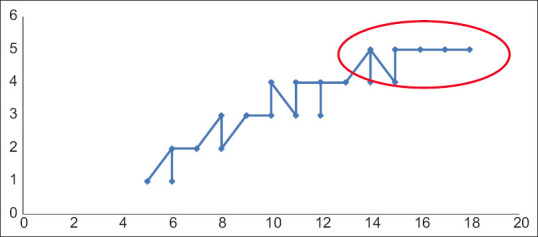
Out of 70 cases studied, the mean age of menarche of the study participants was 10.48 years, the highest age being 12 years and the lowest being 7 years, as depicted. On questioning their mothers, the highest age of menarche was found at 17 years and the lowest at 12 years, as depicted in Figure 1. The X-axis denotes the number of cases. Y axis the age of menarche. Each spike has points denoting one case. Those touching the baseline (0), stand for those cases who had not yet attained menarche [Figure 1]. Tanners Staging 5 for the development of pubic hair was obtained at the age of 14 years as shown in Figure 2. Tanners staging 5 for fully developed breasts was attained at the age of 14 years as shown in Figure 3. The number of permanent teeth increased with age and age 14 or above showed the presence of 28 teeth or more. From 15 years fusion of the distal end of the Radius and Ulna with the parent bone was found to be positive in 25% of cases, by 16 years in 75% of cases, and by 17 years in 100% of the cases.
Figure 1.
Age of menarche of the individuals as compared to the age of menarche of their mothers
Figure 2.
Pubic hair staging (y-axis) with respect to age of the participant (x-axis)
Figure 3.
Breast development staging (y-axis) with respect to age of the participant (x-axis)
Exploratory data analysis insights
Wrist joint fusion and elbow joint are skewed.
Permanent dentition shows a positive correlation between pubic hair stage and breast development.
Documented ages positively correlate with pubic hair stage, breast development, and permanent dentition.
Documented age shows a normal distribution.
The age of menarche shows a skewed distribution.
Insights from elbow joint and wrist joint
The false category has a normal distribution (quartile box and equidistant whiskers) for both the wrist and elbow joints.
For the elbow joint, there are a few true outliers.
For the wrist joint, most of the values are in the quartiles.
There are more false categories in both features than true values, which is alright since the dataset consists of individuals up until 20 years of age. And the percentage difference is alright too because these developments happen in adulthood.
We concluded that both variables have a significant impact on the determination of either age or age of menarche.
Multiple linear regression model
The dependent variable is age against the rest of the variables which are considered as our independent variables. Here, we have built a regression model that can predict the age of an individual based on the other variables. The dataset has been split into a 70:30 ratio. The model is built on 70% of the data and tested against the rest 30%. The linear model is: Y =4.9837 + 0.12805* Age of menarche of individual +0.090311* Age of Menarche of Mother +0.71906* Pubic Hair Stage +0.46661* Breast Development +0.13294* Permanent Dentition +1.85423* Wrist joint Fusion +0.231* Elbow Joint
Highlights of the model
A total of 97.99% of the data fit the regression model.
Mean absolute is 0.56 for the model, mean square error and root mean square error are closer to zero.
If we try to predict the documented age of an individual with the below stats, we get the age as 8.9 years:
Age of menarche of individual =12, Age of menarche of mother =17, Pubic Hair stage =2, Breast Development =2, Permanent Dentition =10, Wrist joint Fusion =0, Elbow Joint =1 (where 0 stands for non-fusion and 1 stands for complete fusion)
Model validation
Our R2 score: 0.9798586098928078 r2 ranges between 0 and 1, where 0 means there is no linear relationship between variables and a value of 1 shows a perfect relationship. A total of 97.9% of our independent variables can be explained using our independent variables. In ANOVA and F stats, R2 is higher than linear regression, 0.981. F-Statistic is 448.3, much greater than 1>. Since our dataset is small, 560 data points, it demonstrates that there is a strong relationship between our independent variables and documented age. Looking at the P-value, we can conclude the statistical significance of the variable, or lack thereof. The age of menarche of the mother and wrist joint is not statistically significant. Removing these predictors might reduce R2 value but we might make better predictions of the age of an individual. These findings can be seen in Figure 4. In linearity, we have found that there is a linear relationship between documented age and independent variables as depicted in Figure 5. Residuals are normally distributed as depicted in Figure 6. When collinearity was checked: (a) pubic hair stage, breast development and permanent dentition have a high positive correlation to each other, along with documented age; (b) Age of menarche of individuals is also highly correlated to these and (c) the age of menarche of the mother is the only feature which shows lower and negative correlation to the other features, as shown in Figure 7. Residuals of our model are randomly scattered across the 0 line, which indicates a well-performing model as shown in Figure 8.
