Abstract
Following the lead of recent studies on the presence of RNA in virions of human cytomegalovirus, we investigated the presence and identity of RNAs from purified virions of herpes simple virus 1. To facilitate these studies, we designed primers for all known open reading frames (ORFs) and also constructed cDNA arrays containing probes designed to detect all known transcripts. In the first series of experiments, labeled DNA made by reverse transcription of poly(A)+ RNA extracted from infected HEp-2 or rabbit skin cells hybridized to all but two of the probes in the cDNA array. A similar analysis of the RNA extracted from purified extracellular virions derived from infected HEp-2 cells hybridized to probes representing 24 of the ORFs. In the second series of analyses, we reverse transcribed and amplified by PCR RNAs from purified intracellular or extracellular virions derived from infected HEp-2 or Vero cell lines. The positive RNAs were retested by PCR with and without prior reverse transcription to ensure that the samples tested were free of contaminating DNA. The results were as follows. (i) Only a fraction of viral ORF transcripts were represented in virion RNA, and only nine RNAs (UL10, UL34/UL35, UL36, UL42, UL48, UL51, US1/US1.5, US8.5, and US10/US11) were positive in all RT PCR assays. Of these, seven were positive by hybridization to cDNA arrays. (ii) RNA extracted from cells infected with a mutant virus lacking the US8 to US12 genes yielded results similar to those described above, indicating that US11, a known RNA binding protein, does not play a role in packaging RNA in virions. (iii) Cellular RNAs detected in virions were representative of the abundant cellular RNAs. Last, RNA extracted from virions was translated in vitro and the translation products were reacted with antibody to αTIF (VIP16). The immune precipitate contained a labeled protein with the apparent molcular weight of αTIF, indicating that at least one mRNA packaged in virions was intact and capable of being translated. The basis for the apparent selectivity in the packaging of the viral RNAs packaged in virions is unknown.
In recent years, both cellular and viral RNAs have been reported to be packaged in virions of human cytomegalovirus (CMV) (1, 4, 7). The observations are of particular interest for several reasons. Foremost, human CMVs are among the largest viruses infecting cells of higher organisms. In addition, these viruses, members of the Herpesviridae family, incorporate into their virions numerous proteins with multiple functions that effectively assist in the creation of an effective intracellular environment for rapid takeover and redirection of cellular functions to the benefit of the virus. In comparison with other members of Herpesviridae family, the functions encoded in their genomes and the ready-made proteins brought into cells during infection should be more than sufficient to render the infected cell a very pliant client. The presence of the RNAs in virions is therefore an unexpected, novel, intriguing facet of herpesvirus biology.
Following the basic premise that viruses do not perform gratuitous functions, we decided to determine whether herpes simplex virus type 1 (HSV-1) virions also contain RNAs and to determine their function. The advantage of HSV-1 is twofold. First, HSV-1 contains fewer open reading frames (ORFs) and the pattern of transcription of the viral genome has been extensively studied. Second, the major functions of HSV-1 gene products are at least in part understood. Hence, if RNAs were packaged in virions, we would have a basis on which to evaluate the significance of the packaged mRNAs.
The purification of HSV-1 virions has been extensively studied and characterized (5, 10). In this report, RNAs extracted from either intracellular or extracellular purified virions after RNase digestion were reverse transcribed, amplified by PCR, and subjected to two kinds of analyses. We report that a fraction of the HSV RNAs are represented in RNAs extracted from purified preparations and that the RNA transcripts of nine ORFs were detected in all purified preparations tested. We also report the presence of cellular RNAs in purified virions. In this instance, the selectivity of the packaged RNAs is less well apparent.
MATERIALS AND METHODS
Cells and viruses.
Vero and HEp-2 cell lines (American Type Culture Collection) and a rabbit skin cell line (originally obtained from J. McLaren) were propagated in Dulbecco's modified Eagle's medium supplemented with 5% newborn calf serum. HSV-1(F) is the prototype HSV-1 strain used in this laboratory (2). Isolation of the mutant virus R7023 was described elsewhere (6). R7023 lacks the genes US8 through US12. Titers of the stocks of HSV-1(F) and R7023 on Vero cells were determined.
Purification of virions.
HSV-1 and R7023 virions were purified as described by Spear and Roizman (10). Briefly, Vero or HEp-2 cells grown in roller bottles or in 150-cm2 flasks were exposed to 5 PFU of virus per cell. The cells were harvested 22 to 24 h after infection and centrifuged at 2,000 rpm for 10 min in an Allegra 6R centrifuge equipped with a GH-3.8 rotor (Beckman Coulter, Inc., Fullerton, Calif.). The supernatant fluids and the infected-cell pellets were collected and processed separately. Viral particles in the supernatant fluids were recovered by centrifugation at 20,000 rpm for 1 h at 4°C in an Optima LE-80K ultracentrifuge equipped with an SW28 rotor (Beckman Coulter, Inc.). The cell pellet was resuspended in 1 mM phosphate buffer and disrupted in a glass homogenizer with three strokes for Vero cells and two strokes for HEp-2 cells. Extracellular and cytoplasmic fractions were individually layered on 36-ml dextran-10 gradients (1.04 to 1.09 g/cm3) in 1 mM phosphate buffer. The gradients were centrifuged for 1 h at 20,000 rpm at 4°C in an Optima LE-80K ultracentrifuge equipped with an SW28 rotor. After centrifugation, virion-containing bands were collected and diluted in 10 mM phosphate buffer. Purified virions were concentrated by pelleting at 25,000 rpm for 2 h as described above. The pellets were resuspended in 200 to 300 μl of 10 mM phosphate buffer and stored at −20°C before processing.
Isolation of RNA from virions.
A 100-μl volume of purified virions was digested with 5 μl of RNase I (100 U/μl) for 3 h at 37°C. After RNase I digestion, RNA was extracted from virions with the aid of RNAquose-4PCR Kit (Ambion, Austin, Tex.) according to the manufacturer's instructions. The purified RNA samples were resuspended in 200 μl of elution buffer (1 mM EDTA; Ambion) and treated with 10 U of DNase I (Life Technologies, Rockville, Md.) for 3 h at 37°C. Fragmented DNA contamination was removed by phenol-chloroform extraction (Fisher Scientific, Huntsville, Ala.), followed by ethanol precipitation. Each sample was dissolved in 20 μl of diethyl pyrocarbonate-treated water.
Preparation of DNA probes from virion RNAs for cDNA arrays.
Ten microliters of RNA purified from viral particles was used to generate radioactively labeled cDNA with the aid of an avian myeloblastosis virus (AMV) reverse transcriptase (RT) system (Promega, Madison, Wis.). The reverse transcription was primed with a mixture of oligo(dT)15 primer and random hexanucleotides and performed in the presence of 1 mM dATP, dGTP, and dTTP (Pharmacia, Piscataway, N.J.) and 10 μl of [32P]dCTP (Amerham Pharmacia; concentration, 10 mCi/ml; specific activity, 3,000 Ci/mmol) in a total reaction volume of 35 μl. The mixture was incubated at 42°C for 45 min, shifted to 52°C for 45 min, and then heat inactivated at 95°C for 5 min. The labeled cDNAs were purified by passage through a Micro BioSpin-6 chromatography column (Bio-Rad, Hercules, Calif.). The efficiency of [32P]dCTP incorporation was determined in a liquid β-spectrometer (Beckman Coulter, Inc.).
