ABSTRACT
Diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes; so, a nerve conduction study (NCS) is conducted to detect the type of neuropathy that is present. To discuss the NCS findings in diabetic patients. An observational study was conducted in the Physiology Department of AIIMS, Bhopal, in collaboration with the Medicine Department of the Institute. Seventy-two diagnosed type 2 diabetes mellitus (T2DM) patients were examined using NCS (Nihon Kohden Neuropack XI Machine). Microsoft Excel was utilized for data compilation and result analysis. Based on NCS, 94% of patients were abnormal, and 6% were normal. Of abnormal patients, 89% had asymmetrical involvement, and 5% had symmetrical involvement. About 74% had mixed neuropathy, 11% had motor neuropathy, and 10% had sensory neuropathy. Mixed involvement was seen in 60% of patients and axonal involvement in 35% of patients, and 5% were normal. Lower limb involvement was seen predominately. The most common bilaterally involved motor nerve was the peroneal nerve, seen in 49% of cases, whereas the most common bilaterally involved sensory nerve was the sural nerve involved in 59% of cases. The left tibial nerve was the most common unilaterally involved motor nerve seen in 32% of cases, and the left sural nerve was the most common sensory nerve involved in 54% of cases. Asymmetric sensorimotor involvement with mixed involvement (axonal + demyelinating) was seen in diabetic patients. Peroneal and sural nerves were the most common bilaterally involved motor and sensory nerves, respectively. Similarly, the left tibial and left sural nerves were the most common unilaterally affected motor and sensory nerves, respectively.
Keywords: Axonal, demyelinating, diabetes mellitus, electrophysiological testing, motor, nerve conduction study, neuropathy, sensory
Introduction
India has observed rising prevalence of diabetes in the last few decades with the diabetic population hitting an alarming mark of 69.9 million by 2025 and 80 million by 2030 deeming it to be the world’s capital of diabetes.[1] Due to chronically high blood sugar in diabetes, major complications known as diabetic peripheral neuropathy (DPN) are caused, which results in nerve damage.
DPN remains asymptomatic in its early stages but as soon as overt deficits occur, they cannot be reversed back; hence, early diagnosis and timely intervention serve as a boon. It still represents an enormous burden for clinicians and health systems across the globe due to difficult diagnosis, high cost of treatment, and multidisciplinary approach for effective treatment. So, there is a need for reliable surrogate markers to monitor the onset and progression of early neuropathic changes.[2]
Nerve conduction study (NCS) plays an important role in the diagnosis of DPN. An abnormality of nerve conduction tests, which is frequently subclinical, appears to be the first objective quantitative indication of the condition. It also plays an important role in assessing the severity of distal sensorimotor polyneuropathy. As per the American Diabetic Association (ADA) 2010,[3] electrophysiological NCS was given importance for diagnosis in clinical practice as well as in research studies; however, according to ADA 2017,[4] electrophysiological testing or referral to a neurologist is rarely needed for screening. In many studies, NCS is taken as the gold standard.[3] Some studies in India have recommended NCS in the diagnosis of DPN.[5,6] In a recent study, sural radial amplitude ratio along with minimal F-wave latency was recommended to assess polyneuropathy along clinical screening in diabetes mellitus patients.[7]
General practitioners come across a wide variety of diabetes mellitus patients. The patients are considered to be the first point of contact with general practitioners whether its government setup or private setup. Electrophysiological studies are generally advised in tertiary setup infrequently. General practitioners should be aware of the common findings of neuropathy in diabetes patients. That is how this study would be useful to general practitioners.
With this study, we attempted to discuss various electrophysiological studies in patients with diabetes mellitus. These electrophysiological findings would not only help general practitioners but also help physician about electrophysiological findings in diabetes mellitus patients. These findings may be useful to prove the utility of various electrophysiological tests in diabetes mellitus patients.