Figure 4.
Regression analysis
Figure 5.
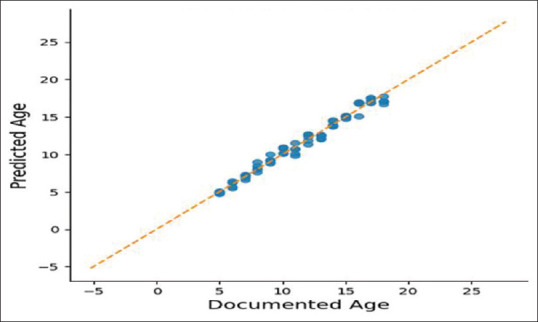
Linearity assumption
Figure 6.
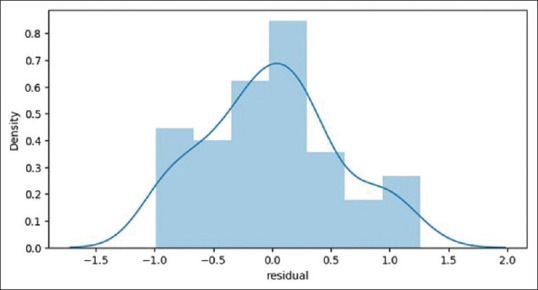
Residual distribution
Figure 7.
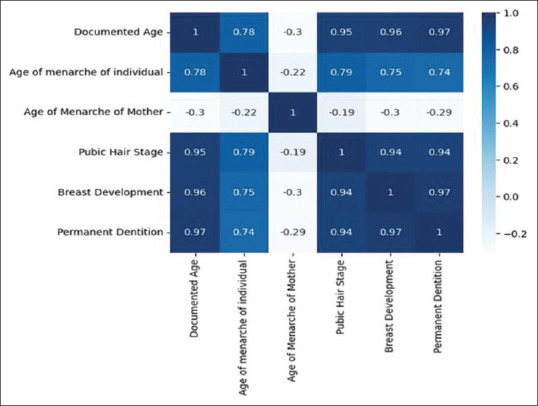
Collinearity between the variables
Figure 8.
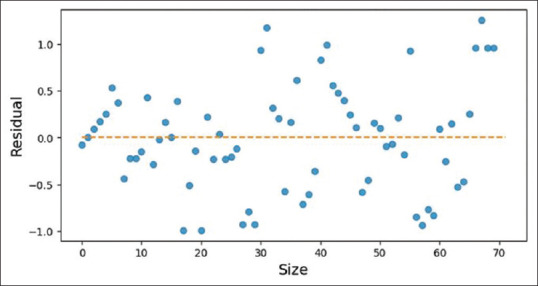
Homescedasticity assumption of the residuals
Discussion
Adolescence marks a period of intricate and swift transformations encompassing hormonal, physical, and cognitive aspects. Tanner Staging, or Sexual Maturity Rating (SMR), serves as an objective system employed by healthcare providers to record and monitor the progression and sequence of secondary sexual characteristics in children during puberty. Originating from a longitudinal study conducted by Marshall and Tanner in England from the 1940s to 1960s, this classification system includes scales for the development of external genitalia, such as the phallus, scrotum, and testes volume in males, as well as breasts in females, and pubic hair in both genders.[3] Many researchers have shown that individuals in the earlier stages of puberty tended to make more hazardous decisions in contrast to those in later Tanner stages.[8]
Changes in how genes are expressed, controlled by DNA methylation, may affect how quickly puberty happens.[9] Research on precocious puberty in China showed the unadjusted and adjusted prevalence rates of precocious puberty were 5.01%.[10] In our study, we have found the mean age of menarche among the participants was 10.48 years while research conducted in other parts of India found it to be 13.13 ± 1.23 years and 13 ± 1.1 years.[11,12] In this study, the participants reached Tanner Stage 5 for both pubic hair and breast development at the age of 14 years, indicating full maturation in these aspects while some research focuses that in females, the duration of breast development, on average, was lengthier, while the pace of development was comparatively slower than that of pubic hair.[13] Regarding Fusion of Radius and Ulna, a radiological study of 149 Northwest Indian schoolgirls aged 11–19 assessed epiphyseal fusion at elbow and wrist joints. Findings suggest girls <16 years have incomplete fusion at the humerus and radius epiphyses, while those >16 years exhibit complete fusion at the radius and ulna distal epiphyses.[14] Another study Analysis of 160 healthy subjects aged 12–20 revealed fusion began at 13–14 years (radius) and 13–14 years (ulna), completing at 17–18 years.[15] In our study, we found 100% fusion of the radius and ulna at the age of 17 years. One study investigates the mean age of menarche among adolescent girls and their mothers in an SRM field practice area in Mamandur, noting a decline in the mean age of menarche among Indian women. Results reveal that adolescent girls experience menarche at 12.5 years while their mothers at 14 years.[16] In our study too we have found a decline in the age of menarche among generations. As for the multiple linear regression model developed, the skewed distribution suggests potential variations in the timing of skeletal maturity among individuals. Permanent dentition, pubic hair stage, breast development, and documented ages show positive correlations, indicating that these factors may influence each other and contribute to overall development. The regression model constructed aims to predict the age of an individual based on various independent variables, including the age of menarche (both individual and maternal), pubic hair stage, breast development, permanent dentition, wrist joint fusion, and elbow joint. The model demonstrates a high fit to the data, with approximately 97.99% of the data fitting the regression model, indicating strong predictive