Isolation and reverse transcription of RNA extracted from infected cells.
HEp-2 and rabbit skin cell lines were infected with HSV-1(F) and harvested 22 and 18 h postinfection (p.i.), respectively. Total RNA was extracted with the aid of TRIZOL reagent according to the manufacturer's instructions (Life Technologies). Poly(A)+ RNAs were purified from total RNA with the aid of an Oligotex mRNA Midi Kit (Qiagen). One microgram of poly(A)+ RNA was used to generate labeled cDNA by reverse transcription in the presence of [32P]dCTP. The reverse transcription was done as described above.
In a separate experiment, HEp-2 cells were infected with HSV-1(F) and harvested 22 h after infection. Total RNA was extracted with the aid of TRIZOL reagent according to the manufacturer's instructions (Life Technologies). DNase I treatment, phenol-chloroform extraction, and ethanol precipitation (Fisher Scientific, Houston, Tex.) were carried out to remove possible DNA contamination. Total RNA (2.5 μg) was reverse transcribed with 60 U of AMV (Promega) in a total reaction volume of 35 μl. The reverse transcription was primed with oligo(dT)15 primer (Promega) and performed in the presence of 1 mM of dGTP, dATP, and dTTP and 10 μl of [33P]dCTP (Amerham Pharmacia). The reverse transcription was done as described above.
Preparation of HSV cDNA arrays.
The HSV-1 arrays were made by printing cDNA probes on nylon membranes (VP-Scientific, Inc., San Diego, Calif.). Briefly, primer pairs were designed to amplify approximately 300- to 400-bp fragments of all the known expressed HSV-1 ORFs. Two rounds of PCR amplifications were done. In the first round, a complete set of HSV-1(F) ORFs was amplified from a set of plasmids and cosmids (Table 1). In the second round, the PCR products obtained in the first round were purified and reamplified with the same pairs of primers. The conditions used were as follows: 1 min at 94°C, 1 min at 60°C, 90 s at 72°C for 35 cycles. The final PCR products were diluted to 100 to 200 ng/ml and denatured in 0.8 M NaOH–20 mM EDTA (pH 8.0) at 94°C for 10 min. The arrays were printed with either 10 or 20 ng of amplified DNA per spot onto nylon membranes with the aid of a 380-pin replicator treated with VP110 surfactant (VP-Scientific). The DNA was cross-linked to the membranes with UV light. To test for the reproducibility of delivery, a second set of spots was printed slightly offset relative to the first one.
TABLE 1.
Sources of DNA sequences to serve as probes for HSV-1 ORFs
| DNA | Plasmid | Cosmid |
|---|---|---|
| UL1–3 | pRB134(E) | |
| UL4–8.5/9 | pRB132(C) | |
| UL9–17 | CS 46 | |
| UL18–19 | pRB125(M) | |
| UL20–21 | pRB4324 | |
| UL22–24 | pRB103(Q) | |
| UL25–29 | CS 50 | |
| UL30 | pRB118(R) | |
| UL31–33 | CS 50 | |
| UL34–36 | pRB101(D) | |
| UL37–38 | CS 69 | |
| UL39–40 | pRB129(O) | |
| UL41–44 | pRB172(I) | |
| UL45–49.5 | CS 69 | |
| UL50 | pRB142(D′) | |
| UL51–53 | pRB126(L) | |
| UL54–56, a0 | pRB112(B) | |
| α4-1 | pRB115(S2P1) | |
| α4-2 | pRB136(Y) | |
| LAT/ORFO/P/γ1 34.5 | pRB4789 | |
| Oris1 | EC | |
| Oris2, Us1–3 | p5210(N) | |
| Us4–8.5 | pRB123(J) | |
| Us9 | pRB124(X) | |
| Us10–12 | pRB421(X(3′)+Z) |
Hybridization of labeled cDNA to HSV-1 and human cDNA arrays.
The membranes imprinted with the HSV-1 cDNA array were preincubated for 4 h at 42°C in 5 ml of MicroHyb prehybridization solution (Research Genetics, Huntsville, Ala.). The prehybridization solution contained 5 μl of poly(dA) (1 μg/μl; Research Genetics) and 5 μl of human Cot-1 DNA (1 μg/μl; Life Technologies). Pure labeled DNAs, derived from viral purified RNAs, were diluted in 5 ml of MicroHyb solution and incubated separately overnight at 42°C. The membranes were then rinsed first with 2× SSC + 1% sodium dodecyl sulfate (SDS) and successively with 0.1× SSC + 1% SDS solutions (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The same membrane was also hybridized with labeled DNAs derived from total RNA extracted from rabbit skin or HEp-2 infected cell lines under the same conditions described above.
In separate experiments designed to detect human genes, one human cDNA array (GF2111; Research Genetics) containing cDNA probes for >4,000 known human genes was hybridized with labeled probes derived from virion RNA. The hybridization procedures followed in these experiments were identical to that described for the HSV-1 cDNA array. For comparison, we used a cDNA array hybridized with labeled probes derived from total RNA of infected HEp-2 cells. The hybridization of labeled DNA to the membranes was quantified in a Storm 860 (General Dynamics) phosphorimager.
Reverse transcription and PCR amplification of RNA extracted from virions.
RNAs extracted from purified viral particles derived from HSV-1 and R7023 was reverse transcribed to yield single-stranded cDNA using 60 U of AMV RT (Promega) in a total reaction volume of 20 μl. The reverse transcription was primed with oligo(dT)15 primer and performed using a pool of nucleotides consisted of 1 mM concentrations (each) of dGTP, dATP, dTTP, and dCTP (Promega). Forty units of RNasin (Promega) were added to each reaction mixture. The mixture containing only the RNA template, and the oligo(dT)15 was first heated at 70°C for 10 min, chilled on ice, and after the addition of the other components, incubated at 42°C for 45 min, shifted at 52°C for 45 min, and then heat inactivated at 95°C for 5 min.