Subject and Methods
This was an observational study conducted among 72 diagnosed type 2 diabetes mellitus (T2DM) patients above the age of 18 years irrespective of gender for more than 1 year symptomatic or asymptomatic of DPN who gave their consent for the study and visited the Medicine Department of AIIMS, Bhopal, for consultation. Standard diagnostic criteria were used to diagnose diabetes.[8]
The study was conducted by the Physiology Department in collaboration with the Medicine Department.
Exclusion criteria were as follows
Patients with end-stage renal disease
Chronic alcoholics
Cancer patients
Patients on neurotoxic medications
Newly diagnosed case of diabetes mellitus
The study was undertaken after due approval from the Institutional Human Ethics Committee of AIIMS, Bhopal. Due consent was obtained from patients in a consent form after explaining the procedures to the participants with the help of participant information sheet. The patient underwent detailed history taking followed by general and systemic examination.
Nerve conduction study procedure
This was conducted using the Nihon Kohden Neuropack X1 Machine.
The nerves tested were median, ulnar, common peroneal, tibial, and sural nerves. The parameters recorded were distal latencies, amplitudes of compound motor action potentials (CMAPs), duration of CMAP, F-wave latencies, and conduction velocities in motor nerves. In sensory nerves, latencies and amplitudes of the sensory nerve action potentials (SNAPs) and their conduction velocities were documented. H reflex was studied. All recordings were made as per the standard procedure[9] as follows:
Subjects were asked to remove any metallic objects/mobiles that may interfere with the procedure. All aseptic precautions were taken during the procedure.
Area where electrodes are to be applied was cleaned with the spirit swab.
Paste/jelly was applied to the ground, reference and recording electrode and electrode were secured at the appropriate place using micropore tape.
Stimulus was given using appropriate voltage.
Standard guidelines were used for the interpretation of NCS.[10]
Statistical analysis was conducted using the Statistical Package for Social Sciences (SPSS) statistical software. The categorical or nominal variable was summarized by count or percentage and the numerical variable by mean and standard deviation (SD) (normally distributed). Microsoft Excel was utilized for data compilation and result analysis.
Results
The study included the sample size of 72 patients with T2DM recruited from the medicine outpatient department (OPD) with disease duration of more than 1 year. Forty-one male and 31 female patients were included in the study. The average duration of diabetes in the study was more than 7 years, and the range was 1 to 30 years.
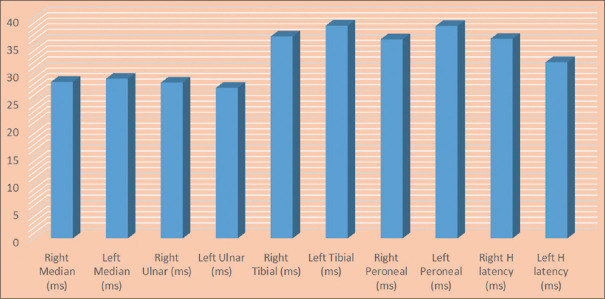
Figure 1 depicts the basic characteristics of patients included in the NCS. Tables 1 and 2 discuss the various motor nerve parameters, such as distal latency, proximal and distal amplitude, and NCS, whereas Tables 3 and 4 depict various upper limb and lower limb sensory nerve parameters. Figure 2 depicts the F-wave latency and H-wave latency in the study participants. The number mentioned in the respective tables is the number of patient where that particular test was recordable, and based on the number of patients in whom recording was possible, statistical parameters of the particular test were calculated.
Figure 1.
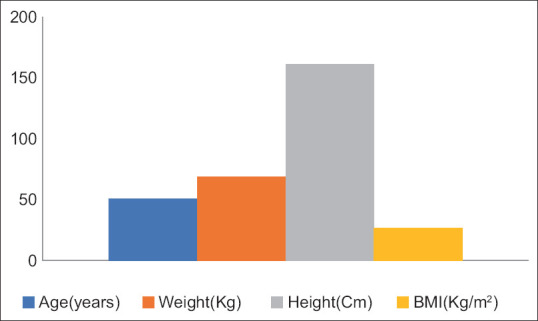
Characteristics of patients (N = 72). BMI = basal metabolic index
Table 1.