power. Mean absolute error, mean square error, and root mean square error are low, indicating good model performance. Using the model, the age of an individual can be predicted based on the provided statistics. The R2 score is high (0.9798586098928078), indicating that a significant portion of the variance in the documented age can be explained by the independent variables. The F-statistic is high, suggesting a strong relationship between the independent variables and the documented age. Some variables, such as the age of menarche of the mother and wrist joint, are found not to be statistically significant. Removing these predictors may enhance the model’s predictive accuracy. The analysis confirms a linear relationship between the documented age and independent variables, and residuals are normally distributed, indicating a well-performing model. There are high positive correlations among pubic hair stage, breast development, permanent dentition, and documented age, suggesting multicollinearity, which should be considered when interpreting the model results. This analysis provides valuable insights into the developmental factors influencing the documented age of individuals, and the regression model offers a robust framework for predicting age based on various developmental parameters. Ongoing refinement and validation of the model could further enhance its predictive accuracy and applicability. Primary care physicians are often the frontline healthcare providers involved in the initial assessment of individuals, particularly children and adolescents, during routine check-ups or medical consultations. Primary care physicians are adept at identifying and monitoring developmental milestones, including signs of sexual maturation, teeth eruption, and bone fusion, which are essential indicators of biological development. Moreover, they may refer individuals to specialists for further evaluation when necessary, such as pediatric endocrinologists or orthodontists. In addition to their clinical role, primary care physicians contribute valuable data, insights, and clinical observations to research initiatives aimed at understanding trends in female development. By applying findings from research studies, primary care physicians can enhance their clinical practice, incorporating evidence-based approaches for more accurate age estimation and appropriate healthcare interventions tailored to individual patients.
Conclusion
The contemporary trends of sexual maturity, teeth eruption patterns, and bone fusion in females, juxtaposed against documented ages, show a decline in the age of menarche, earlier teeth eruption, and bone fusion. Findings reveal a dynamic landscape of biological development, with significant variability observed across different age groups. The multiple linear regression model constructed exhibits strong predictive power, with approximately 97.99% of the data fitting the model. Mean absolute error, mean square error, and root mean square error are low, indicating good model performance. However, some variables, such as the age of menarche of the mother and wrist joint fusion, were found not to be statistically significant, suggesting potential avenues for refining the model. Overall, this analysis provides valuable insights into the intricate interplay of developmental factors influencing the documented age of individuals, offering a robust framework for age prediction based on these parameters. Such notability of change in milestones of the general population can bring change in health policies and laws of the land.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We sincerely acknowledge the departmental staff of Tezpur Medical College and Hospital for their help.