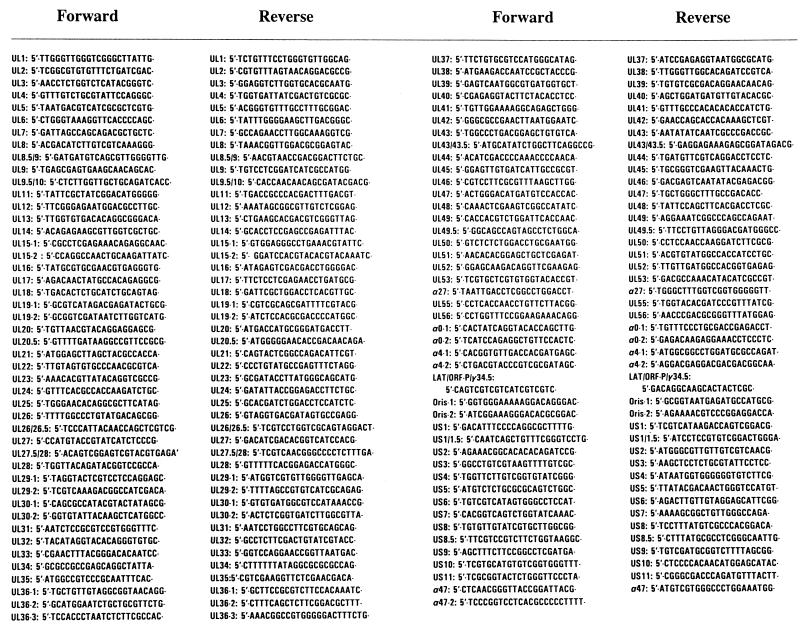
cDNAs obtained from reverse transcription of RNA extracted from virions were amplified by PCR under the following conditions: 1 min at 95°C, 45 s at 60°C, and 2 min at 72°C. The sequences of primers used for PCR are shown in Fig. 1. PCR products were resolved on 2% agarose gel containing ethidium bromide (0.5 μg/ml).
FIG. 1.
Primer sequences.
In vitro translation of purified virion RNAs and immunoprecipitation of UL48 protein.
RNAs extracted from extracellular virions purified from HSV-1(F)-infected HEp-2 cell cultures were translated in vitro in the presence of [35S]methionine with the aid of a rabbit reticulocyte lysate system according to the manufacturer's instructions (Promega). Briefly, 10 μl of RNAs were incubated for 90 min at 30°C in the presence of 40 μCi of [35S]methionine (1,200 Ci/mmol; Amersham) and 35 μl (70% concentration) of the Promega rabbit reticulocyte lysate in a total volume of 50 μl. The labeled translation products were mixed with 250 μl of phosphate-buffered saline buffer containing 1% NP-40 and a cocktail of protease inhibitors (Sigma) and then precleared with preimmune rabbit serum and 50% (vol/vol) protein A (Sigma) for 2 h at 4°C. The UL48 protein were immunoprecipitated from the total translation mixture with a monoclonal antibody specific for UL48 (a kind gift of T. Minson, Cambridge, United Kingdom) and protein A (50%) overnight at 4°C. The immune complexes were rinsed three times in phosphate-buffered saline and disrupted by addition of 40 μl of 1× SDS gel loading buffer (2% SDS; 5% β-mercaptoethanol; 50 mM Tris, pH 6.8; 2.75% sucrose). Samples were heated at 95°C for 4 min, resolved by 10% polyacrylamide gel electrophoresis, and transferred to a nitrocellulose sheet for autoradiography.
RESULTS
Experimental design.
The studies described in this report were done on four virion preparations, which were as follows: (i) HSV-1(F) virions derived from intracellular extracts of Vero cells, (ii) HSV-1(F) virions derived from intracellular extracts of HEp-2 cells, (iii) HSV-1(F) virions derived from extracellular medium of infected HEp-2 cells, and (iv) R7023 virions derived from extracellular medium of infected HEp-2 cells. In order to cover the entire transcribed domain of HSV-1, a set of 90 pairs of primers was designed to amplify all 84 expressed ORFs of HSV (Fig. 1). The analyses were done as described in Materials and Methods.
Detection of viral cDNA derived from DNA-free purified virions by HSV-1 cDNA array.
The objectives of this series of experiments were twofold. The first was to determine what subset of total viral RNAs accumulating in infected cells could be detected by labeled product of reverse transcription of RNA extracted from infected cells under the conditions described in Materials and Methods. The second objective was to verify the presence and identity of RNAs extracted from purified virions by an additional method. Two series of experiments were done.
In the first series of experiments, we hybridized a set of labeled DNAs obtained by reverse transcription of poly(A)+ RNA extracted from either rabbit skin and HEp-2 cells harvested 18 and 22 h after infection, respectively. The results are shown in Fig. 2A and B. The caveat in the interpretation of the results is that a high content of G+C is notoriously difficult to retrotranscribe or amplify. Therefore, the results are valid with respect to identities of the viral RNAs detected in these arrays, but the intensity of the autoradiographic images, while comparable from one panel to the next, may not accurately represent the quantity of that specific RNA relative to the total RNA.
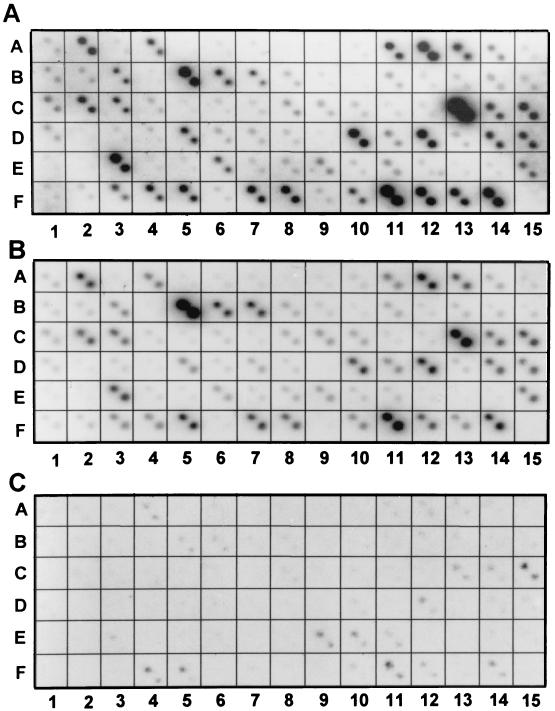
FIG. 2.
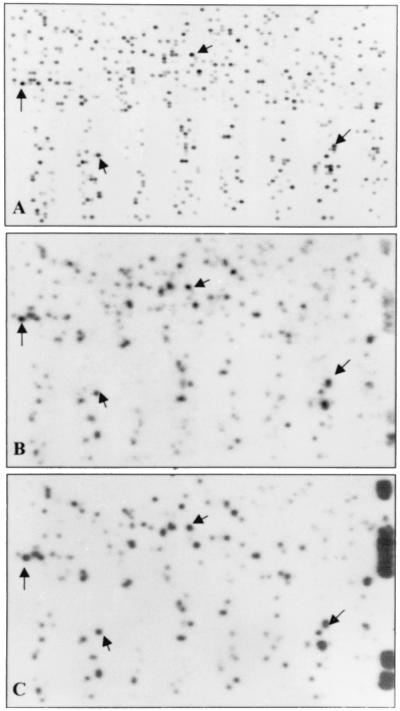
Photographs of autoradiographic images of HSV-1(F) cDNA array. The HSV-1 cDNA arrays representing the known ORFs were constructed as described in Materials and Methods. A total of 75 cDNA arrays were imprinted in duplicate as described, and the identity of each spot is listed in Table 2. (A and B) The arrays were hybridized with 32P-labeled cDNAs derived from poly(A)+ RNA extracted from infected HEp-2 or rabbit skin cells, respectively. (C) The same cDNA array was hybridized with 32P-labeled cDNA derived from RNA extracted from virions purified from extracellular medium of infected HEp-2 cells.