Upper limb motor nerve parameters
| Nerve parameters | Right median nerve (mean±SD) (range) n=70 | Left median nerve (mean±SD) (range) n=72 | Right ulnar nerve (mean±SD) (range) n=72 | Left ulnar nerve (mean±SD) (range) n=68 |
|---|---|---|---|---|
| Distal latency (ms) | 3.65±0.93 (1.9 to 7.4) | 3.86±0.93 (2 to 7.5) | 3.19±0.64 (1.4 to 5.9) | 3.25±0.79 (0 to 5.5) |
| Distal amplitude (mv) | 4.51±2.07 (1 to 10.1) | 5.25±2.37 (0.9 to 11.6) | 6.10±1.79 (1 to 9.6) | 5.76±2.06 (2.4 to 16) |
| Proximal amplitude (mv) | 3.94±1.75 (1.1 to 8.7) | 4.41±1.94 (0.9 to 9.7) | 5.33±1.80 (1.8 to 9.7) | 4.79±1.77 (1.1 to 8.7) |
| Nerve conduction velocity (m/s) | 55.13±8.30 (21.2 to 72.5) | 54.63±7.97 (20.77 to 66) | 55.53±9.94 (18.8 to 76.2) | 56.07±9.31 (18.9 to 79.2) |
Table 2.
Lower limb motor nerve parameters
| Nerve parameters | Right tibial motor nerve (mean±SD) (range) n=45 | Left tibial motor nerve (mean±SD) (range) n=41 | Right peroneal motor nerve (mean±SD) (range) n=30 | Left peroneal motor nerve (mean±SD) (range) n=26 |
|---|---|---|---|---|
| Distal latency (ms) | 5.80±1.71 (1.2 to 11.2) | 6.75±4.41 (3.8 to 30.4) | 4.63±1.87 (1.6 to 10.8) | 4±1.11 (1 to 5.5) |
| Distal amplitude (mv) | 5.05±3.20 (0.9 to 15.6) | 5.74±3.22 (1 to 13.8) | 2.94±2.19 (0.7 to 12.7) | 2.79±1.83 (0.5 to 8.7) |
| Proximal amplitude (mv) | 3.53±3.05 (1 to 14.3) | 3.63±2.57 (1.1 to 10.2) | 2.69±2.22 (1.1 to 12.7) | 2.66±1.71 (1 to 8.7) |
| Nerve conduction velocity (m/s) | 45.25±14.04 (16.3 to 94.5) | 44.04±15.46 (17.3 to 93.5) | 44.17±8.24 (25 to 64.7) | 43.52±6.64 (28.33 to 56.9) |
Table 3.
Upper limb sensory nerve parameters
| Right median nerve (mean±SD) (range) n=61 | Left median nerve (mean±SD) (range) n=54 | Right ulnar nerve (mean±SD) (range) n=59 | Left ulnar nerve (mean±SD) (range) n=60 | |
|---|---|---|---|---|
| Distal latency (ms) | 4.75±4.46 (1.6 to 23.6) | 4.29±5.57 (1.5 to 39.7) | 3.31±2.47 (1.6 to 15.2) | 4±2.70 (1.6 to 13.5) |
| Conduction velocity (m/s) | 44.41±19.26 (6.8 to 86.4) | 49.91±17.25 (3.8 to 79.2) | 48.89±16.64 (5.9 to 78.1) | 44.60±18.35 (9.8 to 86.4) |
Table 4.
Lower limb sensory nerve parameters
| Right sural nerve (mean±SD) (range) n=40 | Left sural nerve (mean±SD) (range) n=42 | |
|---|---|---|
| Distal latency (ms) | 3.88±3.16 (1.8 to 18.2) | 4.24±3.49 (2.1 to 18.5) |
| Conduction velocity (m/s) | 53.99±18.88 (11 to 96.6) | 50.70±17.39 (15.2 to 86.66) |
Figure 2.