References
- 1.Manjunatha BS, Soni NK. Estimation of age from development and eruption of teeth. J Forensic Dent Sci. 2014;6:73–6. doi: 10.4103/0975-1475.132526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manzoor MA, Hassan N, Ahmed A. Bone age assessment methods: A critical review. Pak J Med Sci. 2014;30:211–5. doi: 10.12669/pjms.301.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greil H, Kahl H. Assessment of developmental age: Cross-sectional analysis of secondary sexual characteristics. Anthropol Anz. 2005;63:63–75. [PubMed] [Google Scholar]
- 5.Turp JC, Alt KW. Designating teeth: The advantages of the FDI's two-digit system. Quintessence Int. 1995;26:501–4. [PubMed] [Google Scholar]
- 6.Microsoft Excel Version 10.0. Microsoft Corporation. 2018. [[Last accessed on 2024 Mar 10]]. Available from: https://office.microsoft.com/excel .
- 7.Python 3.9, Delaware. 2020. [[Last accessed on 2024 Mar 10]]. Available from: https://www.python.org/downloads/release/python-390/
- 8.Dir AL, Hummer TA, Aalsma MC, Hulvershorn LA. Pubertal influences on neural activation during risky decision-making in youth with ADHD and disruptive behavior disorders. Dev Cogn Neurosci. 2019 doi: 10.1016/j.dcn.2019.100634. doi: 10.1016/j.dcn.2019.100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Y, Sánchez BN, Goodrich JM, Dolinoy DC, Cantoral A, Mercado-Garcia A, et al. Dietary exposures, epigenetics and pubertal tempo. Environ Epigenet. 2019;5:dvz002. doi: 10.1093/eep/dvz002. doi: 10.1093/eep/dvz002.PMC6404688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Ni J, Zhang L, Yu T, Li X, Xue P, et al. The prevalence of precocious puberty among children in Qufu City, Shandong Province, China, a population-based study. Front Endocrinol. 2022;25(13):910119. doi: 10.3389/fendo.2022.910119. [Google Scholar]
- 11.Bajpai A, Bansal U, Rathoria R, Rathoria E, Singh V, Singh GK, et al. A prospective study of the age at menarche in north Indian girls, its association with the tanner stage, and the secular trend. Cureus. 2023;15:e45383. doi: 10.7759/cureus.45383. doi: 10.7759/cureus.45383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omidvar S, Amiri FN, Bakhtiari A, Begum K. A study on menstruation of Indian adolescent girls in an urban area of South India. J Fam Med Prim Care. 2018;7:698. doi: 10.4103/jfmpc.jfmpc_258_17. doi: 10.4103/jfmpc.jfmpc_258_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunddorf LLH, Ramlau-Hansen CH, Arendt LH, Patton GC, Sawyer SM, Dashti SG, et al. Characteristics of puberty in a population-based sample of danish adolescents. J Adol Health. 2023 doi: 10.1016/j.jadohealth.2023.10.005. doi: 10.1016/j.jadohealth.2023.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Sahini D, Jit I. Time of fusion of epiphyses at the elbow and wrist joints in girls of northwest India. Forensic Sci Int. 1995;74:47–55. doi: 10.1016/0379-0738(95)01736-3. [DOI] [PubMed] [Google Scholar]
- 15.Hasan N, Noor F, Ahmad S, Fazili KM. Age of fusion of the distal radial and ulnar epiphyses from hand radiographs-A study in Kashmiri population. Sci Justice J Forensic Sci Soc. 2016;56:431–6. doi: 10.1016/j.scijus.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Ramraj B, Subramanian VM, Vijayakrishnan G. Study on age of menarche between generations and the factors associated with it. Clin Epidemiol Glob Health. 2021;1:100758. doi: 10.1016/j.cegh.2021.100758. [Google Scholar]