The key features of the results were as follows. (i) The time of harvest of the infected cells represents the end of logarithmic phase of accumulation of infectious virus (15 to 20 h, depending on cell line and multiplicity of infection) which should correspond to the highest rate of maturation of virions. It could be expected therefore that the RNA identified in the DNA arrays would represent the RNAs present in the cell at the time of packaging of most virions and represent the RNA available for packaging. In this respect, both the purpose and the design of the studies represented here differ from the global analyses of temporal patterns of transcript accumulation recently published by Stingley et al. (11).
(ii) Although the autoradiogram of labeled cDNAs derived from infected rabbit skin cell RNAs was somewhat weaker than that of cDNAs derived from infected HEp-2 cell RNAs, the patterns were very similar. This indicates both that infected cells have a similar population of viral RNAs and that the process of reverse transcription and hybridization to the cDNA arrays yielded similar results.
(iii) In the more intense autoradiogram of the cDNA derived from infected HEp-2 cell RNAs, we failed to detect the products of UL8 (A8) and UL50 (E2). In addition, the products of UL8.5/UL9 (A9), UL21 (B10), and UL49.5 (E1) were barely detectable by these procedures.
In the second series of experiments, the labeled DNAs derived from RNA extracted from extracellular virions purified from infected HEp-2 cell cultures were hybridized to the viral cDNA arrays (Fig. 2C). We classified as positive a total of 24 RNAs. The significant aspects of the results that bear on the overall conclusion of the study is that the intensities of the 32P spots in Fig. 2C did not coincide with the intensities of the corresponding spots in either Fig. 2A or B. Thus, UL18 and UL34/UL35 formed the most intense spots in the arrays measuring RNAs derived from infected cells (Fig. 2A and B) but are barely visible in the arrays measuring cDNAs derived from virion RNAs.
Identification of viral RNA species by reverse PCR of DNA-free RNA extracted from purified virions.
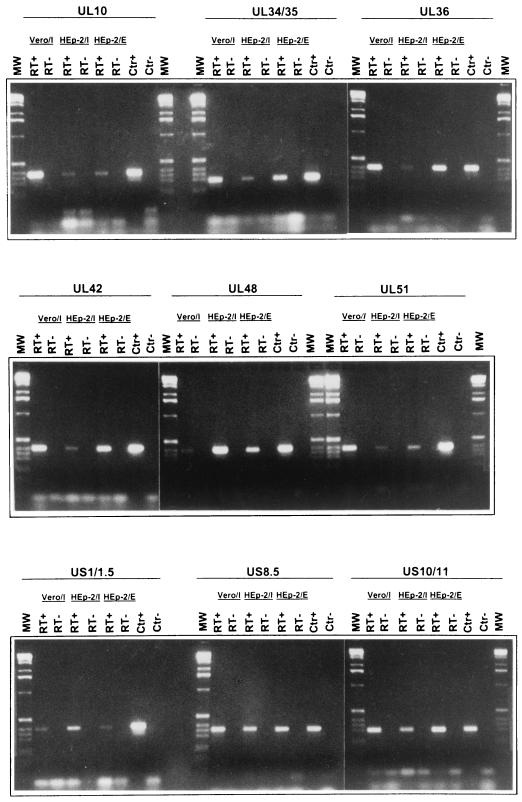
The procedure involved in this series of experiments consisted of two steps. In the first round of analyses, we reverse transcribed RNAs extracted from purified virions and amplified the cDNAs by PCR. In the second step, all RNAs found to be positive were retested both by reverse PCR and by PCR without reverse transcription to eliminate the possibility that the positive results were due to contaminating DNA. A photograph of the bands obtained after reverse transcription and PCR amplification of the nine RNAs reproducibly detected in all three experiments are shown in Fig. 3. In each instance, we detected an amplified DNA band with the correct size only after reverse transcription followed by PCR amplification, indicating that the detected material was RNA and not contaminating DNA.
FIG. 3.
Photographs of DNA bands derived by reverse transcription and PCR amplification of RNAs extracted from HSV-1(F) virions using primers of selected ORFs. RNAs derived from HSV-1(F) virions was reverse transcribed as described in Materials and Methods. The PCR was performed with primers specific for the HSV-1 ORFs indicated. The selected ORFs were those of RNAs detected in all virion preparations. Vero/I and HEp-2/I, virions purified from homogenized infected cells; HEp-2/E, virions purified from extracellular medium. Lanes RT+, presence of RT in the RT mixture; lanes RT−, control reactions in which RT was omitted; lanes CTR+, plasmid DNA containing the sequence of the indicated ORF was used as template; lanes CTR−, no template was added to the PCR mixture; lanes MW, 1-kb DNA ladder used as a size marker.
The results of the three experiments are summarized in the first three columns of Table 2. The key features of the results are those of the 90 primer sets; at least 27 yielded positive results in at least one virion preparation, but only nine viral RNAs were positive in all three virion preparations. The primers selected for the studies were carefully chosen to ensure detection of the RNA, if present. In several instances, as illustrated in part in Fig. 1, we tested several pairs of primers to insure both sensitivity and specificity. Nevertheless, we could not design primers that met our requirements and could differentiate between UL34 and UL35, between US1 and US1.5, and between US10 and US11. On the other hand, we differentiated between US8 and US8.5 and between other sets of 3′ coterminal transcripts (Fig. 1).
TABLE 2.