F-wave and H latencies in upper and lower limbs
Based on NCS, 94% (68) of patients were abnormal, whereas 6% (4) were normal. The nerve modality involved was 73% (50) mixed neuropathy, 15% (10) motor neuropathy, and 12% (8) sensory neuropathy. Neuropathy was axonal in 36% (24), mixed (axonal + demyelinating) in 60% (41), and demyelinating in 4% (3) cases [Figures 3 and 4].
Figure 3.
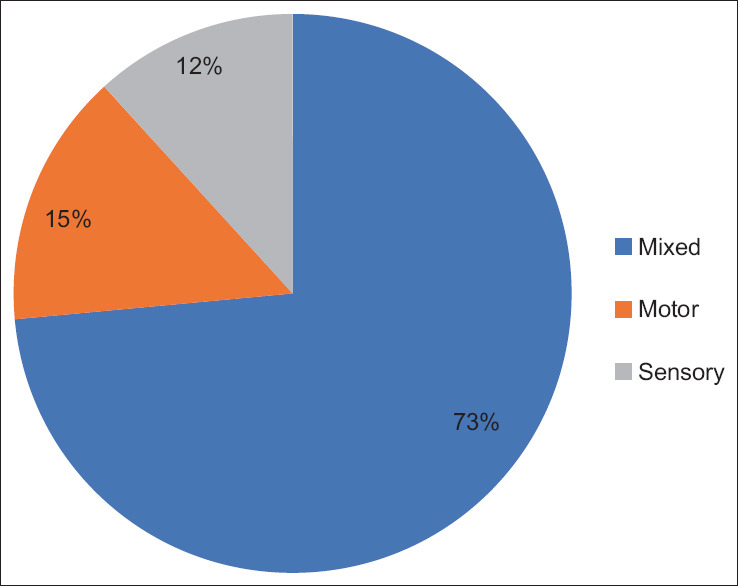
Distribution of mixed, sensory, and motor neuropathy
Figure 4.
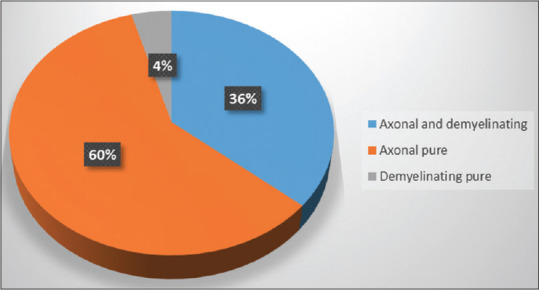
Distribution of mixed (axonal + demyelinating), pure axonal, and pure demyelinating neuropathy
Peroneal nerve (49%) and sural nerve (59%) were the most commonly involved bilateral motor and sensory nerves, respectively, whereas left tibial (32%) and left sural (54%) were the most commonly involved unilateral motor and sensory nerves, respectively.
Discussion
In our study, we found that the NCS was abnormal in 94% of patients showing asymmetrical involvement in 89% of cases and symmetrical involvement in 5% of cases with 73% of patients showing mixed involvement, while 15% and 12%, respectively, show sensory and motor involvement. Significant slowing down of both sensory and motor nerve conduction velocities as well as reduction in CMAP and SNAP was observed.
We had come across certain studies where the result was comparable with ours. Mythili et al.[11] observed distal sensory motor neuropathy in 54% of the patients, while pure sensory and pure motor neuropathy were observed in 11% and 3% of the cases. The mean duration of diabetes was around 8.09 years. The range of diabetes duration in this study was 1 to 30 years, which may be due to the high percentage of neuropathy in this study.
Lower limb involvement was seen predominantly,[6] which is similar to us. In this study, the most common motor nerve involved bilaterally was peroneal nerve in 49% of patients, while sural nerve is the most common bilaterally involved nerve in 59% of cases.