Viral transcripts detected in HSV viral particles by RT-PCR and HSV arraya
| Gene(s) | RT-PCR
|
Array, HSV-1
|
||||
|---|---|---|---|---|---|---|
| HSV-1
|
R7023
|
|||||
| Vero/I | HEp-2/I | HEp-2/E | HEp-2/E | HEp-2/E | Position | |
| UL1 | − | − | − | − | − | A1 |
| UL2 | − | − | − | − | − | A2 |
| UL3 | − | − | − | − | − | A3 |
| UL4 | − | − | − | − | + | A4 |
| UL5 | − | − | − | − | − | A5 |
| UL6 | − | − | − | − | − | A6 |
| UL7 | + | − | − | + | − | A7 |
| UL8 | − | − | − | − | − | A8 |
| UL8.5/9 | − | − | − | − | − | A9 |
| UL9 | − | − | − | − | − | A10 |
| UL9.5/10 | + | + | + | + | + | A11 |
| UL11 | − | − | − | − | + | A12 |
| UL12 | + | − | − | + | + | A13 |
| UL13 | + | − | + | + | − | A14 |
| UL14 | − | − | − | − | − | A15 |
| UL15 | + | − | − | − | − | B1 |
| UL15-2/15.5 | ND | ND | ND | ND | − | B2 |
| UL16 | − | − | − | − | − | B3 |
| UL17 | − | − | − | − | − | B4 |
| UL18 | − | − | − | + | + | B5 |
| UL19-1 | + | − | + | + | + | B6 |
| UL19-2 | ND | ND | ND | ND | − | B7 |
| UL20 | + | − | − | − | − | B8 |
| UL20.5 | − | − | − | + | − | B9 |
| UL21 | + | − | + | + | − | B10 |
| UL22 | − | − | − | − | − | B11 |
| UL23 | − | − | − | − | − | B12 |
| UL24 | − | − | − | − | − | B13 |
| UL25 | − | − | − | − | − | B14 |
| UL26 | ND | ND | ND | ND | − | B15 |
| UL26/26.5 | − | − | − | − | − | C1 |
| UL27 | + | + | − | − | − | C2 |
| UL27.5/28 | − | − | − | − | − | C3 |
| UL28 | ND | ND | ND | ND | − | C4 |
| UL29-1 | + | − | + | + | − | C5 |
| UL29-2 | ND | ND | ND | ND | − | C6 |
| UL30-1 | − | − | − | − | + | C7 |
| UL30-2 | ND | ND | ND | − | C8 | |
| UL31 | + | − | − | − | − | C9 |
| UL32 | − | − | − | − | − | C10 |
| UL33 | − | − | − | − | − | C11 |
| UL34 | + | + | + | + | − | C12 |
| UL34/35 | + | + | + | + | + | C13 |
| UL36-1 | + | + | + | + | + | C14 |
| UL36-2 | ND | ND | ND | ND | + | C15 |
| UL36-3 | ND | ND | ND | ND | − | D1 |
| UL37 | + | − | + | − | − | D2 |
| UL38 | − | − | − | − | − | D3 |
| UL39 | − | − | − | − | − | D4 |
| UL40 | + | + | − | + | − | D5 |
| UL41 | − | − | − | − | − | D6 |
| UL42 | + | + | + | + | − | D7 |
| UL43 | ND | ND | ND | ND | − | D8 |
| UL43/43.5 | − | − | − | − | − | D9 |
| UL44 | − | − | − | − | + | D10 |
| UL45 | + | − | − | − | − | D11 |
| UL46 | − | − | − | − | + | D12 |
| UL47 | − | − | − | − | − | D13 |
| UL48 | + | + | + | + | + | D14 |
| UL49 | − | − | − | − | + | D15 |
| UL49.5 | − | − | + | − | − | E1 |
| UL50 | − | − | − | − | − | E2 |
| UL51 | + | + | + | + | + | E3 |
| UL52 | − | − | − | − | − | E4 |
| UL53 | − | − | − | − | − | E5 |
| UL54 | − | − | − | − | − | E6 |
| UL55 | + | − | + | − | − | E7 |
| UL56 | − | − | + | − | − | E8 |
| α0-1 | ND | ND | ND | ND | + | E9 |
| α0-2 | − | − | − | − | + | E10 |
| α4-1 | + | − | + | + | + | E11 |
| α4-2 | ND | ND | ND | ND | − | E12 |
| LAT/ORFO γ1 34.5 | − | − | − | − | − | E13 |
| Oris-1 | − | − | − | − | − | E14 |
| Oris-2 | ND | ND | ND | ND | − | E15 |
| US1 | ND | ND | ND | ND | − | F1 |
| US1/1.5 | + | + | + | + | − | F2 |
| US2 | − | − | − | − | − | F3 |
| US3 | − | − | − | − | + | F4 |
| US4 | + | + | − | + | + | F5 |
| US5 | − | − | − | − | − | F6 |
| US6 | − | − | − | − | − | F7 |
| US7 | + | − | + | − | − | F8 |
| US8 | − | − | − | − | − | F9 |
| US8.5 | + | + | + | − | + | F10 |
| US9 | − | − | − | − | + | F11 |
| US10/11 | + | + | + | − | + | F12 |
| US11 | + | + | + | − | − | F13 |
| US12 | − | − | − | − | + | F14 |
| US12-2 | − | − | − | − | ND | |
Vero/I and HEp-2/I, virions purified from homogenized infected cells; HEp-2/E, virions purified from supernatant. R7023 is an HSV-1 mutant lacking the genes US8 through US12. Two or three pairs of primers were designed for the genes that contain exons (i.e., α0, UL15) or for the ORFs longer than 3.5 kb (i.e., UL19-1, UL29, UL30, UL36, α4). The position of the ORFs in the HSV array is also reported (see Fig. 2). ND, not done.
Analyses of virion mRNA in deletion mutant R7023 lacking genes US8 though US12.
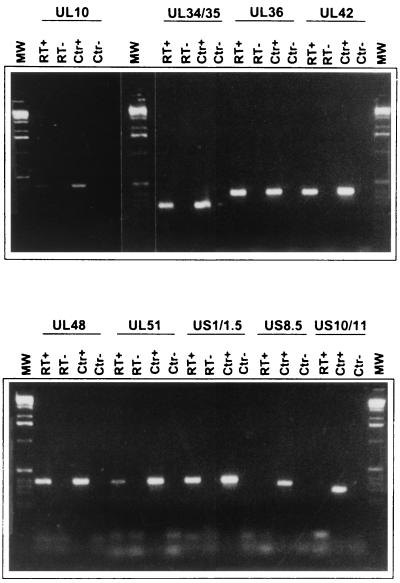
A major component of the virion capable of binding RNA is the US11 protein. This protein was shown earlier to bind RNA in a conformation- and sequence-specific manner. The only RNAs previously shown to bind US11 protein included UL34 mRNA and an RNA mapping antisense to the US11 and US12 ORFs. To test the role of this protein in the packaging of RNA in virions, we analyzed the RNAs extracted from purified extracellular virions of HEp-2 cells infected with the mutant virus R7023. The results of this experiment are included in Table 2. As expected, we did not detect cDNA representing US8 through US12. With these exceptions, all of the RNAs reproducibly detected in wild-type virions were also detected in purified R7023 virions (Fig. 4).
FIG. 4.
Photographs of DNA bands derived by reverse transcription and PCR amplification of RNAs extracted from R7023 mutant virions using primers of selected ORFs. The selected ORFs were those of RNAs detected in all virion preparations. Note that the R7023 mutant lacks the genes US8 through US12. The procedures were the same as those described in the legend to Fig. 3. Lanes RT+, presence of RT in the RT mixture; lanes RT−, control reactions in which RT was omitted; lanes CTR+, plasmid DNA containing the sequence of the indicated ORF was used as template; lanes CTR−, no template was added to the PCR mixture; lanes MW, 1-kb DNA ladder used as a size marker.
Detection of cellular cDNA derived from DNA-free purified virions by a human gene array.
The purpose of this series of experiments was to determine whether cellular RNAs were also represented among RNAs extracted from purified virions. Specifically, the labeled cDNA from purified intracellular and extracellular virions were sequentially hybridized to a Research Genetics cDNA array (GF2111) containing probes for >4,000 known human genes (Fig. 5B and C, respectively). The results obtained with labeled cDNA derived from purified virions may be compared with the results of analyses on similar cDNA array of labeled cDNA derived from HSV-1-infected HEp-2 cells (Fig. 5A). The cDNA derived from these cells hybridized to approximately 1,500 probes. A detailed comparison of the hybridization of the labeled DNA derived from virions and that of RNA derived from the infected cells could not be done for two reasons. First, the cDNA derived from the infected cells RNA was labeled with 33P in contrast to the 32P-labeled DNA prepared from virion RNAs. The consequences were that the signal derived from virion cDNA arrays was more likely to impinge on signals derived from hybridization to nearby probes. Second, the virion RNA lacked the marker RNAs that would permit precise alignment of the autoradiographic images of the cellular and virion cDNA arrays. Nevertheless, the overall patterns are strikingly similar with respect to both position and relative intensity of the signal. Some of the cellular DNAs were readily identified on that basis (Fig. 5A, B, and C, arrows). The relatively abundant cellular genes identified in Fig. 5 are shown in Table 3.
FIG. 5.
Autoradiographic image of a human cDNA array hybridized to labeled cDNAs derived from cellular or virion RNAs. (A) Human cDNA array (GF2111; Research Genetics) membrane was probed with 33P-labeled cDNA generated by reverse transcription of total RNA isolated from HSV-1-infected HEp-2 cells 22 h after infection. The same membrane was probed with 32P-labeled cDNA generated by reverse transcription of RNA isolated from intracellular (B) or extracellular (C) purified HSV-I(F) virions from infected HEp-2 cells. Arrows indicate relatively abundant mRNA species consistently positive in all preparations.
TABLE 3.
Cellular genes packaged in purified HSV-1 virions
| Accession no. |
Namea |
|---|---|
| R55188 | Human pre-T/NK cell-associated protein (3B3) mRNA |
| AA292536 | CCAAT displacement protein CUTL1 |
| AA150487 | Alkaline phosphatase placental, ALPP |
| AA644657 | MHC-1, HLA-A |
| AA411440 | Villin 2, VIL2 |
| AA490256 | Alternative guanine nucleotide-binding regulatory, GNAII |
| AA464755 | Ankyrin 1 erythrocytic, ANK1 |
| AA284528 | Protease, serine 2, PRSS2 |
| AA070997 | Proteasome subunit, beta type 6, PSMB6 |
| R27585 | Proteasome component C2, PSMA2 |
| AA872397 | Galectin-2 |
| N71628 | Spi-B transcription factor, SPIB |
| N55461 | Erythrocyte membrane protein band 4.9, EPB49 |
| N49856 | Na- and Cl-dependent betaine transporter |
| AA505045 | Human L2-9 transcript of unrearranged immunoglobulin V(H)5 |
| AA155913 | Matrix Gla protein, MGP |
| AA669055 | MHC-2 DQ beta 1, HLA-DQB1 |
| AA670347 | Metaxin, MTX |
| AA608988 | Testis-specific protein, Y-linked, TSPY |
| AA243439 | Multiple endocrine neoplasia I, MEN 1 |
| AA894557 | Creatine kinase B, CKB |
| AA279429 | Endothelial converting enzyme I, ECE1 |
| AA397824 | Dopacrome tautomerase, DCT |
| N75028 | Homo sapiens mRNA l-3-phosphoserine-phosphatase homologue |
| AA757170 | Homo sapiens TAX interaction protein 33 mRNA |
| AA699469 | Mitocondrial carbonic anhydrase, CA5 |
| AA664180 | Glutathione peroxidase 3, GPX3 |
| AA235706 | Human TATA-binding protein-associated factor |
| AA459292 | CDC28 protein kinase CKS1 |
| AA878561 | Ubiquitin A-52, UBA52 |
| AA633901 | Transforming growth factor, TGF B1 |
| AA416952 | Calpastatin CAST |
| AA043228 | Calponin 3, acidic, CNN3 |
| AA490911 | Homo sapiens drp1 mRNA |
| AA488979 | Homo sapiens nucleolar protein, MSP58 |
| AA464566 | Human mRNA for LDL receptor |
| AA098896 | Steroid hormone receptor, ERR1 |
| AA029964 | Human ataxin-2-related protein mRNA |
| AA778392 | Human BENE mRNA |
| AA427934 | Cell division cycle 42, CDC42 |
| T81764 | Cell division cycle 27, CDC27 |
| AA872001 | Annexin VI, ANX6 |
| AA076063 | Caldesmon, CALDI |
| AA866113 | Human FE65-like protein (hFE65L) mRNA |
| AA046690 | Kinesin heavy chain |
| AA457739 | Homo sapiens putative OSP-like protein mRNA |
| AA704492 | Transducin-like enhancer of SPLIT 4, TLE4 |
| AA772066 | Human phosphatidylinositol |
| AA281784 | Human phosphatidylinositol 3-kinase catalytic subunit P110 delta mRNA |
| AA709414 | Nidogen, NID |
| AA707922 | cGMP phosphodiesterase, gamma subunit |
| AA488346 | Myosin light chain, alkaline |
| AA282537 | Myocyte-specific enhancer factor 2 |
| N92646 | Immunoglobulin gamma 3, IGHG3 |
| AA127096 | Human enigma gene |
| AA430675 | Human DNA-repairing protein XRCC9 |
| W44860 | Human calmodulin mRNA |
| AA427899 | Human mRNA encoding beta-tubulin |
| AA441895 | Human glutathione S-transferase homolog mRNA |
| AA291490 | Homo sapiens mRNA for processing α-glucosidase I |
| AA253430 | Human C-1 mRNA |
| AA459263 | Human Bcl2-related (Bfl1) mRNA |
| N21546 | Human DNA topoisomerase III mRNA |
| W96450 | Human putative tRNA synthetase-like protein mRNA |
| AA609655 | Homo sapiens mRNA for SCP-1 |
| AA425934 | Human mRNA for S100 alpha protein |
| AA491302 | 69KD ISLET cell autoantigen |
| Accession no. |
Namea |
| AA504342 | Homo sapiens mRNA p115 |
| AA477428 | Human RNA polymerase II subunit hsRPB7 mRNA |
| AA463924 | Factor VIII intron 22 protein |
| AA488072 | Homo sapiens mRNA for cytokine-inducible nuclear protein |
| AA629923 | Human mRNA for pM5 protein |
| AA281635 | Human MDA7 (mda-7) mRNA |
| AA419177 | Integral membrane protein E16 |
| AA490991 | Homo sapiens hnRNP F protein mRNA |
| AA460830 | Homo sapiens RNA polymerase II mRNA |
| AA457153 | Homo sapiens mRNA repressor protein |
| R52548 | Human SOD-1 |
| AA150828 | Mitogen-activated protein kinase kinase kinase 5 (MAPKKK5) mRNA |
| N46828 | Homo sapiens mRNA for inositol-1,4,5-triphosphatase-3-kinase isoenzyme |
| AA486435 | Homo sapiens mRNA for CDEP |
| AA521431 | Human profilin mRNA |
| AA481758 | DNAJ protein homolog I |
| AA682851 | Homo sapiens mRNA for ERp28 |
| AA454926 | Human HBV-X-associated protein (XAP2) mRNA |
| N62179 | Human methylmalonate semialdehyde dehydrogenase |
| N30302 | GTP-binding protein HSRI |
| N78621 | Homo sapiens mRNA for gamma-adaptin |
MHC, major histocompatibility complex.