Sural nerve was affected earlier than median nerve.[5,12] Delayed distal latency, decreased amplitude, and conduction velocity were seen in the tested patients.[12,13,14] Conduction velocity and amplitude in motor as well as sensory nerve were decreased in patients with HbA1C levels of more than 10%.[15] The axonal loss resulted in decreased amplitude, whereas delayed conduction velocity and delayed latency were observed in the demyelinating pattern of neuropathy which could be due to combination of segmental demyelination, loss of fastest conducting axons, and metabolic alteration.[13,16] Decreased nerve conduction velocity was one of the earliest abnormalities at the time of diagnosis.[17] In our study, mixed pattern, that is, axonal as well as demyelinating pattern, could have resulted as a result of long-standing diabetes mellitus. Prolonged minimal F-wave latency was reflected mainly due to decreased excitability of anterior horn cell or due to selective loss of fastest axon.[18]
One of the uniqueness of the study was that all the major nerves were evaluated in the study along with H reflex and F-wave. All parameters of the nerve conduction studies are not evaluated in the same subjects in various studies. All parameters were examined in the patients with diabetes mellitus in this study.
Monitoring of diabetes with the electrophysiological study has been recommended. The correlation was observed between NCS and damage to large motor and sensory nerve.[19] It can be used for routine examination and early intervention in DPN[5] and for the evaluation of large fibre neuropathy.[20] NCS was considered to be the gold standard for grading severity of neuropathy[3,5,12] and can even be used as the gold standard in asymptomatic DPN patients.[14,21] Routine nerve conduction studies should be conducted in diabetics at least on a yearly basis.[14] However, as per the ADA 2017 guidelines, electrophysiological testing is rarely needed for screening, except in situations where the clinical features are atypical, the diagnosis is unclear, or a different aetiology is suspected.[4] Also, a major limitation of NCS is that it only assesses large myelinated nerve fibres and overlooks small nerve fibre dysfunction.[14] In our country, where the patient load is much higher, we also recommend ideal screening test as electrodiagnostic test may not be feasible for every patient. Based on the screening test, the clinician should decide which patient is to be advised NCS.
Limitation of the study
One of the limitations was that the data were not compared with that of healthy adults, and further sample size was less. One of the other limitations was that the study was conducted in the tertiary care hospital where the patient tends to visit when condition is beyond control due to which we may have got biased result. Further, the mean age was around 50 years; so, the age-related effects may not be ruled out.
Future aspect—Neuropathy is common in diabetic patients as evidenced by this study. We recommend further studies where comparison should be performed with healthy adult. Further, longitudinal studies with the role of various factors, including duration, treatment modalities, and lifestyle intervention, would be evaluated.
Conclusion
This study shows that NCS could play a great role in routine examination and early intervention as well as grading and detection of subclinical cases. A few drawbacks of testing have also been discussed. So, screening tests should decide whether patient should undergo NCS testing or not.
Financial support and sponsorship
We would like to thank the Indian Council of Medical Research (ICMR) for thesis funding.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank all technician of the Physiology Department, AIIMS, Bhopal, for their thankless support.
References
- 1.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus. Geneva;World Health Organization. 1999. [[Last accessed on 2022 Sep 20]]. Available from: https://apps.who.int/iris/handle/10665/66040 .