In vitro translation of purified virion RNA and immunoprecipitation of UL48 protein.
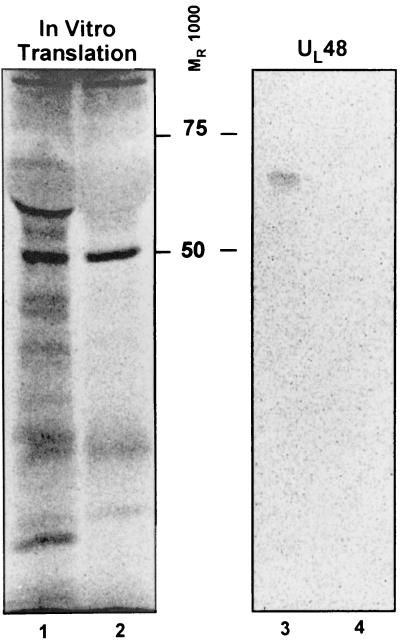
This experiment was designed to determine whether the RNA detected in virions was intact or fragmented oligoribonucleotides. As described in Materials and Methods, the RNAs were translated in vitro in the presence of [35S]methionine. The translation mixture was reacted with monoclonal antibody to UL48 protein, and the immune complexes recovered with protein A were subjected to electrophoresis in denaturing polyacrylamide gels. As shown in Fig. 6, lane 3, the closely migrating labeled doublet had the immune reactivity and electrophoretic mobility of the UL48 protein, suggesting that at least some of the RNAs whose presence we have detected in virions were intact.
FIG. 6.
Autoradiographic images of in vitro translation of purified virions RNAs and subsequent immunoprecipitation with UL48 antibody. Lane 1, autoradiographic image of electrophoretically separated products of in vitro translation of virion RNAs; lane 2, electrophoretic mobility of the in vitro translation mixture to which no exogenous RNA was added (negative control); lane 3, autoradiographic image of the electrophoretically separated immunoprecipitate of the translation products shown in lane 1 obtained with the anti-UL48 antibody; lane 4, the autoradiographic image of electrophoretically separate precipitate obtained from the translation products shown in lane 2 with the anti-UL48 antibody.
DISCUSSION
It is convenient to begin the discussion with a summary of the results and their conclusions. In essence, (i) we designed primers for amplification of probes corresponding to HSV-1 ORFs to detect all of the transcripts expressed in infected cells. Labeled DNA derived from poly(A)-selected RNA purified from infected cell hybridized to all but two of the probes in the cDNA array, validating the primer set.
(ii) We have detected and identified RNA species in extracts of purified intracellular and extracellular virions by two methods: hybridization of labeled DNA strands reverse transcribed from virion-extracted RNA and by PCR-assisted amplification of DNA strands reverse transcribed from virion RNA. The packaged RNA species identified by both methods represented a subset of viral transcripts detected in the population of poly(A)-selected RNA extracted from infected cells. Of this subset, only a small set comprising nine RNAs was reproducibly detected in all preparations tested. In one case, we were able to establish that the RNA was intact and encoded a full-length protein. Several aspects of the data require elaboration since they impinge on the overall conclusions of this report. First, the assays based on RT-PCR yielded unambiguous, i.e., positive or negative, results, but these were not fully reproducible from one preparation to another. Of the 27 RNAs detected at least once, only nine were positive in all preparations. The nine RNAs present in all virion preparations are likely to be a minimal set, defined by an abundance of specific RNAs in either the infected cells or in virions or to as-yet-undefined limitations of the RT-PCR. The cDNA arrays, on the other hand, did not yield discontinuous values. As is the case for all analyses of data derived from microarrays, we set a cutoff threshold based on the intensity of the signal. The 24 mRNAs defined as positive on the basis of this criterion included the seven mRNAs reproducibly detected in virions by RT-PCR. The remaining two were barely detected by hybridization of cDNA derived from total infected-cell DNA and therefore may reflect a defect in the cDNA array rather than an absence from virions. The necessary conclusion, however, is that by both procedures we identified only the most abundant RNAs associated with virions rather than the entire set of mRNAs that may be present. It remains to be determined how the abundance of the mRNAs detected in this study translates into average RNA molecules per virion.
(iii) We could not associate the presence of specific RNAs in the virion with the presence of the US11 protein shown earlier to bind RNA.
(iv) Cellular RNAs were also detected in RNA extracted from virions. In this instance, a superficial analysis of the cellular RNAs suggests that virions contain the more abundant species of cellular RNAs.
The key issues that arise from the data are the specificity and significance of the RNAs extracted from purified virions. Relevant to these issues are the following. (i) The presence of viral RNA in virions does not appear to be the result of simple contamination of virions with cellular debris containing RNA. There are three reasons for this conclusion. First, the major contaminating components of purified virions are membranous vesicles that cosediment in dextran-10 gradients. These vesicles arise in part during homogenization and would be expected to be more numerous in preparations of intracellular virus than in virus released into the extracellular medium. We have not found significant differences between the RNAs packaged in extracellular and intracellular virions. Moreover, the nature of the RNAs detected in the virion preparation argues against the hypothesis that the detected RNAs were derived from cosedimenting cellular vesicles. If membranous vesicles were the source of the RNA, we would expect to see a prevalence of RNAs bound to membranes. Only UL10 (gM), US4 (gG), and possibly UL34 mRNAs were reproducibly found in virions. The failure to find US6 (gD) RNA or, more significantly, the UL44 (gC) RNA expressed late in infection is inconsistent with the hypothesis that membranous vesicles are responsible for the presence of RNA in virion preparations. Second, the results are inconsistent with the hypothesis that the RNA detected in these preparations represents mRNA that is resistant to RNase digestion. Thus, in vitro translation of the RNAs extracted from virions resulted in the synthesis of high-molecular-weight proteins. Analyses of the in vitro translation products revealed the presence of full-length VP16, a finding inconsistent with the hypothesis that the detected RNAs represent incompletely digested RNAs. Lastly, even assuming that all RNAs are present in virions but that the majority are present at an abundance below the level of detection, the nine RNAs reproducibly detected in virion preparations by RT-PCR were not uniformly the most abundant RNAs present in infected cells at the moment of harvest as illustrated in Fig. 2. The firm conclusion is that while the species detected in virions may simply reflect their abundance above the limits of detection, they are not representative of the most abundant viral RNA species accumulating in infected cells.