- 2.Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Front Endocrinol. 2021;12:671257. doi: 10.3389/fendo.2021.671257. doi: 10.3389/fendo.2021.671257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aruna BMK, Haragopal R. Role of electrodiagnostic nerve conduction studies in the early diagnosis of diabetic neuropathy: A case-control study. Int J Sci Study. 2016;4:143–6. [Google Scholar]
- 6.Kakrani A, Gokhale V, Vohra KV, Chaudhary N. Clinical and nerve conduction study correlation in patients of diabetic neuropathy. J Assoc Physicians India. 2014;62:24–7. [PubMed] [Google Scholar]
- 7.Ramanathan S, Thomas R, Chanu AR, Naik D, Jebasingh F, Sivadasan A, et al. Standard clinical screening tests, sural radial amplitude ratio and F wave latency compared to conventional nerve conduction studies in the assessment of sensorimotor polyneuropathy in patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2021;25:509–15. doi: 10.4103/ijem.ijem_426_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harreiter J, Roden M. [Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023)] Wien Klin Wochenschr. 2023;135(Suppl 1):7–17. doi: 10.1007/s00508-022-02122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante MA. Neuromuscular electrodiagnosis. Handb Clin Neurol. 2023;195:251–70. doi: 10.1016/B978-0-323-98818-6.00019-4. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez Do Campo R. Electrodiagnostic assessment of polyneuropathy. Neurol Clin. 2021;39:1015–34. doi: 10.1016/j.ncl.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Mythili A, Kumar KD, Subrahmanyam KAV, Venkateswarlu K, Butchi RG. A comparative study of examination scores and quantitative sensory testing in diagnosis of diabetic polyneuropathy. Int J Diabetes Dev Ctries. 2010;30:43–8. doi: 10.4103/0973-3930.60007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain G, Rizvi SAA, Singhal S, Zubair M, Ahmad J. Cross sectional study to evaluate the effect of duration of type 2 diabetes mellitus on the nerve conduction velocity in diabetic peripheral neuropathy. Diabetes Metab Syndr Clin Res Rev. 2014;8:48–52. doi: 10.1016/j.dsx.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S, Lukhmana S, Kahlon N, Malik P, Nandini H. Nerve conduction study in neurologically asymptomatic diabetic patients and correlation with glycosylated hemoglobin and duration of diabetes. Natl J Physiol Pharm Pharmacol. 2018;8:1533–8. [Google Scholar]
- 14.Yadav N, Shete A, Yadav P, Yadav N, Khan ST. Study of nerve conduction velocity in type II diabetes mellitus. Natl J Integr Res Med. 2015;6 [Google Scholar]
- 15.Muley PA, Muley PP, Sambre AD, Ambad RS. A cross-sectional study of electrophysiological changes occurring in type II diabetes mellitus. Cureus. 2022;14:e28994. doi: 10.7759/cureus.28994. doi: 10.7759/cureus.28994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston DC, Shapiro BE. Electromyography and Neuromuscular Disorders Clinical Electrophysiologic Correlation. 3rd ed. London, New York, Oxford, St Louis, Sydney, Toronto: Elsevier Sauders Publisher; 2013. pp. 19–46. [Google Scholar]
- 17.Viswanathan V, Snehalatha C, Seena R, Ramachandran A. Early recognition of diabetic neuropathy: Evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J. 2002;78:541–2. doi: 10.1136/pmj.78.923.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen H, Stålberg E, Falck B. F-wave latency, the most sensitive nerve conduction parameter in patients with diabetes mellitus. Muscle Nerve. 1997;20:1296–302. doi: 10.1002/(sici)1097-4598(199710)20:10<1296::aid-mus12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Motataianu A, Barcutean L, Bajko Z, Stoian A, Maier S, Voidazan S, et al. Autonomic and somatic nerve functions in type 2 diabetes mellitus patients: Electrophysiological aspects. Diagn Basel Switz. 2021;11:2005. doi: 10.3390/diagnostics11112005. doi: 10.3390/diagnostics11112005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deguchi T, Nishio Y. [Achilles tendon reflex, vibration sensation threshold, and nerve conduction study for the evaluation of the large fiber neuropathy in diabetes mellitus. Nihon Rinsho Jpn J Clin Med. 2016;74(Suppl 2):244–8. [PubMed] [Google Scholar]
- 21.Hu H, Li H, Zheng F ping, Cheng Y, Miao J, Zhang W. [A comparison of clinical effectiveness of different neuropathy scoring systems in screening asymptomatic diabetic peripheral neuropathy. Zhonghua Nei Ke Za Zhi. 2012;51:13–7. [PubMed] [Google Scholar]