(ii) The hypothesis that the RNAs represent a selection of RNAs present in infected cells during the process of viral maturation raises the question as to the basis of the selectivity. We can exclude the hypothesis that they represent a single kinetic class: of the nine RNAs reproducibly found in virion preparations by RT-PCR, one is an α-RNA (US1/1.5), one is a β-RNA (UL42), and the rest are γ-RNAs. We can also exclude high G+C content that could be responsible for the unusual secondary structures since the conserved RNAs are on the average 63 to 67 G+C mol%, and RNAs of much higher G+C content (e.g., UL26, UL43, α0, α4, ORF P, γ134.5, etc., in the range of 71 to 84 G+C mol%) were not reproducibly detected in virion preparations. If sequence or structure were the basis for the selectivity of the packaged RNAs, they have not been identified. To some extent, the sequence and structural basis for the selectivity of the packaged RNA is compromised by the evidence that a large number of cellular RNAs are also packaged, and in this instance, selectivity is less readily demonstrable.
Of the proteins packaged in the virion, US11 is known to bind RNA in a sequence- and conformation-specific fashion (9). Since the repertoire of RNAs packaged in virions of R7023 mutant lacking the genes US8 to US12 was similar to that of HSV-1(F), the results suggest that the packaging of RNAs in virions is not sequence specific and either does not involve US11 protein or involves additional RNA-binding proteins. More recent studies have identified two additional tegument proteins capable of binding RNAs. The specificity of RNAs bound by these proteins is presently under study (M. T. Sciortino and B. Roizman, unpublished data).
(iii) Another criterion for determining the specificity of packaging of the RNAs is functionality. We should point out that the conditions of virus infection and especially spread from cell to cell is vastly different in vivo than it is in cultured cells in vitro. A characteristic of in vivo infection is the establishment after the first round of replication of a microenvironment in which the infected cell releases, in addition to virus particles, a variety of induced cytokines, a large number of cellular products, damaged organelles, and other cell debris. All of these by-products of infection could be expected to influence the cells infected by the progeny of the first infected cell. It is conceivable that, to overcome activated host responses, the virus must bring into cells RNA capable of directing the synthesis of proteins before newly made viral RNA is capable of taking over this process. But, if this is the case, the role of the products of the packaged RNAs in such a process is not readily apparent. In the case of gene-expressing glycoproteins, it could be argued that the protein carried into the cell by the virion may not get to where the virus needs these proteins early in infection. In the case of UL34, it has been shown that this protein interacts with the intermediate chain of the cytoplasmic neuronal dynein (12). One unproven scenario is that newly synthesized UL34 protein could initiate the transport of the virus to the nucleus. A similar argument was made by Brensnahan and Shenk (1) to explain the presence of one RNA in CMV virions. Except for this one scenario, no other could presently be envisioned to account for the presence of the remaining viral RNAs found in purified virions.
(iv) The question as to where the RNAs are packaged into virions remains unresolved. Greijer et al. (4) reported that the RNA packaged in CMV virions was contained in capsids. However, these authors prepared capsids by extraction of virion preparations with Triton X-100 only and the purity of the capsids was not determined. In the experience of this laboratory, this procedure does not strip tegument proteins or even fully remove glycoproteins from HSV virions. To fully remove glycoproteins and tegument proteins, it is necessary to treat virions with nonionic detergents and desoxycholate (3). While we cannot exclude the possibility that RNA is packaged in capsids, the more likely site is the tegument.
Currently, two models for maturation and egress prevail. The single envelopment model predicts that capsid envelopment takes place only at the inner nuclear membrane and that the virus is then transported through the Golgi to the extracellular space. The double envelopment model predicts that virus enveloped at the nuclear membrane is de-enveloped in the cytoplasm and then re-enveloped at a cytoplasmic membrane (8). The packaging of RNAs into virions is compatible with both models, but the sources of the RNA would be different. Greijer et al. (4) also reported that whereas both cellular and viral RNAs were associated with intact capsids, only viral RNAs were detected in their capsid preparations. The authors concluded that the RNAs were packaged in capsids and virions in different compartments. The results however are marred by the observation that the amounts of RNA extracted from capsids were far lower than those extracted from virions.
In essence, there is little doubt that, consistent with other herpesviruses, the HSV-1 virions package RNAs. The central question is the role of these RNAs in the course of the infectious process. While Brensnahan and Shenk (1) found a plausible explanation for at least some of the packaged RNAs, Greiger et al. (4) concluded on the basis of less data that they have no function whatsoever. For heuristic reasons if none other, it is appropriate to assume that viruses do not encode vestigial genes or perform useless functions. Both the studies by Brensnahan and Shenk (1) and those reported here suggest a level of selectivity of incorporation of RNAs that must be explored further.
ACKNOWLEDGMENTS
These studies were aided by grants from the National Cancer Institute (CA87761, CA83939, CA71933, and CA78766), United States Public Health Service.
REFERENCES
- 1.Brensnahan W A, Shenk T. A subset of viral transcripts packaged within human cytomegalovirus particles. Science. 2000;288:2373–2376. doi: 10.1126/science.288.5475.2373. [DOI] [PubMed] [Google Scholar]
- 2.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 3.Gibson W, Roizman B. Proteins specified by herpes simplex virus. VIII. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10:1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greijer A E, Dekkers C A J, Middeldorp J M. Human cytomegalovirus virions differentially incorporate viral and host cell RNA during the assembly process. J Virol. 2000;74:9078–9082. doi: 10.1128/jvi.74.19.9078-9082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heine J W, Honess R W, Cassai E, Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974;14:640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longnecker R, Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986;58:583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prichard M N, Jairath S, Penfold M E T, St. Jeor S, Bohlman M C, Pari G S. Identification of persistent RNA-DNA hybrid structures within the origin of replication of human cytomegalovirus. J Virol. 1998;72:6997–7004. doi: 10.1128/jvi.72.9.6997-7004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roizman B, Knipe D M. The replication of Herpes simplex viruses. In: Knipe D M, Howley P M, Hirsch M S, Monath T P, Roizman B, editors. Fields virology. 4th ed. Philadelphia, Pa: Lippincott-Raven Press; 2001. pp. 2399–2459. [Google Scholar]
- 9.Roller R J, Roizman B. The herpes simplex virus US11 open reading frame encodes a sequence specific RNA binding protein. J Virol. 1990;64:3463–3470. doi: 10.1128/jvi.64.7.3463-3470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spear P, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stingley S W, Garcia Ramirez J J, Aguilar S A, Simmen K, Sandri-Goldin R M, Ghazal P, Wagner E K. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J Virol. 2000;74:9916–9927. doi: 10.1128/jvi.74.21.9916-9927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye G J, Vaughan K T, Vallee R B, Roizman B. The herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]